Coherent Vibrational Anti-Stokes Raman Spectroscopy Assisted by Pulse Shaping
Abstract
1. Introduction
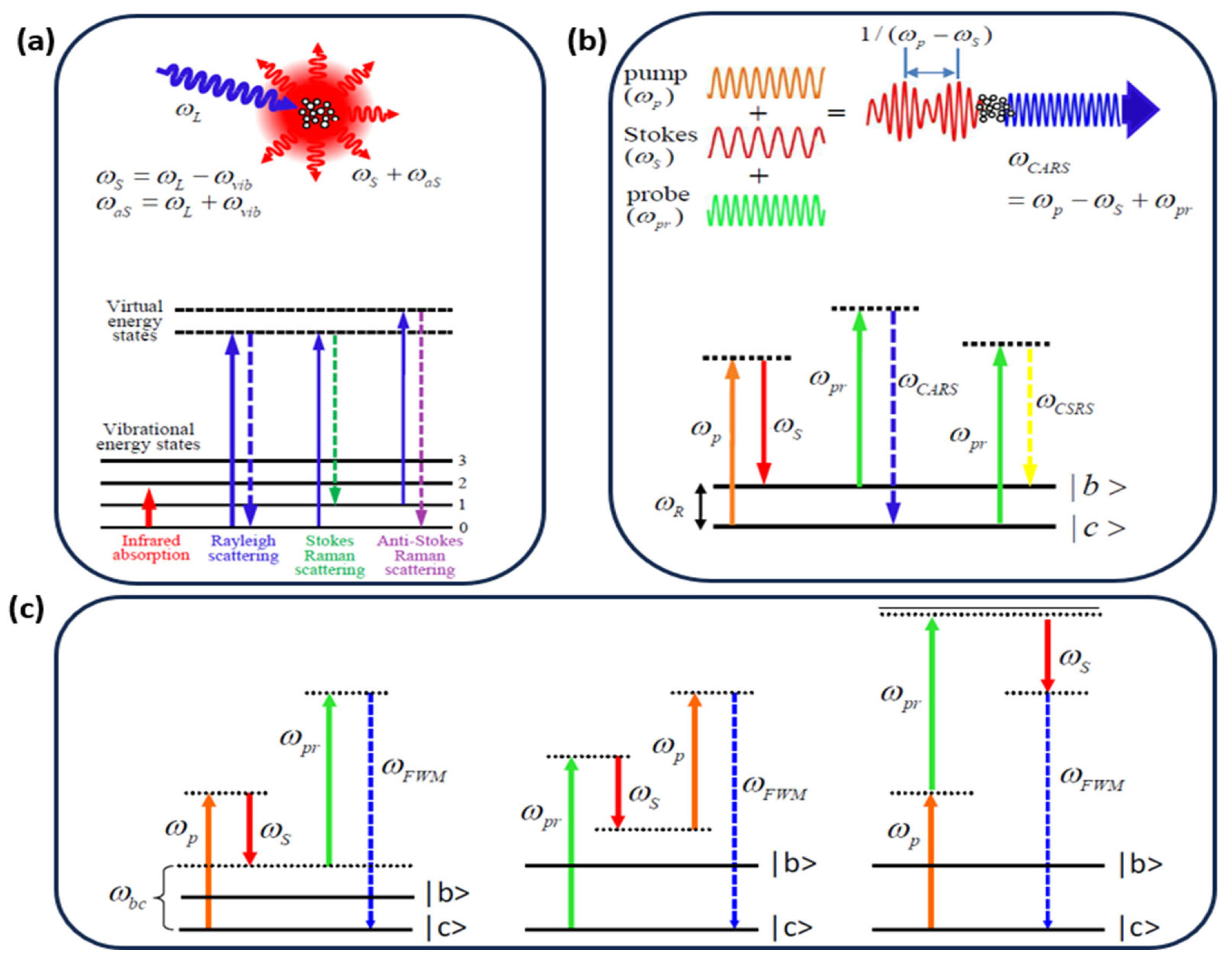
2. Theory of Ultrafast CARS
3. Passive Pulse Shaping for CARS
3.1. Hybrid CARS
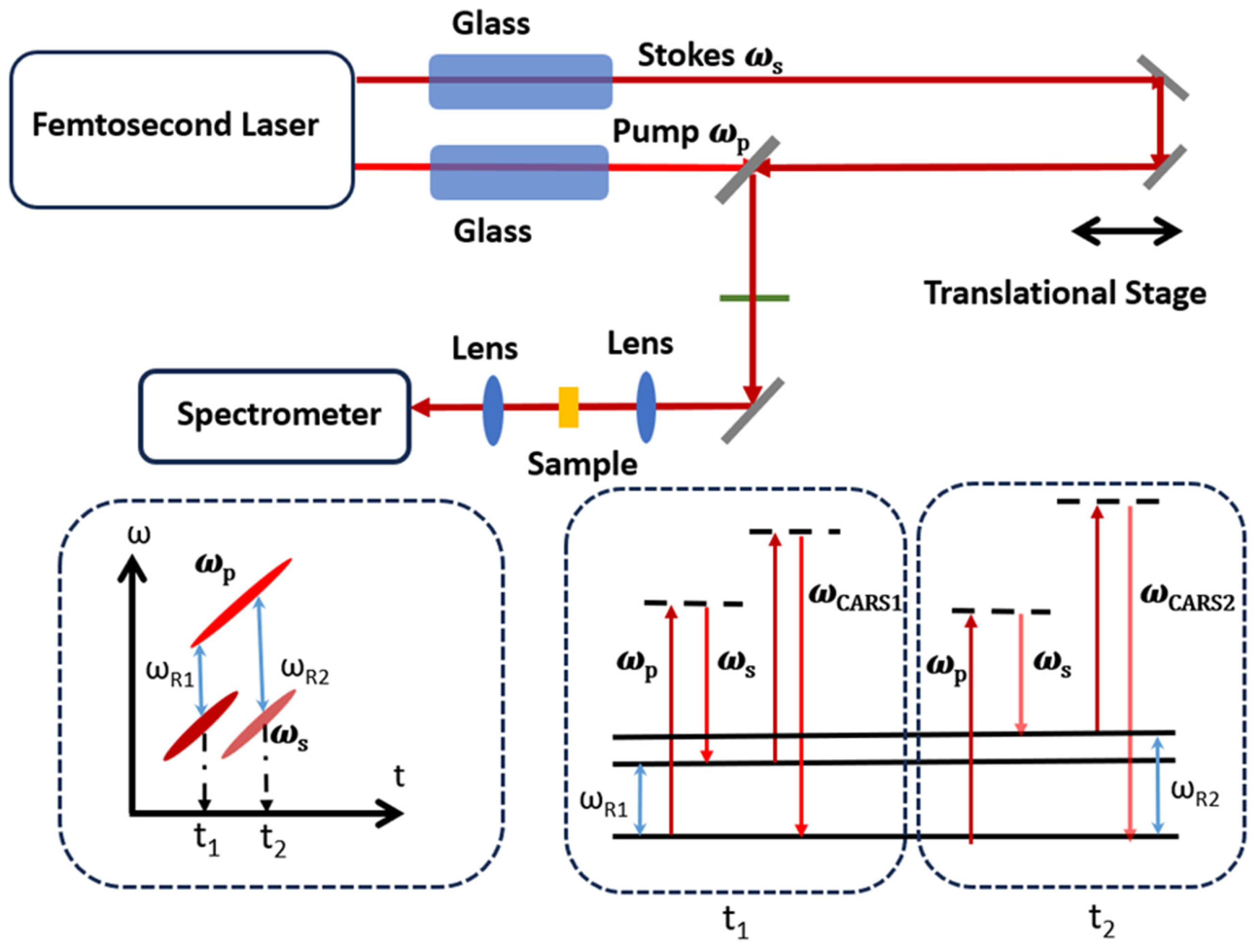
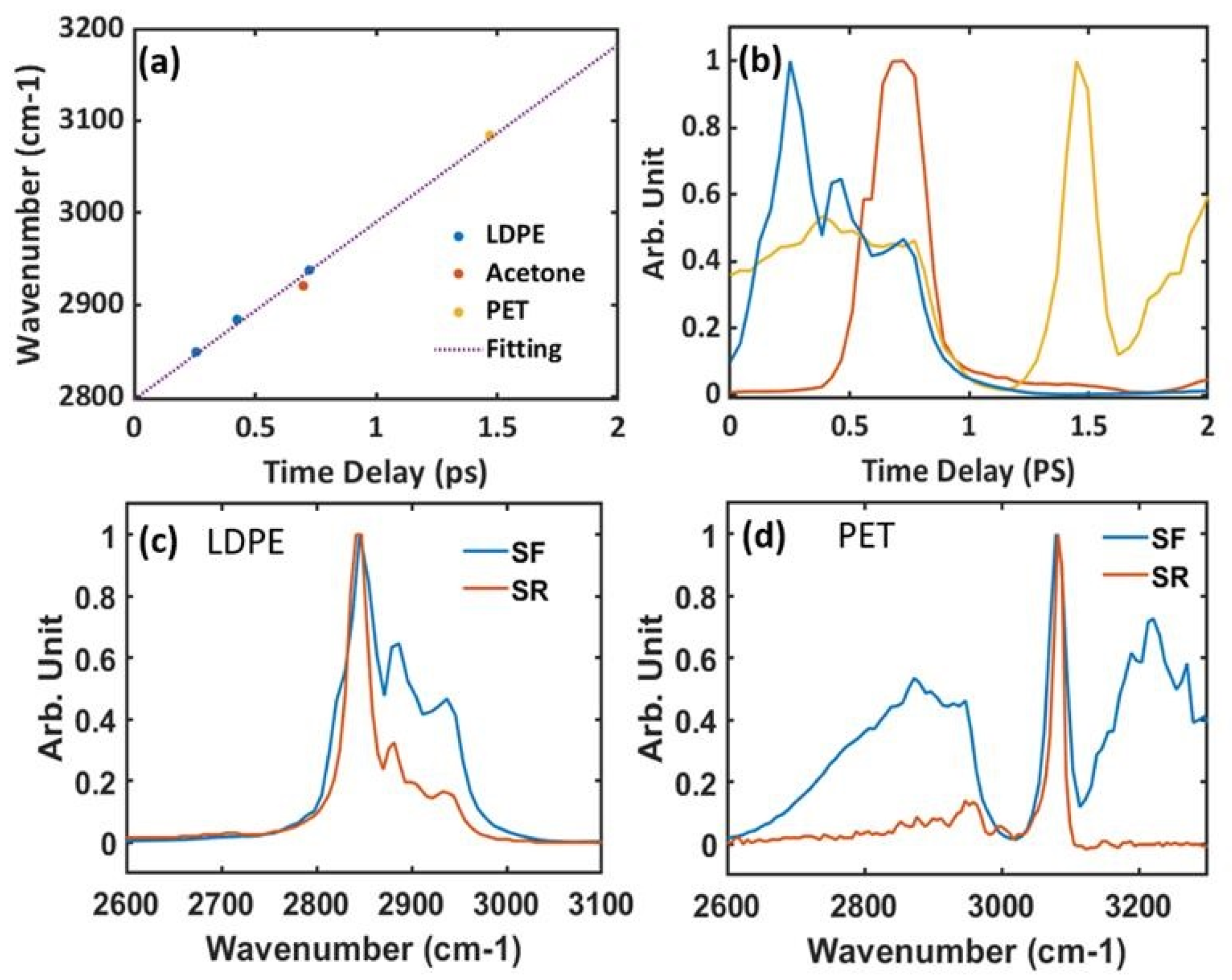
3.2. Spectral Focusing CARS
4. Active Pulse Shaping CARS
4.1. Pulse Shaping with SLM
4.2. AOPDF for CARS
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| CARS | Coherent anti-Stokes Raman scattering |
| AOPDF | Acousto-optic programmable dispersive filters |
| CSRS | Coherent Stokes Raman Scattering |
| NR | Non-resonant |
| FWM | Four wave mixing |
| SNR | Signal-to-noise ratio |
| FASTCARS | Femtosecond adaptive spectroscopic techniques for CARS |
| OPA | Optical parametric amplifier |
| CCD | Charge-coupled device |
| SLM | Spatial light modulator |
| GA | Genetic algorithm |
| X-FROG | Cross-frequency resolved optical gating |
| LDPE | Low density polyethylene |
| PET | Polyethylene terephthalate |
References
- Tolles, W.M.; Nibler, J.W.; McDonald, J.R.; Harvey, A.B. A Review of the Theory and Application of Coherent Anti-Stokes Raman Spectroscopy (CARS). Appl. Spectrosc. 1997, 31, 253–271. [Google Scholar] [CrossRef]
- Petrov, G.I.; Arora, R.; Yakovlev, V.V.; Wang, X.; Sokolov, A.V.; Scully, M.O. Comparison of Coherent and Spontaneous Raman Microspectroscopies for Noninvasive Detection of Single Bacterial Endospores. Proc. Natl. Acad. Sci. USA 2007, 104, 7776–7779. [Google Scholar] [CrossRef] [PubMed]
- Pestov, D.; Ariunbold, G.O.; Wang, X.; Murawski, R.K.; Sautenkov, V.A.; Sokolov, A.V.; Scully, M.O. Coherent versus Incoherent Raman Scattering: Molecular Coherence Excitation and Measurement. Opt. Lett. 2007, 32, 1725–1727. [Google Scholar] [CrossRef] [PubMed]
- Régnier, P.R.; Taran, J.-P.E. Gas Concentration Measurement by Coherent Raman Anti-Stokes Scattering. In Laser Raman Gas Diagnostics; Springer: New York, NY, USA, 1974. [Google Scholar]
- Bohlin, A.; Kliewer, C.J. Communication: Two-Dimensional Gas-Phase Coherent Anti-Stokes Raman Spectroscopy (2D-CARS): Simultaneous Planar Imaging and Multiplex Spectroscopy in a Single Laser Shot. J. Chem. Phys. 2013, 138, 221101. [Google Scholar] [CrossRef]
- Bahari, A.; Sower, K.; Wang, K.; Han, Z.; Florence, J.T.; Wang, Y.Y.; Gao, S.; Lee, H.W.H.; Scully, M.O.; Zheltikov, A.; et al. Background-Penalty-Free Waveguide Enhancement of CARS Signal in Air-Filled Anti-Resonant Hollow-Core Fiber. Opt. Lett. 2022, 47, 4339–4342. [Google Scholar] [CrossRef]
- Zhang, F.; Xie, H.; Yuan, L.; Zhang, Z.; Fu, B.; Yu, S.; Li, G.; Zhang, N.; Lu, X.; Yao, J.; et al. Background-free single-beam coherent Raman spectroscopy assisted by air lasing. Opt. Lett. 2022, 47, 481–484. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, F.; Xu, B.; Xie, H.; Fu, B.; Lu, X.; Zhang, N.; Yu, S.; Yao, J.; Cheng, Y.; et al. High-sensitivity gas detection with air-lasing-assisted coherent Raman spectroscopy. Ultrafast Sci. 2022, 2022, 9761458. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, N.; Lu, X.; Li, Z.; Chen, Y.; Gong, R.; Huang, S.; Wang, Q.; Yao, J. Measurement of Carbon Dioxide Isotopes with Air-Lasing-Based Coherent Raman Spectroscopy. J. Phys. Chem. Lett. 2024, 15, 2944–2950. [Google Scholar] [CrossRef]
- Marko, K.A.; Rimai, L. Space- and Time-Resolved Coherent Anti-Stokes Raman Spectroscopy for Combustion Diagnostics. Opt. Lett. 1979, 4, 211–213. [Google Scholar] [CrossRef]
- Hancock, R.D.; Bertagnolli, K.E.; Lucht, R.P. Nitrogen and Hydrogen CARS Temperature Measurements in a Hydrogen/Air Flame Using a Near-Adiabatic Flat-Flame Burner. Combust. Flame 1997, 109, 323–331. [Google Scholar] [CrossRef]
- Vestin, F.; Sedarsky, D.; Collin, R.; Aldén, M.; Linne, M.; Bengtsson, P.-E. Rotational Coherent Anti-Stokes Raman Spectroscopy (CARS) Applied to Thermometry in High-Pressure Hydrocarbon Flames. Combust. Flame 2008, 154, 143–152. [Google Scholar] [CrossRef]
- Roy, S.; Gord, J.R.; Patnaik, A.K. Recent Advances in Coherent Anti-Stokes Raman Scattering Spectroscopy: Fundamental Developments and Applications in Reacting Flows. Prog. Energy Combust. Sci. 2010, 36, 280–306. [Google Scholar] [CrossRef]
- Song, K.; Xia, M.; Yun, S.; Zhang, Y.; Zhang, S.; Ge, H.; Deng, Y.; Liu, M.; Wang, W.; Zhao, L.; et al. Advances in Femtosecond Coherent Anti-Stokes Raman Scattering for Thermometry. J. Phys. Chem. 2024, 11, 622. [Google Scholar] [CrossRef]
- Vernuccio, F.; Vanna, R.; Ceconello, C.; Bresci, A.; Manetti, F.; Sorrentino, S.; Ghislanzoni, S.; Lambertucci, F.; Motiño, O.; Martins, I.; et al. Full-Spectrum CARS Microscopy of Cells and Tissues with Ultrashort White-Light Continuum Pulses. J. Phys. Chem. B 2023, 127, 4733–4745. [Google Scholar] [CrossRef] [PubMed]
- Nan, X.; Cheng, J.X.; Xie, X.S. Vibrational Imaging of Lipid Droplets in Live Fibroblast Cells with Coherent Anti-Stokes Raman Scattering Microscopy. J. Lipid Res. 2003, 44, 2202. [Google Scholar] [CrossRef]
- Wang, H.; Fu, Y.; Zickmund, P.; Shi, R.; Cheng, J.X. Coherent Anti-Stokes Raman Scattering Imaging of Axonal Myelin in Live Spinal Tissues. Biophys. J. 2005, 89, 581–591. [Google Scholar] [CrossRef]
- Huff, T.B.; Cheng, J.X. In Vivo Coherent Anti-Stokes Raman Scattering Imaging of Sciatic Nerve Tissue. J. Microsc. 2007, 225, 175–182. [Google Scholar] [CrossRef]
- Evans, C.L.; Xie, X.S. Coherent Anti-Stokes Raman Scattering Microscopy: Chemical Imaging for Biology and Medicine. Annu. Rev. Anal. Chem. 2008, 1, 883–909. [Google Scholar] [CrossRef]
- Okuno, M.; Kano, H.; Leproux, P.; Couderc, V.; Day, J.P.; Bonn, M.; Hamaguchi, H.O. Quantitative CARS Molecular Fingerprinting of Single Living Cells with the Use of the Maximum Entropy Method. Angew. Chem. Int. Ed. 2010, 49, 6773–6777. [Google Scholar] [CrossRef]
- Shi, Y.; Kim, S.; Huff, T.B.; Borgens, R.B.; Park, K.; Shi, R.; Cheng, J.X. Effective Repair of Traumatically Injured Spinal Cord by Nanoscale Block Copolymer Micelles. Nat. Nanotechnol. 2010, 5, 80–87. [Google Scholar] [CrossRef]
- Camp, C.H., Jr.; Lee, Y.J.; Heddleston, J.M.; Hartshorn, C.M.; Hight Walker, A.R.; Rich, J.N.; Lathia, J.D.; Cicerone, M.T. High-Speed Coherent Raman Fingerprint Imaging of Biological Tissues. Nat. Photonics 2014, 8, 627–634. [Google Scholar] [CrossRef] [PubMed]
- Tu, H.; Boppart, S.A. Coherent Anti-Stokes Raman Scattering Microscopy: Overcoming Technical Barriers for Clinical Translation. J. Biophotonics 2014, 7, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Li, Y.; Yi, R.; Liu, L.; Qu, J. Coherent Anti-Stokes Raman Scattering Microscopy and Its Applications. Front. Phys. 2020, 8, 598420. [Google Scholar] [CrossRef]
- Baryalay, S.; Byrne, S.; McNamara, T.; Muddiman, R.; Hennelly, B. Broadband Coherent Anti-Stokes Raman Spectroscopy for Small Scale Microplastic Detection. In Nonlinear Optics and Its Applications 2024; SPIE: St Bellingham, WA, USA, 2024; Volume 13004, pp. 165–175. [Google Scholar]
- Choi, D.S.; Jeoung, S.C.; Chon, B.H. Thickness Dependent CARS Measurement of Polymeric Thin Films without Depth-Profiling. Opt. Express 2008, 16, 2604–2613. [Google Scholar] [CrossRef]
- Yahng, J.S.; Jeoung, S.C. Thickness Determination with Chemical Identification of Double-Layered Polymeric Thin Film by Using Multiplex CARS. Opt. Laser Eng. 2011, 49, 66–70. [Google Scholar] [CrossRef]
- Virga, A.; Ferrante, C.; Batignani, G.; De Fazio, D.; Nunn, A.D.G.; Ferrari, A.C.; Cerullo, G.; Scopigno, T. Coherent Anti-Stokes Raman Spectroscopy of Single and Multi-Layer Graphene. Nat. Commun. 2019, 10, 3658. [Google Scholar] [CrossRef]
- Lin, X.-M.; Li, J.-F. Applications of In Situ Raman Spectroscopy on Rechargeable Batteries and Hydrogen Energy Systems. ChemElectroChem 2023, 10, e202201003. [Google Scholar] [CrossRef]
- Takahashi, T.; Pawel, K.; Herdzik, K.; Bourdakos, N.; Arthur, J.; Mahajan, R.S. Selective Imaging of Microplastic and Organic Particles in Flow by Multimodal Coherent Anti-Stokes Raman Scattering and Two-Photon Excited Autofluorescence Analysis. Anal. Chem. 2021, 93, 5234–5240. [Google Scholar] [CrossRef] [PubMed]
- Rhee, H.; Jeong, S.; Lee, H.; Cho, M.G.; Choi, D.S. Rapid Detection and Identification of Microplastics from Nonchemically Treated Soil with CARS Microspectroscopy. Anal. Chim. Acta 2024, 342, 123080. [Google Scholar] [CrossRef]
- Zhu, H.; Xu, C.; Yakovlev, V.V.; Zhang, D. What Is Cooking in Your Kitchen: Seeing “Invisible” with Time-Resolved Coherent Anti-Stokes Raman Spectroscopy. Anal. Bioanal. Chem. 2023, 415, 6471–6480. [Google Scholar] [CrossRef]
- Cheng, J.-X.; Volkmer, A.; Book, L.D.; Xie, X.S. An Epi-Detected Coherent Anti-Stokes Raman Scattering (E-CARS) Microscope with High Spectral Resolution and High Sensitivity. J. Phys. Chem. B 2001, 105, 1277–1280. [Google Scholar] [CrossRef]
- De Vito, G.; Bifone, A.; Piazza, V. Rotating-Polarization CARS Microscopy: Combining Chemical and Molecular Orientation Sensitivity. Opt. Express 2012, 20, 29369–29377. [Google Scholar] [CrossRef]
- Cheng, J.-X. Coherent Anti-Stokes Raman Scattering Microscopy. Appl. Spectrosc. 2007, 61, 197–208. [Google Scholar] [CrossRef]
- Oron, D.; Dudovich, N.; Yelin, D.; Silberberg, Y. Narrow-Band Coherent Anti-Stokes Raman Signals from Broad-Band Pulses. Phys. Rev. Lett. 2002, 88, 063004. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.; Caster, A.G.; Leone, S.R. Single-Pulse Phase-Control Interferometric Coherent Anti-Stokes Raman Scattering Spectroscopy. Phys. Rev. A 2005, 72, 041803. [Google Scholar] [CrossRef]
- Kee, T.W.; Zhao, H.; Cicerone, M.T. One-Laser Interferometric Broadband Coherent Anti-Stokes Raman Scattering. Opt. Express 2006, 14, 3631–3640. [Google Scholar] [CrossRef]
- Potma, E.O.; Evans, C.L.; Xie, X.S. Heterodyne Coherent Anti-Stokes Raman Scattering (CARS) Imaging. Opt. Lett. 2006, 31, 241–243. [Google Scholar] [CrossRef]
- Dudovich, N.; Oron, D.; Silberberg, Y. Single-pulse coherently controlled nonlinear Raman spectroscopy and microscopy. Nature 2002, 418, 51–514. [Google Scholar] [CrossRef] [PubMed]
- Ideguchi, T.; Holzner, S.; Bernhardt, B.; Guelachvili, G.; Picqué, N.; Hänsch, T.W. Coherent Raman Spectro-Imaging with Laser Frequency Combs. Nature 2013, 502, 355–358. [Google Scholar] [CrossRef]
- Weiner, A.M. Ultrafast Optical Pulse Shaping: A Tutorial Review. Opt. Commun. 2011, 284, 3669–3692. [Google Scholar] [CrossRef]
- Pestov, D.S. Detection of Bacterial Endospores by Means of Ultrafast Coherent Raman Spectroscopy; Texas A&M University: College Station, TX, USA, 2008. [Google Scholar]
- Wang, X. Coherent Anti-Stokes Raman Scattering (CARS) Optimized by Exploiting Optical Interference. Ph.D. Dissertation, Texas A&M University, College Station, TX, USA, 2011. [Google Scholar]
- Koch, T.; Ackermann, R.; Stöcker, A.; Meyer-Zedler, T.; Gabler, T.; Lippoldt, T.; Missbach-Güntner, J.; Rußmann, C.; Popp, J.; Nolte, S. Ultrabroadband Two-Beam Coherent Anti-Stokes Raman Scattering and Spontaneous Raman Spectroscopy of Organic Fluids: A Comparative Study. J. Biophotonics 2024, 17, e202300505. [Google Scholar] [CrossRef]
- Warren, W.S.; Rabitz, H.; Dahleh, M. Coherent Control of Quantum Dynamics: The Dream Is Alive. Science 1993, 259, 1581–1588. [Google Scholar] [CrossRef] [PubMed]
- Bernhard, C. Response Function Theory of Time-Resolved CARS and CSRS of Rotating Molecules in Liquids under General Polarization Conditions. Chem. Phys. 1987, 113, 131–147. [Google Scholar]
- Hogiu, S.; Werncke, W.; Pfeiffer, M.; Lau, A.; Steinke, T. Picosecond Time-Resolved CARS Spectroscopy of a Mixed Excited Singlet State of Diphenylhexatriene. Chem. Phys. Lett. 1998, 287, 8–16. [Google Scholar] [CrossRef]
- Pestov, D.; Zhi, M.; Sariyanni, Z.-E.; Kalugin, N.G.; Kolomenskii, A.; Murawski, R.; Rostovtsev, Y.V.; Sautenkov, V.A.; Sokolov, A.V.; Scully, M.O. Femtosecond CARS of Methanol–Water Mixtures. J. Raman Spectrosc. 2006, 37, 392–396. [Google Scholar] [CrossRef]
- Scully, M.O.; Kattawar, G.W.; Lucht, R.P.; Opatrny, T.; Pilloff, H.; Rebane, A.; Sokolov, A.V.; Zubairy, M.S. FAST CARS: Engineering a Laser Spectroscopic Technique for Rapid Identification of Bacterial Spores. Proc. Natl. Acad. Sci. USA 2002, 99, 10994–11001. [Google Scholar] [CrossRef] [PubMed]
- Sidorov-Biryukov, D.A.; Serebryannikov, E.E.; Zheltikov, A.M. Time-Resolved Coherent Anti-Stokes Raman Scattering with a Femtosecond Soliton Output of a Photonic-Crystal Fiber. Opt. Lett. 2006, 31, 2323–2325. [Google Scholar] [CrossRef]
- Seeger, T.; Kiefer, J.; Leipertz, A.; Patterson, B.D.; Kliewer, C.J.; Settersten, T.B. Picosecond Time-Resolved Pure-Rotational Coherent Anti-Stokes Raman Spectroscopy for N2 Thermometry. Opt. Lett. 2009, 34, 3755–3757. [Google Scholar] [CrossRef]
- Pestov, D.; Murawski, R.K.; Ariunbold, G.O.; Wang, X.; Zhi, M.; Sokolov, A.V.; Sautenkov, V.A.; Rostovtsev, Y.V.; Dogariu, A.; Huang, Y.; et al. Optimizing the Laser-Pulse Configuration for Coherent Raman Spectroscopy. Science 2007, 316, 265–268. [Google Scholar] [CrossRef]
- Lim, H.; Caster, A.G.; Leone, S.R. Fourier Transform Spectral Interferometric Coherent Anti-Stokes Raman Scattering (FTSI-CARS) Spectroscopy. Opt. Lett. 2007, 32, 1332–1334. [Google Scholar] [CrossRef]
- Oron, D.; Dudovich, N.; Silberberg, Y. Femtosecond Phase-and-Polarization Control for Background-Free Coherent Anti-Stokes Raman Spectroscopy. Phys. Rev. Lett. 2003, 90, 213902. [Google Scholar] [CrossRef] [PubMed]
- van Rhijn, A.C.W.; Offerhaus, H.L.; van der Walle, P.; Herek, J.L.; Jafarpour, A. Exploring, Tailoring, and Traversing the Solution Landscape of a Phase-Shaped CARS Process. Opt. Express 2010, 18, 2695–2709. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, K.; Welch, G.R.; Sokolov, A.V. Heterodyne coherent anti-Stokes Raman scattering by the phase control of its intrinsic background. Phys. Rev. A 2011, 84, 021801(R). [Google Scholar] [CrossRef]
- Wang, X.; Zhang, A.; Zhi, M.; Sokolov, A.V.; Welch, G.R. Glucose concentration measured by the hybrid coherent anti-Stokes Raman-scattering technique. Phys. Rev. A 2007, 81, 013813. [Google Scholar] [CrossRef]
- Voronine, D.V.; Sinyukov, A.M.; Hua, X.; Wang, K.; Jha, P.K.; Munusamy, E.; Wheeler, S.E.; Welch, G.; Sokolov, A.V.; Scull, M.O. Time-resolved surface-enhanced coherent sensing of nanoscale molecular complexes. Sci. Rep. 2012, 2, 891. [Google Scholar] [CrossRef]
- Prince, B.D.; Chakraborty, A.; Prince, B.M.; Stauffer, H.U. Development of Simultaneous Frequency- and Time-Resolved Coherent Anti-Stokes Raman Scattering for Ultrafast Detection of Molecular Raman Spectra. J. Chem. Phys. 2006, 125, 044502. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Voronine, D.V.; Sokolov, A.V.; Scully, M.O. A versatile setup using femtosecond adaptive spectroscopic techniques for coherent anti-Stokes Raman scattering. Rev. Sci. Instrum. 2015, 86, 083107. [Google Scholar] [CrossRef] [PubMed]
- Shutov, A.; Pestov, D.; Altangerel, N.; Yi, Z.; Wang, X.; Sokolov, A.V.; Scully, M.O. Collinear FAST CARS for chemical mapping of gases. Appl. Sci. 2017, 7, 705. [Google Scholar] [CrossRef]
- Zhao, H.; Tian, Z.; Li, Y.; Wei, H. Hybrid fs/ps Vibrational Coherent Anti-Stokes Raman Scattering for Simultaneous Gas-Phase N2/O2/CO2 Measurements. Opt. Lett. 2021, 46, 1688–1691. [Google Scholar] [CrossRef]
- Miller, J.D.; Slipchenko, M.N.; Meyer, T.R. Probe-Pulse Optimization for Non-Resonant Suppression in Hybrid fs/ps Coherent Anti-Stokes Raman Scattering at High Temperature. Opt. Express 2011, 19, 13326–13333. [Google Scholar] [CrossRef]
- Miller, J.D.; Slipchenko, M.N.; Meyer, T.R.; Stauffer, H.U.; Gord, J.R. Hybrid Femtosecond/Picosecond Coherent Anti-Stokes Raman Scattering for High-Speed Gas-Phase Thermometry. Opt. Lett. 2010, 35, 2430–2432. [Google Scholar] [CrossRef] [PubMed]
- Kerstan, M.; Makos, I.; Nolte, S.; Tünnermann, A.; Ackermann, R. Two-Beam Femtosecond Coherent Anti-Stokes Raman Scattering for Thermometry on CO2. Appl. Phys. Lett. 2017, 110, 021116. [Google Scholar] [CrossRef]
- Santagata, R.; Scherman, M.; Toubeix, M.; Nafa, M.; Tretout, B.; Bresson, A. Ultrafast Background-Free Ro-Vibrational fs/ps-CARS Thermometry Using an Yb:YAG Crystal-Fiber Amplified Probe. Opt. Express 2019, 27, 32924–32937. [Google Scholar] [CrossRef] [PubMed]
- Kearney, S.P.; Scoglietti, D.; Kliewer, C. Hybrid Femtosecond/Picosecond Rotational Coherent Anti-Stokes Raman Scattering Temperature and Concentration Measurements Using Two Different Picosecond-Duration Probes. Opt. Express 2013, 21, 12327–12339. [Google Scholar] [CrossRef]
- Peng, J.H.; Pestov, D.; Scully, M.O.; Sokolov, A.V. Simple Setup for Hybrid Coherent Raman Microspectroscopy. J. Raman Spectrosc. 2009, 40, 795–799. [Google Scholar] [CrossRef]
- Chen, D.; Liu, Z.; Liu, S.; Niu, H. Ultra-Broadband Three-Color Coherent Anti-Stokes Raman Scattering Spectroscopy. Opt. Commun. 2022, 519, 128367. [Google Scholar] [CrossRef]
- Pestov, D.; Wang, X.; Ariunbold, G.O.; Murawski, R.K.; Sautenkov, V.A.; Dogariu, A.; Sokolov, A.V.; Scully, M.O. Single-Shot Detection of Bacterial Endospores via Coherent Raman Spectroscopy. Proc. Natl. Acad. Sci. USA 2008, 105, 422–427. [Google Scholar] [CrossRef]
- Peng, J.; Sokolov, A.V. Epi-detected hybrid coherent Raman micro-spectroscopy. J. Mod. Opt. 2009, 56, 1964–1969. [Google Scholar] [CrossRef]
- Cao, T.; Huang, L.; Peng, J. Simple configurations for hybrid coherent Raman microspectroscopy. In Proceedings of the 2016 Progress in Electromagnetic Research Symposium (PIERS), Shanghai, China, 8–11 August 2016; IEEE: Piscataway, NJ, USA, 2016; p. 7734949. [Google Scholar]
- Miller, J.D.; Roy, S.; Gord, J.R.; Meyer, T.R. Communication: Time-Domain Measurement of High-Pressure N2 and O2 Self-Broadened Linewidths Using Hybrid Femtosecond/Picosecond Coherent Anti-Stokes Raman Scattering. J. Chem. Phys. 2011, 135, 201104. [Google Scholar] [CrossRef]
- Kearney, S.P.; Scoglietti, D.J. Hybrid Femtosecond/Picosecond Rotational Coherent Anti-Stokes Raman Scattering at Flame Temperatures Using a Second-Harmonic Bandwidth-Compressed Probe. Opt. Lett. 2013, 38, 833–835. [Google Scholar] [CrossRef]
- Kearney, S.P.; Guildenbecher, D.R. Temperature Measurements in Metalized Propellant Combustion Using Hybrid fs/ps Coherent Anti-Stokes Raman Scattering. Appl. Opt. 2016, 55, 4958–4966. [Google Scholar] [CrossRef] [PubMed]
- Kearney, S.P. Hybrid fs/ps rotational CARS temperature and oxygen measurements in the product gases of canonical flat flames. Combust. Flame 2015, 162, 1748–1758. [Google Scholar] [CrossRef]
- Kearney, S.P.; Danehy, P.M. Pressure measurements using hybrid femtosecond/picosecond rotational coherent anti-Stokes Raman scattering. Opt. Lett. 2015, 40, 4082–4085. [Google Scholar] [CrossRef]
- Hellerer, T.; Enejder, A.M.; Zumbusch, A. Spectral Focusing: High Spectral Resolution Spectroscopy with Broad-Bandwidth Laser Pulses. Appl. Phys. Lett. 2004, 85, 25–27. [Google Scholar] [CrossRef]
- Gershgoren, E.; Bartels, R.A.; Fourkas, J.T.; Tobey, R.; Murnane, M.M.; Kapteyn, H.C. Simplified setup for high resolution spectroscopy that uses ultrashort pulses. Opt. Lett. 2003, 28, 361–363. [Google Scholar] [CrossRef] [PubMed]
- Pestov, D.; Wang, X.; Murawski, R.K.; Ariunbold, G.O.; Sautenkov, V.A.; Sokolov, A.V. Pulse shaping for mode-selective ultrafast coherent Raman spectroscopy of highly scattering solids. JOSAB 2008, 25, 768–772. [Google Scholar] [CrossRef]
- Pegoraro, A.F.; Ridsdale, A.; Moffatt, D.J.; Jia, Y.; Pezacki, J.P.; Stolow, A. Optimally Chirped Multimodal CARS Microscopy Based on a Single Ti:Sapphire Oscillator. Opt. Express 2009, 17, 2984–2996. [Google Scholar] [CrossRef]
- Langbein, W.; Rocha-Mendoza, I.; Borri, P. Coherent Anti-Stokes Raman Micro-Spectroscopy Using Spectral Focusing: Theory and Experiment. J. Raman Spectrosc. 2009, 40, 800–808. [Google Scholar] [CrossRef]
- Mohseni, M.; Polzer, C.; Hellerer, T. Resolution of Spectral Focusing in Coherent Raman Imaging. Opt. Express 2018, 26, 10230–10241. [Google Scholar] [CrossRef]
- Cole, R.A.; Slepkov, A.D. Interplay of Pulse Bandwidth and Spectral Resolution in Spectral-Focusing CARS Microscopy. J. Opt. Soc. Am. B 2018, 35, 842–850. [Google Scholar] [CrossRef]
- Chen, K.; Wu, T.; Wei, H.; Li, Y. Dual-Soliton Stokes-Based Background-Free Coherent Anti-Stokes Raman Scattering Spectroscopy and Microscopy. Opt. Lett. 2016, 41, 2628–2631. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.C.; Sung, J.; Lim, S.H. Chemical Imaging with Frequency Modulation Coherent Anti-Stokes Raman Scattering Microscopy at the Vibrational Fingerprint Region. J. Phys. Chem. B 2010, 114, 16871–16880. [Google Scholar] [CrossRef] [PubMed]
- Rocha-Mendoza, I.; Langbein, W.; Borri, P. Coherent Anti-Stokes Raman Microspectroscopy Using Spectral Focusing with Glass Dispersion. Appl. Phys. Lett. 2008, 93, 201103. [Google Scholar] [CrossRef]
- Brückner, L.; Buckup, T.; Motzkus, M. Exploring the Potential of Tailored Spectral Focusing. J. Opt. Soc. Am. B 2016, 33, 1482–1491. [Google Scholar] [CrossRef]
- Porquez, J.G.; Cole, R.A.; Tabarangao, J.T.; Slepkov, A.D. Spectrally-broad coherent anti-Stokes Raman scattering hyper-microscopy utilizing a Stokes supercontinuum pumped at 800 nm. Biomed. Opt. Express 2016, 7, 4335–4345. [Google Scholar] [CrossRef]
- Langbein, W.; Rocha-Mendoza, I.; Borri, P. Single source coherent anti-stokes Raman microspectroscopy using spectral focusing. Appl. Phys. Lett. 2009, 95, 081109. [Google Scholar] [CrossRef]
- Chen, K.; Wu, T.; Wei, H.; Zhou, T.; Li, Y. Quantitative chemical imaging with background-free multiplex coherent anti-Stokes Raman scattering by dual-soliton Stokes pulses. Biomed. Opt. Express 2016, 7, 3927–3939. [Google Scholar] [CrossRef]
- Tomita, K.; Kojima, Y.; Kannari, F. Selective coherent anti-Stokes Raman scattering microscopy employing dual-wavelength nanofocused ultrafast plasmon pulses. Nano Lett. 2016, 18, 1366–1372. [Google Scholar] [CrossRef]
- Herdzik, K.P.; Bourdakos, K.N.; Johnson, P.B.; Lister, A.P.; Pitera, A.P.; Guo, C.Y.; Mahajan, S. Multimodal spectral focusing CARS and SFG microscopy with a tailored coherent continuum from a microstructured fiber. Appl. Phys. B 2020, 126, 84. [Google Scholar] [CrossRef]
- Pope, I.; Langbein, W.; Watson, P.; Borri, P. Simultaneous hyperspectral differential-CARS, TPF and SHG microscopy with a single 5 fs Ti:Sa laser. Opt. Express 2013, 21, 7096–7106. [Google Scholar] [CrossRef]
- Kearney, S.P. Bandwidth optimization of femtosecond pure-rotational coherent anti-Stokes Raman scattering by pump/Stokes spectral focusing. Appl. Opt. 2014, 53, 6579–6585. [Google Scholar] [CrossRef] [PubMed]
- Konorov, S.O.; Blades, M.W.; Turner, R.F. Lorentzian Amplitude and Phase Pulse Shaping for Nonresonant Background Suppression and Enhanced Spectral Resolution in Coherent Anti-Stokes Raman Scattering Spectroscopy and Microscopy. Appl. Spectrosc. 2010, 64, 767–774. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.X.; Iyer, R.R.; Sorrells, J.E.; Renteria, C.A.; Boppart, S.A. Temporally Optimized and Spectrally Shaped Hyperspectral Coherent Anti-Stokes Raman Scattering Microscopy. Opt. Express 2024, 32, 11474–11490. [Google Scholar] [CrossRef]
- Mecker, N.T.; Courtney, T.L.; Patterson, B.D.; Escofet-Martin, D.; Peterson, B.; Kliewer, C.J.; Linne, M. Optimising hybrid rotational femtosecond/picosecond coherent anti-Stokes Raman spectroscopy (HR-CARS) in nitrogen at high pressures and temperatures. JOSAB 2020, 37, 1035–1046. [Google Scholar] [CrossRef]
- Sorrells, J.E.; Yang, L.; Iyer, R.R.; Chaney, E.J.; Renteria, C.A.; Boppart, S.A. Programmable hyperspectral coherent anti-Stokes Raman scattering microscopy. Opt. Lett. 2024, 49, 2513. [Google Scholar] [CrossRef]
- Xu, X.G.; Konorov, S.O.; Hepburn, J.W.; Milner, V. Noise autocorrelation spectroscopy with coherent Raman scattering. Nat. Phys. 2007, 4, 125–129. [Google Scholar] [CrossRef]
- Li, B.; Warren, S.W.; Fisher, M.C. Phase-Cycling Coherent Anti-Stokes Raman Scattering Using Shaped Femtosecond Laser Pulses. Opt. Express 2010, 18, 25825–25833. [Google Scholar] [CrossRef]
- Bernhard, V.V.; Motzkus, M.M. Time-Resolved Two-Color Single-Beam CARS Employing Supercontinuum and Femtosecond Pulse Shaping. Opt. Commun. 2006, 264, 488–493. [Google Scholar]
- Bernhard, V.V.; Motzkus, M.M. Time-Resolving Molecular Vibration for Microanalytics: Single Laser Beam Nonlinear Raman Spectroscopy in Simulation and Experiment. Phys. Chem. Chem. Phys. 2008, 10, 681–691. [Google Scholar]
- Isobe, K.; Suda, A.; Tanaka, M.; Hashimoto, H.; Kannari, F.; Kawano, H.; Mizuno, H.; Miyawaki, A.; Midorikawa, K. Single-Pulse Coherent Anti-Stokes Raman Scattering Microscopy Employing an Octave Spanning Pulse. Opt. Express 2009, 17, 11259–11266. [Google Scholar] [CrossRef]
- Postma, S.; van Rhijn, A.C.W.; Korterik, J.P.; Gross, P.; Herek, J.L.; Offerhaus, H.L. Application of Spectral Phase Shaping to High Resolution CARS Spectroscopy. Opt. Express 2008, 16, 7985–7996. [Google Scholar] [CrossRef] [PubMed]
- Konorov, S.O.; Xu, X.G.; Turner, R.F.B.; Blades, M.W.; Hepburn, J.W.; Milner, V. Pulse Optimization for Raman Spectroscopy with Cross-Correlation Frequency Resolved Optical Gating. Opt. Express 2007, 15, 7564–7572. [Google Scholar] [CrossRef]
- Polack, T.; Oron, D.; Silbergerg, Y. Control and measurement of a non-resonant Raman wavepacket using a single ultrashort pulse. Chem. Phys. 2005, 318, 163–169. [Google Scholar] [CrossRef]
- Roy, S.; Wrzesinski, P.J.; Pestov, D.; Dantus, M.; Gord, J.R. Single-Beam Coherent Anti-Stokes Raman Scattering (CARS) Spectroscopy of Gas-Phase CO2 via Phase and Polarization Shaping of a Broadband Continuum. J. Raman Spectrosc. 2009, 41, 1194–1199. [Google Scholar] [CrossRef]
- Roy, S.; Wrzesinski, P.; Pestov, D.; Gunaratne, T.; Dantus, M.; Gord, J.R. Single-Beam Coherent Anti-Stokes Raman Scattering Spectroscopy of N2 Using a Shaped 7 fs Laser Pulse. Appl. Phys. Lett. 2009, 95, 074102. [Google Scholar] [CrossRef]
- Li, H.; Harris, D.A.; Xu, B.; Wrzesinski, P.J.; Lozovoy, V.V.; Dantus, M. Standoff and Arms-Length Detection of Chemicals with Single-Beam Coherent Anti-Stokes Raman Scattering. Appl. Opt. 2009, 48, B17–B22. [Google Scholar] [CrossRef] [PubMed]
- Katz, O.; Natan, A.; Silberberg, Y.; Rosenwaks, S. Standoff Detection of Trace Amounts of Solids by Nonlinear Raman Spectroscopy Using Shaped Femtosecond Pulses. Appl. Phys. Lett. 2008, 92, 171116. [Google Scholar] [CrossRef]
- Oron, D.; Dudovich, N.; Silberberg, Y. All-Optical Processing in Coherent Nonlinear Spectroscopy. Phys. Rev. A 2004, 70, 023415. [Google Scholar] [CrossRef]
- Bernhard, V.V.; Buckup, T.; Motzkus, M. Highly Sensitive Single-Beam Heterodyne Coherent Anti-Stokes Raman Scattering. Opt. Lett. 2006, 31, 2495–2497. [Google Scholar]
- Tournois, P. Acousto-optic programmable dispersive filter for adaptive compensation of group delay time dispersion in laser systems. Opt. Comm. 1997, 140, 245–249. [Google Scholar] [CrossRef]
- Oksenhendler, T.; Forget, N. Pulse-Shaping Techniques: Theory and Experimental Implementations for Femtosecond Pulses. In Advances in Solid State Lasers Development and Applications; Grishin, M., Ed.; IntechOpen: London, UK, 2010. [Google Scholar]
- Forget, N.; Canova, L.; Chen, X.; Jullien, A.; Lopez-Martens, R. Closed-Loop Carrier-Envelope Phase Stabilization with an Acousto-Optic Programmable Dispersive Filter. Opt. Lett. 2009, 34, 3647–3649. [Google Scholar] [CrossRef] [PubMed]
- Canova, L.; Chen, X.; Trisorio, A.; Jullien, A.; Assion, A.; Tempea, G.; Forget, N.; Oksenhendler, T.; Lopez-Martens, R. Carrier-Envelope Phase Stabilization and Control Using a Transmission Grating Compressor and an AOPDF. Opt. Lett. 2009, 34, 1333–1335. [Google Scholar] [CrossRef]
- Ohno, K.; Tanabe, T.; Kannari, F. Adaptive Pulse Shaping of Phase and Amplitude of an Amplified Femtosecond Pulse Laser by Direct Reference to Frequency-Resolved Optical Gating Traces. J. Opt. Soc. Am. B 2002, 19, 2781–2790. [Google Scholar] [CrossRef]
- Schubert, M.; Eisele, M.; Crozatier, V.; Forget, N.; Kaplan, D.; Huber, R. Rapid-Scan Acousto-Optical Delay Line with 34 kHz Scan Rate and 15 as Precision. Opt. Lett. 2013, 38, 2907–2909. [Google Scholar] [CrossRef]
- Cousin, S.L.; Forget, N.; Grün, A.; Bates, P.K.; Austin, D.R.; Biegert, J. Few-Cycle Pulse Characterization with an Acousto-Optic Pulse Shaper. Opt. Lett. 2011, 36, 2803–2805. [Google Scholar] [CrossRef] [PubMed]
- Zhi, M.; Wang, K.; Hua, X.; Sokolov, A.V. Pulse-Shaper-Assisted Phase Control of a Coherent Broadband Spectrum of Raman Sidebands. Opt. Lett. 2011, 36, 4032–4034. [Google Scholar] [CrossRef]
- Zhi, M.; Wang, K.; Hua, X.; Strycker, B.D.; Sokolov, A.V. Shaper-Assisted Phase Optimization of a Broad “Holey” Spectrum. Opt. Express 2011, 19, 23400–23408. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Leng, Y.; Li, R. Further Development of the Short-Pulse Petawatt Laser: Trends, Technologies, and Bottlenecks. Laser Photonics Rev. 2023, 17, 2100705. [Google Scholar] [CrossRef]
- Shivkumar, S.; Ranann, D.; Metais, S.; Suresh, S.; Forget, N.; Bartels, R.; Oron, D.; Rigneault, H. Selective Detection in Impulsive Low-Frequency Raman Imaging Using Shaped Probe Pulses. Phys. Rev. Appl. 2023, 19, 054075. [Google Scholar] [CrossRef]
- Lindley, M.; Gala de Pablo, J.; Kinegawa, R.; Hiramatsu, K.; Goda, K. Highly Sensitive Fourier-Transform Coherent Anti-Stokes Raman Scattering Spectroscopy via Genetic Algorithm Pulse Shaping. Opt. Lett. 2021, 46, 4320–4323. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, L.; Zhang, X.; Ding, L.; Chen, G.; Sun, Z.; Wang, Z. Selective Excitation of CARS by Adaptive Pulse Shaping Based on Genetic Algorithm. Chem. Phys. Lett. 2007, 433, 416–421. [Google Scholar] [CrossRef]








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, K.; Florence, J.T.; Hua, X.; Han, Z.; Shen, Y.; Wang, J.; Wang, X.; Sokolov, A.V. Coherent Vibrational Anti-Stokes Raman Spectroscopy Assisted by Pulse Shaping. Molecules 2025, 30, 2243. https://doi.org/10.3390/molecules30102243
Wang K, Florence JT, Hua X, Han Z, Shen Y, Wang J, Wang X, Sokolov AV. Coherent Vibrational Anti-Stokes Raman Spectroscopy Assisted by Pulse Shaping. Molecules. 2025; 30(10):2243. https://doi.org/10.3390/molecules30102243
Chicago/Turabian StyleWang, Kai, James T. Florence, Xia Hua, Zehua Han, Yujie Shen, Jizhou Wang, Xi Wang, and Alexei V. Sokolov. 2025. "Coherent Vibrational Anti-Stokes Raman Spectroscopy Assisted by Pulse Shaping" Molecules 30, no. 10: 2243. https://doi.org/10.3390/molecules30102243
APA StyleWang, K., Florence, J. T., Hua, X., Han, Z., Shen, Y., Wang, J., Wang, X., & Sokolov, A. V. (2025). Coherent Vibrational Anti-Stokes Raman Spectroscopy Assisted by Pulse Shaping. Molecules, 30(10), 2243. https://doi.org/10.3390/molecules30102243








