Systematic Optimization of Complex Salt Roasting and Leaching Conditions for Efficient Extraction of Lithium, Rubidium and Cesium from Lepidolite
Abstract
1. Introduction
2. Results and Discussion
2.1. Optimization of Complex Salt Roasting Conditions
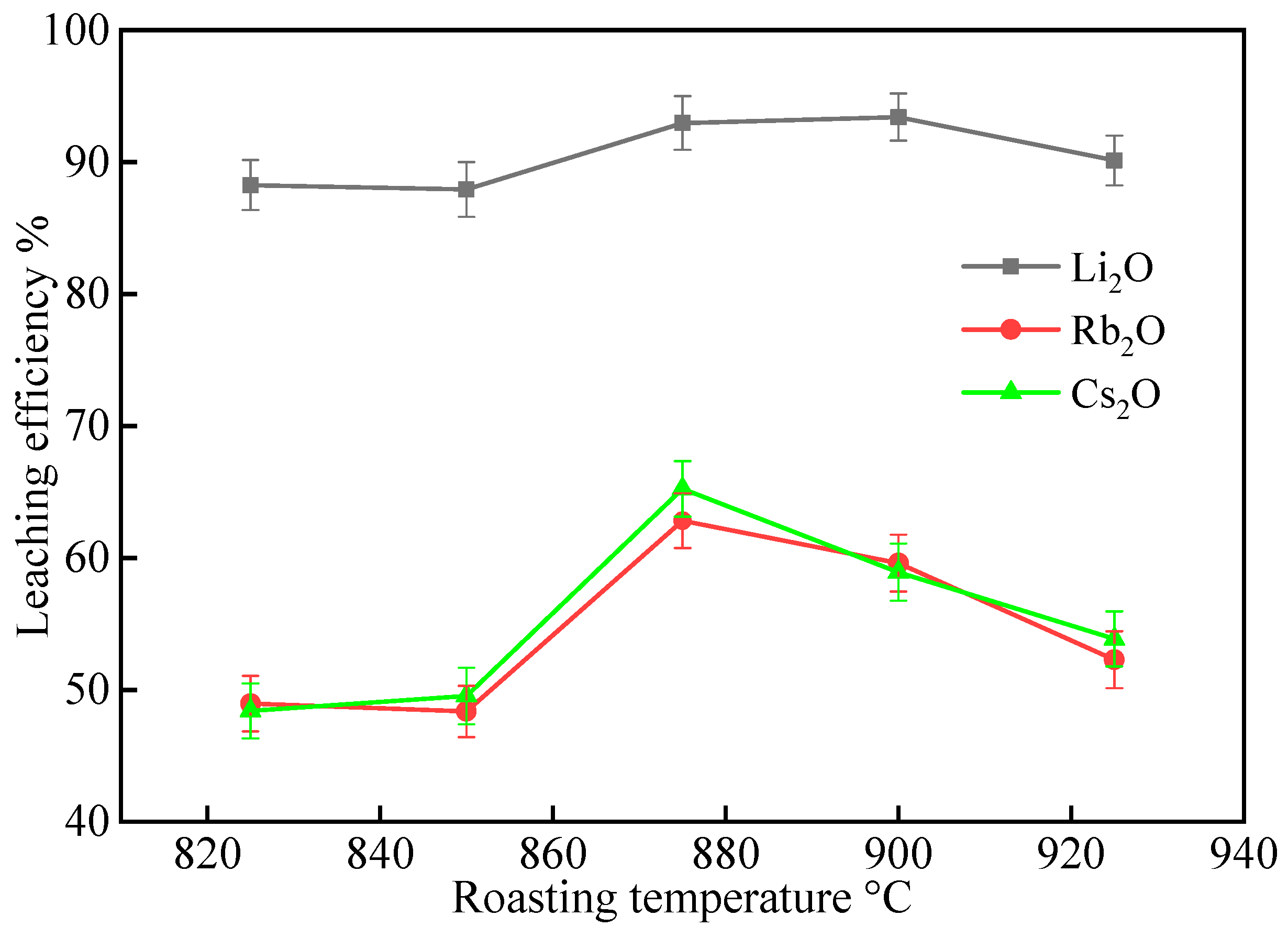
2.1.1. Effect of Roasting Temperature
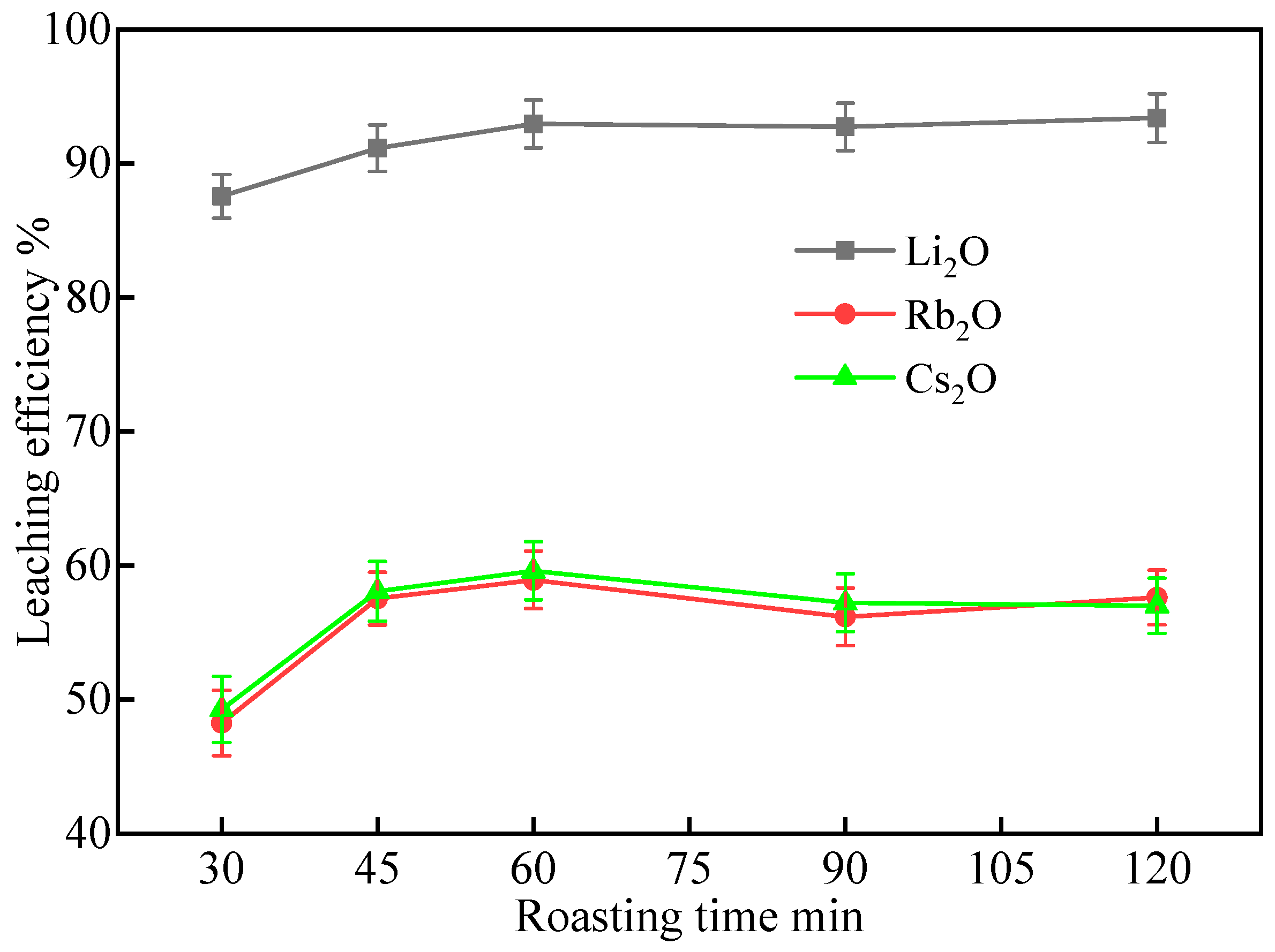
2.1.2. Effect of Roasting Time
2.2. Optimization of Complex Salt Roasting Process
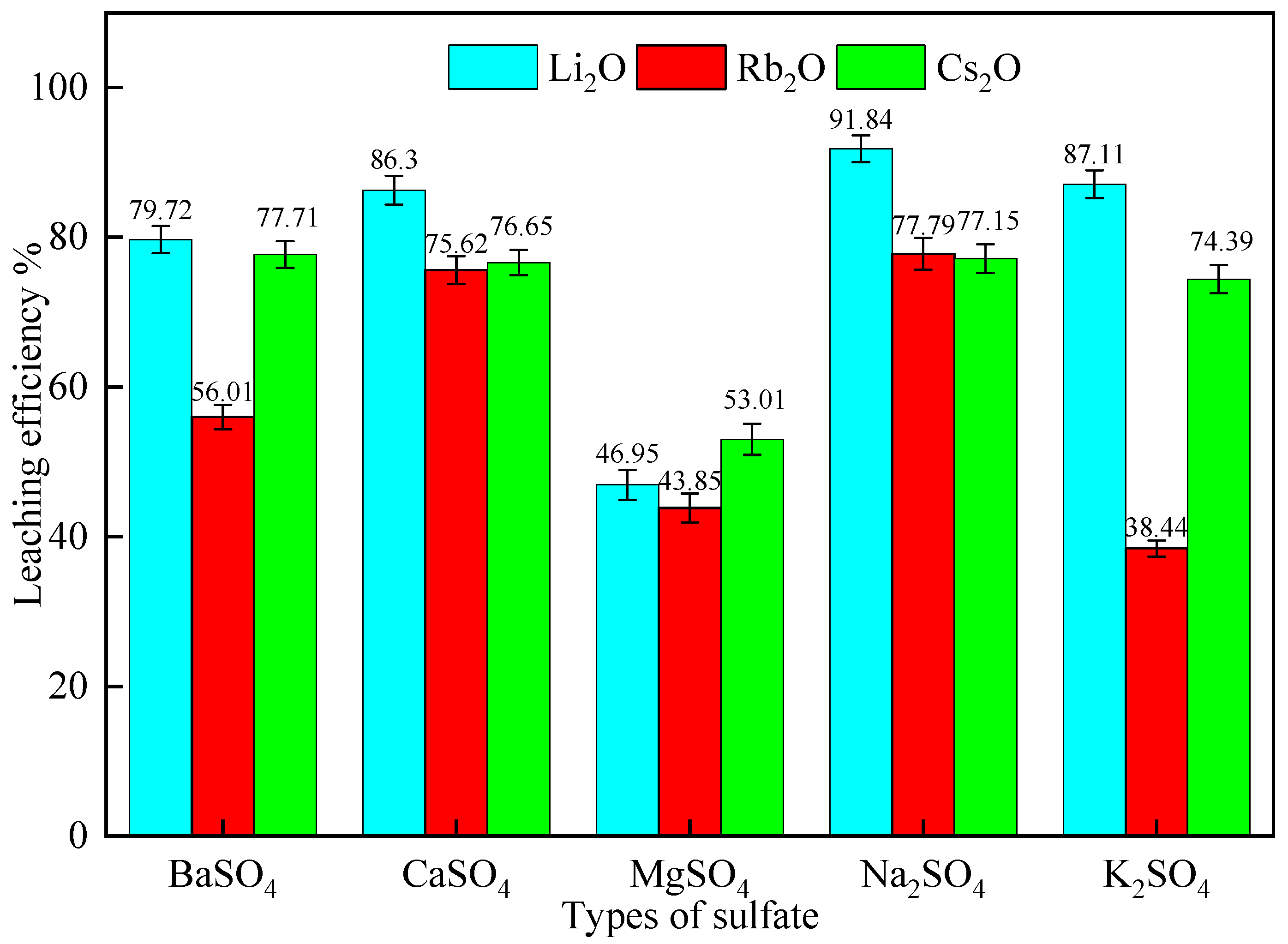
2.2.1. Effect of Different Sulfates
2.2.2. Effect of Na2SO4 Dosage
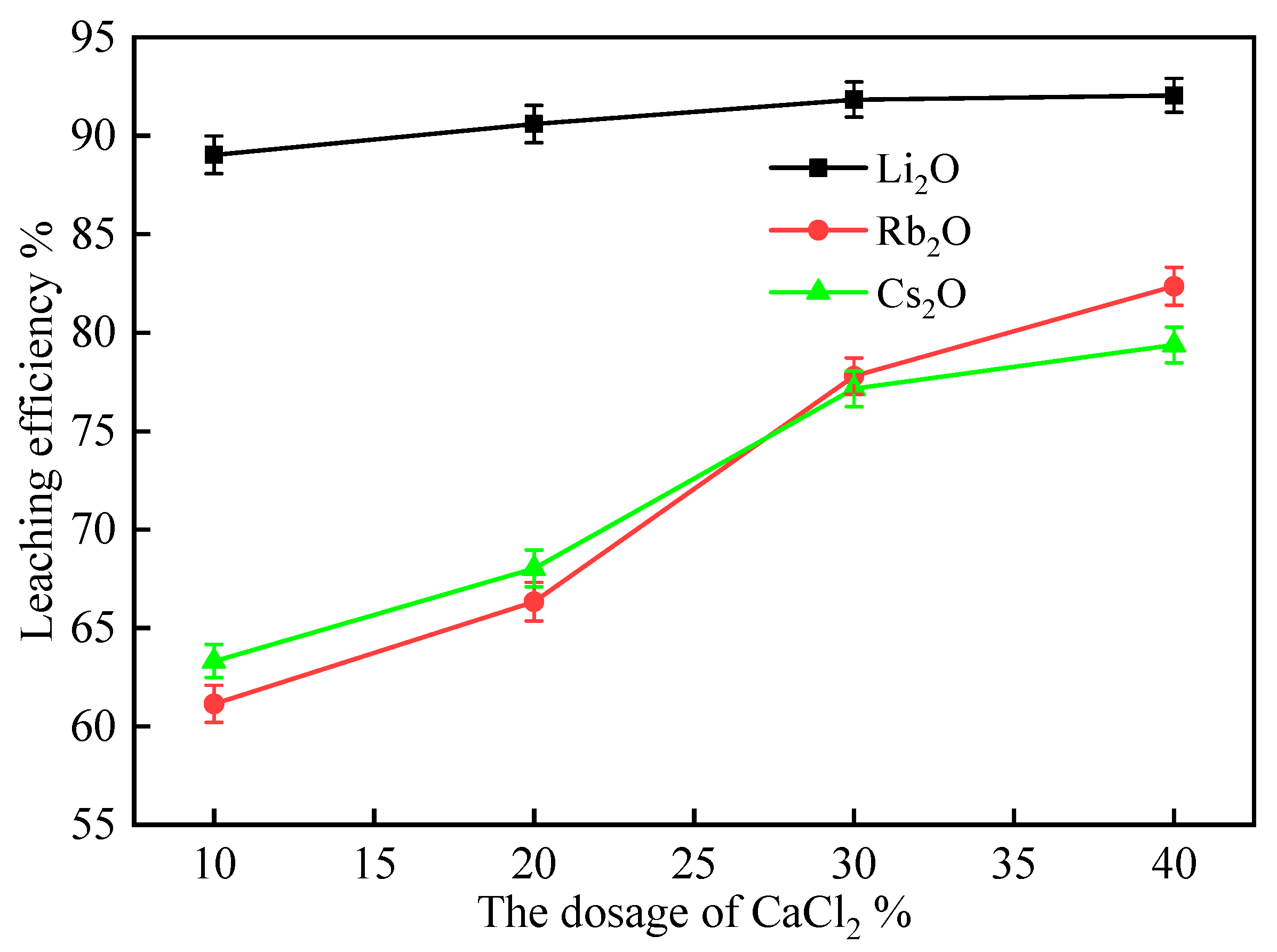
2.2.3. Effect of CaCl2 Dosage
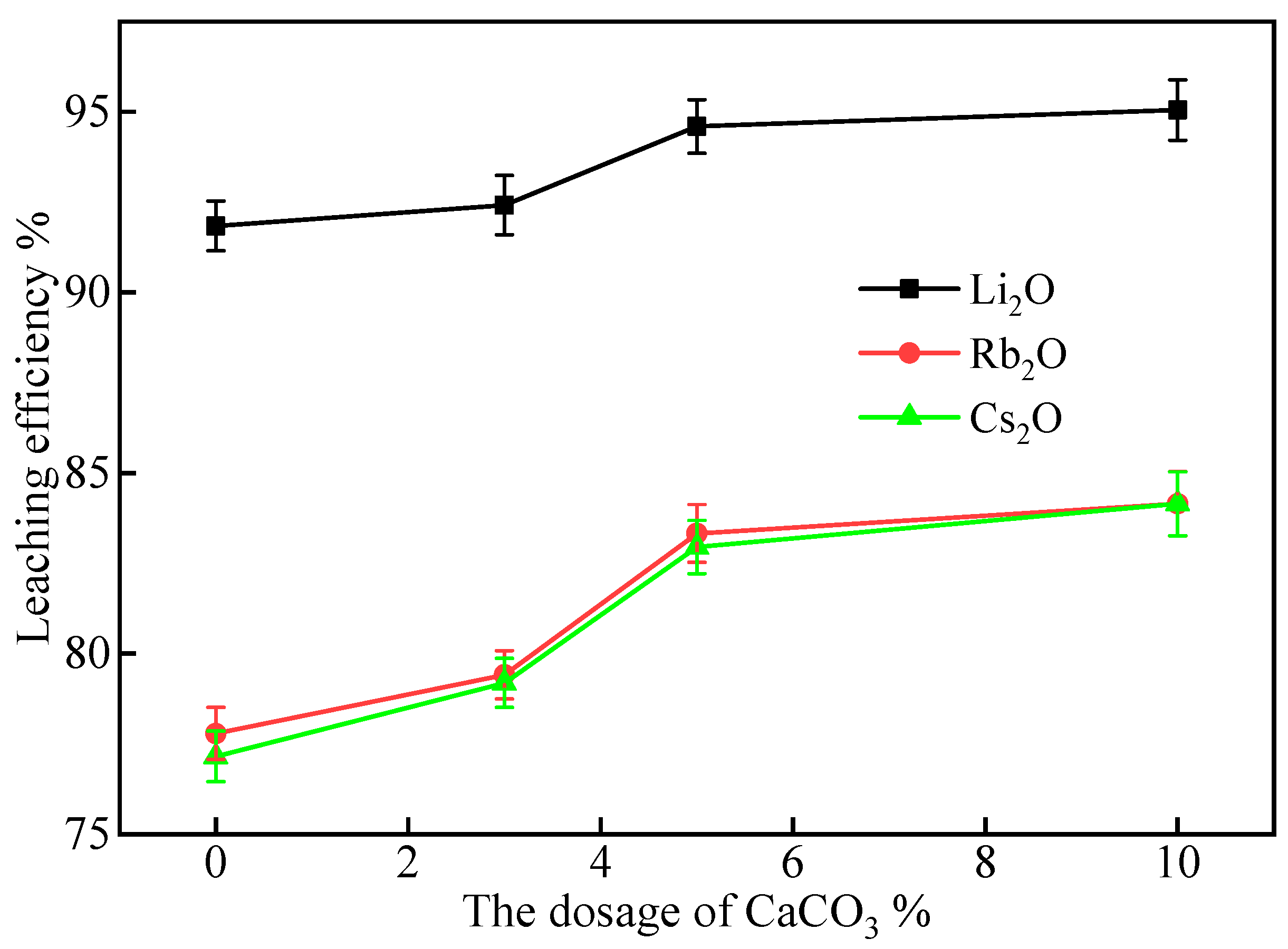
2.2.4. Effect of CaCO3 Addition
2.2.5. Effect of Complex Salt Replacement
2.3. Optimization of Leaching Conditions
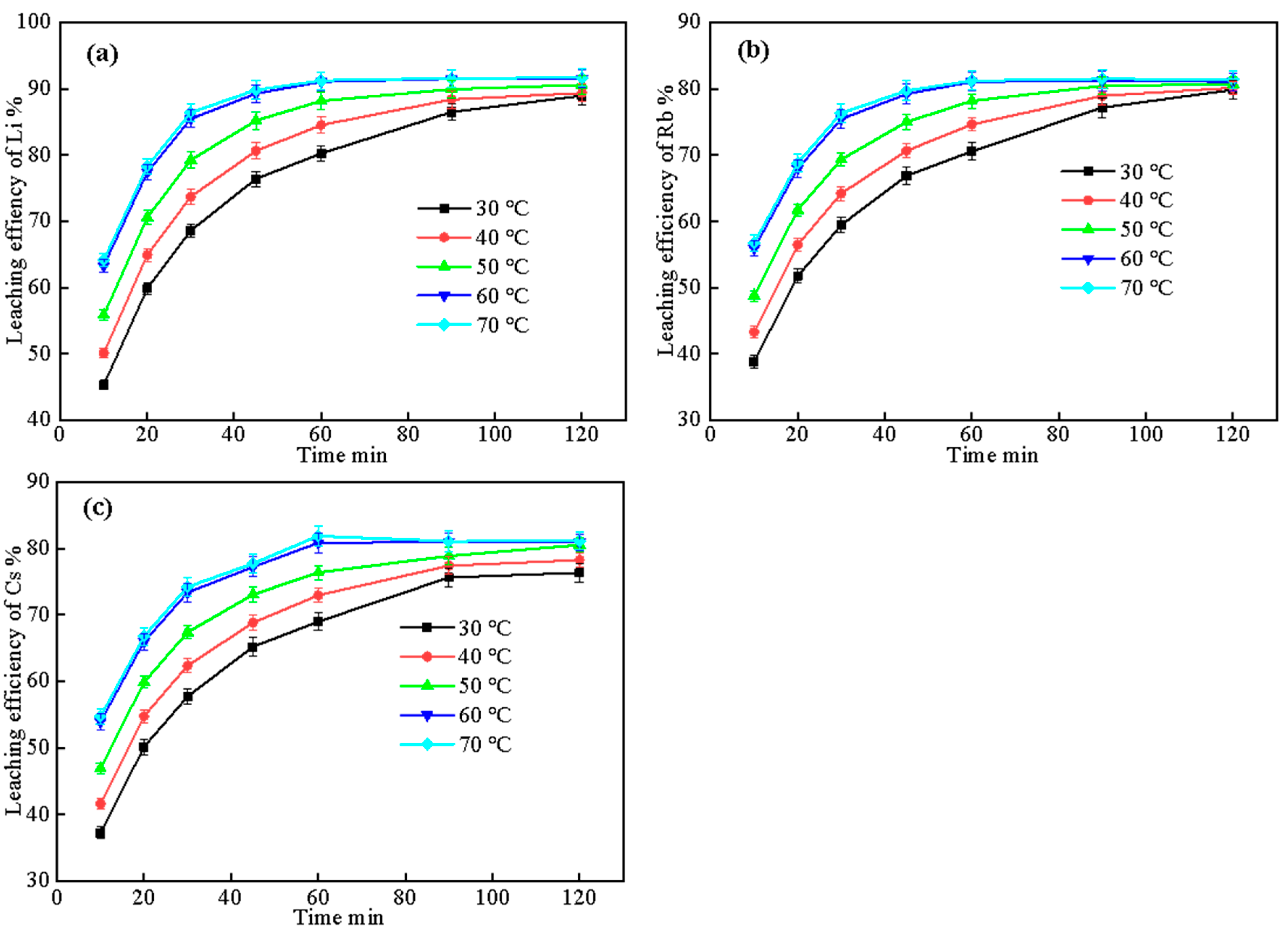
2.3.1. Effect of Leaching Temperature and Time
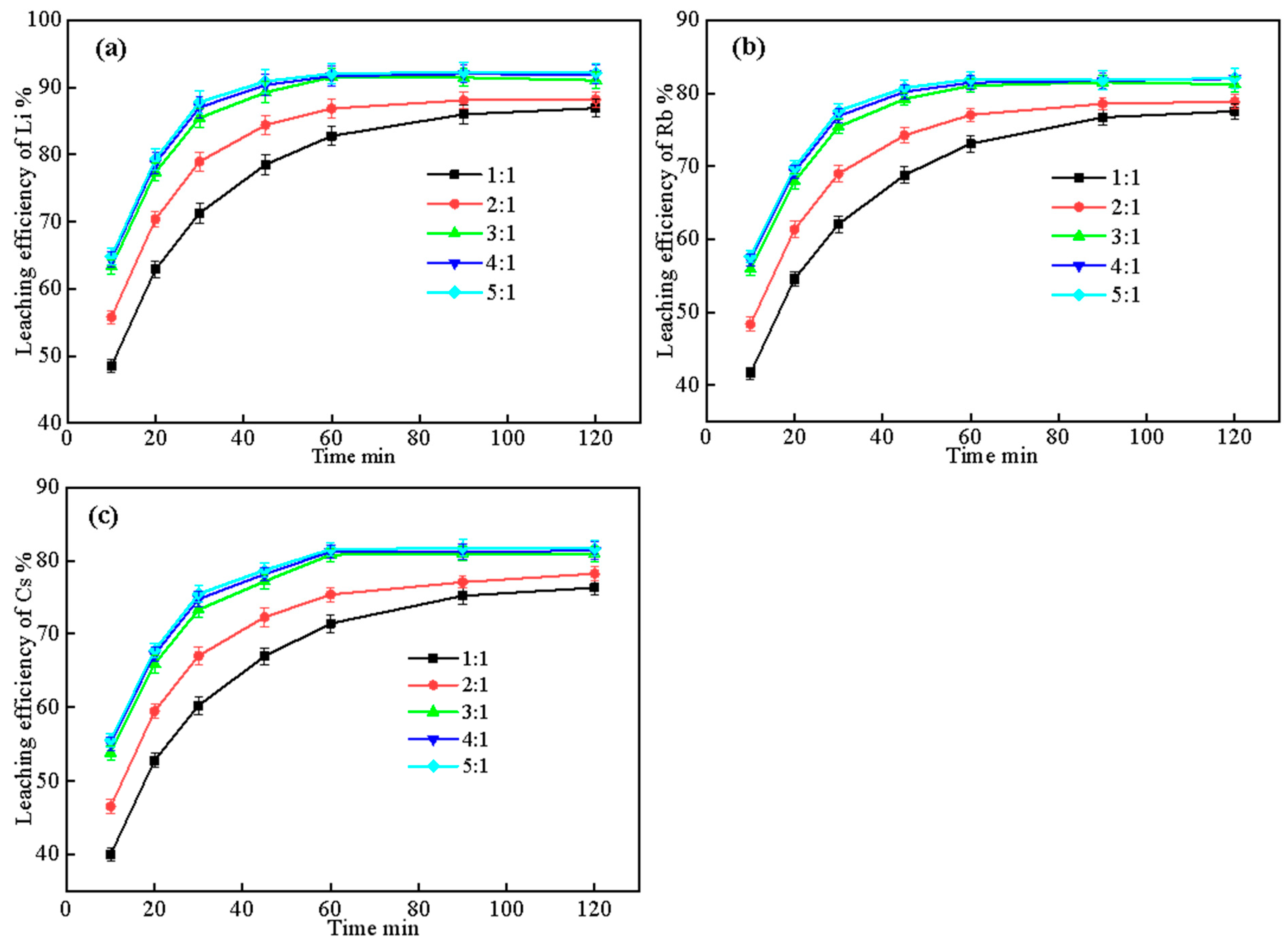
2.3.2. Effect of Liquid–Solid Ratio and Leaching Time
2.4. Characterization of Lepidolite Concentrate, Roasted Product, and Leaching Residue
2.4.1. XRD Analysis
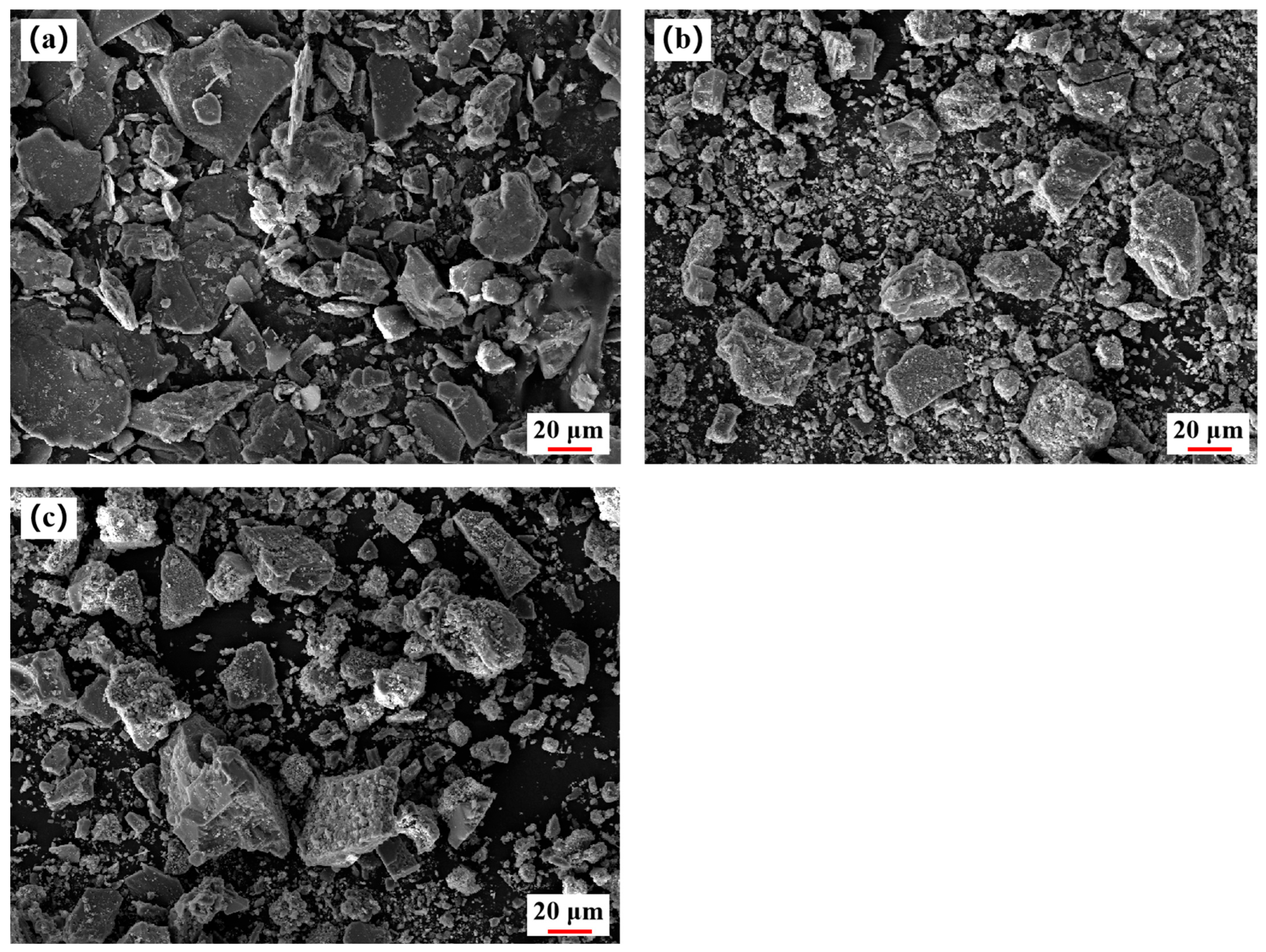
2.4.2. SEM Analysis
3. Materials and Methods
3.1. Materials
3.2. Experimental Methods
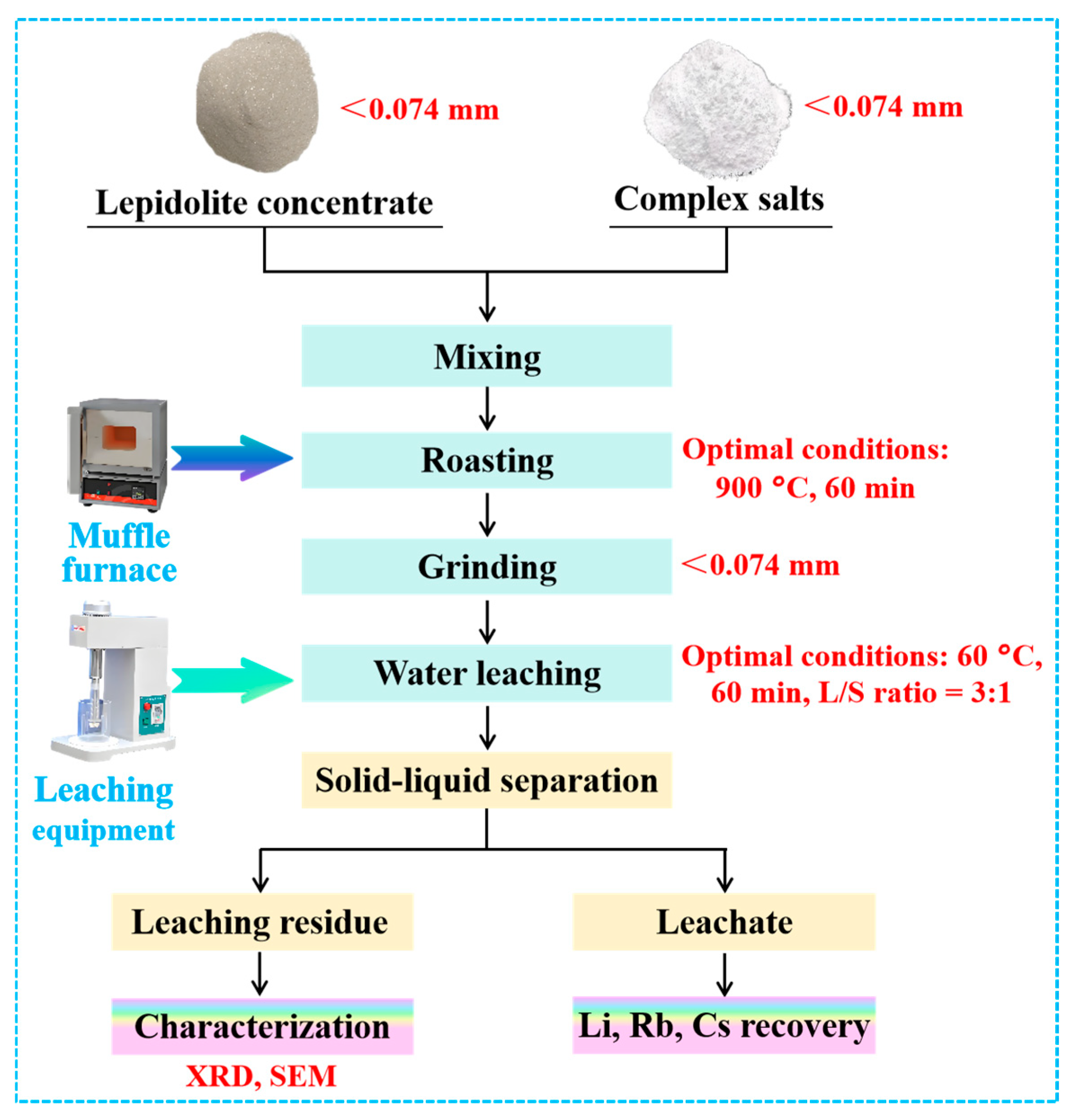
3.2.1. Complex Salt Roasting
3.2.2. Water Leaching
3.2.3. Characterization Techniques
3.2.4. Calculation of Leaching Efficiency
3.3. Experimental Flowchart
4. Conclusions
- (1)
- The optimal roasting conditions for the complex salt roasting–water leaching process were determined to be 900 °C for 60 min, with a complex salt composition of Lepidolite:Na2SO4:CaCl2:CaCO3 = 1:0.5:0.3:0.05. The optimal leaching conditions were found to be 60 °C, 60 min, and a liquid–solid ratio of 3:1. Under these conditions, the highest leaching efficiencies of 94.60%, 83.33%, and 82.95% were achieved for Li2O, Rb2O, and Cs2O, respectively.
- (2)
- The addition of Na2SO4, CaCl2, and CaCO3 to the complex salt mixture significantly enhanced the leaching efficiencies of Li, Rb, and Cs. The optimal dosages of Na2SO4, CaCl2, and CaCO3 were determined to be 50%, 30%, and 5%, respectively. These additives promoted the formation of water-soluble phases and stable compounds during roasting, facilitating the release and extraction of Li+, Rb+, and Cs+ ions from the lepidolite structure.
- (3)
- The XRD and SEM characterization results confirmed the decomposition of lepidolite and the formation of water-soluble phases during roasting, as well as the selective separation of Li from insoluble phases during leaching. The morphological changes observed by SEM revealed the development of a porous structure in the roasted product, which facilitated the dissolution of Li, Rb, and Cs during the leaching process.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Onwe, C.A.; Zangiabadi, M.; Wade, N.; Feeney, M.; Pickert, V.; Chilvers, I.; Jakeman, N. Smart Hubs 1—A Demonstration of Electric Vehicle’s Lithium-Ion Battery Charging Characteristic and Efficient Demand Side Energy Management with Solar Photovoltaic System. In Proceedings of the 2021 12th International Renewable Engineering Conference (IREC), Amman, Jordan, 14 April 2021; IEEE: Piscataway, NJ, USA, 2021; pp. 1–6. [Google Scholar]
- Pražanová, A.; Knap, V.; Stroe, D.-I. Literature Review, Recycling of Lithium-Ion Batteries from Electric Vehicles, Part II: Environmental and Economic Perspective. Energies 2022, 15, 7356. [Google Scholar] [CrossRef]
- Martí-Florences, M.; Cecilia, A.; Costa-Castelló, R. Modelling and Estimation in Lithium-Ion Batteries: A Literature Review. Energies 2023, 16, 6846. [Google Scholar] [CrossRef]
- Zhang, W.; Zhong, M.; Han, J.; Sun, Y.; Wang, Y. Research on the strategy of lithium-ion battery-supercapacitor hybrid energy storage to suppress power fluctuation of direct current microgrid. Int. J. Low-Carbon Technol. 2022, 17, 1012–1017. [Google Scholar] [CrossRef]
- Sankaran, G.; Venkatesan, S. An Overview of Lithium-Ion Batteries for Electric Mobility and Energy Storage Applications. IOP Conf. Ser.Earth Environ. Sci. 2022, 1042. [Google Scholar] [CrossRef]
- Nurpeissova, A.; Seipiyev, A. Preparation of Battery-Grade Lithium Composites from Local Spodumene. ECS Meet. Abstr. 2022, MA2022-02, 2589. [Google Scholar] [CrossRef]
- Djoudi, N.; Mostefa, M.L.P.; Muhr, H. Hydrometallurgical Process to Recover Cobalt from Spent Li-Ion Batteries. Resources 2021, 10, 58. [Google Scholar] [CrossRef]
- Zhang, Y.; Dong, Z.; Liu, S.; Yang, Q.; Jia, Y. Prediction of the Potential Trade Relationship of Lithium-Ion Battery’s Main Element Raw Material Minerals Combined with the Local Characteristics of the Trade Network. Int. J. Energy Res. 2023, 2023, 1–37. [Google Scholar] [CrossRef]
- Bhanu, P.; Mohan, T.V.K.; Amit, R.K.; Shankar, V. Factors affecting the market dynamics of lithium-ion battery for electric mobility: A system dynamics perspective. J. Simul. 2022, 18, 428–445. [Google Scholar] [CrossRef]
- Harun, I.; Bahrudin, F.I.; Daud, N.; Baba Zin, N.F.S.; Mat Yunus, N.F.; Mahat, M.M.; Shaffee, S.N.A. Opportunities and Challenges of Recycling and Reusing Lithium-Ion Batteries for Sustainable Mobility. IOP Conf. Ser. Earth Environ. Sci. 2023, 1281, 012009. [Google Scholar] [CrossRef]
- Habib, A.K.M.A.; Hasan, M.K.; Issa, G.F.; Singh, D.; Islam, S.; Ghazal, T.M. Lithium-Ion Battery Management System for Electric Vehicles: Constraints, Challenges, and Recommendations. Batteries 2023, 9, 152. [Google Scholar] [CrossRef]
- Raugei, M.; Peluso, A.; Leccisi, E.; Fthenakis, V. Life-Cycle Carbon Emissions and Energy Return on Investment for 80% Domestic Renewable Electricity with Battery Storage in California (U.S.A.). Energies 2020, 13, 3934. [Google Scholar] [CrossRef]
- Murphy, O.; Haji, M.N. A review of technologies for direct lithium extraction from low Li+ concentration aqueous solutions. Front. Chem. Eng. 2022, 4, 1008680. [Google Scholar] [CrossRef]
- Disu, B.; Rafati, R.; Haddad, A.S.; Fierus, N. Lithium Extraction from North Sea Oilfield Brines Using Ion Exchange Membranes. In Proceedings of the SPE Offshore Europe Conference & Exhibition, Aberdeen, UK, 5–8 September 2023. [Google Scholar]
- Zeng, Y.; Li, W.; Wan, Z.; Qin, S.; Huang, Q.; Cai, W.; Wang, Q.; Yao, M.; Zhang, Y. Electrochemically Mediated Lithium Extraction for Energy and Environmental Sustainability. Adv. Funct. Mater. 2024, 34, 2400416. [Google Scholar] [CrossRef]
- Park, T.; Shin, J.; Kim, S.; Ryu, T.; Kim, B.; Chang, H. An effective lithium extraction route from lepidolite. Hydrometallurgy 2023, 222, 106202. [Google Scholar] [CrossRef]
- Zhang, X.; Aldahri, T.; Tan, X.; Liu, W.; Zhang, L.; Tang, S. Efficient co-extraction of lithium, rubidium, cesium and potassium from lepidolite by process intensification of chlorination roasting. Chem. Eng. Process. Process. Intensif. 2020, 147, 107777. [Google Scholar] [CrossRef]
- Zhang, X.; Tan, X.; Li, C.; Yi, Y.; Liu, W.; Zhang, L. Energy-efficient and simultaneous extraction of lithium, rubidium and cesium from lepidolite concentrate via sulfuric acid baking and water leaching. Hydrometallurgy 2019, 185, 244–249. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, B.; Lv, Y.; Wang, C.; Chen, Y. Selective recovery and efficient separation of lithium, rubidium, and cesium from lepidolite ores. Sep. Purif. Technol. 2022, 288, 120667. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, B.; Lv, Y.; Wang, C.; Chen, Y. Thorough extraction of lithium and rubidium from lepidolite via thermal activation and acid leaching. Miner. Eng. 2022, 178, 107407. [Google Scholar] [CrossRef]
- Kong, X.Z.; Ye, H.; Qin, Y. Factors of Extracting Lithium from Lepidolite by Sulfate Roasting and Dilute Sulphuric Acid Leaching. Appl. Mech. Mater. 2014, 522-524, 1467–1470. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, B.; Lü, Y.; Wang, C.; Chen, Y. A review of lithium extraction from natural resources. Int. J. Miner. Met. Mater. 2022, 30, 209–224. [Google Scholar] [CrossRef]
- Barbosa, L.I.; González, J.A.; Ruiz, M.d.C. Extraction of lithium from β-spodumene using chlorination roasting with calcium chloride. Thermochim. Acta 2015, 605, 63–67. [Google Scholar] [CrossRef]
- Ertan, B. Chlorination roasting process for extraction of valuable metals in boron clays. Pamukkale Univ. J. Eng. Sci. 2020, 26, 1267–1272. [Google Scholar] [CrossRef]
- Su, H.; Ju, J.; Zhang, J.; Yi, A.; Lei, Z.; Wang, L.; Zhu, Z.; Qi, T. Lithium recovery from lepidolite roasted with potassium compounds. Miner. Eng. 2020, 145, 106087. [Google Scholar] [CrossRef]
- Üçerler, Z.; Güney, A. TheiInvestigation of lithium extraction by roasting with sulfating agent and water leaching from nepheline syenite rocks. Physicochem. Probl. Miner. Process. 2022, 58, 146154. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, B.; Lv, Y.; Wang, C.; Chen, Y. Thermodynamics analysis and response surface methodology to investigate decomposition behaviors for lepidolite sulfation products in presence of coal. Sci. Total. Environ. 2023, 888, 164089. [Google Scholar] [CrossRef]
- Plyushchev, V.E. On the Interaction of Spodumene and Alkaline Metal Sulphates. Doklady Akad. Nauk S.S.S.R. 1959, 124. [Google Scholar]
- He, F.; Chen, Y.; Zhao, B.; Chen, C.; Huang, S.; Peng, S. H2 Reduction of Na2SO4 to Na2S Based on Dilute-Phase Fluidization. Processes 2024, 12, 776. [Google Scholar] [CrossRef]
- González, Y.C.; Alcaraz, L.; Alguacil, F.J.; González, J.; Barbosa, L.; López, F.A. Study of the Carbochlorination Process with CaCl2 and Water Leaching for the Extraction of Lithium from Spent Lithium-Ion Batteries. Batteries 2022, 9, 12. [Google Scholar] [CrossRef]
- Shen, X.-Y.; Shao, H.-M.; Ding, J.-W.; Liu, Y.; Gu, H.-M.; Zhai, Y.-C. Zinc extraction from zinc oxidized ore using (NH4)2SO4 roasting-leaching process. Int. J. Miner. Met. Mater. 2020, 27, 1471–1481. [Google Scholar] [CrossRef]
- Alkan, G.; Yagmurlu, B.; Cakmakoglu, S.; Hertel, T.; Kaya, Ş.; Gronen, L.; Stopic, S.; Friedrich, B. Novel Approach for Enhanced Scandium and Titanium Leaching Efficiency from Bauxite Residue with Suppressed Silica Gel Formation. Sci. Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, H.; Sun, M.; Zhang, Y.; Meng, X.; Zhang, L.; Lv, X.; Davaasambuu, S.; Qiu, G. Scandium extraction from silicates by hydrometallurgical process at normal pressure and temperature. J. Mater. Res. Technol. 2020, 9, 709–717. [Google Scholar] [CrossRef]
- Chang, J.; Pan, A.; Ma, Y.; Sun, Y.; Hu, S. Behaviors of Silicon, Aluminum and Iron and Kinetics of Silicon from the Roasted Clinker of Silver Tailings in Water–Acid Leaching Process. Minerals 2023, 13, 105. [Google Scholar] [CrossRef]
- Su, A.; Yang, J.; Bai, K.; Tang, C.; Zeng, W. Efficient Extraction of Lithium and Rubidium from Lepidolite by Medium-Temperature Chlorination Roasting-Water Leaching Process. JOM 2024, 77, 3167–3176. [Google Scholar] [CrossRef]
- Mulwanda, J.; Senanayake, G.; Oskierski, H.C.; Altarawneh, M.; Dlugogorski, B.Z. Extraction of lithium from lepidolite by sodium bisulphate roasting, water leaching and precipitation as lithium phosphate from purified leach liquors. Hydrometallurgy 2023, 222, 106139. [Google Scholar] [CrossRef]
- Yan, Q.; Li, X.; Wang, Z.; Wu, X.; Wang, J.; Guo, H.; Hu, Q.; Peng, W. Extraction of lithium from lepidolite by sulfation roasting and water leaching. Int. J. Miner. Process. 2012, 110-111, 1–5. [Google Scholar] [CrossRef]
- Vieceli, N.; Nogueira, C.A.; Pereira, M.F.C.; Durão, F.O.; Guimarães, C.; Margarido, F. Optimization of Lithium Extraction from Lepidolite by Roasting Using Sodium and Calcium Sulfates. Miner. Process. Extr. Met. Rev. 2016, 38, 62–72. [Google Scholar] [CrossRef]


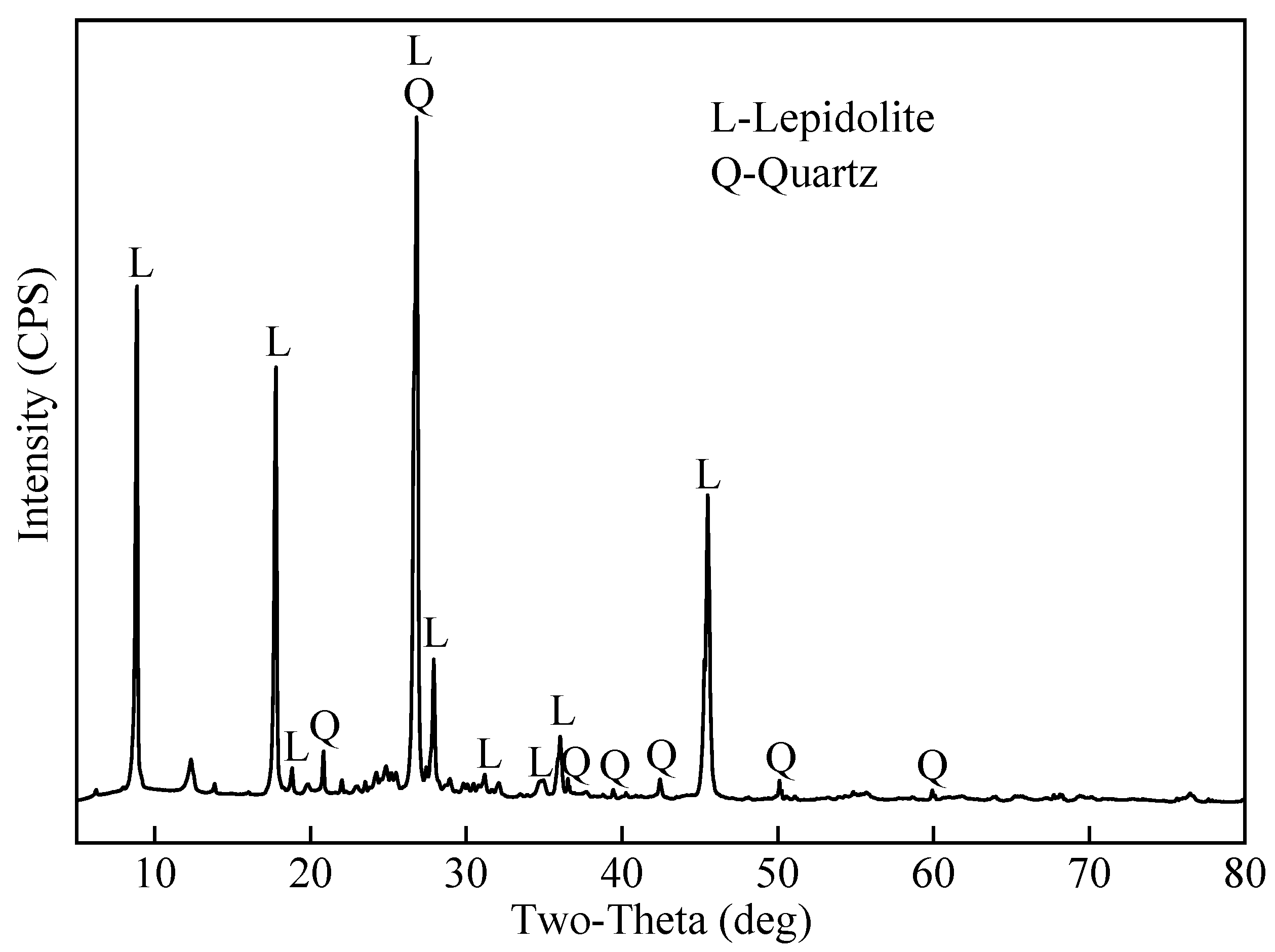
| Roasting Temperature and Time | Composition (Mass Ratio) | Leaching Efficiency % | ||
|---|---|---|---|---|
| Li2O | Rb2O | Cs2O | ||
| 900 °C, 60 min | Lepidolite:Na2SO4:CaCl2: CaCO3 = 1:0.5:0.3:0.05 | 94.60 | 83.33 | 82.95 |
| Lepidolite:Na2SO4:NaCl:CaCO3 = 1:0.5:0.3:0.05 | 82.46 | 67.25 | 65.39 | |
| Lepidolite:CaSO4:NaCl:CaCO3 = 1:0.5:0.3:0.05 | 87.71 | 73.95 | 72.54 | |
| Lepidolite:Na2SO4:CaSO4:NaCl:CaCO3 = 1:0.2:0.3:0.3:0.05 | 84.26 | 70.24 | 68.25 | |
| Lepidolite:Na2SO4:CaSO4:CaCl2:NaCl:CaCO3 = 1:0.2:0.2:0.1:0.2:0.05 | 90.25 | 78.68 | 77.31 | |
| Lepidolite:Na2SO4:CaSO4:CaCl2:NaCl:CaCO3 = 1:0.2:0.2:0.1:0.1:0.05 | 87.81 | 74.52 | 72.87 | |
| Lepidolite:Na2SO4:CaSO4:CaCl2:NaCl:CaCO3 = 1:0.2:0.1:0.1:0.2:0.05 | 86.74 | 71.58 | 70.16 | |
| Li2O | Al2O3 | SiO2 | Fe2O3 | Na2O | K2O | CaO | MgO | MnO | Rb2O | Cs2O | F | P2O5 | BeO | Loss |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3.92 | 23.73 | 54.33 | 0.31 | 1.80 | 8.66 | 0.18 | 0.24 | 0.25 | 1.29 | 0.34 | 5.60 | 0.40 | 0.09 | 3.14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, J.; Liang, B.; Luo, X.; Yuan, W.; Xiao, B.; Tang, X. Systematic Optimization of Complex Salt Roasting and Leaching Conditions for Efficient Extraction of Lithium, Rubidium and Cesium from Lepidolite. Molecules 2025, 30, 2244. https://doi.org/10.3390/molecules30102244
Gu J, Liang B, Luo X, Yuan W, Xiao B, Tang X. Systematic Optimization of Complex Salt Roasting and Leaching Conditions for Efficient Extraction of Lithium, Rubidium and Cesium from Lepidolite. Molecules. 2025; 30(10):2244. https://doi.org/10.3390/molecules30102244
Chicago/Turabian StyleGu, Jihan, Binjun Liang, Xianping Luo, Weiquan Yuan, Bin Xiao, and Xuekun Tang. 2025. "Systematic Optimization of Complex Salt Roasting and Leaching Conditions for Efficient Extraction of Lithium, Rubidium and Cesium from Lepidolite" Molecules 30, no. 10: 2244. https://doi.org/10.3390/molecules30102244
APA StyleGu, J., Liang, B., Luo, X., Yuan, W., Xiao, B., & Tang, X. (2025). Systematic Optimization of Complex Salt Roasting and Leaching Conditions for Efficient Extraction of Lithium, Rubidium and Cesium from Lepidolite. Molecules, 30(10), 2244. https://doi.org/10.3390/molecules30102244







