Proanthocyanidin-Conjugated NIR-ΙΙ Nano-Prodrugs for Reversing Drug Resistance in Photothermal Therapy
Abstract
1. Introduction
2. Results
2.1. Effects of Polyphenol Structure on Gold Nanoparticles
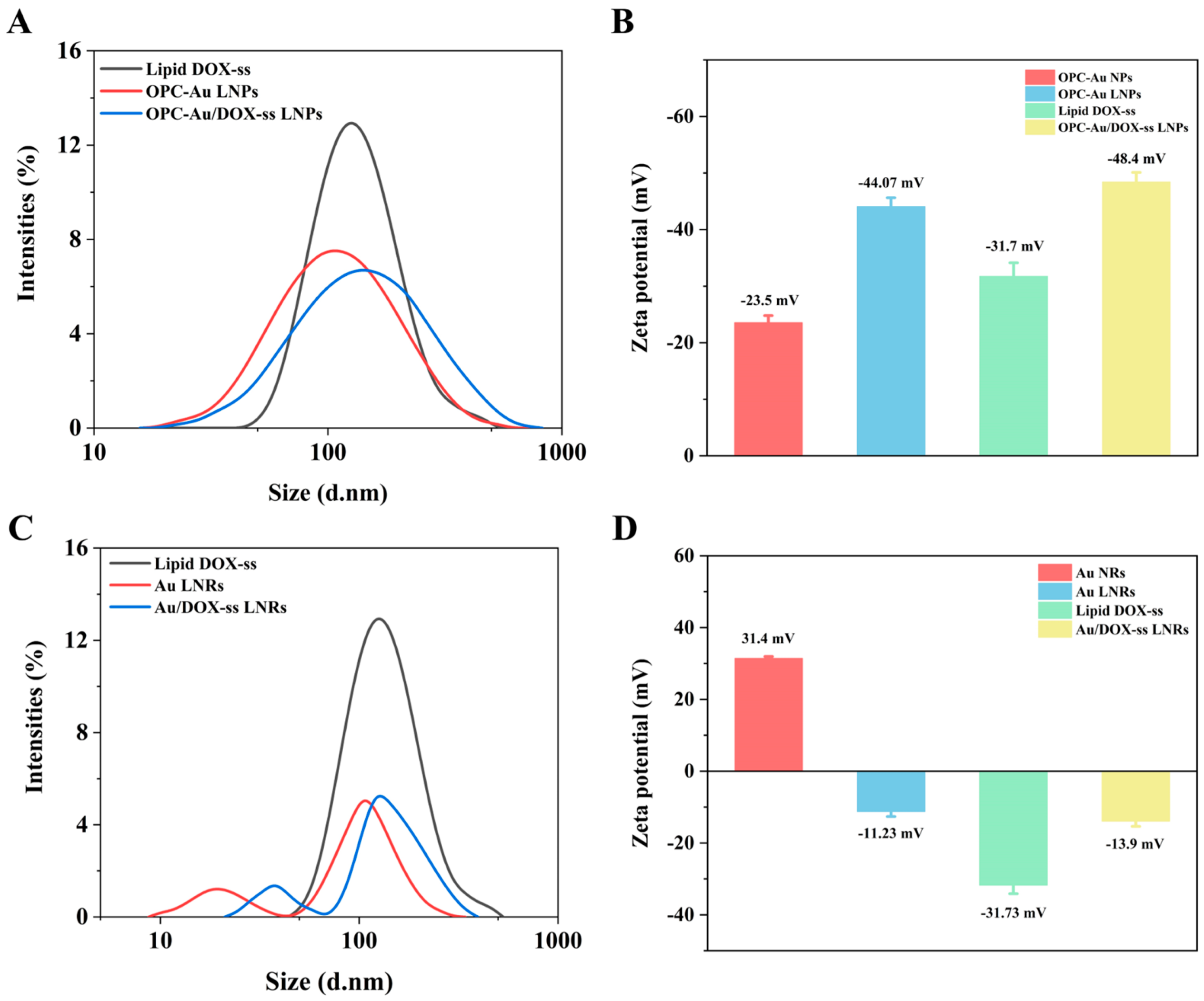
2.2. Chemical Characteristics of Polyphenol-Conjugated Gold Nano-Prodrugs
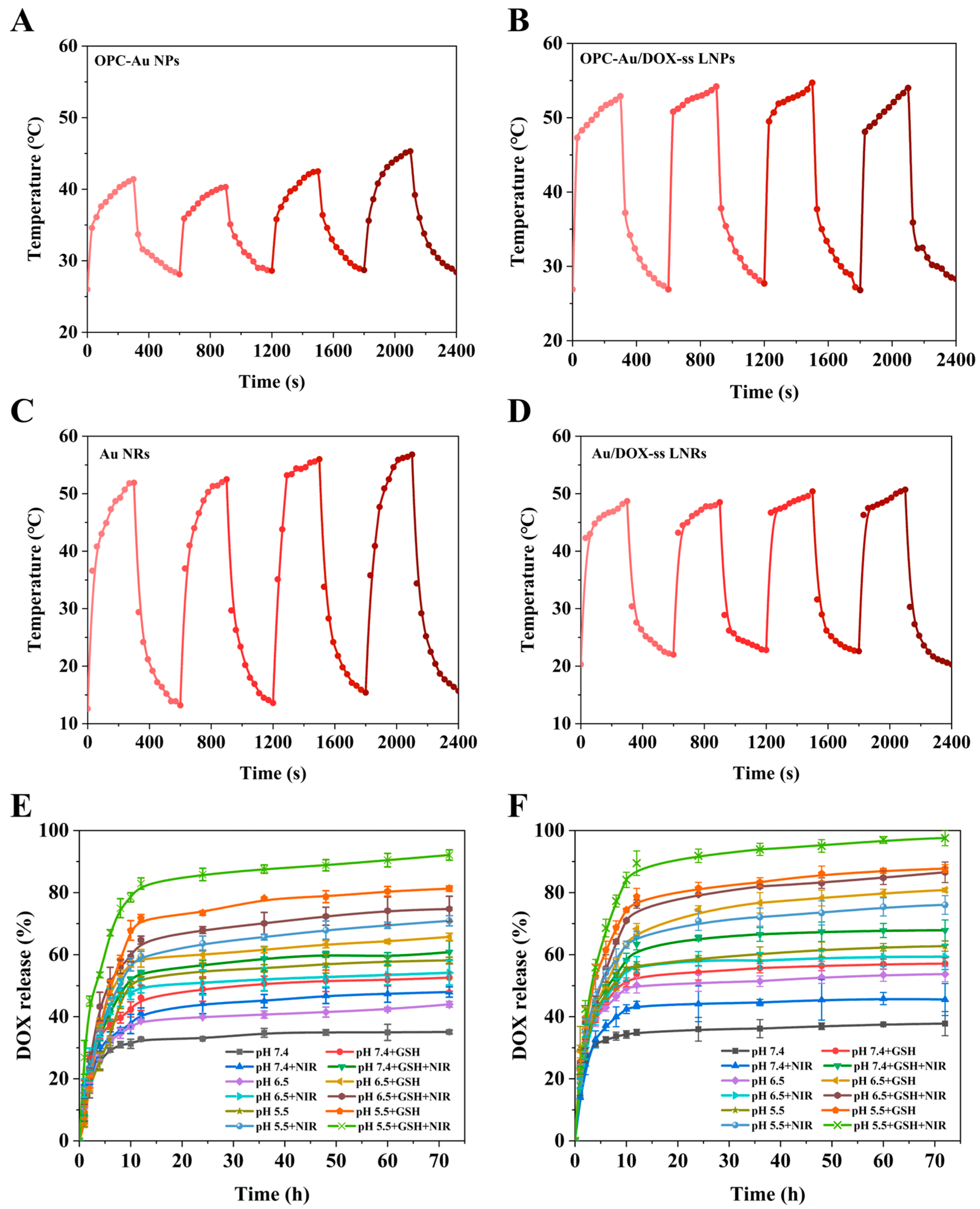
2.3. Effects of Polyphenol Structure on Photothermal Conversion and Drug Release
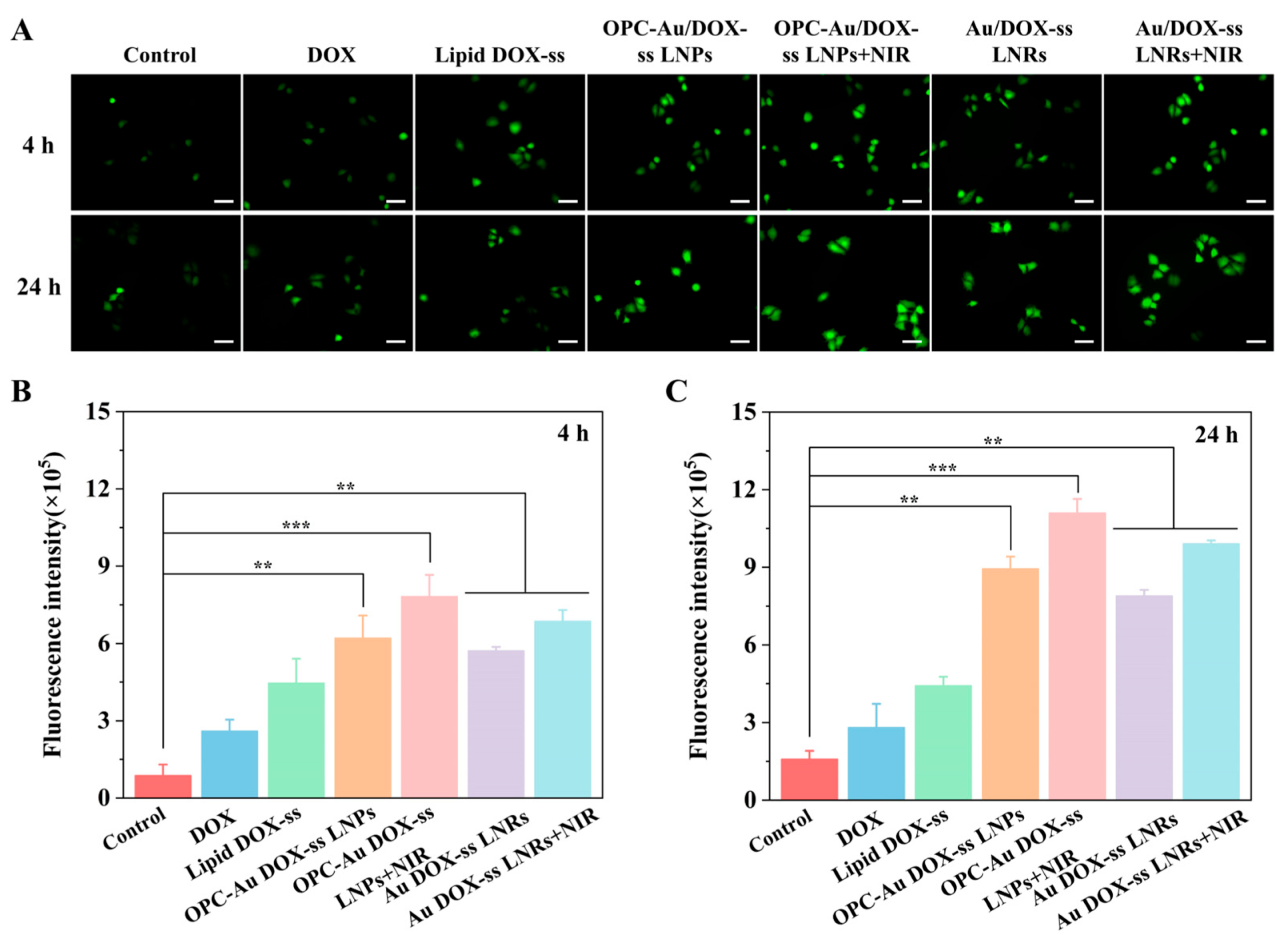
2.4. Targeting Selectivity to Multidrug-Resistant Breast Cancer Cells
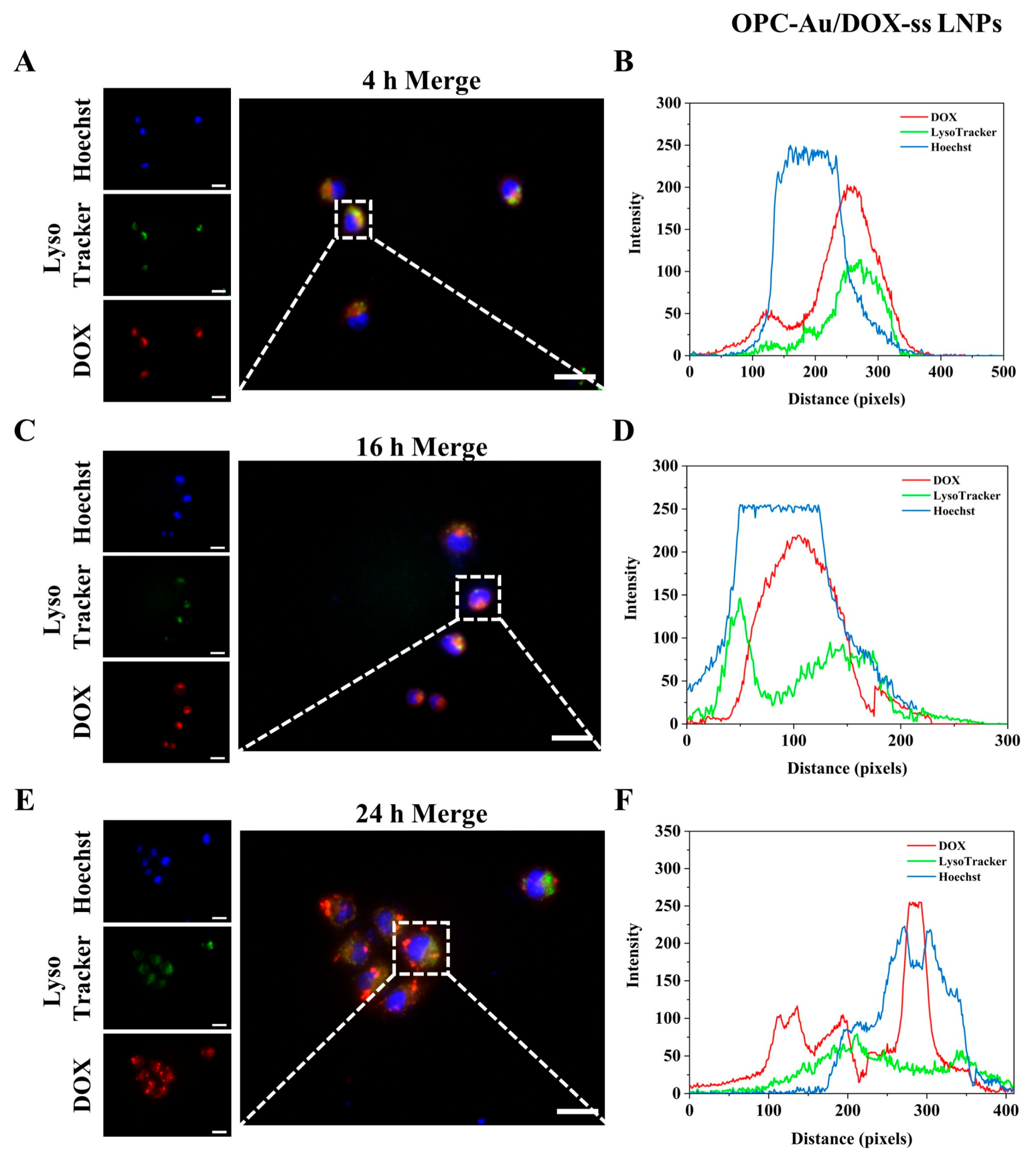
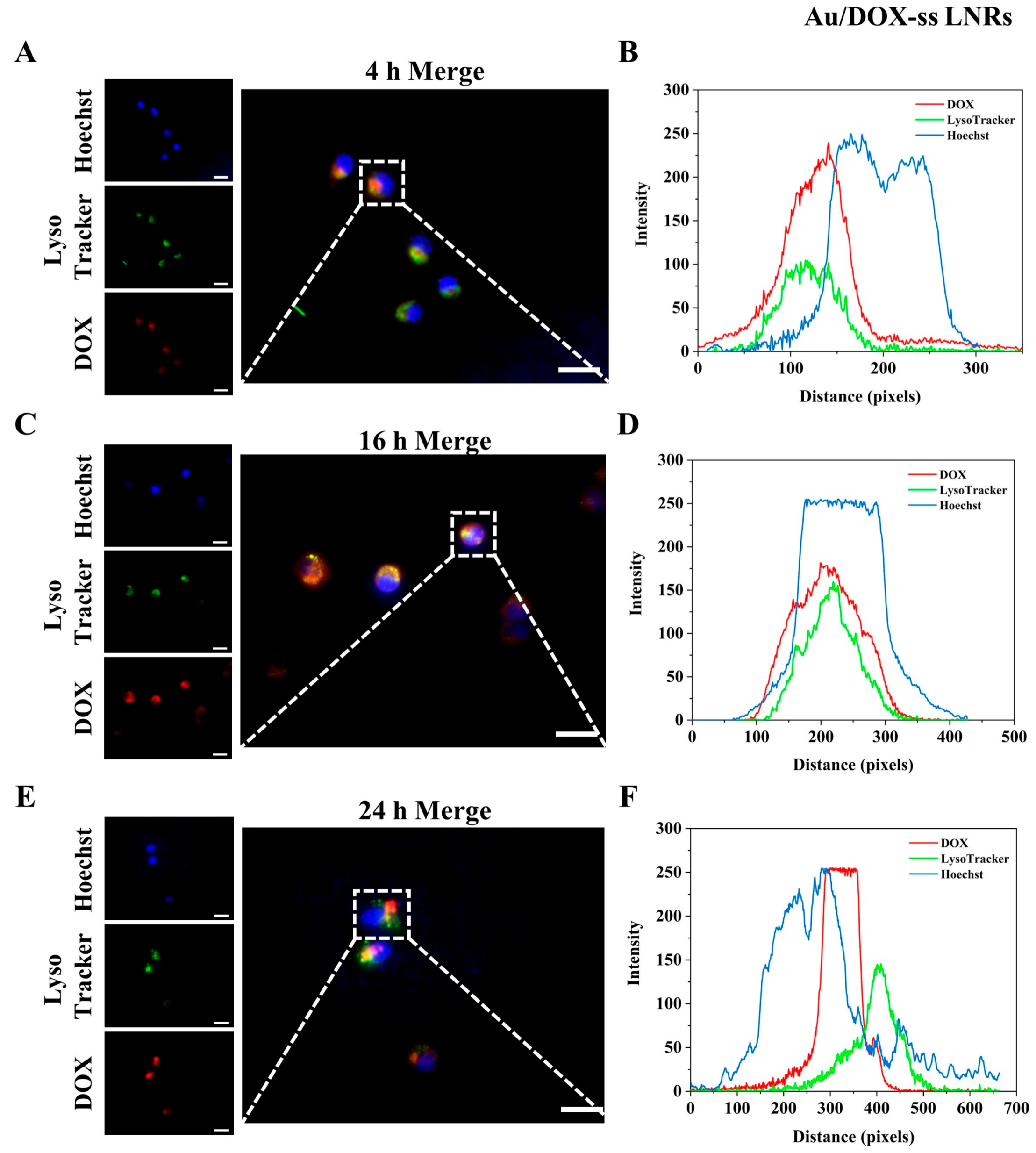
2.5. Intracellular Lysosomal Escape
2.6. Intracellular ROS Detection
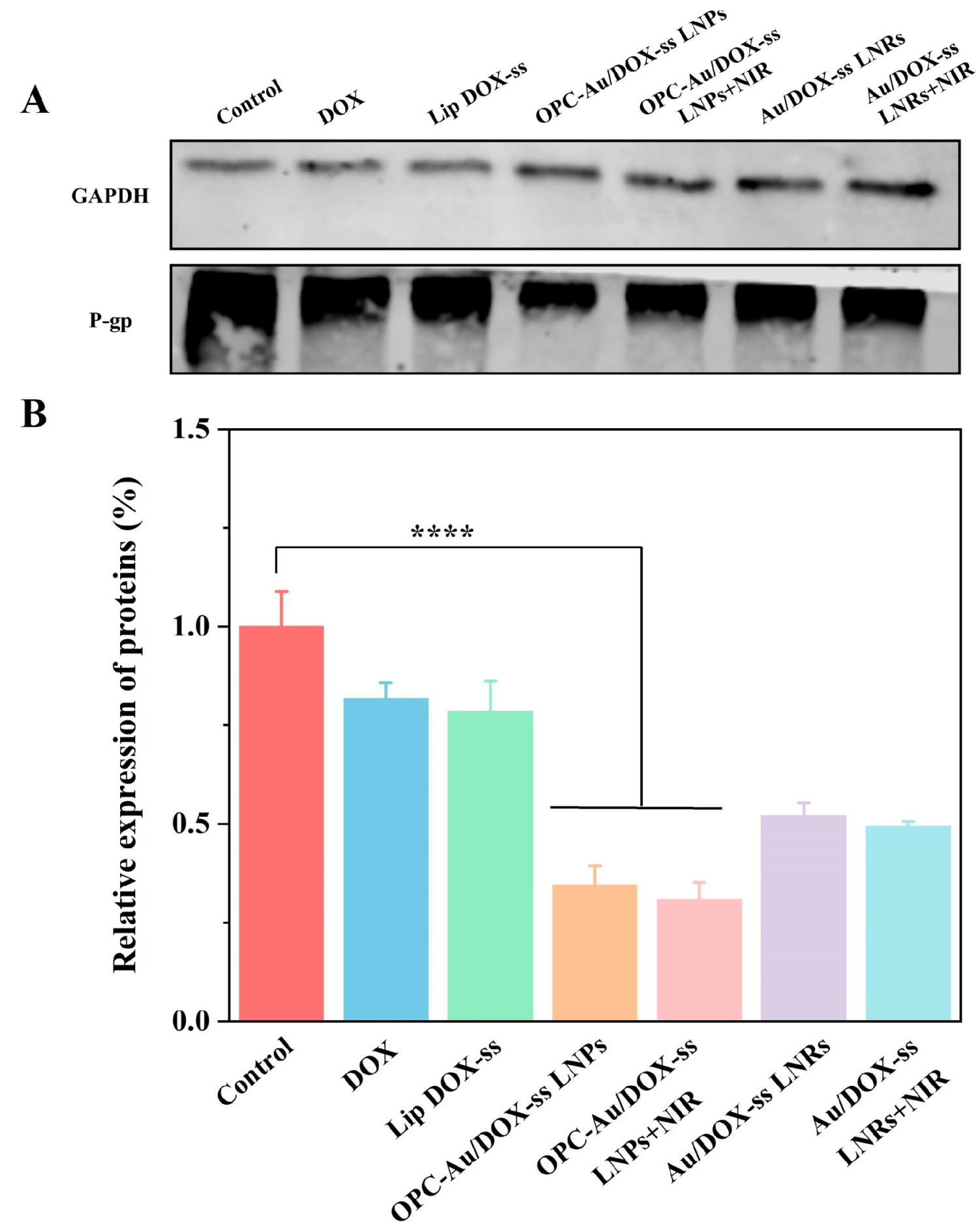
2.7. Effects of Polyphenol Structure on P-gp Inhibition in Multidrug-Resistant Breast Cancer Cells
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Au NRs
3.3. Synthesis of OPC-Au NPs
3.4. Synthesis of Lipid DOX-ss
3.5. Synthesis of Polyphenol-Conjugated Gold NIR-ΙΙ Nano-Prodrugs
3.6. Particle Size and Zeta Potential Analysis
3.7. UV–Vis Analysis
3.8. FT-IR Analysis
3.9. Examination of Photothermal Conversion Performance
3.10. In Vitro Drug Release
3.11. Hemolytic Experiments
3.12. In Vitro Cytotoxicity
3.13. Lysosome Escape
3.14. ROS Detection
3.15. Expression of P-Glycoprotein (P-gp) Through Western Blot Examination
3.16. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liang, Y.; Lei, P.; An, R.; Du, P.; Liu, S.; Wei, Y.; Zhang, H. Biodegradable Monometallic Aluminum as a Biotuner for Tumor Pyroptosis. Angew. Chem. Int. Ed. Engl. 2024, 63, e202317304. [Google Scholar] [CrossRef]
- Shu, G.; Chen, K.; Li, J.; Liu, B.; Chen, X.; Wang, J.; Hu, X.; Lu, W.; Huang, H.; Zhang, S. Galangin Alleviated Doxorubicin-Induced Cardiotoxicity by Inhibiting through GSTP1/JNK Pathway. Phytomedicine 2024, 134, 155989. [Google Scholar] [CrossRef]
- Magar, K.T.; Boafo, G.F.; Li, X.; Chen, Z.; He, W. Liposome-Based Delivery of Biological Drugs. Chin. Chem. Lett. 2022, 33, 587–596. [Google Scholar] [CrossRef]
- Large, D.E.; Abdelmessih, R.G.; Fink, E.A.; Auguste, D.T. Liposome Composition in Drug Delivery Design, Synthesis, Characterization, and Clinical Application. Adv. Drug Deliv. Rev. 2021, 176, 113851. [Google Scholar] [CrossRef]
- Li, C.; Gao, D.; Gao, Y.; Zhang, R.; Qu, X.; Li, S.; Xing, C. NIR-II Regulation of Mitochondrial Potassium Channel with Dual-Targeted Conjugated Oligomer Nanoparticles for Efficient Cancer Theranostics In Vivo. Adv. Heal. Mater. 2023, 12, e2301954. [Google Scholar] [CrossRef]
- Fu, S.; Chang, L.; Liu, S.; Gao, T.; Sang, X.; Zhang, Z.; Mu, W.; Liu, X.; Liang, S.; Yang, H.; et al. Temperature Sensitive Liposome Based Cancer Nanomedicine Enables Tumour Lymph Node Immune Microenvironment Remodelling. Nat. Commun. 2023, 14, 2248. [Google Scholar] [CrossRef]
- Amin, M.; Lammers, T.; Hagen, T.L.T. Temperature-Sensitive Polymers to Promote Heat-Triggered Drug Release from Liposomes: Towards Bypassing EPR. Adv. Drug Deliv. Rev. 2022, 189, 114503. [Google Scholar] [CrossRef]
- Bian, J.; Xu, Y.; Sun, M.; Ma, Z.; Li, H.; Sun, C.; Xiong, F.; Zhao, X.; Yao, W.; Chen, Y.; et al. Engineering AIEgens-Tethered Gold Nanoparticles with Enzymatic Dual Self-Assembly for Amplified Cancer-Specific Phototheranostics. ACS Nano 2024, 18, 26784–26798. [Google Scholar] [CrossRef]
- Liu, X.; Zhou, W.; Wang, T.; Miao, S.; Lan, S.; Wei, Z.; Meng, Z.; Dai, Q.; Fan, H. Highly Localized, Efficient, and Rapid Photothermal Therapy Using Gold Nanobipyramids for liver Cancer Cells Triggered by Femtosecond Laser. Sci. Rep. 2023, 13, 3372. [Google Scholar] [CrossRef]
- Gong, L.; Chen, Y.; He, K.; Liu, J. Surface Coverage-Regulated Cellular Interaction of Ultrasmall Luminescent Gold Nanoparticles. ACS Nano 2019, 13, 1893–1899. [Google Scholar] [CrossRef]
- Rauf, A.; Imran, M.; Abu-Izneid, T.; Iahtisham-Ul-Haq; Patel, S.; Pan, X.; Naz, S.; Silva, A.S.; Saeed, F.; Suleria, H.A.R. Proanthocyanidins: A Comprehensive Review. Biomed. Pharmacother. 2019, 116, 108999. [Google Scholar] [CrossRef]
- Rutkowska, M.; Balcerczak, E.; Świechowski, R.; Dubicka, M.; Olszewska, M.A. Seasonal Variation in Phenylpropanoid Biosynthesis and in Vitro Antioxidant Activity of Sorbus Domestica Leaves: Harvesting Time Optimisation for Medicinal Application. Ind. Crop. Prod. 2020, 156, 112858. [Google Scholar] [CrossRef]
- Yin, Y.; Yang, X.; Li, L. Noncovalent Interaction between Proanthocyanidins and Soy Protein Isolate Fibers: Structure, Functionality and Interaction Mechanism. Food Hydrocoll. 2025, 160, 110663. [Google Scholar] [CrossRef]
- Zhao, Y.; Jiang, C.; Lu, J.; Sun, Y.; Cui, Y. Research Progress of Proanthocyanidins and Anthocyanidins. Phytotherapy Res. 2023, 37, 2552–2577. [Google Scholar] [CrossRef]
- Nie, F.; Liu, L.; Cui, J.; Zhao, Y.; Zhang, D.; Zhou, D.; Wu, J.; Li, B.; Wang, T.; Li, M.; et al. Oligomeric Proanthocyanidins: An Updated Review of Their Natural Sources, Synthesis, and Potentials. Antioxidants 2023, 12, 1004. [Google Scholar] [CrossRef]
- Zheng, W.; Feng, Y.; Bai, Y.; Feng, Z.; Yang, X.; Dang, B.; Xiao, M.; Zhang, J.; Han, S.-Q. Proanthocyanidins Extracted from Grape Seeds Inhibit the Growth of Hepatocellular Carcinoma Cells and Induce Apoptosis through the MAPK/AKT Pathway. Food Biosci. 2022, 45, 101337. [Google Scholar] [CrossRef]
- Wang, T.K.; Xu, S.; Li, S.; Zhang, Y. Proanthocyanidins Should Be a Candidate in the Treatment of Cancer, Cardiovascular Diseases and Lipid Metabolic Disorder. Molecules 2020, 25, 5971. [Google Scholar] [CrossRef]
- Wang, H.; Wang, D.; Huangfu, H.; Chen, S.; Qin, Q.; Ren, S.; Zhang, Y.; Fu, L.; Zhou, Y. Highly Efficient Photothermal Branched Au–Ag Nanoparticles Containing Procyanidins for Synergistic Antibacterial and Anti-Inflammatory Immunotherapy. Biomater. Sci. 2023, 11, 1335–1349. [Google Scholar] [CrossRef]
- Shaikh, S.; Younis, M.; Yuan, L. Functionalized DNA Nanostructures for Bioimaging. Co-ord. Chem. Rev. 2022, 469, 214648. [Google Scholar] [CrossRef]
- Cai, Y.; Chai, T.; Nguyen, W.; Liu, J.; Xiao, E.; Ran, X.; Ran, Y.; Du, D.; Chen, W.; Chen, X. Phototherapy in Cancer Treatment: Strategies and Challenges. Signal Transduct. Target. Ther. 2025, 10, 115. [Google Scholar] [CrossRef]
- Chen, Y.-S.; Zhao, Y.; Yoon, S.J.; Gambhir, S.S.; Emelianov, S. Miniature Gold Nanorods for Photoacoustic Molecular Imaging in the Second Near-Infrared Optical Window. Nat. Nanotechnol. 2019, 14, 465–472. [Google Scholar] [CrossRef]
- Liu, D.; Lin, R.; Wu, H.; Ji, J.; Wang, D.; Xue, Z.; Feng, S.; Chen, X. Green Synthesis, Characterization of Procyanidin-Mediated Gold Nanoparticles and Its Application in Fluorescence Detection of Prazosin. Microchem. J. 2022, 173, 107045. [Google Scholar] [CrossRef]
- Cui, L.; Xu, Q.; Lou, W.; Wang, Y.; Xi, X.; Chen, Y.; Sun, M.; Wang, Z.; Zhang, P.; Yang, S.; et al. Chitosan Oligosaccharide-Functionalized Nano-Prodrug for Cascade Chemotherapy through Oxidative Stress Amplification. Int. J. Biol. Macromol. 2024, 268, 131641. [Google Scholar] [CrossRef]
- Xi, J.; Li, M.; Jing, B.; An, M.; Yu, C.; Pinnock, C.B.; Zhu, Y.; Lam, M.T.; Liu, H. Long-Circulating Amphiphilic Doxorubicin for Tumor Mitochondria-Specific Targeting. ACS Appl. Mater. Interfaces 2018, 10, 43482–43492. [Google Scholar] [CrossRef]
- Yang, S.; Zhang, B.; Zhao, X.; Zhang, M.; Zhang, M.; Cui, L.; Zhang, L. Enhanced Efficacy against Drug-Resistant Tumors Enabled by Redox-Responsive Mesoporous-Silica-Nanoparticle-Supported Lipid Bilayers as Targeted Delivery Vehicles. Int. J. Mol. Sci. 2024, 25, 5553. [Google Scholar] [CrossRef]
- Nguyen, Q.N.; Wang, C.; Shang, Y.; Janssen, A.; Xia, Y. Colloidal Synthesis of Metal Nanocrystals: From Asymmetrical Growth to Symmetry Breaking. Chem. Rev. 2023, 123, 3693–3760. [Google Scholar] [CrossRef]
- Zhu, J.; Lennox, R.B. Insight into the Role of Ag in the Seed-Mediated Growth of Gold Nanorods: Implications for Biomedical Applications. ACS Appl. Nano Mater. 2021, 4, 3790–3798. [Google Scholar] [CrossRef]
- Zhang, Q.; Deng, T.-S.; Wei, M.-Z.; Chen, X.; Cheng, Z.; Li, S.; Gu, Y.-J. Symmetric and Asymmetric Overgrowth of a Ag Shell onto Gold Nanorods Assisted by Pt Pre-Deposition. RSC Adv. 2021, 11, 34516–34524. [Google Scholar] [CrossRef]
- Roach, L.; Coletta, P.L.; Critchley, K.; Evans, S.D. Controlling the Optical Properties of Gold Nanorods in One-Pot Syntheses. J. Phys. Chem. C 2022, 126, 3235–3243. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Guo, Y.; Shen, Y.; Chen, R.; Wang, F.; Zhou, D. HCl-Retarded Gold Nanorod Growth for Aspect Ratio and Shape Tuning. J. Nanosci. Nanotechnol. 2016, 16, 1194–1201. [Google Scholar] [CrossRef] [PubMed]
- Mannino, G.; Chinigò, G.; Serio, G.; Genova, T.; Gentile, C.; Munaron, L.; Bertea, C.M. Proanthocyanidins and Where to Find Them: A Meta-Analytic Approach to Investigate Their Chemistry, Biosynthesis, Distribution, and Effect on Human Health. Antioxidants 2021, 10, 1229. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Song, M.; Fang, Z.; Zheng, L.; Huang, X.; Liu, K. Applications and Challenges of Ultra-Small Particle Size Nanoparticles in Tumor Therapy. J. Control. Release 2023, 353, 699–712. [Google Scholar] [CrossRef] [PubMed]
- Di, J.; Gao, X.; Du, Y.; Zhang, H.; Gao, J.; Zheng, A. Size, Shape, Charge and “Stealthy” Surface: Carrier Properties Affect the Drug Circulation Time in Vivo. Asian J. Pharm. Sci. 2021, 16, 444–458. [Google Scholar] [CrossRef]
- Zhang, A.; Meng, K.; Liu, Y.; Pan, Y.; Qu, W.; Chen, D.; Xie, S. Absorption, Distribution, Metabolism, and Excretion of Nanocarriers in Vivo and their Influences. Adv. Colloid Interface Sci. 2020, 284, 102261. [Google Scholar] [CrossRef]
- Zhang, S.-Q.; Fu, Q.; Zhang, Y.-J.; Pan, J.-X.; Zhang, L.; Zhang, Z.-R.; Liu, Z.-M. Surface Loading of Nanoparticles on Engineered or Natural Erythrocytes for Prolonged Circulation Time: Strategies and Applications. Acta Pharmacol. Sin. 2021, 42, 1040–1054. [Google Scholar] [CrossRef]
- Andersen-Civil, A.I.S.; Leppä, M.M.; Thamsborg, S.M.; Salminen, J.-P.; Williams, A.R. Structure-Function Analysis of Purified Proanthocyanidins Reveals a Role for Polymer Size in Suppressing Inflammatory Responses. Commun. Biol. 2021, 4, 896. [Google Scholar] [CrossRef] [PubMed]
- Biao, L.; Tan, S.; Zhang, X.; Gao, J.; Liu, Z.; Fu, Y. Synthesis and Characterization of Proanthocyanidins-Functionalized Ag Nanoparticles. Colloids Surfaces B Biointerfaces 2018, 169, 438–443. [Google Scholar] [CrossRef]
- Meng, L.; Ren, J.; Li, L. Hyaluronic Acid-Targeted Mixed Micelles Encapsulating Hypericin for Breast Cancer Photodynamic Therapy. J. Drug Deliv. Sci. Technol. 2022, 78, 103961. [Google Scholar] [CrossRef]
- Liang, J.; Yang, X.; Liu, D.; Cong, M.; Song, Y.; Bai, S. Lipid/Hyaluronic Acid—Coated Doxorubicin-Fe3O4 as a Dual-Targeting Nanoparticle for Enhanced Cancer Therapy. Aaps Pharmscitech 2020, 21, 235. [Google Scholar] [CrossRef]
- Xue, Y.; Li, X.; Li, H.; Zhang, W. Quantifying Thiol–Gold Interactions towards the Efficient Strength Control. Nat. Commun. 2014, 5, 4348. [Google Scholar] [CrossRef]
- Abu Dayyih, A.; Alawak, M.; Ayoub, A.M.; Amin, M.U.; Abu Dayyih, W.; Engelhardt, K.; Duse, L.; Preis, E.; Brüßler, J.; Bakowsky, U. Thermosensitive Liposomes Encapsulating Hypericin: Characterization and Photodynamic Efficiency. Int. J. Pharm. 2021, 609, 121195. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Nie, T.; Fang, Y.; Huang, H.; Wu, J. Poly(Disulfide)s: From Synthesis to Drug Delivery. Biomacromolecules 2022, 23, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Oda, C.M.R.; Fernandes, R.S.; Lopes, S.C.d.A.; de Oliveira, M.C.; Cardoso, V.N.; Santos, D.M.; Pimenta, A.M.d.C.; Malachias, A.; Paniago, R.; Townsend, D.M.; et al. Synthesis, Characterization and Radiolabeling of Polymeric Nano-Micelles as a Platform for Tumor Delivering. Biomed. Pharmacother. 2017, 89, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Dasary, S.S.; Jones, Y.K.; Barnes, S.L.; Ray, P.; Singh, A.K. Alizarin Dye Based Ultrasensitive Plasmonic SERS Probe for Trace Level Cadmium Detection in Drinking Water. Sensors Actuators B Chem. 2016, 224, 65–72. [Google Scholar] [CrossRef]
- Tian, F.; Li, S.; Yang, X.; Fan, K.; Zhou, M.; Gao, D.; Hu, D.; Ju, S. Molecular Etching-Derived High-Brightness NIR-II Gold Nanoclusters for High-Resolution Bioimaging and Photothermal Therapy. Adv. Funct. Mater. 2025, 2418867. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, L.; Lou, W.; Wei, X.; Li, M.; Sun, M.; Wang, S.; Yang, S.; Zhang, L.; Zhou, G.; Li, P.; et al. Proanthocyanidin-Conjugated NIR-ΙΙ Nano-Prodrugs for Reversing Drug Resistance in Photothermal Therapy. Molecules 2025, 30, 2334. https://doi.org/10.3390/molecules30112334
Cui L, Lou W, Wei X, Li M, Sun M, Wang S, Yang S, Zhang L, Zhou G, Li P, et al. Proanthocyanidin-Conjugated NIR-ΙΙ Nano-Prodrugs for Reversing Drug Resistance in Photothermal Therapy. Molecules. 2025; 30(11):2334. https://doi.org/10.3390/molecules30112334
Chicago/Turabian StyleCui, Lan, Weishuang Lou, Xin Wei, Mengdi Li, Mengyao Sun, Siyue Wang, Shuoye Yang, Lu Zhang, Guangzhou Zhou, Peng Li, and et al. 2025. "Proanthocyanidin-Conjugated NIR-ΙΙ Nano-Prodrugs for Reversing Drug Resistance in Photothermal Therapy" Molecules 30, no. 11: 2334. https://doi.org/10.3390/molecules30112334
APA StyleCui, L., Lou, W., Wei, X., Li, M., Sun, M., Wang, S., Yang, S., Zhang, L., Zhou, G., Li, P., & Qu, L. (2025). Proanthocyanidin-Conjugated NIR-ΙΙ Nano-Prodrugs for Reversing Drug Resistance in Photothermal Therapy. Molecules, 30(11), 2334. https://doi.org/10.3390/molecules30112334





