Ultrasound-Assisted Osmotic Dehydration of Apples in Xylitol Solution: Effects on Kinetics, Physicochemical Properties and Antioxidant Activity
Abstract
1. Introduction
2. Results and Discussion
2.1. Kinetic Parameters of the Osmotic Dehydration Process
2.2. Physicochemical and Antioxidant Properties of Osmotically Dehydrated Apples
2.2.1. Effect of Osmotic Dehydration Process on Dry Matter Content, Water Activity, Total Acidity, pH, and Sugar Profile of Analyzed Apples
2.2.2. Effect of Osmotic Dehydration Process on Total Phenolic Content Vitamin C Content and Antioxidant Activity in the Analyzed Apples
2.2.3. Effect of Osmotic Dehydration Process on Color Parameters of Apples
2.3. Principal Component Analysis
3. Materials and Methods
3.1. Chemicals
3.2. Materials
3.3. Ultrasonic Pretreatment Osmotic Dehydration Procedure
3.4. Kinetics Parameters
3.5. Scanning Electron Microscopy (SEM)
3.6. Determination of Physicochemical Properties
3.7. Determination of Sugar Profile
3.8. Procedure of Ethanolic Extraction
3.9. Determination of Total Phenolic Content
3.10. Determination of DPPH Radical-Scavenging Activity
3.11. Determination of ABTS Cation Radical-Scavenging Activity
3.12. Determination of Vitamin C Content
3.13. Color Parameters
3.14. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Matuska, M.; Lenart, A.; Lazarides, H.N. On the Use of Edible Coatings to Monitor Osmotic Dehydration Kinetics for Minimal Solids Uptake. J. Food Eng. 2006, 72, 85–91. [Google Scholar] [CrossRef]
- Salehi, F. Recent Advances in the Ultrasound-Assisted Osmotic Dehydration of Agricultural Products: A Review. Food Biosci. 2023, 51, 102307. [Google Scholar] [CrossRef]
- Nowacka, M.; Tylewicz, U.; Laghi, L.; Dalla Rosa, M.; Witrowa-Rajchert, D. Effect of Ultrasound Treatment on the Water State in Kiwifruit during Osmotic Dehydration. Food Chem. 2014, 144, 18–25. [Google Scholar] [CrossRef]
- Giraldo, G.; Talens, P.; Fito, P.; Chiralt, A. Influence of Sucrose Solution Concentration on Kinetics and Yield during Osmotic Dehydration of Mango. J. Food Eng. 2003, 58, 33–43. [Google Scholar] [CrossRef]
- Pluta, M.; Klewicki, R. Mass Transport and Changes in the Saccharide Profile during Osmotic Dehydration of Apricot and Chokeberry Fruits. Biotechnol. Food Sci. 2022, 84, 19–32. [Google Scholar] [CrossRef]
- Brochier, B.; Inácio, J.M.; Noreña, C.P.Z. Study of Osmotic Dehydration of Kiwi Fruit Using Sucrose Solution. Braz. J. Food Technol. 2019, 22, e2018146. [Google Scholar] [CrossRef]
- Nowacka, M.; Tylewicz, U.; Tappi, S.; Siroli, L.; Lanciotti, R.; Romani, S.; Witrowa-Rajchert, D. Ultrasound Assisted Osmotic Dehydration of Organic Cranberries (Vaccinium Oxycoccus): Study on Quality Parameters Evolution during Storage. Food Control 2018, 93, 40–47. [Google Scholar] [CrossRef]
- Boudhrioua Mihoubi Nourh, D.M.N.; Courtois Franci, K.N. Effect of Osmo-Dehydration Conditions on the Quality Attributes of Pears. J. Food Process. Technol. 2013, 4, 100256. [Google Scholar] [CrossRef]
- Wiktor, A.; Chadzynska, M.; Rybak, K.; Dadan, M.; Witrowa-Rajchert, D.; Nowacka, M. The Influence of Polyols on the Process Kinetics and Bioactive Substance Content in Osmotic Dehydrated Organic Strawberries. Molecules 2022, 27, 1376. [Google Scholar] [CrossRef]
- Grembecka, M. Sugar Alcohols—Their Role in the Modern World of Sweeteners: A Review. Eur. Food Res. Technol. 2015, 241, 1–14. [Google Scholar] [CrossRef]
- Cichowska, J.; Woźniak, Ł.; Figiel, A.; Witrowa-Rajchert, D. The Influence of Osmotic Dehydration in Polyols Solutions on Sugar Profiles and Color Changes of Apple Tissue. Period. Polytech. Chem. Eng. 2019, 64, 530–538. [Google Scholar] [CrossRef]
- Pobiega, K.; Matys, A.; Trusinska, M.; Rybak, K.; Witrowa-Rajchert, D.; Nowacka, M. The Effect of Ultrasound and Pulsed Electric Field on the Osmotic Dehydration Process of Strawberries. Appl. Sci. 2023, 13, 12335. [Google Scholar] [CrossRef]
- Cichowska, J.; Witrowa-Rajchert, D.; Stasiak-Różańska, L.; Figiel, A. Ultrasound-Assisted Osmotic Dehydration of Apples in Polyols and Dihydroxyacetone (DHA) Solutions. Molecules 2019, 24, 3429. [Google Scholar] [CrossRef]
- Wiktor, A.; Sledz, M.; Nowacka, M.; Rybak, K.; Witrowa-Rajchert, D. The Influence of Immersion and Contact Ultrasound Treatment on Selected Properties of the Apple Tissue. Appl. Acoust. 2016, 103, 136–142. [Google Scholar] [CrossRef]
- Fernandes, F.A.N.; Gallão, M.I.; Rodrigues, S. Effect of Osmosis and Ultrasound on Pineapple Cell Tissue Structure during Dehydration. J. Food Eng. 2009, 90, 186–190. [Google Scholar] [CrossRef]
- Fernandes, F.A.N.; Gallão, M.I.; Rodrigues, S. Effect of Osmotic Dehydration and Ultrasound Pre-Treatment on Cell Structure: Melon Dehydration. LWT-Food Sci. Technol. 2008, 41, 604–610. [Google Scholar] [CrossRef]
- Prithani, R.; Dash, K.K. Mass Transfer Modelling in Ultrasound Assisted Osmotic Dehydration of Kiwi Fruit. Innov. Food Sci. Emerg. Technol. 2020, 64, 102407. [Google Scholar] [CrossRef]
- Rahaman, A.; Zeng, X.-A.; Kumari, A.; Rafiq, M.; Siddeeg, A.; Manzoor, M.F.; Baloch, Z.; Ahmed, Z. Influence of Ultra-sound-Assisted Osmotic Dehydration on Texture, Bioactive Compounds and Metabolites Analysis of Plum. Ultrason. Sonochem. 2019, 58, 104643. [Google Scholar] [CrossRef]
- Osae, R.; Zhou, C.; Xu, B.; Tchabo, W.; Tahir, H.E.; Mustapha, A.T.; Ma, H. Effects of Ultrasound, Osmotic Dehydration, and Osmosonication Pretreatments on Bioactive Compounds, Chemical Characterization, Enzyme Inactivation, Color, and Anti-oxidant Activity of Dried Ginger Slices. J. Food Biochem. 2019, 43, e12832. [Google Scholar] [CrossRef]
- Corrêa, J.L.G.; Justus, A.; De Oliveira, L.F.; Alves, G.E. Osmotic Dehydration of Tomato Assisted by Ultrasound: Evaluation of the Liquid Media on Mass Transfer and Product Quality. Int. J. Food Eng. 2015, 11, 505–516. [Google Scholar] [CrossRef]
- Asghari, A.; Zongo, P.A.; Osse, E.F.; Aghajanzadeh, S.; Raghavan, V.; Khalloufi, S. Review of Osmotic Dehydration: Promising Technologies for Enhancing Products’ Attributes, Opportunities, and Challenges for the Food Industries. Comp. Rev. Food Sci. Food Saf. 2024, 23, e13346. [Google Scholar] [CrossRef] [PubMed]
- Kowalska, H.; Lenart, A. Mass Exchange during Osmotic Pretreatment of Vegetables. J. Food Eng. 2001, 49, 137–140. [Google Scholar] [CrossRef]
- Assis, F.R.; Morais, R.M.S.C.; Morais, A.M.M.B. Mass Transfer in Osmotic Dehydration of Food Products: Comparison Between Mathematical Models. Food Eng. Rev. 2016, 8, 116–133. [Google Scholar] [CrossRef]
- Brochier, B.; Marczak, L.D.F.; Noreña, C.P.Z. Use of Different Kinds of Solutes Alternative to Sucrose in Osmotic Dehydration of Yacon. Braz. Arch. Biol. Technol. 2014, 58, 34–40. [Google Scholar] [CrossRef]
- Cichowska, J.; Żubernik, J.; Czyżewski, J.; Kowalska, H.; Witrowa-Rajchert, D. Efficiency of Osmotic Dehydration of Apples in Polyols Solutions. Molecules 2018, 23, 446. [Google Scholar] [CrossRef]
- Łata, B. Relationship between Apple Peel and the Whole Fruit Antioxidant Content: Year and Cultivar Variation. J. Agric. Food Chem. 2007, 55, 663–671. [Google Scholar] [CrossRef]
- Ticha, A.; Salejda, A.M.; Hyspler, R.; Matejicek, A.; Paprstein, F.; Zadak, Z. Sugar Composition Of Apple Cultivars And Its Relationship To Sensory Evaluation. Żywność Nauka Technol. Jakość 2015, 22, 137–150. [Google Scholar] [CrossRef]
- Nowacka, M.; Fijalkowska, A.; Wiktor, A.; Dadan, M.; Tylewicz, U.; Dalla Rosa, M.; Witrowa-Rajchert, D. Influence of Power Ultrasound on the Main Quality Properties and Cell Viability of Osmotic Dehydrated Cranberries. Ultrasonics 2018, 83, 33–41. [Google Scholar] [CrossRef]
- Butkeviciute, A.; Viskelis, J.; Viskelis, P.; Liaudanskas, M.; Janulis, V. Changes in the Biochemical Composition and Physico-chemical Properties of Apples Stored in Controlled Atmosphere Conditions. Appl. Sci. 2021, 11, 6215. [Google Scholar] [CrossRef]
- Khoualdia, B.; Ben-Ali, S.; Hannachi, A. Pomegranate Arils Osmotic Dehydration: Effect of Pre-Drying on Mass Transfer. J. Food Sci. Technol. 2020, 57, 2129–2138. [Google Scholar] [CrossRef]
- Fasogbon, B.M.; Gbadamosi, S.O.; Taiwo, K.A. Studies on the Osmotic Dehydration and Rehydration Characteristics of Pineapple Slices. J. Food Process Technol. 2013, 4, 1000220. [Google Scholar] [CrossRef]
- Garcia-Noguera, J.; Oliveira, F.I.P.; Gallão, M.I.; Weller, C.L.; Rodrigues, S.; Fernandes, F.A.N. Ultrasound-Assisted Osmotic Dehydration of Strawberries: Effect of Pretreatment Time and Ultrasonic Frequency. Dry. Technol. 2010, 28, 294–303. [Google Scholar] [CrossRef]
- Wojtyś, A.; Pietrzyk, S.; Bogacz, S.; Witkowicz, R. Osmotic Dehydration of Japanese Quince (Chaenomeles Japonica) Fruits in Erythritol Solutions: Impact of Processing Conditions on the Kinetic Parameters and on Physicochemical and Antioxidant Properties of the Fruits. Molecules 2024, 29, 5524. [Google Scholar] [CrossRef]
- Lindley, M.G.; Birch, G.G.; Khan, R. Sweetness of Sucrose and Xylitol. Structural Considerations. J. Sci. Food Agric. 1976, 27, 140–144. [Google Scholar] [CrossRef]
- Mussatto, S.I. Application of Xylitol in Food Formulations and Benefits for Health. In D-Xylitol; Da Silva, S.S., Chandel, A.K., Eds.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 309–323. ISBN 978-3-642-31886-3. [Google Scholar]
- Baranowski, D.; Rutkowska, J.; Antoniewska, A. Ksylitol—Rola technologiczna i żywieniowa. Żywność Nauka Technol. Jakość 2020, 123, 5–21. [Google Scholar] [CrossRef]
- Schaefer, E.J.; Gleason, J.A.; Dansinger, M.L. Dietary Fructose and Glucose Differentially Affect Lipid and Glucose Homeostasis. J. Nutr. 2009, 139, 1257S–1262S. [Google Scholar] [CrossRef] [PubMed]
- Tetzloff, W.; Dauchy, F.; Medimagh, S.; Carr, D.; Bär, A. Tolerance to Subchronic, High-Dose Ingestion of Erythritol in Human Volunteers. Regul. Toxicol. Pharmacol. 1996, 24, S286–S295. [Google Scholar] [CrossRef]
- Lee, B.D.; Park, M.K. Effects and safety of xylitol on middle ear epithelial cells. J. Int. Adv. Otol. 2014, 10, 19–24. [Google Scholar] [CrossRef]
- Livesey, G. Health potential of polyols as sugar replacers, with emphasis on low glycaemic properties. Nutr. Res. Rev. 2003, 16, 163–191. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition and Allergy (NDA). Scientific Opinion on the substantiation of health claims related to the sugar replacers xylitol, sorbitol, mannitol, maltitol, lactitol, isomalt, erythritol, D-tagatose, isomaltulose, sucralose and polydextrose and maintenance of tooth mineralisation by decreasing tooth demineralisation. EFSA J. 2011, 9, 2076. [Google Scholar]
- Yu, Y.; Jin, T.Z.; Fan, X.; Wu, J. Biochemical Degradation and Physical Migration of Polyphenolic Compounds in Osmotic Dehydrated Blueberries with Pulsed Electric Field and Thermal Pretreatments. Food Chem. 2018, 239, 1219–1225. [Google Scholar] [CrossRef] [PubMed]
- Kroehnke, J.; Szadzińska, J.; Radziejewska-Kubzdela, E.; Biegańska-Marecik, R.; Musielak, G.; Mierzwa, D. Osmotic Dehy-dration and Convective Drying of Kiwifruit (Actinidia Deliciosa)—The Influence of Ultrasound on Process Kinetics and Product Quality. Ultrason. Sonochem. 2021, 71, 105377. [Google Scholar] [CrossRef] [PubMed]
- Pirce, F.; Vieira, T.M.F.S.; Augusto-Obara, T.R.; Alencar, S.M.; Romero, F.; Scheuermann, E. Effects of Convective Drying Assisted by Ultrasound and Osmotic Solution on Polyphenol, Antioxidant and Microstructure of Murtilla (Ugni Molinae Turcz) Fruit. J. Food Sci. Technol. 2021, 58, 138–146. [Google Scholar] [CrossRef]
- Kowalska, H.; Marzec, A.; Kowalska, J.; Ciurzyńska, A.; Czajkowska, K.; Cichowska, J.; Rybak, K.; Lenart, A. Osmotic De-hydration of Honeoye Strawberries in Solutions Enriched with Natural Bioactive Molecules. LWT-Food Sci. Technol. 2017, 85, 500–505. [Google Scholar] [CrossRef]
- Siucińska, K.; Mieszczakowska-Frąc, M.; Połubok, A.; Konopacka, D. Effects of Ultrasound Assistance on Dehydration Pro-cesses and Bioactive Component Retention of Osmo-Dried Sour Cherries. J. Food Sci. 2016, 81, C1654–C1661. [Google Scholar] [CrossRef]
- Amami, E.; Khezami, W.; Mezrigui, S.; Badwaik, L.S.; Bejar, A.K.; Perez, C.T.; Kechaou, N. Effect of Ultrasound-Assisted Osmotic Dehydration Pretreatment on the Convective Drying of Strawberry. Ultrason. Sonochem. 2017, 36, 286–300. [Google Scholar] [CrossRef] [PubMed]
- Lepaus, B.M.; Valiati, B.S.; Machado, B.G.; Domingos, M.M.; Silva, M.N.; Faria-Silva, L.; Bernardes, P.C.; da Silva Oliveira, D.; de São José, J.F.B. Impact of Ultrasound Processing on the Nutritional Components of Fruit and Vegetable Juices. Trends Food Sci. Technol. 2023, 138, 752–765. [Google Scholar] [CrossRef]
- Wiktor, A.; Nowacka, M.; Anuszewska, A.; Rybak, K.; Dadan, M.; Witrowa-Rajchert, D. Drying Kinetics and Quality of De-hydrated Cranberries Pretreated by Traditional and Innovative Techniques. J. Food Sci. 2019, 84, 1820–1828. [Google Scholar] [CrossRef] [PubMed]
- Nowacka, M.; Fijalkowska, A.; Dadan, M.; Rybak, K.; Wiktor, A.; Witrowa-Rajchert, D. Effect of Ultrasound Treatment during Osmotic Dehydration on Bioactive Compounds of Cranberries. Ultrasonics 2018, 83, 18–25. [Google Scholar] [CrossRef]
- Sakooei-Vayghan, R.; Peighambardoust, S.H.; Hesari, J.; Peressini, D. Effects of Osmotic Dehydration (with and without Son-ication) and Pectin-Based Coating Pretreatments on Functional Properties and Color of Hot-Air Dried Apricot Cubes. Food Chem. 2020, 311, 125978. [Google Scholar] [CrossRef]
- Rojas, M.L.; Kubo, M.T.K.; Caetano-Silva, M.E.; Augusto, P.E.D. Ultrasound Processing of Fruits and Vegetables, Structural Modification and Impact on Nutrient and Bioactive Compounds: A Review. Int. J. Food Sci. Technol. 2021, 56, 4376–4395. [Google Scholar] [CrossRef]
- Yin, X.; Chen, K.; Cheng, H.; Chen, X.; Feng, S.; Song, Y.; Liang, L. Chemical Stability of Ascorbic Acid Integrated into Com-mercial Products: A Review on Bioactivity and Delivery Technology. Antioxidants 2022, 11, 153. [Google Scholar] [CrossRef]
- Landim, A.P.M.; Barbosa, M.I.M.J.; Júnior, J.L.B. Influence of Osmotic Dehydration on Bioactive Compounds, Antioxidant Capacity, Color and Texture of Fruits and Vegetables: A Review. Cienc. Rural 2016, 46, 1714–1722. [Google Scholar] [CrossRef]
- Bhagya Raj, G.V.S.; Dash, K.K. Ultrasound Assisted Osmotic Dehydration of Dragon Fruit Slices: Modeling and Optimization Using Integrated Artificial Neural Networks and Genetic Algorithms. J. Food Process. Preserv. 2022, 46, e16993. [Google Scholar] [CrossRef]
- Radojčin, M.; Babić, M.; Babić, L.; Pavkov, I.; Bukurov, M.; Bikić, S.; Mitrevski, V. Effects of Osmotic Pretreatment on Quality and Physical Properties of Dried Quinces (Cydonia Oblonga). J. Food Nutr. Res. 2015, 54, 142–155. [Google Scholar]
- Zhu, L.; Zhu, L.; Murtaza, A.; Liu, Y.; Liu, S.; Li, J.; Iqbal, A.; Xu, X.; Pan, S.; Hu, W. Ultrasonic Processing Induced Activity and Structural Changes of Polyphenol Oxidase in Orange (Citrus Sinensis Osbeck). Molecules 2019, 24, 1922. [Google Scholar] [CrossRef]
- Zawawi, N.A.F.; Hazmi, N.A.M.; How, M.S.; Kantono, K.; Silva, F.V.M.; Sulaiman, A. Thermal, High Pressure, and Ultrasound Inactivation of Various Fruit Cultivars’ Polyphenol Oxidase: Kinetic Inactivation Models and Estimation of Treatment Energy Requirement. Appl. Sci. 2022, 12, 1864. [Google Scholar] [CrossRef]
- Silva, L.C.A.; Almeida, P.S.; Rodrigues, S.; Fernandes, F.A.N. Inactivation of Polyphenoloxidase and Peroxidase in Apple Cubes and in Apple Juice Subjected to High Intensity Power Ultrasound Processing. J. Food Process. Preserv. 2015, 39, 2081–2087. [Google Scholar] [CrossRef]
- Iqbal, A.; Murtaza, A.; Marszałek, K.; Iqbal, M.A.; Chughtai, M.F.J.; Hu, W.; Barba, F.J.; Bi, J.; Liu, X.; Xu, X. Inactivation and Structural Changes of Polyphenol Oxidase in Quince (CYDONIA OBLONGA Miller) Juice Subjected to Ultrasonic Treatment. J. Sci. Food Agric. 2020, 100, 2065–2073. [Google Scholar] [CrossRef]
- Mokrzycki, W.; Tatol, M. Color difference Delta E—A survey. Mach. Graph. Vis. 2011, 20, 383–411. [Google Scholar]
- Pathare, P.B.; Opara, U.L.; Al-Said, F.A.-J. Colour Measurement and Analysis in Fresh and Processed Foods: A Review. Food Bioprocess Technol. 2013, 6, 36–60. [Google Scholar] [CrossRef]
- Kowalska, H.; Woźniak, Ł.; Masiarz, E.; Stelmach, A.; Salamon, A.; Kowalska, J.; Piotrowski, D.; Marzec, A. The Impact of Using Polyols as Osmotic Agents on Mass Exchange during Osmotic Dehydration and Their Content in Osmodehydrated and Dried Apples. Dry. Technol. 2020, 38, 1620–1631. [Google Scholar] [CrossRef]
- Czajkowska, K.; Kowalska, H.; Cichowska, J.; Wojnowski, M. Osmotic dehydration of apple in chokeberry juice concentrate. Technol. Prog. Food Process. 2016, 2, 5–11. [Google Scholar]
- AOAC International. Official Methods of Analysis of AOAC International, 21st ed.; AOAC International: Rockville, MD, USA, 2019. [Google Scholar]
- Bogdanov, S.; Martin, P.; Lullmann, C. Harmonised Methods of the International Honey Commission; Swiss Bee Research Centre, FAM: Liebefeld, Switzerland, 2002. [Google Scholar]
- Ainsworth, E.A.; Gillespie, K.M. Estimation of Total Phenolic Content and Other Oxidation Substrates in Plant Tissues Using Folin–Ciocalteu Reagent. Nat. Protoc. 2007, 2, 875–877. [Google Scholar] [CrossRef]
- Larrauri, J.A.; Sánchez-Moreno, C.; Rupérez, P.; Saura-Calixto, F. Free Radical Scavenging Capacity in the Aging of Selected Red Spanish Wines. J. Agric. Food Chem. 1999, 47, 1603–1606. [Google Scholar] [CrossRef] [PubMed]
- Baltrušaitytė, V.; Venskutonis, P.R.; Čeksterytė, V. Radical Scavenging Activity of Different Floral Origin Honey and Beebread Phenolic Extracts. Food Chem. 2007, 101, 502–514. [Google Scholar] [CrossRef]
- Chauhan, O.P.; Singh, A.; Singh, A.; Raju, P.S.; Bawa, A.S. Effects of Osmotic Agents on Colour, Textural, Structural, Thermal, and Sensory Properties of Apple Slices. Int. J. Food Prop. 2011, 14, 1037–1048. [Google Scholar] [CrossRef]
- Wojdyło, A.; Figiel, A.; Lech, K.; Nowicka, P.; Oszmiański, J. Effect of Convective and Vacuum–Microwave Drying on the Bioactive Compounds, Color, and Antioxidant Capacity of Sour Cherries. Food Bioprocess Technol. 2014, 7, 829–841. [Google Scholar] [CrossRef]
- Buera, M.P.; Lozano, R.D.; Petriella, C. Definition of Color in the Non-enzymatic Browning Process. Die Farbe 1985, 32, 318–322. [Google Scholar]
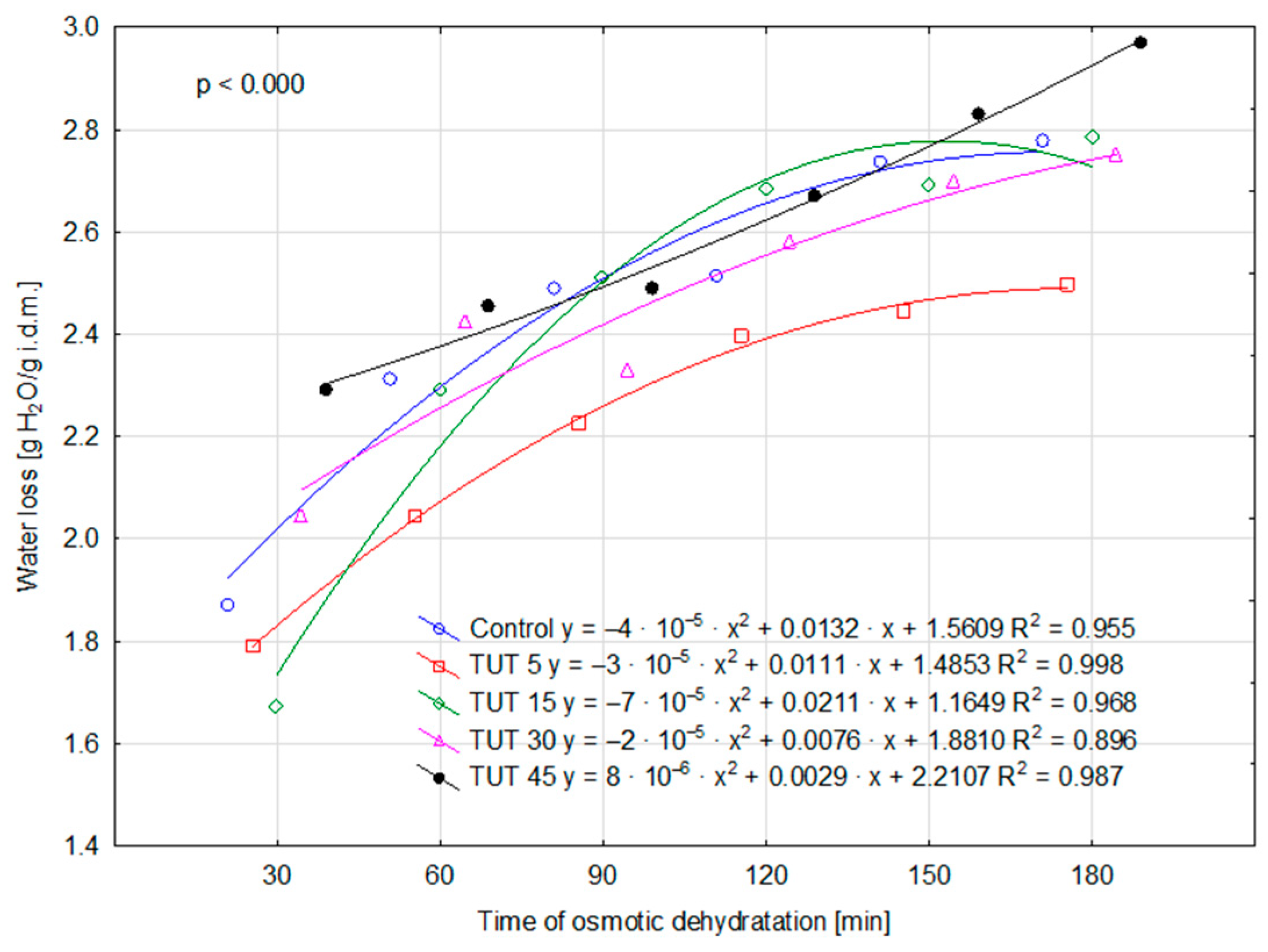

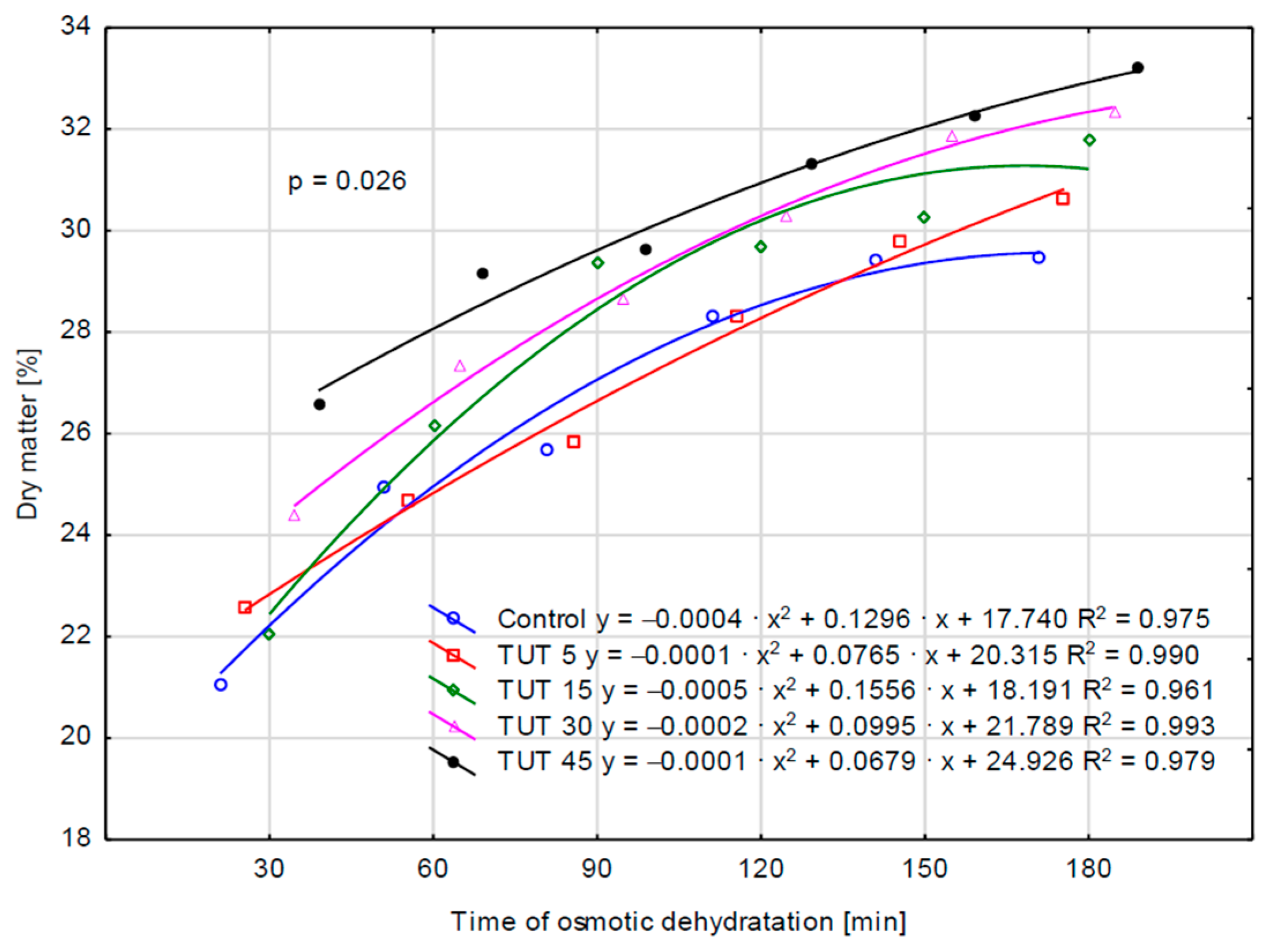


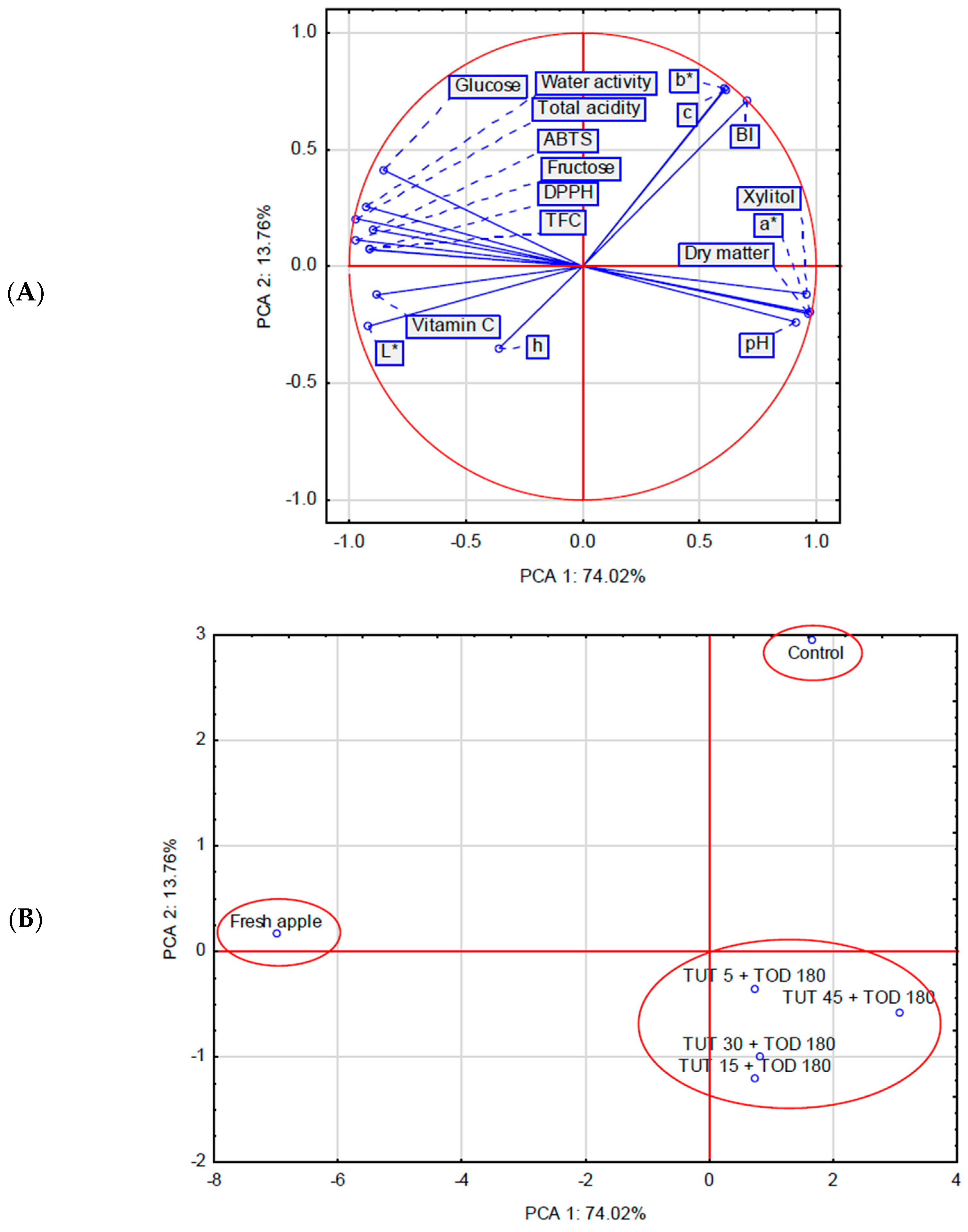
| Factor | Factor Level | Water Loss [g H2O2/g i.d.m.] 1 | Mass Loss [%] | Solid Gain [g d. m./g i.d.m.] | Dry Matter [%] |
|---|---|---|---|---|---|
| Time of ultrasound treatment (TUT) [min] | 5 (TUT 5) | 2.232 a | 28.563 a | 0.300 a | 26.973 a |
| 15 (TUT 15) | 2.438 b | 31.702 b | 0.294 a | 28.216 b | |
| 30 (TUT 30) | 2.470 b | 31.318 ab | 0.352 b | 29.175 c | |
| 45 (TUT 45) | 2.617 c | 33.257 b | 0.367 b | 30.370 d | |
| Control (TUT 0) | 2.449 b | 33.463 b | 0.284 a | 26.481 a | |
| Time of osmotic dehydration (TOD) [min] | 30 (TOD 30) | 1.933 a | 26.148 a | 0.167 a | 23.343 a |
| 60 (TOD 60) | 2.305 b | 30.482 b | 0.257 b | 26.454 b | |
| 90 (TOD 90) | 2.408 c | 31.216 bc | 0.311 c | 27.844 c | |
| 120 (TOD 120) | 2.568 d | 32.884 bcd | 0.372 d | 29.603 d | |
| 150 (TOD 150) | 2.679 e | 34.162 cd | 0.398 d | 30.716 e | |
| 180 (TOD 180) | 2.755 e | 35.072 d | 0.412 d | 31.498 e |
| Parameters | Fresh Apple | Control | TUT 5 + TOD 180 | TUT 15 + TOD 180 | TUT 30 + TOD 180 | TUT 45 + TOD 180 |
|---|---|---|---|---|---|---|
| Dry matter [%] | 14.78 a | 29.46 b | 30.63 c | 31.81 d | 32.38 de | 33.22 e |
| Water activity | 0.929 c | 0.884 b | 0.875 b | 0.874 b | 0.875 b | 0.840 a |
| Total acidity [%] | 0.818 d | 0.222 c | 0.122 ab | 0.118 ab | 0.136 b | 0.043 a |
| pH | 3.79 a | 4.62 b | 4.86 d | 4.89 d | 4.77 c | 4.74 c |
| Fructose [g/100 g d.m.] | 43.09 e | 5.65 d | 4.18 c | 3.44 b | 3.16 b | 2.00 a |
| Glucose [g/100 g d.m.] | 9.78 e | 8.16 d | 7.34 c | 7.42 c | 6.55 b | 5.56 a |
| Xylitol [g/100 g d.m.] | – | 57.84 a | 60.74 c | 59.30 b | 65.86 d | 61.38 c |
| Total sugar [g/100 g d.m.] | 52.87 a | 71.65 d | 72.26 d | 70.16 c | 75.57 e | 68.94 b |
| Parameters | Fresh Apple | Control | TUT 5 + TOD 180 | TUT 15 + TOD 180 | TUT 30 + TOD 180 | TUT 45 + TOD 180 |
|---|---|---|---|---|---|---|
| TFC [mg/g d.m.] | 4.52 c | 2.97 b | 3.38 b | 3.15 b | 3.02 b | 1.89 a |
| ABTS [μM TE/ g d.m.] | 1543 d | 1052 bc | 1207 c | 964 b | 1052 bc | 694 a |
| DPPH [μM TE/ g d.m.] | 221 c | 145 b | 165 b | 148 b | 154 b | 92 a |
| Vitamin C [mg/g d.m.] | 0.52 c | 0.04 a | 0.04 a | 0.05 ab | 0.11 b | 0.08 ab |
| Parameters | Fresh Apple | Control | TUT 5 + TOD 180 | TUT 15 + TOD 180 | TUT 30 + TOD 180 | TUT 45 + TOD 180 |
|---|---|---|---|---|---|---|
| L* | 73.74 f | 62.67 a | 63.49 b | 66.41 e | 66.17 d | 64.93 c |
| A* | 0.18 a | 0.80 b | 0.85 c | 0.88 d | 0.92 e | 0.99 f |
| B* | 22.36 a | 30.30 e | 24.11 b | 24.25 b | 25.15 c | 25.99 d |
| C* | 22.37 a | 30.26 e | 24.13 b | 24.25 b | 25.15 c | 26.01 d |
| h° | 89.56 c | 88.55 b | 87.99 a | 90.14 d | 90.41 d | 87.82 a |
| BI | 34.99 a | 63.71 f | 46.86 d | 43.53 b | 45.65 c | 50.16 e |
| ΔE | – | 13.94 e | 10.59 d | 7.72 a | 8.37 b | 9.60 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wojtyś, A.; Pietrzyk, S.; Grzesińska, K.; Witkowicz, R. Ultrasound-Assisted Osmotic Dehydration of Apples in Xylitol Solution: Effects on Kinetics, Physicochemical Properties and Antioxidant Activity. Molecules 2025, 30, 2304. https://doi.org/10.3390/molecules30112304
Wojtyś A, Pietrzyk S, Grzesińska K, Witkowicz R. Ultrasound-Assisted Osmotic Dehydration of Apples in Xylitol Solution: Effects on Kinetics, Physicochemical Properties and Antioxidant Activity. Molecules. 2025; 30(11):2304. https://doi.org/10.3390/molecules30112304
Chicago/Turabian StyleWojtyś, Angelika, Sławomir Pietrzyk, Karolina Grzesińska, and Robert Witkowicz. 2025. "Ultrasound-Assisted Osmotic Dehydration of Apples in Xylitol Solution: Effects on Kinetics, Physicochemical Properties and Antioxidant Activity" Molecules 30, no. 11: 2304. https://doi.org/10.3390/molecules30112304
APA StyleWojtyś, A., Pietrzyk, S., Grzesińska, K., & Witkowicz, R. (2025). Ultrasound-Assisted Osmotic Dehydration of Apples in Xylitol Solution: Effects on Kinetics, Physicochemical Properties and Antioxidant Activity. Molecules, 30(11), 2304. https://doi.org/10.3390/molecules30112304






