Harvesting Friction Energy on Zinc Oxide and Zinc Oxide/Europium Oxide Sol-Gel Catalysts for Tribocatalytic Paracetamol Degradation
Abstract
1. Introduction
2. Results and Discussion
2.1. Structure Analysis
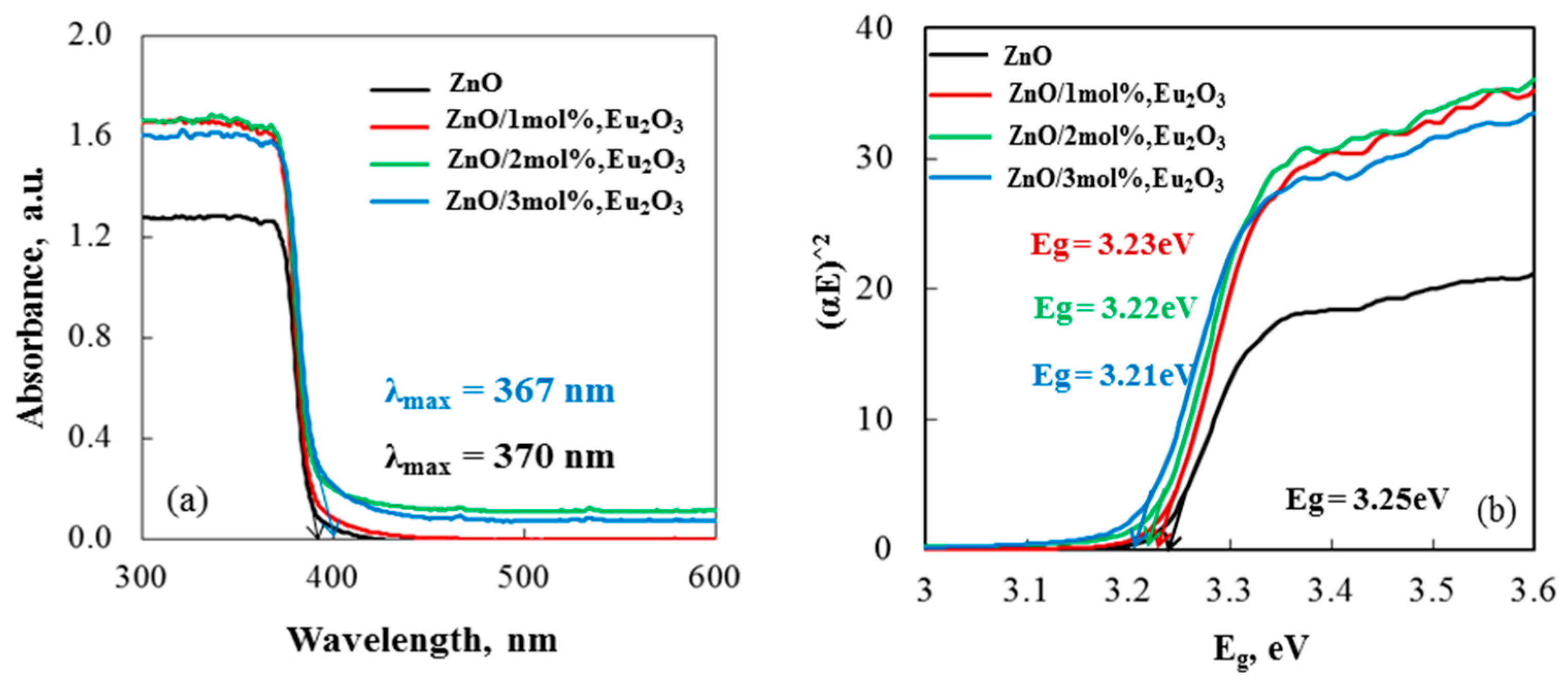
2.2. Optical Analysis
2.3. Tribocatalytic Decomposition of Paracetamol
3. Materials and Methods
3.1. Chemicals
3.2. Synthesis of Sol-Gel ZnO and ZnO/Eu2O3 Particles
3.3. Methods
3.4. Tribocatalysis for Degradation of Paracetamol
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ashraf, M.; Ayaz, M.; Khan, M.; Adil, S.; Faroog, W.; Ullah, N.; Tahir, M. Recent Trends in Sustainable Solar Energy Conversion Technologies: Mechanisms, Prospects, and Challenges. Energy Fuels 2023, 37, 6283–6301. [Google Scholar] [CrossRef]
- Imran, S.; Hussain, M. Emerging trends in water splitting innovations for solar hydrogen production: Analysis, comparison, and economical insights. Int. J. Hydrogen Energy 2024, 77, 975–996. [Google Scholar] [CrossRef]
- Wu, S.; Hu, H.; Lin, Y.; Zhang, J.; Hu, Y. Visible light photocatalytic degradation of tetracycline over TiO2. Chem. Eng. J. 2020, 382, 122842. [Google Scholar] [CrossRef]
- Domínguez, C.; García, J.; Pedraz, M.; Torres, A.; Galán, M. Photocatalytic oxidation of organic pollutants in water. Catal. Today 1998, 40, 85–101. [Google Scholar] [CrossRef]
- Pattnaik, A.; Sahu, J.; Poonia, A.; Ghosh, P. Current perspective of nano-engineered metal oxide based photocatalysts in advanced oxidation processes for degradation of organic pollutants in wastewater. Chem. Eng. Res. Des. 2023, 190, 667–686. [Google Scholar] [CrossRef]
- Acher, A. Sunlight Photooxidation of Organic Pollutants in Wastewater. Water Sci. Technol. 1985, 17, 623–632. [Google Scholar] [CrossRef]
- Yaqoob, A.; Noor, N.; Serrà, A.; Ibrahim, M. Advances and Challenges in Developing Efficient Graphene Oxide-Based ZnO Photocatalysts for Dye Photo-Oxidation. Nanomaterials 2020, 10, 932. [Google Scholar] [CrossRef]
- Antuch, M.; Rouby, W.; Millet, P. A comparison of water photo-oxidation and photo-reduction using photoelectrodes surface-modified by deposition of co-catalysts: Insights from photo-electrochemical impedance spectroscopy. Int. J. Hydrogen Energy 2019, 44, 9970–9977. [Google Scholar] [CrossRef]
- Ojha, N.; Pant, K.; Coy, E. Photocatalytic Conversion of Carbon Dioxide and Nitrogen Dioxide: Current Developments, Challenges, and Perspectives. Ind. Eng. Chem. Res. 2023, 62, 21885–21908. [Google Scholar] [CrossRef]
- Lu, Z.; Wang, Z. Complete Photooxidation of Formaldehyde to CO2 via Ni-Dual-Atom Decorated Crystalline Triazine Frameworks: A DFT Study. Toxics 2024, 12, 242. [Google Scholar] [CrossRef]
- Saravanan, A.; Kumar, P.; Jeevanantham, S.; Karishma, S.; Kiruthika, A. Photocatalytic disinfection of micro-organisms: Mechanisms and applications. Environ. Technol. Innov. 2021, 24, 101909. [Google Scholar] [CrossRef]
- Feng, M.; Lv, S.; Deng, J.; Guo, Y.; Wu, Y.; Shi, G.; Zhang, M. An overview of environmental energy harvesting by thermoelectric generators. Renew. Sustain. Energy Rev. 2023, 187, 113723. [Google Scholar] [CrossRef]
- Myers, A.; Hodges, R.; Jur, J. Human and environmental analysis of wearable thermal energy harvesting. Energy Convers. Manage. 2017, 143, 218–226. [Google Scholar] [CrossRef]
- Oturan, M.A.; Aaron, J.J. Advanced oxidation processes in water/wastewater treatment: Principles and applications. A review. Crit. Rev. Environ. Sci. Technol. 2014, 44, 2577–2641. [Google Scholar] [CrossRef]
- Niu, S.; Wang, Z.L. Theoretical systems of triboelectric nanogenerators. Nano Energy 2015, 14, 161–192. [Google Scholar] [CrossRef]
- Liu, C.; Wang, Y.; Zhang, N.; Yang, X.; Wang, Z.; Zhao, L.; Yang, W.; Dong, L.; Che, L.; Wang, G.; et al. A self-powered and high sensitivity acceleration sensor with V-Q-a model based on triboelectric nanogenerators. Nano Energy 2020, 67, 104228. [Google Scholar] [CrossRef]
- Li, X.; Tong, W.; Shi, J.; Chen, Y.; Zhang, Y.; An, Q. Tribocatalysis mechanisms: Electron transfer and transition. J. Mater. Chem. A 2023, 11, 4458–4472. [Google Scholar] [CrossRef]
- Xiao, X.; Zhang, X.; Wang, S.; Ouyang, H.; Chen, P.; Song, L.; Yuan, H.; Ji, Y.; Wang, P.; Li, Z.; et al. Honeycomb structure inspired triboelectric nanogenerator for highly effective vibration energy harvesting and selfpowered engine condition monitoring. Adv. Energy Mater. 2019, 9, 1902460. [Google Scholar] [CrossRef]
- Zhao, P.; Soin, N.; Prashanthi, K.; Chen, J.; Dong, S.; Zhou, E.; Zhu, Z.; Narasimulu, A.; Montemagno, C.; Yu, L.; et al. Emulsion electrospinning of polytetrafluoroethylene (PTFE) nanofibrous membranes for high-performance triboelectric nanogenerators. ACS Appl. Mater. Inter. 2018, 10, 5880–5891. [Google Scholar] [CrossRef]
- Sharma, D.; Shukla, S.; Sharma, K.; Kumar, V. A review on ZnO: Fundamental properties and applications. Mater. Today 2022, 49, 3028–3035. [Google Scholar] [CrossRef]
- Chong, J.; Tai, B.; Zhang, Y. Tribocatalysis effect based on ZnO with various specific surface areas for dye degradation. Chem. Phys. Lett. 2024, 835, 140998. [Google Scholar] [CrossRef]
- Lei, H.; Cui, X.; Jia, X.; Qi, J.; Wang, J.; Chen, W. Enhanced Tribocatalytic Degradation of Organic Pollutants by ZnO Nanoparticles of High Crystallinity. Nanomaterials 2023, 13, 46. [Google Scholar] [CrossRef]
- Zhao, J.; Chen, L.; Luo, W.; Li, H.; Wu, Z.; Xu, Z.; Zhang, Y.; Zhang, H.; Yuan, G.; Gao, J.; et al. Strong tribo-catalysis of zinc oxide nanorods via triboelectrically-harvesting friction energy. Ceram. Int. 2020, 46, 25293–25298. [Google Scholar] [CrossRef]
- Kumbhakar, P.; Mishra, S.; Kumbhakar, P.; Barik, R.; Tiwary, C.; Singh, A. Strain-Induced Tribocatalytic Activity of 2D ZnO Quantum Dots. J. Phys. Chem. C 2024, 128, 10733–10741. [Google Scholar] [CrossRef]
- Xiao, L.; Xu, X.; Wu, Z.; Sun, T.; He, X.; Xu, X.; Qin, L.; Chen, D. Recent progress and prospect of friction-driven-tribocatalysis: From basic principle to material design. Surf. Interfaces 2025, 56, 105557. [Google Scholar] [CrossRef]
- Mohamed, W.; Abu-Dief, A. Synthesis, characterization and photocatalysis enhancement of Eu2O3-ZnO mixed oxide nanoparticles. J. Phys. Chem. Solids 2018, 116, 375–385. [Google Scholar] [CrossRef]
- Dong, X.U.; Jiang, B.; Jiao, L.; Cui, F.-D.; Xu, H.-X.; Yang, Y.-T.; Yu, R.-H.; Cheng, X.-N. Sol–gel synthesis of Y2O3-doped ZnO thin films varistors and their electrical properties. Trans. Nonferrous Met. Soc. China 2012, 22 (Suppl. S1), s110–s114. [Google Scholar]
- Chao, L.C.; Huang, J.W.; Chang, C.W. Annealing effects on the properties of Nd containing ZnO nanoparticles prepared by sol-gel process. Physica B 2009, 404, 1301. [Google Scholar] [CrossRef]
- Wang, R.H.; Xin, J.H.Z.; Yang, Y.; Liu, H.F.; Xu, L.M.; Hu, J.H. The characteristics and photocatalytic activities of silver doped ZnO nanocrystallites. Appl. Surf. Sci. 2004, 227, 312. [Google Scholar] [CrossRef]
- Aydın, C.; El-Sadek, M.S.A.; Zheng, K.; Yahia, I.S.; Yakuphanoglu, F. Synthesis, diffused reflectance and electrical properties of nanocrystalline Fe-doped ZnO via sol-gel calcination technique. Opt. Laser Technol. 2013, 48, 447–452. [Google Scholar] [CrossRef]
- Yang, L.; Wang, Z.; Zhang, Z.; Sun, Y.; Gao, M.; Yang, J.; Yan, Y. Surface effects on the optical and photocatalytic properties of graphene-like ZnO:Eu3+ nanosheets. J. Appl. Phys. 2013, 113, 33514. [Google Scholar] [CrossRef]
- Najafi, M.; Haratizadeh, H. Synthesize and optical properties of ZnO: Eu microspheres based nano-sheets at direct and indirect excitation. Int. J. Nanosci. Nanotechnol. 2015, 11, 101–113. [Google Scholar]
- Gao, M.; Yan, C.; Li, B.; Zhou, L.; Yao, J.; Zhang, Y.; Liu, H.; Cao, L.; Cao, Y.; Yang, J.; et al. Strong red emission and catalytic properties of ZnO by adding Eu2O3 shell. J. Alloys Compd. 2017, 724, 537–542. [Google Scholar] [CrossRef]
- Ivanova, D.; Kolev, H.; Stefanov, B.I.; Kaneva, N. Enhanced Tribodegradation of a Tetracycline Antibiotic by Rare-Earth-Modified Zinc Oxide. Molecules 2024, 29, 3913. [Google Scholar] [CrossRef] [PubMed]
- Brunckova, H.; Rocha, L.A.; Nassar, E.J.; Moscardini, S.B.; Kolev, H. Luminescence properties of neodymium, samarium, and europium niobate and tantalate thin films. Luminescence 2022, 37, 642. [Google Scholar] [CrossRef]
- Singh, V.; Sivaramaiah, G.; Rao, J.L.; Dhoble, S.J.; Kim, S.H. Mn2+, Eu2+ and Eu3+ emission in co-doped LaAl11O18 phosphors. Mater. Chem. Phys. 2015, 149–150, 202–208. [Google Scholar] [CrossRef]
- Dehelean, A.; Rada, S.; Popa, A.; Suciu, R.C.; Culea, E. Raman, photoluminescence and EPR spectroscopic characterization of europium(III) oxide-lead dioxide-tellurite glassy network. J. lumin. 2016, 177, 65–70. [Google Scholar] [CrossRef]
- Somasundarama, K.; Girija, K.G.; Christopher, P.S.; Sudarsan, V.; Kadam, R.M.; Vatsa, R.K. Blue electroluminescence from ZnGa2O4:Eu powder samples. J. Lumin. 2017, 185, 145–150. [Google Scholar] [CrossRef]
- Petrosyan, A.G.; Asatryan, H.R.; Hovhannesyan, K.L.; Derdzyan, M.V.; Feofilov, S.P.; Eganyan, A.V.; Sargsyan, R.S. Growth, optical and EPR studies of 151 Eu2+:YAG single crystals. Mater. Chem. Phys. 2017, 185, 39–43. [Google Scholar] [CrossRef]
- Reddy, J.A.; Kokila, M.K.; Nagabhushana, H.; Shivakumara, C.; Chakradhar, R.P.S.; Nagabhushana, B.M.; Krishna, H.R. Luminescence studies and EPR investigation of solution combustion derived Eu doped ZnO. Spectrochim Acta Part A 2014, 132, 305–312. [Google Scholar] [CrossRef]
- Brückner, A.; Bentrup, U.; Zanthoff, H.; Maschmeyer, D. The role of different Ni sites in supported nickel catalysts for butene dimerization under industry-like conditions. J. Catal. 2009, 266, 120–128. [Google Scholar] [CrossRef]
- Micic, O.I.; Zhang, Y.; Cromack, K.R.; Trifunac, A.D.; Thurmauer, M.C. Trapped holes on TiO2 colloids studied by Electron Paramagnetic Resonance. J. Phys. Chem. 1993, 97, 7277–7283. [Google Scholar] [CrossRef]
- Li, W.; Zhang, H.; Zhang, X.; Qin, G.; Li, H.; Xiong, Y.; Ye, L.; Ruan, H.; Tong, C.; Kong, C.; et al. Non-axial NO-VZn shallow acceptor complexes in nitrogen implanted p-type ZnO thin films. Appl. Surf. Sci. 2020, 529, 147168. [Google Scholar] [CrossRef]
- Ammar, A.; Yildirim, I.; Aleinawi, M.; BulduAkturk, M.; Turhan, N.; Nadupalli, S.; Rostas, A.; Erdem, E. Multifrequency EPR spectroscopy study of Mn, Fe, and Cu doped nanocrystalline ZnO. Mat. Res. Bull. 2023, 160, 112117. [Google Scholar] [CrossRef]
- Krasilinikov, V.; Dyachkova, T.; Tyutyunnik, A.; Gyrdasova, O.; Melkozerova, M.; Baklanova, I.; Perevozchikova, Y.A.; Emelyanova, S.M.; Weber, H.; Marchenkov, V. Magnetic and optical properties as well as EPR studies of polycrystalline ZnO synthesized from different precursors. Mat. Res. Bull. 2018, 97, 553–559. [Google Scholar] [CrossRef]
- Decremps, F.; Pellicer-Porres, J.; Saitta, A.; Chervin, J.; Polian, A. High-pressure Raman sprectroscopy study of wurtzite ZnO. Phys. Rev. B 2002, 65, 092101. [Google Scholar] [CrossRef]
- Kumar, S.; Kumar, T.; Kaur, H.; Kumar, A.; Kumar, A. Optimizing photocatalysis: Tuning europium concentration in zinc oxide nanoparticles for superior performance. Phys. B Condens. Matters 2025, 697, 416699. [Google Scholar]
- AlAbdulaal, T.; Ganesh, V.; AlShadidi, M.; Hussien, M.; Bouzidi, A.; Algarni, H.; Zahran, H.; Abdel-wahab, M.; Yahia, I.; Nasr, S. The Auto-Combustion Method Synthesized Eu2O3- ZnO Nanostructured Composites for Electronic and Photocatalytic Applications. Materials 2022, 15, 3257. [Google Scholar] [CrossRef]
- Kumar, M.; Chauhan, M.; Akhtar, M.; Umar, A. Effect of cerium ions in Ce-Doped ZnO nanostructures on their photocatalytic and picric acid chemical sensing. Ceram. Int. 2021, 47, 3089–3098. [Google Scholar] [CrossRef]
- Chandrasekhar, M.; Nagabhushana, H.; Sharma, S.; Sudheer kumar, K.; Dhananjaya, N.; Sunitha, D.; Shivakumara, C.; Nagabhushana, B. Particle size, morphology and color tunable ZnO:Eu3+ nanophosphors via plant latex mediated green combustion synthesis. J. Alloys Compd. 2014, 584, 417–424. [Google Scholar] [CrossRef]
- Ntwaeaborwa, O.; Mofokeng, S.; Kumar, V.; Kroon, R. Structural, optical and photoluminescence properties of Eu3+ doped ZnO nanoparticles. Spectrochim. Acta A 2017, 182, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Röder, R.; Geburt, S.; Zapf, M.; Franke, D.; Lorke, M.; Frauenheim, T.; Luisa da Rosa, A.; Ronning, C. Transition metal and rare earth element doped zinc oxide nanowires for optoelectronics. Phys. Status Solidi B 2019, 256, 1800604. [Google Scholar] [CrossRef]
- Kumar, V.; Som, S.; Kumar, V.; Kumar, V.; Ntwaeaborwa, O.; Coetsee, E.; Swart, H. Tunable and white emission from ZnO:Tb3+ nanophosphors for solid state lighting applications. Chem. Eng. J. 2014, 255, 541–552. [Google Scholar] [CrossRef]
- Marin, R.; Oussta, F.; Katea, S.; Prabhudev, S.; Botton, G.; Westin, G.; Hemmer, E. Europium-doped ZnO nanosponges—Controlling optical properties and photocatalytic activity. J. Mater. Chem. C 2019, 7, 3909–3919. [Google Scholar] [CrossRef]
- Wu, M.; Xu, Y.; He, Q.; Sun, P.; Weng, X.; Dong, X. Tribocatalysis of homogeneous material with multi-size granular distribution for degradation of organic pollutants. J. Colloid Interface Sci. 2022, 622, 602–611. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Yin, R.; Zhang, Y.; Zhou, B.; Sun, P.; Dong, X. Unveiling the Mechanism of Frictional Catalysis in Water by Bi12TiO20: A Charge Transfer and Contaminant Decomposition Path Study. Langmuir 2022, 38, 14153–14161. [Google Scholar] [CrossRef]
- Dash, D.; Panda, N.; Sahu, D. Photoluminescence and photocatalytic properties of europium doped ZnO nanoparticles. Appl. Surf. Sci. 2019, 494, 666–674. [Google Scholar] [CrossRef]
- Kumar, M.; Singh, G.; Chauhan, M. Europium (Eu3+)—Doped ZnO nanostructures: Synthesis, characterization, and photocatalytic, chemical sensing and preliminary assessment of magnetic properties. Ceram. Int. 2021, 47, 17023–17033. [Google Scholar] [CrossRef]
- Kraus, W.; Nolze, G. POWDER CELL—A program for the representation and manipulation of crystal structures and calculation of the resulting X-ray powder patterns. J. Appl. Crystallogr. 1996, 29, 301–303. [Google Scholar] [CrossRef]
- Dollase, W.A. Correction of intensities for preferred orientation in powder diffractometry: Application of the March model. J. Appl. Crystallogr. 1986, 19, 267–272. [Google Scholar] [CrossRef]
- Shirley, D. High-Resolution X-Ray Photoemission Spectrum of the Valence Bands of Gold. Phys. Rev. B 1972, 5, 4709–4714. [Google Scholar] [CrossRef]
- Scofield, J. Hartree-Slater subshell photoionization cross-sections at 1254 and 1487 eV. J. Electron Spectrosc. Relat. Phenom. 1976, 8, 129. [Google Scholar] [CrossRef]
- Moulder, F.; Sticke, W.F.; Sobol, P.E.; Bombel, K.D. Handbook of X-Ray Photoelectron Spectroscopy, 2nd ed.; Castain, J., Ed.; Physical Electron Division; Perkin-Elmer Corporation: Eden Prairie, MN, USA, 1992. [Google Scholar]
- Jia, X.; Wanga, H.; Lei, H.; Mao, C.; Cui, X.; Liu, Y.; Jia, Y.; Yao, W.; Chen, W. Boosting tribocatalytic conversion of H2O and CO2 by Co3O4 nanoparticles through metallic coatings in reactors. J. Adv. Ceram. 2023, 12, 1833–1843. [Google Scholar] [CrossRef]









| Tribocatalysts | ZnO Phase | Eu2O3 Phase | ||
|---|---|---|---|---|
| Crystal. Size Parameters | Vol. Mi- % Crostrain | Crystal. Size Parameters | Vol. At. % % | Mi- Crostrain |
| ZnO: 42.4 a, b: 3.2407 c: 5.2017 | 100 1.8 × 10−3 | ― | ― | ― |
| ZnO/1 mol% Eu2O3 39.0 a, b: 3.2484 c: 5.2043 ZnO/2 mol% Eu2O3 36.0 a, b: 3.2475 c: 5.2032 ZnO/3 mol% Eu2O3 30.2 a, b: 3.2485 c: 5.2039 | 97.8 3.3 × 10−4 94.8 2.1 × 10−4 93.7 9.2 × 10−4 | 33.4 a, b, c: 10.8485 34.1 a, b, c: 10.8592 26.8 a, b, c: 10.8587 | 2.2 0.68 5.2 1.65 6.3 2.01 | 1.5 × 10−3 9.9 × 10−4 2.5 × 10−1 |
| Before O 1s Tribocatalysts | Zn2p | Eu3d5/2 | ||
|---|---|---|---|---|
| BE, eV | Conc., BE, at% eV | Conc., at% | BE, eV | Conc., at% |
| ZnO 530.6 531.6 532.5 | 27.67 1021.7 15.78 6.76 | 49.80 | ― | 0.00 |
| ZnO/1 mol% Eu2O3 530.7 531.6 532.5 ZnO/2 mol% Eu2O3 530.7 531.7 532.7 ZnO/3 mol% Eu2O3 530.7 531.5 532.4 | 29.40 1021.7 14.27 6.21 28.88 1021.7 14.75 5.79 28.32 1021.7 15.30 8.32 | 50.16 50.35 42.73 | ~1135.0 ~1135.9 ~1136.0 | 0.17 0.23 0.32 |
| After O 1s Tribocatalysts | Zn2p | Eu3d5/2 | ||
|---|---|---|---|---|
| BE, eV | Conc., BE, at% eV | Conc., at% | BE, eV | Conc., at% |
| ZnO 530.7 531.6 533.0 | 24.46 1021.7 19.19 7.28 | 49.06 | ― | 0.00 |
| ZnO/1 mol% Eu2O3 530.7 531.7 532.7 ZnO/2 mol% Eu2O3 530.7 531.7 532.9 ZnO/3 mol% Eu2O3 530.7 531.6 532.9 | 28.70 1021.7 16.12 7.07 31.76 1021.7 13.59 8.30 28.05 1021.7 19.00 6.70 | 47.89 46.08 46.09 | ~1135.6 ~1135.4 ~1134.5 | 0.21 0.27 0.16 |
| Tribocatalysts 1 Rod | 2 Rods | 3 Rods | ||
|---|---|---|---|---|
| k, h−1 | D, % k, h−1 | D, % | k, h−1 | D, % |
| ZnO 0.0383 | 70.45 0.0564 | 74.07 | 0.0671 | 74.07 |
| ZnO/1 mol%, Eu2O3 0.0492 ZnO/2 mol%, Eu2O3 0.0559 ZnO/3 mol%, Eu2O3 0.0698 | 75.62 0.0647 81.20 0.0795 85.12 0.0939 | 78.20 85.02 89.67 | 0.0778 0.0969 0.1054 | 81.71 89.15 92.15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ivanova, D.; Kolev, H.; Mladenova, R.; Stefanov, B.I.; Kaneva, N. Harvesting Friction Energy on Zinc Oxide and Zinc Oxide/Europium Oxide Sol-Gel Catalysts for Tribocatalytic Paracetamol Degradation. Molecules 2025, 30, 2265. https://doi.org/10.3390/molecules30112265
Ivanova D, Kolev H, Mladenova R, Stefanov BI, Kaneva N. Harvesting Friction Energy on Zinc Oxide and Zinc Oxide/Europium Oxide Sol-Gel Catalysts for Tribocatalytic Paracetamol Degradation. Molecules. 2025; 30(11):2265. https://doi.org/10.3390/molecules30112265
Chicago/Turabian StyleIvanova, Dobrina, Hristo Kolev, Ralitsa Mladenova, Bozhidar I. Stefanov, and Nina Kaneva. 2025. "Harvesting Friction Energy on Zinc Oxide and Zinc Oxide/Europium Oxide Sol-Gel Catalysts for Tribocatalytic Paracetamol Degradation" Molecules 30, no. 11: 2265. https://doi.org/10.3390/molecules30112265
APA StyleIvanova, D., Kolev, H., Mladenova, R., Stefanov, B. I., & Kaneva, N. (2025). Harvesting Friction Energy on Zinc Oxide and Zinc Oxide/Europium Oxide Sol-Gel Catalysts for Tribocatalytic Paracetamol Degradation. Molecules, 30(11), 2265. https://doi.org/10.3390/molecules30112265











