Biological Activity Evaluation Against Fusarium oxysporum, Fusarium circinatum, and Meloidogyne incognita of Bioactives-Enriched Extracts of Ruta graveolens L.
Abstract
1. Introduction
2. Results
2.1. Biological Activity of Enriched Extracts of Ruta graveolens L.
2.1.1. Selection of the Enriched Extracts for the Biological Activity Analysis
2.1.2. Antifungal Activity Against Fusarium oxysporum and Fusarium circinatum
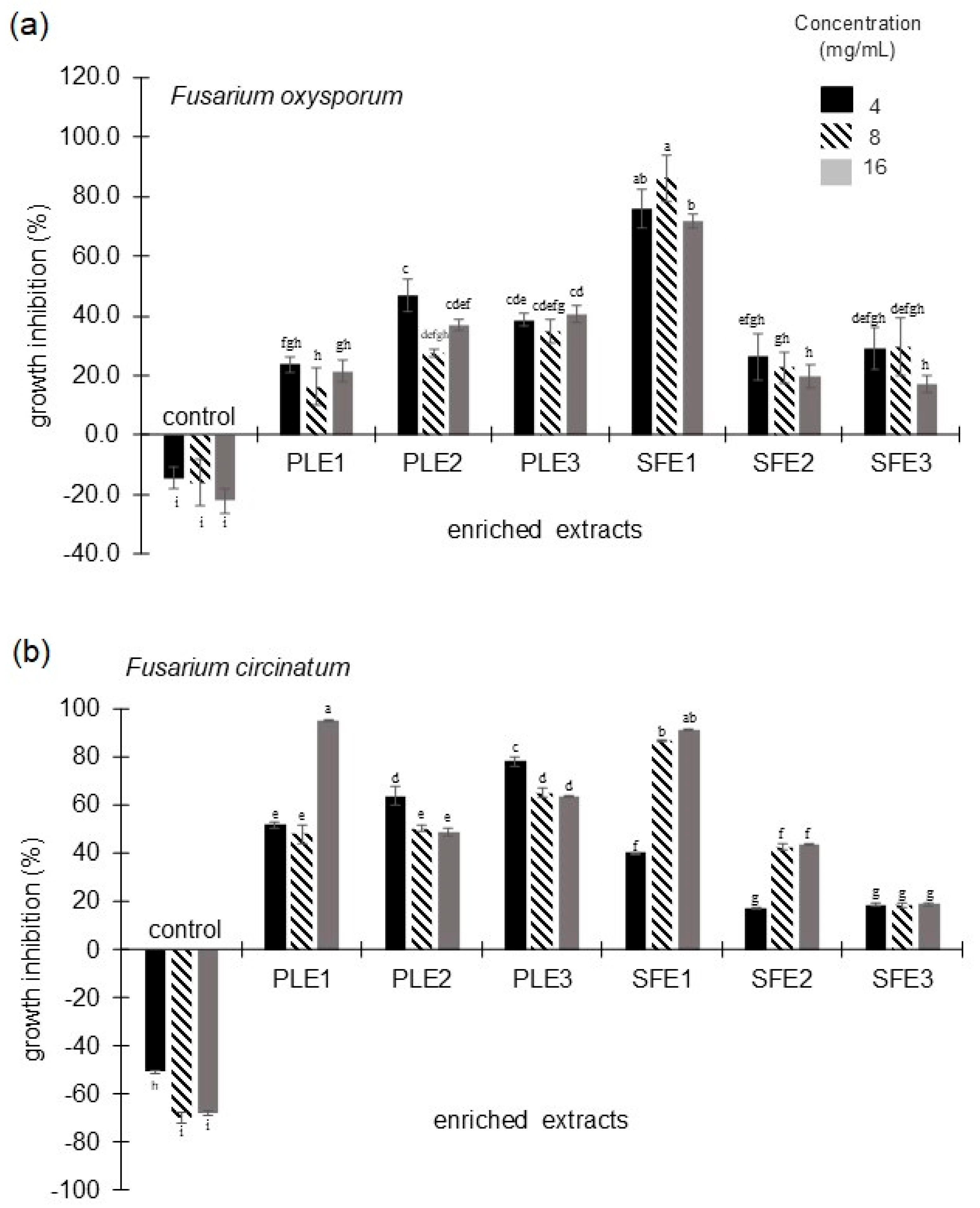
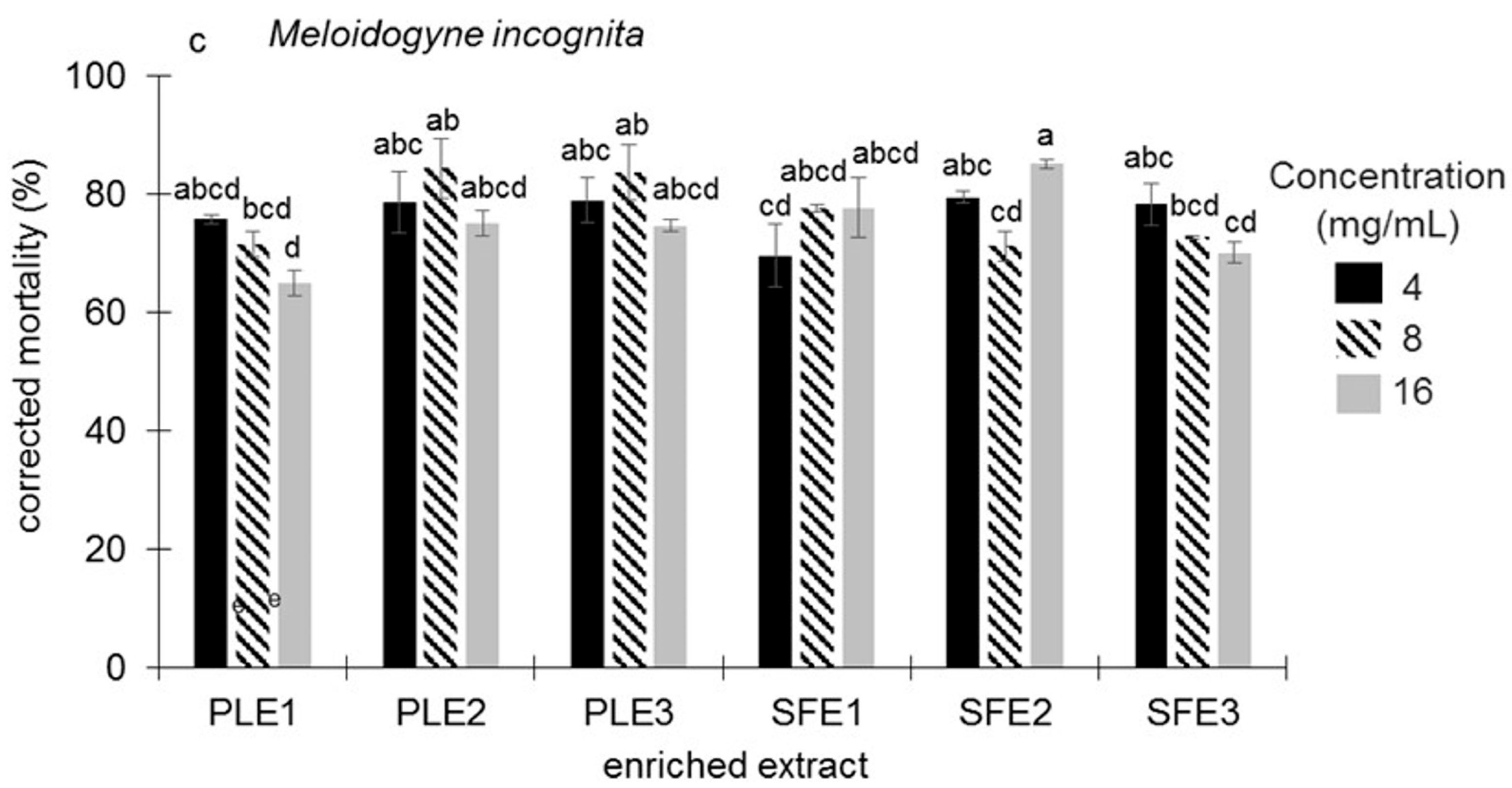
2.1.3. Nematicidal Activity Against Meloidogyne incognita
2.1.4. Biological Combined Action
2.2. Relationship Between Chemical Composition of the Selected Extracts and Biological Activity
3. Discussion
4. Materials and Methods
4.1. Samples of Ruta graveolens L.
4.2. Pressurized Liquid Extraction
- PLE1 extract was obtained from the aerial part using D-limonene-ethyl acetate (50:50, v/v) as solvent at 40 °C;
- PLE2 was obtained from the aerial part using ethyl acetate (100%) as solvent at 105 °C;
- PLE3 was obtained from roots using ethyl acetate (100%) as solvent at 105 °C.
4.3. Supercritical Fluid Extraction
- SFE1 extract was obtained from the dry aerial parts extraction during the first 30 min at 200 bar;
- SFE2 was obtained from the moistened aerial parts sample during the last 60 min of the process at 350 bar;
- SFE3 was also obtained from the moistened sample at 350 bar, but this time only during the first 15 min from a sample of rue roots.
4.4. Chromatography—Mass Spectrometry Analysis (GC–q-TOF-MS)
4.5. Evaluation of Biological Activity Against Phytopathogenic Microorganisms
4.5.1. Antifungal Activity Against Fusarium oxysporum and Fusarium circinatum
4.5.2. Nematicidal Activity Against Meloidogyne incognita
4.5.3. Theoretical Activity Factor and Theoretical Combined Action
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Al | Alkaloids |
| Am | Amides |
| ANOVA | ANalysis Of VAriance |
| CM | Corrected Mortality |
| C | Coumarins |
| FA | Fatty Acids |
| FC | FuranoCoumarins |
| GC–q-TOF-MS | Gas Chromatography—Mass Spectrometry analysis |
| GI | Growth Inhibition |
| J2 | Second-Stage Juveniles |
| K | Ketones |
| O | Other compounds |
| Pc | Phenolic compounds |
| PLE | Pressurized Liquid Extraction |
| PCA | Principal Component Analysis |
| PC | Principal Component |
| scCO2 | Supercritical CO2 |
| SFE | Supercritical Fluid Extraction |
| T | Terpenes |
References
- Sawicka, B.; Egbuna, C.; Nayak, A.K.; Kala, S. Chapter 2—Plant Diseases, Pathogens and Diagnosis. In Natural Remedies for Pest, Disease and Weed Control; Egbuna, C., Sawicka, B., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 17–28. [Google Scholar]
- Munkvold, G.P. Fusarium Species and Their Associated Mycotoxins. In Mycotoxigenic Fungi: Methods and Protocols; Moretti, A., Susca, A., Eds.; Methods in Molecular Biology; Humana Press: New York, NY, USA, 2017; Volume 1542, pp. 51–106. [Google Scholar]
- Joshi, R. A review of Fusarium oxysporum on its plant interaction and industrial use. J. Med. Plants Stud. 2018, 6, 112–115. [Google Scholar] [CrossRef]
- Gordon, T.R. Fusarium oxysporum and the Fusarium Wilt Syndrome. Annu. Rev. Phytopathol. 2017, 55, 23–39. [Google Scholar] [CrossRef] [PubMed]
- Di, X.; Takken, F.L.W.; Tintor, N. How Phytohormones Shape Interactions between Plants and the Soil-Borne Fungus Fusarium oxysporum. Front. Plant Sci. 2016, 7, 170. [Google Scholar] [CrossRef]
- Edel-Hermann, V.; Lecomte, C. Current Status of Fusarium oxysporum Formae Speciales and Races. Phytopathology 2019, 109, 512–530. [Google Scholar] [CrossRef]
- Siddique, S.; Radakovic, Z.S.; Hiltl, C.; Pellegrin, C.; Baum, T.J.; Beasley, H.; Bent, A.F.; Chitambo, O.; Chopra, D.; Danchin, E.G.J.; et al. The genome and lifestage-specific transcriptomes of a plant-parasitic nematode and its host reveal susceptibility genes involved in trans-kingdom synthesis of vitamin B5. Nat. Commun. 2022, 13, 6190. [Google Scholar] [CrossRef]
- Savary, S.; Willocquet, L.; Pethybridge, S.J.; Esker, P.; McRoberts, N.; Nelson, A. The global burden of pathogens and pests on major food crops. Nat. Ecol. Evol. 2019, 3, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Hallmann, J.; Meressa, B.H. Nematode parasites of vegetables. In Plant Parasitic Nematodes in Subtropical and Tropical Agriculture; Sikora, R.A., Coyne, D., Hallmann, J., Timper, P., Eds.; CABI: Cambridge, UK, 2018; pp. 346–410. [Google Scholar]
- Abubakar, Y.; Tijjani, H.; Egbuna, C.; Adetunji, C.O.; Kala, S.; Kryeziu, T.L.; Ifemeje, J.C.; Patrick-Iwuanyanwu, K.C. Pesticides, history, and classification. In Natural Remedies for Pest, Disease and Weed Control; Egbuna, C., Sawicka, B., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 29–42. [Google Scholar]
- Malik, S.; Coutinho Moraes, D.F.; Mendonca do Amaral, F.M.; Sousa Ribeiro, M.N. Ruta graveolens: Phytochemistry, Pharmacology, and Biotechnology. In Transgenesis and Secondary Metabolism; Jha, S., Ed.; Reference Series in Phytochemistry; Springer Nature: Gewerbestrasse, Switzerland, 2017; pp. 177–204. [Google Scholar]
- Reyes-Vaquero, L.; Bueno, M.; Ventura-Aguilar, R.I.; Aguilar-Guadarrama, A.B.; Robledo, N.; Sepulveda-Jimenez, G.; Vanegas-Espinoza, P.E.; Ibáñez, E.; Del Villar-Martinez, A.A. Seasonal variation of chemical profile of Ruta graveolens extracts and biological activity against Fusarium oxysporum, Fusarium proliferatum and Stemphylium vesicarium. Biochem. Syst. Ecol. 2021, 95, 104223. [Google Scholar] [CrossRef]
- Stashenko, E.E.; Acosta, R.; Martinez, J.R. High-resolution gas-chromatographic analysis of the secondary metabolites obtained by subcritical-fluid extraction from Colombian rue (Ruta graveolens L.). J. Biochem. Bioph. Meth. 2000, 43, 379–390. [Google Scholar] [CrossRef]
- da Silva, F.G.E.; Mendes, F.R.d.S.; Assuncao, J.C.d.C.; Pinheiro Santiago, G.M.; Xavier Bezerra, M.A.; Barbosa, F.G.; Mafezoli, J.; Rocha, R.R. Seasonal variation, larvicidal and nematicidal activities of the lef essential oil of Ruta graveolens L. J. Essent. Oil Res. 2014, 26, 204–209. [Google Scholar] [CrossRef]
- Faria, J.M.S.; Barbosa, P.; Bennett, R.N.; Mota, M.; Cristina Figueiredo, A. Bioactivity against Bursaphelenchus xylophilus: Nematotoxics from essential oils, essential oils fractions and decoction waters. Phytochemistry 2013, 94, 220–228. [Google Scholar] [CrossRef]
- Meepagala, K.A.; Schrader, K.K.; Wedge, D.E.; Duke, S.O. Algicidal and antifungal compounds from the roots of Ruta graveolens and synthesis of their analogs. Phytochemistry 2005, 66, 2689–2695. [Google Scholar] [CrossRef] [PubMed]
- Oliva, A.; Meepagala, K.M.; Wedge, D.E.; Harries, D.; Hale, A.L.; Aliotta, G.; Duke, S.O. Natural fungicides from Ruta graveolens L. leaves, including a new quinolone alkaloid. J. Agr. Food Chem. 2003, 51, 890–896. [Google Scholar] [CrossRef]
- Reis, K.B.; de Carvalho Barros Cortes, M.V.; Martins, F.S.; Corsi de Filippi, M.C.; de Paula, J.R.; da Conceicao, E.C. Characterization of rue extract and its potential for controlling rice blast. Pesqui. Agropecu. Bras. 2015, 50, 1121–1130. [Google Scholar] [CrossRef]
- Herrero, M.; Sanchez-Camargo, A.d.P.; Cifuentes, A.; Ibáñez, E. Plants, seaweeds, microalgae and food by-products as natural sources of functional ingredients obtained using pressurized liquid extraction and supercritical fluid extraction. TrAC-Trends Anal. Chem. 2015, 71, 26–38. [Google Scholar] [CrossRef]
- Bueno, M. Compressed fluids for food by-products biorefinery. In Advanced Nanotechnology and Application of Supercritical Fluids; Inamuddin, Asiri, A.M., Eds.; Springer Nature: Gewerbestrasse, Switzerland, 2020; pp. 219–238. [Google Scholar]
- Herrero, M.; Ibanez, E. Green processes and sustainability: An overview on the extraction of high added-value products from seaweeds and microalgae. J. Supercrit. Fluids 2015, 96, 211–216. [Google Scholar] [CrossRef]
- Jadeja, G.C.; Maheshwari, R.C.; Naik, S.N. Extraction of natural insecticide azadirachtin from neem (Azadirachta indica A. Juss) seed kernels using pressurized hot solvent. J. Supercrit. Fluids 2011, 56, 253–258. [Google Scholar] [CrossRef]
- Schoss, K.; Kocevar Glavac, N.; Dolenc Koce, J.; Anzlovar, S. Supercritical CO2 Plant Extracts Show Antifungal Activities against Crop-Borne Fungi. Molecules 2022, 27, 1132. [Google Scholar] [CrossRef]
- Wu, H.-b.; Guo, P.-x.; Ma, L.-h.; Li, X.-m.; Liu, T.-t. Nematicidal, antifungal and insecticidal activities of Artemisia halodendron extracts: New polyacetylenes involved. Ind. Crop Prod. 2021, 170, 113825. [Google Scholar] [CrossRef]
- Reyes-Vaquero, L.; Álvarez-Rivera, G.; Mendiola, J.A.; Del Villar-Martínez, A.A.; Ibáñez, E.; Bueno, M. Utilizing green solvents in compressed fluids technologies for extracting bioactive compounds from Ruta graveolens L. Ind. Crop Prod. 2024, 216, 118717. [Google Scholar] [CrossRef]
- Melough, M.M.; Cho, E.; Chun, O.K. Furocoumarins: A review of biochemical activities, dietary sources and intake, and potential health risks. Food Chem. Toxicol. 2018, 113, 99–107. [Google Scholar] [CrossRef]
- Liu, S.; Ruan, W.; Li, J.; Xu, H.; Wang, J.; Gao, Y.; Wang, J. Biological control of phytopathogenic fungi by fatty acids. Mycopathologia 2008, 166, 93–102. [Google Scholar] [CrossRef]
- Amaral, D.R.; Oliveira, D.F.; Campos, V.P.; Pantaleão, J.A.; de Carvalho, D.A.; Nunes, A.d.S. Effect of plant and fungous metabolites on Meloidogyne exigua. Cienci. Agrotec. 2009, 33, 1861–1865. [Google Scholar] [CrossRef]
- Contreras, L. Evaluación In Vitro de la Acción Nematicida de un Grupo de Plantas Seleccionadas Para Controlar Xiphinema spp. Bachelor’s Thesis, Universidad de Chile, Santiago, Chile, 2000. [Google Scholar]
- Insunza, V.; Aballay, E.; Macaya, J. Nematicidal activity of aqueus plant extracts on Xiphinema index. Nematol. Medit. 2001, 29, 35–40. [Google Scholar]
- Sasanelli, N.; D’Addabbo, T. Effect to Cineraria maritima, Ruta graveolens and Tagetes erecta leaf and root extracts on Italian population of Meloidogyne species. Nematol. Medit. 1993, 21, 49–51. [Google Scholar]
- Aballay, E.; Sepulveda, R.; Insunza, V. Evaluation of five nematode-antagonistic plants used as green manure to control Xiphinema index Thorne et Allen on Vitis vinifera L. Nematropica 2004, 34, 45–51. [Google Scholar]
- Caboni, P.; Saba, M.; Oplos, C.; Aissani, N.; Maxia, A.; Menkissoglu-Spiroudi, U.; Casu, L.; Ntalli, N. Nematicidal activity of furanocoumarins from parsley against Meloidogyne spp. Pest Manag. Sci. 2015, 71, 1099–1105. [Google Scholar] [CrossRef]
- Ntalli, N.G.; Ferrari, F.; Giannakou, I.; Menkissoglu-Spiroudi, U. Synergistic and antagonistic interactions of terpenes against Meloidogyne incognita and the nematicidal activity of essential oils from seven plants indigenous to Greece. Pest Manag. Sci. 2011, 67, 341–351. [Google Scholar] [CrossRef]
- Diwan, R.; Malpathak, N. Ruta graveolens cultures as screening resources for phyto-pharmaceuticals: Bio-prospecting, metabolic phenotyping and multivariate analysis. Bioremediation Biodivers. Bioavailab. 2011, 5, 1–9. [Google Scholar]
- Cantón, E.; Martín, E.; Espinel-Ingroff, A. Métodos estandarizados por el CLSI para el estudio de la sensibilidad a los antifúngicos (documentos M27-A3, M38-A y M44-A). In Guía Práctica de Identificación y Diagnóstico en Micología Clínica; Pemán, J., Martín-Mazuelos, E., Rubio, C.M.C., Eds.; Revista Iberoamericana de Micología: Bilbao, Spain, 2007. [Google Scholar]
- Pryor, S.W.; Gibson, D.M.; Krasnoff, S.B.; Walker, L.P. Identification of antifungal compounds in a biological control product using a microplate inhibition bioassay. Trans. Asabe 2006, 49, 1643–1649. [Google Scholar] [CrossRef]
- Ntalli, N.G.; Caboni, P. Botanical Nematicides: A Review. J. Agr. Food Chem. 2012, 60, 9929–9940. [Google Scholar] [CrossRef]

| Group of Metabolites | PLE1 | PLE2 | PLE3 | SFE1 | SFE2 | SFE3 |
|---|---|---|---|---|---|---|
| Alkaloids (Al) | 11.6 ± 0.7 D | 23.8 ± 1.5 C | 48.6 ± 3.2 A | 14.5 ± 3.8 D | 41.7 ± 3.7 AB | 40.4 ± 2.1 B |
| Coumarins (C) | - | - | 10.2 ± 0.5 A | - | - | 6.9 ± 0.2 B |
| Furanocoumarins (FC) | 21.9 ± 2.9 B | 34.7 ± 4.5 A | 16.6 ± 1.6 C | 23.5 ± 2.6 B | 41.5 ± 2.6 A | 23.5 ± 3.1 B |
| Terpenes (T) | 59.7 ± 3.8 A | 30.2 ± 1.9 B | 3.5 ± 2.0 C | 4.6 ± 0.6 C | 0.8 ± 0.2 D | 4.3 ± 0.7 C |
| Fatty acids (FA) | 4.2 ± 0.3 D | 6.5 ± 0.5 C | 0.03 ± 0.02 E | 52.6 ± 3.1 A | 7.6 ± 2.0 BC | 10.6 ± 1.5 B |
| Amides (Am) | 2.1 ± 1.2 B | 4.3 ± 3.1 AB | 9.2 ± 5.4 A | 1.3 ± 0.3 B | 1.6 ± 0.7 B | 2.0 ± 0.4 B |
| Ketones (K) | - | - | - | 0.2 ± 0.1 B | 0.1 ± 0.1 B | 1.7 ± 0.1 A |
| Phenolic compounds (Pc) | - | - | - | 0.2 ± 0.1 B | 0.4 ± 0.1 B | 5.8 ± 0.2 A |
| Others (O) | 0.5 ± 0.2 D | 0.6 ± 0.2 D | 11.8 ± 2.1 A | 3.2 ± 1.0 B | 6.4 ± 1.5 C | 4.7 ± 0.6 BC |
| RT (min) | Tentative Identification | Match Factor | Monoisotopic Mass | PLE1 | PLE2 | PLE3 | SFE1 | SFE2 | SFE3 |
|---|---|---|---|---|---|---|---|---|---|
| Alkaloids | |||||||||
| 21.36 | 3-Methyl-2-nonyl-1H-quinolin-4-one | 85 | 285.2093 | − | − | − | − | − | + |
| 22.16 | Dictamnine | 92 | 199.0633 | − | − | + | + | + | + |
| 23.60 | 2-Methyl-3-undecyl-1H-quinolin-4-one | 83 | 313.2406 | − | − | − | − | − | + |
| 28.29 | Fagarine | 84 | 229.0739 | + | + | + | + | + | − |
| 28.94 | Pteleine | 88 | 229.0739 | − | − | + | + | + | + |
| 32.75 | Skimmianine | 92 | 259.0845 | + | + | + | + | + | + |
| 34.34 | Kokusaginine | 89 | 259.0845 | + | + | + | + | + | + |
| 34.66 | 1-Hydroxy-10-methyl-9(10H)-acridinone | 90 | 225.0790 | − | − | + | − | − | + |
| 34.80 | 3-Methyl-2-undecyl-1H-quinolin-4-one | 86 | 313.2406 | − | − | + | − | − | + |
| 37.87 | 1-Hydroxy-3-methoxy-10-methyl-9(10H)-acridinone | 92 | 255.0895 | − | − | + | − | − | + |
| 40.03 | Furofoline I | 80 | 265.0739 | − | − | + | − | − | − |
| 40.17 | Arborinine | 90 | 285.1001 | + | + | − | + | + | − |
| 45.19 | 3-Methyl-2-pentyl-1H-quinolin-4-one | 85 | 229.1467 | − | − | + | − | − | + |
| 47.15 | Graveoline | 80 | 279.0895 | − | − | + | − | − | + |
| Furanocoumarins | |||||||||
| 19.63 | Psoralen | 92 | 186.0317 | + | + | + | + | + | + |
| 22.19 | 4-(1,1-Dimethylallyl)-9-methoxy-7H-furo[3,2-g][1]benzopyran-7-one | 85 | 284.1049 | − | − | − | − | − | + |
| 24.06 | Xanthotoxin | 90 | 216.0423 | − | − | + | − | −- | + |
| 25.67 | Bergapten | 90 | 216.0423 | + | + | + | + | + | + |
| 28.03 | Chalepensin | 80 | 254.0943 | + | + | + | + | + | + |
| 29.39 | Isopimpinellin | 91 | 246.0528 | − | − | + | + | + | + |
| 35.82 | Chalepin | 82 | 314.1518 | + | + | + | + | + | + |
| 37.80 | Rutamarin | 90 | 356.1624 | + | + | + | + | + | + |
| Terpenes | |||||||||
| 6.34 | Sabinene | 81 | 136.1252 | − | − | + | − | − | − |
| 6.40 | p-Cymene | 91 | 134.1096 | − | − | + | − | − | − |
| 6.76 | m-Cymenene | 97 | 132.0939 | + | + | + | − | − | − |
| 6.88 | p-Mentha-1,5,8-triene | 91 | 134.1096 | − | − | + | − | − | − |
| 7.22 | p-Mentha-2,8-dien-1-ol | 93 | 152.1201 | + | + | + | − | − | − |
| 7.24 | trans-Verbenol | 80 | 152.1201 | + | + | − | − | − | − |
| 7.28 | Citronellal | 86 | 154.1358 | − | − | + | − | − | − |
| 7.50 | p-Mentha-1(7),8-dien-2-ol | 84 | 152.1201 | − | − | + | − | − | − |
| 7.87 | trans-3-Caren-2-ol | 80 | 152.1201 | + | + | − | − | − | − |
| 7.92 | trans-3(10)-Caren-2-ol | 80 | 152.1201 | + | + | − | − | − | − |
| 8.04 | trans-Carveol | 93 | 152.1201 | + | + | + | − | − | − |
| 8.06 | trans-2-Caren-4-ol | 80 | 152.1201 | + | + | − | − | − | − |
| 8.07 | Carveol | 80 | 152.1201 | + | + | − | − | − | − |
| 8.15 | Pulegone | 80 | 152.1201 | + | + | − | − | − | − |
| 8.30 | Carvenone | 87 | 152.1201 | + | + | − | − | − | − |
| 8.49 | trans-Ascaridolglycol | 88 | 170.1307 | + | − | + | − | − | − |
| 9.98 | Isoascaridol | 80 | 168.1150 | + | + | + | − | − | − |
| 11.68 | α-Ionone | 80 | 192.1514 | − | − | + | − | − | − |
| 12.28 | Calarene | 81 | 204.1878 | − | − | − | + | + | − |
| 12.34 | trans-β-Ionone | 80 | 192.1514 | − | − | − | − | − | + |
| 14.48 | α-Eudesmol | 81 | 222.1984 | + | + | − | − | − | − |
| 22.48 | Limonen-6-ol, pivalate | 80 | 236.1776 | + | + | − | − | − | − |
| 26.40 | Phytol | 92 | 296.3079 | + | + | − | + | + | − |
| 43.13 | Tocopherol | 85 | 430.3811 | + | + | − | + | + | − |
| 45.29 | Campesterol | 88 | 400.3705 | + | + | − | + | + | + |
| 47.56 | γ-Sitosterol | 89 | 414.3862 | + | + | − | + | + | + |
| Fatty acids | − | ||||||||
| 9.37 | Nonanoic acid | 80 | 158.1307 | − | − | − | + | + | + |
| 10.76 | n-Decanoic acid | 85 | 172.1463 | − | − | − | + | + | − |
| 11.82 | 2,5-Octadecadiynoic acid, methyl ester | 82 | 290.2246 | − | − | − | − | − | + |
| 13.30 | Fumaric acid, ethyl 2-methylallyl ester | 82 | 198.0892 | − | − | − | + | + | − |
| 15.31 | Dodecanoic acid | 84 | 200.1776 | − | − | + | + | + | + |
| 18.44 | Myristic acid | 88 | 228.2089 | − | − | − | + | + | + |
| 21.94 | Palmitic acid, methyl ester | 80 | 270.2559 | − | − | − | + | + | − |
| 22.40 | Palmitic acid | 90 | 256.2402 | + | + | − | + | + | + |
| 23.94 | Palmitic acid, ethyl ester | 80 | 284.2715 | − | − | − | + | + | + |
| 25.51 | 8,11-Octadecadienoic acid, methyl ester | 83 | 294.2559 | − | − | − | + | + | − |
| 26.51 | Linoleic acid | 87 | 280.2402 | + | + | − | + | + | + |
| 27.18 | Oleic Acid | 89 | 282.2559 | − | − | − | + | + | − |
| 27.25 | Linolenic acid | 88 | 280.2402 | − | − | − | + | + | − |
| 27.87 | Stearic acid | 86 | 284.2715 | − | − | − | + | + | − |
| 28.38 | Linolenic acid, ethyl ester | 80 | 306.2559 | − | − | − | + | + | − |
| Coumarins | − | ||||||||
| 18.05 | 6,7,8-Trimethoxycoumarin | 85 | 236.0685 | − | − | − | − | − | + |
| 18.17 | 7-Methoxycoumarin | 89 | 176.0473 | − | − | − | + | + | − |
| 23.89 | 5,7-Dimethoxycoumarin | 87 | 206.0579 | − | − | − | − | − | + |
| 25.32 | Seselin | 80 | 228.0786 | − | − | + | − | − | − |
| 27.05 | Ostol | 87 | 244.1099 | − | − | + | − | − | + |
| 31.34 | 5-Hydroxy-7-methoxy-2-methyl-6-(3-methyl-2-butenyl)- chromone | 83 | 274.1205 | − | − | − | − | − | + |
| 32.46 | 3-(1,1-dimethylallyl) scopoletin | 87 | 260.1049 | − | − | + | − | − | + |
| Phenolic compounds | |||||||||
| 8.18 | 1,3-bis(1,1-dimethylethyl)-benzene | 80 | 190.1722 | − | − | − | − | − | + |
| 11.46 | 4-Tert-butyl-2-(2-methylbutan-2-yl)phenol | 83 | 220.1827 | − | − | + | − | − | − |
| 14.55 | 2,4-Di-tert-butylphenol | 83 | 206.1671 | − | − | − | + | + | + |
| 18.26 | Turmeronol A | 80 | 232.1463 | + | + | − | + | + | + |
| 18.33 | €-Coniferyl alcohol | 83 | 180.0786 | − | − | − | − | − | + |
| 20.27 | Isogentisin | 80 | 258.0528 | + | + | − | − | − | − |
| Amides | |||||||||
| 21.47 | Myristamide | 84 | 227.2249 | − | − | + | − | − | − |
| 33.04 | Oleamide | 90 | 281.2719 | + | + | + | + | + | + |
| Ketones | |||||||||
| 9.79 | 2-Undecanone | 80 | 170.1671 | − | − | − | + | + | + |
| 13.42 | 2-Tridecanone | 85 | 198.1984 | − | − | − | + | + | + |
| Others | |||||||||
| 7.40 | Myrtenyl methyl ether | 80 | 166.1358 | − | − | + | − | − | − |
| 10.39 | Piperonal | 80 | 150.0317 | − | − | − | − | − | + |
| 10.55 | Tricycloekasantalal | 85 | 178.1358 | − | − | − | + | + | + |
| 10.61 | 7-Tetradecene | 83 | 196.2191 | − | − | − | + | + | − |
| 11.09 | 1,9-Nonanediol | 87 | 160.1463 | − | − | − | + | + | − |
| 11.78 | Tricyclo[4.4.1.1(3,8)]dodeca-4,9-diene | 84 | 160.1252 | − | − | − | + | + | − |
| 11.97 | 2,6,10-Trimethyltetradecane | 81 | 240.2817 | − | − | − | + | + | − |
| 14.35 | Heptacosane | 80 | 380.4382 | + | + | − | + | + | + |
| 14.41 | β-Acorenol | 80 | 222.1984 | − | − | − | + | + | − |
| 14.99 | 5,6,7,7a-tetrahydro-4,4,7a-trimethyl-2(4H)-Benzofuranone | 80 | 180.1150 | − | − | − | + | + | + |
| 15.41 | Syringaldehyde | 82 | 182.0579 | − | − | − | + | + | − |
| 15.80 | cis,α-Santalol | 81 | 234.1984 | + | + | − | − | − | − |
| 16.19 | 2,5,5,8a-Tetramethyl-6,7,8,8a-tetrahydro-5H-naphthalen-1-one | 83 | 204.1514 | − | − | − | − | − | + |
| 16.28 | Illudol | 80 | 252.1725 | − | − | − | + | + | − |
| 17.41 | 2-Hexadecanol | 83 | 242.2610 | − | − | − | + | + | − |
| 18.57 | Santalcamphor | 84 | 236.1776 | + | + | − | + | + | − |
| 18.69 | 6-Hydroxy-4,4,7a-trimethyl-5,6,7,7a-tetrahydro2(4H)benzofuran | 83 | 196.1099 | − | − | − | + | + | − |
| 19.96 | Heptadecane-2,4-dione | 82 | 268.2402 | + | + | − | − | − | − |
| 21.29 | 7-Methyl-Z-tetradecen-1-ol acetate | 80 | 268.2402 | − | − | − | + | + | − |
| 22.05 | 11,13-Dimethyl-12-tetradecen-1-ol acetate | 81 | 282.2559 | − | − | − | + | + | − |
| 23.76 | Tetratetracontane | 85 | 618.7043 | − | − | − | + | + | − |
| 24.95 | α-Tocospiro A | 82 | 462.3709 | − | − | − | + | + | − |
| 41.95 | 3,4-bis(1,3-benzodioxol-5-ylmethyl)dihydro-(3R-trans)-2(3H)-Furanone | 83 | 354.1103 | − | − | + | − | − | + |
| Extract | Fusarium oxysporum | Fusarium circinatum | Meloidogyne incognita | Theoretical Combined Action | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Concentration (mg/mL) | ||||||||||||
| 4 | 8 | 16 | 4 | 8 | 16 | 4 | 8 | 16 | 4 | 8 | 16 | |
| PLE1 | 0.28 ± 0.02 | 0.09 ± 0.02 | 0.06 ± 0.01 | 0.61 ± 0.01 | 0.28 ± 0.01 | 0.28 ± 0.00 | 0.89 ± 0.01 | 0.42 ± 0.01 | 0.19 ± 0.01 | 1.77 ± 0.02 | 0.79 ± 0.02 | 0.53 ± 0.01 |
| PLE2 | 0.26 ± 0.02 | 0.08 ± 0.01 | 0.05 ± 0.01 | 0.36 ± 0.01 | 0.14 ± 0.03 | 0.07 ± 0.00 | 0.44 ± 0.01 | 0.24 ± 0.01 | 0.11 ± 0.01 | 1.06 ± 0.03 | 0.46 ± 0.01 | 0.23 ± 0.01 |
| PLE3 | 1.16 ± 0.03 | 0.52 ± 0.03 | 0.31 ± 0.01 | 2.35 ± 0.03 | 0.98 ± 0.01 | 0.48 ± 0.01 | 2.38 ± 0.04 | 1.26 ± 0.03 | 0.56 ± 0.01 | 5.89 ± 0.02 | 2.76 ± 0.03 | 1.35 ± 0.01 |
| SFE1 | 2.44 ± 0.10 | 1.39 ± 0.06 | 0.58 ± 0.01 | 1.29 ± 0.01 | 1.40 ± 0.01 | 0.73 ± 0.01 | 2.24 ± 0.07 | 1.25 ± 0.01 | 0.62 ± 0.01 | 5.97 ± 0.13 | 4.03 ± 0.07 | 1.93 ± 0.01 |
| SFE2 | 1.10 ± 0.16 | 0.47 ± 0.06 | 0.21 ± 0.01 | 0.71 ± 0.01 | 0.88 ± 0.02 | 0.45 ± 0.00 | 3.30 ± 0.02 | 1.48 ± 0.02 | 0.88 ± 0.01 | 5.10 ± 0.19 | 2.83 ± 0.06 | 1.54 ± 0.02 |
| SFE3 | 0.57 ± 0.07 | 0.29 ± 0.05 | 0.08 ± 0.01 | 0.36 ± 0.01 | 0.18 ± 0.01 | 0.09 ± 0.00 | 1.53 ± 0.03 | 0.71 ± 0.01 | 0.34 ± 0.01 | 2.46 ± 0.08 | 1.18 ± 0.04 | 0.52 ± 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reyes-Vaquero, L.; Ibáñez, E.; Sanz-Alférez, S.; Nombela, G.; Del Villar-Martínez, A.A.; Bueno, M. Biological Activity Evaluation Against Fusarium oxysporum, Fusarium circinatum, and Meloidogyne incognita of Bioactives-Enriched Extracts of Ruta graveolens L. Molecules 2025, 30, 2240. https://doi.org/10.3390/molecules30102240
Reyes-Vaquero L, Ibáñez E, Sanz-Alférez S, Nombela G, Del Villar-Martínez AA, Bueno M. Biological Activity Evaluation Against Fusarium oxysporum, Fusarium circinatum, and Meloidogyne incognita of Bioactives-Enriched Extracts of Ruta graveolens L. Molecules. 2025; 30(10):2240. https://doi.org/10.3390/molecules30102240
Chicago/Turabian StyleReyes-Vaquero, Lorena, Elena Ibáñez, Soledad Sanz-Alférez, Gloria Nombela, Alma Angélica Del Villar-Martínez, and Mónica Bueno. 2025. "Biological Activity Evaluation Against Fusarium oxysporum, Fusarium circinatum, and Meloidogyne incognita of Bioactives-Enriched Extracts of Ruta graveolens L." Molecules 30, no. 10: 2240. https://doi.org/10.3390/molecules30102240
APA StyleReyes-Vaquero, L., Ibáñez, E., Sanz-Alférez, S., Nombela, G., Del Villar-Martínez, A. A., & Bueno, M. (2025). Biological Activity Evaluation Against Fusarium oxysporum, Fusarium circinatum, and Meloidogyne incognita of Bioactives-Enriched Extracts of Ruta graveolens L. Molecules, 30(10), 2240. https://doi.org/10.3390/molecules30102240









