De Novo Terpenes Emitted from Juvenile Leaves of Eucalyptus globulus Labill. subsp. globulus
Abstract
1. Introduction
2. Results
2.1. Origin of 13C-Labelled Terpenes
2.2. Identification and Kinetics of 13C-Labelled Compounds
2.3. Monoterpene Synthase Activity
3. Discussion
3.1. The Origin of Labelled Terpenes
3.2. Identification of 13C Labelling
3.3. The Putative Importance of De Novo Synthesis
4. Materials and Methods
4.1. Plant Material
4.2. Gas Exchange System
4.3. BVOC Sampling and Analysis
4.4. Extraction of Terpenes from Individual Oil Glands and Leaf Tissue
4.5. GC-MS Analysis of Individual Terpenes
4.6. Monoterpene Synthase Assay
4.7. Data Analysis and Error Determination
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BVOC | Biogenic volatile organic compounds |
| DMADP | Dimethylally diphosphate |
| GC-MS | Gas chromatography–mass spectrometry |
| GDP | Geranyl diphosphate |
| MVA | Mevalonic acid |
| MEP | Methylerithritol phosphate |
| PAR | Photosynthetically active radiation |
| PTR-MS | Proton transfer reaction–mass spectrometer |
| RT | Retention time |
| SIM | Selected ion monitoring |
| TPS | Terpene synthase |
References
- Guenther, A.; Hewitt, C.N.; Erickson, D.; Fall, R.; Geron, C.; Graedel, T.; Harley, P.; Klinger, L.; Lerdau, M.; McKay, W.A.; et al. A global model of natural volatile organic compound emissions. J. Geophys. Res. Atmos. 1995, 100, 8873–8892. [Google Scholar] [CrossRef]
- Atkinson, R. Atmospheric chemistry of VOCs and NOx. Atmos. Environ. 2000, 34, 2063–2101. [Google Scholar] [CrossRef]
- Fuentes, J.D.; Gu, L.; Lerdau, M.; Atkinson, R.; Baldocchi, D.; Bottenheim, J.W.; Ciccioli, P.; Lamb, B.; Geron, C.; Guenther, A.; et al. Biogenic hydrocarbons in the atmospheric boundary layer: A review. Bull. Am. Meteorol. Soc. 2000, 81, 1537–1575. [Google Scholar] [CrossRef]
- Claeys, M.; Graham, B.; Vas, G.; Wang, W.; Vermeylen, R.; Pashynska, V.; Cafmeyer, J.; Guyon, P.; Andreae, M.O.; Artaxo, P.; et al. Formation of secondary organic aerosols through photooxidation of isoprene. Science 2004, 303, 1173–1176. [Google Scholar] [CrossRef]
- Guenther, A.; Karl, T.; Harley, P.; Wiedinmyer, C.; Palmer, P.I.; Geron, C. Estimates of global terrestrial isoprene emissions using MEGAN (Model of Emissions of Gases and Aerosols from Nature). Atmos. Chem. Phys. 2006, 6, 3181–3210, Corrected in Atmos. Chem. Phys. 2006, 6, 3181–3210. [Google Scholar] [CrossRef]
- Külheim, C.; Yeoh, S.H.; Wallis, I.R.; Laffan, S.; Moran, G.F.; Foley, W.J. The molecular basis of quantitative variation in foliar secondary metabolites in Eucalyptus globulus. New Phytol. 2011, 191, 1041–1053. [Google Scholar] [CrossRef]
- Poisson, N.; Kanakidou, M.; Crutzen, P.J. Impact of non-methane hydrocarbons on tropospheric chemistry and the oxidizing power of the global troposphere: 3-dimensional modelling results. J. Atmos. Chem. 2000, 36, 157–230. [Google Scholar] [CrossRef]
- Bell, N.; Heard, D.E.; Pilling, M.J.; Tomlin, A.S. Atmospheric lifetime as a probe of radical chemistry in the boundary layer. Atmos. Environ. 2003, 37, 2193–2205. [Google Scholar] [CrossRef]
- Loreto, F.; Schnitzler, J.-P. Abiotic stresses and induced BVOCs. Trends Plant Sci. 2010, 15, 154–166. [Google Scholar] [CrossRef]
- Ghirardo, A.; Koch, K.; Taipale, R.; Zimmer, I.; Schnitzler, J.; Rinne, J. Determination of de novo and pool emissions of terpenes from four common boreal/alpine trees by 13CO2 labelling and PTR-MS analysis. Plant Cell Environ. 2010, 33, 781–792. [Google Scholar] [CrossRef]
- Loreto, F.; Ciccioli, P.; Cecinato, A.; Brancaleoni, E.; Frattoni, M.; Tricoli, D. Influence of environmental factors and air composition on the emission of [α]-pinene from Quercus ilex leaves. Plant Physiol. 1996, 110, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, H.; Kotzias, D.; Steinbrecher, R.; Schürmann, W.; Schönwitz, R. Emission of biosynthesized monoterpenes from needles of Norway spruce. Sci. Nat. 1993, 80, 276–278. [Google Scholar] [CrossRef]
- Brooker, M.I.H.; Kleinig, D.A. Field Guide to Eucalypts. Volume 1, South-Eastern Australia; Bloomings Books: Victoria, Australia, 1999. [Google Scholar]
- Wilson, P.G.; O’Brien, M.M.; Gadek, P.A.; Quinn, C.J. Myrtaceae revisited: A reassessment of infrafamilial groups. Am. J. Bot. 2001, 88, 2013–2025. [Google Scholar] [CrossRef]
- Guenther, A.B.; Monson, R.K.; Fall, R. Isoprene and monoterpene emission rate variability: Observations with eucalyptus and emission rate algorithm development. J. Geophys. Res. Atmos. 1991, 96, 10799–10808. [Google Scholar] [CrossRef]
- Guenther, A.B.; Zimmerman, P.R.; Harley, P.C.; Monson, R.K.; Fall, R. Isoprene and monoterpene emission rate variability: Model evaluations and sensitivity analyses. J. Geophys. Res. Atmos. 1993, 98, 12609–12617. [Google Scholar] [CrossRef]
- Coppen, J.J.W. (Ed.) Estimates of eucalypt plantations worldwide. In Eucalyptus: The Genus Eucalyptus; Taylor and Francis: New York, NY, USA, 2002; pp. 408–411. [Google Scholar]
- Wiedinmyer, C.; Tie, X.; Guenther, A.; Neilson, R.; Granier, C. Future Changes in Biogenic Isoprene Emissions: How Might They Affect Regional and Global Atmospheric Chemistry? Earth Interact. 2006, 10, 1–19. [Google Scholar] [CrossRef]
- Owen, S.; Harley, P.; Guenther, A.; Hewitt, C. Light dependency of VOC emissions from selected Mediterranean plant species. Atmos. Environ. 2002, 36, 3147–3159. [Google Scholar] [CrossRef]
- Niinemets, Ü.; Reichstein, M.; Staudt, M.; Seufert, G.; Tenhunen, J.D. Stomatal constraints may affect emission of oxygenated monoterpenoids from the foliage of Pinus pinea. Plant Physiol. 2002, 130, 1371–1385. [Google Scholar] [CrossRef]
- Grote, R.; Niinemets, Ü. Modeling volatile isoprenoid emissions—A story with split ends. Plant Biol. 2008, 10, 8–28. [Google Scholar] [CrossRef]
- Tingey, D.T.; Manning, M.; Grothaus, L.C.; Burns, W.F. Influence of light and temperature on monoterpene emission rates from slash pine. Plant Physiol. 1980, 65, 797–801. [Google Scholar] [CrossRef]
- Werner, C.; Fasbender, L.; Romek, K.M.; Yáñez-Serrano, A.M.; Kreuzwieser, J. Heat waves change plant carbon allocation among primary and secondary metabolism altering CO2 assimilation, respiration, and VOC emissions. Front. Plant Sci. 2020, 11, 1242. [Google Scholar] [CrossRef] [PubMed]
- Byron, J.; Kreuzwieser, J.; Purser, G.; van Haren, J.; Ladd, S.N.; Meredith, L.K.; Werner, C.; Williams, J. Chiral monoterpenes reveal forest emission mechanisms and drought responses. Nature 2022, 609, 307–312. [Google Scholar] [CrossRef]
- Kreuzwieser, J.; Meischner, M.; Grün, M.; Yáñez-Serrano, A.M.; Fasbender, L.; Werner, C. Drought affects carbon partitioning into volatile organic compound biosynthesis in Scots pine needles. New Phytol. 2021, 232, 1930–1943. [Google Scholar] [CrossRef]
- Delwiche, C.F.; Sharkey, T.D. Rapid appearance of 13C in biogenic isoprene when 13CO2 is fed to intact leaves. Plant Cell Environ. 1993, 16, 587–591. [Google Scholar] [CrossRef]
- Loreto, F.; Ciccioli, P.; Brancaleoni, E.; Cecinato, A.; Frattoni, M.; Sharkey, T.D. Different sources of reduced carbon contribute to form three classes of terpenoid emitted by Quercus ilex L. leaves. Proc. Natl. Acad. Sci. USA 1996, 93, 9966–9969. [Google Scholar] [CrossRef]
- Loreto, F.; Ciccioli, P.; Brancaleoni, E.; Frattoni, M.; Delfine, S. Incomplete 13C labelling of α-pinene content of Quercus ilex leaves and appearance of unlabelled C in α-pinene emission in the dark. Plant Cell Environ. 2000, 23, 229–234. [Google Scholar] [CrossRef]
- Fasbender, L.; Yáñez-Serrano, A.M.; Kreuzwieser, J.; Dubbert, D.; Werner, C. Real-time carbon allocation into biogenic volatile organic compounds (BVOCs) and respiratory carbon dioxide (CO2) traced by PTR-TOF-MS, 13CO2 laser spectroscopy and 13C-pyruvate labelling. PLoS ONE 2018, 13, e0204398. [Google Scholar] [CrossRef]
- Schnitzler, J.-P.; Graus, M.; Kreuzwieser, J.; Heizmann, U.; Rennenberg, H.; Wisthaler, A.; Hansel, A. Contribution of different carbon sources to isoprene biosynthesis in poplar leaves. Plant Physiol. 2004, 135, 152–160. [Google Scholar] [CrossRef]
- Kreuzwieser, J.; Graus, M.; Wisthaler, A.; Hansel, A.; Rennenberg, H.; Schnitzler, J. Xylem-transported glucose as an additional carbon source for leaf isoprene formation in Quercus robur. New Phytol. 2002, 156, 171–178. [Google Scholar] [CrossRef]
- Schnitzler, J.; Steinbrecher, R.; Zimmer, I.; Steigner, D.; Fladung, M. Hybridization of European oaks (Quercus ilex × Q. robur) results in a mixed isoprenoid emitter type. Plant Cell Environ. 2004, 27, 585–593. [Google Scholar] [CrossRef]
- Yáñez-Serrano, A.M.; Mahlau, L.; Fasbender, L.; Byron, J.; Williams, J.; Kreuzwieser, J.; Werner, C. Heat stress increases the use of cytosolic pyruvate for isoprene biosynthesis. J. Exp. Bot. 2019, 70, 5827–5838. [Google Scholar] [CrossRef] [PubMed]
- Shao, M.; Czapiewski, K.V.; Heiden, A.C.; Kobel, K.; Komenda, M.; Koppmann, R.; Wildt, J. Volatile organic compound emissions from Scots pine: Mechanisms and description by algorithms. J. Geophys. Res. Atmos. 2001, 106, 20483–20491. [Google Scholar] [CrossRef]
- Tani, A.; Hayward, S.; Hansel, A.; Hewitt, C.N. Effect of water vapour pressure on monoterpene measurements using proton transfer reaction-mass spectrometry (PTR-MS). Int. J. Mass Spectrom. 2004, 239, 161–169. [Google Scholar] [CrossRef]
- Brophy, J.J.; House, A.P.N.; Boland, D.J.; Lassak, E.V. Digests of the essential oils of 111 species from northern and eastern Australia. In Eucalyptus Leaf Oils: Use, Chemistry, Distillation and Marketing; Boland, D.J., Brophy, J.J., Eds.; Inkarta Press: Melbourne, Australia, 1991; pp. 29–156. [Google Scholar]
- Harley, P.; Fridd-Stroud, V.; Greenberg, J.; Guenther, A.; Vasconcellos, P. Emission of 2-methyl-3-buten-2-ol by pines: A potentially large natural source of reactive carbon to the atmosphere. J. Geophys. Res. Atmos. 1998, 103, 25479–25486. [Google Scholar] [CrossRef]
- Baker, B.; Guenther, A.; Greenberg, J.; Goldstein, A.; Fall, R. Canopy fluxes of 2-methyl-3-buten-2-ol over a ponderosa pine forest by relaxed eddy accumulation: Field data and model comparison. J. Geophys. Res. Atmos. 1999, 104, 26107–26114. [Google Scholar] [CrossRef]
- Gray, D.W.; Goldstein, A.H.; Lerdau, M.T. The influence of light environment on photosynthesis and basal methylbutenol emission from Pinus ponderosa. Plant Cell Environ. 2005, 28, 1463–1474. [Google Scholar] [CrossRef]
- Fischbach, R.J.; Zimmer, I.; Steinbrecher, R.; Pfichner, A.; Schnitzler, J.-P. Monoterpene synthase activities in leaves of Picea abies (L.) Karst. and Quercus ilex L. Phytochemistry 2000, 54, 257–265. [Google Scholar] [CrossRef]
- Bernard-Dagan, C.; Carde, J.P.; Gleizes, M. Etude des composés terpéniques au cours de la croissance des aiguilles du Pin maritime: Comparaison de données biochimiques et ultrastructurales. Can. J. Bot. 1979, 57, 255–263. [Google Scholar] [CrossRef]
- Laule, O.; Fürholz, A.; Chang, H.-S.; Lange, M. Crosstalk between cytosolic and plastidial pathways of isoprenoid biosyn-thesis in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2003, 100, 6866–6871. [Google Scholar] [CrossRef]
- Piel, J.; Atzorn, R.; Gäbler, R.; Kühnemann, F.; Boland, W. Cellulysin from the plant parasitic fungus Trichoderma viride elicits volatile biosynthesis in higher plants via the octadecanoid signalling cascade. FEBS Lett. 1997, 416, 143–148. [Google Scholar] [CrossRef]
- Karl, T.; Fall, R.; Rosenstiel, T.; Prazeller, P.; Larsen, B.; Seufert, G.; Lindinger, W. On-line analysis of the 13 CO 2 labeling of leaf isoprene suggests multiple subcellular origins of isoprene precursors. Planta 2002, 215, 894–905. [Google Scholar] [CrossRef] [PubMed]
- Trowbridge, A.M.; Asensio, D.; Eller, A.S.D.; Way, D.A.; Wilkinson, M.J.; Schnitzler, J.-P.; Jackson, R.B.; Monson, R.K. Contribution of various carbon sources toward isoprene biosynthesis in poplar leaves mediated by altered atmospheric CO2 concentrations. PLoS ONE 2012, 7, e32387. [Google Scholar] [CrossRef]
- Dalal, K.; Salunkhe, D.; Olson, L.; Do, J.; Yu, M.H. Volatile components of developing tomato fruit grown under field and greenhouse conditions. Plant Cell Physiol. 1968, 9, 389–400. [Google Scholar] [CrossRef]
- Nimitkeatkai, H.; Ueda, Y.; Furukawa, H.; Inamoto, K.; Doi, M. Emission of methylbutyric acid from Gypsophila paniculata L. during bud opening: Changes in amino acid catabolism. Sci. Hortic. 2005, 106, 370–380. [Google Scholar] [CrossRef]
- Yoshimoto, H.; Fukushige, T.; Yonezawa, T.; Sakai, Y.; Okawa, K.; Iwamatsu, A.; Sone, H.; Tamai, Y. Pyruvate decarboxylase encoded by the PDC1 gene contributes, at least partially, to the decarboxylation of α-ketoisocaproate for isoamyl alcohol formation in Saccharomyces cerevisiae. J. Biosci. Bioeng. 2001, 92, 83–85. [Google Scholar] [CrossRef]
- Gray, D.W.; Lerdau, M.T.; Goldstein, A.H. Influences of temperature history, water stress and needle age on methyl butenol emissions. Ecology 2003, 84, 765–776. [Google Scholar] [CrossRef]
- Hakola, H.; Tarvainen, V.; Bäck, J.; Ranta, H.; Bonn, B.; Rinne, J.; Kulmala, M. Seasonal variation of mono- and sesquiterpene emission rates of Scots pine. Biogeosciences 2006, 3, 93–101, Discussions 2, 1697–1717. [Google Scholar] [CrossRef]
- Turner, G.; Gershenzon, J.; Nielson, E.E.; Froehlich, J.E.; Croteau, R. Limonene synthase, the enzyme responsible for monoterpene biosynthesis in peppermint, is localized to leucoplasts of oil gland secretory cells. Plant Physiol. 1999, 120, 879–886. [Google Scholar] [CrossRef]
- McConkey, M.E.; Gershenzon, J.; Croteau, R.B. Developmental regulation of monoterpene biosynthesis in the glandular trichomes of peppermint. Plant Physiol. 2000, 122, 215–224. [Google Scholar] [CrossRef]
- Schnitzler, J.-P.; Zimmer, I.; Bachl, A.; Arend, M.; Fromm, J.; Fischbach, R.J. Biochemical properties of isoprene synthase in poplar (Populus × canescens). Planta 2005, 222, 777–786. [Google Scholar] [CrossRef]
- Dörffel, R.A. Statistik in der Analytischen Chemie. 3 Auflage; Verlag Chemie: Weinheim, Germany, 1984; pp. 51–72. [Google Scholar]
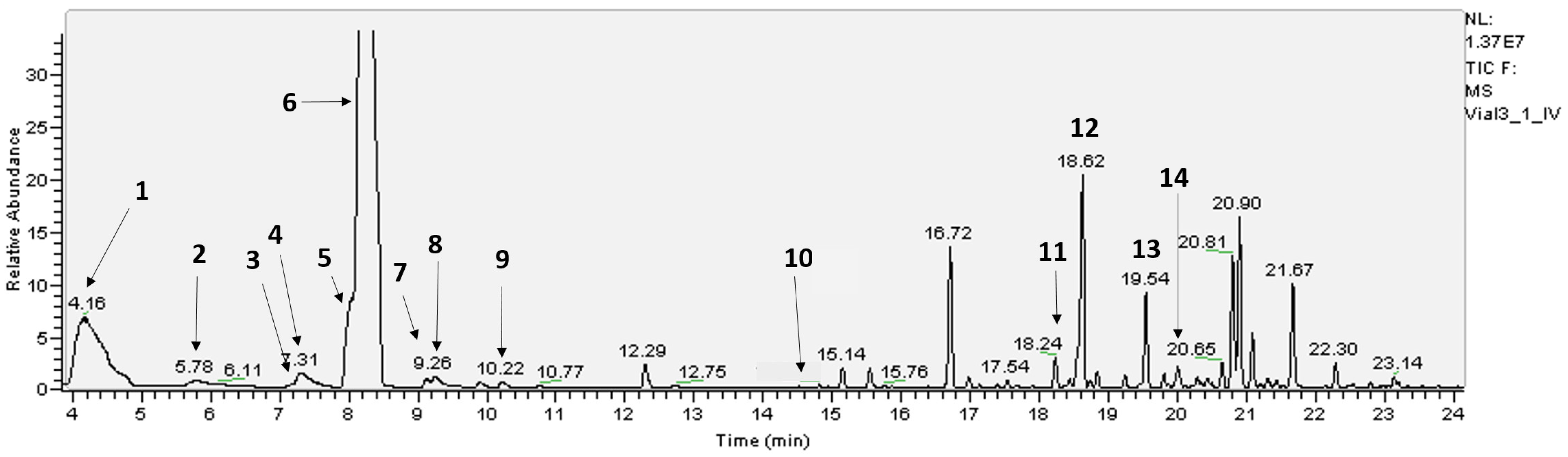


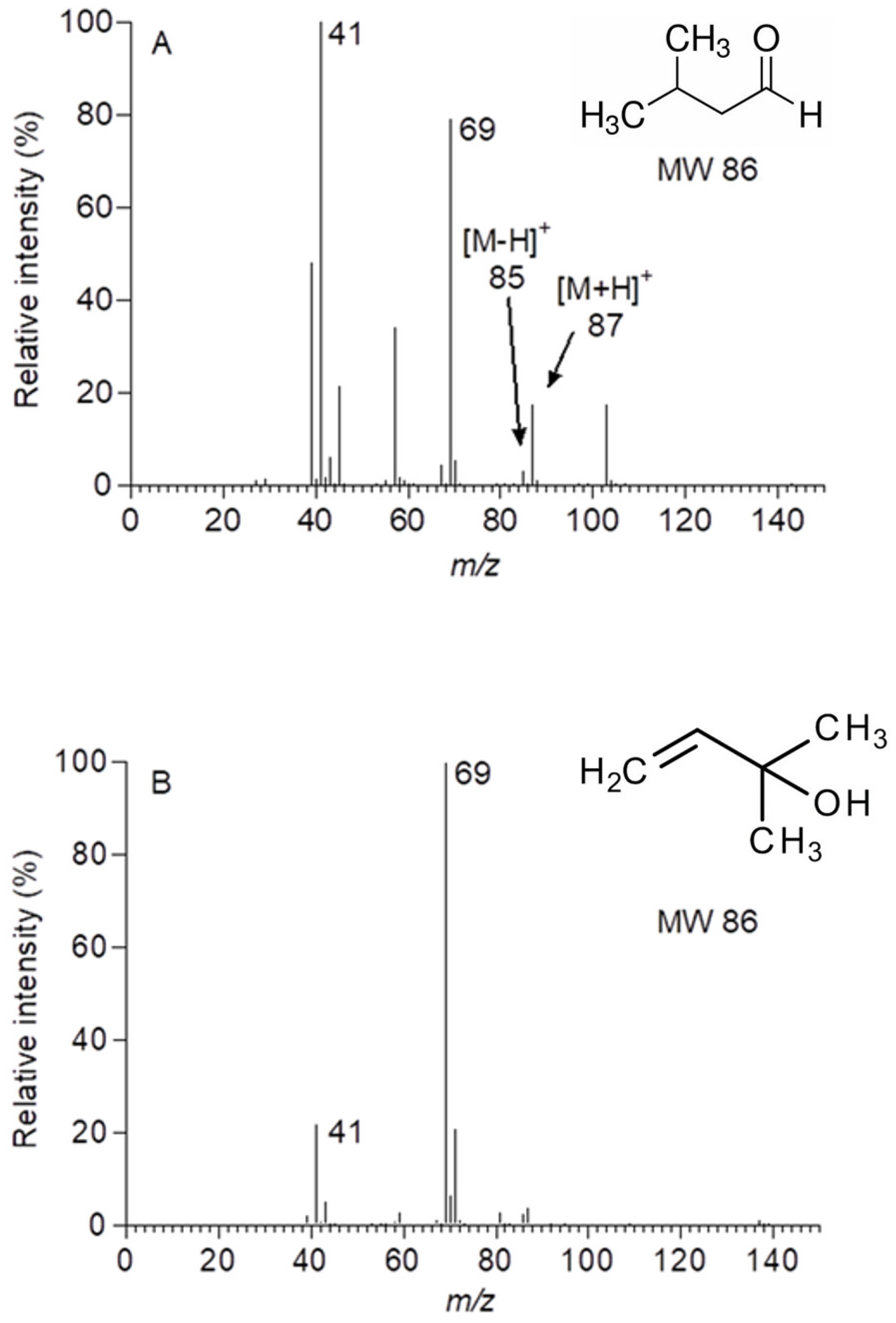
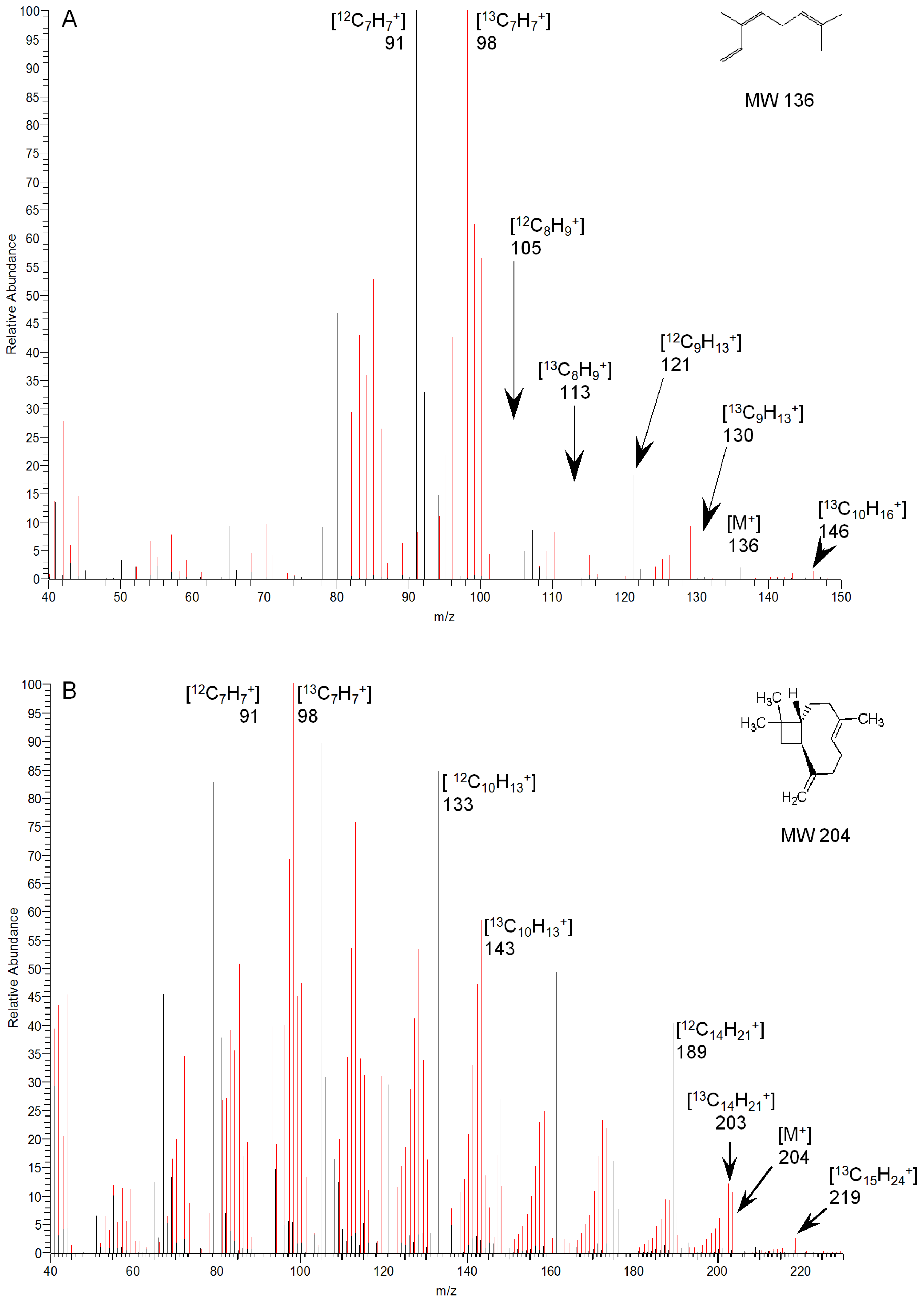

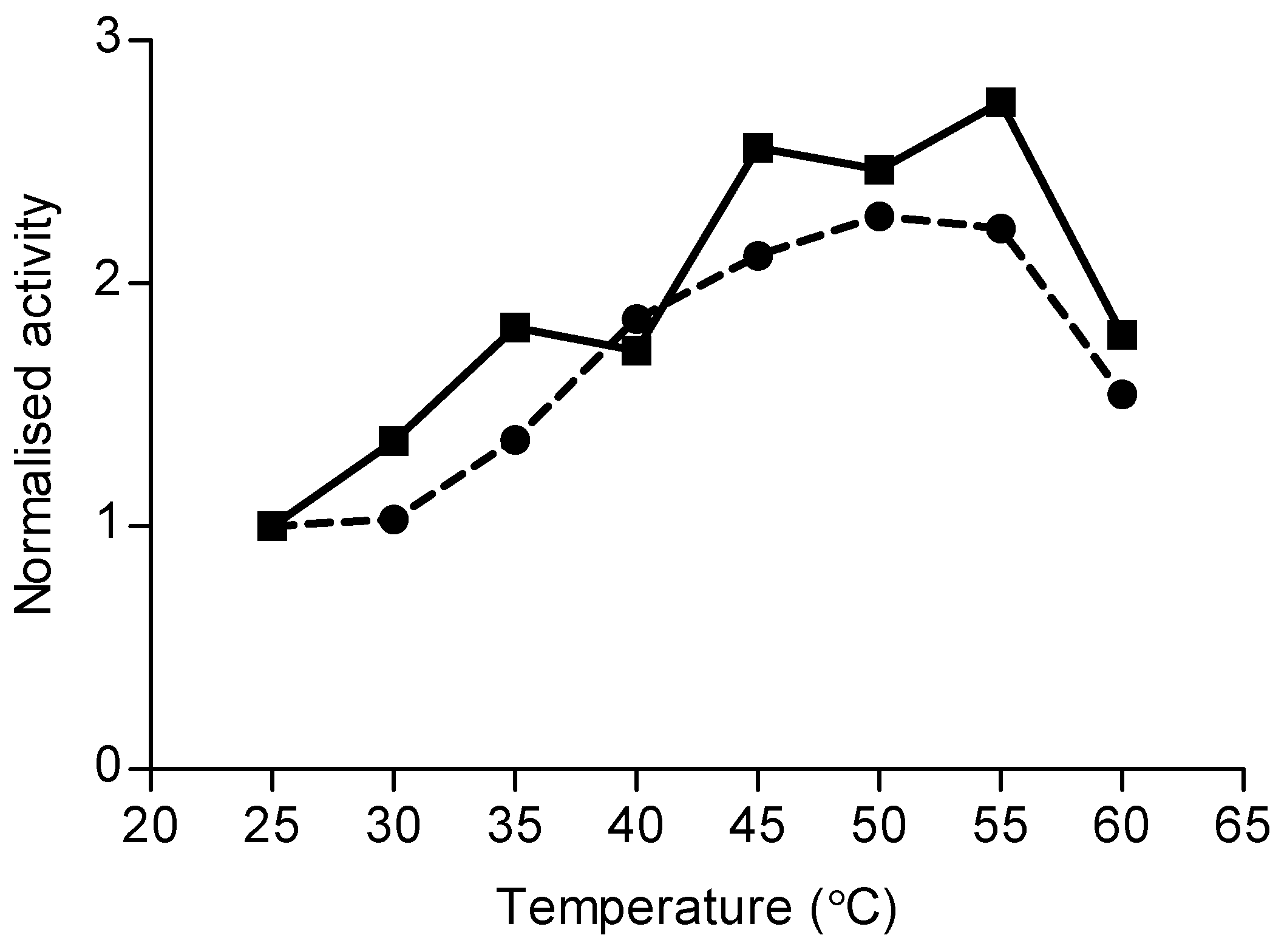
| Oil Glands | Whole Leaf | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Compound | RT | Light | Dark | Light | Dark | ||||
| (min) | Mean | SE | Mean | SE | Mean | SE | Mean | SE | |
| 1. α-pinene | 4.16 | 8.43 | ±0.45 | 9.62 | ±0.59 | 9.44 | ±0.34 | 8.82 | ±0.36 |
| 2. β-pinene | 5.78 | 0.49 | ±0.02 | 0.52 | ±0.01 | 0.59 | ±0.02 | 0.52 | ±0.02 |
| 3. R-(-)-α-phellandrene | 7.17 | 0.06 | ±0.01 | 0.06 | ±0.01 | 0.21 | ±0.15 | 0.07 | ±0.01 |
| 4. myrcene | 7.31 | 0.93 | ±0.06 | 0.97 | ±0.04 | 1.13 | ±0.08 | 1.01 | ±0.04 |
| 5. limonene | 8.04 | 2.96 | ±0.09 | 3.09 | ±0.09 | 2.72 | ±0.09 | 3.12 | ±0.20 |
| 6. 1,8-cineole * | 8.22 | 59.1 | ±0.90 | 58.55 | ±1.21 | 56.43 | ±0.79 | 58.31 | ±3.18 |
| 7. cis-ocimene | 9.10 | 0.23 | ±0.04 | 0.14 | ±0.04 | 0.28 | ±0.05 | 0.32 | ±0.02 |
| 8. p-cymene | 9.26 | 0.32 | ±0.03 | 0.34 | ±0.03 | 0.31 | ±0.02 | 0.35 | ±0.03 |
| 9. trans-ocimene | 10.2 | 0.04 | ±0.01 | 0.04 | ±0.01 | 0.05 | ±0.01 | 0.04 | ±0.01 |
| C10H16 | 10.74 | 0.07 | ±0.01 | 0.08 | ±0.02 | 0.07 | ±0.01 | 0.06 | ±0.01 |
| 10. p-α-dimethylstyrene | 14.5 | 0.02 | ±0.01 | 0.02 | ±0.01 | 0.03 | ±0.01 | 0.02 | ±0.01 |
| C15H24 | 14.93 | 0.03 | ±0.01 | 0.03 | ±0.01 | 0.05 | ±0.01 | 0.04 | ±0.01 |
| C15H24 | 15.134 | 0.60 | ±0.03 | 0.57 | ±0.04 | 0.60 | ±0.03 | 0.59 | ±0.06 |
| C15H24 | 15.53 | 0.37 | ±0.06 | 0.35 | ±0.07 | 0.56 | ±0.07 | 0.45 | ±0.11 |
| C15H24 | 15.85 | 0.02 | ±0.01 | 0.03 | ±0.01 | 0.03 | ±0.01 | 0.03 | ±0.01 |
| C15H24 | 16.36 | 0.01 | ±0.01 | 0.01 | ±0.01 | 0.01 | ±0.01 | 0.01 | ±0.01 |
| C10H16O | 16.72 | 4.43 | ±0.25 | 4.19 | ±0.36 | 4.70 | ±0.22 | 4.55 | ±0.45 |
| C15H24 | 16.97 | 0.23 | ±0.01 | 0.22 | ±0.02 | 0.26 | ±0.01 | 0.24 | ±0.03 |
| C15H24 | 17.21 | 0.04 | ±0.01 | 0.05 | ±0.01 | 0.08 | ±0.01 | 0.05 | ±0.01 |
| C15H24 | 17.40 | 0.05 | ±0.01 | 0.04 | ±0.01 | 0.05 | ±0.01 | 0.04 | ±0.01 |
| C15H24 | 17.54 | 0.02 | ±0.01 | 0.02 | ±0.01 | 0.04 | ±0.01 | 0.03 | ±0.01 |
| C15H24 | 17.65 | 0.06 | ±0.01 | 0.06 | ±0.01 | 0.07 | ±0.01 | 0.06 | ±0.01 |
| C15H24 | 17.88 | 0.01 | ±0.01 | 0.01 | ±0.01 | 0.02 | ±0.01 | 0.02 | ±0.01 |
| 11. trans-caryophyllene | 18.24 | 0.65 | ±0.03 | 0.63 | ±0.05 | 0.73 | ±0.03 | 0.71 | ±0.01 |
| C15H24 | 18.43 | 0.12 | ±0.01 | 0.12 | ±0.01 | 0.13 | ±0.01 | 0.13 | ±0.01 |
| 12. aromadendrene | 18.612 | 5.37 | ±0.28 | 5.10 | ±0.28 | 4.90 | ±0.38 | 5.47 | ±0.02 |
| C15H24 | 18.72 | 0.12 | ±0.01 | 0.11 | ±0.01 | 0.10 | ±0.01 | 0.12 | ±0.63 |
| C15H24 | 18.83 | 0.24 | ±0.01 | 0.22 | ±0.02 | 0.24 | ±0.02 | 0.24 | ±0.02 |
| C15H24 | 19.23 | 0.29 | ±0.01 | 0.30 | ±0.02 | 0.31 | ±0.01 | 0.30 | ±0.03 |
| 13. alloaromadendrene | 19.54 | 1.61 | ±0.36 | 1.78 | ±0.33 | 1.78 | ±0.23 | 2.42 | ±0.04 |
| C15H24 | 19.8 | 0.69 | ±0.36 | 0.72 | ±0.39 | 0.75 | ±0.40 | 0.54 | ±0.05 |
| C15H24 | 19.99 | 0.19 | ±0.02 | 0.15 | ±0.02 | 0.17 | ±0.03 | 0.22 | ±0.30 |
| C15H26O | 20.11 | 0.06 | ±0.03 | 0.05 | ±0.02 | 0.06 | ±0.02 | 0.05 | ±0.01 |
| 14. a-terpineol | 20.02 | 0.01 | ±0.01 | 0.02 | ±0.01 | 0.02 | ±0.01 | 0.02 | ±0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Winters, A.J.; Hocart, C.H.; Schnitzler, J.-P.; Zimmer, I.; Adams, M.A.; Rennenberg, H.; Kreuzwieser, J.; Keitel, C. De Novo Terpenes Emitted from Juvenile Leaves of Eucalyptus globulus Labill. subsp. globulus. Molecules 2025, 30, 2234. https://doi.org/10.3390/molecules30102234
Winters AJ, Hocart CH, Schnitzler J-P, Zimmer I, Adams MA, Rennenberg H, Kreuzwieser J, Keitel C. De Novo Terpenes Emitted from Juvenile Leaves of Eucalyptus globulus Labill. subsp. globulus. Molecules. 2025; 30(10):2234. https://doi.org/10.3390/molecules30102234
Chicago/Turabian StyleWinters, Anthony J., Charles H. Hocart, Jörg-Peter Schnitzler, Ina Zimmer, Mark A. Adams, Heinz Rennenberg, Jürgen Kreuzwieser, and Claudia Keitel. 2025. "De Novo Terpenes Emitted from Juvenile Leaves of Eucalyptus globulus Labill. subsp. globulus" Molecules 30, no. 10: 2234. https://doi.org/10.3390/molecules30102234
APA StyleWinters, A. J., Hocart, C. H., Schnitzler, J.-P., Zimmer, I., Adams, M. A., Rennenberg, H., Kreuzwieser, J., & Keitel, C. (2025). De Novo Terpenes Emitted from Juvenile Leaves of Eucalyptus globulus Labill. subsp. globulus. Molecules, 30(10), 2234. https://doi.org/10.3390/molecules30102234








