A Natural Inhibitor, 1′S-1′-Acetoxychavicol Acetate, Against Testosterone-Induced Alopecia via NADPH Oxidase Regulation
Abstract
1. Introduction
2. Results
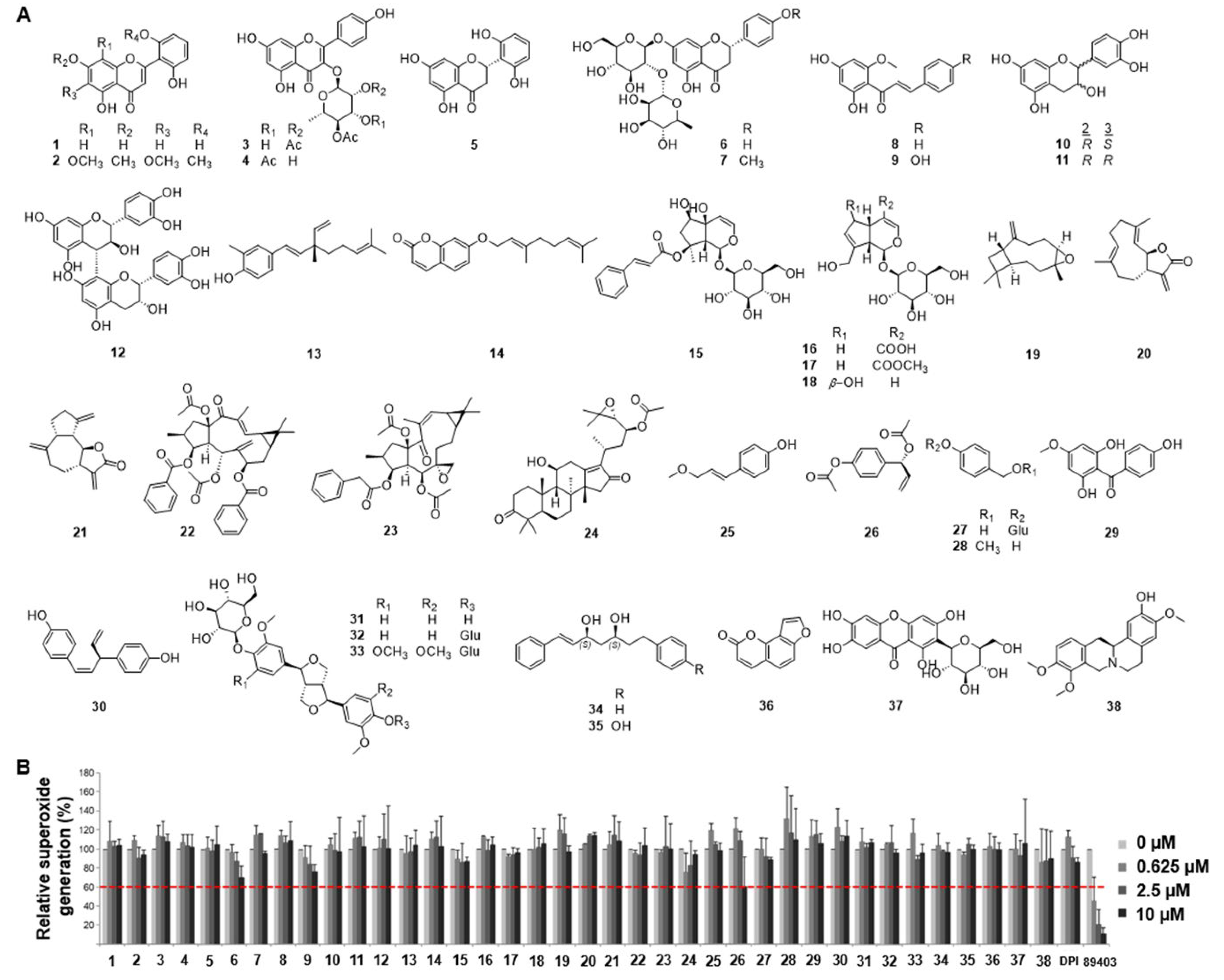
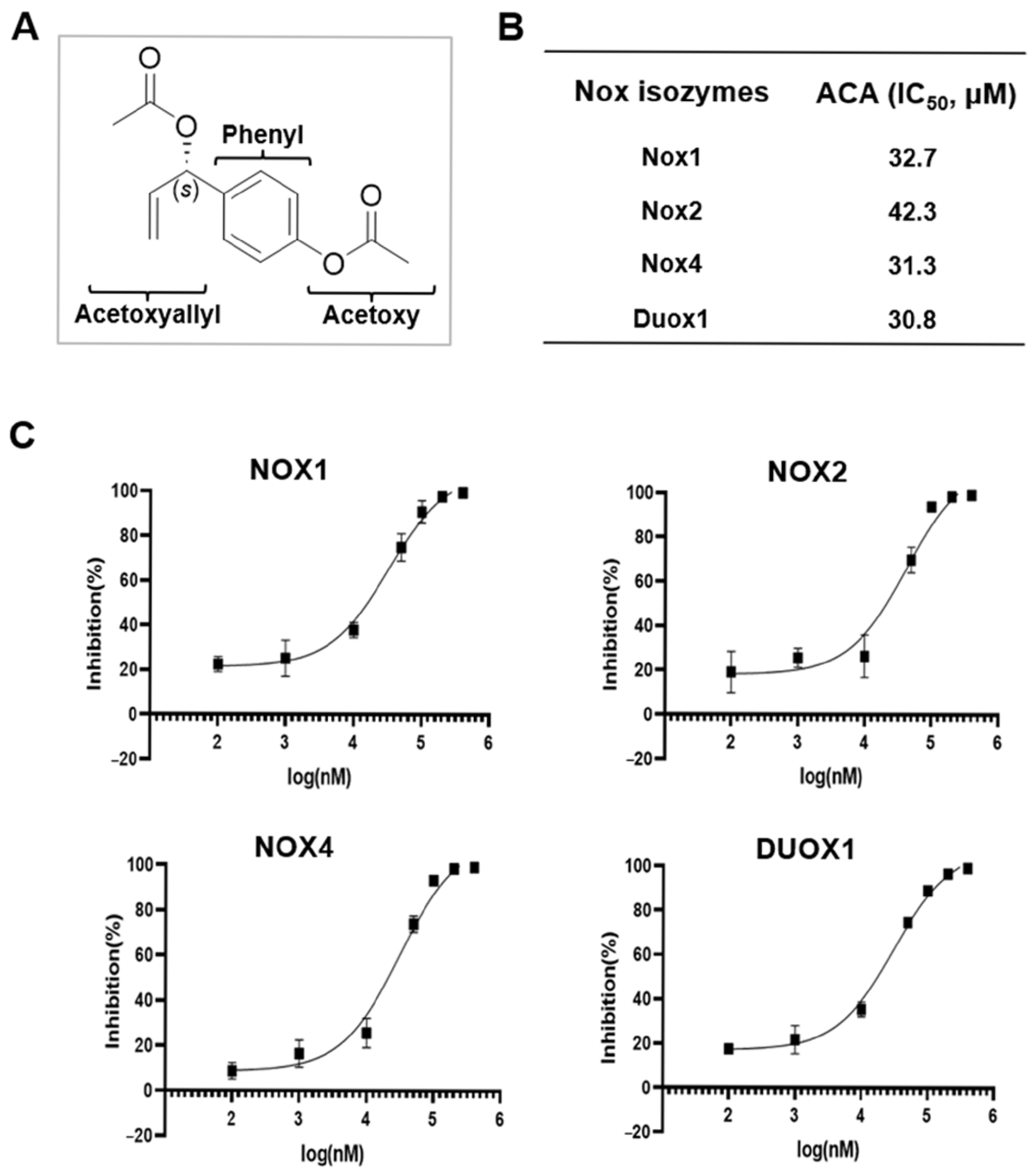
2.1. Identification of ACA as an Inhibitor of Nox from a Library of Natural Compounds
2.2. Molecular Modeling of ACA with Nox5
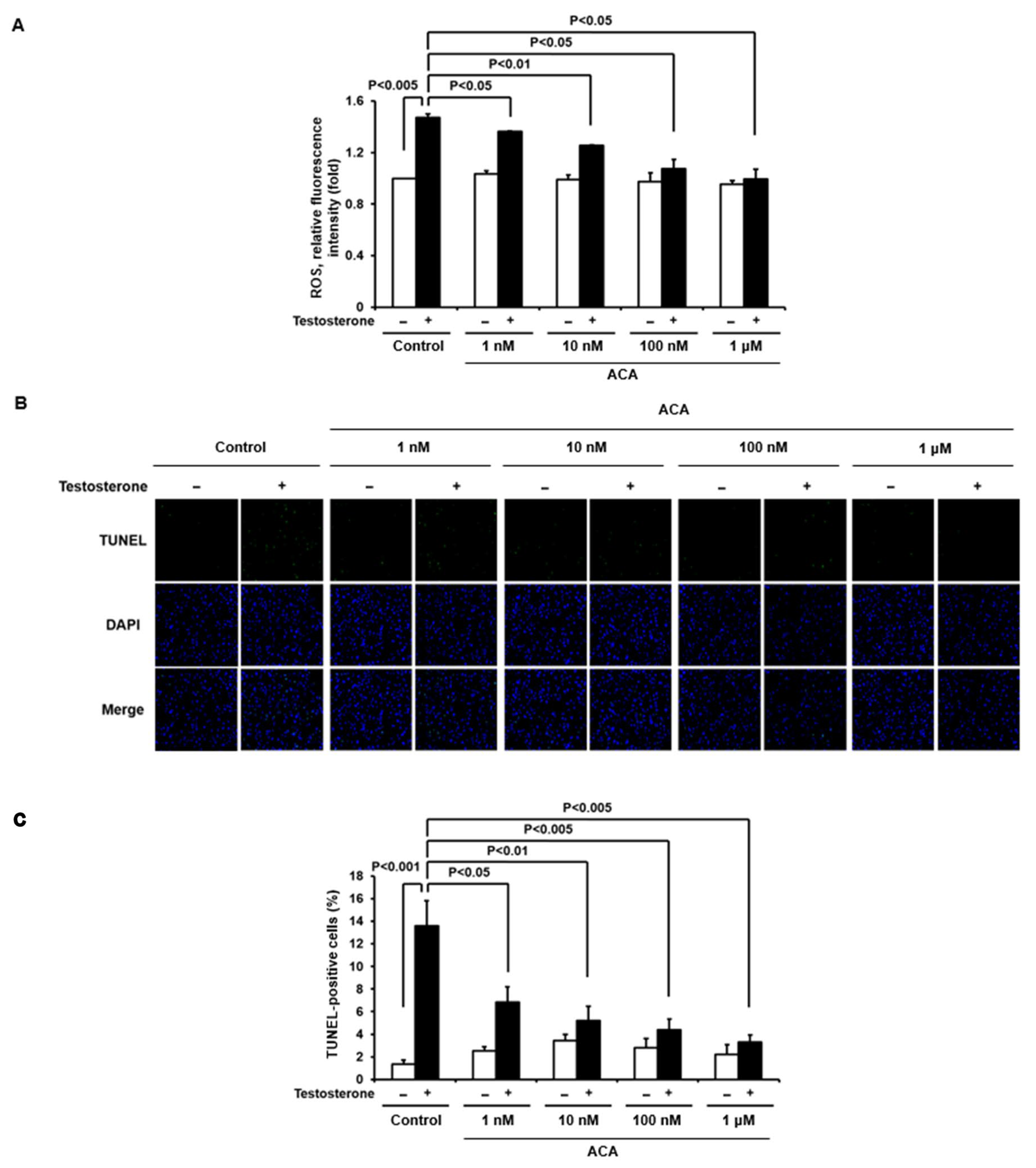
2.3. ACA Suppresses Testosterone-Mediated ROS Generation in Skin Keratinocytes
2.4. ACA Reduces Testosterone-Induced Apoptosis in Skin Keratinocytes
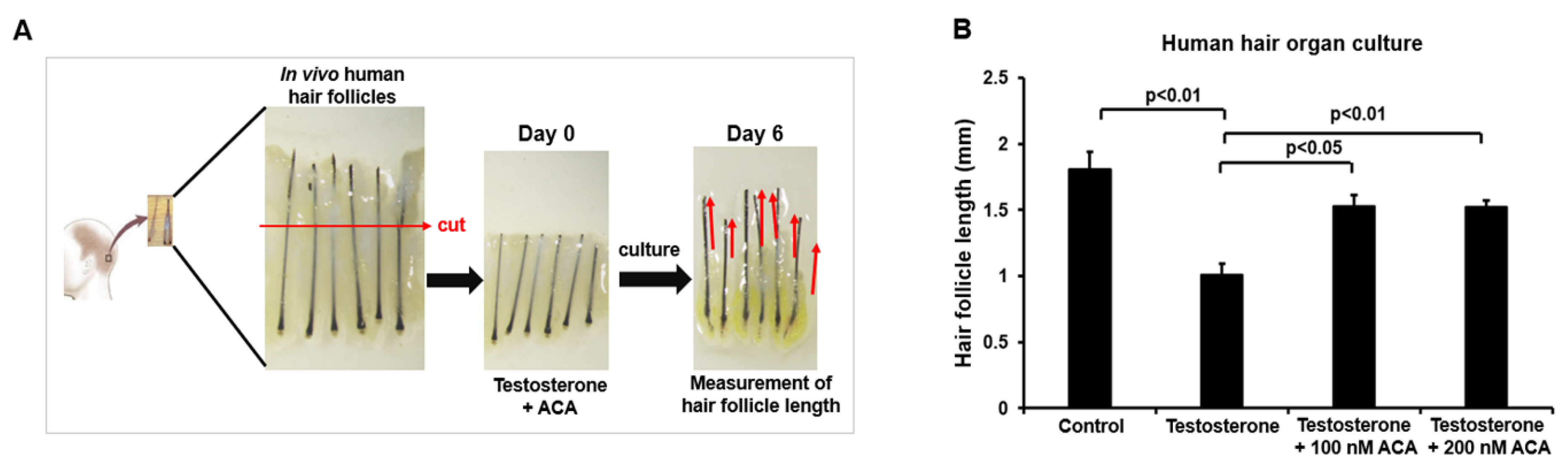
2.5. Function of ACA in Human Hair Follicle Organ Culture
2.6. ACA Regulates Testosterone-Induced Hair Loss
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Nox Inhibitor Screening
4.3. In Silico Model of the Dehydrogenase Domain of hNox5 in Complex with ACA
4.4. Cell Culture
4.5. Measurement of Intracellular ROS by DCF-DA
4.6. TdT-UDP Nick End Labeling (TUNEL) Assay
4.7. Function of ACA in Testosterone-Treated Human Hair Follicle Organ Culture
4.8. ACA Efficacy Test in Testosterone-Treated Dorsal Skin of C57BL/6J Male Mice
4.9. Statistical Analyses
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACA | 1′S-1′-Acetoxychavicol acetate |
| AGA | Androgenetic alopecia |
| DP | Dermal papilla |
| Ker-CT | hTERT/CDK4 immortalized human keratinocytes |
| Nox | NADPH oxidase |
| ORS | Outer root sheath |
| ROS | Reactive oxygen species |
| TUNEL | TdT-UDP nick end labeling |
| DCF-DA | 2′,7′-dichlorodihydrofluorescein diacetate |
References
- Ntshingila, S.; Oputu, O.; Arowolo, A.T.; Khumalo, N.P. Androgenetic alopecia: An update. JAAD Int. 2023, 13, 150–158. [Google Scholar] [CrossRef]
- Park, A.M.; Khan, S.; Rawnsley, J. Hair biology: Growth and pigmentation. Facial Plast. Surg. Clin. North Am. 2018, 26, 415–424. [Google Scholar] [CrossRef]
- Buffoli, B.; Rinaldi, F.; Labanca, M.; Sorbellini, E.; Trink, A.; Guanziroli, E.; Rezzani, R.; Rodella, L.F. The human hair: From anatomy to physiology. Int. J. Dermatol. 2014, 53, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Muller-Rover, S.; Handjiski, B.; van der Veen, C.; Eichmuller, S.; Foitzik, K.; McKay, I.A.; Stenn, K.S.; Paus, R. A comprehensive guide for the accurate classification of murine hair follicles in distinct hair cycle stages. J. Invest. Dermatol. 2001, 117, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Hong, Y.; Weng, C.; Tan, C.; Imperato-McGinley, J.; Zhu, Y.S. Androgen stimulates endothelial cell proliferation via an androgen receptor/VEGF/cyclin A-mediated mechanism. Am. J. Physiol. Heart Circ. Physiol. 2011, 300, H1210–H1221. [Google Scholar] [CrossRef] [PubMed]
- Kohan-Ivani, K.; Gabler, F.; Selman, A.; Vega, M.; Romero, C. Role of dihydrotestosterone (DHT) on TGF-β1 signaling pathway in epithelial ovarian cancer cells. J. Cancer Res. Clin. Oncol. 2016, 142, 47–58. [Google Scholar] [CrossRef]
- Kwack, M.H.; Sung, Y.K.; Chung, E.J.; Im, S.U.; Ahn, J.S.; Kim, M.K.; Kim, J.C. Dihydrotestosterone-inducible Dickkopf 1 from balding dermal papilla cells causes apoptosis in follicular keratinocytes. J. Invest. Dermatol. 2008, 128, 262–269. [Google Scholar] [CrossRef]
- Pi, M.; Faber, P.; Ekema, G.; Jackson, P.D.; Ting, A.; Wang, N.; Fontilla-Poole, M.; Mays, R.W.; Brunden, K.R.; Harrington, J.J.; et al. Identification of a novel extracellular cation-sensing G-protein-coupled receptor. J. Biol. Chem. 2005, 280, 40201–40209. [Google Scholar] [CrossRef]
- Pi, M.; Quarles, L.D. Multiligand specificity and wide tissue expression of GPRC6A reveals new endocrine networks. Endocrinology 2012, 153, 2062–2069. [Google Scholar] [CrossRef]
- Ko, E.; Choi, H.; Kim, B.; Kim, M.; Park, K.N.; Bae, I.H.; Sung, Y.K.; Lee, T.R.; Shin, D.W.; Bae, Y.S. Testosterone stimulates Duox1 activity through GPRC6A in skin keratinocytes. J. Biol. Chem. 2014, 289, 28835–28845. [Google Scholar] [CrossRef]
- Sies, H.; Berndt, C.; Jones, D.P. Oxidative stress. Annu. Rev. Biochem. 2017, 86, 715–748. [Google Scholar] [CrossRef] [PubMed]
- Forman, H.J.; Zhang, H. Targeting oxidative stress in disease: Promise and limitations of antioxidant therapy. Nat. Rev. Drug Discov. 2021, 20, 689–709. [Google Scholar] [CrossRef]
- Maraldi, T. Natural compounds as modulators of NADPH oxidases. Oxidative Med. Cell. Longev. 2013, 2013, 271602. [Google Scholar] [CrossRef]
- Du, F.; Li, J.; Zhang, S.; Zeng, X.; Nie, J.; Li, Z. Oxidative stress in hair follicle development and hair growth: Signalling pathways, intervening mechanisms and potential of natural antioxidants. J. Cell. Mol. Med. 2024, 28, e18486. [Google Scholar] [CrossRef]
- Magnani, F.; Nenci, S.; Millana Fananas, E.; Ceccon, M.; Romero, E.; Fraaije, M.W.; Mattevi, A. Crystal structures and atomic model of NADPH oxidase. Proc. Natl. Acad. Sci. USA 2017, 114, 6764–6769. [Google Scholar] [CrossRef]
- Adil, A.; Godwin, M. The effectiveness of treatments for androgenetic alopecia: A systematic review and meta-analysis. J. Am. Acad. Dermatol. 2017, 77, 136–141.e5. [Google Scholar] [CrossRef]
- Choi, J.Y.; Boo, M.Y.; Boo, Y.C. Can plant extracts help prevent hair loss or promote hair growth? A review comparing their therapeutic efficacies, phytochemical components, and modulatory targets. Molecules 2024, 29, 2288. [Google Scholar] [CrossRef]
- Lee, T.-K.; Kim, B.; Kim, D.W.; Ahn, J.H.; Sim, H.; Lee, J.-C.; Yang, G.E.; Her, Y.; Park, J.H.; Kim, H.S. Effects of decursin and Angelica gigas nakai root extract on hair growth in mouse dorsal skin via regulating inflammatory cytokines. Molecules 2020, 25, 3697. [Google Scholar] [CrossRef]
- Park, G.-H.; Park, K.-Y.; Cho, H.-I.; Lee, S.-M.; Han, J.S.; Won, C.H.; Chang, S.E.; Lee, M.W.; Choi, J.H.; Moon, K.C. Red ginseng extract promotes the hair growth in cultured human hair follicles. J. Med. Food 2015, 18, 354–362. [Google Scholar] [CrossRef]
- Aldieri, E.; Riganti, C.; Polimeni, M.; Gazzano, E.; Lussiana, C.; Campia, I.; Ghigo, D. Classical inhibitors of NOX NAD (P) H oxidases are not specific. Curr. Drug Metab. 2008, 9, 686–696. [Google Scholar] [CrossRef]
- Heumüller, S.; Wind, S.; Barbosa-Sicard, E.; Schmidt, H.H.; Busse, R.; Schröder, K.; Brandes, R.P. Apocynin is not an inhibitor of vascular NADPH oxidases but an antioxidant. Hypertension 2008, 51, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Xu, J.; Qu, W.; Peng, X.; Xin, P.; Yang, X.; Ying, C.; Sun, X.; Hao, L. Resveratrol reduces vascular cell senescence through attenuation of oxidative stress by SIRT1/NADPH oxidase-dependent mechanisms. J. Nutr. Biochem. 2012, 23, 1410–1416. [Google Scholar] [CrossRef] [PubMed]
- Paik, S.; Song, G.Y.; Jo, E.-K. Ginsenosides for therapeutically targeting inflammation through modulation of oxidative stress. Int. Immunopharmacol. 2023, 121, 110461. [Google Scholar] [CrossRef]
- Woo, A.; Min, B.; Ryoo, S. Piceatannol-3′-O-β-D-glucopyranoside as an active component of rhubarb activates endothelial nitric oxide synthase through inhibition of arginase activity. Exp. Mol. Med. 2010, 42, 524–532. [Google Scholar] [CrossRef]
- Bae, Y.S.; Oh, H.; Rhee, S.G.; Yoo, Y.D. Regulation of reactive oxygen species generation in cell signaling. Mol. Cells 2011, 32, 491–509. [Google Scholar] [CrossRef]
- Lee, S.R.; An, E.J.; Kim, J.; Bae, Y.S. Function of NADPH oxidases in diabetic nephropathy and development of Nox inhibitors. Biomol. Ther. 2020, 28, 25–33. [Google Scholar] [CrossRef]
- Haslam, I.S.; Jadkauskaite, L.; Szabo, I.L.; Staege, S.; Hesebeck-Brinckmann, J.; Jenkins, G.; Bhogal, R.K.; Lim, F.L.; Farjo, N.; Farjo, B.; et al. Oxidative damage control in a human (mini-) organ: Nrf2 activation protects against oxidative stress-induced hair growth inhibition. J. Invest. Dermatol. 2017, 137, 295–304. [Google Scholar] [CrossRef]
- Sasaki, M.; Shinozaki, S.; Shimokado, K. Sulforaphane promotes murine hair growth by accelerating the degradation of dihydrotestosterone. Biochem. Biophys. Res. Commun. 2016, 472, 250–254. [Google Scholar] [CrossRef]
- Nam, J.-W.; Kim, S.-J.; Han, A.-R.; Lee, S.-K. Cytotoxic phenylpropanoids from the rhizomes of Alpinia galanga. Biomol. Ther. 2005, 13, 263–266. [Google Scholar]
- Ha, E.-M.; Oh, C.-T.; Bae, Y.S.; Lee, W.-J. A direct role for dual oxidase in Drosophila gut immunity. Science 2005, 310, 847–850. [Google Scholar] [CrossRef]
- Rhee, S.G.; Chang, T.-S.; Jeong, W.; Kang, D. Methods for detection and measurement of hydrogen peroxide inside and outside of cells. Mol. Cells 2010, 29, 539–549. [Google Scholar] [CrossRef] [PubMed]
- Bordoli, L.; Kiefer, F.; Arnold, K.; Benkert, P.; Battey, J.; Schwede, T. Protein structure homology modeling using SWISS-MODEL workspace. Nat. Protoc. 2009, 4, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Magerl, M.; Kauser, S.; Paus, R.; Tobin, D.J. Simple and rapid method to isolate and culture follicular papillae from human scalp hair follicles. Exp. Dermatol. 2002, 11, 381–385. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, K.; Youn, I.; Suh, J.M.; Choi, M.H.; Bae, D.-W.; Park, S.-B.; Kwack, M.H.; Cha, S.-S.; Jang, D.S.; Sung, Y.K.; et al. A Natural Inhibitor, 1′S-1′-Acetoxychavicol Acetate, Against Testosterone-Induced Alopecia via NADPH Oxidase Regulation. Molecules 2025, 30, 2246. https://doi.org/10.3390/molecules30102246
Park K, Youn I, Suh JM, Choi MH, Bae D-W, Park S-B, Kwack MH, Cha S-S, Jang DS, Sung YK, et al. A Natural Inhibitor, 1′S-1′-Acetoxychavicol Acetate, Against Testosterone-Induced Alopecia via NADPH Oxidase Regulation. Molecules. 2025; 30(10):2246. https://doi.org/10.3390/molecules30102246
Chicago/Turabian StylePark, Kkotnara, Isoo Youn, Jung Min Suh, Min Hye Choi, Da-Woon Bae, Soo-Bong Park, Mi Hee Kwack, Sun-Shin Cha, Dae Sik Jang, Young Kwan Sung, and et al. 2025. "A Natural Inhibitor, 1′S-1′-Acetoxychavicol Acetate, Against Testosterone-Induced Alopecia via NADPH Oxidase Regulation" Molecules 30, no. 10: 2246. https://doi.org/10.3390/molecules30102246
APA StylePark, K., Youn, I., Suh, J. M., Choi, M. H., Bae, D.-W., Park, S.-B., Kwack, M. H., Cha, S.-S., Jang, D. S., Sung, Y. K., Bae, Y. S., & Seo, E. K. (2025). A Natural Inhibitor, 1′S-1′-Acetoxychavicol Acetate, Against Testosterone-Induced Alopecia via NADPH Oxidase Regulation. Molecules, 30(10), 2246. https://doi.org/10.3390/molecules30102246





