AS-IV Attenuates Oxidative Stress-Induced Apoptosis in Zebrafish via Modulation of the AKT/NRF2/HO-1/Caspase-3 Signaling Axis
Abstract
1. Introduction
2. Results
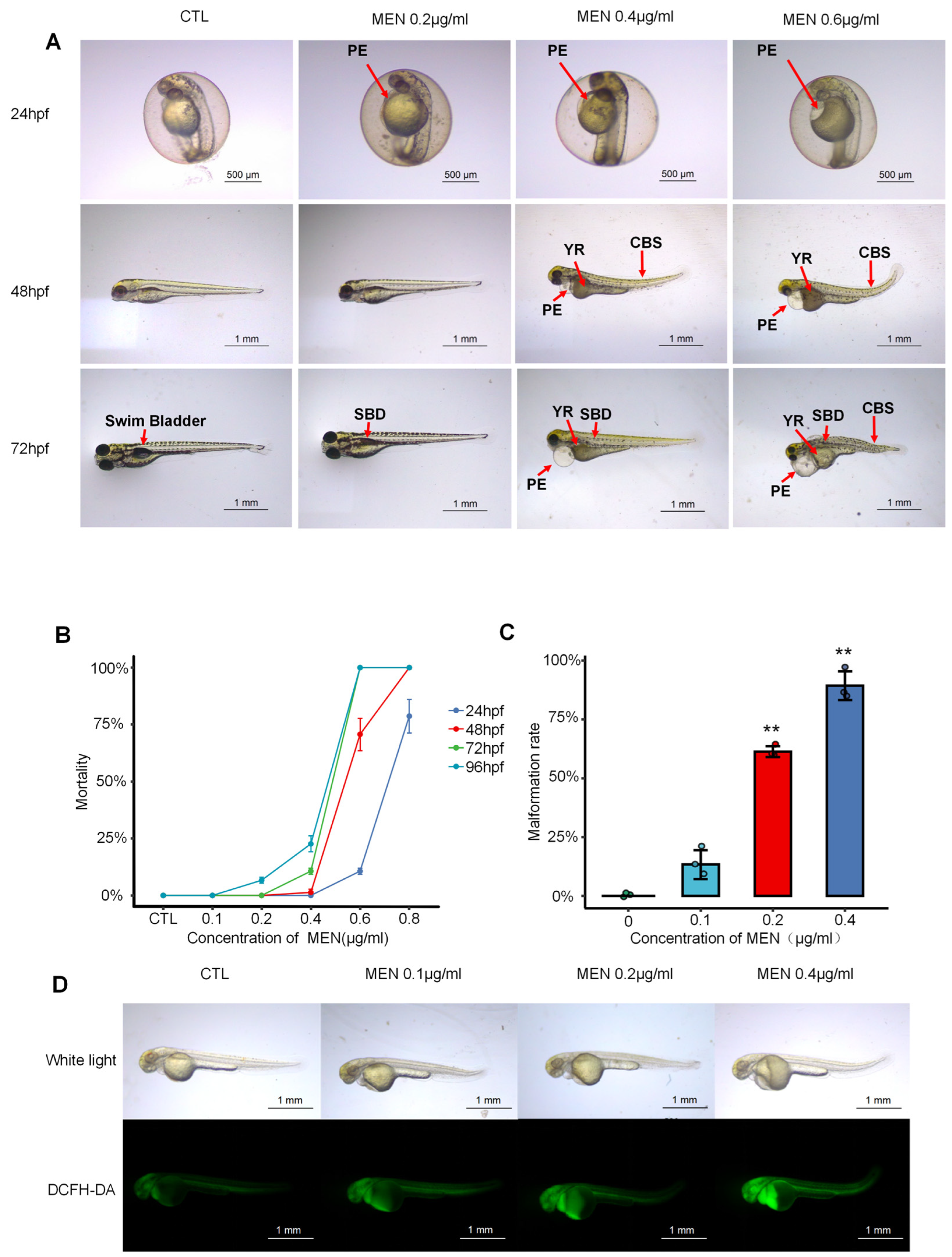
2.1. Menaquinone Triggered Oxidative Stress and Developmental Abnormalities in Zebrafish Embryos and Larvae
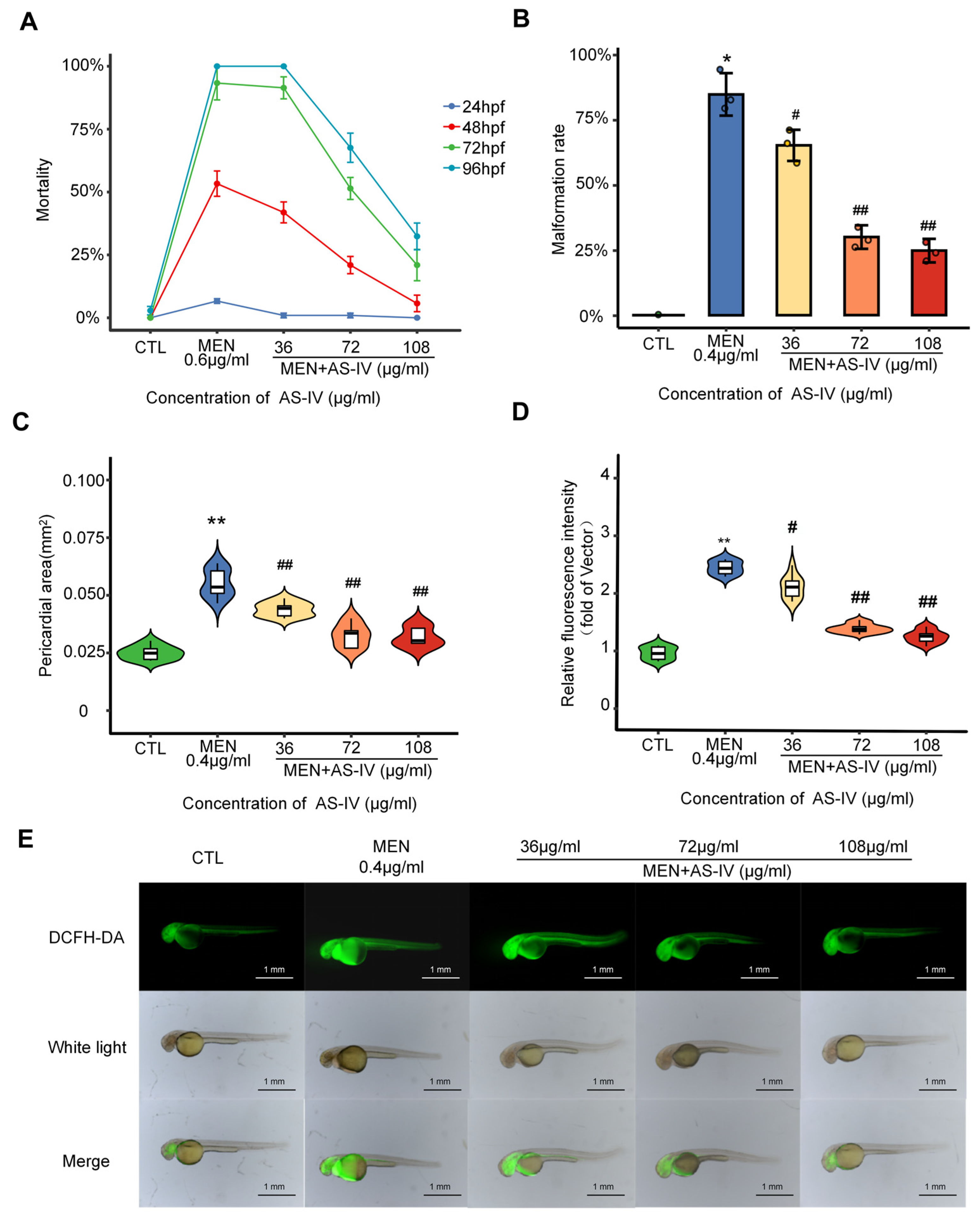
2.2. Antioxidant Properties of Astragaloside IV
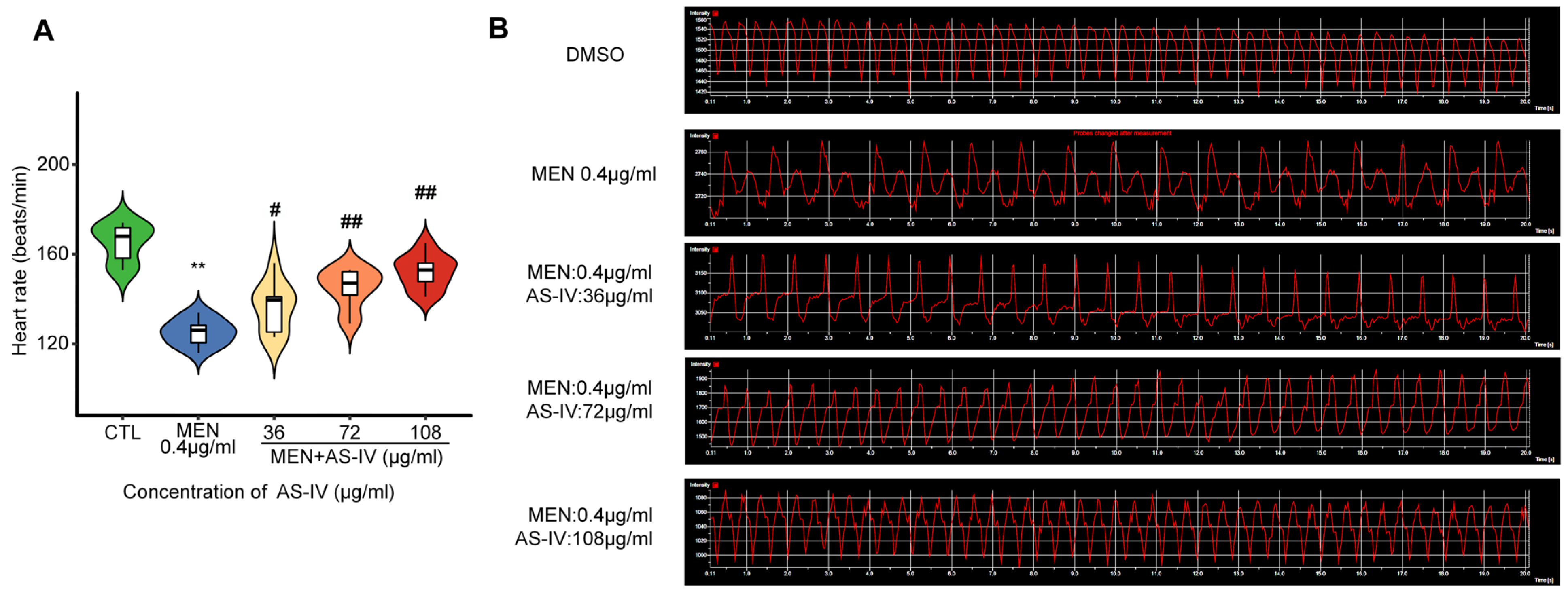
2.3. Astragaloside IV Suppresses Toxicity in Developing Hearts
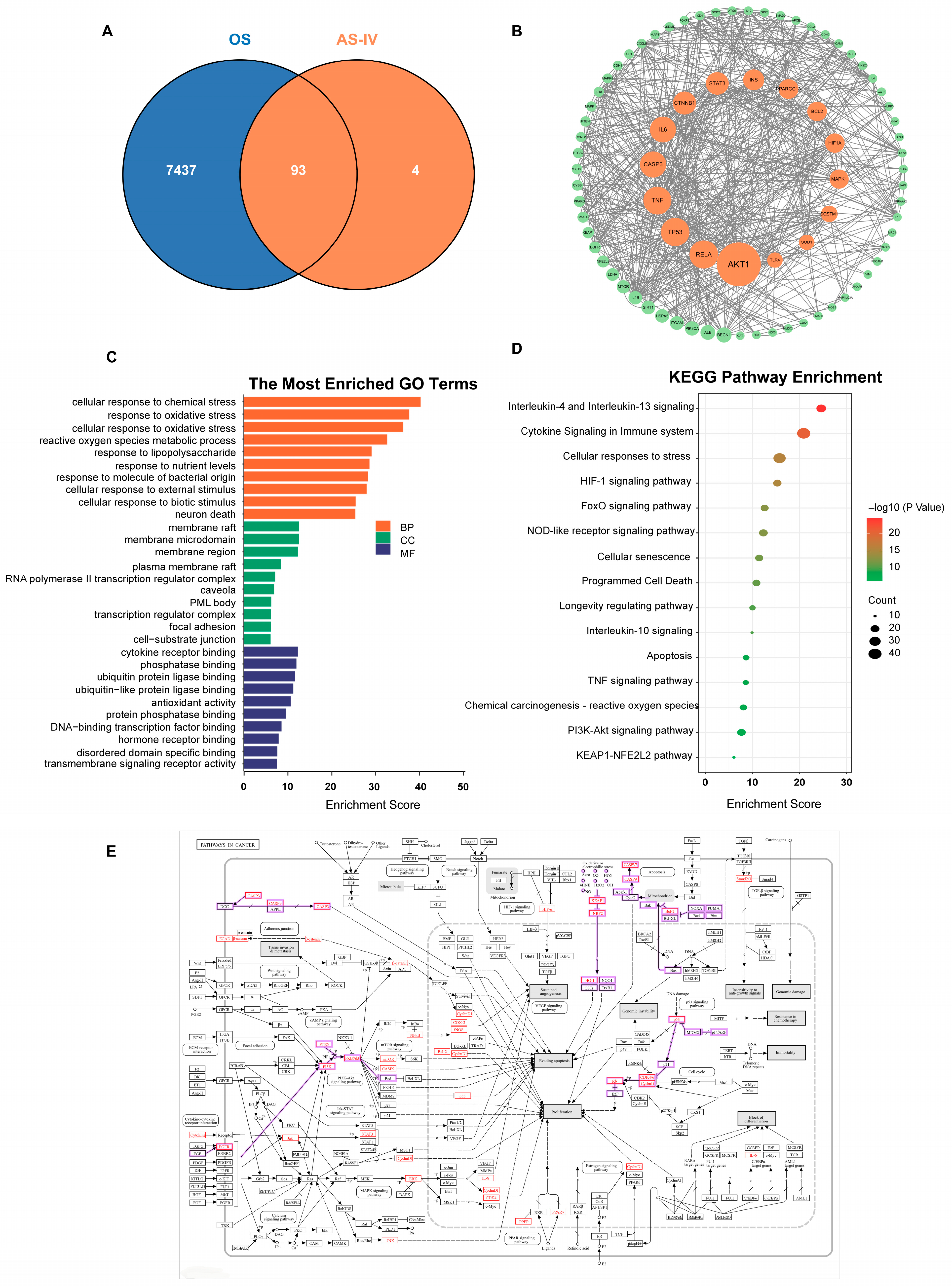
2.4. Network Pharmacology Analysis of the Antioxidant Targets of Astragaloside IV
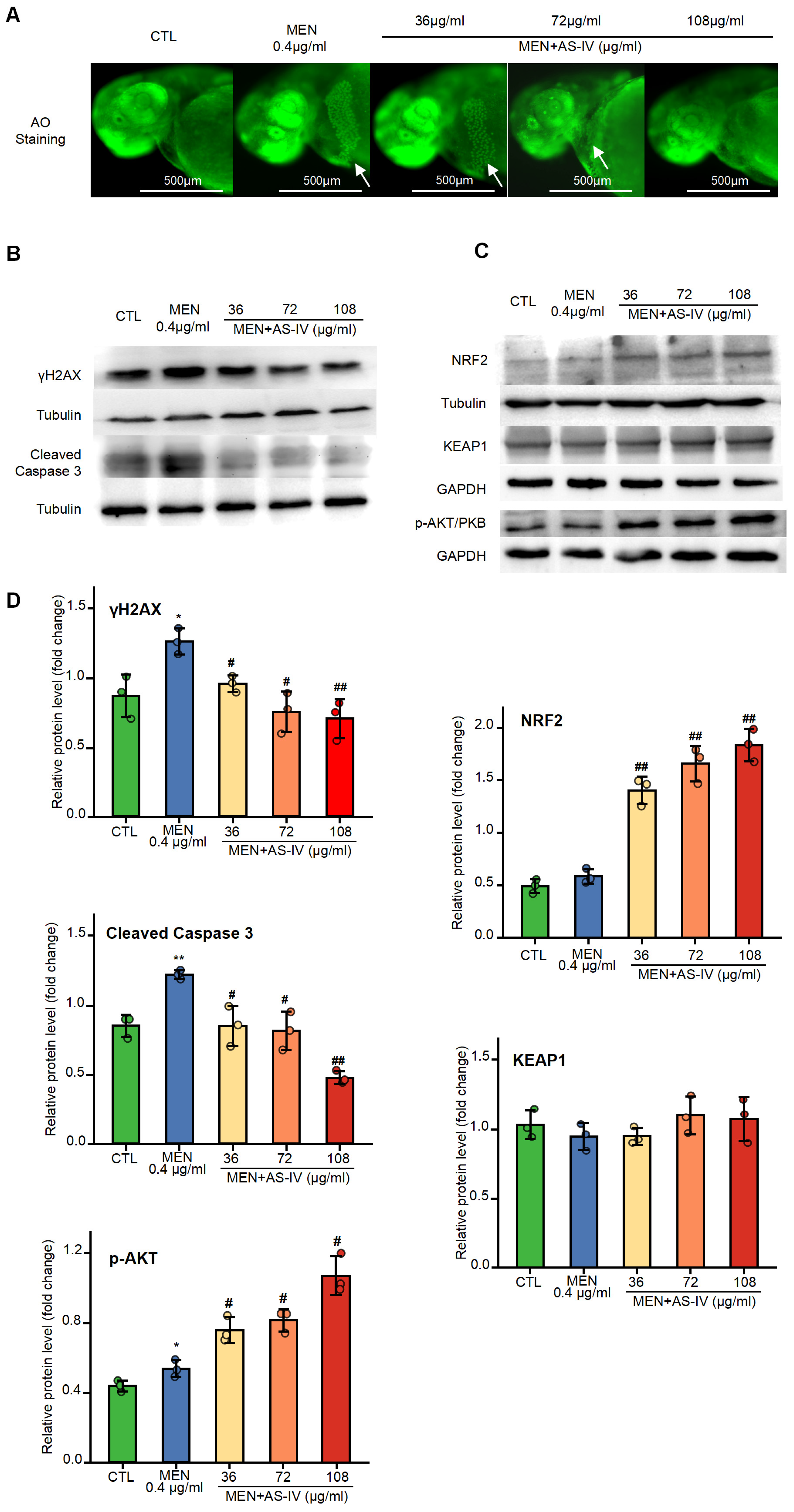
2.5. The Impact of AS-IV on Apoptosis and DNA Damage Triggered by Oxidative Stress
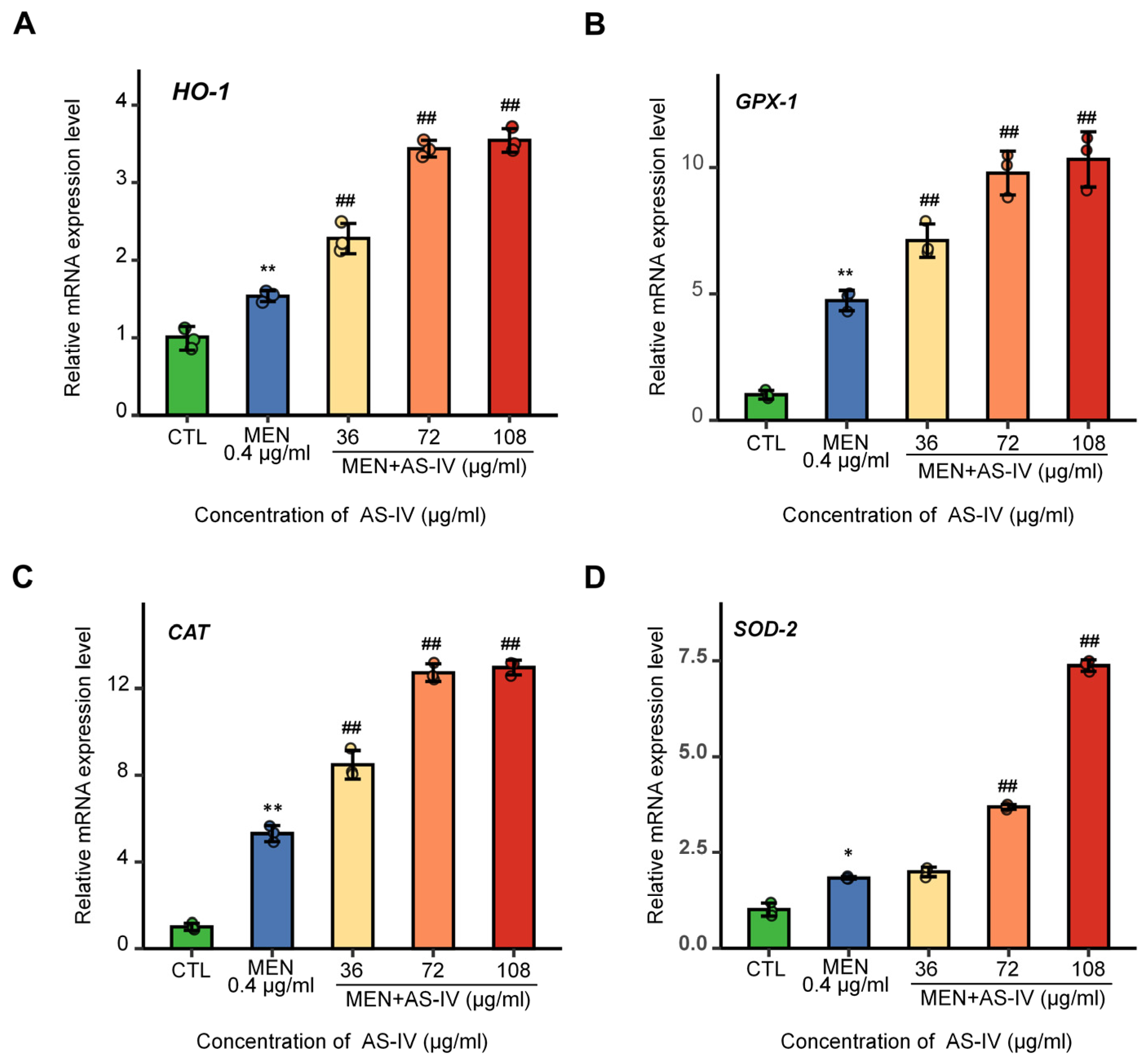
2.6. The Effects of AS-IV on Antioxidant Gene Expression
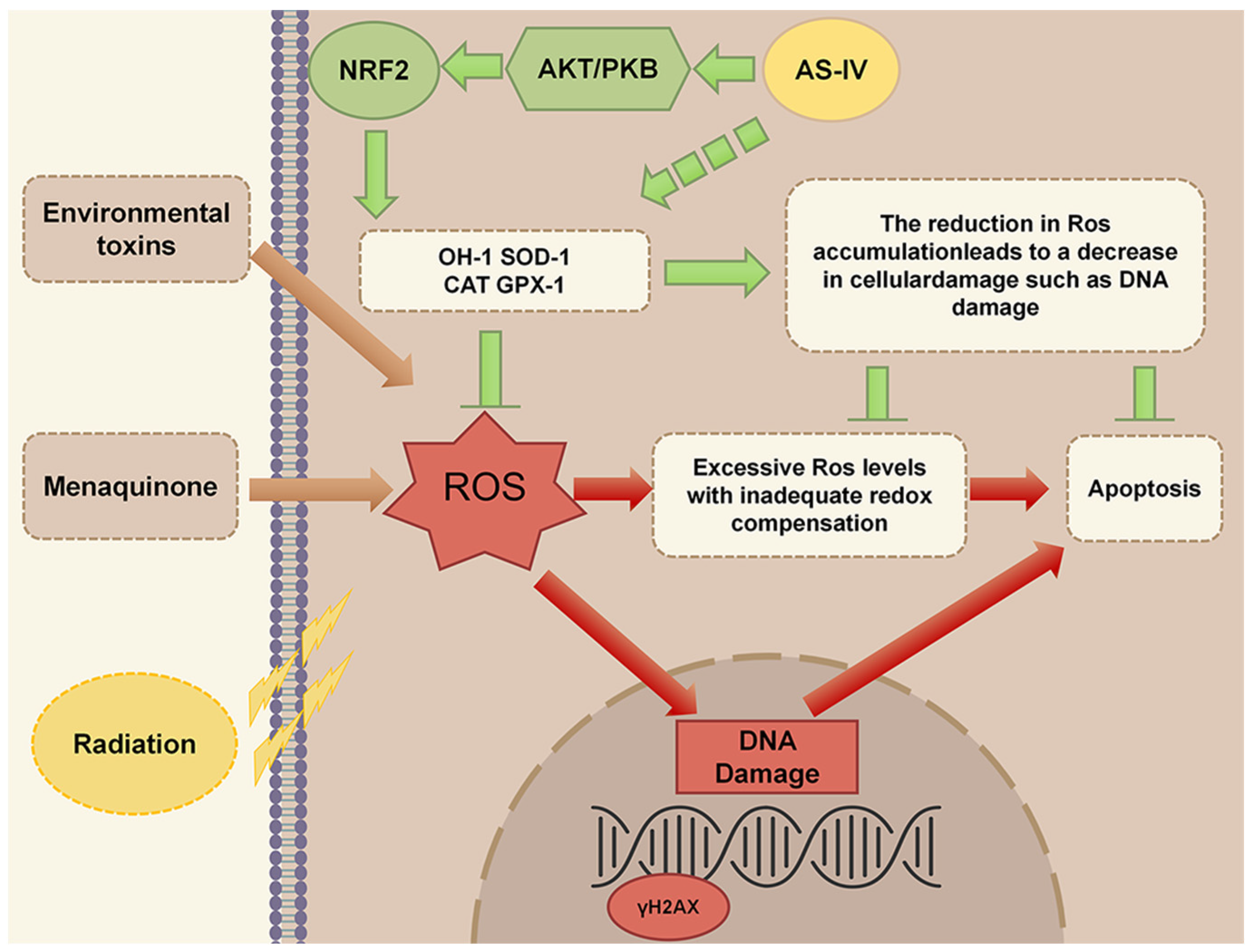
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Rearing Zebrafish and Collecting Samples
4.3. Modeling Oxidative Stress and Treatment with AS-IV
4.4. Detection of Reactive Oxygen Species Levels
4.5. Acridine Orange (AO) Staining
4.6. Real-Time Quantitative PCR Analysis
4.7. Western Blot Assay of Proteins
4.8. Web-Based Pharmacology Analysis
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AKT/PKB | Protein kinase B |
| AS-IV | Astragaloside IV |
| AO | Acridine Orange 10-nonyl bromide |
| ARE | Antioxidant response element |
| CAT | Antioxidant response element |
| DMSO | Dimethyl sulfoxide |
| DCFH-DA | 2′-7′dichlorofluorescein diacetate |
| DAVID | Database for Annotation, Visualization, and Integrated Discovery |
| GPX | Glutathione peroxidase |
| GO | Gene Ontology Enrichment Analysis |
| HO-1 | Heme oxygenase-1 |
| Keapl | Kelch-like ECH-associated protein 1 |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| MEN | Menaquinone |
| NRF2 | Nuclear factor erythroid 2-related factor 2 |
| OS | Oxidative stress |
| PPI | Protein–protein interaction |
| ROS | Reactive oxygen species |
| SOD | Superoxide dismutase |
| SPSS | Statistical Package for the Social Sciences |
References
- Zhang, Y.; Chen, Z.; Chen, L.; Dong, Q.; Yang, D.H.; Zhang, Q.; Zeng, J.; Wang, Y.; Liu, X.; Cui, Y.; et al. Astragali Radix (Huangqi): A Time-Honored Nourishing Herbal Medicine. Chin. Med. 2024, 19, 119. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.X.; Song, X.M.; Dan, L.W.; Tang, J.M.; Jiang, Y.; Deng, C.; Zhang, D.D.; Li, Y.Z.; Wang, W. Astragali Radix: Comprehensive Review of Its Botany, Phytochemistry, Pharmacology, and Clinical Application. Arch. Pharm. Res. 2024, 47, 165–218. [Google Scholar] [CrossRef]
- Fu, J.; Wang, Z.; Huang, L.; Zheng, S.; Wang, D.; Chen, S.; Zhang, H.; Yang, S. Review of the Botanical Characteristics, Phytochemistry, and Pharmacology of Astragalus membranaceus (Huangqi). Phytother. Res. 2014, 28, 1275–1283. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhang, Y.; Luo, Y.; Meng, X.; Pan, G.; Zhang, H.; Li, Y.; Zhang, B. The Molecular Basis of the Anti-Inflammatory Property of Astragaloside IV for the Treatment of Diabetes and Its Complications. Drug Des. Dev. Ther. 2023, 17, 771–790. [Google Scholar] [CrossRef]
- Xu, Z.; Zhou, H.; Zhang, Y.; Cheng, Z.; Wan, M.; Qin, W.; Li, P.; Feng, J.; Shao, S.; Xue, W.; et al. Recent Pharmacological Advances in the Treatment of Cardiovascular Events with Astragaloside IV. Biomed. Pharmacother. 2023, 168, 115752. [Google Scholar] [CrossRef]
- Wang, S.; Fu, J.L.; Hao, H.F.; Jiao, Y.N.; Li, P.P.; Han, S.Y. Metabolic Reprogramming by Traditional Chinese Medicine and Its Role in Effective Cancer Therapy. Pharmacol. Res. 2021, 170, 105728. [Google Scholar] [CrossRef] [PubMed]
- Gong, L.; Wang, R.; Wang, X.; Liu, J.; Han, Z.; Li, Q.; Jin, Y.; Liao, H. Research Progress of Natural Active Compounds on Improving Podocyte Function to Reduce Proteinuria in Diabetic Kidney Disease. Ren. Fail. 2023, 45, 2290930. [Google Scholar] [CrossRef]
- Packer, M. Qiliqiangxin: A Multifaceted Holistic Treatment for Heart Failure or a Pharmacological Probe for the Identification of Cardioprotective Mechanisms? Eur. J. Heart Fail. 2023, 25, 2130–2143. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, A.; Zhao, K.; Gao, H.; Shi, P.; Chen, Y.; Cheng, Z.; Zhou, W.; Zhang, Y. The Role of Oxidative Stress in Intervertebral Disc Degeneration: Mechanisms and Therapeutic Implications. Ageing Res. Rev. 2024, 98, 102323. [Google Scholar] [CrossRef]
- Deng, X.; Lin, B.; Wang, F.; Xu, P.; Wang, N. Mangiferin Attenuates Osteoporosis by Inhibiting Osteoblastic Ferroptosis Through Keap1/Nrf2/SLC7A11/GPX4 Pathway. Phytomedicine 2024, 124, 155282. [Google Scholar] [CrossRef]
- Arfin, S.; Jha, N.K.; Jha, S.K.; Kesari, K.K.; Ruokolainen, J.; Roychoudhury, S.; Rathi, B.; Kumar, D. Oxidative Stress in Cancer Cell Metabolism. Antioxidants 2021, 10, 642. [Google Scholar] [CrossRef]
- Hao, Y.; Zhu, Y.J.; Zou, S.; Zhou, P.; Hu, Y.W.; Zhao, Q.X.; Gu, L.N.; Zhang, H.Z.; Wang, Z.; Li, J. Metabolic Syndrome and Psoriasis: Mechanisms and Future Directions. Front. Immunol. 2021, 12, 711060. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B. Understanding Mechanisms of Antioxidant Action in Health and Disease. Nat. Rev. Mol. Cell Biol. 2024, 25, 13–33. [Google Scholar] [CrossRef]
- Allegra, M. Redox Systems, Oxidative Stress, and Antioxidant Defenses in Health and Disease. Antioxidants 2021, 10, 1955. [Google Scholar] [CrossRef]
- Taysi, S.; Tascan, A.S.; Ugur, M.G.; Demir, M. Radicals, Oxidative/Nitrosative Stress and Preeclampsia. Mini-Rev. Med. Chem. 2019, 19, 178–193. [Google Scholar] [CrossRef] [PubMed]
- Forman, H.J.; Zhang, H. Targeting Oxidative Stress in Disease: Promise and Limitations of Antioxidant Therapy. Nat. Rev. Drug Discov. 2021, 20, 689–709. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Ni, J.; Cao, Y.; Xing, X.; Wu, Q.; Fan, G. Astragaloside IV Attenuates Myocardial Ischemia-Reperfusion Injury from Oxidative Stress by Regulating Succinate, Lysophospholipid Metabolism, and ROS Scavenging System. Oxidative Med. Cell. Longev. 2019, 2019, 9137654. [Google Scholar] [CrossRef]
- Chowdhury, S.; Saikia, S.K. Use of Zebrafish as a Model Organism to Study Oxidative Stress: A Review. Zebrafish 2022, 19, 165–176. [Google Scholar] [CrossRef]
- Howe, K.; Clark, M.D.; Torroja, C.F.; Torrance, J.; Berthelot, C.; Muffato, M.; Collins, J.E.; Humphray, S.; McLaren, K.; Matthews, L.; et al. The Zebrafish Reference Genome Sequence and Its Relationship to the Human Genome. Nature 2013, 496, 498–503. [Google Scholar] [CrossRef]
- Fuse, Y.; Kobayashi, M. Conservation of the Keap1-Nrf2 System: An Evolutionary Journey through Stressful Space and Time. Molecules 2017, 22, 436. [Google Scholar] [CrossRef]
- Kobayashi, M.; Itoh, K.; Suzuki, T.; Osanai, H.; Nishikawa, K.; Katoh, Y.; Takagi, Y.; Yamamoto, M. Identification of the Interactive Interface and Phylogenic Conservation of the Nrf2-Keap1 System. Genes Cells. 2002, 7, 807–820. [Google Scholar] [CrossRef] [PubMed]
- Mukaigasa, K.; Nguyen, L.T.P.; Li, L.; Nakajima, H.; Yamamoto, M.; Kobayashi, M. Genetic Evidence of an Evolutionarily Conserved Role for Nrf2 in the Protection against Oxidative Stress. Mol. Cell. Biol. 2012, 32, 4455–4461. [Google Scholar] [CrossRef] [PubMed]
- Fuse, Y.; Nguyen, V.T.; Kobayashi, M. Nrf2-Dependent Protection against Acute Sodium Arsenite Toxicity in Zebrafish. Toxicol. Appl. Pharmacol. 2016, 305, 136–142. [Google Scholar] [CrossRef]
- Nguyen, V.T.; Fuse, Y.; Tamaoki, J.; Akiyama, S.; Muratani, M.; Tamaru, Y.; Kobayashi, M. Conservation of the Nrf2-Mediated Gene Regulation of Proteasome Subunits and Glucose Metabolism in Zebrafish. Oxid. Med. Cell Longev. 2016, 2016, 5720574. [Google Scholar] [CrossRef] [PubMed]
- Bladen, C.L.; Kozlowski, D.J.; Dynan, W.S. Effects of Low-Dose Ionizing Radiation and Menadione, an Inducer of Oxidative Stress, Alone and in Combination in a Vertebrate Embryo Model. Radiat. Res. 2012, 178, 499–503. [Google Scholar] [CrossRef]
- Thor, H.; Smith, M.T.; Hartzell, P.; Bellomo, G.; Jewell, S.A.; Orrenius, S. The Metabolism of Menadione (2-Methyl-1,4-naphthoquinone) by Isolated Hepatocytes: A Study of the Implications of Oxidative Stress in Intact Cells. J. Biol. Chem. 1982, 257, 12419–12425. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Y.; Zhou, Y.; Zhang, X.; Faulkner, S.; Liu, H.; Wang, L. Toxic Effects of Triphenyltin on the Development of Zebrafish (Danio rerio) Embryos. Sci. Total Environ. 2023, 885, 163783. [Google Scholar] [CrossRef]
- Yamaga, S.; Aziz, M.; Murao, A.; Brenner, M.; Wang, P. DAMPs and Radiation Injury. Front. Immunol. 2024, 15, 1353990. [Google Scholar] [CrossRef]
- Ma, M.; Jiang, W.; Zhou, R. DAMPs and DAMP-Sensing Receptors in Inflammation and Diseases. Immunity 2024, 57, 752–771. [Google Scholar] [CrossRef]
- Van Loenhout, J.; Peeters, M.; Bogaerts, A.; Smits, E.; Deben, C. Oxidative Stress-Inducing Anticancer Therapies: Taking a Closer Look at Their Immunomodulating Effects. Antioxidants 2020, 9, 1188. [Google Scholar] [CrossRef]
- Cacciatore, S.; Andaloro, S.; Bernardi, M.; Oterino Manzanas, A.; Spadafora, L.; Figliozzi, S.; Asher, E.; Rana, J.S.; Ecarnot, F.; Gragnano, F.; et al. Chronic Inflammatory Diseases and Cardiovascular Risk: Current Insights and Future Strategies for Optimal Management. Int. J. Mol. Sci. 2025, 26, 3071. [Google Scholar] [CrossRef] [PubMed]
- Sule, R.O.; Rivera, G.D.T.; Vaidya, T.; Gartrell, E.; Gomes, A.V. Environmental Toxins and Oxidative Stress: The Link to Cardiovascular Diseases. Antioxidants 2025, 14, 604. [Google Scholar] [CrossRef]
- Tang, J.; Gao, Y.; Fu, Y.; Han, Z.; Xu, P.; Li, X.; Wang, S.; Wang, X. Astragaloside IV Mitigates Influenza-Induced Inflammatory Responses by Suppressing the Wnt/β-Catenin Signalling Pathway in Alveolar Macrophages. Molecules 2025, 30, 1234. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Yu, J.; He, W.; Qiao, Y.; Cheng, X.; Huang, H.; Lai, S.; Yin, D.; He, H. Astragaloside IV Intervenes Multi-Regulatory Cell Death Forms against Doxorubicin-Induced Cardiotoxicity by Regulating AMPKα2 Pathway. Int. Immunopharmacol. 2024, 142, 113078. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, M.; Xu, Y.; Lu, M.; Zhang, L.; Li, C. Effect of Astragaloside IV on Improving Cardiac Function in Rats with Heart Failure: A Preclinical Systematic Review and Meta-Analysis. Front. Pharmacol. 2023, 14, 1226008. [Google Scholar] [CrossRef]
- Siddiqui, M.S.; François, M.; Fenech, M.F.; Leifert, W.R. Persistent γH2AX: A Promising Molecular Marker of DNA Damage and Aging. Mutat. Res. Rev. Mutat. Res. 2015, 766, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q. Role of Nrf2 in Oxidative Stress and Toxicity. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 401–426. [Google Scholar] [CrossRef]
- Crisman, E.; Duarte, P.; Dauden, E.; Cuadrado, A.; Rodríguez-Franco, M.I.; López, M.G.; León, R. KEAP1-NRF2 Protein-Protein Interaction Inhibitors: Design, Pharmacological Properties and Therapeutic Potential. Med. Res. Rev. 2023, 43, 237–287. [Google Scholar] [CrossRef]
- Zhang, Q.; Yao, M.; Qi, J.; Song, R.; Wang, L.; Li, J.; Zhou, X.; Chang, D.; Huang, Q.; Li, L.; et al. Puerarin Inhibited Oxidative Stress and Alleviated Cerebral Ischemia-Reperfusion Injury through PI3K/Akt/Nrf2 Signaling Pathway. Front. Pharmacol. 2023, 14, 1134380. [Google Scholar] [CrossRef]
- Maruyama, A.; Mimura, J.; Harada, N.; Itoh, K. Nrf2 Activation Is Associated with Z-DNA Formation in the Human HO-1 Promoter. Nucleic Acids Res. 2013, 41, 5223–5234. [Google Scholar] [CrossRef]
- Li, Y.; Huang, J.; Wang, J.; Xia, S.; Ran, H.; Gao, L.; Feng, C.; Gui, L.; Zhou, Z.; Yuan, J. Human Umbilical Cord-Derived Mesenchymal Stem Cell Transplantation Supplemented with Curcumin Improves the Outcomes of Ischemic Stroke via AKT/GSK-3β/β-TrCP/Nrf2 Axis. J. Neuroinflamm. 2023, 20, 49. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dai, J.; E, Z.; Bi, Y.; Yin, Z.; Wang, Y.; Wang, X.; Jia, X.; Zou, B. AS-IV Attenuates Oxidative Stress-Induced Apoptosis in Zebrafish via Modulation of the AKT/NRF2/HO-1/Caspase-3 Signaling Axis. Molecules 2025, 30, 2355. https://doi.org/10.3390/molecules30112355
Dai J, E Z, Bi Y, Yin Z, Wang Y, Wang X, Jia X, Zou B. AS-IV Attenuates Oxidative Stress-Induced Apoptosis in Zebrafish via Modulation of the AKT/NRF2/HO-1/Caspase-3 Signaling Axis. Molecules. 2025; 30(11):2355. https://doi.org/10.3390/molecules30112355
Chicago/Turabian StyleDai, Jili, Zhizhou E, Yannan Bi, Zetao Yin, Yanfang Wang, Xingyu Wang, Xiaoe Jia, and Bo Zou. 2025. "AS-IV Attenuates Oxidative Stress-Induced Apoptosis in Zebrafish via Modulation of the AKT/NRF2/HO-1/Caspase-3 Signaling Axis" Molecules 30, no. 11: 2355. https://doi.org/10.3390/molecules30112355
APA StyleDai, J., E, Z., Bi, Y., Yin, Z., Wang, Y., Wang, X., Jia, X., & Zou, B. (2025). AS-IV Attenuates Oxidative Stress-Induced Apoptosis in Zebrafish via Modulation of the AKT/NRF2/HO-1/Caspase-3 Signaling Axis. Molecules, 30(11), 2355. https://doi.org/10.3390/molecules30112355




