Towards a Rational Design of Biosensors: Engineering Covalently Grafted Interfacial Adlayers as a Testbed Platform for Electrochemical Detection of Epinephrine
Abstract
1. Introduction
2. Results and Discussion
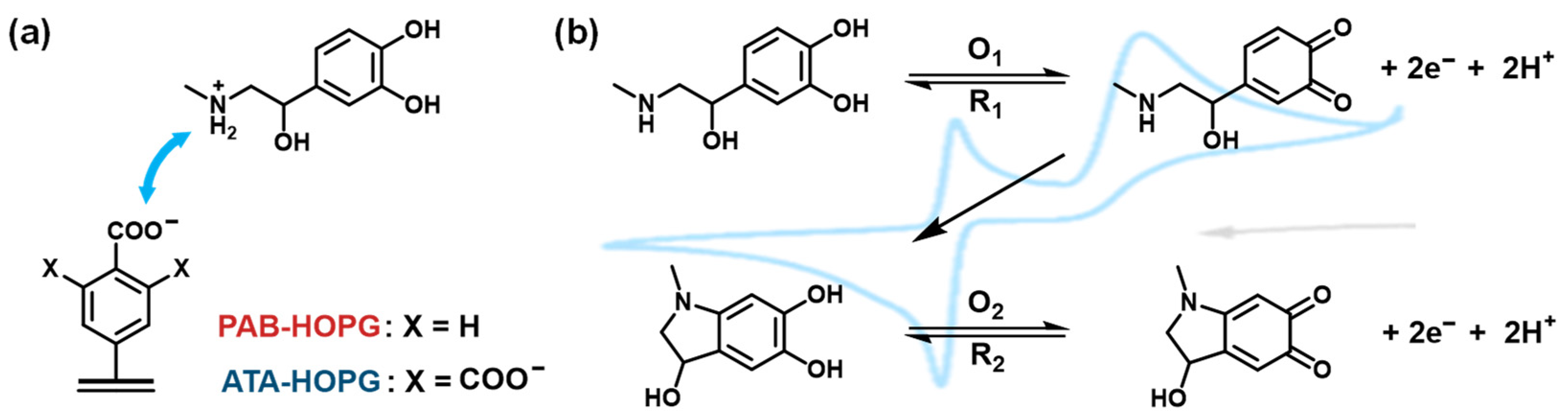
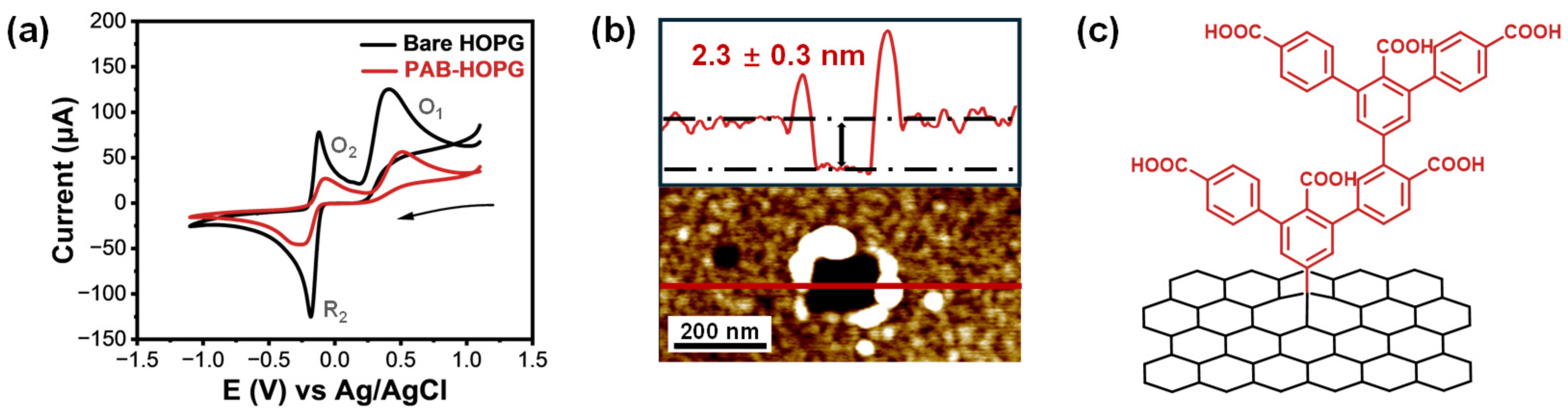
2.1. Electrochemical Grafting of PAB onto Graphite
2.2. Electrochemical Response of PAB-Modified Graphite to EP and AFM
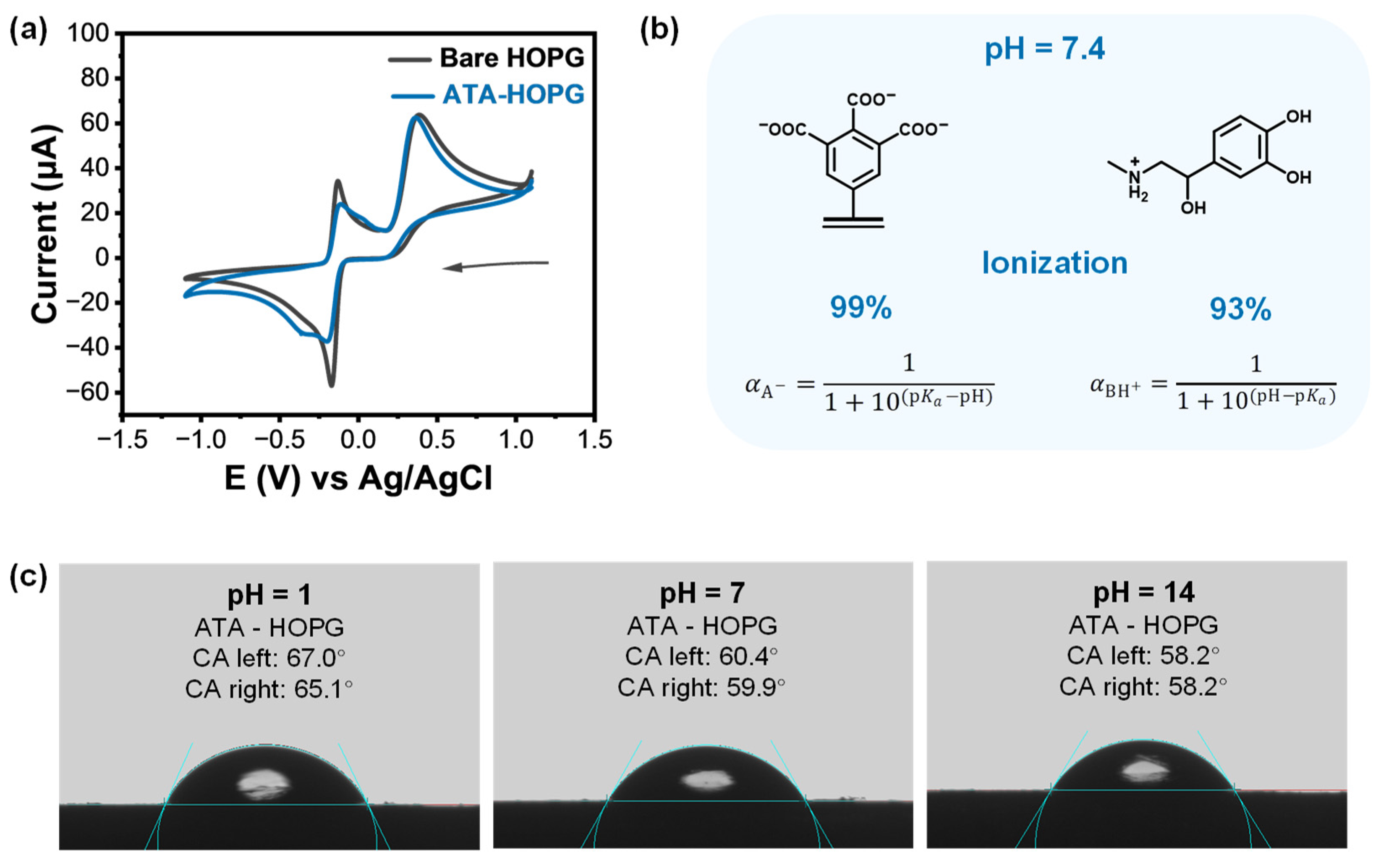
2.3. Molecular Design and Electrochemical Grafting of ATA onto Graphite
2.4. ATA-Modified Graphite Electrodes: CV of EP and pH-Dependent Surface Ionization
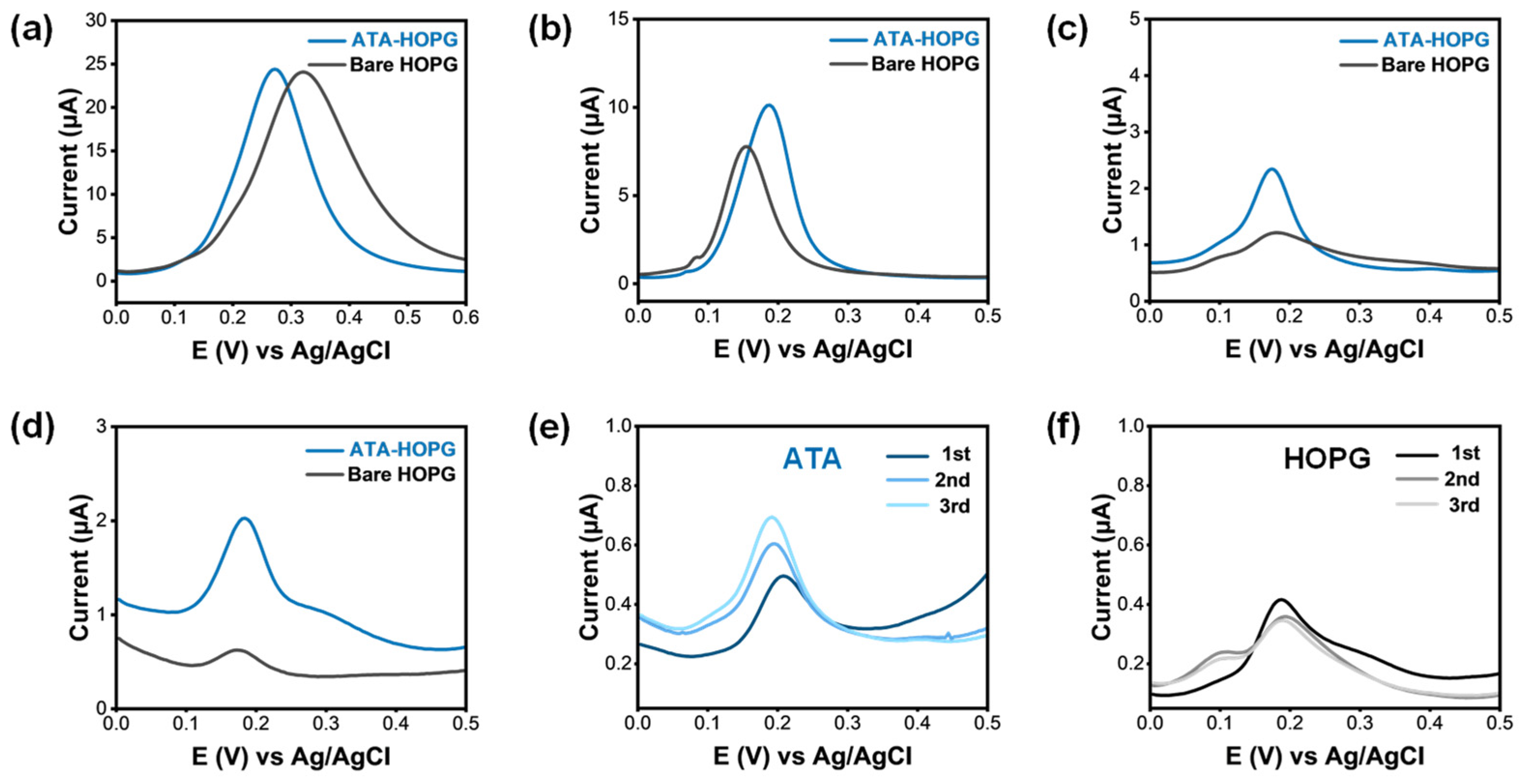
2.5. ATA-Modified Graphite Electrodes: DPV Sensing at Low EP Concentration
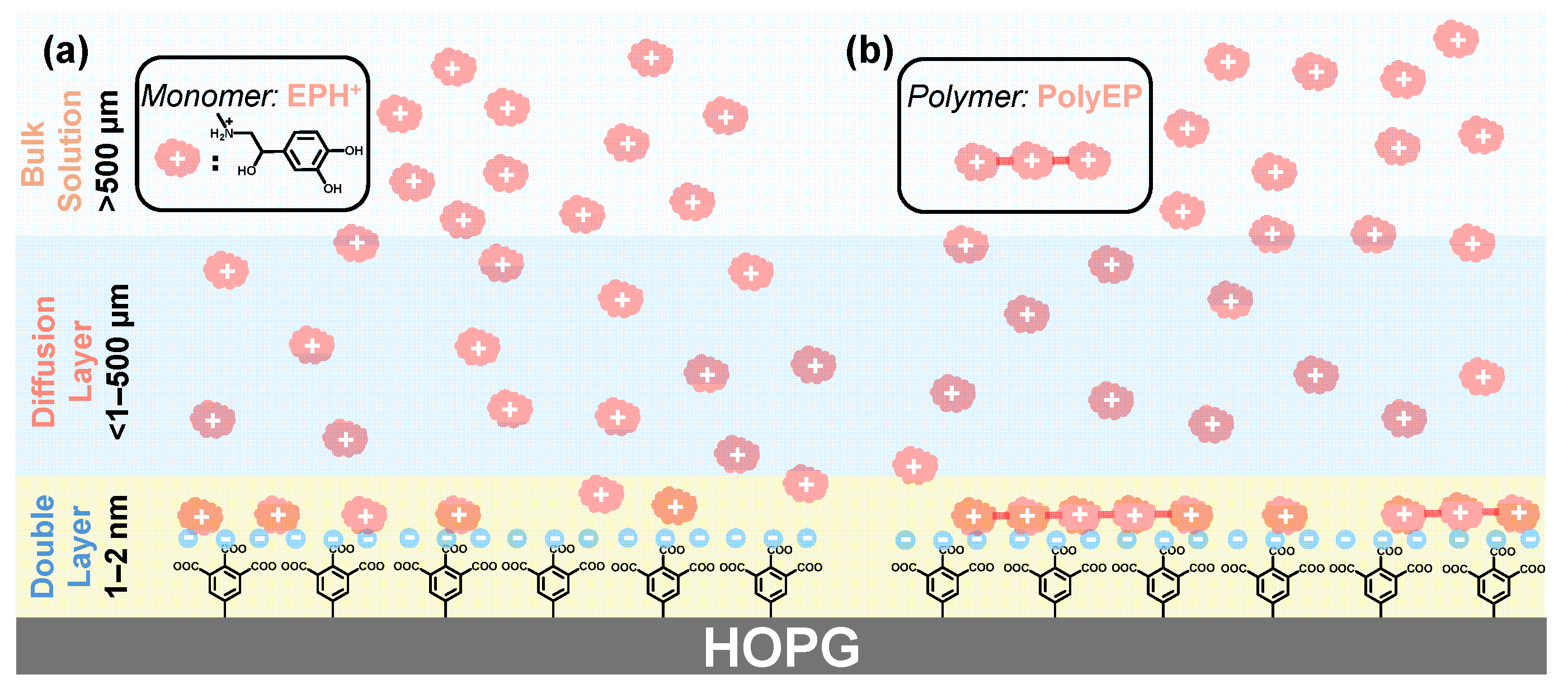
2.6. Interfacial Recognition and Accumulation Processes on Carboxylated Graphite Electrodes
3. Materials and Methods
3.1. Materials
3.2. Preparation and Cleaning of Covalently Grafted Samples on Graphite Surfaces
3.3. Material Characterization and Electrochemical Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| EP | Epinephrine |
| PAB | Grafts based on para-aminobenzoic acid |
| ATA | Grafts based on 3,4,5-tricarboxybenzenediazonium |
| HOPG | Highly oriented pyrolytic graphite |
| GO | Graphene oxide |
| CQDs | Carbon quantum dots |
| CNTs | Carbon nanotubes |
| rGO | Reduced graphene oxide |
| CV | Cyclic voltammetry |
| AFM | Atomic force microscopy |
| PBS | Phosphate-buffered saline |
| DPV | Differential pulse voltammetry |
| TBD | Grafts based on 3,5-bis-tert-butylbenzenediazonium |
References
- Turner, A.P.F. Biosensors: Sense and sensibility. Chem. Soc. Rev. 2013, 42, 3184–3196. [Google Scholar] [CrossRef] [PubMed]
- Naresh, V.; Lee, N. A Review on Biosensors and Recent Development of Nanostructured Materials-Enabled Biosensors. Sensors 2021, 21, 1109. [Google Scholar] [CrossRef]
- Xu, J.J.; Zhao, W.W.; Song, S.P.; Fan, C.H.; Chen, H.Y. Functional nanoprobes for ultrasensitive detection of biomolecules: An update. Chem. Soc. Rev. 2014, 43, 1601–1611. [Google Scholar] [CrossRef]
- Bhalla, N.; Pan, Y.W.; Yang, Z.G.; Payam, A.F. Opportunities and Challenges for Biosensors and Nanoscale Analytical Tools for Pandemics: COVID-19. ACS Nano 2020, 14, 7783–7807. [Google Scholar] [CrossRef]
- Kowalczyk, A. Trends and perspectives in DNA biosensors as diagnostic devices. Curr. Opin. Electrochem. 2020, 23, 36–41. [Google Scholar] [CrossRef]
- Xiao, F.; Wang, L.; Duan, H.W. Nanomaterial based electrochemical sensors for in vitro detection of small molecule metabolites. Biotechnol. Adv. 2016, 34, 234–249. [Google Scholar] [CrossRef] [PubMed]
- Kabay, G.; DeCastro, J.; Altay, A.; Smith, K.; Lu, H.W.; Capossela, A.M.; Moarefian, M.; Aran, K.; Dincer, C. Emerging Biosensing Technologies for the Diagnostics of Viral Infectious Diseases. Adv. Mater. 2022, 34, e2201085. [Google Scholar] [CrossRef]
- Nnachi, R.C.; Sui, N.; Ke, B.W.; Luo, Z.H.; Bhalla, N.; He, D.P.; Yang, Z.G. Biosensors for rapid detection of bacterial pathogens in water, food and environment. Environ. Int. 2022, 166, 107357. [Google Scholar] [CrossRef]
- Felix, F.S.; Angnes, L. Electrochemical immunosensors—A powerful tool for analytical applications. Biosens. Bioelectron. 2018, 102, 470–478. [Google Scholar] [CrossRef]
- Wang, K.; Lin, X.G.; Zhang, M.X.; Li, Y.; Luo, C.F.; Wu, J.E. Review of Electrochemical Biosensors for Food Safety Detection. Biosensors 2022, 12, 959. [Google Scholar] [CrossRef]
- Ye, Y.L.; Ji, J.; Sun, Z.Y.; Shen, P.L.; Sun, X.L. Recent advances in electrochemical biosensors for antioxidant analysis in foodstuff. TrAC Trends Anal. Chem. 2020, 122, 115718. [Google Scholar] [CrossRef]
- Aydin, E.B.; Aydin, M.; Sezginturk, M.K. Biosensors in Drug Discovery and Drug Analysis. Curr. Anal. Chem. 2019, 15, 467–484. [Google Scholar] [CrossRef]
- Giuliano, K.A.; Taylor, D.L. Fluorescent-protein biosensors: New tools for drug discovery. Trends Biotechnol. 1998, 16, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.; Shin, M.; Lee, T.; Choi, J.W. Highly Sensitive Biosensors Based on Biomolecules and Functional Nanomaterials Depending on the Types of Nanomaterials: A Perspective Review. Materials 2020, 13, 299. [Google Scholar] [CrossRef]
- Akkilic, N.; Geschwindner, S.; Höök, F. Single-molecule biosensors: Recent advances and applications. Biosens. Bioelectron. 2020, 151, 111944. [Google Scholar] [CrossRef]
- Hemdan, M.; Ali, M.A.; Doghish, A.S.; Mageed, S.S.A.; Elazab, I.M.; Khalil, M.M.; Mabrouk, M.; Das, D.B.; Amin, A.S. Innovations in Biosensor Technologies for Healthcare Diagnostics and Therapeutic Drug Monitoring: Applications, Recent Progress, and Future Research Challenges. Sensors 2024, 24, 5143. [Google Scholar] [CrossRef] [PubMed]
- Walther, B.K.; Dinu, C.Z.; Guldi, D.M.; Sergeyev, V.G.; Creager, S.E.; Cooke, J.P.; Guiseppi-Elie, A. Nanobiosensing with graphene and carbon quantum dots: Recent advances. Mater. Today 2020, 39, 23–46. [Google Scholar] [CrossRef]
- Liang, X.; Li, N.; Zhang, R.H.; Yin, P.G.; Zhang, C.M.; Yang, N.; Liang, K.; Kong, B. Carbon-based SERS biosensor: From substrate design to sensing and bioapplication. NPG Asia Mater. 2021, 13, 8. [Google Scholar] [CrossRef]
- Rezaei, B.; Jamei, H.R.; Ensafi, A.A. An ultrasensitive and selective electrochemical aptasensor based on rGO-MWCNTs/Chitosan/carbon quantum dot for the detection of lysozyme. Biosens. Bioelectron. 2018, 115, 37–44. [Google Scholar] [CrossRef]
- Zhu, X.L.; Wu, G.L.; Lu, N.; Yuan, X.; Li, B.K. A miniaturized electrochemical toxicity biosensor based on graphene oxide quantum dots/carboxylated carbon nanotubes for assessment of priority pollutants. J. Hazard. Mater. 2017, 324, 272–280. [Google Scholar] [CrossRef]
- Zhu, Z.Z. An Overview of Carbon Nanotubes and Graphene for Biosensing Applications. Nano-Micro Lett. 2017, 9, 25. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, L.; Manoharan, R.; Mohanta, K.; Bhattacharjee, R.R. Conducting carbon quantum dots—A nascent nanomaterial. J. Mater. Chem. A 2015, 3, 1580–1586. [Google Scholar] [CrossRef]
- Castro Neto, A.H.; Guinea, F.; Peres, N.M.R.; Novoselov, K.S.; Geim, A.K. The electronic properties of graphene. Rev. Mod. Phys. 2009, 81, 109–162. [Google Scholar] [CrossRef]
- Ali, A.; Koloor, S.S.R.; Alshehri, A.H.; Arockiarajan, A. Carbon nanotube characteristics and enhancement effects on the mechanical features of polymer-based materials and structures—A review. J. Mater. Res. Technol. 2023, 24, 6495–6521. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Fal’ko, V.I.; Colombo, L.; Gellert, P.R.; Schwab, M.G.; Kim, K. A roadmap for graphene. Nature 2012, 490, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Wei, X.D.; Kysar, J.W.; Hone, J. Measurement of the elastic properties and intrinsic strength of monolayer graphene. Science 2008, 321, 385–388. [Google Scholar] [CrossRef]
- Peigney, A.; Laurent, C.; Flahaut, E.; Bacsa, R.R.; Rousset, A. Specific surface area of carbon nanotubes and bundles of carbon nanotubes. Carbon 2001, 39, 507–514. [Google Scholar] [CrossRef]
- Ma, Y.F.; Chang, H.C.; Zhang, M.; Chen, Y.S. Graphene-Based Materials for Lithium-Ion Hybrid Supercapacitors. Adv. Mater. 2015, 27, 5296–5308. [Google Scholar] [CrossRef]
- Eivazzadeh-Keihan, R.; Noruzi, E.B.; Chidar, E.; Jafari, M.; Davoodi, F.; Kashtiaray, A.; Gorab, M.G.; Hashemi, S.M.; Javanshir, S.; Cohan, R.A.; et al. Applications of carbon-based conductive nanomaterials in biosensors. Chem. Eng. J. 2022, 442, 136183. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhou, S.G.; Zhao, B.; Zhuang, L.; Wang, Y.Q. Microbially-reduced graphene scaffolds to facilitate extracellular electron transfer in microbial fuel cells. Bioresour. Technol. 2012, 116, 453–458. [Google Scholar] [CrossRef]
- Dai, L.M. Functionalization of Graphene for Efficient Energy Conversion and Storage. Acc. Chem. Res. 2013, 46, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Li, D.P.; Zhang, W.S.; Yu, X.Q.; Wang, Z.P.; Su, Z.Q.; Wei, G. When biomolecules meet graphene: From molecular level interactions to material design and applications. Nanoscale 2016, 8, 19491–19509. [Google Scholar] [CrossRef]
- Zhang, L.M.; Xia, J.G.; Zhao, Q.H.; Liu, L.W.; Zhang, Z.J. Functional Graphene Oxide as a Nanocarrier for Controlled Loading and Targeted Delivery of Mixed Anticancer Drugs. Small 2010, 6, 537–544. [Google Scholar] [CrossRef]
- Xu, Y.X.; Wu, Q.O.; Sun, Y.Q.; Bai, H.; Shi, G.Q. Three-Dimensional Self-Assembly of Graphene Oxide and DNA into Multifunctional Hydrogels. ACS Nano 2010, 4, 7358–7362. [Google Scholar] [CrossRef]
- Lei, J.P.; Ju, H.X. Signal amplification using functional nanomaterials for biosensing. Chem. Soc. Rev. 2012, 41, 2122–2134. [Google Scholar] [CrossRef]
- Liu, X.J.; Cheng, H.; Zhao, Y.C.; Wang, Y.; Li, F. Portable electrochemical biosensor based on laser-induced graphene and MnO2 switch-bridged DNA signal amplification for sensitive detection of pesticide. Biosens. Bioelectron. 2022, 199, 113906. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.Y.; Dai, Z.H. Carbon nanomaterial-based electrochemical biosensors: An overview. Nanoscale 2015, 7, 6420–6431. [Google Scholar] [CrossRef] [PubMed]
- Sanati, A.; Jalali, M.; Raeissi, K.; Karimzadeh, F.; Kharaziha, M.; Mahshid, S.S.; Mahshid, S. A review on recent advancements in electrochemical biosensing using carbonaceous nanomaterials. Microchim. Acta 2019, 186, 773. [Google Scholar] [CrossRef]
- Campuzano, S.; Yáñez-Sedeño, P.; Pingarrón, J.M. Carbon Dots and Graphene Quantum Dots in Electrochemical Biosensing. Nanomaterials 2019, 9, 634. [Google Scholar] [CrossRef]
- Qin, Y.Q.; Cui, J.W.; Zhang, Y.; Wang, Y.; Zhang, X.Y.; Zheng, H.M.; Shu, X.; Fu, B.W.; Wu, Y.C. Integration of microfluidic injection analysis with carbon nanomaterials/gold nanowire arrays-based biosensors for glucose detection. Sci. Bull. 2016, 61, 473–480. [Google Scholar] [CrossRef]
- Nie, H.G.; Yao, Z.; Zhou, X.M.; Yang, Z.; Huang, S.M. Nonenzymatic electrochemical detection of glucose using well-distributed nickel nanoparticles on straight multi-walled carbon nanotubes. Biosens. Bioelectron. 2011, 30, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Balkourani, G.; Brouzgou, A.; Tsiakaras, P. A review on recent advancements in electrochemical detection of dopamine using carbonaceous nanomaterials. Carbon 2023, 213, 118281. [Google Scholar] [CrossRef]
- Cho, Y.W.; Park, J.H.; Lee, K.H.; Lee, T.; Luo, Z.T.; Kim, T.H. Recent advances in nanomaterial-modified electrical platforms for the detection of dopamine in living cells. Nano Converg. 2020, 7, 40. [Google Scholar] [CrossRef] [PubMed]
- Suriyaprakash, J.; Gupta, N.; Shan, L.W.; Wu, L.J. Immobilized Molecules’ Impact on the Efficacy of Nanocarbon Organic Sensors for Ultralow Dopamine Detection in Biofluids. Adv. Mater. Technol. 2022, 7, 2200099. [Google Scholar] [CrossRef]
- Yola, M.L.; Atar, N. Development of molecular imprinted sensor including graphitic carbon nitride/N-doped carbon dots composite for novel recognition of epinephrine. Compos. Part B Eng. 2019, 175, 107113. [Google Scholar] [CrossRef]
- Suriyaprakash, J.; Gupta, N.; Wu, L.J.; Shan, L.W. Engineering of all solution/substrate processable biosensors for the detection of epinephrine as low as pM with rapid readout. Chem. Eng. J. 2022, 436, 135254. [Google Scholar] [CrossRef]
- Sun, H.J.; Ren, J.S.; Qu, X.G. Carbon Nanomaterials and DNA: From Molecular Recognition to Applications. Acc. Chem. Res. 2016, 49, 461–470. [Google Scholar] [CrossRef]
- Guo, Y.J.; Guo, S.J.; Ren, J.T.; Zhai, Y.M.; Dong, S.J.; Wang, E.K. Cyclodextrin Functionalized Graphene Nanosheets with High Supramolecular Recognition Capability: Synthesis and Host-Guest Inclusion for Enhanced Electrochemical Performance. ACS Nano 2010, 4, 4001–4010. [Google Scholar] [CrossRef]
- Jiang, C.; Alam, M.T.; Silva, S.M.; Taufik, S.; Fan, S.J.; Gooding, J.J. Unique Sensing Interface That Allows the Development of an Electrochemical Immunosensor for the Detection of Tumor Necrosis Factor α in Whole Blood. ACS Sens. 2016, 1, 1432–1438. [Google Scholar] [CrossRef]
- Mondal, A.; Jana, N.R. Fluorescent detection of cholesterol using β-cyclodextrin functionalized graphene. Chem. Commun. 2012, 48, 7316–7318. [Google Scholar] [CrossRef]
- Bartholomeusz, G.; Cherukuri, P.; Kingston, J.; Cognet, L.; Lemos, R.; Leeuw, T.K.; Gumbiner-Russo, L.; Weisman, R.B.; Powis, G. In Vivo Therapeutic Silencing of Hypoxia-Inducible Factor 1 Alpha (HIF-1α) Using Single-Walled Carbon Nanotubes Noncovalently Coated with siRNA. Nano Res. 2009, 2, 279–291. [Google Scholar] [CrossRef] [PubMed]
- Siu, K.S.; Chen, D.; Zheng, X.F.; Zhang, X.S.; Johnston, N.; Liu, Y.L.; Yuan, K.; Koropatnick, J.; Gillies, E.R.; Min, W.P. Non-covalently functionalized single-walled carbon nanotube for topical siRNA delivery into melanoma. Biomaterials 2014, 35, 3435–3442. [Google Scholar] [CrossRef]
- Liu, Y.X.; Dong, X.C.; Chen, P. Biological and chemical sensors based on graphene materials. Chem. Soc. Rev. 2012, 41, 2283–2307. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.J.; Zhu, Y.F.; Kaskel, S. Porphyrin-Based Metal-Organic Frameworks for Biomedical Applications. Angew. Chem. Int. Ed. 2021, 60, 5010–5035. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Van Guyse, J.F.R.; de la Rosa, V.R.; Van Gorp, H.; Walke, P.; González, M.C.R.; Uji-i, H.; Hoogenboom, R.; De Feyter, S.; Mertens, S.F.L. One-Step Covalent Immobilization of β-Cyclodextrin on sp2 Carbon Surfaces for Selective Trace Amount Probing of Guests. Adv. Funct. Mater. 2019, 29, 1901488. [Google Scholar] [CrossRef]
- Freddi, S.; Rodriguez Gonzalez, M.C.; Casotto, A.; Sangaletti, L.; De Feyter, S. Machine-learning-aided NO2 discrimination with an array of graphene chemiresistors covalently functionalized by diazonium chemistry. Chem. Eur. J. 2023, 29, e202302154. [Google Scholar] [CrossRef]
- Thomas, P.A.; Wu, F.; Kravets, V.; Ivasenko, O.; Day, P.; Grigorenko, A. Graphene-based plasmonic biosensing. In Proceedings of the 2017 Conference on Lasers and Electro-Optics Europe & European Quantum Electronics Conference (CLEO/Europe-EQEC), Munich, Germany, 25–29 June 2017; p. 1613. [Google Scholar]
- Wu, F.; Singh, J.; Thomas, P.; Ivasenko, O.; De Feyter, S.; Kravets, V.; Day, P.; Grigorenko, A. Ultrasensitive plasmonic biosensing. In Proceedings of the 2018 International Conference Laser Optics (ICLO), Saint Petersburg, Russia, 4–8 June 2018; p. 530. [Google Scholar]
- Wu, F.; Thomas, P.; Kravets, V.; Arola, H.O.; Soikkeli, M.; Iljin, K.; Kim, G.; Kim, M.; Shin, H.; Andreeva, D.; et al. Layered material platform for surface plasmon resonance biosensing. Sci. Rep. 2019, 9, 20286. [Google Scholar] [CrossRef]
- Wu, F.; Singh, J.; Thomas, P.A.; Ge, Q.; Kravets, V.G.; Day, P.J.; Grigorenko, A.N. Ultrasensitive and rapid detection of malaria using graphene-enhanced surface plasmon resonance. Chem. Commun. 2020, 7, 045019. [Google Scholar] [CrossRef]
- Daelemans, B.; Bernaerts, S.; Eyley, S.; Thielemans, W.; Dehaen, W.; De Feyter, S. Covalent immobilization of N-heterocyclic carbenes on pristine carbon substrates: From nanoscale characterization to bulk catalysis. Chem. Commun. 2024, 60, 1432–1435. [Google Scholar] [CrossRef]
- Zhu, Z.X.; Lu, W.Y.; Li, N.; Xu, T.F.; Chen, W.X. Pyridyl-containing polymer blends stabilized iron phthalocyanine to degrade sulfonamides by enzyme-like process. Chem. Eng. J. 2017, 321, 58–66. [Google Scholar] [CrossRef]
- Wang, Q.H.; Jin, Z.; Kim, K.K.; Hilmer, A.J.; Paulus, G.L.C.; Shih, C.-J.; Ham, M.-H.; Sanchez-Yamagishi, J.D.; Watanabe, K.; Taniguchi, T.; et al. Understanding and controlling the substrate effect on graphene electron-transfer chemistry via reactivity imprint lithography. Nat. Chem. 2012, 4, 724–732. [Google Scholar] [CrossRef]
- Yang, X.; Zhao, P.C.; Xie, Z.G.; Ni, M.J.; Wang, C.X.; Yang, P.P.; Xie, Y.X.; Fei, J.J. Selective determination of epinephrine using electrochemical sensor based on ordered mesoporous carbon/nickel oxide nanocomposite. Talanta 2021, 233, 122545. [Google Scholar] [CrossRef]
- Huynh, T.M.T.; Tahara, K.; De Feyter, S.; Phan, T.H. On the role of functional groups in the formation of diazonium based covalent attachments: Dendritic vs. layer-by-layer growth. RSC Adv. 2023, 13, 24576–24582. [Google Scholar] [CrossRef] [PubMed]
- Greenwood, J.; Phan, T.H.; Fujita, Y.; Li, Z.; Lvasenko, O.; Vanderlinden, W.; Van Gorp, H.; Frederickx, W.; Lu, G.; Tahara, K.; et al. Covalent Modification of Graphene and Graphite Using Diazonium Chemistry: Tunable Grafting and Nanomanipulation. ACS Nano 2015, 9, 5520–5535. [Google Scholar] [CrossRef] [PubMed]
- Tahara, K.; Kubo, Y.; Lindner, B.; Hashimoto, S.; Hirose, S.; Brown, A.; Hirsch, B.; Daukiya, L.; De Feyter, S.; Tobe, Y. Steric and Electronic Effects of Electrochemically Generated Aryl Radicals on Grafting of the Graphite Surface. Langmuir 2019, 35, 2089–2098. [Google Scholar] [CrossRef]
- Radic, N.; Prkic, A. Historical remarks on the Henderson-Hasselbalch equation: Its advantages and limitations and a novel approach for exact pH calculation in buffer region. Rev. Anal. Chem. 2012, 31, 93–98. [Google Scholar] [CrossRef]
- Maxwell, W.R.; Partington, J.R. The dissociation constants of some polybasic acids. Part III. Trans. Faraday Soc. 1937, 33, 0670–0677. [Google Scholar] [CrossRef]
- Riegelman, S.; Fischer, E.Z.; Strait, L.A. Acid Dissociation Constants of Phenylalkanolamines. J. Pharm. Sci. 1962, 51, 129. [Google Scholar] [CrossRef]
- Gong, J.M.; Lin, X.Q. A glassy carbon supported bilayer lipid-like membrane of 5,5-ditetradecyl-2-(2-trimethyl-ammonioethyl)-1,3-dioxane bromide for electrochemical sensing of epinephrine. Electrochim. Acta 2004, 49, 4351–4357. [Google Scholar] [CrossRef]
- Patel, A.N.; McKelvey, K.; Unwin, P.R. Nanoscale Electrochemical Patterning Reveals the Active Sites for Catechol Oxidation at Graphite Surfaces. J. Am. Chem. Soc. 2012, 134, 20246–20249. [Google Scholar] [CrossRef]
- Patel, A.N.; Tan, S.Y.; Unwin, P.R. Epinephrine electro-oxidation highlights fast electrochemistry at the graphite basal surface. Chem. Commun. 2013, 49, 8776–8778. [Google Scholar] [CrossRef] [PubMed]
- Fredj, Z.; Sawan, M. Advanced Nanomaterials-Based Electrochemical Biosensors for Catecholamines Detection: Challenges and Trends. Biosensors 2023, 13, 211. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, X.; Fang, Y.; Ivasenko, O. Towards a Rational Design of Biosensors: Engineering Covalently Grafted Interfacial Adlayers as a Testbed Platform for Electrochemical Detection of Epinephrine. Molecules 2025, 30, 2236. https://doi.org/10.3390/molecules30102236
Chang X, Fang Y, Ivasenko O. Towards a Rational Design of Biosensors: Engineering Covalently Grafted Interfacial Adlayers as a Testbed Platform for Electrochemical Detection of Epinephrine. Molecules. 2025; 30(10):2236. https://doi.org/10.3390/molecules30102236
Chicago/Turabian StyleChang, Xiaoli, Yuan Fang, and Oleksandr Ivasenko. 2025. "Towards a Rational Design of Biosensors: Engineering Covalently Grafted Interfacial Adlayers as a Testbed Platform for Electrochemical Detection of Epinephrine" Molecules 30, no. 10: 2236. https://doi.org/10.3390/molecules30102236
APA StyleChang, X., Fang, Y., & Ivasenko, O. (2025). Towards a Rational Design of Biosensors: Engineering Covalently Grafted Interfacial Adlayers as a Testbed Platform for Electrochemical Detection of Epinephrine. Molecules, 30(10), 2236. https://doi.org/10.3390/molecules30102236





