Abstract
This perspective highlights current trends and recent advances in the introduction of fluorine and fluoroalkyl moieties into the furanic core of biobased furfural-derived furans. Existing and potential applications of these fluorinated building blocks in the development of pharmaceuticals and advanced materials are also discussed.
1. Introduction
While naturally occurring organofluorine compounds are rare, [1] these synthetic products have a significant impact on academic and industrial fields. Fluorine is the most electronegative element that is sterically similar to hydrogen [2]; therefore, carbon–fluorine bonds are characterized by high stability, low polarization and a strong electron-withdrawing ability [3]. Consequently, the introduction of a fluorine atom does not substantially increase the volume of the molecule, yet it often leads to significant changes in physical, chemical, stereochemical, physicochemical, and biological properties of small organic molecules and polymers [4,5,6,7,8].
Due to their unique advantages, fluorinated compounds are widely used, particularly in the development of advanced pharmaceuticals, agrochemicals, and materials [9,10,11,12]. The importance of fluorine in the development of pharmaceuticals and agrochemicals is mostly a consequence of the considerable influence of fluorination on bioactivity profiles, metabolic stability, and physicochemical properties such as acidity/basicity, lipophilicity, and solubility [2,5,13,14,15,16,17]. Additionally, fluorine atoms can participate in hydrogen bonding as electron pair donors, thereby stabilizing certain conformations [18]. These advantages of fluorination contribute to fluorine-containing compounds accounting for approximately 20–25% of approved small molecule pharmaceuticals, with 30–40% of agrochemicals containing at least one fluorine atom in their structure [19,20,21,22,23,24,25]. Numerous fluorinated derivatives with notable biological activity have been synthesized, including indoles [26,27], quinazolines [28,29], steroids [30], and morphinans [31,32,33].
Notably, the trifluoromethyl group is the most commonly used fluorinated group [34]. In biological studies and materials, it is particularly attractive due to the greater chemical stability of trifluoromethylated products compared to difluoromethyl- and monofluoromethyl-containing analogues, as well as its relatively low toxicity [35]. The steric demand of a trifluoromethyl group is comparable to that of an isopropyl group, and its electronegativity is similar to that of oxygen [18]. Incorporating trifluoromethyl groups into organic compounds can significantly modify their chemical reactivity, acidity, polarity, lipophilicity, metabolic stability, and binding selectivity [34,36].
Direct trifluoromethylation with or without transition metal catalysts offers an important pathway for synthesizing fluorinated targets, but it often suffers from poor selectivity, low yields, harsh reaction conditions, and the usage of corrosive and expensive CF3 sources, which restricts the feasibility of this approach [37,38,39]. Over the past decade, substantial efforts have been made to address these limitations. Consequently, numerous efficient methods for the direct incorporation of trifluoromethyl groups into heterocyclic molecules have been developed, utilizing electrophilic, nucleophilic, and radical trifluoromethylating reagents [34,40,41,42]. Compared to nucleophilic and electrophilic trifluoromethylation, radical trifluoromethylation allows for the direct introduction of CF3 into organic molecules without the need for additional steps to prepare functionalized substrates (such as organohalides and organometallic reagents), thereby providing a more efficient route to access trifluoromethylated compounds [43,44].
Carbo- and heterocycles containing fluorine or fluoroalkyl moieties are common structural motifs of many bioactive substances [9,20,45]. On the other hand, furans are perhaps one of the most prominent classes of heteroaromatic compounds with widespread occurrence in nature [46]. Numerous bioactive natural and synthetic products such as pharmaceuticals, agrochemicals and flavouring agents contain a furan ring in their structure [47,48,49,50,51,52]. The insufficient stability of many furanic compounds significantly limits their synthetic utility [53,54,55]. The presence of strong electron-withdrawing F-containing substituents at the α-carbon (C2 or C5) positions markedly improves the furan ring stability under acidic conditions [56,57]. Consequently, fluorine-functionalized furans have emerged as particularly valuable scaffolds in fine organic synthesis and prospective biologically active compounds [56,57].
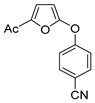
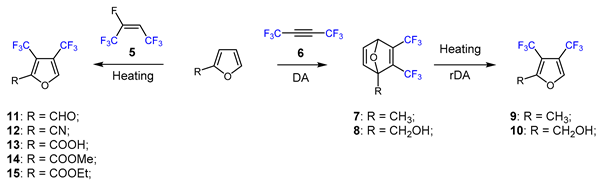
Fluorine-containing furans have been utilized in the design of drugs for treatment of various diseases [57,58,59,60,61,62,63]. For instance, β-fluorofuran derivative 1, a structural analogue of trovirdine (LY 300046), exhibits high amounts of activity against the human immunodeficiency viruses (HIV) (Figure 1) [64]. Compound 2 with a α-fluorofuran moiety is a potent and selective inhibitor of the induced myeloid leukemia cell differentiation protein MCL1 and is considered a promising candidate for targeted cancer therapy [65]. Trifluoromethylated furan subunits can often be found in novel structures for drug development and agrochemicals (examples of biologically active α-trifluoromethylated furans 3 and 4 are presented on Figure 1) [21,23,60,66]; they have also found prospective applications in various materials including liquid crystals, photoresist polymers, and self-assembling monolayers [60].
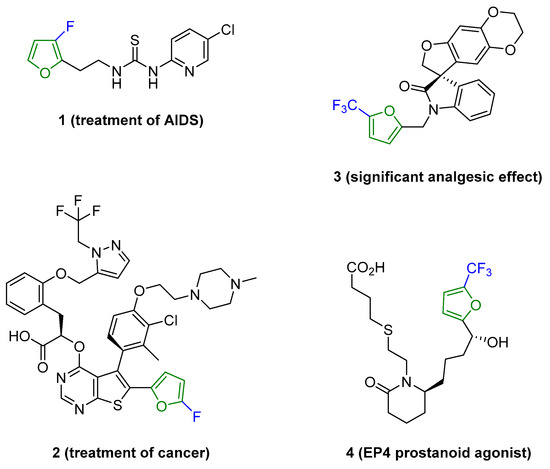
Figure 1.
Representative examples of bioactive compounds with fluorofuran and trifluoromethylfuran functionalities. Color indicates the fluorine-functionalized furan fragment.
Fluorinated and fluoroalkylated furans may serve as valuable synthetic intermediates. While numerous methods for the nucleophilic or transition-metal-catalyzed defluorinative C−F bond functionalization of fluorinated heterocycles are reported [67,68,69], information regarding the application of these methods for modifying fluorine-functionalized furans is limited [70]. On the other hand, the high electronegativity of the fluorine atom can make the adjacent C–H bond in a furan ring more susceptible to nucleophilic attacks that may facilitate the transformation of fluorine-containing furans into more complex fluorinated structures [71,72]. Furthermore, fluorofurans and fluoroalkylfurans can be utilized as building blocks for synthesizing non-furanic cyclic or acyclic compounds [73].
Furfural is a key renewable furanic compound produced industrially from plant biomass [74,75,76]. Due to its availability and diverse reactivity, furfural has been selected as a platform chemical [77] and is actively used as a renewable building block for biofuels, fine chemicals, monomers, and polymers [78,79,80,81]. One of the actual and promising areas of application of furfural and other bioderived furans is the sustainable production of pharmaceuticals and other bioactive compounds [82,83].
In contrast to traditional aromatic polymers, furfural-derived polymers enable the transformation into three-dimensional dynamic thermosets by utilizing the reactivity of the furan ring in Diels–Alder reactions with maleimides [84,85]. Dynamic thermosets, also known as covalent adaptable networks (CANs), dynamers or vitrimers, combine the advantages of traditional thermosets such as heat, chemical, and creep resistance, high mechanical strength, and electrical insulation with the ability to be reprocessed like thermoplastics through the activation of dynamic covalent bonds [86,87]. Current research on furan–maleimide Diels–Alder (FMDA) thermosets focuses on developing sustainable and biobased smart materials, which combine excellent mechanical properties with effective self-healing efficiency and recycling capabilities [88,89]. The self-healing mechanism in FMDA polymers relies on the reversibility of the furan–maleimide Diels–Alder reaction, which can be initiated by various external stimuli such as temperature, light, and mechanical or magnetic forces [84,85].
The modification of furfural into fluorinated furanic building blocks presents an intriguing approach to linking the biomass conversion with the production of fluorinated fine chemicals, advanced dynamic polymers, and pharmaceuticals (Scheme 1). Application of biobased furans as starting materials makes the production of targeted fluorinated products more sustainable and environmentally friendly in agreement with modern trends towards green chemical industry. In this perspective, we analyze the current challenges and recent advancements in introducing fluorine or fluoroalkyl functionalities into the furanic core of biobased furfural and most important derivatives. We also discuss the progress in applications of these fluorinated furanic building blocks in the development of pharmaceuticals and dynamic polymers.
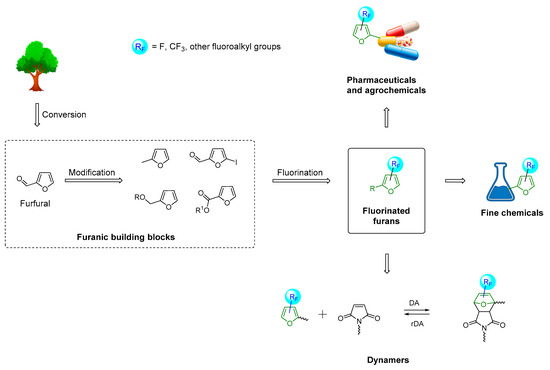
Scheme 1.
Synthetic pathways for fluorinated fine chemicals, bioactive compounds, and dynamic polymers using furfural as a renewable building block.
2. Fluorination and Fluoroalkylation of Furfural-Derived Furans
While numerous methods for the incorporation of fluorine or fluorine-containing functional groups into various aromatic and heteroaromatic structures have been reported, [10,34,41,90,91,92], the functionalization of furanic building blocks remains underdeveloped. The scientific literature includes a limited number of reviews focused on the synthesis of fluorinated furans, with the most comprehensive reviews addressing the advances in the preparation of fluorofuran and fluoroalkylfuran derivatives covering the corresponding publications only up to 2015 [93,94]. In this paper, we discuss studies on the synthesis of fluorofuran and fluoroalkylfuran building blocks, primarily published over the last decade.
The carbon–fluorine bond formation is a challenging chemical transformation [37]. Two fundamentally distinct strategies exist for introducing fluorine-containing functional groups into furans and other heterocycles: (1) direct substitution of a hydrogen or functional group in the heterocyclic ring with fluorinated reagents or (2) cyclocondensation/cycloaddition reactions with fluorinated acyclic building blocks [18,56,66,95]. The direct introduction of fluorine and various fluoroalkyl functional groups into heteroaromatic cores can be performed with or without a catalyst through electrophilic, nucleophilic, or radical mechanisms [37,43,96,97,98,99,100]. Various environmental approaches such as the use of organocatalysts [101], electrocatalysis [40], or in-water fluorination [102] are also actively explored. The introduction of fluorinated functional groups via C–H activation eliminates the requirement for pre-functionalizing the substrate. This also enables selective functionalization at a late stage in the synthesis, which is particularly advantageous when developing new drugs and other high-value chemical products [99,103].
While approaches for the direct introduction of fluorinated functional groups into heterocycles look preferable and conceptually simpler than the use of fluorinated acyclic precursors, these methods often face challenges [18,56,57,66]. Despite the longstanding appreciation of fluorine utility, many fluorination methods still lack generality, practicality, and predictability [37,103]. Moreover, most fluorinating agents are costly, toxic, corrosive, and potentially explosive or exhibit too high reactivity, which can lead to undesired modifications in the existing functional groups [36,57,104,105,106]. Due to the electron-rich nature of the furan ring, the direct introduction of fluorinated functional groups into the furanic core predominantly occurs through electrophilic or radical substitution [93,94]. This functionalization of 2-substituted furans typically takes place selectively at the C5 position, owing to the greater stabilization of the corresponding carbocations and radicals (Scheme 2) [76,94,107,108].

Scheme 2.
Possible pathways for introduction of fluorinated functional groups into 2-substituted furan derivatives by electrophilic or radical mechanisms. RF+ = fluorinated electrophile, •RF = fluorinated radical. Here and below, blue color indicates fluorine or a fluorinated functional group.
2.1. Synthesis of Fluorofurans
Although methods for the synthesis of fluorofurans (compounds with the Cfur-F bond) and fluoroalkylated furans have been developed, these methodologies often rely on non-furanic cyclic or acyclic fluorinated building blocks [93,94,109,110,111,112,113,114]. This approach has gained popularity due to its convenience and selective formation of target fluorinated molecules [57]. However, many of these methods require multi-step procedures for synthesizing specific substrates or involve costly reagents, which limits their synthetic applicability [57].
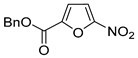
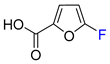
Preparation of representative fluorofurans through direct fluorination of furfural-derived substrates is summarized in Table 1. Nucleophilic fluorination via reactions with gaseous fluorine is not suitable for furan fluorination [94]. Nevertheless, some α-fluorofurans have been obtained from electron-poor furfural-derived substrates using nucleophilic fluorodenitration [78], through fluorodecarboxylation using the electrophilic fluorinating agent Selectfluor [115,116], or by employing rhodium-catalyzed heteroaryl exchange (Table 1) [117,118]. Another potential approach is the metalation-fluorination strategy, which has been effectively utilized for fluorination at both the α- and β-positions of the furan ring [119,120,121].

Table 1.
Synthesis of fluorofurans from furfural-derived furanic building blocks.
2.2. Synthesis of Fluoroalkylfurans
The recent methods for fluoroalkylation reactions involving furfural-derived furans can be classified into two main categories based on the mechanisms of generation and reactivity of fluoroalkyl species: (1) radical fluoroalkylation and (2) transition metal-catalyzed (cross)coupling. The mechanism of radical fluoroalkylation typically consists of three key stages (Scheme 3). The first stage involves the generation of the fluoroalkyl radical (•RF), which is a strong electrophile that readily reacts with electron-rich substrates such as furans I. These radicals can be produced from various fluorinated reagents, including halogenated fluoro-sources, fluorinated acids, anhydrides, sulfonyl chlorides, or hypervalent iodine reagents, typically through single electron transfer (SET) oxidation. In the subsequent stage, the •RF radical inserts itself into the C5 position of the 2-substituted furanic core, resulting in the formation of a fluoroalkylated furanic radical II. Finally, the rearomatization of the furan ring leads to the desired 5-fluoroalkylated furans IV. This process generally involves a one-electron oxidation of II into the corresponding furanic cation III via an SET process, followed by deprotonation (Scheme 3). Various strategies have been employed for the radical fluoroalkylation of furans, including visible-light-mediated photocatalysis and photoredox catalysis, transition metal catalysis, and alternating current electrolysis (Table 2, Table 3, Table 4, Table 5 and Table 6).
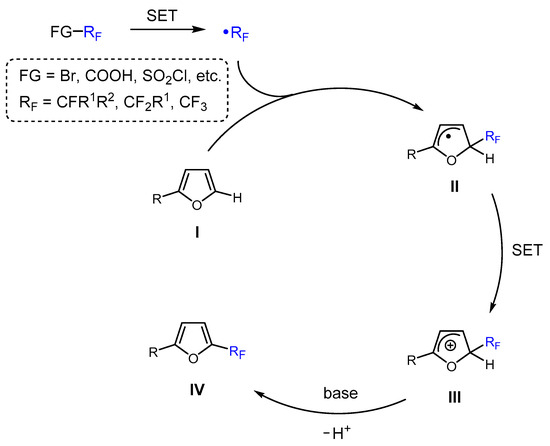
Scheme 3.
The general proposed mechanism for the radical fluoroalkylation of 2-substituted furans.
Among the various methods for trifluoromethylation of furans, visible light-mediated radical trifluoromethylation, with or without photoredox catalysis, has recently garnered the most attention (Table 2 and Table 3) [92,122]. Photoredox-catalyzed trifluoromethylation, achieved through the generation of the electrophilic radical •CF3 using trifluoroacetic acid [123], trifluoroacetic anhydride (TFAA) [124,125], perfluoroarene iodine(III) trifluoroacetate [105], or trifluoromethanesulfonic anhydride [43] as CF3 sources has demonstrated high efficiency for the trifluoromethylation of acceptor-substituted furans such as 2-acetylfuran and 2-furoic acid derivatives (Table 2). Furfural-derived furans containing electron-donor substituents at the C2 position were trifluoromethylated through the transition-metal-mediated generation of trifluoromethyl radicals, with [38,39,106,126] or without [127,128,129] photoredox catalysis (Table 3). Zhong et al. reported a method for trifluoromethylation using perfluorocarboxylic anhydrides under metal-free conditions in the presence of urea and hydrogen peroxide [3]. This approach produced two α-trifluoromethylated furanic esters in good yields (Table 3, entries 9, 10).

Table 2.
Radical trifluoromethylation of electron-poor furfural-derived furans.
Table 2.
Radical trifluoromethylation of electron-poor furfural-derived furans.
 | ||||
| № | R | Reaction Conditions | Yield | Ref. |
| 1 | Me | CF3COOH, (4-ClPh)2SO (2 eq.), Ru(bpy)3Cl2 (1 mol. %), DCE, RT, 427 nm LED | 62 | [123] |
| 2 | Me | TFAA, Me2C=NOH (2 eq.), Ru(bpy)3Cl2, DCE, RT, 390 nm LED | 62 | [125] |
| 3 | OMe | CF3COOH, (4-ClPh)2SO (2 eq.), Ru(bpy)3Cl2 (1 mol. %), DCE, RT, 427 nm LED | 59 | [123] |
| 4 | OMe | TFAA, Me2C=NOH (2 eq.), Ru(bpy)3Cl2, DCE, RT, 390 nm LED | 74 | [125] |
| 5 | OMe | TFAA, Ir(ppy)3 (3 mol. %), EtOAc, 40 °C, 30 W blue LED | 64 | [124] |
| 6 | OMe | C6F5I(OCOCF3)2, Ru(bpy)3(PF6)2 (2 mol. %), CH3CN, 35 °C, blue LED | 41 | [105] |
| 7 | OMe | (CF3SO2)2O, Ru(bpy)3Cl2 (2 mol. %), pyridine, DCE, RT, LED | 74 | [43] |
| 8 | OMe | CF3SO2Na, [Ru(bpy)3][PF6]2, LiClO4, CH3CN, RT, blue LED, CCE at 4.0 mA | 77 | [130] |
| 9 | OMe | CF3SO2Cl, K2HPO4, 100 Hz, (+)C/(−)C, 4.8 V, CH3CN, 0.125 M LiClO4 | 32 | [131] |
| 10 | OC8H17 | CF3COOH, (4-ClPh)2SO (2 eq.), Ru(bpy)3Cl2 (1 mol. %), DCE, RT, 427 nm LED | 58 | [123] |
| 11 | OC8H17 | TFAA, Me2C=NOH (2 eq.), Ru(bpy)3Cl2, DCE, RT, 390 nm LED | 60 | [125] |
| 12 | NHMe | CF3SO2Na, [Ru(bpy)3][PF6]2, LiClO4, CH3CN, RT, blue LED, CCE at 4.0 mA | 69 | [130] |
| 13 | NH(CH2COOEt) | CF3SO2Na, [Ru(bpy)3][PF6]2, LiClO4, CH3CN, RT, blue LED, CCE at 4.0 mA | 70 | [130] |
| 14 | OH | TFAA, Me2C=NOH (2 eq.), Ru(bpy)3Cl2, DCE, RT, 390 nm LED | 68 | [125] |
| 15 | OH | (CF3SO2)2O, Ru(bpy)3Cl2 (2 mol. %), pyridine, DCE, RT, LED | 72 | [43] |

Table 3.
Radical trifluoromethylation of electron-rich furfural-derived furans.
Table 3.
Radical trifluoromethylation of electron-rich furfural-derived furans.
 | ||||
| № | R | Reaction Conditions | Yield | Ref. |
| 1 | CH3 | CF3SO2Cl, Ru-cat., Mg acetate, CH3CN, RT, blue light | 86 | [106] |
| 2 | CH3 | CF3SO2Cl, Ru(phen)3Cl2 (1 mol. %), K2HPO4, CH3CN, RT, 26 W light | 87 | [38] |
| 3 | CH3 | Co(III)-CF3 complex, NMP, RT, 16 W light | 87 | [127] |
| 4 | CH3 | CF3I, Ru(bpy)3Cl2 (1 mol. %), TMEDA, CH3CN, blue LED | 75 | [36] |
| 5 | CH3 | TMSCF3 (2eq.), PhI(OAc) (2 eq.), AgF (25 mol. %), DMSO, RT | 51 | [128] |
| 6 | CH3 | 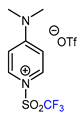 , Ir(ppy)3 (2 mol.%), KBr, CH2Cl2, RT, blue LED , Ir(ppy)3 (2 mol.%), KBr, CH2Cl2, RT, blue LED | 63 | [132] |
| 7 | CH3 | CF3SO2Cl (2 eq.), K2HPO4, 100 Hz, (+)C/(−)C, CH3CN, 0.125 M LiClO4 | 43 | [131] |
| 8 | CH2S(CHO) | CF3SO2Cl, CdSe (10 mol. %), K2HPO4, CH3CN, RT, blue LED | 68 | [39] |
| 9 |  | TFAA, urea-hydrogen peroxide, CH2Cl2, 0 °C | 49 | [3] |
| 10 | CH2OBn | (C2F5CO)2O, urea-hydrogen peroxide, CH2Cl2, 0 °C | 46 | [3] |
| 11 | 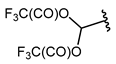 | CF3COOH, XeF2, CH2Cl2, 0 → 20 °C, then H2O, CF3COOH | 30 1 | [133] |
1 Yield of 5-(trifluoromethyl)-2-furaldehyde.
Mono-, di-, and perfluoroalkyl-functionalized heterocycles are less common than aryl-fluorinated (Ar-F) or aryl-trifluoromethylated (Ar-CF3) compounds, but they remain of interest due to their biological activity and applications in materials science [134,135]. Representative and efficient methods for fluoroalkylation of both electron-poor (Table 4 and Table 5) and electron-rich (Table 6) furans are outlined below. The nucleophilic fluorinating reagent Deoxo-Fluor exhibited significant selectivity in the deoxyfluorination of 5-nitro-2-furaldehyde to produce 2-(difluoromethyl)-5-nitrofuran (Table 5, entry 8), as well as in the fluorination of several 2,5-disubstituted furanic aldehydes [136]. Additionally, Zhang et al. utilized another nucleophilic fluorinating reagent DAST for the direct difluoromethylation of furfuryl acetate at the C5 position (Table 6, entry 13) [137].

Table 4.
Representative and most efficient methods of introduction of CF-, CF2- and perfluoroalkyl groups to alkyl 2-furoates.
Table 4.
Representative and most efficient methods of introduction of CF-, CF2- and perfluoroalkyl groups to alkyl 2-furoates.
| № | Furan | Reaction Conditions | Product | Yield | Ref. |
|---|---|---|---|---|---|
| 1 | Methyl 2-furoate | ICF2SO2F, [Ir(dFCF3ppy)2(bpy)]PF6 (1 mol. %), AgOTf (1 eq.), DMC, RT, blue LED |  | 80 | [138] |
| 2 | Methyl 2-furoate | BrCF2SO2(4-Cl-Ph), Mn2(CO)10 (10 mol. %), Davephos (15 mol. %), K2CO3, DCM, white LED | 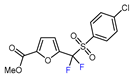 | 86 | [139] |
| 3 | Methyl 2-furoate | BrCF2CO2Et, Pd(PPh3)4 (5 mol. %), Xantphos (10 mol. %), K2CO3, dioxane, Ar, 110 °C | 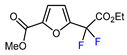 | 67 | [140] |
| 4 | Methyl 2-furoate | C3F7,XeF2, CH2Cl2, RT |  | 33 | [141] |
| 5 | Methyl 2-furoate | IC4F9 (30 eq.), CuI (10 mol. %), phen (20 mol. %), 2,4,6-collidine, CH2Cl2, 110 °C | 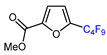 | 46 | [142] |
| 6 | Methyl 2-furoate | IC4F9, [Cu(bcp)DPEphos]PF6 (0.1 eq.), KOAc, CH2Cl2, blue LED | 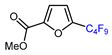 | 51 | [143] |
| 7 | Methyl 2-furoate | 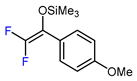 , Cu(OTf)2, CH3CN-H2O, 0 °C , Cu(OTf)2, CH3CN-H2O, 0 °C | 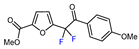 | 61 | [144] |
| 8 | Methyl 2-furoate | BrCF(CO2Et)2, fac-[Ir(ppy)3] (1mol. %), K2CO3, DMF, RT blue, LED | 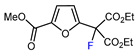 | 72 | [145] |
| 9 |  | [Ph4P]+[Cu(CF2H)2]– (1 eq.), DMAc, 90 °C | 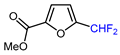 | 56 | [146] |
| 10 | Ethyl 2-furoate | BrCF2CN, [Ir(dtbbpy)(ppy)2][PF6], DMF, blue LED | 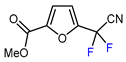 | 74 | [147] |
| 11 | 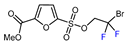 | [Ru(bpy)3]Cl2 6H2O (0.1 mol. %), NBu3 (1.5 eq.), HCOOH (1.5 eq.), DMSO, RT, blue LED | 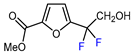 | 73 | [148] |
| 12 | 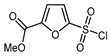 |  (1.75 eq.), Pd(OAc)2 (5 mol. %), SIPr•Cl (10 mol. %), CuCl, Li2CO3, dioxane, 120 °C (1.75 eq.), Pd(OAc)2 (5 mol. %), SIPr•Cl (10 mol. %), CuCl, Li2CO3, dioxane, 120 °C | 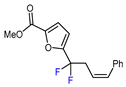 | 50 | [149] |
Transition metal-mediated C-H functionalization and radical visible light-mediated fluoroalkylation, with or without photoredox catalysis, demonstrated high efficiency for the introduction of fluoroalkyl groups into furans. Using these methods, mono-, di-, and perfluoroalkylated furanic building blocks were obtained with notable efficiency from electron-poor furans including 2-furoates (Table 4), furfural, 2-furoic acid, and other derivatives (Table 5) [14,140,150,151] and electron-rich furans such as 2-methylfuran, furfural-based dioxolane acetal, furfuryl alcohol and derivatives (Table 6). Coupling strategies involving furyl iodides [146,152,153] or sulfonyl chlorides [149] were also effectively employed for the fluorination of acceptor-substituted furans (Table 4, entries 9 and 12, and Table 5, entries 4–7).

Table 5.
Representative and most efficient methods of introduction of CF-, CF2- and perfluoroalkyl groups to other electron-poor furfural-derived furans.
Table 5.
Representative and most efficient methods of introduction of CF-, CF2- and perfluoroalkyl groups to other electron-poor furfural-derived furans.
| № | Furan | Reaction Conditions | Product | Yield | Ref. |
|---|---|---|---|---|---|
| 1 | Furfural | BrCF2CO2Et, CuI (10 mol. %), PMDETA (1.5 eq.), CH3CN, 80 °C | 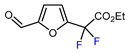 | 50 | [154] |
| 2 | Furfural | BrCF2CO2Et, Ir(ppy)3 (0.5 mol. %), phen (20 mol. %), NaOAc (2 eq.), CH3CN, RT, 3 W blue LED | 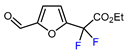 | 44 | [155] |
| 3 | Furfural | BrCF2CO2Et, [Ru(p-cymene)Cl2]2 (5 mol. %), Na2CO3, AgTFA (2 eq.), N-Ac-L-Iso (30 mol. %), tert-butylamine (0.5 eq.), DCE, 155 °C | 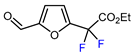 | 62 | [156] |
| 4 | 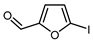 | TMSCF2Br (7.2 eq.), CuBr (5.2 eq.), ArSH (12 eq.), phen, 18-crown-6, NaH, C6F6, NMP, 60 °C | 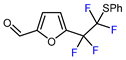 | 81 | [153] |
| 5 | 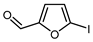 | TMSCF3 (1.8 eq.), C6F5TMS, CuCl (5.2 eq.), KF (12 eq.), 60 °C | 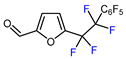 | 75 | [152] |
| 6 | 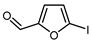 | (DMPU)2Zn(CHF2)2, (dppf)Ni(COD) (15 mol. %), DMSO, RT | 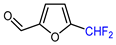 | 78 | [157] |
| 7 | 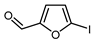 | 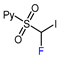 , Et2Zn (0.6 eq.), CuI (1.3 eq.), DMF, RT , Et2Zn (0.6 eq.), CuI (1.3 eq.), DMF, RT | 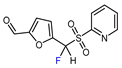 | 67 | [158] |
| 8 | 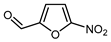 | Deoxo-Fluor (1.5 eq.) CH2Cl2, RT | 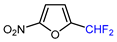 | 68 | [136] |
| 9 | 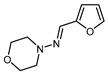 | BrCF2CO2Et, CuBr2 (10 mol. %), DTBDPy (10 mol. %), B2pin2 (30 mol. %), NaHCO3, dioxane, 80 °C | 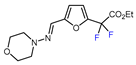 | 51 | [159] |
| 10 | 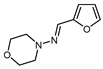 |  (0.33 eq.), NaHCO3, CH3CN, RT, 462 nm LED (0.33 eq.), NaHCO3, CH3CN, RT, 462 nm LED | 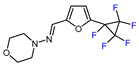 | 69 | [160] |
| 11 | 2-Acetylfuran | BrCF2CO2Et, CuI (10 mol. %), PMDETA (1.5 eq.), CH3CN, 80 °C | 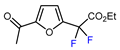 | 53 | [154] |
| 12 | 2-Acetylfuran | BrCF(CO2Et)2, fac-[Ir(ppy)3] (1mol. %), K2CO3, DMF, RT, blue LED | 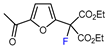 | 65 | [145] |
| 13 | 2-Furonitrile | ICF2SO2F, [Ir(dFCF3ppy)2(bpy)]PF6 (1 mol. %), AgOTf (1 eq.), DMC, RT, blue LED | 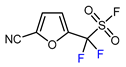 | 85 | [138] |
As shown in Table 2, Table 3, Table 4, Table 5 and Table 6, most fluoroalkylation methods were tested on either C2 donor- or acceptor-substituted furanic substrates. Some methodologies, such as Pd-catalyzed difluoromethylation using ethyl 2-bromo-2,2-difluoroacetate [140], Cu-catalyzed perfluoroalkylation with IC4F9 [142], and electrocatalytic trifluoromethylation [131] demonstrated considerable efficiency for both donor- and acceptor-substituted furans. However, the applicability of many reported fluoroalkylation systems of furans is not fully established, as the range of tested furanic substrates was often limited to highly stable or non-functionalized compounds like 2-methylfuran [14,36,106,126,127,129,134,161,162], methyl 2-furoate [146,148], or 2-acetylfuran [151]. In some cases, fluoroalkylation resulted in significantly lower yields compared to other heteroaromatic substrates, or even failed [22,163,164,165].

Table 6.
Representative and most efficient methods of introduction of CF-, CF2- and perfluoroalkyl groups to electron-rich furfural-derived furans.
Table 6.
Representative and most efficient methods of introduction of CF-, CF2- and perfluoroalkyl groups to electron-rich furfural-derived furans.
| № | Furan | Reaction Conditions | Product | Yield | Ref. |
|---|---|---|---|---|---|
| 1 | 2-Methylfuran | BrCF2CO2Et, CuI (10 mol. %), K2CO3, DMF, 60 °C | 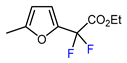 | 61 | [150] |
| 2 | 2-Methylfuran | BrCF2CO2Et, Pd(PPh3)4 (5 mol. %), Xantphos (10 mol. %), K2CO3, dioxane, Ar, 110 °C | 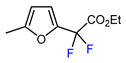 | 57 | [140] |
| 3 | 2-Ethylfuran | BrCF2CO2Et, fac-[Ir(ppy)3], K3PO4, DMF, RT, 7 W blue LED | 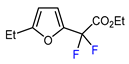 | 83 | [166] |
| 4 | 2-Ethylfuran | PhSO2CF2I, Pd2(dba)3 (5 mol. %), Xantphos (20 mol. %), Cs2CO3, CHCl3, 60 °C | 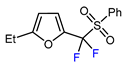 | 96 | [167] |
| 5 | 2-Ethylfuran | PhSO2CF2I, Ru(bpy)3Cl2•6H2O (1 mol. %), K2HPO4, CH2Cl2, 40 °C, 26W light | 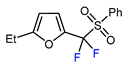 | 96 | [168] |
| 6 | 2-Ethylfuran | BrCF2CO2Et, Pd(PPh3)4 (5 mol. %), Xantphos (10 mol. %), K2CO3, dioxane, Ar, 110 °C | 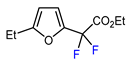 | 63 | [140] |
| 7 | 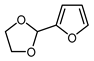 | PhSO2CF2I, Ru(bpy)3Cl2•6H2O (1 mol. %), K2HPO4, CH2Cl2, 26 W light, 40 °C | 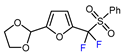 | 90 | [168] |
| 8 | 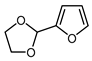 | BrCF2CO2Et, Pd(PPh3)4 (5 mol. %), Xantphos (10 mol. %), K2CO3, dioxane, Ar, 110 °C | 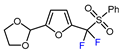 | 80 | [167] |
| 9 | Furfuryl alcohol | IC4F9 (30 eq.), CuI (10 mol. %), 1,10 phenantroline (20 mol. %), 2,4,6-collidine, CH2Cl2, 110 °C | 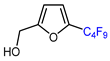 | 74 | [142] |
| 10 | 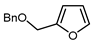 | BrCF2CO2Et, CuI (10 mol. %), K2CO3, DMF, 80 °C | 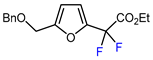 | 64 | [150] |
| 11 | 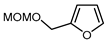 | BrCF2CO2Et, CuI (10 mol. %), K2CO3, DMF, 80 °C | 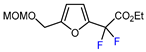 | 65 | [150] |
| 12 | 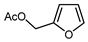 | BrCF2CO2Et, CuI (10 mol. %), K2CO3, DMF, 80 °C | 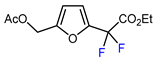 | 63 | [150] |
| 13 | 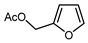 | DAST (3.5 eq.), CH2Cl2, RT | 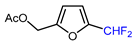 | 60 | [137] |
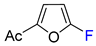
Synthesis of β-fluorinated and β-fluoroalkylated furans through direct fluoroalkylation presents challenges; however, it can be achieved using fluorinated acyclic precursors [70,169,170,171,172,173]. On the other hand, the dienic nature of the furan ring offers the construction of fluorofurans via Diels–Alder chemistry. Regioselective hydrogenation of the unsubstituted double bond in Diels–Alder adducts 7 and 8 formed from electron-rich furans and hexafluorobutyne, followed by temperature-induced elimination of ethylene, resulted in β,β-bis-trifluoromethylated furans 9 and 10 in good overall yields (Table 7) [174].

Table 7.
Indirect synthesis of β,β-bis-trifluoromethylated furans using Diels–Alder chemistry [174].
In the same study, bis-trifluoromethylated furans 11–15 containing electron-accepting functional groups at the C2 position were synthesized in a single step through high-temperature reactions with heptafluorobutene (Table 7). This alkene served as a strong dienophile reacting with 2-furonitrile, 2-furoic acid or its esters, and even with furfural, producing the desired fluorinated trisubstituted furans in good yields [174].
3. Application of Furfural-Derived Fluorofurans as Building Blocks in Drug Development
Introduction of fluorinated furan derivatives into biologically active scaffolds has garnered significant attention [62,63]. The polyfunctional nature of furfural-derived furans, which allow several reactivity patterns such as the modification of the substituents at the C2 position, the functionalization of the C5-H bond, and the dienic reactivity of the furan ring, provides efficient and diverse chemical access to various fluorofuran or fluoroalkylfuran derivatives. A possible reaction map for the synthesis of fluorinated furanic building blocks starting from furfural is presented in Scheme 4. The introduction of fluorinated furan moieties into biologically active scaffolds can be achieved through reductive amination with fluorinated furanic aldehydes (pathway A). The 2-(bromomethyl)-5-(fluoroalkyl)furans, obtainable through the bromination of corresponding 2-(methyl)-5-(fluoroalkyl)furans, can serve as an alkylating agent or substrate in various coupling reactions (pathway B) [175,176,177,178,179]. C5-fluorinated furoic acids can also act as convenient building blocks for the introduction of fluorofuran or fluoroalkylfuran groups via acylation (pathway C). Another potential approach involves the use of halogenated or sulfonated α-fluorofurans in cross-coupling reactions (pathway D). Functionalized furans containing fluorinated functional groups at the β-positions may be potentially synthesized using the Diels–Alder strategy (pathway E). Recent and representative examples of the application of fluorofuran and fluoroalkylfuran reagents in drug development using these approaches are discussed below.
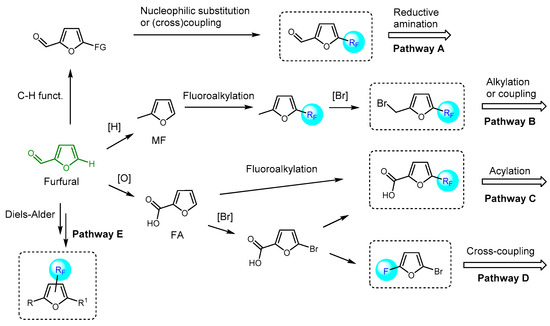
Scheme 4.
Representative reaction map for the synthesis of fluorinated and fluoroalkylated furanic building blocks for drug development (possible structures of these building blocks are enclosed in dashed squares).
α-Fluorofuran 16 with a boronic acid pinacol ester group can be synthesized from 5-bromo-2-furoic acid through decarbonylative fluorination, followed by a reaction with pinacol diborate [180]. Cross-coupling of 16 was employed to introduce the 2-fluorofuran fragment into targeted molecules in the development of inhibitors of MCL1 for cancer therapy (Scheme 5) [65,181]. Compound 2 (Table 1) binds to the BH3-binding groove of MCL1 with high selectively and affinity [65]. In later studies, a number of similar 4-fluorofur-2-yl (17) and 4-fluorophenyl (18) derivatives were discovered. Despite the lower activity of 4-fluorophenyl derivatives 18 in vitro, a fluorophenyl analogue demonstrated the in vivoefficacy comparable to that of fluorofuryl derivatives 17, prompting further research on fluorophenyl derivatives 18 [181].
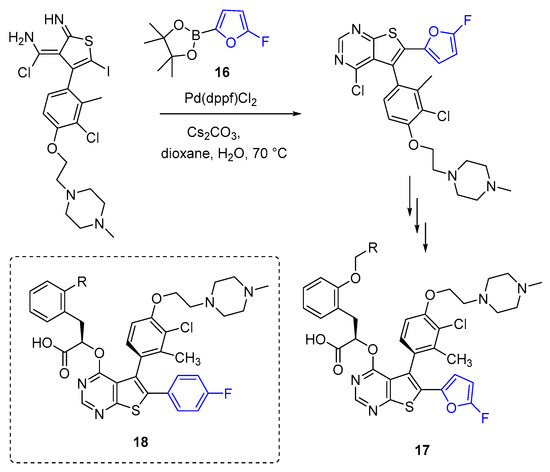
Scheme 5.
Application of fluorofuranic pinacol ester 16 in the synthesis of MCL1 inhibitors. Here and below, blue color indicates the fluorine-functionalized furan or other fluorinated fragment.
Pinacol boronic ester 16 was also utilized in the synthesis of compound 19a, which is a α-fluorofuran analogue of manogepix (APX001A), the active moiety of a novel experimental antifungal drug fosmanogepix (Scheme 6). The antifungal activity of 19a was evaluated against Cryptococcus neoformans and Cryptococcus gattii [182]. Compound 19a exhibited notably lower MIC values and a longer half-life compared to manogepix and their furanic nonfluorinated analogue 19b. Therefore, it can be concluded that the incorporation of a fluorine atom into the furan fragment of the manogepix analogue 19b enhances its metabolic stability.
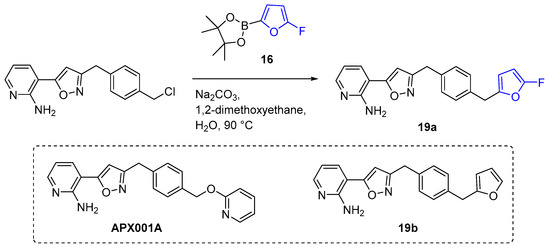
Scheme 6.
Synthesis of α-fluorofuryl analogue of manogepix.
Various 1,2,3,4-tetrahydroisoquinoline-3-carboxylic acids, including 3-(5-fluorofuryl)-substituted derivatives 21(a,b), were synthesized and evaluated for their ability to partially activate peroxisome proliferator-activated receptor gamma (PPARγ), which plays a key role in lowering the blood sugar level in patients with type 2 diabetes [183]. In this series, compound 21b containing an indanyl group exhibited reduced affinity for the PPARγ receptor in comparison to its non-fluorinated counterpart that was selected as the lead compound for further studies. The compounds 21(a,b) were synthesized from 3-(5-fluorofuryl)acrylic acid 20, which was obtained from ethyl 5-bromofuran-2-carboxylate through decarbonylative fluorination using Selectfluor (Scheme 7) [183].
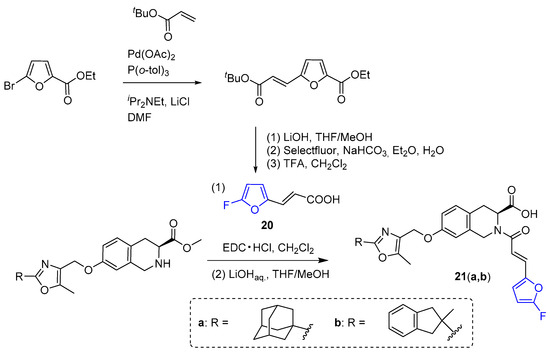
Scheme 7.
Synthesis of acrylic 3-(5-fluorofuryl)-substituted 1,2,3,4-tetrahydroisoquinoline-3-carboxylic acids using 3-(5-fluorofuryl)-acrylic acid 20.
5-Fluorofuranic amides 22 and 23 were synthesized with the aim of searching for new effective selective agonists of α4β2 nicotinic acetylcholine receptors (nAChR) [184]. These amides were obtained through the acylation of corresponding Boc-protected amines with 5-fluoro-2-furoic acid using HBTU as a carboxylic group activator, followed by Boc deprotection (Scheme 8) [184]. SAR studies indicated that furoamide 22 exhibited sufficiently high efficacy as an α4β2 nAChR-selective agonist among other studied heterocyclic amides. However, the significant undesirable activation of nAChR receptors specifically located in the ganglia hindered the potential of 5-fluoro-2-furyl derivatives 22 and 23 as lead compounds for further in vivotesting.
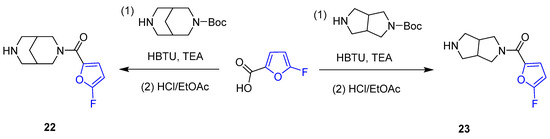
Scheme 8.
Application of 5-fluorofuranic acid for the synthesis of amides 22 and 23.
To mitigate potential genotoxicity associated with the formation of nitrofuran metabolites, the nitro group in furanic orally available UT inhibitor 24 was replaced with more stable fluorine or difluoromethyl group [185]. These new compounds 25(a,b) were synthesized by condensing an N-(4-aminophenyl)acetamide with fluorinated furan-2-carboxylic acids in the presence of a base and HATU (Scheme 9). Unfortunately, the substitution of the nitro group with fluorine or difluoromethyl group resulted in a significant reduction in biological activity for this class of compounds.
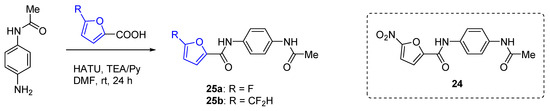
Scheme 9.
Synthesis of amides 25(a,b) by acylation with C5-fluorinated furoic acids.
The α-trifluoromethylfuran derivatives 27(a–d) displayed significantly higher antifungal activity against C. neoformans compared to their non-fluorinated furanic analogs. However, these compounds were less potent than their thiophene counterparts. Although in subsequent tests on fungicidal or fungistatic activity, as well as on selectivity against fungi/human cells, none of trifluoromethylated furans were sufficiently active and selective to be included in the leader compound set, the results of these experiments indicate the importance of substituents in furan moieties for high antifungal activity [186]. The abovementioned derivatives 27(a–d) were obtained from the corresponding 5-trifluoromethylfurane hydrazide 26, which was synthesized starting from 2-trifluoromethylfuran-5-carboxylic acid (Scheme 10).
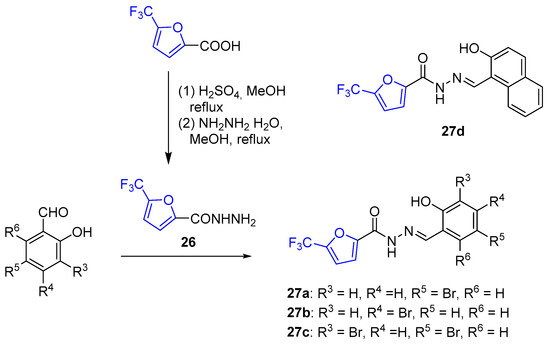
Scheme 10.
Synthesis of α-trifluoromethylated furanic hydrazides 27(a–c).
Using a series of 5-(5-furan-2-yl-pyrazol-1-yl)-1H-benzimidazole derivatives that inhibited the assembly of the HIV-1 capsid, the effect of site-selective modifications at N1, C2 and C16 of this scaffold was clarified. Replacing the methyl substituent at C16 with a trifluoromethyl group (compound 33) produced a six-fold gain in the antiviral potency significantly affecting the IC50 values [187]. The key stage of the synthesis of these 5-(5-furan-2-ylpyrazol-1-yl)-1H-benzimidazoles was the acidic condensation of hydrazine 31 with fluorinated furanic diketone 30, which in turn was obtained from 5-(trifluoromethyl)furan derivative 28 (Scheme 11).
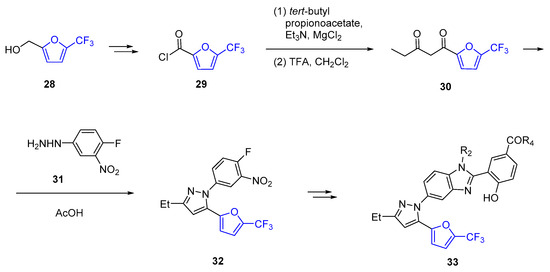
Scheme 11.
Synthesis of HIV-1 inhibitor 33 containing α-trifluoromethylated furan.
Phthalazin-1(2H)-ones with furan as a side substituent showed the highest levels of antiproliferative activities against olaparib- and talazoparib-resistant Capan-1 cells [61]. In attempts to find the optimal substituents at the α-carbon of the furan ring, derivatives 38(a-e) containing the furan substituted at the α-carbon position by various fluoroalkyl moieties were obtained (Scheme 12).
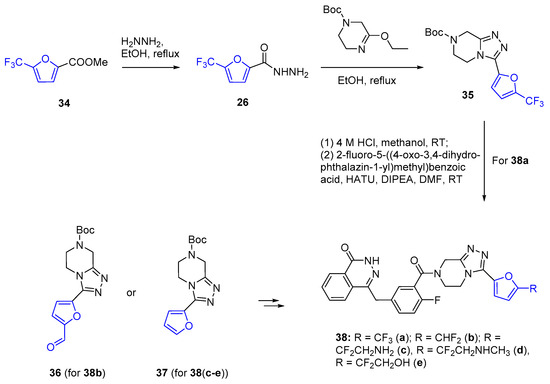
Scheme 12.
Synthesis of α-fluoroalkylated furanic phthalazin-1(2H)-ones 38(a–e).
The initial building block for the synthesis of 38a was α-trifluoromethylated furanic ester 34. This compound was converted into triazole 35 through a two-step process involving modification to hydrazide 26, followed by cyclization. The α-difluoromethyl fragments in 38b and 38(c-e) were introduced via fluorination at α-position of the furanic ring in intermediates 36 and 37, respectively. Among these compounds, water-soluble derivatives 38(c-e) demonstrated improved inhibitory activity, but reduced potency towards drug-resistant cells (olaparib- and talazoparib-resistant Capan-1 cells) [61]. Thus, unfortunately, none of these fluorinated furan structures emerged as the leader compound in this study.
4. Conclusions
Although numerous methods have been reported for introducing fluorine or fluorine-containing functional groups into various aromatic and heteroaromatic compounds, direct functionalization approaches for furfural-derived furans remain underdeveloped. Current methodologies for synthesizing α-fluorofurans and α-fluoroalkylfurans still suffer from low yields, limited generality, or reliance on an expensive reagent. Furthermore, furans containing fluorinated functional groups at the β-positions were synthesized from furfural-derived substrates only through indirect methods. The existing fluoroalkylation techniques were typically tested on a limited range of relatively stable furfural-derived furan substrates, such as 2-methylfuran, methyl 2-furoate, and 2-acetylfuran.
Fluorinated and fluoroalkylated furans, benefiting from the unique properties of both furan and fluorine functionalities, hold significant promise for drug design. However, none of these compounds have yet been approved for clinical use by the Food and Drug Administration (FDA). On the other hand, fluorination significantly increases the exothermic effect of Diels–Alder reactions and lowers the activation energy barriers, which is more pronounced at the α-position of furan compared to the β-position [188,189]. Consequently, incorporating fluorofurans or fluoroalkylfurans into the structures of Diels–Alder-based dynamers could substantially influence their thermal and chemical stability. However, our literature review revealed no reported examples of fluorinated furans being applied in the development of Diels–Alder dynamers.
To achieve more sustainable fluorinated polymers and pharmaceuticals, it is essential to develop more versatile and efficient methods for introducing fluorinated functional groups into furan derivatives. Key considerations include the applicability of these methods to a range of furfural-derived donor- or acceptor-substituted furan substrates, including those containing sensitive functional groups. Establishing such methodologies would not only expand synthetic possibilities but also promote the active and efficient utilization of plant biomass in drug design and materials science through the development of fluoro-functionalized furans.
Author Contributions
Conceptualization and supervision, K.I.G.; writing and editing, K.I.G. and I.V.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the Russian Science Foundation (grant number 23-73-00003).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
This work was carried out within the framework of a P.L. Kapitsa Grant Program (Phase III) at Moscow Polytechnic University. I.V.S. is grateful to the Ministry of Science and Higher Education of the Russian Federation (Contract No. 075-00276-25-00) for providing access to the scientific literature.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| AIDS | Acquired immunodeficiency syndrome |
| BH3 | B-cell lymphoma homology 3 |
| bpy | 2,2′-Bipyridine |
| CAN | Covalent adaptable network |
| CCE | Constant current electrolysis |
| COD | 1,3-Cyclooctadiene |
| DA | Diels–Alder |
| DAST | Diethylaminosulfur trifluoride |
| dba | Dibenzylideneacetone |
| DCE | 1,2-Dichloroethane |
| Deoxo-Fluor | Bis(2-methoxyethyl)aminosulfur trifluoride |
| DIPEA | N,N-Diisopropylethylamine |
| DMC | Dimethyl carbonate |
| DMF | N,N-Dimethylformamide |
| DMPU | 1,3-Dimethyl-3,4,5,6-tetrahydro-2-pyrimidinone |
| DMSO | Dimethyl sulfoxide |
| dppBz | 1,2-Bis(diphenylphosphino)benzene |
| dppf | 1,1′-Bis(diphenylphosphino)ferrocene |
| DTBDPy | 4,4′-Di-tert-butyl-2,2′-dipyridyl |
| EDC | 1-Ethyl-3-(3-dimethylaminopropyl)carbodiimide |
| FDA | Food and Drug Administration |
| FMDA | Furan–maleimide Diels–Alder |
| HATU | Hexafluorophosphate azabenzotriazole tetramethyl uronium |
| HBTU | O-(benzotriazol-1-yl)-N,N,N,N-tetramethyluronium hexafluorophosphate |
| HIV | Human immunodeficiency virus |
| IC50 | Half maximal inhibitory concentration |
| LED | Light-emitting diode |
| MCL1 | Myeloid cell leukemia 1 |
| MIC | Minimum inhibitory concentration |
| MOM | Methoxymethyl |
| nAChR | α4β2 Nicotinic acetylcholine receptors |
| NMP | N-Methyl-2-pyrrolidone |
| phen | 1,10-Phenanthroline |
| PMDETA | N,N,N′,N″,N″-Pentamethyldiethylenetriamine |
| PPARγ | Peroxisome proliferator-activated receptor gamma |
| ppy | 2-Phenylpyridine |
| rDA | Retro Diels–Alder |
| RT | Room temperature |
| SAR | Structure—activity relationship |
| SelectFluor | 1-(Chloromethyl)-4-fluoro-1,4-diazabicyclo [2.2.2]octane-1,4-diium ditetrafluoroborate |
| SET | Single electron transfer |
| SIPr•Cl | 1,3-Bis [2,6-bis(1-methylethyl) phenyl]-1H-imidazolium chloride |
| TEA | Triethylamine |
| TFA | Trifluoroacetic acid |
| TFAA | Trifluoroacetic anhydride |
| THF | Tetrahydrofuran |
| TMEDA | Tetramethylethylenediamine |
References
- Budisa, N.; Kubyshkin, V.; Schulze-Makuch, D. Fluorine-rich planetary environments as possible habitats for life. Life 2014, 4, 374–385. [Google Scholar] [CrossRef] [PubMed]
- Ismail, F.M.D. Important fluorinated drugs in experimental and clinical use. J. Fluorine Chem. 2002, 118, 27–33. [Google Scholar] [CrossRef]
- Zhong, S.; Hafner, A.; Hussal, C.; Nieger, M.; Bräse, S. Metal-free radical perfluoroalkylation of (hetero)arenes. RSC Adv. 2015, 5, 6255–6258. [Google Scholar] [CrossRef]
- Rizzo, C.; Amata, S.; Pibiri, I.; Pace, A.; Buscemi, S.; Palumbo Piccionello, A. FDA-Approved Fluorinated Heterocyclic Drugs from 2016 to 2022. Int. J. Mol. Sci. 2023, 24, 7728. [Google Scholar] [CrossRef]
- Han, J.; Kiss, L.; Mei, H.; Remete, A.M.; Ponikvar-Svet, M.; Sedgwick, D.M.; Roman, R.; Fustero, S.; Moriwaki, H.; Soloshonok, V.A. Chemical Aspects of Human and Environmental Overload with Fluorine. Chem. Rev. 2021, 121, 4678–4742. [Google Scholar] [CrossRef]
- Gillis, E.P.; Eastman, K.J.; Hill, M.D.; Donnelly, D.J.; Meanwell, N.A. Applications of Fluorine in Medicinal Chemistry. J. Med. Chem. 2015, 58, 8315–8359. [Google Scholar] [CrossRef] [PubMed]
- Shah, P.; Westwell, A.D. The role of fluorine in medicinal chemistry. J. Enzyme Inhib. Med. Chem. 2007, 22, 527–540. [Google Scholar] [CrossRef] [PubMed]
- Bohm, H.J.; Banner, D.; Bendels, S.; Kansy, M.; Kuhn, B.; Muller, K.; Obst-Sander, U.; Stahl, M. Fluorine in medicinal chemistry. ChemBioChem 2004, 5, 637–643. [Google Scholar] [CrossRef]
- Niu, Z.X.; Hu, J.; Sun, J.F.; Wang, Y.T. Fluorine in the pharmaceutical industry: Synthetic approaches and application of clinically approved fluorine-enriched anti-infectious medications. Eur. J. Med. Chem. 2024, 271, 116446. [Google Scholar] [CrossRef]
- Moskalik, M.Y. Monofluoromethylation of N-Heterocyclic Compounds. Int. J. Mol. Sci. 2023, 24, 17593. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, J.; Gu, Z.; Wang, S.; Zhu, W.; Acena, J.L.; Soloshonok, V.A.; Izawa, K.; Liu, H. Next Generation of Fluorine-Containing Pharmaceuticals, Compounds Currently in Phase II-III Clinical Trials of Major Pharmaceutical Companies: New Structural Trends and Therapeutic Areas. Chem. Rev. 2016, 116, 422–518. [Google Scholar] [CrossRef] [PubMed]
- Jeschke, P. Recent developments in fluorine-containing pesticides. Pest Manag. Sci. 2024, 80, 3065–3087. [Google Scholar] [CrossRef]
- Grygorenko, O.O.; Melnykov, K.P.; Holovach, S.; Demchuk, O. Fluorinated Cycloalkyl Building Blocks for Drug Discovery. ChemMedChem 2022, 17, e202200365. [Google Scholar] [CrossRef]
- Mao, T.; Ma, M.J.; Zhao, L.; Xue, D.P.; Yu, Y.; Gu, J.; He, C.Y. A general and green fluoroalkylation reaction promoted via noncovalent interactions between acetone and fluoroalkyl iodides. Chem. Commun. 2020, 56, 1815–1818. [Google Scholar] [CrossRef]
- Meanwell, N.A. Fluorine and Fluorinated Motifs in the Design and Application of Bioisosteres for Drug Design. J. Med. Chem. 2018, 61, 5822–5880. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Sanchez-Rosello, M.; Acena, J.L.; del Pozo, C.; Sorochinsky, A.E.; Fustero, S.; Soloshonok, V.A.; Liu, H. Fluorine in pharmaceutical industry: Fluorine-containing drugs introduced to the market in the last decade (2001–2011). Chem. Rev. 2014, 114, 2432–2506. [Google Scholar] [CrossRef]
- Purser, S.; Moore, P.R.; Swallow, S.; Gouverneur, V. Fluorine in medicinal chemistry. Chem. Soc. Rev. 2008, 37, 320–330. [Google Scholar] [CrossRef] [PubMed]
- Burger, K.; Helmreich, B.; Jendrewski, O.; Hecht, R.; Maier, G.; Nuyken, O. New strategies for synthesis of partially fluorinated heterocycles as precursors for trifluoromethyl-substituted polymers. Macromol. Symp. 2011, 82, 143–160. [Google Scholar] [CrossRef]
- Jeanmart, S.; Edmunds, A.J.F.; Lamberth, C.; Pouliot, M.; Morris, J.A. Synthetic Approaches to the 2019–2020 New Agrochemicals. Synthesis 2023, 56, 357–367. [Google Scholar] [CrossRef]
- Ali, S.; Zhou, J. Highlights on U.S. FDA-approved fluorinated drugs over the past five years (2018–2022). Eur. J. Med. Chem. 2023, 256, 115476. [Google Scholar] [CrossRef]
- Wang, Q.; Song, H.; Wang, Q. Fluorine-containing agrochemicals in the last decade and approaches for fluorine incorporation. Chin. Chem. Lett. 2022, 33, 626–642. [Google Scholar] [CrossRef]
- Hong, C.M.; Whittaker, A.M.; Schultz, D.M. Nucleophilic Fluorination of Heteroaryl Chlorides and Aryl Triflates Enabled by Cooperative Catalysis. J. Org. Chem. 2021, 86, 3999–4006. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, Y.; Tokunaga, E.; Kobayashi, O.; Hirai, K.; Shibata, N. Current Contributions of Organofluorine Compounds to the Agrochemical Industry. iScience 2020, 23, 101467. [Google Scholar] [CrossRef] [PubMed]
- Inoue, M.; Sumii, Y.; Shibata, N. Contribution of Organofluorine Compounds to Pharmaceuticals. ACS Omega 2020, 5, 10633–10640. [Google Scholar] [CrossRef] [PubMed]
- Champagne, P.A.; Desroches, J.; Hamel, J.D.; Vandamme, M.; Paquin, J.F. Monofluorination of Organic Compounds: 10 Years of Innovation. Chem. Rev. 2015, 115, 9073–9174. [Google Scholar] [CrossRef]
- Nosova, E.V.; Lipunova, G.N.; Charushin, V.N.; Chupakhin, O.N. Fluorine-containing indoles: Synthesis and biological activity. J. Fluorine Chem. 2018, 212, 51–106. [Google Scholar] [CrossRef]
- Budovská, M.; Krochtová, K.; Kuba, M.; Tischlerová, V.; Mojžiš, J. Synthesis and cytotoxicity evaluation of novel 5-fluorinated indoles. J. Fluorine Chem. 2021, 250, 109879. [Google Scholar] [CrossRef]
- Zayed, M.F. New fluorinated quinazolinone derivatives as anticonvulsant agents. J. Taibah Univ. Med. Sci. 2014, 9, 104–109. [Google Scholar] [CrossRef]
- Balakumar, C.; Lamba, P.; Kishore, D.P.; Narayana, B.L.; Rao, K.V.; Rajwinder, K.; Rao, A.R.; Shireesha, B.; Narsaiah, B. Synthesis, anti-inflammatory evaluation and docking studies of some new fluorinated fused quinazolines. Eur. J. Med. Chem. 2010, 45, 4904–4913. [Google Scholar] [CrossRef]
- Jasem, Y.A.; Thiemann, T.; Gano, L.; Oliveira, M.C. Fluorinated steroids and their derivatives. J. Fluorine Chem. 2016, 185, 48–85. [Google Scholar] [CrossRef]
- Sandulenko, I.V.; Ambartsumyan, A.A.; Moiseev, S.K. Fluorinated and [(18)F]fluorinated morphinan based opioid ligands. Org. Biomol. Chem. 2020, 18, 5533–5557. [Google Scholar] [CrossRef] [PubMed]
- Sandulenko, I.V.; Belozertseva, I.V.; Zvartau, E.E.; Zelentsova, M.V.; Ambartsumyan, A.A.; Smol’yakov, A.F.; Moiseev, S.K. C(21)-fluorinated thevinol scaffold for opioid ligands. 21,21,21-Trifluoro-6-O-nororvinols: Design, synthesis and analgesic activity. Eur. J. Med. Chem. 2023, 252, 115296. [Google Scholar] [CrossRef] [PubMed]
- Ambartsumyan, A.A.; Belozertseva, I.V.; Dravolina Ocapital, A.C.; Zvartau, E.E.; Sandulenko, I.V.; Zelentsova, M.V.; Peregudov, A.S.; Moiseev, S.K. Orvinol-based opioid receptor antagonist fluorinated at C(20)-pharmacophore. Eur. J. Med. Chem. 2025, 284, 117189. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Tian, Q.; Ge, J.; Wu, X.; Li, Z.; Cheng, G. Recent advances in the synthesis of trifluoromethyl-containing heterocyclic compounds via trifluoromethyl building blocks. Org. Biomol. Chem. 2024, 22, 6246–6276. [Google Scholar] [CrossRef]
- Burger, K.; Helmreich, B.; Hennig, L.; Spengler, J.; Albericio, F.; Fuchs, A. Partially Fluorinated Heterocycles from 4,4-Bis(trifluoromethyl)-hetero-1,3-dienes via C–F Bond Activation—Synthesis of 2-Fluoro-3-(trifluoromethyl)furans. Monatsh. Chem. 2007, 138, 227–236. [Google Scholar] [CrossRef]
- Straathof, N.J.; Gemoets, H.P.; Wang, X.; Schouten, J.C.; Hessel, V.; Noel, T. Rapid trifluoromethylation and perfluoroalkylation of five-membered heterocycles by photoredox catalysis in continuous flow. ChemSusChem 2014, 7, 1612–1617. [Google Scholar] [CrossRef]
- Liang, T.; Neumann, C.N.; Ritter, T. Introduction of fluorine and fluorine-containing functional groups. Angew. Chem. Int. Ed. 2013, 52, 8214–8264. [Google Scholar] [CrossRef]
- Nagib, D.A.; MacMillan, D.W. Trifluoromethylation of arenes and heteroarenes by means of photoredox catalysis. Nature 2011, 480, 224–228. [Google Scholar] [CrossRef]
- Muralirajan, K.; Kancherla, R.; Bau, J.A.; Taksande, M.R.; Qureshi, M.; Takanabe, K.; Rueping, M. Exploring the Structure and Performance of Cd–Chalcogenide Photocatalysts in Selective Trifluoromethylation. ACS Catal. 2021, 11, 14772–14780. [Google Scholar] [CrossRef]
- Shaw, R.; Sihag, N.; Bhartiya, H.; Yadav, M.R. Harnessing photocatalytic and electrochemical approaches for C–H bond trifluoromethylation and fluoroalkylation. Org. Chem. Front. 2024, 11, 954–1014. [Google Scholar] [CrossRef]
- Baishya, G.; Dutta, N.B. Recent Advances in Direct C−H Trifluoromethylation of N-Heterocycles. ChemistrySelect 2021, 6, 13384–13408. [Google Scholar] [CrossRef]
- Mandal, D.; Maji, S.; Pal, T.; Sinha, S.K.; Maiti, D. Recent advances in transition metal-mediated trifluoromethylation reactions. Chem. Commun. 2022, 58, 10442–10468. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, Y.; Xu, X.H.; Qing, F.L. Trifluoromethanesulfonic Anhydride as a Low-Cost and Versatile Trifluoromethylation Reagent. Angew. Chem. Int. Ed. 2018, 57, 6926–6929. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Zhang, Z.; Fang, Y.; Zhu, L.; Li, C. Radical trifluoromethylation. Chem. Soc. Rev. 2021, 50, 6308–6319. [Google Scholar] [CrossRef] [PubMed]
- O’Hagan, D.; Young, R.J. Future challenges and opportunities with fluorine in drugs? Med. Chem. Res. 2023, 32, 1231–1234. [Google Scholar] [CrossRef]
- Sham, H.L.; Betebenner, D.A. A new and concise synthesis of 3-fluoro-2,5-disubstituted furans. J. Chem. Soc. Chem. Commun. 1991, 16, 1134–1135. [Google Scholar] [CrossRef]
- Kushwaha, P.; Rashi, R.; Bhardwaj, A.; Khan, D. Advances in Synthesis and Anti-Alzheimer’s Disease Potential of Functionalized Benzofurans: A Recent Overview. Synlett 2025. [Google Scholar] [CrossRef]
- Patel, P.; Shakya, R.; Vishakha; Asati, V.; Kurmi, B.D.; Verma, S.K.; Gupta, G.D.; Rajak, H. Furan and benzofuran derivatives as privileged scaffolds as anticancer agents: SAR and docking studies (2010 to till date). J. Mol. Struct. 2024, 1299, 137098. [Google Scholar] [CrossRef]
- Arce-Ramos, L.; Castillo, J.C.; Becerra, D. Synthesis and Biological Studies of Benzo[b]furan Derivatives: A Review from 2011 to 2022. Pharmaceuticals 2023, 16, 1265. [Google Scholar] [CrossRef]
- Banerjee, R.; Hks, K.; Banerjee, M. Medicinal significance of furan derivatives: A Review. Int. J. Res. Phytochem. Pharmacol. 2015, 5, 48–57. [Google Scholar]
- Nevagi, R.J.; Dighe, S.N.; Dighe, S.N. Biological and medicinal significance of benzofuran. Eur. J. Med. Chem. 2015, 97, 561–581. [Google Scholar] [CrossRef] [PubMed]
- Chen, V.Y.; Kwon, O. Unified Approach to Furan Natural Products via Phosphine-Palladium Catalysis. Angew. Chem. Int. Ed. 2021, 60, 8874–8881. [Google Scholar] [CrossRef] [PubMed]
- Galkin, K.I.; Ananikov, V.P. When Will 5-Hydroxymethylfurfural, the "Sleeping Giant" of Sustainable Chemistry, Awaken? ChemSusChem 2019, 12, 2976–2982. [Google Scholar] [CrossRef]
- Galkin, K.I.; Krivodaeva, E.A.; Romashov, L.V.; Zalesskiy, S.S.; Kachala, V.V.; Burykina, J.V.; Ananikov, V.P. Critical Influence of 5-Hydroxymethylfurfural Aging and Decomposition on the Utility of Biomass Conversion in Organic Synthesis. Angew. Chem. Int. Ed. 2016, 55, 8338–8342. [Google Scholar] [CrossRef] [PubMed]
- Kolykhalov, D.A.; Golysheva, A.N.; Erokhin, K.S.; Karlinskii, B.Y.; Ananikov, V.P. The Stability Challenge of Furanic Platform Chemicals in Acidic and Basic Conditions. ChemSusChem 2025, 18, e202401849. [Google Scholar] [CrossRef]
- Placais, C.; Donnard, M.; Panossian, A.; Vors, J.P.; Bernier, D.; Pazenok, S.; Leroux, F.R. Synthesis of 3-Amino-5-fluoroalkylfurans by Intramolecular Cyclization. Org. Lett. 2021, 23, 4915–4919. [Google Scholar] [CrossRef]
- Wang, J.; Chen, S.; Wu, W.; Wen, S.; Weng, Z. Base-Mediated Tunable Synthesis of 2-Trifluoromethylated Furans and Dihydrofuranols: Extraordinary Stable in Sulfuric Acid. J. Org. Chem. 2019, 84, 15685–15696. [Google Scholar] [CrossRef] [PubMed]
- Taj, M.B.; Raheel, A.; Ayub, R.; Alnajeebi, A.M.; Abualnaja, M.; Habib, A.H.; Alelwani, W.; Noor, S.; Ullah, S.; Al-Sehemi, A.G.; et al. Exploring novel fluorine-rich fuberidazole derivatives as hypoxic cancer inhibitors: Design, synthesis, pharmacokinetics, molecular docking, and DFT evaluations. PLoS ONE 2023, 18, e0262790. [Google Scholar] [CrossRef]
- Zhou, W.; Yue, Z.; Zhang, J. A highly efficient one-pot trifluoromethylation/cyclization reaction of electron-deficient 1,3-conjugated enynes: Modular access to trifluoromethylated furans and 2,3-dihydrofurans. Org. Chem. Front. 2016, 3, 1416–1419. [Google Scholar] [CrossRef]
- Chong, Q.; Xin, X.; Wang, C.; Wu, F.; Wang, H.; Shi, J.C.; Wan, B. DABCO-catalyzed synthesis of trifluoromethylated furans from propargyl alcohols and methyl 2-perfluoroalkynoate. J. Org. Chem. 2014, 79, 2105–2110. [Google Scholar] [CrossRef]
- Sun, Y.; Yang, H.; Yuan, J.; Wang, L.; Song, S.; Chen, R.; Bao, X.; Jia, L.; Yang, T.; Zhang, X.; et al. YCH1899, a Highly Effective Phthalazin-1(2H)-one Derivative That Overcomes Resistance to Prior PARP Inhibitors. J. Med. Chem. 2023, 66, 12284–12303. [Google Scholar] [CrossRef] [PubMed]
- Abbas, A.A.; Farghaly, T.A.; Dawood, K.M. Recent advances on anticancer and antimicrobial activities of directly-fluorinated five-membered heterocycles and their benzo-fused systems. RSC Adv. 2024, 14, 19752–19779. [Google Scholar] [CrossRef]
- Abbas, A.A.; Farghaly, T.A.; Dawood, K.M. Recent progress in therapeutic applications of fluorinated five-membered heterocycles and their benzo-fused systems. RSC Adv. 2024, 14, 33864–33905. [Google Scholar] [CrossRef]
- Cantrell, A.S.; Engelhardt, P.; Hogberg, M.; Jaskunas, S.R.; Johansson, N.G.; Jordan, C.L.; Kangasmetsa, J.; Kinnick, M.D.; Lind, P.; Morin, J.M., Jr.; et al. Phenethylthiazolylthiourea (PETT) compounds as a new class of HIV-1 reverse transcriptase inhibitors. 2. Synthesis and further structure-activity relationship studies of PETT analogs. J. Med. Chem. 1996, 39, 4261–4274. [Google Scholar] [CrossRef] [PubMed]
- Kotschy, A.; Szlavik, Z.; Murray, J.; Davidson, J.; Maragno, A.L.; Le Toumelin-Braizat, G.; Chanrion, M.; Kelly, G.L.; Gong, J.N.; Moujalled, D.M.; et al. The MCL1 inhibitor S63845 is tolerable and effective in diverse cancer models. Nature 2016, 538, 477–482. [Google Scholar] [CrossRef]
- Li, M.; Zhou, W. Transition-Metal-Free Synthesis of Trifluoromethylated Furans via a Bu3P-Mediated Tandem Acylation–Wittig Reaction. Synlett 2020, 31, 2035–2038. [Google Scholar] [CrossRef]
- Xu, W.; Zhang, Q.; Shao, Q.; Xia, C.; Wu, M. Photocatalytic C−F Bond Activation of Fluoroarenes, gem-Difluoroalkenes and Trifluoromethylarenes. Asian J. Org. Chem. 2021, 10, 2454–2472. [Google Scholar] [CrossRef]
- Hooker, L.V.; Bandar, J.S. Synthetic Advantages of Defluorinative C-F Bond Functionalization. Angew. Chem. Int. Ed. 2023, 62, e202308880. [Google Scholar] [CrossRef]
- Simur, T.T.; Ye, T.; Yu, Y.-J.; Zhang, F.-L.; Wang, Y.-F. C–F bond functionalizations of trifluoromethyl groups via radical intermediates. Chin. Chem. Lett. 2022, 33, 1193–1198. [Google Scholar] [CrossRef]
- Burger, K.; Helmreich, B. Synthesis of 3-trifluoromethylfurans from β,β-bis(trifluoromethyl)α,β-unsaturated ketones and tin(II) chloride. J. Chem. Soc. Chem. Commun. 1992, 4, 348–349. [Google Scholar] [CrossRef]
- Li, P.; Chai, Z.; Zhao, G.; Zhu, S.-Z. Synthesis of 3-Fluoro-2,5-Disubstituted Furans and Further Derivative Reactions to Access Fluorine-Containing 3,3′-Bifurans and Tetrasubstituted Furans. Synlett 2008, 2008, 2547–2551. [Google Scholar] [CrossRef]
- Li, Y.; Wheeler, K.A.; Dembinski, R. Room temperature syntheses of entirely diverse substituted beta-fluorofurans. Org. Biomol. Chem. 2012, 10, 2395–2408. [Google Scholar] [CrossRef][Green Version]
- Burger, K.; Hennig, L.; Fuchs, A.; Greif, D.; Spengler, J.; Albericio, F. Domino Reactions with Fluorinated Five-membered Heterocycles—Syntheses of Trifluoromethyl Substituted Butenolides and γ-Ketoacids. Monatsh. Chem. 2005, 136, 1763–1779. [Google Scholar] [CrossRef]
- Xu, C.; Paone, E.; Rodriguez-Padron, D.; Luque, R.; Mauriello, F. Recent catalytic routes for the preparation and the upgrading of biomass derived furfural and 5-hydroxymethylfurfural. Chem. Soc. Rev. 2020, 49, 4273–4306. [Google Scholar] [CrossRef]
- Li, X.; Jia, P.; Wang, T. Furfural: A Promising Platform Compound for Sustainable Production of C4 and C5 Chemicals. ACS Catal. 2016, 6, 7621–7640. [Google Scholar] [CrossRef]
- Mori, A.; Curpanen, S.; Pezzetta, C.; Perez-Luna, A.; Poli, G.; Oble, J. C−H Activation Based Functionalization of Furfural Derivatives. Eur. J. Org. Chem. 2022, 2022, e202200727. [Google Scholar] [CrossRef]
- Bozell, J.J.; Petersen, G.R. Technology development for the production of biobased products from biorefinery carbohydrates—The US Department of Energy’s “Top 10” revisited. Green Chem. 2010, 12, 539. [Google Scholar] [CrossRef]
- Song, R.; Lin, W.; Jiang, Q. A convenient synthesis of 5-fluorofuran-2-carboxylic acid. Tetrahedron Lett. 2011, 52, 4965–4966. [Google Scholar] [CrossRef]
- Gandini, A. The furan/maleimide Diels–Alder reaction: A versatile click–unclick tool in macromolecular synthesis. Prog. Polym. Sci. 2013, 38, 1–29. [Google Scholar] [CrossRef]
- Gevrek, T.N.; Sanyal, A. Furan-containing polymeric Materials: Harnessing the Diels-Alder chemistry for biomedical applications. Eur. Polym. J. 2021, 153, 110514. [Google Scholar] [CrossRef]
- Kucherov, F.A.; Romashov, L.V.; Galkin, K.I.; Ananikov, V.P. Chemical Transformations of Biomass-Derived C6-Furanic Platform Chemicals for Sustainable Energy Research, Materials Science, and Synthetic Building Blocks. ACS Sustain. Chem. Eng. 2018, 6, 8064–8092. [Google Scholar] [CrossRef]
- Espro, C.; Paone, E.; Mauriello, F.; Gotti, R.; Uliassi, E.; Bolognesi, M.L.; Rodriguez-Padron, D.; Luque, R. Sustainable production of pharmaceutical, nutraceutical and bioactive compounds from biomass and waste. Chem. Soc. Rev. 2021, 50, 11191–11207. [Google Scholar] [CrossRef]
- Luque, R.; ZA, A.L.; Balu, A.M.; Voskressensky, L. Heterogeneous Catalysis to Drive the Waste-to-Pharma Concept: From Furanics to Active Pharmaceutical Ingredients. Molecules 2021, 26, 6738. [Google Scholar] [CrossRef]
- Galkin, K.I.; Sandulenko, I.V.; Polezhaev, A.V. Diels–Alder Cycloadditions of Bio-Derived Furans with Maleimides as a Sustainable «Click» Approach towards Molecular, Macromolecular and Hybrid Systems. Processes 2022, 10, 30. [Google Scholar] [CrossRef]
- Briou, B.; Améduri, B.; Boutevin, B. Trends in the Diels–Alder reaction in polymer chemistry. Chem. Soc. Rev. 2021, 50, 11055–11097. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Dam, M.A.; Ono, K.; Mal, A.; Shen, H.; Nutt, S.R.; Sheran, K.; Wudl, F. A thermally re-mendable cross-linked polymeric material. Science 2002, 295, 1698–1702. [Google Scholar] [CrossRef]
- Scheutz, G.M.; Lessard, J.J.; Sims, M.B.; Sumerlin, B.S. Adaptable Crosslinks in Polymeric Materials: Resolving the Intersection of Thermoplastics and Thermosets. J. Am. Chem. Soc. 2019, 141, 16181–16196. [Google Scholar] [CrossRef]
- Lucherelli, M.A.; Duval, A.; Avérous, L. Biobased vitrimers: Towards sustainable and adaptable performing polymer materials. Prog. Polym. Sci. 2022, 127, 101515. [Google Scholar] [CrossRef]
- Mariani, A.; Malucelli, G. Biobased vitrimers: Towards sustainability and circularity. Chem. Commun. 2025, 61, 2173–2189. [Google Scholar] [CrossRef]
- Purushotam; Bera, A.; Banerjee, D. Recent advances on non-precious metal-catalysed fluorination, difluoromethylation, trifluoromethylation, and perfluoroalkylation of N-heteroarenes. Org. Biomol. Chem. 2023, 21, 9298–9315. [Google Scholar] [CrossRef]
- Alonso, C.; Martinez de Marigorta, E.; Rubiales, G.; Palacios, F. Carbon trifluoromethylation reactions of hydrocarbon derivatives and heteroarenes. Chem. Rev. 2015, 115, 1847–1935. [Google Scholar] [CrossRef] [PubMed]
- Kliś, T. Visible-Light Photoredox Catalysis for the Synthesis of Fluorinated Aromatic Compounds. Catalysts 2023, 13, 94. [Google Scholar] [CrossRef]
- O’Leary, E.M.; Jones, D.J.; O’Donovan, F.P.; O’Sullivan, T.P. Synthesis of fluorinated oxygen- and sulfur-containing heteroaromatics. J. Fluorine Chem. 2015, 176, 93–120. [Google Scholar] [CrossRef]
- Serdyuk, O.; Butin, A.; Abaev, V. Synthesis of fluorofurans and perfluoroalkylfurans. J. Fluorine Chem. 2010, 131, 296–319. [Google Scholar] [CrossRef]
- Kondratov, I.S.; Tolmachova, N.A.; Haufe, G. Diels–Alder Reaction in the Synthesis of Fluorinated (Hetero)Aromatic Compounds. Eur. J. Org. Chem. 2018, 2018, 3618–3647. [Google Scholar] [CrossRef]
- Nyffeler, P.T.; Duron, S.G.; Burkart, M.D.; Vincent, S.P.; Wong, C.H. Selectfluor: Mechanistic insight and applications. Angew. Chem. Int. Ed. 2004, 44, 192–212. [Google Scholar] [CrossRef]
- Alic, B.; Petrovcic, J.; Jelen, J.; Tavcar, G.; Iskra, J. Renewable Reagent for Nucleophilic Fluorination. J. Org. Chem. 2022, 87, 5987–5993. [Google Scholar] [CrossRef]
- Campbell, M.G.; Ritter, T. Modern carbon-fluorine bond forming reactions for aryl fluoride synthesis. Chem. Rev. 2015, 115, 612–633. [Google Scholar] [CrossRef]
- Yerien, D.E.; Bonesi, S.; Postigo, A. Fluorination methods in drug discovery. Org. Biomol. Chem. 2016, 14, 8398–8427. [Google Scholar] [CrossRef]
- Fernandes, A.J.; Giri, R.; Houk, K.N.; Katayev, D. Review and Theoretical Analysis of Fluorinated Radicals in Direct C(Ar)-H Functionalization of (Hetero)arenes. Angew. Chem. Int. Ed. 2024, 63, e202318377. [Google Scholar] [CrossRef]
- Jin, Z.; Zhang, F.; Xiao, X.; Wang, N.; Lv, X.; Zhou, L. Recent advances in N-heterocyclic carbene (NHC)-catalyzed fluorination and fluoroalkylation. Org. Chem. Front. 2024, 11, 2112–2133. [Google Scholar] [CrossRef]
- Yang, L.; Dong, T.; Revankar, H.M.; Zhang, C.-P. Recent progress on fluorination in aqueous media. Green Chem. 2017, 19, 3951–3992. [Google Scholar] [CrossRef]
- Britton, R.; Gouverneur, V.; Lin, J.-H.; Meanwell, M.; Ni, C.; Pupo, G.; Xiao, J.-C.; Hu, J. Contemporary synthetic strategies in organofluorine chemistry. Nat. Rev. Methods Primers 2021, 1, 47. [Google Scholar] [CrossRef]
- Bui, T.T.; Hong, W.P.; Kim, H.-K. Recent Advances in Visible Light-mediated Fluorination. J. Fluorine Chem. 2021, 247, 109794. [Google Scholar] [CrossRef]
- Yang, B.; Yu, D.; Xu, X.-H.; Qing, F.-L. Visible-Light Photoredox Decarboxylation of Perfluoroarene Iodine(III) Trifluoroacetates for C–H Trifluoromethylation of (Hetero)arenes. ACS Catal. 2018, 8, 2839–2843. [Google Scholar] [CrossRef]
- Lemmens, V.; Vos, C.; Bugaev, A.L.; Vercammen, J.; Van Velthoven, N.; Gascon, J.; De Vos, D.E. Ru-Bipyridine Entrapped in the Supercages of EMC-1 Faujasite as Catalyst for the Trifluoromethylation of Arenes. ACS Appl. Mater. Interfaces 2022, 14, 971–977. [Google Scholar] [CrossRef]
- Gandini, A.; Lacerda, T.M. Furan Polymers: State of the Art and Perspectives. Macromol. Mater. Eng. 2022, 307, 2100902. [Google Scholar] [CrossRef]
- Iroegbu, A.O.C.; Ray, S.S. On the chemistry of furfuryl alcohol polymerization: A review. J. Polym. Sci. 2023, 62, 1044–1060. [Google Scholar] [CrossRef]
- Yang, X.; Fang, X.; Shao, T. One-Pot Synthesis of α-Fluoroketones and 3-Fluoro-2,4-diarylfurans from Trifluoromethyl β-Diketones via Decarboxylation. Synlett 2015, 26, 1835–1840. [Google Scholar] [CrossRef]
- Panferova, L.I.; Tsymbal, A.V.; Levin, V.V.; Struchkova, M.I.; Dilman, A.D. Reactions of gem-Difluorinated Phosphonium Salts Induced by Light. Org. Lett. 2016, 18, 996–999. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Kuroyanagi, E.; Suzuki, H.; Yasui, T. Catalyst-Free Csp−Csp3 Cross-Coupling of Bromodifluoroacetamides with 1-Iodoalkynes under Visible-Light Irradiation. Adv. Synth. Catal. 2021, 363, 4932–4940. [Google Scholar] [CrossRef]
- Miyazaki, D.; Kudo, K.; Fujiki, Y.; Watanabe, N.; Matsui, T.; Ichikawa, J.; Fuchibe, K. Gold(I)-Catalyzed [2 + 2] and [3 + 2] Cycloadditions of 1,1-Difluoroallenes with Aldehydes: Switchable Syntheses of Fluorinated Oxetanes and Furans. Org. Lett. 2025, 27, 3807–3812. [Google Scholar] [CrossRef] [PubMed]
- Sugiishi, T.; Matsumura, C.; Amii, H. Synthesis of 3-fluoro-2,5-disubstituted furans through ring expansion of gem-difluorocyclopropyl ketones. Org. Biomol. Chem. 2020, 18, 3459–3462. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Maekawa, H. Synthesis of 4-(Trifluoromethyl)cyclopentenones and 2-(Trifluoromethyl)furans by Reductive Trifluoroacetylation of Ynones. Org. Lett. 2017, 19, 6602–6605. [Google Scholar] [CrossRef]
- Forrest, A.K.; O’Hanlon, P.J. The preparation and lithiation of bromofluorofurans via a novel fluorodecarboxylation. Tetrahedron Lett. 1995, 36, 2117–2118. [Google Scholar] [CrossRef]
- Yuan, X.; Yao, J.F.; Tang, Z.Y. Decarboxylative Fluorination of Electron-Rich Heteroaromatic Carboxylic Acids with Selectfluor. Org. Lett. 2017, 19, 1410–1413. [Google Scholar] [CrossRef]
- Arisawa, M. Rhodium-catalyzed synthesis of unsymmetric di(heteroaryl) compounds via heteroaryl exchange reactions. Phosphorus Sulfur Silicon Rel. Elem. 2019, 194, 643–648. [Google Scholar] [CrossRef]
- Arisawa, M.; Tanii, S.; Tazawa, T.; Yamaguchi, M. Rhodium-catalyzed transformation of heteroaryl aryl ethers into heteroaryl fluorides. Chem. Commun. 2016, 52, 11390–11393. [Google Scholar] [CrossRef]
- Yamada, S.; Gavryushin, A.; Knochel, P. Convenient electrophilic fluorination of functionalized aryl and heteroaryl magnesium reagents. Angew. Chem. Int. Ed. 2010, 49, 2215–2218. [Google Scholar] [CrossRef]
- Yamada, S.; Knochel, P. Large-Scale Preparation of Aromatic Fluorides via Electrophilic Fluorination with Functionalized Aryl- or Heteroarylmagnesium Reagents. Synthesis 2010, 2010, 2490–2494. [Google Scholar] [CrossRef]
- Dvornikova, E.; Bechcicka, M.; Kamieńska-Trela, K.; Krówczyński, A. Synthesis and NMR studies of 2- and 3-fluorosubstitued five-membered heterocycles. J. Fluorine Chem. 2003, 124, 159–168. [Google Scholar] [CrossRef]
- Monsigny, L.; Doche, F.; Besset, T. Transition-metal-catalyzed C-H bond activation as a sustainable strategy for the synthesis of fluorinated molecules: An overview. Beilstein J. Org. Chem. 2023, 19, 448–473. [Google Scholar] [CrossRef] [PubMed]
- Yin, D.; Su, D.; Jin, J. Photoredox Catalytic Trifluoromethylation and Perfluoroalkylation of Arenes Using Trifluoroacetic and Related Carboxylic Acids. Cell Rep. Phys. Sci. 2020, 1, 100141. [Google Scholar] [CrossRef]
- Song, Y.; Zheng, B.; Yang, S.; Li, Y.; Liu, Q.; Pan, L. Trifluoromethylations of (Hetero)arenes and Polarized Alkenes Using Trifluoroacetic Anhydride under Photoredox Catalysis. Org. Lett. 2023, 25, 2372–2376. [Google Scholar] [CrossRef]
- Zhang, M.; Chen, J.; Huang, S.; Xu, B.; Lin, J.; Su, W. Photocatalytic fluoroalkylations of (hetero)arenes enabled by the acid-triggered reactivity umpolung of acetoxime esters. Chem Catal. 2022, 2, 1793–1806. [Google Scholar] [CrossRef]
- Deolka, S.; Govindarajan, R.; Khaskin, E.; Fayzullin, R.R.; Roy, M.C.; Khusnutdinova, J.R. Photoinduced Trifluoromethylation of Arenes and Heteroarenes Catalyzed by High-Valent Nickel Complexes. Angew. Chem. Int. Ed. 2021, 60, 24620–24629. [Google Scholar] [CrossRef]
- Harris, C.F.; Kuehner, C.S.; Bacsa, J.; Soper, J.D. Photoinduced Cobalt(III)-Trifluoromethyl Bond Activation Enables Arene C-H Trifluoromethylation. Angew. Chem. Int. Ed. 2018, 57, 1311–1315. [Google Scholar] [CrossRef]
- Seo, S.; Taylor, J.B.; Greaney, M.F. Silver-catalysed trifluoromethylation of arenes at room temperature. Chem. Commun. 2013, 49, 6385–6387. [Google Scholar] [CrossRef] [PubMed]
- Chang, B.; Shao, H.; Yan, P.; Qiu, W.; Weng, Z.; Yuan, R. Quinone-Mediated Trifluoromethylation of Arenes and Heteroarenes with Visible Light. ACS Sustain. Chem. Eng. 2016, 5, 334–341. [Google Scholar] [CrossRef]
- Qiu, Y.; Scheremetjew, A.; Finger, L.H.; Ackermann, L. Electrophotocatalytic Undirected C-H Trifluoromethylations of (Het)Arenes. Chemistry 2020, 26, 3241–3246. [Google Scholar] [CrossRef]
- Rodrigo, S.; Um, C.; Mixdorf, J.C.; Gunasekera, D.; Nguyen, H.M.; Luo, L. Alternating Current Electrolysis for Organic Electrosynthesis: Trifluoromethylation of (Hetero)arenes. Org. Lett. 2020, 22, 6719–6723. [Google Scholar] [CrossRef]
- Xiao, F.; Lin, J.-H.; Hao, F.; Zheng, X.; Guo, Y.; Xiao, J.-C. Visible light mediated C–H trifluoromethylation of (hetero)arenes. Org. Chem. Front. 2022, 9, 1982–1985. [Google Scholar] [CrossRef]
- Popkov, S.V.; Kuzenkov, A.V. Synthesis of trifluoromethyl-substituted heteroaromatic aldehydes. Russ. Chem. Bull. 2005, 54, 1672–1674. [Google Scholar] [CrossRef]
- Surapanich, N.; Kuhakarn, C.; Pohmakotr, M.; Reutrakul, V. Palladium-Mediated Heck-Type Reactions of [(Bromodifluoromethyl)sulfonyl]benzene: Synthesis of α-Alkenyl- and α-Heteroaryl-Substituted α,α-Difluoromethyl Phenyl Sulfones. Eur. J. Org. Chem. 2012, 2012, 5943–5952. [Google Scholar] [CrossRef]
- Zhou, C.; Hao, X.; Chen, Z.; Zhang, R.; Zhou, Q.; Fan, Z.; Zheng, M.; Hou, H.; Zhang, S.; Guo, H. Synthesis and Biological Evaluation of beta-Lactam Derivatives Targeting Speckle-Type POZ Protein (SPOP). ACS Med. Chem. Lett. 2024, 15, 270–279. [Google Scholar] [CrossRef]
- Zhang, Z.K.; Xu, W.Y.; Gong, T.J.; Fu, Y. Modular Synthesis of Fluoro-Substituted Furan Compounds via Controllable Fluorination of Biomass-Based 5-HMF and Its Derivatives. ChemSusChem 2024, 17, e202301072. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Ling, L.; Luo, M.; Zeng, X. Accessing Difluoromethylated and Trifluoromethylated cis-Cycloalkanes and Saturated Heterocycles: Preferential Hydrogen Addition to the Substitution Sites for Dearomatization. Angew. Chem. Int. Ed. 2019, 58, 16785–16789. [Google Scholar] [CrossRef]
- He, X.B.; Jia, X.; Zhao, P.Q.; Fang, Z.; Qing, F.L. Photoredox-Catalysis Fluorosulfonyldifluoromethylation of Unactivated Alkenes and (Hetero)arenes with ICF(2)SO(2)F. Org. Lett. 2024, 26, 6900–6904. [Google Scholar] [CrossRef]
- Zhou, K.; Xia, S.; Xiao, Y.; Huang, Z.; Zhang, J.; Zhao, Y. Ligand-assisted manganese-enabled direct C–H difluoromethylation of arenes. Org. Chem. Front. 2024, 11, 4874–4881. [Google Scholar] [CrossRef]
- Shao, C.; Shi, G.; Zhang, Y.; Pan, S.; Guan, X. Palladium-Catalyzed C–H Ethoxycarbonyldifluoromethylation of Electron-Rich Heteroarenes. Org. Lett. 2015, 17, 2652–2655. [Google Scholar] [CrossRef]
- Tanabe, Y.; Matsuo, N.; Ohno, N. Direct perfluoroalkylation including trifluoromethylation of aromatics with perfluoro carboxylic acids mediated by xenon difluoride. J. Org. Chem. 1988, 53, 4582–4585. [Google Scholar] [CrossRef]
- Baguia, H.; Evano, G. Copper-Catalyzed Direct Perfluoroalkylation of Heteroarenes. Chem.-Eur. J. 2022, 28, e202103599. [Google Scholar] [CrossRef]
- Baguia, H.; Beaudelot, J.; Moucheron, C.; Evano, G. Photoinduced, copper-catalysed direct perfluoroalkylation of heteroarenes. Chem. Commun. 2022, 58, 9080–9083. [Google Scholar] [CrossRef] [PubMed]
- Uneyama, K.; Tanaka, H.; Kobayashi, S.; Shioyama, M.; Amii, H. Oxidative cross-coupling of beta,beta-difluoroenol silyl ethers with nucleophiles: A dipole-inversion method to difluoroketones. Org. Lett. 2004, 6, 2733–2736. [Google Scholar] [CrossRef]
- Li, Y.; Deng, Z.; Tan, J.; Liu, Q.; Zhang, J.; Zhao, Y. Visible light-induced monofluoroalkylation of (hetero)arenes with diethyl 2-bromo-2-fluoromalonate. Asian J. Org. Chem. 2023, 12, e202300391. [Google Scholar] [CrossRef]
- Zhao, H.; Leng, X.B.; Zhang, W.; Shen, Q. [Ph4P]+[Cu(CF2H)2]−: A Powerful Difluoromethylating Reagent Inspired by Mechanistic Investigation. Angew. Chem. Int. Ed. 2022, 61, e202210151. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.J.; Lin, J.H.; Xiao, J.C. BrCF(2)CN for photocatalytic cyanodifluoromethylation. Nat. Commun. 2025, 16, 445. [Google Scholar] [CrossRef]
- Douglas, J.J.; Albright, H.; Sevrin, M.J.; Cole, K.P.; Stephenson, C.R. A Visible-Light-Mediated Radical Smiles Rearrangement and its Application to the Synthesis of a Difluoro-Substituted Spirocyclic ORL-1 Antagonist. Angew. Chem. Int. Ed. 2015, 54, 14898–14902. [Google Scholar] [CrossRef]
- Yuan, K.; Feoktistova, T.; Cheong, P.H.; Altman, R.A. Arylation of gem-difluoroalkenes using a Pd/Cu Co-catalytic system that avoids beta-fluoride elimination. Chem. Sci. 2020, 12, 1363–1367. [Google Scholar] [CrossRef]
- Belhomme, M.C.; Poisson, T.; Pannecoucke, X. Copper-catalyzed direct C-2 difluoromethylation of furans and benzofurans: Access to C-2 CF2H derivatives. J. Org. Chem. 2014, 79, 7205–7211. [Google Scholar] [CrossRef]
- Lin, D.; Krishnamurti, V.; Prakash, G.K.S. Visible Light-Mediated Metal-Free Chlorodifluoromethylation of Arenes and Heteroarenes by a Hypervalent Iodine EDA Complex. Eur. J. Org. Chem. 2022, 2022, e202200607. [Google Scholar] [CrossRef]
- Xie, Q.; Zhu, Z.; Li, L.; Ni, C.; Hu, J. Controllable double CF(2)-insertion into sp(2) C-Cu bond using TMSCF(3): A facile access to tetrafluoroethylene-bridged structures. Chem. Sci. 2019, 11, 276–280. [Google Scholar] [CrossRef] [PubMed]
- Pan, S.; Xie, Q.; Wang, X.; Huang, R.; Lu, Y.; Ni, C.; Hu, J. Controllable Double Difluoromethylene Insertions into S-Cu Bonds: (Arylthio)tetrafluoroethylation of Aryl Iodides with TMSCF(2)Br. Angew. Chem. Int. Ed. 2024, 63, e202400839. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhao, S.; Liu, J.; Zhu, D.; Guo, M.; Tang, X.; Wang, G. Copper-Catalyzed C-H Difluoroalkylations and Perfluoroalkylations of Alkenes and (Hetero)arenes. Org. Lett. 2017, 19, 4187–4190. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, F.; Lin, Z.; Cheng, C.; Zhang, Q.; Li, J. Visible-Light-Induced para-Selective C(sp(2))-H Difluoroalkylation of Diverse (Hetero)aromatic Carbonyls. Org. Lett. 2020, 22, 68–72. [Google Scholar] [CrossRef]
- Cheng, Y.; He, Y.; Zheng, J.; Yang, H.; Liu, J.; An, G.; Li, G. Ruthenium(II)-catalyzed para-selective C H difluoroalkylation of aromatic aldehydes and ketones using transient directing groups. Chin. Chem. Lett. 2021, 32, 1437–1441. [Google Scholar] [CrossRef]
- Xu, L.; Vicic, D.A. Direct Difluoromethylation of Aryl Halides via Base Metal Catalysis at Room Temperature. J. Am. Chem. Soc. 2016, 138, 2536–2539. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Gao, B.; Ni, C.; Hu, J. Copper-mediated fluoroalkylation of aryl iodides enables facile access to diverse fluorinated compounds: The important role of the (2-pyridyl)sulfonyl group. Org. Lett. 2012, 14, 6080–6083. [Google Scholar] [CrossRef]
- Ke, M.; Song, Q. Copper-Catalyzed C(sp(2))-H Difluoroalkylation of Aldehyde Derived Hydrazones with Diboron as Reductant. J. Org. Chem. 2016, 81, 3654–3664. [Google Scholar] [CrossRef]
- Feng, Z.; Riemann, L.; Guo, Z.; Herrero, D.; Simon, M.; Golz, C.; Mata, R.A.; Alcarazo, M. Pentafluorocyclopropanation of (Hetero)arenes Using Sulfonium Salts: Applications in Late-Stage Functionalization. Angew. Chem. Int. Ed. 2023, 62, e202306764. [Google Scholar] [CrossRef]
- Deolka, S.; Govindarajan, R.; Vasylevskyi, S.; Roy, M.C.; Khusnutdinova, J.R.; Khaskin, E. Ligand-free nickel catalyzed perfluoroalkylation of arenes and heteroarenes. Chem. Sci. 2022, 13, 12971–12979. [Google Scholar] [CrossRef] [PubMed]
- Jud, W.; Maljuric, S.; Kappe, C.O.; Cantillo, D. Cathodic C-H Trifluoromethylation of Arenes and Heteroarenes Enabled by an in Situ-Generated Triflyltriethylammonium Complex. Org. Lett. 2019, 21, 7970–7975. [Google Scholar] [CrossRef]
- Fernandez-Garcia, S.; Chantzakou, V.O.; Julia-Hernandez, F. Direct Decarboxylation of Trifluoroacetates Enabled by Iron Photocatalysis. Angew. Chem. Int. Ed. 2024, 63, e202311984. [Google Scholar] [CrossRef]
- Pang, Y.; Lee, J.W.; Kubota, K.; Ito, H. Solid-State Radical C-H Trifluoromethylation Reactions Using Ball Milling and Piezoelectric Materials. Angew. Chem., Int. Ed. 2020, 59, 22570–22576. [Google Scholar] [CrossRef] [PubMed]
- Jacquet, J.; Blanchard, S.; Derat, E.; Desage-El Murr, M.; Fensterbank, L. Redox-ligand sustains controlled generation of CF(3) radicals by well-defined copper complex. Chem. Sci. 2016, 7, 2030–2036. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.; Kim, E.; You, Y.; Cho, E.J. Visible Light-Induced Aromatic Difluoroalkylation. Adv. Synth. Catal. 2014, 356, 2741–2748. [Google Scholar] [CrossRef]
- Liu, Z.-E.; Lin, T.; Zhao, Y.; Cao, J. Pd-catalyzed C H (phenylsulfonyl)difluoromethylation of electron-rich heteroarenes. Tetrahedron Lett. 2025, 158, 155486. [Google Scholar] [CrossRef]
- Su, Y.M.; Hou, Y.; Yin, F.; Xu, Y.M.; Li, Y.; Zheng, X.; Wang, X.S. Visible light-mediated C-H difluoromethylation of electron-rich heteroarenes. Org. Lett. 2014, 16, 2958–2961. [Google Scholar] [CrossRef]
- Mao, Y.; Liu, Y.; Wang, X.; Ni, S.; Pan, Y.; Wang, Y. Acylfluorination of enynes via phosphine and silver catalysis. Chin. Chem. Lett. 2024, 35, 109443. [Google Scholar] [CrossRef]
- Lozovskiy, S.V.; Vasilyev, A.V. Catalyst-Free Preparation of Perfluoroalkyl-Phosphoryl Substituted Furans from 1-Perfluoroalkyl 1,3-Diketones in Two Steps. Adv. Synth. Catal. 2020, 362, 3121–3125. [Google Scholar] [CrossRef]
- Gao, S.J.; Huang, X.Y.; Li, X.Y.; Ge, D.; Ma, M.; Shen, Z.L.; Chu, X.Q. Three-Component Sulfonylation and Heteroannulation Enabled by 3-Fold Defluorofunctionalization of Trifluoromethyl Enones. J. Org. Chem. 2025, 90, 3373–3383. [Google Scholar] [CrossRef]
- Huang, X.Y.; Gao, S.J.; Ge, D.; Ma, M.; Shen, Z.L.; Chu, X.Q. Modular Synthesis of Furans with Four Nonidentical Substituents by Aqueous Defluorinative Reaction of Trifluoromethyl Enones with Two Nucleophiles. Org. Lett. 2025, 27, 462–469. [Google Scholar] [CrossRef] [PubMed]
- Nikolaev, V.A.; Supurgibekov, M.B.; Davies, H.M.; Sieler, J.; Zakharova, V.M. Influence of an internal trifluoromethyl group on the rhodium(II)-catalyzed reactions of vinyldiazocarbonyl compounds. J. Org. Chem. 2013, 78, 4239–4244. [Google Scholar] [CrossRef] [PubMed]
- Chambers, R.D.; Roche, A.J.; Rock, M.H. Polyhalogenated heterocyclic compounds. Part 41. Cycloaddition reactions involving hexafluorobut-2-yne and 1,1,1,2,4,4,4-heptafluorobut-2-ene. J. Chem. Soc. Perkin Trans. 1 1996, 11, 1095–1100. [Google Scholar] [CrossRef]
- Freund, P.; Pauls, M.; Babushkina, D.; Pickl, T.; Bannwarth, C.; Bach, T. Photochemical Deracemization of 4,7-Diaza-1-isoindolinones by Unidirectional Hydrogen Atom Shuttling. J. Am. Chem. Soc. 2025, 147, 1434–1439. [Google Scholar] [CrossRef]
- Zhang, H.; Lu, C.J.; Cai, G.H.; Xi, L.L.; Feng, J.; Liu, R.R. Palladium-catalyzed asymmetric carbene coupling en route to inherently chiral heptagon-containing polyarenes. Nat. Commun. 2024, 15, 3353. [Google Scholar] [CrossRef] [PubMed]
- Aoyama, H.; Davies, C.; Liu, J.; Pahl, A.; Kirchhoff, J.L.; Scheel, R.; Sievers, S.; Strohmann, C.; Grigalunas, M.; Waldmann, H. Collective Synthesis of Sarpagine and Macroline Alkaloid-Inspired Compounds. Chem.-Eur. J. 2024, 30, e202303027. [Google Scholar] [CrossRef]
- Liang, S.; Ma, L.; Guo, Z.; Liu, F.; Lin, Z.; Yi, W. Synthesis of Unsymmetrical Trisulfides from S-Substituted Sulphenylthiosulphates. Angew. Chem. Int. Ed. 2024, 63, e202404139. [Google Scholar] [CrossRef]
- Rajasekhar, M.; Prasad Reddy, T.S.V.; Dadhich, A.S.; Ram, B.; Balram, B. Synthesis, characterisation and antibacterial evaluation of chalcone derivatives linked with 2-trifluoromethyl furan. Der Pharma Chem. 2015, 7, 177–183. [Google Scholar]
- Lu, B.W.; Zhang, S.; He, C.; Feng, T.; Wei, K. 2-Aminopyrimidine Derivative, Preparation Method and Medical Application. Patent CN110684020 A, 14 January 2020. [Google Scholar]
- Szlavik, Z.; Csekei, M.; Paczal, A.; Szabo, Z.B.; Sipos, S.; Radics, G.; Proszenyak, A.; Balint, B.; Murray, J.; Davidson, J.; et al. Discovery of S64315, a Potent and Selective Mcl-1 Inhibitor. J. Med. Chem. 2020, 63, 13762–13795. [Google Scholar] [CrossRef]
- Trzoss, M.C.J.; Shaw, K.J.; Webb, P. Heterocycle Substituted Pyridine Derivative Antifungal Agents. Patent WO2019113542, 13 June 2019. [Google Scholar]
- Otake, K.; Azukizawa, S.; Takeda, S.; Fukui, M.; Kawahara, A.; Kitao, T.; Shirahase, H. Novel 2,7-Substituted (S)-1,2,3,4-Tetrahydroisoquinoline-3-carboxylic Acids: Peroxisome Proliferator-Activated Receptor gamma Partial Agonists with Protein-Tyrosine Phosphatase 1B Inhibition. Chem. Pharm. Bull. 2015, 63, 998–1014. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mazurov, A.A.; Miao, L.; Bhatti, B.S.; Strachan, J.P.; Akireddy, S.; Murthy, S.; Kombo, D.; Xiao, Y.D.; Hammond, P.; Zhang, J.; et al. Discovery of 3-(5-chloro-2-furoyl)-3,7-diazabicyclo[3.3.0]octane (TC-6683, AZD1446), a novel highly selective alpha4beta2 nicotinic acetylcholine receptor agonist for the treatment of cognitive disorders. J. Med. Chem. 2012, 55, 9181–9194. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Xu, Y.; Zhao, Y.; Zhang, S.; Li, M.; Li, X.; He, J.; Zhou, H.; Ge, Z.; Li, R.; et al. N-(4-acetamidophenyl)-5-acetylfuran-2-carboxamide as a novel orally available diuretic that targets urea transporters with improved PD and PK properties. Eur. J. Med. Chem. 2021, 226, 113859. [Google Scholar] [CrossRef]
- Sun, Y.; Kim, S.; Shin, S.; Takemura, K.; Matos, G.S.; Lazzarini, C.; Haranahalli, K.; Zambito, J.; Garg, A.; Del Poeta, M.; et al. SAR study of N′-(Salicylidene)heteroarenecarbohydrazides as promising antifungal agents. Bioorg. Med. Chem. 2024, 100, 117610. [Google Scholar] [CrossRef] [PubMed]
- Tremblay, M.; Bonneau, P.; Bousquet, Y.; DeRoy, P.; Duan, J.; Duplessis, M.; Gagnon, A.; Garneau, M.; Goudreau, N.; Guse, I.; et al. Inhibition of HIV-1 capsid assembly: Optimization of the antiviral potency by site selective modifications at N1, C2 and C16 of a 5-(5-furan-2-yl-pyrazol-1-yl)-1H-benzimidazole scaffold. Bioorg. Med. Chem. Lett. 2012, 22, 7512–7517. [Google Scholar] [CrossRef]
- Pieniazek, S.N.; Houk, K.N. The origin of the halogen effect on reactivity and reversibility of Diels-Alder cycloadditions involving furan. Angew. Chem. Int. Ed. 2006, 45, 1442–1445. [Google Scholar] [CrossRef]
- De Pascalis, L.; Yau, M.K.; Svatunek, D.; Tan, Z.; Tekkam, S.; Houk, K.N.; Finn, M.G. The Influence of Substitution on Thiol-Induced Oxanorbornadiene Fragmentation. Org. Lett. 2021, 23, 3751–3754. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).