Ecofriendly Extraction of Polyphenols from Ampelopsis grossedentata Leaves Coupled with Response Surface Methodology and Artificial Neural Network–Genetic Algorithm
Abstract
1. Introduction
2. Results
2.1. Screening of Suitable DESs System for AGPL Extraction
2.1.1. The Optimal DESs System
2.1.2. Screening of Optimal Molar Ratio and Water Content
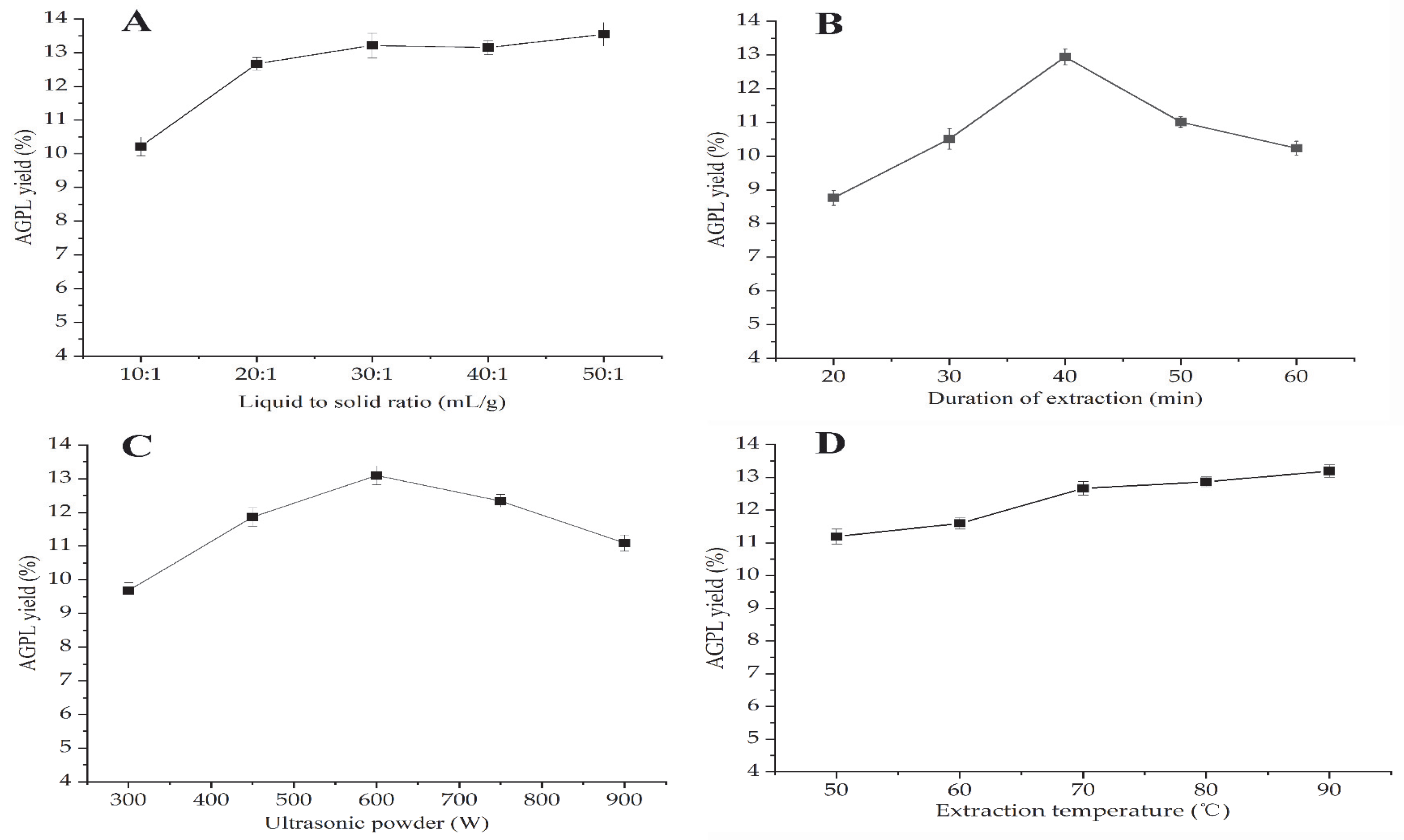
2.2. One-Factor-at-a-Time Evaluation
2.2.1. Screening of Liquid to Solid Ratio
2.2.2. Screening of Extraction Duration
2.2.3. Screening of Ultrasonic Power
2.2.4. Screening of Extraction Temperature
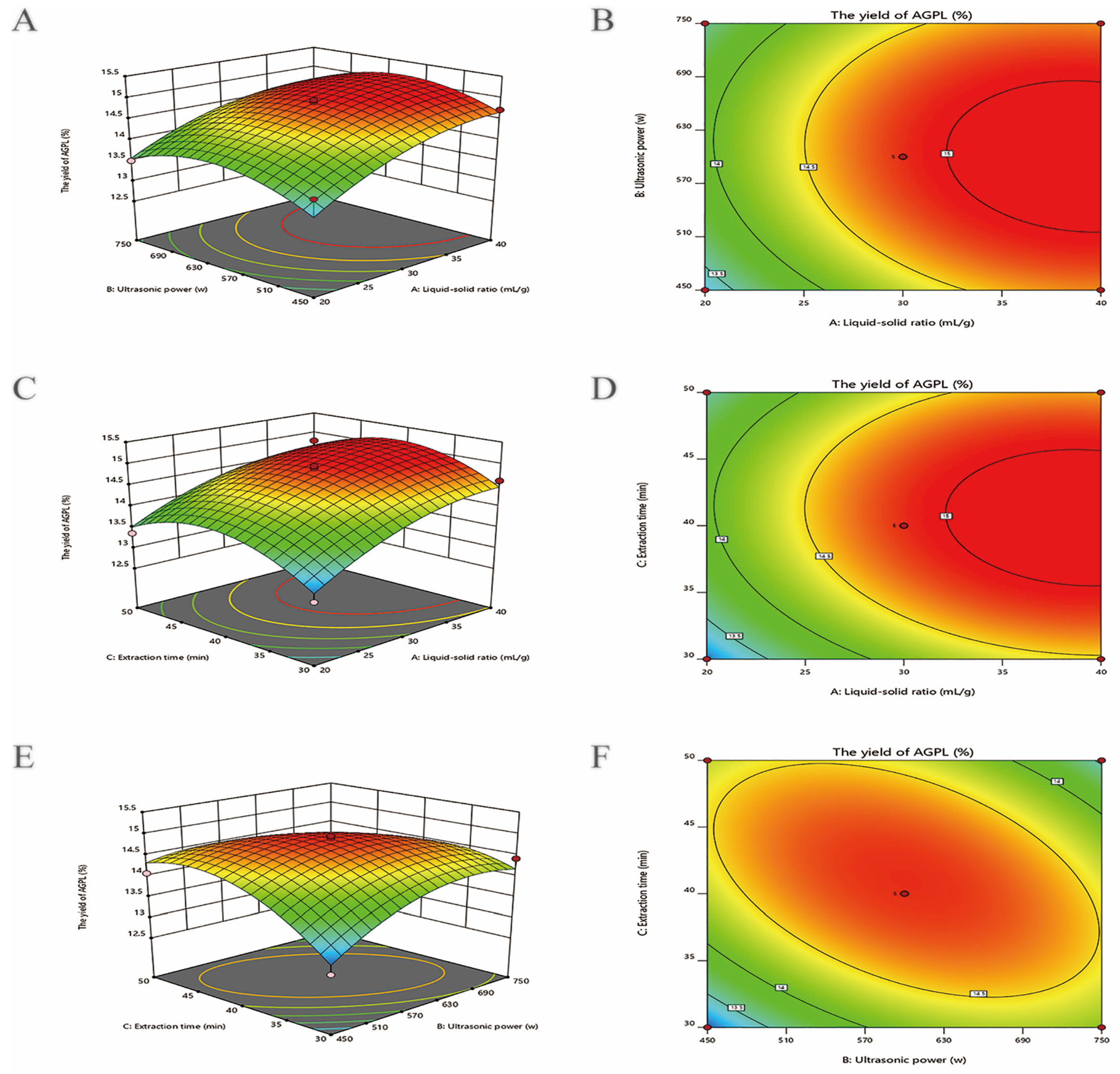
2.3. Optimization by RSM
2.3.1. BBD Analysis
2.3.2. Interactive Effects on AGPL Yield
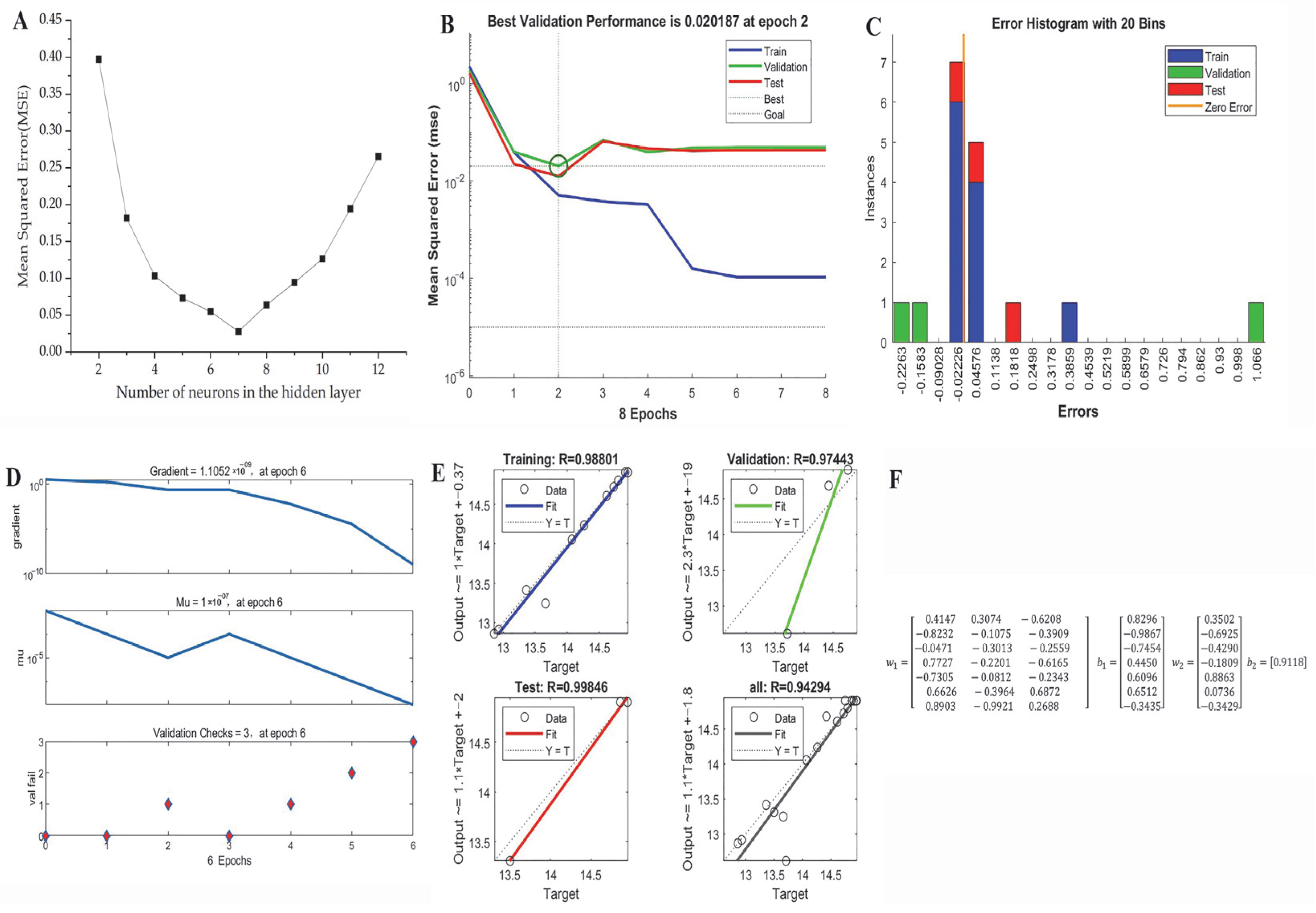
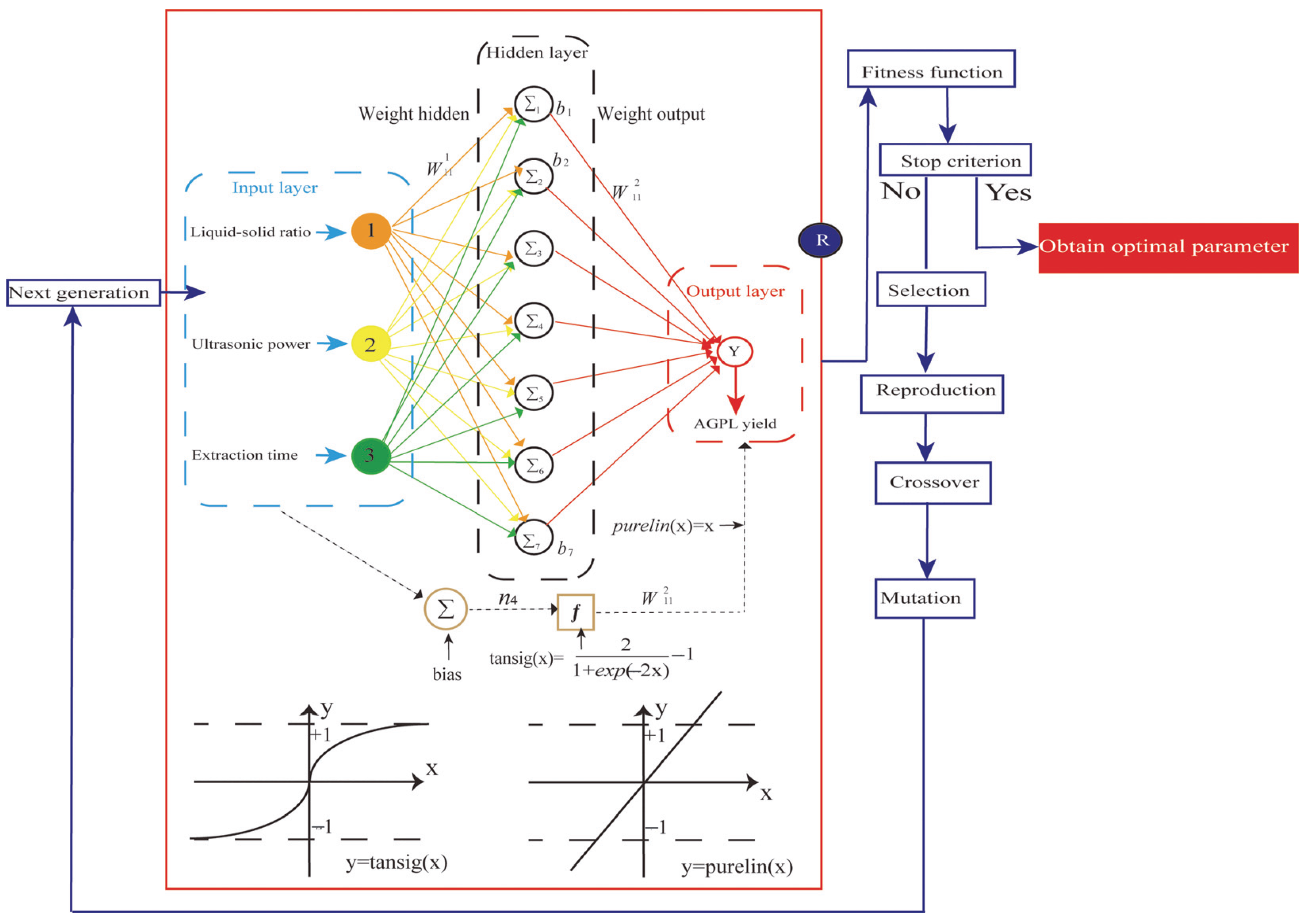
2.4. ANN Model Establishment
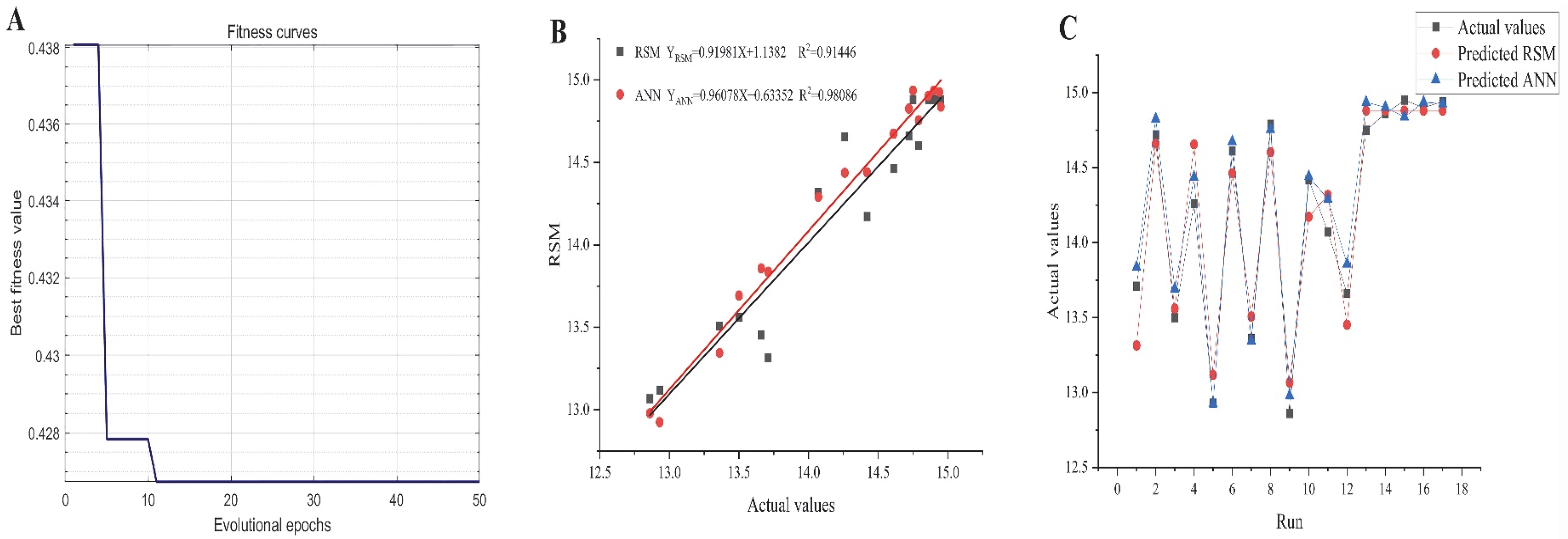
2.5. GA-Oriented Optimization
2.6. Comparative Analysis of RSM and ANN
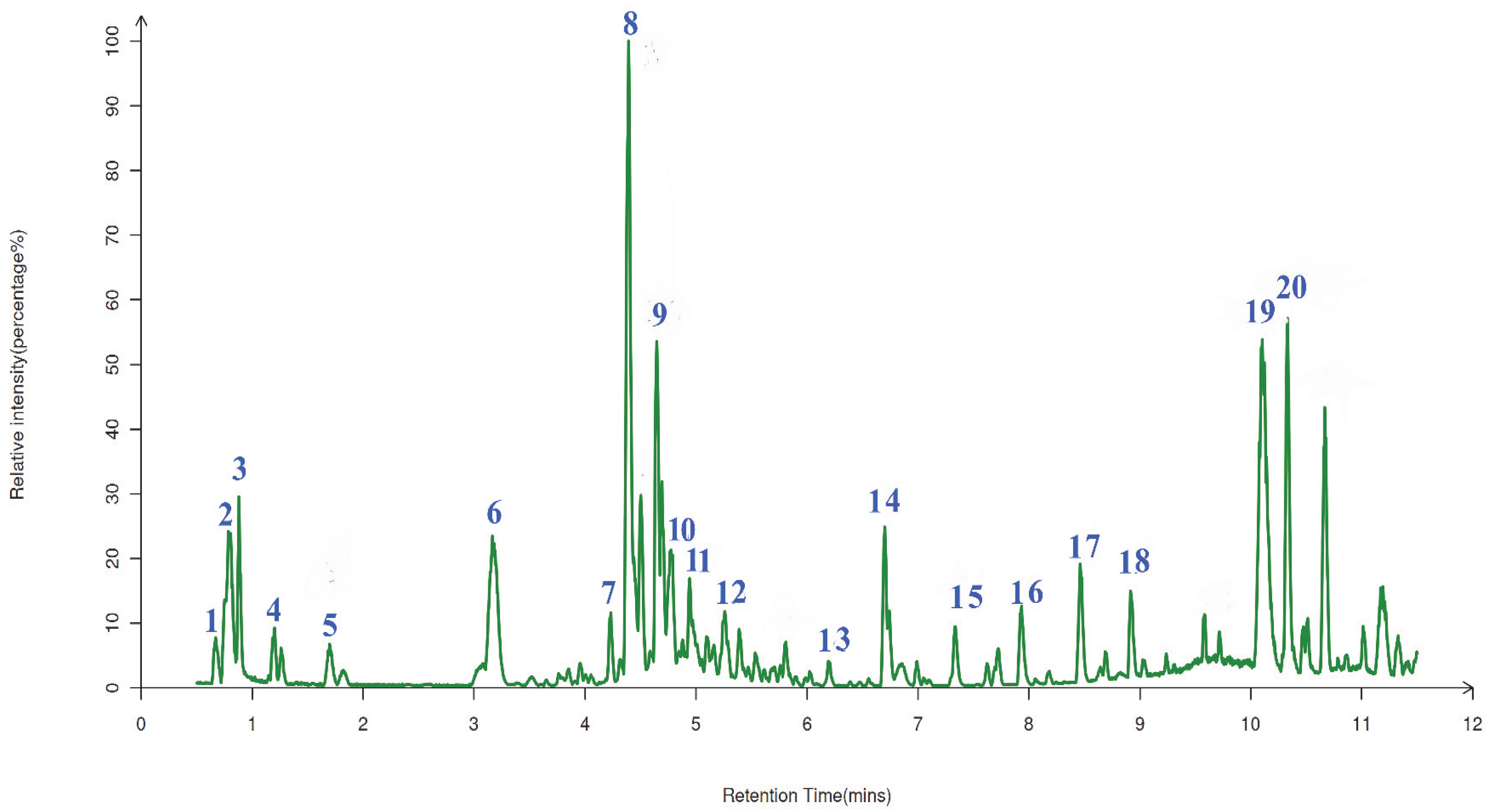
2.7. The Qualitative Analysis of UPLC-ESI-QTOF-MS
2.8. Antioxidant Activity Analysis of AGPL In Vitro
3. Materials and Methods
3.1. Materials and Reagents
3.2. Preparation of DESs Formula
3.3. DESs-Based Extraction and Contents Determination of AGPL
3.4. The Effects of Molar Ratio and Water Content on Polarity of DESs
3.5. RSM-Based Model of AGPL Extraction Process
3.6. ANN Model
3.7. Model Analysis
3.8. Optimization of the Extraction Process
3.9. Phenolic Composition Determination by UPLC/ESI-QTOF-MS/MS
3.10. In-Vitro ORAC Activities of AGPL
3.11. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, Y.Q.; Wang, T.T.; Wu, S.S.; Zhang, Z.H.; Zhang, Y.; Liu, Q.; Gou, Y.X.; Guan, H.N.; Wang, D.X.; Dong, R.X.; et al. Vine Tea (Ampelopsis grossedentata)—A Different Kind of Tea Hidden Deep in the Mountains of China: A Comprehensive Review of the Nutritional Profile, Functional Effect, and Diverse Applications as a Novel Raw Material in Food Practices. Trends Food Sci Tech. 2025, 159, 104939. [Google Scholar] [CrossRef]
- Chen, L.; Yin, S.X.; Dong, S.Q.; Xu, P.; Liu, Y.L.; Xiang, X.L.; Huang, Q.; Ye, L. A New Insight into the Key Matrix Components for Aftertaste in Ampelopsis grossedentata (Vine Tea) Infusion: From the Intensity and Duration of Taste Profiles Using Non-targeted Metabolomics and Molecular Simulation. Food Chem. 2024, 450, 139236. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wu, Y.C.; Zou, J.W.; Gao, K. α-Glucosidase Inhibition and Antihyperglycemic Activity of Flavonoids from Ampelopsis grossedentata and the Flavonoid Derivatives. Bioorg. Med. Chem. 2016, 24, 1488–1494. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, R.C.V.; Ye, L.Y.; Baek, N.; Teixeira, G.H.A.; O’Keefe, S.F. Vine Tea (Ampelopsis grossedentata): A Review of Chemical Composition, Functional Properties, and Potential Food Applications. J. Funct. Foods 2021, 76, 104317. [Google Scholar] [CrossRef]
- Jia, C.H.; Li, J.H.; Zhang, M.X.; Ma, W.B.; Zhao, S.M.; Liu, R.; Rong, J.H.; Li, X.H. Antioxidant Properties of the Extracts of Vine Tea (Ampelopsis grossedentata) with the Different Color Characteristics and Inhibition of Rapeseed and Sunflower Oil Oxidation. LWT 2020, 136, 110292. [Google Scholar] [CrossRef]
- Zeng, T.X.; Song, Y.J.; Qi, S.Y.; Zhang, R.Y.; Xu, L.J.; Xiao, P.G. A Comprehensive Review of Vine Tea: Origin, Research on Materia Medica, Phytochemistry and Pharmacology. J. Ethnopharmacol. 2023, 317, 116788. [Google Scholar] [CrossRef]
- Liu, C.Y.; Sun, Y.Y.; Wang, S.Q.; Jia, Y.Q.; Wang, H.X.; Pan, L.C.; Zhu, Z.Y. Dihydromyricetin from Ampelopsis grossedentata and Its Derivatives: Structural Characterization and Anti-hepatocellular Carcinoma Activity. J. Mol. Struct. 2022, 1258, 132677. [Google Scholar] [CrossRef]
- Saimaiti, A.; Huang, S.Y.; Xiong, R.G.; Wu, S.X.; Zhou, D.D.; Yang, Z.J.; Luo, M.; Gan, R.Y.; Li, H.B. Antioxidant Capacities and Polyphenol Contents of Kombucha Beverages Based on Vine Tea and Sweet Tea. Antioxidants 2022, 11, 1655. [Google Scholar] [CrossRef]
- Li, X.L.; Li, B.Y.; Meng, X.Y.; Yu, F.; Yu, X.; Zhao, W.Q.; Wang, Y.J.; Gao, H.Q.; Cheng, M.; Zhong, L.H. Integrating Network Pharmacology and Experimental Validation to Reveal the Mechanism of Vine Grape Tea Polyphenols on Hyperuricemia-Induced Renal Injury in Mice. J. Med. Food 2024, 27, 312–329. [Google Scholar] [CrossRef]
- Wang, L.L.; Zhou, Y.F.; Wang, Y.B.; Qin, Y.C.; Liu, B.T.; Bai, M.G. Two Green Approaches for Extraction of Dihydromyricetin from Chinese Vine Tea Using β-Cyclodextrin-based and Ionic Liquid-based Ultrasonic-assisted Extraction Methods. Food Bioprod. Process. 2019, 116, 1–9. [Google Scholar] [CrossRef]
- Makris, D.P.; Lalas, S. Glycerol and Glycerol-Based Deep Eutectic Mixtures as Emerging Green Solvents for Polyphenol Extraction: The Evidence So Far. Molecules 2020, 25, 5842. [Google Scholar] [CrossRef] [PubMed]
- Zannou, O.; Pashazadeh, H.; Ghellam, M.; Koca, I.; Kaddour, A.A.; Leriche, F. Appraisal of Phenolic Compounds, Antioxidant Activity and in Vitro Gastrointestinal Digestion of Borage (Echium amoenum) Flowers Using Natural Deep Eutectic Solvent (NADES). Biomass. Convers. Biorefin. 2024, 14, 8523–8535. [Google Scholar] [CrossRef]
- Serna-Vázquez, J.; Ahmad, M.Z.; Boczkaj, G.; Castro-Muñoz, R. Latest Insights on Novel Deep Eutectic Solvents (DES) for Sustainable Extraction of Phenolic Compounds from Natural Sources. Molecules 2021, 26, 5037. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.Y.; Ou, H.; Gregersen, H.; Zou, J. Deep Eutectic Solvent-based Ultrasound-assisted Extraction of Polyphenols from Cosmos sulphureus. JARMAP 2023, 32, 100444. [Google Scholar] [CrossRef]
- Al-Moubaraki, A.H.; Obot, I.B. Corrosion challenges in petroleum refinery operations: Sources, mechanisms, mitigation, and future outlook. J. Saudi Chem. Soc. 2021, 25, 101370. [Google Scholar] [CrossRef]
- Yao, C.; Hou, Y.; Ren, S.; Wu, W.; Zhang, K.; Ji, Y.; Liu, H. Efficient separation of phenol from model oils using environmentally benign quaternary ammonium- based zwitterions via forming deep eutectic solvents. Chem. Eng. J. 2017, 326, 620–626. [Google Scholar] [CrossRef]
- Wang, D.T.; Wang, Y.Y.; Ma, R.; Ma, J.R.; Xu, M.J.; Ai, L.L.; Leng, C.Y.; Ma, Q.T.; Jia, D.Z.; Wang, L.X.; et al. Efficient and green separation of phenolics by halogen free deep eutectic solvents. J. Mol. Liq. 2025, 417, 126602. [Google Scholar] [CrossRef]
- Champati, B.B.; Padhiari, B.M.; Ray, A.; Halder, T.; Jena, S.; Sahoo, A.; Kar, B.; Kamila, P.K.; Panda, P.C.; Ghosh, B.; et al. Application of a Multilayer Perceptron Artificial Neural Network for the Prediction and Optimization of the Andrographolide Content in Andrographis paniculata. Molecules 2022, 27, 2765. [Google Scholar] [CrossRef]
- Alrugaibah, M.; Yagiz, Y.; Gu, L.W. Use Natural Deep Eutectic Solvents as Efficient Green Reagents to Extract Procyanidins and Anthocyanins from Cranberry Pomace and Predictive Modeling by RSM and Artificial Neural Networking. Sep. Purif. Technol. 2021, 255, 117720. [Google Scholar] [CrossRef]
- Yu, H.C.; Huang, S.M.; Lin, W.M.; Kuo, C.H.; Shieh, C.J. Comparison of Artificial Neural Networks and Response Surface Methodology towards an Efficient Ultrasound-Assisted Extraction of Chlorogenic Acid from Lonicera Japonica. Molecules 2019, 24, 2304. [Google Scholar] [CrossRef]
- Li, C.Q.; Cui, Y.P.; Lu, J.; Liu, C.Y.; Chen, S.T.; Ma, C.Y.; Liu, Z.H.; Wang, J.M.; Kang, W.Y. Ionic Liquid-Based Ultrasonic-Assisted Extraction Coupled with HPLC and Artificial Neural Network Analysis for Ganoderma lucidum. Molecules 2020, 25, 1309. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Brewer, D.; Quintana, S.E.; García-Zapateiro, L.A. Modeling and Optimization of Microwave-assisted Extraction of Total Phenolics Content from Mango (Mangifera indica) Peel Using Response Surface Methodology (RSM) and Artificial Neural Networks (ANN). Food Chem. X 2024, 22, 101420. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.Y.; Su, J.Q.; Chu, X.L.; Wang, X.Y. A Green Method of Extracting and Recovering Flavonoids from Acanthopanax senticosus Using Deep Eutectic Solvents. Molecules 2022, 27, 923. [Google Scholar] [CrossRef]
- Dai, Y.T.; Witkamp, G.J.; Verpoorte, R.; Choi, Y.H. Tailoring Properties of Natural Deep Eutectic Solvents with Water to Facilitate Their Applications. Food Chem. 2015, 187, 14–19. [Google Scholar] [CrossRef]
- Liu, Y.; Friesen, J.B.; Mcalpine, J.B.; Lankin, D.C.; Chen, S.N.; Pauli, G.F. Natural Deep Eutectic Solvents: Properties, Applications, and Perspectives. J. Nat. Prod. 2018, 81, 679–690. [Google Scholar] [CrossRef] [PubMed]
- Martins, M.A.R.; Pinho, S.P.; Coutinho, J.A.P. Insights into the Nature of Eutectic and Deep Eutectic Mixtures. J. Solut. Chem. 2019, 48, 962–982. [Google Scholar] [CrossRef]
- Li, Z.; Lee, P.I. Investigation on Drug Solubility Enhancement Using Deep Eutectic Solvents and Their Derivatives. Int. J. Pharm. 2016, 505, 283–288. [Google Scholar] [CrossRef]
- Zdanowicz, M.; Wilpiszewska, K.; Spychaj, T. Deep Eutectic Solvents for Polysaccharides Processing: A review. Carbohydr. Polym. 2018, 200, 361–380. [Google Scholar] [CrossRef]
- Zhang, L.J.; Wang, M.S. Optimization of Deep Eutectic Solvent-based Ultrasound-assisted Extraction of Polysaccharides from Dioscorea opposite Thunb. Int. J. Biol. Macromol. 2017, 95, 675–681. [Google Scholar] [CrossRef]
- Montes, L.; Gisbert, M.; Hinojosa, I.; Sineiro, J.; Moreira, R. Impact of Drying on the Sodium Alginate Obtained after Polyphenols Ultrasound-assisted Extraction from Ascophyllum nodosum Seaweeds. Carbohydr. Polym. 2021, 272, 118455. [Google Scholar] [CrossRef]
- Bashir, I.; Dar, A.H.; Dash, K.K.; Pandey, V.K.; Fayaz, U.; Shams, R.; Srivastava, S.; Singh, R. Deep Eutectic Solvents for Extraction of Functional Components from Plant-based Products: A Promising Approach. Sustain. Chem. Pharm. 2023, 33, 101102. [Google Scholar] [CrossRef]
- Vilkhu, K.; Mawson, R.; Simons, L.; Bates, D. Applications and Opportunities for Ultrasound Assisted Extraction in the Food Industry—A Review. IFSET 2008, 9, 161–169. [Google Scholar] [CrossRef]
- Marianne, L.C.; Lucía, A.G.; de Jesús, M.S.M.; Leonardo, H.M.E.; Mendoza-Sánchez, M. Optimization of the Green Extraction Process of Antioxidants Derived from Grape Pomace. Sustain. Chem. Pharm. 2024, 37, 101396. [Google Scholar] [CrossRef]
- Chemat, F.; Huma, Z.; Khan, M.K. Applications of Ultrasound in Food Technology: Processing, Preservation and Extraction. Ultrason. Sonochem. 2011, 18, 813–835. [Google Scholar] [CrossRef]
- Pätzold, M.; Siebenhaller, S.; Kara, S.; Liese, A.; Syldatk, C.; Holtmann, D. Deep Eutectic Solvents as Efficient Solvents in Biocatalysis. Trends Biotechnol. 2019, 37, 943–959. [Google Scholar] [CrossRef]
- Tang, Z.H.; Xu, Y.; Cai, C.Y.; Tan, Z.J. Extraction of Lycium barbarum Polysaccharides Using Temperature-switchable Deep Eutectic Solvents: A Sustainable Methodology for Recycling and Reuse of the Extractant. J. Mol. Liq. 2023, 383, 122063. [Google Scholar] [CrossRef]
- Suleiman, J.; Shamsudin, R.; Hamzah, M.H.; Basri, M.S.M.; Jimoh, K.A. Extraction Optimization and Characterization of Durian (Durio zibethinus) Rind Pectin Extracted by Subcritical Water. Food Chem. 2025, 474, 143123. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Sun, X.Q.; Fang, L.T.; Jiang, X.X.; Zhang, X.N.; Ge, Z.J.; Wang, R.B.; Wang, C.Q. Optimization of Ultrasound-Assisted Extraction of Polyphenols from Ilex latifolia Using Response Surface Methodology and Evaluation of Their Antioxidant Activity. Molecules 2022, 27, 3999. [Google Scholar] [CrossRef]
- Jiao, P.F.; He, X.M.; Ma, S.H.; Wang, S.P.; Niu, Q.H. Ultrasonic-Assisted Extraction of Antioxidants from Perilla frutescens Leaves Based on Tailor-Made Deep Eutectic Solvents: Optimization and Antioxidant Activity. Molecules 2023, 28, 7554. [Google Scholar] [CrossRef]
- Beeler, N.; Hühn, T.; Rohn, S.; Colombi, R. Development and Optimization of a Cocoa Extraction Treatment by Means of the Response Surface Methodology (RSM) and Artificial Neural Network (ANN). Ind. Crop. Prod. 2024, 222, 119610. [Google Scholar] [CrossRef]
- Gammoudi, N.; Mabrouk, M.; Bouhemda, T.; Nagaz, K.; Ferchichi, A. Modeling and Optimization of Capsaicin Extraction from Capsicum annuum L. Using Response Surface Methodology (RSM), Artificial Neural Network (ANN), and Simulink Simulation. Ind. Crop. Prod. 2021, 171, 113869. [Google Scholar] [CrossRef]
- Xue, H.K.; Xu, J.Q.; Zhang, J.L.; Wei, Y.N.; Cai, X.; Tan, J.Q. Modeling, Optimization, Purification, and Characterization of Polysaccharide from Lilium lancifolium Thunb. LWT 2022, 162, 113491. [Google Scholar] [CrossRef]
- Wu, J.K.; Yang, F.; Guo, L.Y.; Sheng, Z.L. Modeling and Optimization of Ellagic Acid from Chebulae Fructus Using Response Surface Methodology Coupled with Artificial Neural Network. Molecules 2024, 29, 3953. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Feng, F.; Jiang, J.; Qiao, Y.; Wu, T.; Voglmeir, J.; Chen, Z. Green and efficient extraction of rutin from tartary buckwheat hull by using natural deep eutectic solvents. Food Chem. 2017, 221, 1400–1405. [Google Scholar] [CrossRef]
- Chen, H.Y.; Wang, B.; Li, J.P.; Xu, J.; Zeng, J.S.; Gao, W.H.; Chen, K.F. Comparative Study on the Extraction Efficiency, Characterization, and Bioactivities of Bletilla striata Polysaccharides Using Response Surface Methodology (RSM) and Genetic Algorithm-artificial Neural Network (GA-ANN). Int. J. Biol. Macromol. 2022, 226, 982–995. [Google Scholar] [CrossRef]
- Subramani, V.; Tomer, V.; Balamurali, G.; Mansingh, P. Artificial Neural Network in Optimization of Bioactive Compound Extraction: Recent Trends and Performance Comparison with Response Surface Methodology. Anal. Sci. 2025, 41, 101–117. [Google Scholar] [CrossRef]
- Xue, H.K.; Wang, W.L.; Wu, J.P.; Xie, K.F.; Ge, S.Q.; Tan, J.Q. Ultrasound Assisted Aqueous Two-phase Extraction of Polysaccharides from Corn Stigma: Process Optimization, Structure Characterization, and Immunomodulatory Activity. IFSET 2024, 91, 103531. [Google Scholar] [CrossRef]
- Wang, X.Y.; Liu, X.Y.; Shi, N.W.; Zhang, Z.Y.; Chen, Y.M.; Yan, M.Y.; Li, Y.P. Response Surface Methodology Optimization and HPLC-ESI-QTOF-MS/MS Analysis on Ultrasonic-assisted Extraction of Phenolic Compounds from Okra (Abelmoschus esculentus) and Their Antioxidant Activity. Food Chem. 2023, 405, 134966. [Google Scholar] [CrossRef]
- Cao, G.H.; Alessio, H.M.; Cutler, R.G. Oxygen-radical Absorbance Capacity Assay for Antioxidants. Free Radic. Biol. Med. 1993, 14, 303–311. [Google Scholar] [CrossRef]


| No. | X1, Liquid to Solid Ratio (mL/g) | X2, Ultrasonic Power (W) | X3, Duration of Extraction (min) | Y, AGPL Yield (%) |
|---|---|---|---|---|
| 1 | 20 | 600 | 50 | 13.36 |
| 2 | 30 | 600 | 40 | 14.94 |
| 3 | 30 | 600 | 40 | 14.86 |
| 4 | 30 | 600 | 40 | 14.75 |
| 5 | 20 | 600 | 30 | 12.93 |
| 6 | 30 | 750 | 30 | 14.42 |
| 7 | 40 | 600 | 30 | 14.61 |
| 8 | 20 | 450 | 40 | 13.71 |
| 9 | 40 | 750 | 40 | 14.26 |
| 10 | 30 | 450 | 50 | 14.07 |
| 11 | 30 | 450 | 30 | 12.86 |
| 12 | 40 | 450 | 40 | 14.72 |
| 13 | 30 | 600 | 40 | 14.95 |
| 14 | 40 | 600 | 50 | 14.79 |
| 15 | 20 | 750 | 40 | 13.50 |
| 16 | 30 | 750 | 50 | 13.66 |
| 17 | 30 | 600 | 40 | 14.90 |
| Source | Sum of Squares | Df | Mean Square | F-Value | p-Value | |
|---|---|---|---|---|---|---|
| Model | 7.66 | 9 | 0.8512 | 8.92 | 0.0043 | ** |
| X1-Liquid solid ratio | 2.98 | 1 | 2.98 | 31.20 | 0.0018 | ** |
| X2-Ultrasonic power | 0.4095 | 1 | 0.4095 | 23.66 | 0.0008 | ** |
| X3-Extraction time | 0.1405 | 1 | 0.1405 | 1.47 | 0.2644 | |
| X1X2 | 0.2256 | 1 | 0.2256 | 13.03 | 0.0086 | ** |
| X1X3 | 0.0156 | 1 | 0.0156 | 0.1638 | 0.0153 | * |
| X2X3 | 0.9702 | 1 | 0.9702 | 10.17 | 0.6978 | |
| X12 | 0.4620 | 1 | 0.4620 | 4.84 | 0.0637 | |
| X22 | 1.06 | 1 | 1.06 | 11.09 | 0.0126 | * |
| X32 | 1.65 | 1 | 1.65 | 17.31 | 0.0042 | ** |
| Residual | 0.6679 | 7 | 0.0954 | |||
| Lack of Fit | 0.0950 | 3 | 0.0317 | 4.83 | 0.082 | |
| Pure Error | 0.0262 | 4 | 0.0065 | |||
| Cor Total | 8.33 | 16 |
| Model | Process Parameter | AGPL Yield(%) | Coefficient | ||||||
|---|---|---|---|---|---|---|---|---|---|
| X1 | X2 | X3 | Actual | Predicted | R2 | MSE | RMSE | AAD (%) | |
| RSM | 39.170 | 595.054 | 40.736 | 14.83 | 14.9049 | 0.9145 | 0.0393 | 0.1982 | 0.1641 |
| ANN | 35.492 | 696.796 | 46.124 | 15.32 | 15.2822 | 0.9809 | 0.0279 | 0.1669 | 0.1336 |
| Compound No. | RT (min) | Formula | Molecular Weight (m/z) | MS2 Fragment Ions (m/z) | Percentage (%) | Name |
|---|---|---|---|---|---|---|
| Flavonols | ||||||
| 1 | 0.652 | C15H14O8 | 305.0542 | 259.06; 231.07; 153.02; 149.02; 123.04 | 1.71% | Leucodelphinidin |
| 2 | 0.784 | C15H14O7 | 306.0674 | 259.06; 231.07; 153.02; 123.04 | 6.74% | Leucocyanidin |
| 3 | 0.913 | C15H12O6 | 289.0706 | 243.07; 215.07; 153.02; 107.05 | 4.29% | Aromadendrin |
| 5 | 1.683 | C15H10O7 | 303.0491 | 107.182; 121.173; 151.072; 179.058 | 2.02% | Quercetin |
| 6 | 3.116 | C15H10O6 | 287.0523 | 269.23; 257.23; 187.15 | 7.80% | Fisetin |
| 7 | 4.189 | C15H12O7 | 305.1982 | 259.06; 231.07; 153.02; 149.02 | 3.10% | Taxifolin |
| 8 | 4.232 | C15H14O7 | 307.0832 | 197.25; 169.11; 139.04 | 10.98% | (−)-Epigallocatechin |
| 9 | 4.623 | C15H14O6 | 291.0844 | 197.25; 167.12; 139.04 | 7.99% | Epicatechin |
| 12 | 5.176 | C16H12O6 | 299.058 | 207.48; 165.10; 134.09 | 2.51% | Kaempferide |
| 19 | 10.075 | C15H12O8 | 319.04 | 193.01; 163.14; 125.02 | 10.37% | Dihydromyricetin |
| 20 | 10.114 | C21H20O12 | 463.0909 | 317.03; 316.02 | 9.22% | Myricitrin |
| Flavone glycosides | ||||||
| 4 | 1.251 | C17H22O10 | 369.1184 | 151.06; 133.05; 110.04; 101.03 | 1.96% | 4-O-beta-D-Glucosyl-sinapate |
| 10 | 4.722 | C27H30O16 | 609.1377 | 528.21; 301.04; 179.00 | 6.11% | Quercetin 3-O-rhamnoside 7-O-glucoside |
| 16 | 7.930 | C21H20O10 | 431.0996 | 399.22299.21; 153.00; 99.01 | 2.87% | Apigenin 7-O-beta-D-glucoside |
| 17 | 8.418 | C21H20O12 | 463.0815 | 300.03; 271.02; 255.03 | 2.97% | Quercetin 3-O-glucoside |
| Acids | ||||||
| 11 | 4.920 | C18H30O3 | 294.4297 | 277.22; 197.11; 151.11; 107.09 | 5.38% | Colneleic acid |
| 13 | 6.088 | C20H32O2 | 303.2413 | 258.23; 198.14; 59.01 | 1.92% | Arachidonic acid |
| 14 | 6.674 | C19H38O2 | 297.2509 | 279.06; 249.24; 189.03 | 5.31% | Nonadecanoic acid |
| 15 | 7.231 | C20H28O2 | 300.2644 | 282.12; 251.03; 170.10 | 2.28% | all-trans-Retinoic acid |
| 18 | 8.901 | C16H18O9 | 353.0882 | 191.06; 179.04; 173.05; 113.21 | 5.33% | Neochlorogenic acid |
| NO. | HBA | HBD | Molar Ratio | Water Content | Abbreviation |
|---|---|---|---|---|---|
| DES-1 | choline chloride | 1,2-propylene glycol | 1:2 | 10% | Chcl-Pro |
| DES-2 | choline chloride | malonic acid | 1:2 | 10% | Chcl-Ma |
| DES-3 | choline chloride | urea | 1:2 | 10% | Chcl-Ur |
| DES-4 | choline chloride | 1,4-butanediol | 1:2 | 10% | Chcl-Bu4 |
| DES-5 | choline chloride | 1,3-butanediol | 1:2 | 10% | Chcl-Bu3 |
| DES-6 | choline chloride | ethylene glycol | 1:2 | 10% | Chcl-Eg |
| DES-7 | L-carnitine | 1,3-butanediol | 1:2 | 10% | Carn-Bu3 |
| DES-8 | L-carnitine | urea | 1:2 | 10% | Carn-Ur |
| DES-9 | L-carnitine | 1,4-butanediol | 1:2 | 10% | Carn-Bu4 |
| DES-10 | L-carnitine | 1,2-propylene glycol | 1:2 | 10% | Carn-Pro |
| DES-11 | L-carnitine | ethylene glycol | 1:2 | 10% | Carn-Eg |
| DES-12 | L-carnitine | malonic acid | 1:2 | 10% | Carn-Ma |
| Variable | Units | Coded Levels | |||
|---|---|---|---|---|---|
| Symbol | −1 | 0 | 1 | ||
| Liquid–solid ratio | mL/g | X1 | 20 | 30 | 40 |
| Ultrasonic power | W | X2 | 450 | 600 | 750 |
| Extraction time | min | X3 | 30 | 40 | 50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, X.; Li, C.; Wang, Y.; Jiang, J.; Wu, W.; Wang, S.; Lin, M.; He, L. Ecofriendly Extraction of Polyphenols from Ampelopsis grossedentata Leaves Coupled with Response Surface Methodology and Artificial Neural Network–Genetic Algorithm. Molecules 2025, 30, 2354. https://doi.org/10.3390/molecules30112354
Huang X, Li C, Wang Y, Jiang J, Wu W, Wang S, Lin M, He L. Ecofriendly Extraction of Polyphenols from Ampelopsis grossedentata Leaves Coupled with Response Surface Methodology and Artificial Neural Network–Genetic Algorithm. Molecules. 2025; 30(11):2354. https://doi.org/10.3390/molecules30112354
Chicago/Turabian StyleHuang, Xubo, Chen Li, Yanbin Wang, Jinrong Jiang, Weizhi Wu, Shifeng Wang, Ming Lin, and Liang He. 2025. "Ecofriendly Extraction of Polyphenols from Ampelopsis grossedentata Leaves Coupled with Response Surface Methodology and Artificial Neural Network–Genetic Algorithm" Molecules 30, no. 11: 2354. https://doi.org/10.3390/molecules30112354
APA StyleHuang, X., Li, C., Wang, Y., Jiang, J., Wu, W., Wang, S., Lin, M., & He, L. (2025). Ecofriendly Extraction of Polyphenols from Ampelopsis grossedentata Leaves Coupled with Response Surface Methodology and Artificial Neural Network–Genetic Algorithm. Molecules, 30(11), 2354. https://doi.org/10.3390/molecules30112354







