Design, Synthesis, and Biological Evaluations of a Novel Resveratrol-Type Analogue Against VEGF
Abstract
1. Introduction
2. Results
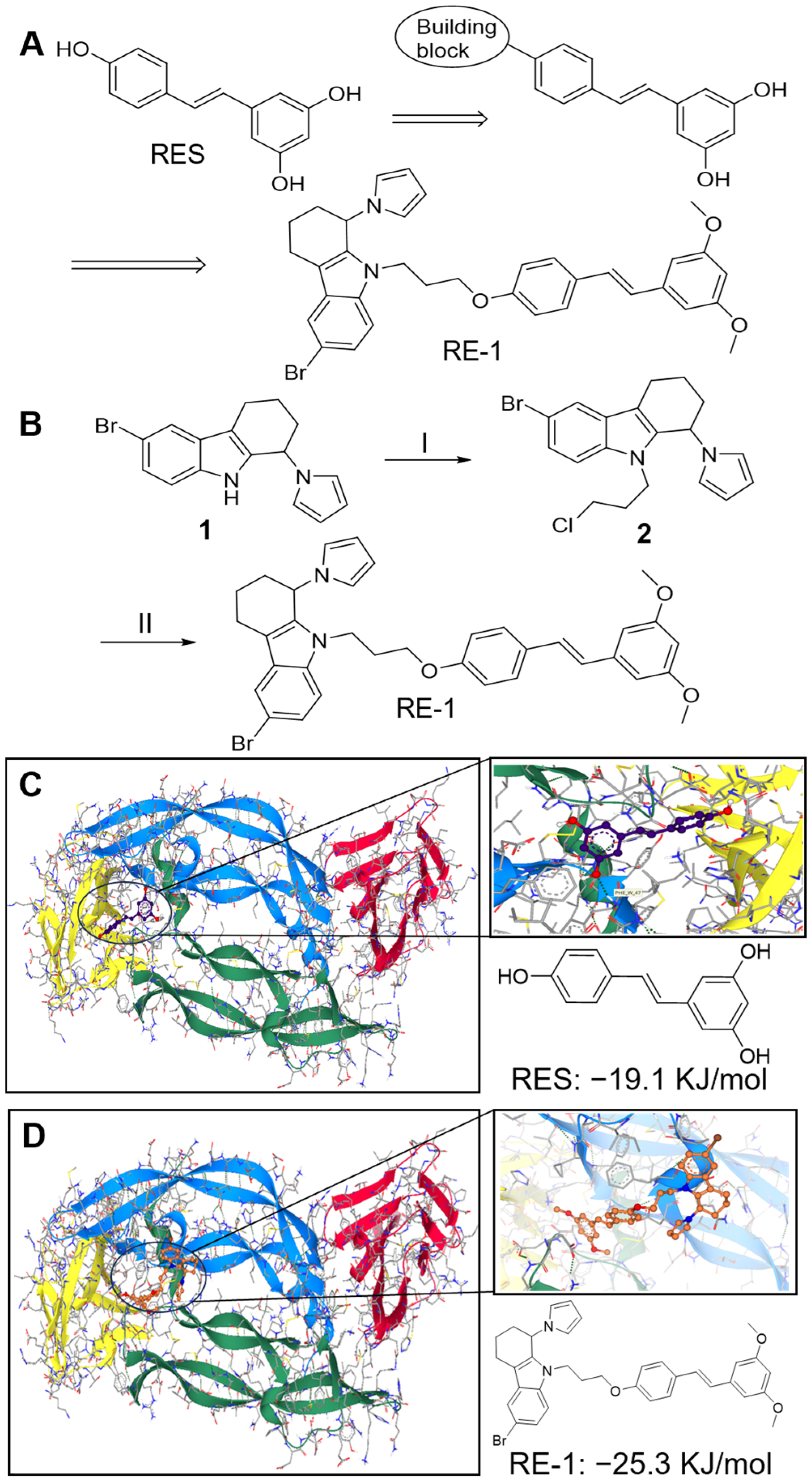
2.1. RE-1 Expresses Good Binding Affinity to VEGF Protein
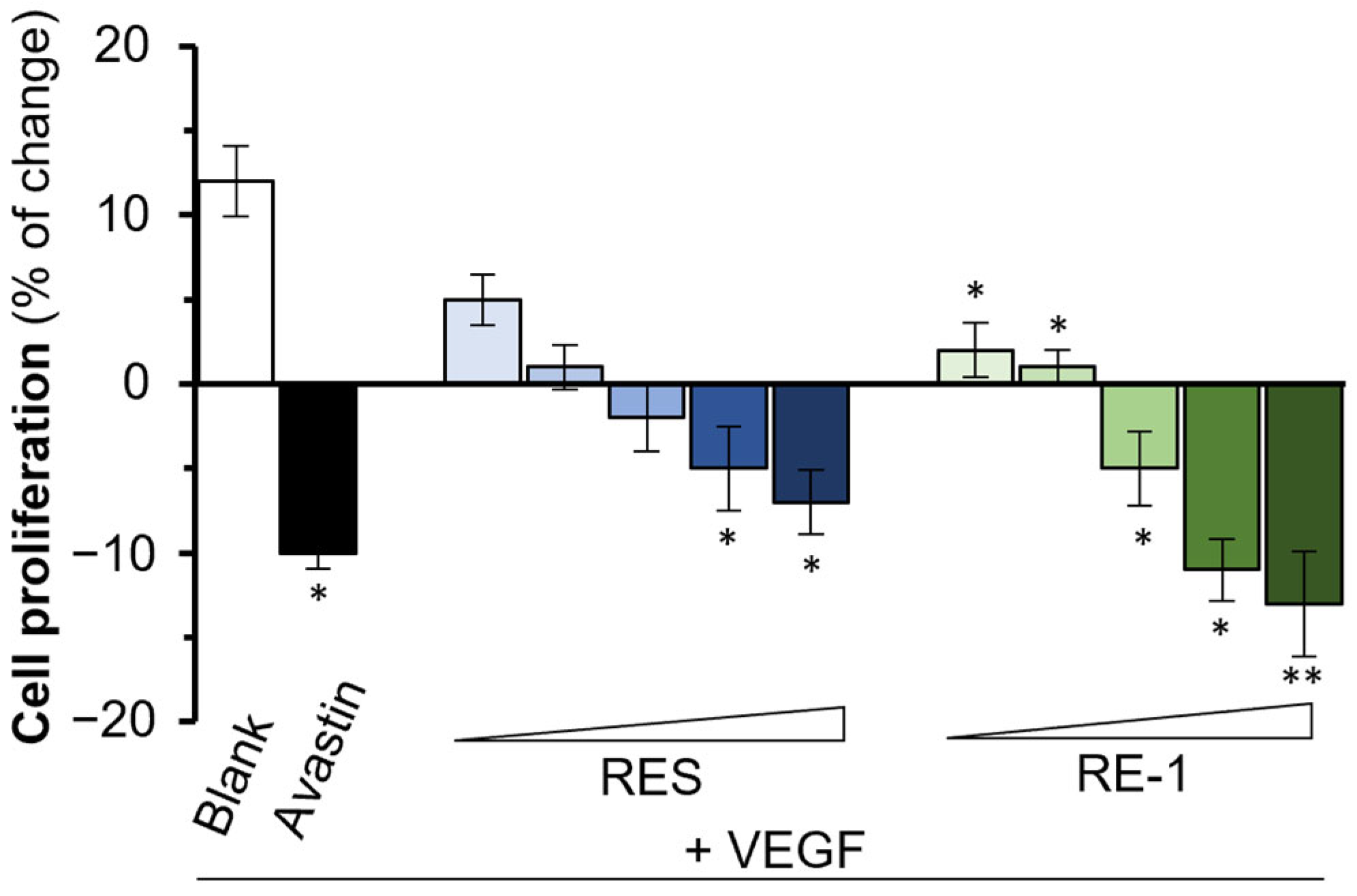
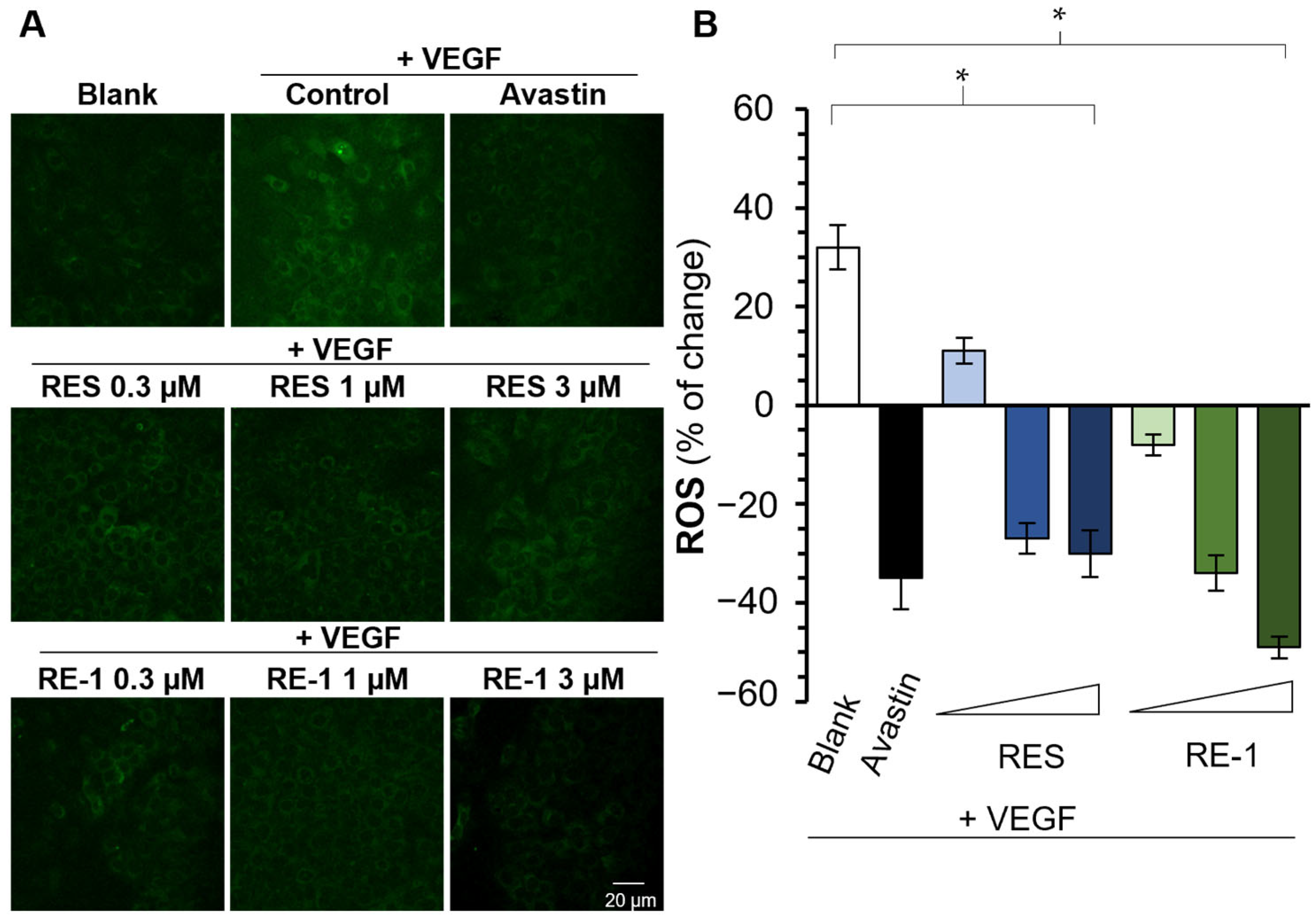
2.2. RE-1 Inhibits VEGF-Mediated Bioactivities
2.3. Pharmacological Properties of RE-1
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Synthesis of Chemicals
4.3. Cell Cultures
4.4. Binding Affinity Assay
4.5. Cell Viability
4.6. Wound Closure Assay
4.7. Measurement of Intracellular ROS
4.8. SDS/PAGE and Western Blotting
4.9. Cell Proliferation
4.10. Computational Docking Study
4.11. Stability Test in HPLC
4.12. Trans-Epithelial Permeability of Chemical Transport
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ball, S.; Shuttleworth, A.; Kielty, C. Vascular endothelial growth factor can signal through platelet-derived growth factor receptors. J. Cell Biol. 2007, 177, 489–500. [Google Scholar] [CrossRef] [PubMed]
- Mandal, K.; Kent, S. Total chemical synthesis of biologically active vascular endothelial growth factor. Angew. Chem. Int. Ed. 2011, 50, 8029–8033. [Google Scholar] [CrossRef]
- Guzmán-Hernández, M.L.; Potter, G.; Egervári, K.; Kiss, J.; Balla, T. Secretion of VEGF-165 has unique characteristics, including shedding from the plasma membrane. Mol. Biol. Cell 2014, 25, 1061–1072. [Google Scholar] [CrossRef]
- Apte, R.; Chen, D.; Ferrara, N. VEGF in signaling and disease: Beyond discovery and development. Cell 2019, 176, 1248–1264. [Google Scholar] [CrossRef]
- Ferrara, N.; Adamis, A. Ten years of anti-vascular endothelial growth factor therapy. Nat. Rev. Drug Discov. 2016, 15, 385–403. [Google Scholar] [CrossRef]
- Yang, Y.; Cao, Y. The impact of VEGF on cancer metastasis and systemic disease. Semin. Cancer Biol. 2022, 86, 251–261. [Google Scholar] [CrossRef] [PubMed]
- Shahidatul-Adha, M.; Zunaina, E.; Aini-Amalina, M. Evaluation of vascular endothelial growth factor (VEGF) level in the tears and serum of age-related macular degeneration patients. Sci. Rep. 2022, 12, 4423–4431. [Google Scholar] [CrossRef] [PubMed]
- Vinge, E.; Bro, T. Treatment burden on patients receiving intravitreal anti-VEGF for wet age-related macular degeneration. Acta Ophthalmol. 2023, 102, 478–482. [Google Scholar] [CrossRef]
- Min, H.-Y.; Lee, H.-Y. Molecular targeted therapy for anticancer treatment. Exp. Mol. Med. 2022, 54, 1670–1694. [Google Scholar] [CrossRef]
- Lee, Y.T.; Tan, Y.J.; Oon, C.E. Molecular targeted therapy: Treating cancer with specificity. Eur. J. Pharmacol. 2018, 834, 188–196. [Google Scholar] [CrossRef]
- Nwibo, D.; Levi, C.; Nwibo, M. Small molecule drugs; down but not out: A future for medical research and therapeutics. J. Dent. Med. Sci. 2015, 14, 70–77. [Google Scholar]
- Hu, W.; Duan, R.; Xia, Y.; Xiong, Q.; Wang, H.; Chan, G.; Liu, S.; Dong, T.; Qin, Q.; Tsim, W. Binding of resveratrol to vascular endothelial growth factor suppresses angiogenesis by inhibiting the receptor signaling. J. Agric. Food Chem. 2019, 67, 1127–1137. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Zhang, X.; Leung, K.W.; Duan, R.; Dong, T.; Qin, Q.; Tsim, K. Resveratrol, an inhibitor binding to VEGF, restores the pathology of abnormal angiogenesis in retinopathy of prematurity (ROP) in mice: Application by intravitreal and topical Instillation. Int. J. Mol. Sci. 2022, 23, 6455. [Google Scholar] [CrossRef]
- Hu, W.; Chan, G.; Duan, R.; Wang, H.; Kong, X.; Dong, T.; Tsim, K. Synergy of Ginkgetin and resveratrol in suppressing VEGF-induced angiogenesis: A therapy in treating colorectal cancer. Cancers 2019, 11, 1828. [Google Scholar] [CrossRef]
- Romanelli, G.P.; Jios, J.L.; Guaymas, O.; Piovoso, R.; Autino, J.C. A simple method for n-phenoxyethylation of anilines. Molecules 2000, 5, 562–563. [Google Scholar] [CrossRef]
- Kelly, R.; Rixe, O. Axitinib—A selective inhibitor of the vascular endothelial growth factor (VEGF) receptor. Targ. Oncol. 2009, 4, 297–305. [Google Scholar] [CrossRef]
- Almalki, S.; Agrawal, D. ERK signaling is required for VEGF-A/VEGFR2-induced differentiation of porcineadipose-derived mesenchymal stem cells into endothelial cells. Stem Cell Res. Ther. 2017, 8, 113. [Google Scholar] [CrossRef] [PubMed]
- Abhinand, C.; Galipon, J.; Mori, M.; Ramesh, P.; Prasad, T.S.K.; Raju, R.; Sudhakaran, P.; Tomita, M. Temporal phosphoproteomic analysis of VEGF-A signaling in HUVECs: An insight into early signaling events associated with angiogenesis. J. Cell Commun. Signal. 2023, 17, 1067–1079. [Google Scholar] [CrossRef]
- Maraldi, T.; Prata, C.; Caliceti, C.; Sega, F.V.D.; Zambonin, L.; Fiorentini, D.; Hakim, G. VEGF-induced ROS generation from NAD(P)H oxidases protects human leukemic cells from apoptosis. Int. J. Oncol. 2010, 36, 1581–1589. [Google Scholar]
- Beneta, L.; Hosey, C.; Ursu, O.; Oprea, T. BDDCS, the Rule of 5 and Drugability. Adv. Drug Deliv. Rev. 2016, 101, 89–98. [Google Scholar] [CrossRef]
- Juvale, I.I.A.; Hamid, A.A.A.; Halim, K.B.A.; Has, A.T.C. P-glycoprotein: New insights into structure, physiological function, regulation and alterations in disease. Heliyon 2022, 8, e09777. [Google Scholar] [CrossRef] [PubMed]
- Hou, T.; Wang, J.; Li, Y. ADME evaluation in drug discovery. 8. the prediction of human intestinal absorption by a support vector machine. J. Chem. Inf. Model. 2007, 47, 2408–2415. [Google Scholar] [CrossRef]
- Ahmed, I.; Leach, D.; Wohlmuth, H.; De Voss, J.; Blanchfield, J. Caco-2 cell permeability of flavonoids and saponins from Gynostemma Pentaphyllum: The immortal herb. ACS Omega 2020, 5, 21561–21569. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.Y.; Yeoh, Y.; Low, T.Y. A recent update on small-molecule kinase inhibitors for targeted cancer therapy and their therapeutic insights from mass spectrometry-based proteomic analysis. FEBS J. 2023, 290, 2845–2864. [Google Scholar] [CrossRef] [PubMed]
- Shuel, S. Targeted cancer therapies: Clinical pearls for primary care. Can. Fam. Physician 2022, 68, 515–518. [Google Scholar] [CrossRef]
- Choi, H.Y.; Chang, J.-E. Targeted therapy for cancers: From ongoing clinical trials to FDA-approved drugs. Int. J. Mol. Sci. 2023, 24, 13618. [Google Scholar] [CrossRef]
- Wang, L.; Liu, W.-Q.; Broussy, S.; Han, B.; Fang, H. Recent advances of anti-angiogenic inhibitors targeting VEGF/VEGFR axis. Front. Pharmacol. 2024, 14, 1307860. [Google Scholar] [CrossRef]
- Escalante, C.; Zalpour, A. Vascular endothelial growth factor inhibitor-induced hypertension: Basics for primary care providers. Cardiol. Res. Pract. 2011, 2011, 816897. [Google Scholar] [CrossRef]
- Elebiyo, T.; Rotimi, D.; Evbuomwan, I.; Maimako, R.F.; Iyobhebhe, M.; Ojo, O.A.; Oluba, O.; Adeyemi, O. Reassessing vascular endothelial growth factor (VEGF) in anti-angiogenic cancer therapy. Cancer Treat. Res. Commun. 2022, 32, 100620. [Google Scholar] [CrossRef]
- Szczepanska, P.; Rychlicka, M.; Groborz, S.; Kruszynska, A.; Ledesma-Amaro, R.; Rapak, A.; Gliszczynska, A.; Lazar, Z. Studies on the anticancer and antioxidant activities of resveratrol and long-chain fatty acid esters. Int. J. Mol. Sci. 2023, 24, 7167. [Google Scholar] [CrossRef]
- Liu, X.; Pei, J.; Li, J.; Zhu, H.; Zheng, X.; Zhang, X.; Ruan, B.; Chen, L. Recent advances in resveratrol derivatives: Structural modifications and biological activities. Molecules 2025, 30, 958. [Google Scholar] [CrossRef] [PubMed]
- Hughes, J.P.; Rees, S.; Kalindjian, S.B.; Philpott, K.L. Principles of early drug discovery. Br. J. Pharmacol. 2010, 162, 1239–1249. [Google Scholar] [CrossRef] [PubMed]
- Chan, B.; Lynch, N.; Tran, W.; Joyce, J.; Savage, P.; Meutermans, W.; Montgomery, A.; Kassiou, M. Fragment-based drug discovery for disorders of the central nervous system: Designing better drugs piece by piece. Front. Chem. 2010, 12, 1379518. [Google Scholar] [CrossRef] [PubMed]
- Papaioannou, C. Advancements in the treatment of age-related macular degeneration: A comprehensive review. Postgrad. Med. J. 2024, 100, 445–450. [Google Scholar] [CrossRef]
- Khanani, A.M.; Patel, S.; Ferrone, P.J.; Osborne, A.; Sahni, J.; Grzeschik, S.; Basu, K.; Ehrlich, J.S.; Haskova, Z.; Dugel, P.U. Efficacy of every four monthly and quarterly dosing of faricimab vs ranibizumab in neovascular age-related macular degeneration the stairway phase 2 randomized clinical trial. JAMA Ophthalmol. 2020, 138, 964–972. [Google Scholar] [CrossRef]
- Guo, M.S.; Gao, X.; Hu, W.; Wang, X.; Dong, T.; Tsim, K. Scutellarin potentiates the skin regenerative function of self growth colony, an optimized platelet-rich plasma extract, in cultured keratinocytes through VEGF receptor and MAPK signaling. J. Cosmet. Dermatol. 2021, 21, 4836–4845. [Google Scholar] [CrossRef]
- Lin, S.; Tang, R.; Ye, Y.; Xia, C.; Wu, J.; Duan, R.; Leung, K.-W.; Dong, T.; Tsim, K. Drug screening of flavonoids as potential VEGF inhibitors through computational docking and cell models. Molecules 2025, 30, 257. [Google Scholar] [CrossRef]
- Zheng, K.; Choi, R.; Guo, A.; Bi, C.; Zhu, K.; Du, C.; Zhang, Z.; Lau, D.; Dong, T.; Tsim, K. The membrane permeability of Astragali Radix-derived formononetin and calycosin is increased by Angelicae Sinensis Radix in Caco-2 cells: A synergistic action of an ancient herbal decoction Danggui Buxue Tang. J. Pharm. Biomed. Anal. 2012, 70, 671–679. [Google Scholar] [CrossRef]





| Analytes | Papp Values (×10−6 cm/s) a | SD b |
|---|---|---|
| RES c | 18.2 | 1.3 |
| RES d | 15.1 | 0.8 |
| RE-1 c | 20.9 | 1.6 |
| RE-1 d | 15.8 | 1.2 |
| Analytes | Precision | |||
|---|---|---|---|---|
| Intra-day (n = 6) a | Inter-day (n = 6) b | |||
| Mean Peak Area c | RSD (%) | Mean Peak Area c | RSD (%) | |
| RE-1 (acidic) | 1316.9 | 5.84 | 1403.9 | 8.83 |
| RE-1 (neutral) | 1002.8 | 4.93 | 1046.6 | 9.21 |
| RE-1 (basic) | 755.8 | 5.81 | 747.2 | 9.35 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, S.; Guo, M.S.; Tang, R.W.-L.; Ye, Y.; Wu, J.; Ho, Y.M.; Duan, R.; Leung, K.W.; Dong, T.T.-X.; Tsim, K.W.-K. Design, Synthesis, and Biological Evaluations of a Novel Resveratrol-Type Analogue Against VEGF. Molecules 2025, 30, 2345. https://doi.org/10.3390/molecules30112345
Lin S, Guo MS, Tang RW-L, Ye Y, Wu J, Ho YM, Duan R, Leung KW, Dong TT-X, Tsim KW-K. Design, Synthesis, and Biological Evaluations of a Novel Resveratrol-Type Analogue Against VEGF. Molecules. 2025; 30(11):2345. https://doi.org/10.3390/molecules30112345
Chicago/Turabian StyleLin, Shengying, Maggie Suisui Guo, Roy Wai-Lun Tang, Yutong Ye, Jiahui Wu, Yuen Man Ho, Ran Duan, Ka Wing Leung, Tina Ting-Xia Dong, and Karl Wah-Keung Tsim. 2025. "Design, Synthesis, and Biological Evaluations of a Novel Resveratrol-Type Analogue Against VEGF" Molecules 30, no. 11: 2345. https://doi.org/10.3390/molecules30112345
APA StyleLin, S., Guo, M. S., Tang, R. W.-L., Ye, Y., Wu, J., Ho, Y. M., Duan, R., Leung, K. W., Dong, T. T.-X., & Tsim, K. W.-K. (2025). Design, Synthesis, and Biological Evaluations of a Novel Resveratrol-Type Analogue Against VEGF. Molecules, 30(11), 2345. https://doi.org/10.3390/molecules30112345









