Impact of Geographical Origin on the Contents of Inorganic Elements and Bioactive Compounds in Polygonum perfoliatum L.
Abstract
1. Introduction
2. Results and Discussion
2.1. HPLC Analysis of Bioactive Compounds in P. perfoliatum
2.1.1. Method Validation
2.1.2. Quantitative Analysis of KP Bioactive Compounds
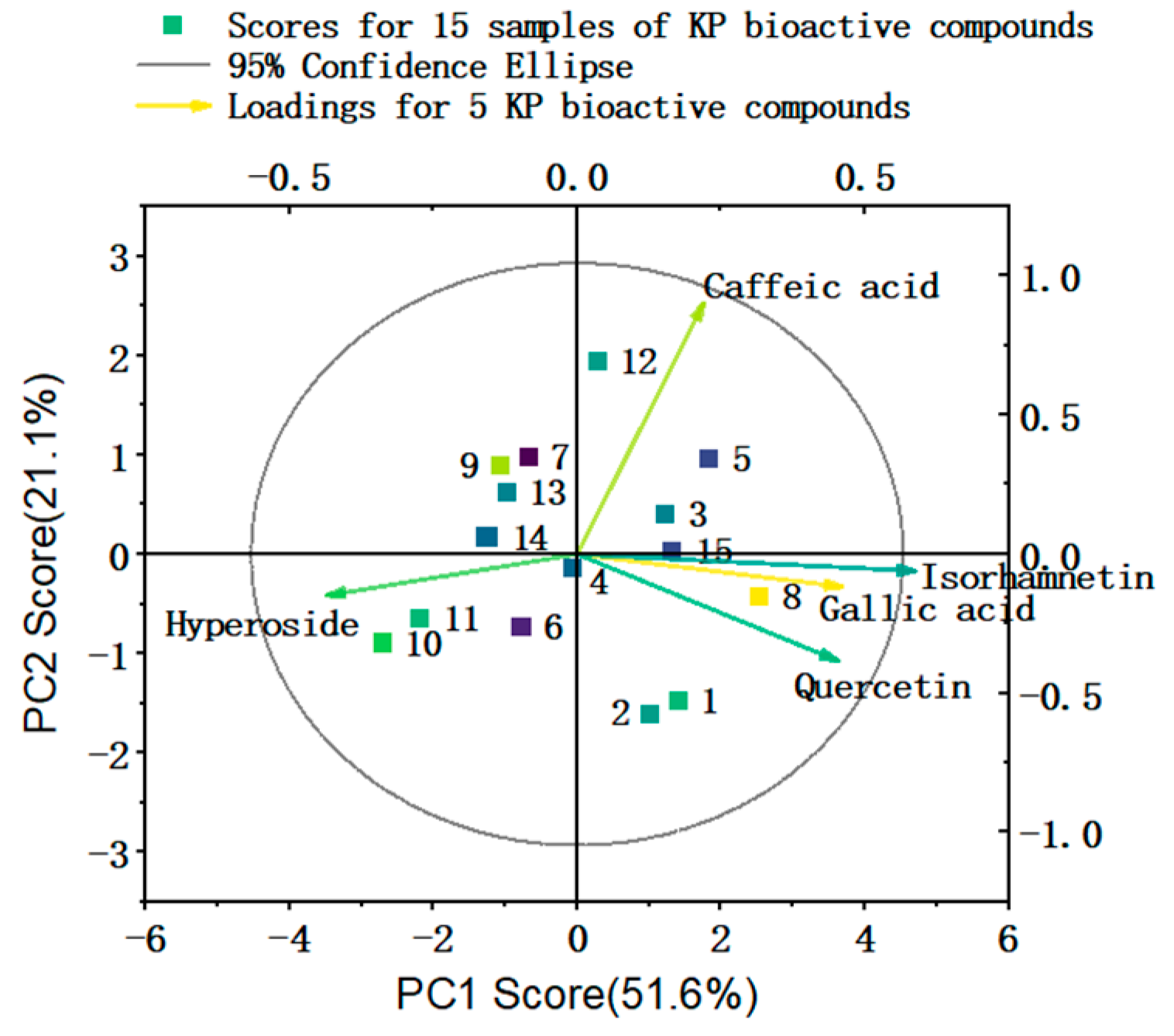
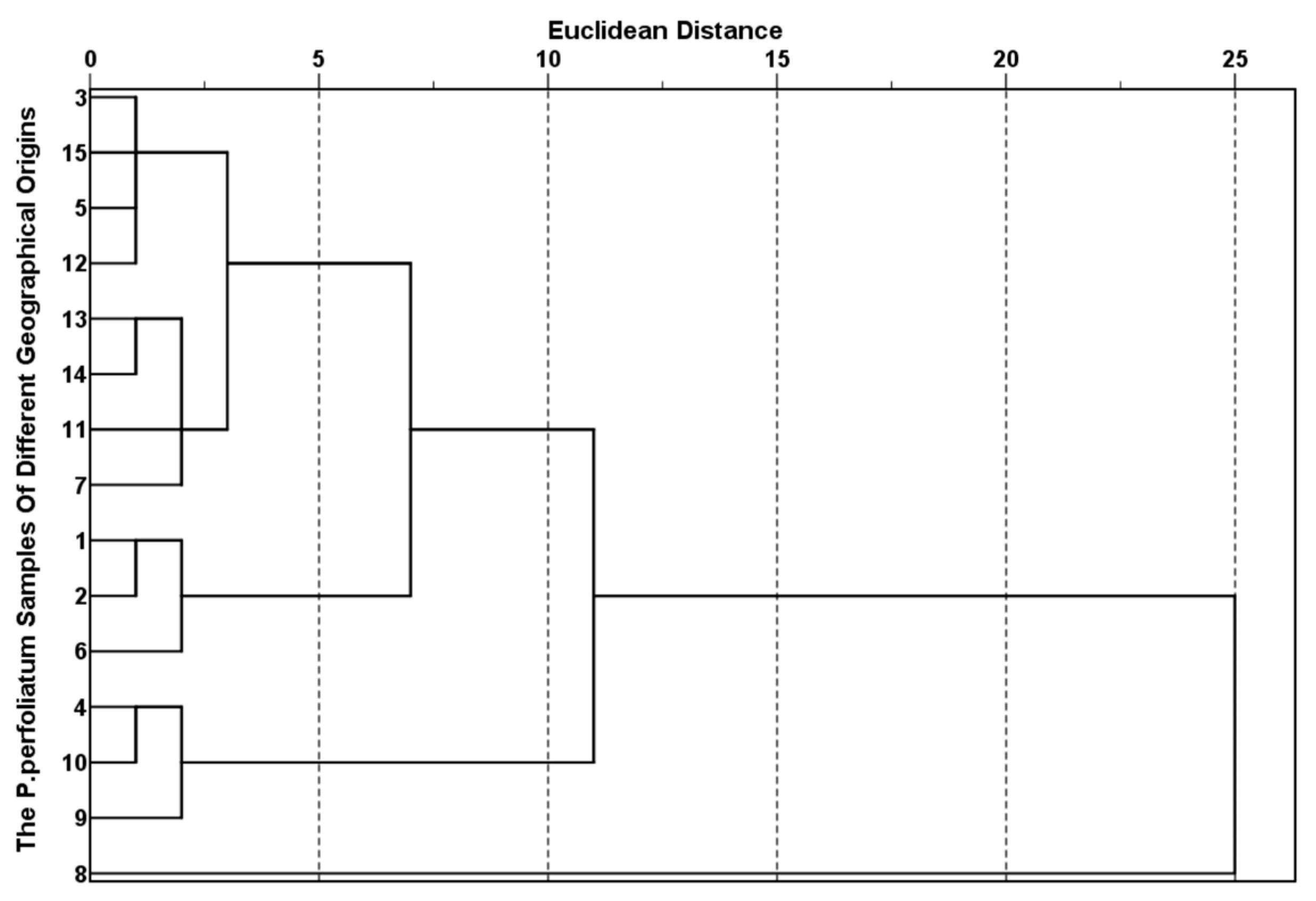
2.1.3. Results and Analysis of PCA and HCA of KP Bioactive Compounds
2.2. Analysis of Inorganic Elements
2.2.1. Quantitative Analysis of Inorganic Elements
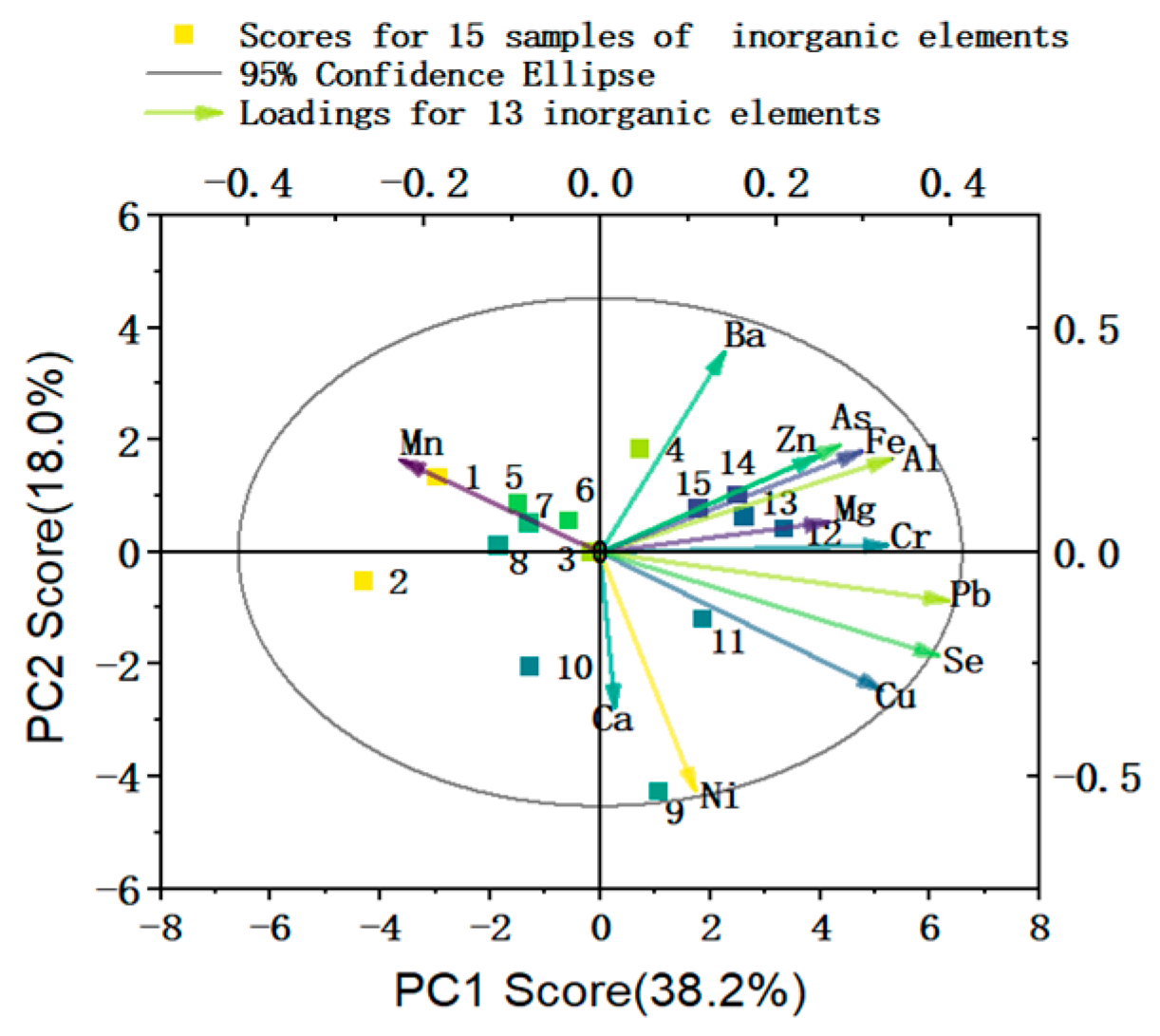
2.2.2. Results of HCA and PCA of Inorganic Elements
2.3. Correlation Analysis
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. HPLC Analysis
3.2.1.1. HPLC Sample Preparation
3.2.1.2. Standard Solution Preparation and Method Validation
3.2.1.3. Sample Analysis
3.2.2. ICP-AES Analysis
3.2.2.1. ICP-AES Sample Preparation
3.2.2.2. Quantitative Analysis
3.2.3. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Committee, N.P. Pharmacopoeia of the People’s Republic of China; China Medical Science and Technology Press: Beijing, China, 2020. [Google Scholar]
- Liu, J.; Zeng, Y.; Sun, G.; Yu, S.; Xu, Y.; He, C.; Li, Z.; Jin, S.; Qin, X. Polygonum perfoliatum L., an Excellent Herbal Medicine Widely Used in China: A Review. Front. Pharmacol. 2020, 11, 581266. [Google Scholar] [CrossRef]
- Lei, J.; Yao, N.; Wang, K.-W. Phytochemical and chemotaxomic study on Polygonum perfoliatum L. Biochem. Syst. Ecol. 2013, 48, 186–188. [Google Scholar] [CrossRef]
- Wang, K.W.; Zhu, J.R.; Shen, L.Q. A new lignan with anti-tumour activity from Polygonum perfoliatum L. Nat. Prod. Res. 2013, 27, 568–573. [Google Scholar] [CrossRef]
- Cheng, H.B.; Liu, X.Q.; Chen, K.L. Chemical constituents of ethyl acetate extract from Polygonum perfoliatum. Zhong Yao Cai 2012, 35, 1088–1090. [Google Scholar]
- Fan, D.; Zhao, Y.; Zhou, X.; Gong, X.; Zhao, C. Simultaneous determination of esculetin, quercetin-3-O-β-D-glucuronide, quercetin-3-O-β -D-glucuronopyranside methyl ester and quercetin in effective part of Polygonum perfoliatum L. using high performace liquid chromatography. Pharmacogn. Mag. 2014, 10, 359–366. [Google Scholar] [CrossRef]
- Liu, X.-X. Study on Effective Composition Analysis and Antibacterial Effects of Herb Polygonum perfoliatum. Prog. Vet. Med. 2008, 29, 45–49. [Google Scholar] [CrossRef]
- Liu, P.; Tang, X.; Gong, C.; Xu, G. Manganese tolerance and accumulation in six Mn hyperaccumulators or accumulators. Plant Soil 2010, 335, 385–395. [Google Scholar] [CrossRef]
- Xue, S.; Wang, J.; Wu, C.; Li, S.; Hartley, W.; Wu, H.; Zhu, F.; Cui, M. Physiological response of Polygonum perfoliatum L. following exposure to elevated manganese concentrations. Environ. Sci. Pollut. Res. 2018, 25, 132–140. [Google Scholar] [CrossRef]
- Shen, L.; Pang, S.; Zhong, M.; Sun, Y.; Qayum, A.; Liu, Y.; Rashid, A.; Xu, B.; Liang, Q.; Ma, H.; et al. A comprehensive review of ultrasonic assisted extraction (UAE) for bioactive components: Principles, advantages, equipment, and combined technologies. Ultrason. Sonochem. 2023, 101, 106646. [Google Scholar] [CrossRef]
- Zhang, J.; Yu, X.; Yang, R.; Zheng, B.Q.; Zhang, Y.Q.; Zhang, F. Quality evaluation of Lonicerae Japonicae Flos from different origins based on high-performance liquid chromatography (HPLC) fingerprinting and multicomponent quantitative analysis combined with chemical pattern recognition. Phytochem. Anal. 2024, 35, 647–663. [Google Scholar] [CrossRef]
- Mrmošanin, J.; Pavlović, A.; Rašić Mišić, I.; Tošić, S.; Petrović, S.; Mitić, Z.; Pecev-Marinković, E.; Arsić, B. Evaluation of an Inductively Coupled Plasma–Atomic Emission Spectrometry (ICP-AES) Method for the Determination of Macro and Microelements in Trifolium L. Species. Anal. Lett. 2024, 57, 558–571. [Google Scholar] [CrossRef]
- Xu, D.; Huang, L.; Chen, Y.; Ye, S.; Tang, Y. Research Progress on Chemical Constituents, Pharmacological Action and Quality Standard of Polygonum perfoliatum L. Chin. Wild Plant Resour. 2021, 40, 31–34. [Google Scholar]
- Long, S.; Zao, Y.; Zhou, X.; Gong, X.; Chen, H.; Zhao, C. Determination of Heavy Metal in Polygonum perfoliatum and Evaluation on its Quality. Chin. J. Exp. Tradit. Med. Formulae 2011, 17, 52–55. [Google Scholar] [CrossRef]
- Huang, G.; Zhao, W.; Liao, Z.; Wang, J.; Xu, L. Determination of Content of Total Flavonoids in Polygonum perfoliatum from Different Areas. Guangzhou Chem. Ind. 2022, 50, 102–104. [Google Scholar]
- Zhang, D.; Zhang, X.; Li, Y.; Huang, H.; Lin, S.; Liu, H. Determination and Comparison of Content of Trace Elements in Polygonum perfoliatum L. from Different Origins. J. Anhui Agric. Sci. 2018, 46, 152–154. [Google Scholar]
- Pan, Y.; Zhang, H.; Zhang, P.; Pei, S.; Fan, J.; Liu, S. Prediction of Suitable Distribution Area of Polygonum perfoliatum L. Based on MaxEnt Model and ArcGIS. Chin. J. Inf. Tradit. Chin. Med. 2021, 28, 1–4. [Google Scholar] [CrossRef]
- Sun, X.; Chen, H.; Zhou, X. Research progress on chemical composition, quality control and pharmacological action of Polygonum perfoliatum L. Shandong Med. J. 2017, 57, 110–113. [Google Scholar]
- Huang, Y.; Shi, T.; Luo, X.; Xiong, H.; Min, F.; Chen, Y.; Nie, S.; Xie, M. Determination of multi-pesticide residues in green tea with a modified QuEChERS protocol coupled to HPLC-MS/MS. Food Chem. 2019, 275, 255–264. [Google Scholar] [CrossRef]
- Chiang, M.C.; Tsai, T.Y.; Wang, C.J. The Potential Benefits of Quercetin for Brain Health: A Review of Anti-Inflammatory and Neuroprotective Mechanisms. Int. J. Mol. Sci. 2023, 24, 6328. [Google Scholar] [CrossRef]
- Gao, J.; Hu, J.; Hu, D.; Yang, X. A Role of Gallic Acid in Oxidative Damage Diseases: A Comprehensive Review. Nat. Prod. Commun. 2019, 14, 1934578X19874174. [Google Scholar] [CrossRef]
- Xie, Y.; Huang, B.; Yu, K.; Shi, F.; Liu, T.; Xu, W. Caffeic acid derivatives: A new type of influenza neuraminidase inhibitors. Bioorganic Med. Chem. Lett. 2013, 23, 3556–3560. [Google Scholar] [CrossRef] [PubMed]
- Plaper, A.; Golob, M.; Hafner, I.; Oblak, M.; Solmajer, T.; Jerala, R. Characterization of quercetin binding site on DNA gyrase. Biochem. Biophys. Res. Commun. 2003, 306, 530–536. [Google Scholar] [CrossRef]
- Samoilova, Z.; Tyulenev, A.; Muzyka, N.; Smirnova, G.; Oktyabrsky, O. Tannic and gallic acids alter redox-parameters of the medium and modulate biofilm formation. AIMS Microbiol. 2019, 5, 379–392. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Fu, H.; Xu, Y.; Niu, Y.; An, X. Hyperoside reduces albuminuria in diabetic nephropathy at the early stage through ameliorating renal damage and podocyte injury. J. Nat. Med. 2016, 70, 740–748. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.; Chen, X.; Xu, W.; Wu, Y. Fingerprints Study on the Epimedium acuminaum Franch.in Different Areas of Guizhou Province Based on Principal. Seed 2018, 37, 5–11. [Google Scholar] [CrossRef]
- Li, M.; Zhang, Y.; Xi, H.; Fu, Y.; Wang, H.; Zhang, Y.; Sun, S. Characterization of Rose Essential Oils by Double-Region Atmospheric Pressure Chemical Ionization Mass Spectrometry (DRAPCI-MS) with Principal Component Analysis (PCA), Hierarchical Cluster Analysis (HCA), and Heatmap Analysis. Anal. Lett. 2022, 55, 2382–2393. [Google Scholar] [CrossRef]
- Proceedings of the 11th International Conference on Innovation and Management; University Press of Wuhan University of Technology: Wuhan, China, 2014.
- Zhou, H.; Liu, F.; Zhang, X. Study on climate regionalization of Longtan pearl plum planting in Hechi City based on GIS. Hubei Agric. Sci. 2022, 61, 93–99. [Google Scholar] [CrossRef]
- Li, Y.; Lu, X. Research on the Issues and Countermeasures of Mulberry Silkworm Industry in Yizhou District, Hechi City. Guangdong Seric. 2024, 58, 45–47. [Google Scholar]
- Kalogiouri, N.P.; Manousi, N.; Zachariadis, G.A. Determination of the Toxic and Nutrient Element Content of Almonds, Walnuts, Hazelnuts and Pistachios by ICP-AES. Separations 2021, 8, 28. [Google Scholar] [CrossRef]
- Wu, H.; Chang, X.; Sang, X.; Qu, B.; Cui, H.; Peng, X. Comprehensive evaluation of twenty-seven varieties of mineral elements in Tetrastigma hemsleyanum from different growing areas. Chin. Tradit. Pat. Med. 2018, 40, 2475–2480. [Google Scholar]
- Feng, W.; Kita, D.; Peaucelle, A.; Cartwright, H.N.; Doan, V.; Duan, Q.; Liu, M.C.; Maman, J.; Steinhorst, L.; Schmitz-Thom, I.; et al. The FERONIA Receptor Kinase Maintains Cell-Wall Integrity during Salt Stress through Ca(2+) Signaling. Curr. Biol. 2018, 28, 666–675.e5. [Google Scholar] [CrossRef] [PubMed]
- Dandan, M. Functional Analyses of AtMGT6 in Mg-Transport in Arabidopsis. Ph.D. Thesis, Hunan Normal University, Changsha, China, 2011. [Google Scholar]
- Koletzko, B.; Goulet, O.; Hunt, J.; Krohn, K.; Shamir, R. 1. Guidelines on Paediatric Parenteral Nutrition of the European Society of Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) and the European Society for Clinical Nutrition and Metabolism (ESPEN), Supported by the European Society of Paediatric Research (ESPR). J. Pediatr. Gastroenterol. Nutr. 2005, 41 (Suppl. S2), S1–S87. [Google Scholar] [CrossRef]
- Lötscher, J.; Martí, I.L.A.A.; Kirchhammer, N.; Cribioli, E.; Giordano Attianese, G.M.P.; Trefny, M.P.; Lenz, M.; Rothschild, S.I.; Strati, P.; Künzli, M.; et al. Magnesium sensing via LFA-1 regulates CD8(+) T cell effector function. Cell 2022, 185, 585–602.e29. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Lei, X.; Che, S. Coordination bonding based pH-responsive albumin nanoparticles for anticancer drug delivery. Dalton Trans. 2012, 41, 3714–3719. [Google Scholar] [CrossRef] [PubMed]
- Ahamed, M.; Khan, M.A.M. Enhanced Photocatalytic and Anticancer Activity of Zn-Doped BaTiO3 Nanoparticles Prepared Through a Green Approach Using Banana Peel Extract. Catalysts 2023, 13, 985. [Google Scholar] [CrossRef]
- Schümann, K.; Ettle, T.; Szegner, B.; Elsenhans, B.; Solomons, N.W. On risks and benefits of iron supplementation recommendations for iron intake revisited. J. Trace Elem Med. Biol. 2007, 21, 147–168. [Google Scholar] [CrossRef]
- Dambiec, M.; Polechońska, L.; Klink, A. Levels of essential and non-essential elements in black teas commercialized in Poland and their transfer to tea infusion. J. Food Compos. Anal. 2013, 31, 62–66. [Google Scholar] [CrossRef]
- Zuo, T.; Shen, M.; Zhang, L.; Jin, H.; Ma, S. Formulation of limit standards for heavy metals and harmful elements in TCMs and related reflections. Chin. J. Pharm. Anal. 2023, 43, 701–711. [Google Scholar] [CrossRef]
- Vitali, D.; Vedrina Dragojević, I.; Šebečić, B. Bioaccessibility of Ca, Mg, Mn and Cu from whole grain tea-biscuits: Impact of proteins, phytic acid and polyphenols. Food Chem. 2008, 110, 62–68. [Google Scholar] [CrossRef]
- World Health Organization. WHO Guidelines for Assessing Quality of Herbal Medicines with Reference to Contaminants and Residues; World Health Organization: Geneva, Switzerland, 2007. [Google Scholar]
- Liu, P.; Wang, C.N.; Song, X.; Wu, Y.N. Dietary intake of lead and cadmium by children and adults—Result calculated from dietary recall and available lead/cadmium level in food in comparison to result from food duplicate diet method. Int. J. Hyg. Environ. Health 2010, 213, 450–457. [Google Scholar] [CrossRef]
- Li, M.; Liu, Y.; Zhou, R.; Lin, Q.; Wu, B. Analysis on Limit Standards for Heavy Metals and Arsenic Salts in Traditional Chinese Medicine Both at Home and Abroad. Lishizhen Med. Mater. Medica Res. 2007, 18, 2859–2860. [Google Scholar]
- Zoroddu, M.A.; Aaseth, J.; Crisponi, G.; Medici, S.; Peana, M.; Nurchi, V.M. The essential metals for humans: A brief overview. J. Inorg. Biochem. 2019, 195, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Prihantono; Irfandi, R.; Raya, I. Warsinggih, Potential anticancer activity of Mn (II) complexes containing arginine dithiocarbamate ligand on MCF-7 breast cancer cell lines. Ann. Med. Surg. 2020, 60, 396–402. [Google Scholar] [CrossRef]
- Wang, M.; Li, M.; Wang, Y.; Shao, Y.; Zhu, Y. Efficient Antibacterial Activity of Hydroxyapatite through ROS Generation Motivated by Trace Mn(III) Coupled H Vacancy. J. Mater. Chem. B 2021, 9, 3401–3411. [Google Scholar] [CrossRef]
- Stanojković-Sebić, A.; Maksimović, J.; Dinić, Z.; Poštić, D.; Ilićić, R.; Stanojković, A. Microelements and Heavy Metals Content in Frequently Utilized Medicinal Plants Collected from the Power Plant Area. Nat. Prod. Commun. 2017, 12, 185–188. [Google Scholar] [CrossRef]
- Köhrle, J. Selenium in Endocrinology-Selenoprotein-Related Diseases, Population Studies, and Epidemiological Evidence. Endocrinology 2021, 162, bqaa228. [Google Scholar] [CrossRef]
- Wang, S.; Kang, X.; Dai, J.; Dai, W.; Zhang, J.; Ji, J. Evaluation of Areca Quality Based on Principal Component and Hierarchical Cluster Analyses in Hainan, China. HortScience 2023, 58, 699–703. [Google Scholar] [CrossRef]
- Singh, D. Principal Component Analysis in Bitter Gourd (Momordica charantia L.). Bangladesh J. Bot. 2019, 51, 1–7. [Google Scholar] [CrossRef]
- Zhang, Q.; Ma, X.; Wei, W. Evaluation of climate comfort of human settlements in Meizhou city. Guangdong Meteorol. 2017, 39, 57–59. [Google Scholar]
- Zhao, Q.; Zhao, X.; Huang, P.; Pu, J.; Zhou, S.; Feng, Y.; Gu, Z.; Shi, X.; Chu, B. Soil Carbon Changes and Its Influencing Factors in Major Forest Types in the Subtropical Area of Yunnan Province. For. Res. 2024, 37, 63–72. [Google Scholar]
- Yang, Q.; You, X.; Zhang, H.; Mwenda, K.; Wang, Y.; Huang, Y. A New Method to Predict Erythrocyte Sedimentation Rate with Natural Geographical Factors and Location by Case-based Reasoning: A Case Study of China. Chin. Geogr. Sci. 2020, 30, 157. [Google Scholar] [CrossRef]
- Lu, Y.; Xin, Y.; Li, J.; Wang, J. Geochronological and Geochemical Constraints of Leucogranites inJianning Area, Central Wuyishan, and Their Geological Implications. Acta Geosci. Sin. 2024, 45, 926–940. [Google Scholar]
- Li, J.; Zhang, M.; Wang, J.; Fu, Z. Effects of Isorhamnetin on intracelluar free calcium concentration in cultured rabbit aortic smooth muscle cells. J. Southwest Med. Univ. 1999, 22, 188–190. [Google Scholar]
- Liu, G.; Li, D.; Mai, H.; Lin, X.; Lu, X.; Chen, K.; Wang, R.; Riaz, M.; Tian, J.; Liang, C. GmSTOP1-3 regulates flavonoid synthesis to reduce ROS accumulation and enhance aluminum tolerance in soybean. J. Hazard. Mater. 2024, 480, 136074. [Google Scholar] [CrossRef]
- Pandey, N. Antioxidant Defense System in Plants Exposed to Metal Toxicity. In Plants Under Metal and Metalloid Stress: Responses, Tolerance and Remediation; Hasanuzzaman, M., Nahar, K., Fujita, M., Eds.; Springer: Singapore, 2018; pp. 107–148. [Google Scholar]
- Lv, M.; Chen, M.; Zhang, R.; Zhang, W.; Wang, C.; Zhang, Y.; Wei, X.; Guan, Y.; Liu, J.; Feng, K.; et al. Manganese is critical for antitumor immune responses via cGAS-STING and improves the efficacy of clinical immunotherapy. Cell Res. 2020, 30, 966–979. [Google Scholar] [CrossRef]
- Al Zahrani, N.A.; El-Shishtawy, R.M.; Asiri, A.M. Recent developments of gallic acid derivatives and their hybrids in medicinal chemistry: A review. Eur. J. Med. Chem. 2020, 204, 112609. [Google Scholar] [CrossRef] [PubMed]
- Prasad, N.R.; Karthikeyan, A.; Karthikeyan, S.; Reddy, B.V. Inhibitory effect of caffeic acid on cancer cell proliferation by oxidative mechanism in human HT-1080 fibrosarcoma cell line. Mol. Cell. Biochem. 2011, 349, 11–19. [Google Scholar] [CrossRef]
- Choudhary, N.; Collignon, T.E.; Tewari, D.; Bishayee, A. Hypericin and its anticancer effects: From mechanism of action to potential therapeutic application. Phytomedicine 2022, 105, 154356. [Google Scholar] [CrossRef]
- Bhosale, P.B.; Ha, S.E.; Vetrivel, P.; Kim, H.H.; Kim, S.M.; Kim, G.S. Functions of polyphenols and its anticancer properties in biomedical research: A narrative review. Transl. Cancer Res. 2020, 9, 7619–7631. [Google Scholar] [CrossRef]
- Biswas, P.; Abu Kaium, M.; Tareq, M.M.I.; Tauhida, S.J.; Hossain, M.R.; Siam, L.S.; Parvez, A.; Bibi, S.; Hasan, M.H.; Rahman, M.M.; et al. The experimental significance of isorhamnetin as an effective therapeutic option for cancer: A comprehensive analysis. Biomed. Pharmacother. 2024, 176, 116860. [Google Scholar] [CrossRef]
- Km, C. Dietary Flavonoid Quercetin in Relation to Cataract. Ph.D. Thesis, University of East Anglia, Norwich, UK, 2002. [Google Scholar]
- Milner, M.J.; Seamon, J.; Craft, E.; Kochian, L.V. Transport properties of members of the ZIP family in plants and their role in Zn and Mn homeostasis. J. Exp. Bot. 2013, 64, 369–381. [Google Scholar] [CrossRef]
- Lee, M.Y.; Ojeda-Britez, S.; Ehrbar, D.; Samwer, A.; Begley, T.J.; Melendez, J.A. Selenoproteins and the senescence-associated epitranscriptome. Exp. Biol. Med. 2022, 247, 2090–2102. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.Z.; Ding, J.; Yu, P.Z.; Lei, C.; Zheng, X.J.; Wang, Y. Composition and distribution of the main active components in selenium-enriched fruit bodies of Cordyceps militaris link. Food Chem. 2013, 137, 164–167. [Google Scholar] [CrossRef]
- Kostic, D.; Velickovic, J.; Mitic, S.; Mitić, M.; Randjelovic, S.; Arsic, B.; Pavlović, A. Correlation among phenolic, toxic metals and antioxidant activity of the extracts of plant species from Southeast Serbia. Bull. Chem. Soc. Ethiop. 2012, 27, 169–178. [Google Scholar] [CrossRef][Green Version]
- Tokalıoğlu, Ş. Determination of trace elements in commonly consumed medicinal herbs by ICP-MS and multivariate analysis. Food Chem. 2012, 134, 2504–2508. [Google Scholar] [CrossRef]
- Chen, L.; Chang, L.; Cao, F.; Wang, G.; Dong, X. Effects of Temperature and Soil Water Deficit on the Flavonoid Content and Activities of Enzymes Involved in Ginkgo Leaves. Acta Bot. Boreali-Occident. Sin. 2013, 33, 755–762. [Google Scholar]
- Wang, G.; Cao, F.; Chang, L.; Guo, X.; Wang, J. Temperature has more effects than soil moisture on biosynthesis of flavonoids in Ginkgo (Ginkgo biloba L.) leaves. New For. 2014, 45, 797–812. [Google Scholar] [CrossRef]
- Meng, J.-F.; Ning, P.-F.; Xu, T.-F.; Zhang, Z.-W. Effect of Rain-Shelter Cultivation of Vitis vinifera cv. Cabernet Gernischet on the Phenolic Profile of Berry Skins and the Incidence of Grape Diseases. Molecules 2013, 18, 381–397. [Google Scholar] [CrossRef]
- Zhang, X.; Zhou, T.; Guo, L.; Zhu, S.; Huang, L. Ecology suitability of Polygonum capitatum in Guizhou province based on topographical conditions. Zhongguo Zhong Yao Za Zhi 2011, 36, 311–315. [Google Scholar]
- Min, R. Effects of Water Stress on Tannin Content and Flavonoid Content of Lespedeza davurica. Master’s Thesis, Shanxi Agricultural University, Jinzhong, China, 2018. [Google Scholar]
- Salami, M.; Heidari, B.; Batley, J.; Wang, J.; Tan, X.-L.; Richards, C.; Tan, H. Integration of genome-wide association studies, metabolomics, and transcriptomics reveals phenolic acid-and flavonoid-associated genes and their regulatory elements under drought stress in rapeseed flowers. Front. Plant Sci. 2024, 14, 1249142. [Google Scholar] [CrossRef]
- Oruc, H.; Sorucu, A.; Ünal, H.; Aydin, L. Effects of season and altitude on biological active certain phenolic compounds levels and partial standardization of propolis. Vet. J. Ank. Univ. 2017, 64, 13–20. [Google Scholar] [CrossRef]
- Sardans, J.; Peñuelas, J.; Prieto, P.; Estiarte, M. Changes in Ca, Fe, Mg, Mo, Na, and S content in a Mediterranean shrubland under warming and drought. J. Geophys. Res. Biogeosci. 2008, 113, G03039. [Google Scholar] [CrossRef]
- Feng, J.; Zhou, Y.; Bai, Y.; Fan, M.; Wang, Y.; Tang, F.; Feng, J. Changes in rainfall impact the release of metal elements in the litter of a subtropical mixed forest. Environ. Res. 2025, 274, 121293. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Y.; Li, R.; Zhang, Q.; Qi, M.; Lu, B.; Huang, L.; Xu, X. Effects of Temperature and Arsenic on Growth and Arsenic Uptake of Different Rice Varieties during Seedlings Stage. Acta Pedol. Sin. 2024, 61, 1156–1165. [Google Scholar]
- Kabata-Pendias, A. Trace Elements in Soils and Plants, 4th ed.; CRC Press: Boca Raton, FL, USA, 2010; pp. 1–520. [Google Scholar]
- Xu, L.; Lu, A.; Wang, J. Research Progress on the Effects of Temperature Changes on the Availability of Heavy Metals in Plants. Jiangsu Agric. Sci. 2016, 44, 26–30. [Google Scholar] [CrossRef]
- Ma, J. Laws of Soil Vertical Variations on Southern Slope of Funiu Mt.: Simultaneous Study on Subtropical Zone. Acta Geogr. Sin. 2004, 59, 998–1011. [Google Scholar] [CrossRef]
- Montgomery, B.L.; Oh, S.; Karakkat, B. Molecular basis and fitness implications of the interplay between light and the regulation of iron homeostasis in photosynthetic organisms. Environ. Exp. Bot. 2015, 114, 48–56. [Google Scholar] [CrossRef]
- Lingyan, Z.; Yubin, Z.; Zonggeng, L.; Wenke, L. Effect of Continuous Red/Blue LED Light and Its Light Intensity on Growth and Mineral Elements Absorption of Lettuce. Spectrosc. Spectr. Anal. 2019, 39, 2474–2480. [Google Scholar]
- Sharma, M.; Kothari, C.; Sherikar, O.; Mehta, P. Concurrent estimation of amlodipine besylate, hydrochlorothiazide and valsartan by RP-HPLC, HPTLC and UV-spectrophotometry. J. Chromatogr. Sci. 2014, 52, 27–35. [Google Scholar] [CrossRef]
- Harshit, D.; Charmy, K.; Nrupesh, P. Organophosphorus pesticides determination by novel HPLC and spectrophotometric method. Food Chem. 2017, 230, 448–453. [Google Scholar] [CrossRef]
- Kowalska, M.; Woźniak, M.; Kijek, M.; Mitrosz, P.; Szakiel, J.; Turek, P. Management of validation of HPLC method for determination of acetylsalicylic acid impurities in a new pharmaceutical product. Sci. Rep. 2022, 12, 1. [Google Scholar] [CrossRef] [PubMed]
- Pereira, J.B.; Dantas, K.G.F. Evaluation of inorganic elements in cat’s claw teas using ICP OES and GF AAS. Food Chem. 2016, 196, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Du, Q.; Cai, Y.; Chen, Z.; Wei, D.; Cao, Y.; Chen, Y.; Yu, S.; Zhao, Q.; Wu, J.; Liu, M. Determination of Trace Elements in Corydalis conspersa and Corydalis linarioides by ICP-AES. J. Chem. 2020, 2020, 6567015. [Google Scholar] [CrossRef]


| P. perfoliatum Samples | Sampling Locations | Geographic Coordinates | Annual Average Temperature (°C) | Annual Average Maximum Temperature (°C) | Annual Average Minimum Temperature (°C) | Annual Average Rainfall (mm) | Elevation (m) | Average Annual Sunshine Duration (h) |
|---|---|---|---|---|---|---|---|---|
| P. perfoliatum-1 | Fujian Wuyishan | E 118°02′, N 27°39′ | 19 | 24 | 14 | 1926 | 350 | 1650.11 |
| P. perfoliatum-2 | Gangxi Hechi | E 107°43′, N 24°32′ | 20 | 25 | 17 | 1493 | 203 | 1372.76 |
| P. perfoliatum-3 | Anhui Bozhou | E 116°19′, N 33°31′ | 14.7 | 22 | 11 | 800 | 32 | 2141.76 |
| P. perfoliatum-4 | Zhejiang Taizhou | E 120°17′, N 28°14′ | 19.4 | 24 | 15 | 1632 | 141 | 1813.98 |
| P. perfoliatum-5 | Jiangxi Ganzhou | E 115°50′, N 26°05′ | 20.2 | 24 | 16 | 1447 | 107 | 1713.21 |
| P. perfoliatum-6 | Guizhou Guiyang | E 106°37′, N 26°21′ | 15.3 | 19 | 12 | 1130 | 1275 | 1173.17 |
| P. perfoliatum-7 | Guangdong Qingyuan | E 113°05′, N 24°26′ | 20.7 | 27 | 19 | 1900 | 105 | 1613.8 |
| P. perfoliatum-8 | Henan Luoyang | E 112°05′, N 34°10′ | 14.5 | 20 | 9 | 603 | 144 | 2151.33 |
| P. perfoliatum-9 | Guangxi Yizhou | E 24°09′, N 109°11′ | 20.4 | 26 | 17 | 1455.4 | 255 | 1696.9 |
| P. perfoliatum-10 | Guangdong Meizhou | E 115°58′, N 24°45′ | 21.2 | 28 | 18 | 1472.9 | 96 | 1847.24 |
| P. perfoliatum-11 | Fujian Fuzhou | E 118°46′, N 26°01′ | 19.7 | 24 | 16 | 1359 | 258 | 1677.31 |
| P. perfoliatum-12 | Yunnan Kunming | E 102°28, N 24°56′ | 15 | 23 | 11 | 924 | 1892 | 2173.98 |
| P. perfoliatum-13 | Hunan Changsha | E 114°10′, N 28°20′ | 17.2 | 21 | 14 | 1368 | 63 | 1577.08 |
| P. perfoliatum-14 | Hebei Baoding | E 114°55′, N 39°22′ | 13.4 | 18 | 8 | 498.9 | 338 | 2549.78 |
| P. perfoliatum-15 | Sichuan Chengdu | E 104°20, N 30°32′ | 16 | 20 | 13 | 904 | 500 | 1087.48 |
| Components | Linear Equation | Determination Coefficients (R2) |
|---|---|---|
| Gallic acid | Y = 53777X − 14524 | R2 = 0.9992 |
| Caffeic acid | Y = 36739X − 8649 | R2 = 0.9991 |
| Hyperoside | Y = 683275X − 438920 | R2 = 0.9990 |
| Quercetin | Y = 796966X − 634788 | R2 = 0.9993 |
| Isorhamnetin | Y = 550994X − 1.45741 × 106 | R2 = 0.9996 |
| The Source | Gallic Acid | Caffeic Acid | Hyperoside | Quercetin | Isorhamnetin |
|---|---|---|---|---|---|
| 1 | 2.21 ± 0.10 a | 10.64 ± 0.31 f | 11.87 ± 0.34 d | 50.19 ± 1.04 b | 0.96 ± 0.02 e |
| 2 | 1.11 ± 0.03 e | 6.54 ± 0.26 g | 5.33 ± 0.16 f | 50.19 ± 1.09 b | 1.02 ± 0.01 d |
| 3 | 1.47 ± 0.05 c | 20.1 ± 0.65 d | 5.23 ± 0.12 f | 30.66 ± 0.63 d | 0.98 ± 0.02 d |
| 4 | 1.04 ± 0.04 e | 10.65 ± 0.33 f | 5.44 ± 0.14 f | ND | 1.01 ± 0.01 d |
| 5 | 1.24 ± 0.03 d | 23.3 ± 0.11 c | 3.22 ± 0.08 g | 23.82 ± 0.47 f | 1.43 ± 0.03 a |
| 6 | ND | 10.63 ± 032 f | 10.37 ± 0.30 e | 37.19 ± 0.76 c | 0.78 ± 0.02 f |
| 7 | 1.10 ± 0.04 e | 25.64 ± 0.99 b | 18.31 ± 0.54 a | 26.44 ± 0.44 e | 0.54 ± 0.01 h |
| 8 | 1.28 ± 0.05 d | 20.03 ± 0.81 d | 5.34 ± 0.11 f | 73.62 ± 1.48 a | 1.32 ± 0.02 b |
| 9 | 1.04 ± 0.03 e | 20.45 ± 0.83 c | 15.38 ± 0.41 b | ND | 0.65 ± 0.01 g |
| 10 | 0.62 ± 0.01 fg | 4.43 ± 0.11 h | 13.24 ± 0.35 c | ND | ND |
| 11 | ND | 10.37 ± 0.04 f | 17.43 ± 0.52 a | 24.09 ± 0.43 f | 0.41 ± 0.01 j |
| 12 | 0.54 ± 0.01 g | 28.35 ± 1.04 a | ND | 25.46 ± 0.51 ef | 0.48 ± 0.01 i |
| 13 | 0.74 ± 0.02 f | 20.36 ± 0.75 d | 15.89 ± 0.36 b | 15.99 ± 0.35 g | 0.66 ± 0.02 g |
| 14 | 0.49 ± 0.01 g | 15.43 ± 0.52 e | 12.34 ± 0.37 d | 15.67 ± 0.32 g | 0.53 ± 0.01 h |
| 15 | 1.60 ± 0.05 b | 18.64 ± 0.73 d | 9.85 ± 0.23 e | 30.66 ± 0.54 d | 1.24 ± 0.02 c |
| CV (%) | 43.2 | 43.7 | 50.0 | 50.0 | 38.2 |
| Elements | Analytical Lines (nm) | Detection Limits (DL) µg/mL | Linear Equation | Determination Coefficients (R2) |
|---|---|---|---|---|
| Al | 167.081 | 0.015 | y = 95.422x + 33.836 | R2 = 0.9998 |
| As | 228.812 | 0.002 | y = 2127.9x + 514.61 | R2 = 0.9999 |
| Ba | 230.424 | 0.002 | y = 22133x + 1177 | R2 = 0.9999 |
| Ca | 220.861 | 0.017 | y = 19148x + 25226 | R2 = 0.9993 |
| Cr | 267.716 | 0.0022 | y = 15277x + 817.33 | R2 = 1 |
| Cu | 324.754 | 0.0072 | y = 26348x + 3810.9 | R2 = 0.9999 |
| Fe | 238.204 | 0.0022 | y = 19253x + 1375 | R2 = 0.9999 |
| Mg | 285.213 | 0.004 | y = 36173x + 2678 | R2 = 0.9998 |
| Mn | 259.373 | 0.0071 | y = 61869x + 1397.1 | R2 = 1 |
| Ni | 221.647 | 0.0071 | y = 10925x + 563.2 | R2 = 1 |
| Pb | 220.353 | 0.0035 | y = 1546.9x + 294.25 | R2 = 1 |
| Se | 203.985 | 0.0028 | y = 153.27x + 106.33 | R2 = 1 |
| Zn | 213.856 | 0.0051 | y = 8665x + 694.4 | R2 = 0.9998 |
| Samples | Al | As | Ba | Ca | Cr | Cu | Fe | Mg | Mn | Ni | Pb | Se | Zn |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 34.8 ± 0.10 l | 6.5 ± 0.01 i | 9.2 ± 0.10 b | 631.0 ± 2.00 h | 4.9 ± 0.03 g | 0.5 ± 0.01 k | 18.0 ± 0.10 l | 113.0 ± 0.69 m | 37.8 ± 0.23 c | 0.6 ± 0.24 i | 1.2 ± 0.14 | 5.8 ± 0.43 l | 4.7 ± 0.42 h |
| 2 | 21.9 ± 0.10 n | 5.2 ± 0.01 j | 1.1 ± 0.05 m | 545.00 ± 3.00 j | 4.6 ± 0.02 h | 0.5 ± 0.02 l | 12.5 ± 0.30 m | 83.9 ± 1.31 n | 11.5 ± 0.13 g | 0.6 ± 0.13 i | 1.0 ± 0.17 | 4.9 ± 0.33 n | 2.8 ± 0.14 j |
| 3 | 54.1 ± 0.10 f | 9.3 ± 0.03 de | 6.4 ± 0.08 f | 765.0 ± 2.00 c | 4.8 ± 0.02 g | 3.0 ± 0.02 e | 39.9 ± 0.20 g | 172.0 ± 1.55 e | 0.0 | 0.9 ± 0.13 f | 1.7 ± 0.18 | 6.7 ± 0.23 g | 6.0 ± 0.48 d |
| 4 | 110.0 ± 0.2 b | 8.8 ± 0.03 e | 8.3 ± 0.08 c | 584.0 ± 4.00 i | 5.8 ± 0.01 e | 2.4 ± 0.003 h | 118.0 ± 0.32 c | 158.0 ± 1.50 i | 32.3 ± 0.10 d | 0.7 ± 0.10 h | 2.1 ± 0.10 | 6.7 ± 0.42 g | 5.4 ± 0.10 e |
| 5 | 49.7 ± 0.20 g | 8.5 ± 0.02 f | 6.6 ± 0.10 e | 643.0 ± 2.00 g | 5.0 ± 0.01 f | 2.2 ± 0.02 i | 37.0 ± 0.13 h | 153.0 ± 2.36 k | 51.3 ± 0.36 a | 0.8 ± 0.20 g | 1.7 ± 0.20 | 6.3 ± 0.10 j | 5.0 ± 0.45 f |
| 6 | 49.2 ± 0.10 h | 10.3 ± 0.03 c | 6.2 ± 0.10 g | 719.0 ± 1.00 e | 5.0 ± 0.01 f | 2.5 ± 0.02 g | 42.6 ± 0.33 f | 201.0 ± 1.75 c | 30.2 ± 0.21 e | 0.9 ± 0.32 f | 1.7 ± 0.11 | 6.4 ± 0.54 i | 4.3 ± 0.32 i |
| 7 | 49.0 ± 0.10 h | 8.9 ± 0.01 e | 6.3 ± 0.05 f | 722.0 ± 0.50 e | 4.9 ± 0.02 g | 2.7 ± 0.03 f | 36.2 ± 0.50 h | 156.0 ± 1.27 j | 50.7 ± 0.63 b | 0.9 ± 0.13 f | 1.7 ± 0.14 | 6.5 ± 0.63 h | 5.0 ± 0.40 f |
| 8 | 28.2 ± 0.30 m | 9.5 ± 0.07 d | 5.9 ± 0.03 h | 648.0 ± 2.00 g | 4.6 ± 0.02 h | 2.2 ± 0.02 i | 19.0 ± 0.25 k | 178.0 ± 2.23 d | 7.4 ± 0.11 h | 0.8 ± 0.10 g | 1.3 ± 0.17 | 5.6 ± 0.73 m | 2.1 ± 0.43 l |
| 9 | 47.2 ± 0.95 i | 7.2 ± 0.07 h | 3.1 ± 0.05 k | 780.0 ± 1.00 b | 5.9 ± 0.04 e | 4.7 ± 0.02 b | 17.9 ± 0.17 l | 170.0 ± 2.55 f | 12.7 ± 0.10 f | 4.9 ± 0.70 a | 2.7 ± 0.16 | 9.6 ± 0.41 a | 2.5 ± 0.34 k |
| 10 | 37.1 ± 0.63 k | 8.0 ± 0.10 g | 2.9 ± 0.06 l | 1200.0 ± 4.00 a | 6.0 ± 0.01 d | 2.0 ± 0.12 j | 23.3 ± 0.230 j | 166.0 ± 1.15 g | 3.7 ± 0.20 i | 1.2 ± 0.23 b | 1.8 ± 0.43 | 6.1 ± 0.56 k | 2.5 ± 0.24 k |
| 11 | 72.6 ± 0.73 d | 7.9 ± 0.10 g | 4.1 ± 0.04 j | 741.0 ± 1.00 d | 6.0 ± 0.02 d | 4.9 ± 0.03 a | 61.4 ± 0.21 d | 134.0 ± 1.52 l | 0.0 | 1.1 ± 0.13 c | 2.4 ± 0.78 | 9.5 ± 0.68 b | 9.2 ± 0.65 b |
| 12 | 109.0 ± 0.70 c | 9.5 ± 0.05 d | 6.5 ± 0.05 e | 734.0 ± 3.00 d | 6.4 ± 0.03 b | 3.6 ± 0.02 d | 190.0 ± 0.31 a | 163.0 ± 1.98 h | 0.0 | 1.0 ± 0.21 de | 2.8 ± 0.45 | 9.4 ± 0.44 c | 6.7 ± 0.41 c |
| 13 | 65.4 ± 0.10 e | 11.1 ± 0.10 b | 10.7 ± 0.05 a | 696.0 ± 3.00 | 6.0 ± 0.002 d | 3.8 ± 0.01 c | 50.0 ± 0.90 e | 230.0 ± 1.63 b | 0.0 | 1.0 ± 0.10 d | 3.6 ± 0.61 | 7.5 ± 0.31 f | 4.8 ± 0.61 g |
| 14 | 115.0 ± 0.20 a | 9.0 ± 0.10 e | 5.4 ± 0.02 i | 504.0 ± 2.00 k | 8.7 ± 0.03 a | 2.0 ± 0.02 j | 128.0 ± 0.63 b | 166.0 ± 2.01 g | 0.0 | 1.0 ± 0.14 e | 2.4 ± 0.32 | 8.2 ± 0.49 d | 5.5 ± 0.47 e |
| 15 | 41.1 ± 0.10 j | 11.8 ± 0.10 a | 7.2 ± 0.02 d | 775.0 ± 1.00 b | 6.2 ± 0.01 c | 2.3 ± 0.03 i | 27.6 ± 0.31 i | 257.0 ± 1.10 a | 0.0 | 1.1 ± 0.15 c | 2.1 ± 0.10 | 7.7 ± 0.89 e | 10.8 ± 0.72 a |
| average | 58.95 | 8.77 | 5.98 | 712.47 | 5.66 | 2.61 | 54.76 | 166.73 | 15.84 | 1.16 | 2.02 | 7.13 | 5.14 |
| CV (%) | 50.92 | 26.4 | 42.1 | 28.7 | 28.9 | 48.2 | 75.5 | 29.4 | 130 | 90.5 | 34.0 | 20.7 | 51.1 |
| Gallic Acid | Caffeic Acid | Hyperoside | Quercetin | Isorhamnetin | |
|---|---|---|---|---|---|
| Al | −0.425 | 0.214 | −0.137 | −0.590 * | −0.291 |
| As | −0.168 | 0.445 | −0.005 | −0.215 | 0.112 |
| Ba | 0.3 | 0.356 | −0.017 | −0.146 | 0.281 |
| Ca | −0.16 | −0.176 | 0.253 | −0.335 | −0.557 * |
| Cr | −0.393 | 0.025 | 0.184 | −0.522 * | −0.441 |
| Cu | −0.508 | 0.404 | 0.323 | −0.229 | −0.358 |
| Fe | −0.401 | 0.296 | −0.354 | −0.444 | −0.274 |
| Mg | −0.1 | 0.35 | 0.131 | −0.192 | 0.074 |
| Mn | 0.282 | 0.089 | 0.008 | 0.034 | 0.314 |
| Ni | −0.054 | 0.174 | 0.32 | 0.074 | −0.202 |
| Pb | −0.434 | 0.372 | 0.275 | −0.453 | −0.396 |
| Se | −0.433 | 0.376 | 0.24 | −0.28 | −0.41 |
| Zn | −0.053 | 0.181 | 0.047 | −0.277 | 0.062 |
| Annual Average Temperature | Annual Average Maximum Temperature | Annual Average Minimum Temperature | Annual Average Rainfall | Elevation | Average Annual Sunshine Duration | |
|---|---|---|---|---|---|---|
| Gallic acid | 0.101 | 0.135 | 0.036 | 0.226 | −0.37 | −0.072 |
| Caffeic acid | −0.298 | −0.143 | −0.221 | −0.238 | 0.238 | 0.252 |
| Hyperoside | 0.354 | 0.209 | 0.411 | 0.364 | −0.372 | −0.24 |
| Quercetin | −0.359 | −0.354 | −0.379 | −0.228 | 0.088 | −0.086 |
| Isorhamnetin | −0.2 | −0.363 | −0.256 | −0.14 | −0.172 | −0.21 |
| Annual Average Temperature | Annual Average Maximum Temperature | Annual Average Minimum Temperature | Annual Average Rainfall | Elevation | Average Annual Sunshine Duration | |
|---|---|---|---|---|---|---|
| Al | −0.331 | −0.266 | −0.364 | −0.263 | 0.349 | 0.531 * |
| As | −0.578 * | −0.607 * | −0.45 | −0.488 | 0.24 | −0.073 |
| Ba | −0.294 | −0.383 | −0.302 | 0.064 | 0.053 | −0.022 |
| Ca | 0.309 | 0.471 | 0.377 | 0.12 | −0.013 | −0.114 |
| Cr | −0.337 | −0.337 | −0.358 | −0.426 | 0.176 | 0.445 |
| Cu | −0.009 | 0.035 | 0.056 | −0.135 | 0.131 | 0.105 |
| Fe | −0.403 | −0.245 | −0.42 | −0.32 | 0.594 * | 0.528 * |
| Mg | −0.444 | −0.508 * | −0.316 | −0.422 | 0.132 | −0.2 |
| Mn | 0.47 | 0.328 | 0.426 | 0.648 ** | −0.123 | −0.28 |
| Ni | 0.242 | 0.267 | 0.244 | 0.062 | −0.048 | −0.024 |
| Pb | −0.164 | −0.175 | −0.104 | −0.164 | 0.208 | 0.136 |
| Se | −0.145 | −0.086 | −0.113 | −0.23 | 0.367 | 0.211 |
| Zn | −0.22 | −0.286 | −0.141 | −0.177 | 0.216 | −0.197 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Zhao, L.; Wang, X.; Zhang, C.; Zuo, H.; Gao, D. Impact of Geographical Origin on the Contents of Inorganic Elements and Bioactive Compounds in Polygonum perfoliatum L. Molecules 2025, 30, 2231. https://doi.org/10.3390/molecules30102231
Zhang Y, Zhao L, Wang X, Zhang C, Zuo H, Gao D. Impact of Geographical Origin on the Contents of Inorganic Elements and Bioactive Compounds in Polygonum perfoliatum L. Molecules. 2025; 30(10):2231. https://doi.org/10.3390/molecules30102231
Chicago/Turabian StyleZhang, Yanping, Liyuan Zhao, Xinsheng Wang, Chenxi Zhang, Haichao Zuo, and Di Gao. 2025. "Impact of Geographical Origin on the Contents of Inorganic Elements and Bioactive Compounds in Polygonum perfoliatum L." Molecules 30, no. 10: 2231. https://doi.org/10.3390/molecules30102231
APA StyleZhang, Y., Zhao, L., Wang, X., Zhang, C., Zuo, H., & Gao, D. (2025). Impact of Geographical Origin on the Contents of Inorganic Elements and Bioactive Compounds in Polygonum perfoliatum L. Molecules, 30(10), 2231. https://doi.org/10.3390/molecules30102231






