Purification of Indole Contained in Wash Oil by Combination of Extraction and Crystallization (Part 2: Highly Efficient Purification of Indole in Indole-Concentrated Oil via Solute Crystallization)
Abstract
1. Introduction
2. Experimental Procedure
2.1. Manufacturing of Model ICO
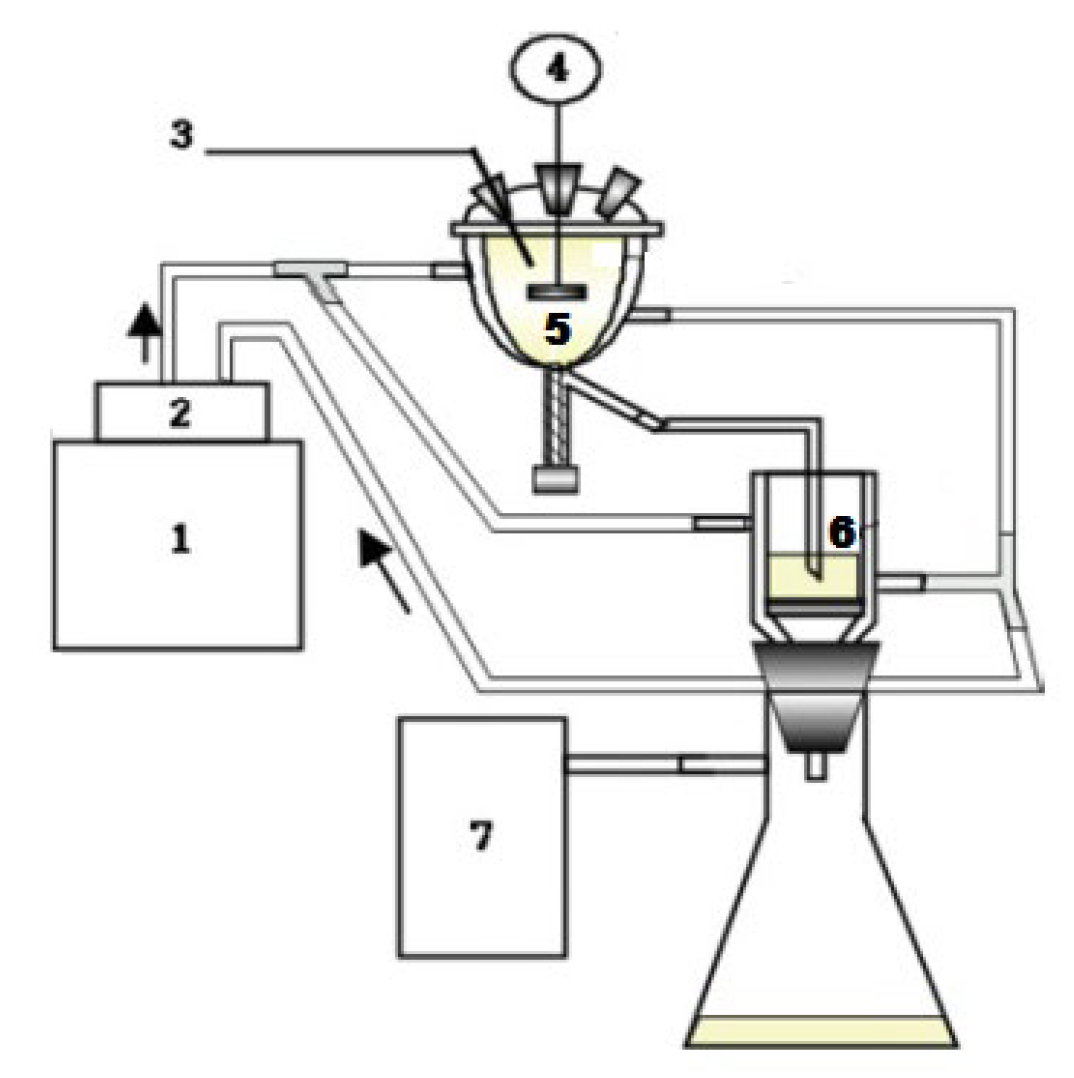
2.2. SC Operation
2.3. Material System and Experimental Conditions
3. Results and Discussion
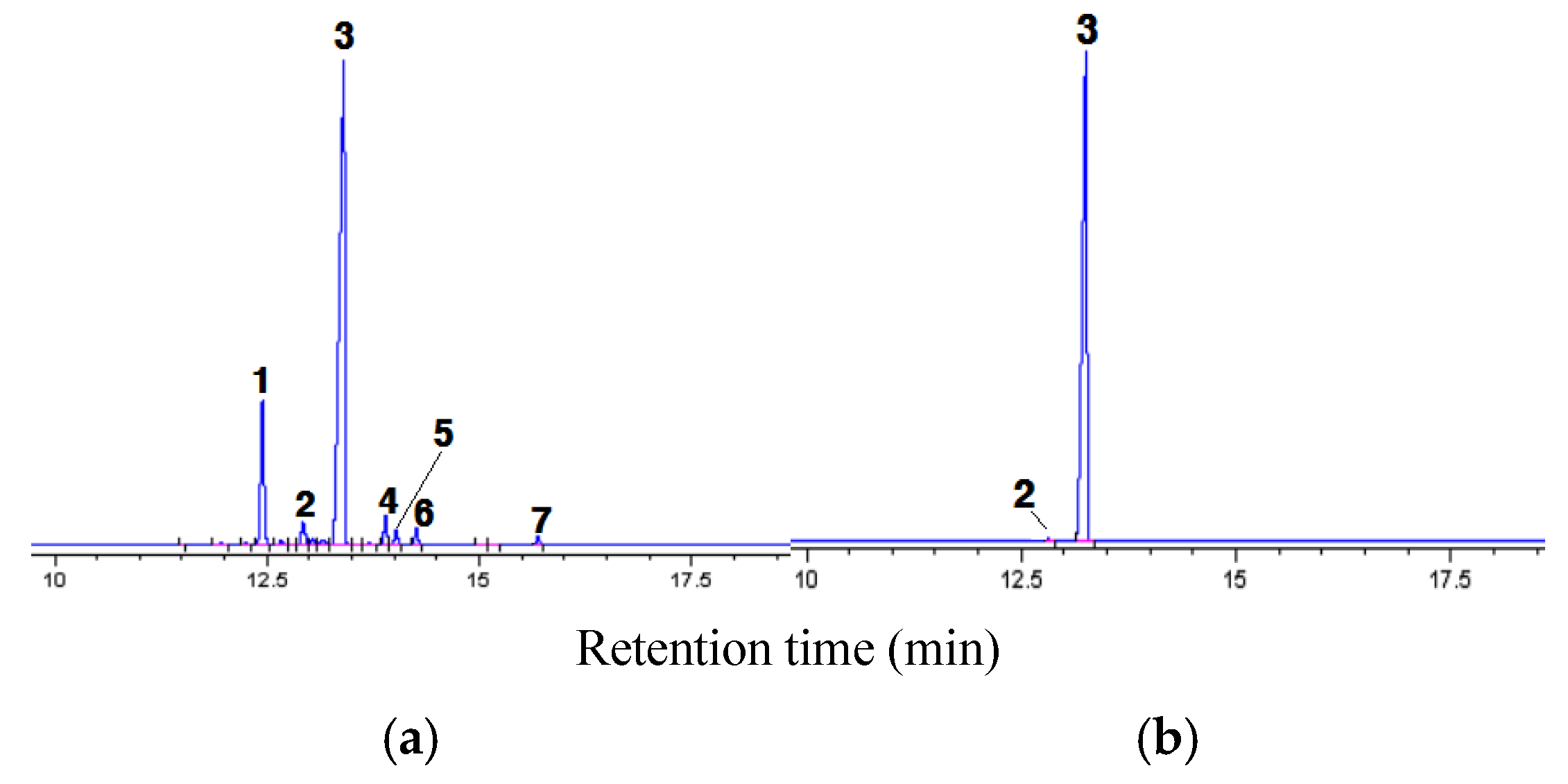
3.1. Gas Chromatogram of Model ICO
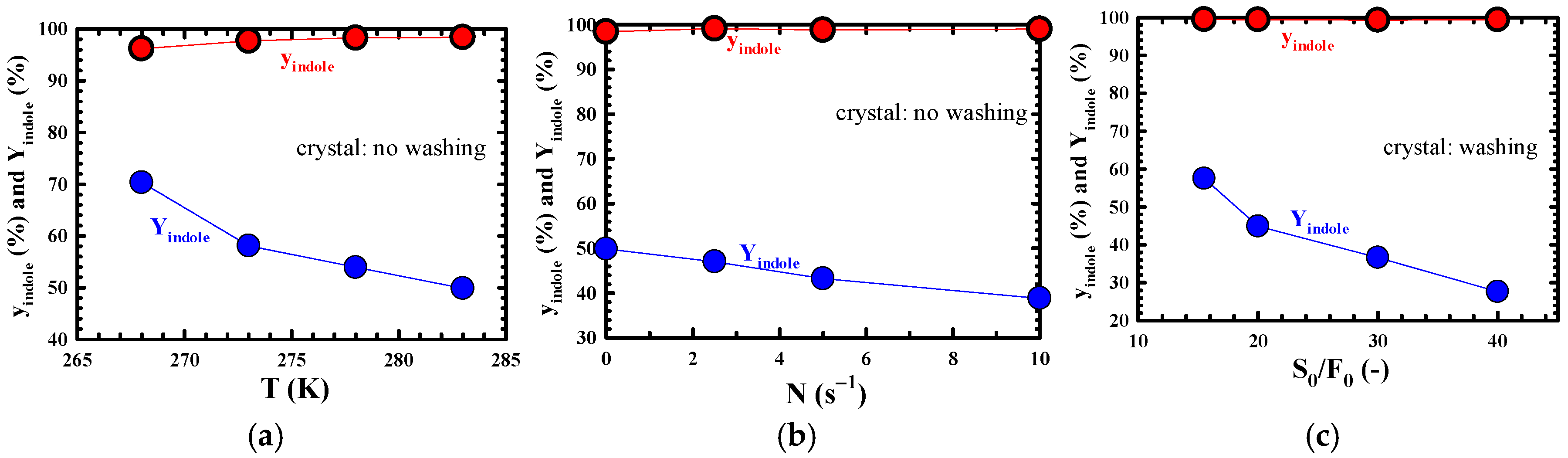
3.2. Highly Efficient Purification Performance of Indole
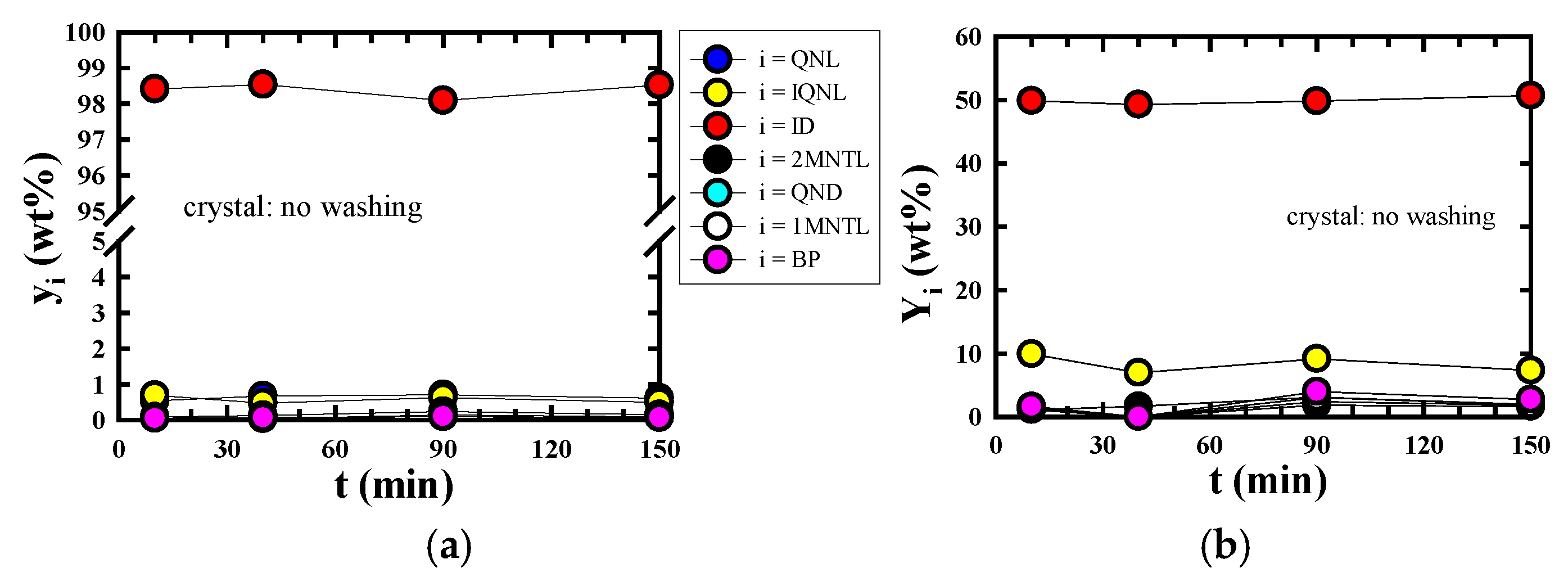
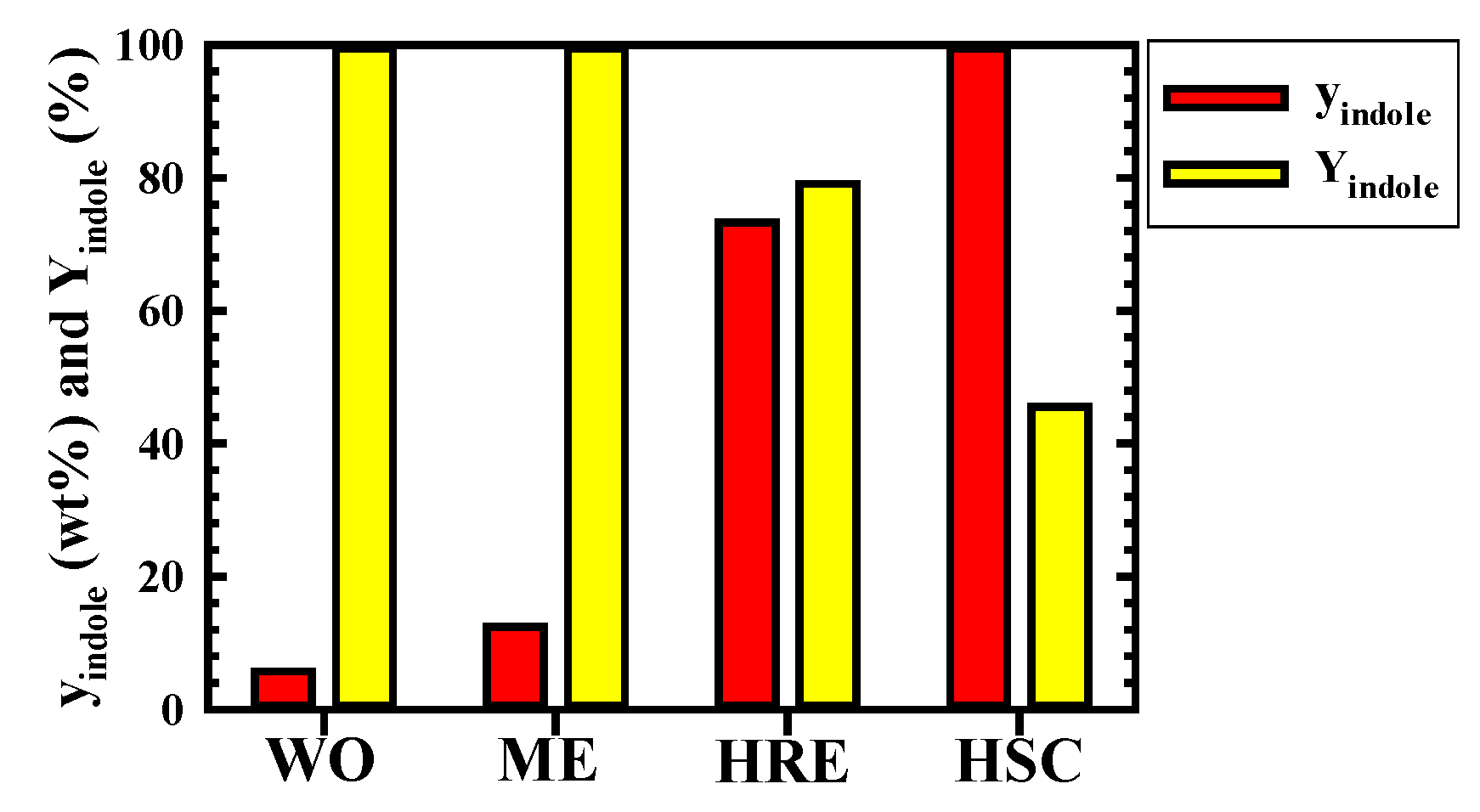
3.3. Changes in yindole and Yindole According to Each Process
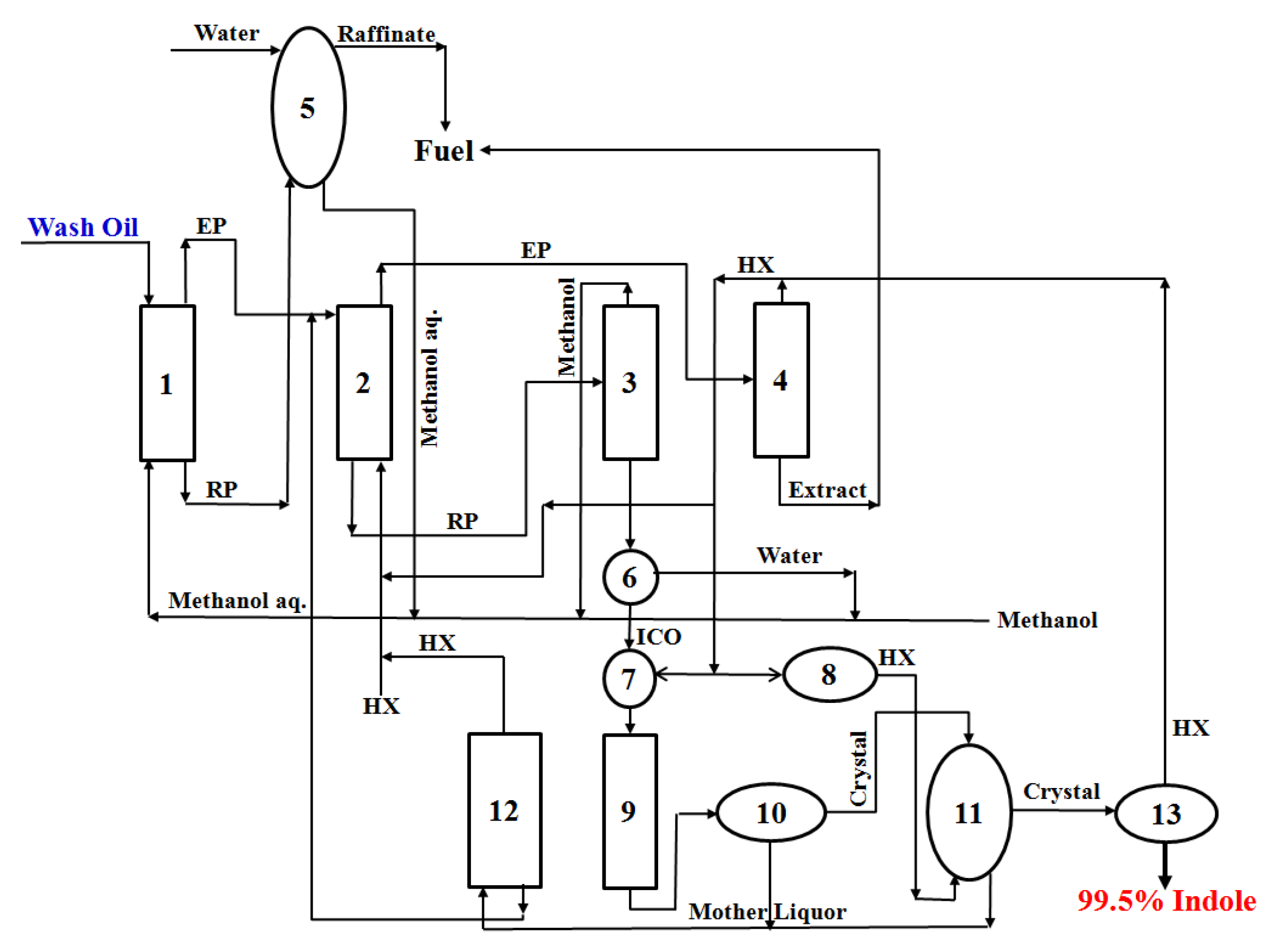
3.4. Proposal of a Novel Process for the Highly Efficient Purification of Indole in Wash Oil
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Miroshnichenko, D.; Bannikov, A.; Bannikov, L.; Borisenko, O.; Shishkin, A.; Gavrilovs, P.; Tertychnyi, V. Impact of wash oil composition on degradation: A comparative analysis of “light” and “heavy” oils. Resources 2025, 14, 5. [Google Scholar] [CrossRef]
- Lobovich, D.V.; Zinov’eva, I.V.; Milevskii, N.A.; Kostanyan, A.E.; Zakhodyaeva, Y.A.; Voshkin, A.A. Extraction kinetics of pyridine, quinoline, and indole from the organic phase with natural deep eutectic solvents and separation study using a centrifugal extractor. Processes 2024, 12, 488. [Google Scholar] [CrossRef]
- Kim, S.J. Study on removal of nitrogen-containing heterocyclic compounds contained in crude methylnaphthalene oil by formamide extraction. Processes 2024, 12, 1550. [Google Scholar] [CrossRef]
- Sakanishi, K.; Obata, H.; Mochida, I.; Sakaki, T. Capture and recovery of indole from methylnaphthalene oil in a continuous supercritical CO2 extraction apparatus over a fixed bed of anion-exchange resin. Ind. Eng. Chem. Res. 1996, 35, 335–337. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, J.; Shen, J.; Wang, Y.; Liu, G.; Niu, Y.; Sheng, Q. Highly efficient extraction of indole from model wash oil using environmentally benign deep eutectic solvents. Sep. Purif. Technol. 2022, 285, 120381. [Google Scholar] [CrossRef]
- Kim, S.J. Separation and purification of indole in model coal tar fraction of 9 compounds system. Polycycl. Aromat. Compd. 2019, 39, 60–72. [Google Scholar] [CrossRef]
- Mindt, M.; Beyraghdar Kashkooli, A.; Suarez-Diez, M.; Ferrer, L.; Jilg, T.; Bosch, D.; Martins dos Santos, V.; Wendisch, V.F.; Cankar, K. Production of indole by corynebacterium glutamicum microbial cell factories for flavor and fragrance applications. Microb. Cell Factories 2022, 21, 45. [Google Scholar] [CrossRef]
- Kim, S.J. Purification of indole contained in wash oil by combination of extraction and crystallization (Part 1: Recovery and concentration of indole contained in wash oil by solvent extraction). Molecules 2022, 27, 5331. [Google Scholar] [CrossRef]
- Sakanishi, K.; Obata, H.; Mochida, I.; Sakaki, T. Removal and recovery of quinoline bases from methylnaphthalene oil in a semicontinuous supercritical CO2 separation apparatus with a fixed bed of supported aluminum sulfate. Ind. Eng. Chem. Res. 1995, 34, 4118–4124. [Google Scholar] [CrossRef]
- Wang, F.W. Extraction of washing oil distillate from tar and its application in fine chemical industry. Coal Chem. Ind. 2004, 2, 26–28. [Google Scholar]
- Xiao, R.H.; Gao, W.H. Study on the recovery of indole from coal tar wash oil. Coal Convers. 1998, 21, 59–61. [Google Scholar]
- Mamoru, Y.; Tomonori, K. Separation and purification of indole from coal tar by supercritical fluid extraction. J. Chem. Eng. Jpn. 1993, 26, 153–158. [Google Scholar]
- Uemasu, I.; Nakayama, T. Concentration of indole in coal tar using α-cyclodextrin as the host for inclusion complexation. J. Incl. Phenom. Mol. Recognit. Chem. 1989, 7, 327–331. [Google Scholar] [CrossRef]
- Uemasu, I. Effect of methanol-water mixture solvent on concentration of indole in coal tar using α-cyclodextrin as complexing agent. J. Jpn. Pet. Inst. 1991, 34, 371–374. [Google Scholar] [CrossRef][Green Version]
- Yamamoto, Y.; Sato, Y.; Ebina, T.; Yokoyama, C.; Takahasi, S.; Mito, Y.; Tanabe, H.; Nishiguchi, N.; Nagaoka, K. Separation of high purity indole from coal tar by high pressure crystallization. Fuel 1991, 70, 565–566. [Google Scholar] [CrossRef]
- Gao, P.; Zhang, J.; Guo, Z.; Gao, J.; Xu, D.; Ma, Y.; Zhang, L.; Wang, Y. Experimental and quantum chemical calculations investigations of morpholine-based ionic liquids as extractants for efficient extraction of nitrogen heterocyclic neutral compounds. Fuel 2023, 333, 126446. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, T.; Gao, P.; Gao, J.; Xu, D.; Zhao, P.; Zhang, L.; Wang, Y. Separation of indole by designed ionic liquids with dual functional chemical sites: Mechanism exploration and experimental validation. J. Environ. Chem. Eng. 2021, 9, 105971. [Google Scholar] [CrossRef]
- Ji, Y.; Hou, Y.; Ren, S.; Wu, W. Highly efficient separation of indole from model wash oil using tetraethyl ammonium amino acid ionic liquids. Sep. Purif. Technol. 2021, 258, 117997. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, D.; Gao, J.; Zhou, S.; Zhao, L.; Zhang, Z. Extraction and mechanism for the separation of neutral N-compounds from coal tar by ionic liquids. Fuel 2017, 194, 27–35. [Google Scholar] [CrossRef]
- Xu, D.; Zhang, M.; Gao, J.; Zhang, L.; Zhou, S.; Wang, Y. Separation of heterocyclic nitrogen compounds from coal tar fractions via ionic liquids: COSMO-SAC screening and experimental study. Chem. Eng. Commun. 2019, 206, 1199–1217. [Google Scholar] [CrossRef]
- Jiao, T.; Zhuang, X.; He, H.; Zhao, L.; Li, C.; Chen, H.; Zhang, S. An ionic liquid extraction process for the separation of indole from wash oil. Green Chem. 2015, 17, 3783–3790. [Google Scholar] [CrossRef]
- Ji, Y.; Hou, Y.; Ren, S.; Niu, M.; Yao, C.; Wu, W. Efficient extraction of indole from wash oil by quaternary ammonium salts via forming deep eutectic solvents. Fuel 2018, 215, 330–338. [Google Scholar] [CrossRef]
- Jiao, T.; Ren, C.; Lin, S.; Zhang, L.; Xu, X.; Zhang, Y.; Zhang, W.; Liang, P. The extraction mechanism research for the separation of indole through the formation of deep eutectic solvents with quaternary ammonium salts. J. Mol. Liq. 2022, 347, 118325. [Google Scholar] [CrossRef]
| Compound | Physical Property | Composition (wt%) | |
|---|---|---|---|
| b.p. * [K] | m.p. ** [K] | Model ICO | |
| Quinoline (C9H7N) | 511 | 257 | 14.34 |
| Iso-quinoline (C9H7N) | 515 | 299–301 | 2.59 |
| Indole (C8H7N) | 526 | 325 | 73.31 |
| 2-Methylnaphthalene (C11H10) | 514–515 | 307–309 | 2.58 |
| Quinaldine (C10H9N) | 521 | 271 | 1.36 |
| 1-Methylnaphthalene (C11H10) | 513–516 | 251 | 1.56 |
| Biphenyl (C12H10) | 528 | 342 | 0.80 |
| Others | 3.46 | ||
| Material System | |
| Raw material | model indole-concentrated oil (model ICO) |
| Crystallization solvent | n-hexane |
| Washing solvent | n-hexane |
| Coolant | 50 vol% methanol aqueous solution |
| Experimental conditions | |
| Crystallization time, t [min] | 10–150 |
| Crystallization temperature, T [K] | 268–283 |
| Impeller speed, N [s−1] | 10–150 |
| Initial volume ratio of solvent to model ICO, S0/F0 (-) | 15.5–40 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.J. Purification of Indole Contained in Wash Oil by Combination of Extraction and Crystallization (Part 2: Highly Efficient Purification of Indole in Indole-Concentrated Oil via Solute Crystallization). Molecules 2025, 30, 2327. https://doi.org/10.3390/molecules30112327
Kim SJ. Purification of Indole Contained in Wash Oil by Combination of Extraction and Crystallization (Part 2: Highly Efficient Purification of Indole in Indole-Concentrated Oil via Solute Crystallization). Molecules. 2025; 30(11):2327. https://doi.org/10.3390/molecules30112327
Chicago/Turabian StyleKim, Su Jin. 2025. "Purification of Indole Contained in Wash Oil by Combination of Extraction and Crystallization (Part 2: Highly Efficient Purification of Indole in Indole-Concentrated Oil via Solute Crystallization)" Molecules 30, no. 11: 2327. https://doi.org/10.3390/molecules30112327
APA StyleKim, S. J. (2025). Purification of Indole Contained in Wash Oil by Combination of Extraction and Crystallization (Part 2: Highly Efficient Purification of Indole in Indole-Concentrated Oil via Solute Crystallization). Molecules, 30(11), 2327. https://doi.org/10.3390/molecules30112327





