Enzymatic Extraction of Hawthorn Pectin Under Ultrahigh Pressure: Optimization and Characterization
Abstract
1. Introduction
2. Results
2.1. Optimization of the Process for Extracting Hawthorn Pectin
2.1.1. Single Factor Experiment Results
2.1.2. Response Surface Analysis
2.2. Characterization Analysis of Properties
2.2.1. Basic Indicators
| Basic Indicators | UPAEE | EE | WE | AE | UPAAE |
|---|---|---|---|---|---|
| Degree of esterification/% | 38.20 ± 1.49 c | 60.43 ± 2.42 a | 56.90 ± 2.44 b | 58.42 ± 3.97 a | 55.84 ± 2.12 b |
| Total galacturonic acid content/% | 58.09 ± 0.64 a | 55.81 ± 1.22 b | 55.83 + 0.90 b | 52.57 + 0.74 c | 53.28 + 0.44 c |
| Total polyphenols/% | 2.16 ± 0.03 a | 1.02 ± 0.02 b | 0.96 + 0.03 c | 0.98 + 0.04 c | 0.92 + 0.02 d |
| Molecular weight/kDa | 121.34 e | 622.67 b | 664.98 a | 508.36 d | 558.45 c |
2.2.2. Monosaccharide Composition
2.2.3. FT-IR
2.2.4. SEM
2.2.5. Rheological Property Analysis
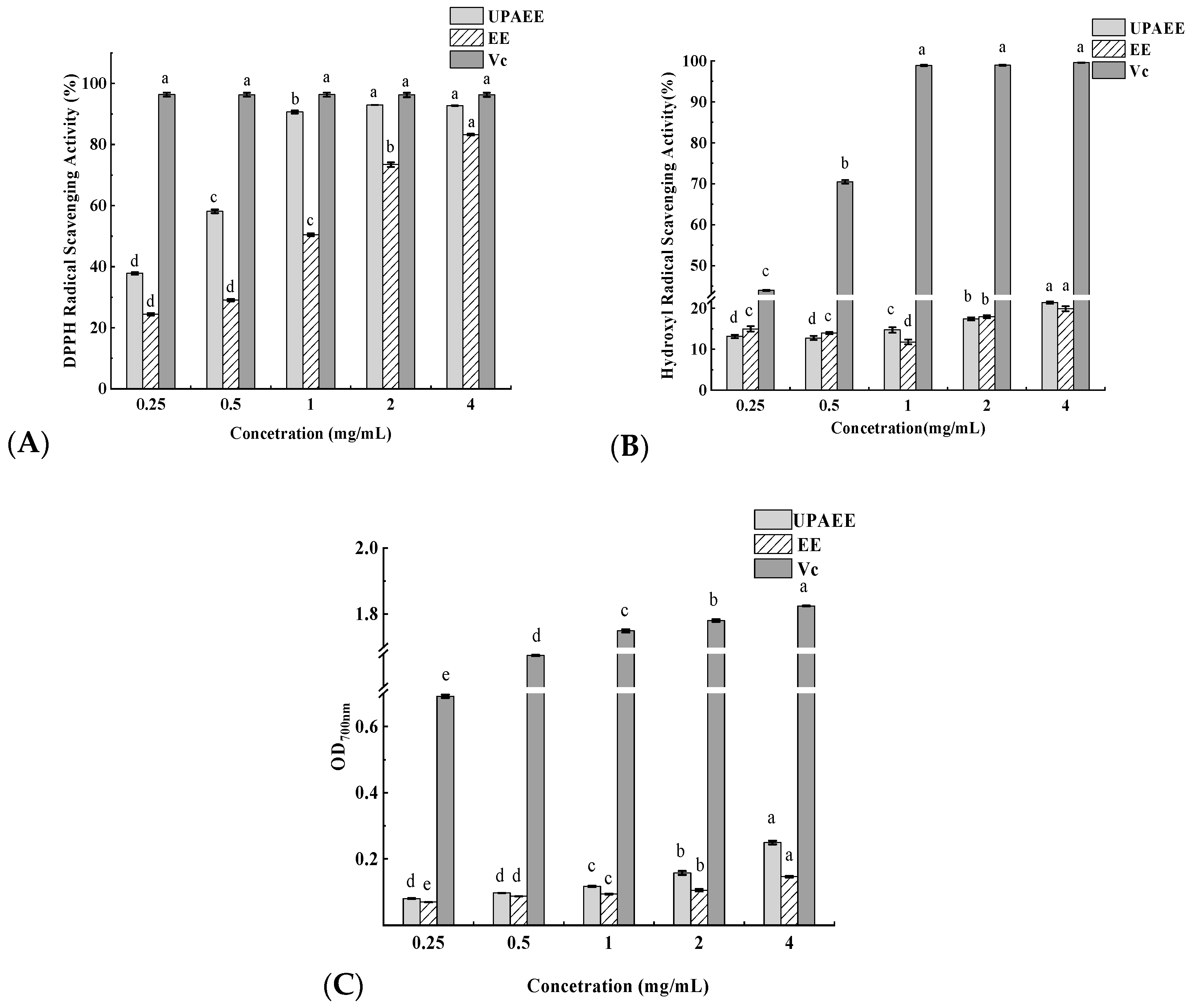
2.2.6. Analysis of Antioxidant Activity
3. Materials and Methods
3.1. Reagents and Instruments
3.2. Raw Material Pretreatment
3.3. Extraction and Purification of Hawthorn Pectin
3.4. Single-Factor and Response Surface Methodology Experiments
3.4.1. Single-Factor Experimental Design
3.4.2. RSM
3.4.3. Calculation of Hawthorn Pectin Yield
3.5. Basic Indices
3.5.1. Degree of Esterification (DE) of Pectin
3.5.2. Determination of Total Galacturonic Acid Content
3.5.3. Total Polyphenols
3.5.4. Molecular Weight Distribution
3.6. Structural Characterization
3.6.1. Monosaccharide Composition
3.6.2. FT-IR
3.6.3. SEM
3.7. Rheological Properties
3.7.1. Viscosity Measurement
3.7.2. Dynamic Viscoelasticity Measurement
3.8. Determination of In Vitro Antioxidant Activity
3.8.1. DPPH Radical Scavenging Capacity
3.8.2. Hydroxyl Radical Scavenging Capacity
3.8.3. Total Reducing Power
3.9. Data Processing
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Feng, R.Y.; Zhu, X.Y.; Zhang, M.M. Progress in Study on Pharmacological Effects of Hawthorn in the Recent Five Years. J. Basic Chin. Med. 2019, 25, 715–718. [Google Scholar] [CrossRef]
- Zhai, J.C.; Shen, Z.T.; Wu, L.B.; Li, L.; Wang, S.Y.; Wei, Y.J.; Qi, H.Y.; Xu, C.H.; Han, J.M.; Li, X.S. Geographical Indication Characteristics and Production Technology of Tianbaoshan Hawthorn. China Fruit Veg. 2023, 43, 62–66. [Google Scholar] [CrossRef]
- Dong, N.G.; Wang, Y.; Zheng, S.Q.; Wang, S.D. Current Situation and Development Suggestions of Hawthorn Industry in China. China Fruits 2022, 5, 87–91. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, Y.; Liu, J.N.; Lan, Y.B.; Qian, X.; Zhu, B.Q. Comprehensive study of chemical and sensory profiles of hawthorn wines from China. Food Chem. X 2025, 26, 102277. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.X.; Zhou, M.; Li, J.; Bi, J.F.; Chen, J.X.; Zhang, X.J.; Zhang, J. Effects of Pectin Structure Differences in Hawthorn from Different Provinces on Rheological Properties. J. Chin. Inst. Food Sci. Technol. 2023, 23, 39–48. [Google Scholar] [CrossRef]
- Ting, Z.; Hao, Z.; Min, W.; Zhu, C.H. Effects of enzymatic treatment on the physicochemical properties and antioxidant activity of hawthorn pectin. Mater. Today Commun. 2022, 30, 103225. [Google Scholar] [CrossRef]
- Li, T.; Ji, W.; Dong, H.; Wu, Y.; Guo, L.; Chen, L.; Wang, X. A Comprehensive Review on the Isolation, Bioactivities, and Structure-Activity Relationship of Hawthorn Pectin and Its Derived Oligosaccharides. Foods 2024, 13, 2750. [Google Scholar] [CrossRef]
- Yi, W.; Yu, Z.; Yi, L.; Shan, G.; Zhang, B.J.; Cai, Q.; Lou, J.Y.; Qu, Y. The lipid-lowering effects of fenugreek gum, hawthorn pectin, and burdock inulin. Front. Nutr. 2023, 10, 1149094. [Google Scholar] [CrossRef]
- Wei, B.A.; Huang, Y.H.; Pan, Q.D.; Wang, W.R.; Liang, Z.M.; Xu, Y.Y.; Zhao, L.Y. Study on the optimization of pectin extraction from large fruit hawthorn pomace by response surface methodology and its hypoglycemic activity. J. Food Saf. Qual. 2024, 15, 304–312. [Google Scholar] [CrossRef]
- Sun, D.; Chen, X.; Zhu, C. Physicochemical properties and antioxidant activity of pectin from hawthorn wine pomace: A comparison of different extraction methods. Int. J. Biol. Macromol. 2020, 158, 1239–1247. [Google Scholar] [CrossRef]
- Wei, X.; Xu, K.; Qin, W.; Lv, S.; Guo, M.M. Hawthorn (Crataegus pinnatifida) berries ripeness induced pectin diversity: A comparative study in physicochemical properties, structure, function and fresh-keeping potential. Food Chem. 2024, 455, 139703. [Google Scholar] [CrossRef]
- Zhang, S.; Waterhouse, G.I.N.; Xu, F.Y.; He, Z.Y.; Du, Y.Y.; Lian, Y.J.; Wu, P.; Sun, D.X. Recent advances in utilization of pectins in biomedical applications: A review focusing on molecular structure-directing health-promoting properties. Crit. Rev. Food Sci. Nutr. 2021, 63, 3386–3419. [Google Scholar] [CrossRef]
- Ren, D.D.; Jiang, W.; Sun, Y.S.; Chen, J.B.; Hua, M.; Li, S.S. Research progress on the classification, function and application of pectin in the food industry. Sci. Technol. Food Ind. 2022, 43, 438–446. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, H.; Zhang, T.; Zhang, X.Y.; Zhu, C.H. Characterization, antioxidant activity and in vitro digestion of hawthorn pectin prepared by gradient ethanol precipitation. Int. J. Biol. Macromol. 2024, 267, 131278. [Google Scholar] [CrossRef] [PubMed]
- Fu, M.; Sun, X.; Fei, C.; Li, D.D.; Zhang, D.; Tuo, X.Q.; Gao, S.; Han, X.; Xiu, J.H.; Wang, J.H.; et al. Optimization and characterization of pectin extracted from hawthorn by deep eutectic solvent. Int. J. Biol. Macromol. 2024, 256, 128688. [Google Scholar] [CrossRef]
- Wang, X.; Sun, Y.; Yu, Y.; Huang, D.J.; Liu, Y.Y.; Huang, M.M.; Jiang, Y.; Li, D.P. Sequential extraction of hawthorn pectin: An attempt to reveal their original mode of being in plants and functional properties. Int. J. Biol. Macromol. 2024, 282, 136756. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.L.; Song, N.N.; Yi, Y.X.; Aytuxun, A. Effects of Different Extraction Methods on the Physicochemical Properties and Antioxidant Activity of Apocynum venetum Polysaccharides. Sci. Technol. Food Ind. 2024, 45, 40–48. [Google Scholar] [CrossRef]
- Wang, J.W.; Shen, X.J.; Liang, K.Y.; Qi, X.Z.; Yu, D.H.; Wu, C.L.; Wu, F.H.; Liu, X.Q.; Wang, P. Effects of Different Extraction Processes on the Structural Characteristics and Biological Activities of Polygonatum filipes Polysaccharides. J. Chin. Cereals Oils Assoc. 2025, 1–13. [Google Scholar] [CrossRef]
- Lu, J.H.; Yuan, Q.X.; Yang, Z.Z.; Huang, C.X.; Tang, P.L.; Li, Z.; Liao, Y.W.; Zhang, J.; Zhao, L.Y.; Hou, X.T. Research Progress on Extraction, Isolation, Purification, Structural Characterization, and Biological Activities of Sugarcane Leaf and Bagasse Polysaccharides. Sci. Technol. Food Ind. 2025, 1–20. [Google Scholar] [CrossRef]
- Wang, J.X.; Zhao, Q.Q.; Xu, X.Y.; Xie, Q.; Chen, Q.X. Study on the Optimization of Ultrasound-Assisted Acid Extraction Process of Passion Fruit Peel Pectin by Response Surface Methodology and Its Antioxidant Activity. Sci. Technol. Food Ind. 2022, 43, 162–170. [Google Scholar] [CrossRef]
- Singhal, S.; Deka, C.S.; Koidis, A.; Swami Hulle, N.R. Standardization of extraction of pectin from Assam lemon (Citrus limon Burm f.) peels using novel technologies and quality characterization. Biomass Convers. Biorefinery 2024, 1–12. [Google Scholar] [CrossRef]
- Vargas, G.C.; Ponce, A.M.N.; Stortz, A.C.; Fissore, E.N.; Bonelli, P.; Otálora González, C.M.; Gerschenson, L.N. Pectin obtention from agroindustrial wastes of Malus domestica using green solvents (citric acid and natural deep eutectic solvents). Chemical, thermal, and rheological characterization. Front. Chem. 2025, 12, 1504582. [Google Scholar] [CrossRef]
- Zhao, Z.X.; Ji, Y.H.; Liu, X.M.; Zhao, L.S. Research Progress on Extraction and Separation Technology Based on Deep Eutectic Solvents and Its Application. Chin. J. Chromatogr. 2021, 39, 152–161. [Google Scholar] [CrossRef]
- Casado, N.; Perestrelo, R.; Silva, L.C.; Sierra, I.; Câmara, J.S. Comparison of high-throughput microextraction techniques, MEPS and μ-SPEed, for the determination of polyphenols in baby food by ultrahigh pressure liquid chromatography. Food Chem. 2019, 292, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Ouyang, H.; Li, Z.; Hong, T.; Zhu, Y.B.; Yang, Y.F.; Guo, X.M.; Ni, H.; Jiang, Z.D. Ultra-high pressure assisted extraction of polysaccharide from Bangia fusco-purpurea: Structure and in vitro hypolipidemic activity. Int. J. Biol. Macromol. 2024, 280, 135687. [Google Scholar] [CrossRef]
- Zheng, M.; Tian, X.; Li, Z.; Hong, T.; Zhu, Y.B.; Yang, Y.F.; Li, Q.B.; Ni, H.; Jiang, Z.D. Effects of ultra-high pressure assisted extraction on the structure, antioxidant and hypolipidemic activities of Porphyra haitanensis polysaccharides. Food Chem. 2023, 437, 137856. [Google Scholar] [CrossRef]
- Wang, B.; Liu, S.; Wang, R. Ultra-high pressure assisted extraction of water-soluble polysaccharides from Moringa oleifera seed husks and their antioxidant activities. Int. J. Food Prop. 2024, 27, 1–14. [Google Scholar] [CrossRef]
- Hongwei, Z.; Supan, C.; Longfei, Z.; Han, Z.P.; Hu, X.J.; Yang, J.; Li, H.; Tan, W.H.; Huang, H.Y. Ultra-high-pressure-assisted extraction of wedelolactone and isodemethylwedelolactone from Ecliptae Herba and purification by high-speed counter-current chromatography. Biomed. Chromatogr. 2019, 33, e4497. [Google Scholar] [CrossRef]
- Chen, R.; Luo, S.; Wang, C.; Bai, H.L.; Lu, J.; Tian, L.; Gao, M.; Wu, J.; Bai, C.L.; Sun, H. Effects of ultra-high pressure enzyme extraction on characteristics and functional properties of red pitaya (Hylocereus polyrhizus) peel pectic polysaccharides. Food Hydrocoll. 2021, 121, 107016. [Google Scholar] [CrossRef]
- Choudhary, B.; Patel, J.; Mishra, A. Physicochemical, radical–scavenging, and anti-proliferative analyses of enzyme-assisted extracted polysaccharides unravel the potential of tropical green seaweeds. Algal Res. 2025, 87, 103973. [Google Scholar] [CrossRef]
- Wei, Y.Y.; Li, M.M.; Yang, M.M.; Xu, W.T.; Zhu, J.; Cheng, Z. Optimization of compound enzyme extraction process and analysis of product properties of pectin from papaya peel. J. Food Saf. Qual. 2022, 13, 381–387. [Google Scholar] [CrossRef]
- Maran, J.P.; Prakash, K.A. Process variables influence on microwave assisted extraction of pectin from waste Carica papaya L. peel. Int. J. Biol. Macromol. 2015, 73, 202–206. [Google Scholar] [CrossRef] [PubMed]
- Nadar, S.S.; Rao, P.; Rathod, K.V. Enzyme-assisted extraction of biomolecules as an approach to novel extraction technology: A review. Food Res. Int. 2018, 108, 309–330. [Google Scholar] [CrossRef]
- Wei, W.; Li, Y.W.; Liu, F.X.; Xu, X.F.; Liu, Z.J. Optimization of ultra-high pressure extraction process of polygonatum sibiricum polysaccharides by response surface methodology. Fine Chem. 2019, 36, 875–881. [Google Scholar] [CrossRef]
- Ruan, W.H.; Wang, Y.; Hao, R. Optimization of ultra-high pressure extraction process of sparassis crispa polysaccharides. J. Food Saf. Qual. 2022, 13, 1732–1739. [Google Scholar] [CrossRef]
- Guo, Y.; Yu, J.J.; Xiao, P.; Li, R. Study on the process of ultra-high pressure extraction of pueraria lobata polysaccharides. J. Tianjin Agric. Univ. 2020, 27, 66–69. [Google Scholar] [CrossRef]
- Lin, M.; Chen, N.N. Process optimization of microwave-assisted extraction of grapefruit peel pectin. China Food Saf. Mag. 2022, 155–158+163. [Google Scholar] [CrossRef]
- Yang, R.P.; Chen, R.Z.; Wang, C.X.; Ren, X.; Luo, S.J.; Gao, M.; Wu, J. Study on the physicochemical characteristics and antioxidant activity of polysaccharides from red pitaya (Hylocereus polyrhizus) peel extracted by ultra-high pressure. J. Mol. Sci. 2022, 38, 265–276. [Google Scholar] [CrossRef]
- Ma, K.K.; Bi, J.F.; Yi, J.Y. Effects of Different Extraction Methods on the Structure and Physicochemical Properties of Fig Pectin. J. Chin. Inst. Food Sci. Technol. 2025, 1–13. Available online: http://kns.cnki.net/kcms/detail/11.4528.ts.20250327.1601.004.html (accessed on 28 March 2025).
- Swarup, R.; Ruchir, P.; Łukasz, Ł.; Biswas, D.; Chandel, V.; Rhim, J.W. Recent progress in pectin extraction, characterization, and pectin-based films for active food packaging applications: A review. Int. J. Biol. Macromol. 2023, 239, 124248. [Google Scholar] [CrossRef]
- Lei, S.J.; Ouyang, N.F.; Yang, D.D.; Liu, H.Y.; Gao, W.Y. Effects of ultra-high pressure treatment on pectin in food. Food Ferment. Ind. 2025, 51, 1–9. [Google Scholar] [CrossRef]
- Tian, H.; Xiong, J.; Yu, H.; Chen, C.; Xu, H.D.; Lou, X.M. Characterize the physicochemical properties and microstructure of pectin from high-pressure and thermal processed cloudy hawthorn (Crataegus pinnatifida) juice based on acid heating extraction. Food Chem. 2023, 407, 135199. [Google Scholar] [CrossRef]
- Zhaowen, T.; Cheng, Y.; Ma, Y.; Wang, D.; Zhao, X.Y. Effects of ultra-high pressure on the structure of serum pectin in tomato paste. Food Sci. Technol. 2019, 44, 85–91. [Google Scholar] [CrossRef]
- Yao, Y.; Eiji, M.; Haruo, K. High pressure facilitates delignification of Japanese cedar in supercritical methanol. J. Wood Sci. 2023, 69, 26. [Google Scholar] [CrossRef]
- Oliveira, C.F.d.; Gurak, P.D.; Cladera-Olivera, F.; Marczak, L.D.F.; Karwe, M.V. Combined Effect of High-Pressure and Conventional Heating on Pectin Extraction from Passion Fruit Peel. Food Bioprocess Technol. 2016, 9, 1021–1030. [Google Scholar] [CrossRef]
- Luo, W.; Tappi, S.; Wang, C.; Yu, Y.; Zhu, S.M.; Dalla Rosa, M.; Rocculi, P. Effect of High Hydrostatic Pressure (HHP) on the Antioxidant and Volatile Properties of Candied Wumei Fruit (Prunus mume) During Osmotic Dehydration. Food Bioprocess Technol. 2019, 12, 98–109. [Google Scholar] [CrossRef]
- Zhao, R.N.; Wen, J.T.; Fan, G.Z.; Li, A.X.; Guo, Z.B.; Shi, X.X. Optimization of ultra-high pressure-assisted extraction process of grapefruit peel pectin by response surface methodology and analysis of its physicochemical properties. Food Ferment. Ind. 2024, 50, 64–71. [Google Scholar] [CrossRef]
- Peng, X.-y.; Mu, T.-h.; Zhang, M.; Sun, H.N.; Chen, J.W.; Yu, M. Effects of pH and high hydrostatic pressure on the structural and rheological properties of sugar beet pectin. Food Hydrocoll. 2016, 60, 161–169. [Google Scholar] [CrossRef]
- Lv, D.; Chen, J.; Yang, C. The Physicochemical Properties and Antioxidant Activities of the Hawthorn Pectin Extracted Using Ultra-High Pressure Assisted Acid Extraction (UPAAE). Foods 2024, 13, 983. [Google Scholar] [CrossRef]
- Spinei, M.; Oroian, M. Structural, functional and physicochemical properties of pectin from grape pomace as affected by different extraction techniques. Int. J. Biol. Macromol. 2023, 224, 739–753. [Google Scholar] [CrossRef]
- Zheng, J.Q. Study on the Gelling Mechanism and Application of RG-I Pectin from Citrus. Master’s Thesis, Zhejiang University, Hangzhou, China, 2021. [Google Scholar] [CrossRef]
- M’sakni, N.H.; Majdoub, H.; Roudesli, S.; Picton, L.; Le Cerf, D.; Rihouey, C.; Morvan, C. Composition, structure and solution properties of polysaccharides extracted from leaves of Mesembryanthemum crystallinum. Eur. Polym. J. 2006, 42, 786–795. [Google Scholar] [CrossRef]
- Li, Z.Q.; Xi, J.; Chen, H.M.; Chen, W.J.; Chen, W.X.; Zhong, Q.P.; Zhang, M. Effect of glycosylation with apple pectin, citrus pectin, mango pectin and sugar beet pectin on the physicochemical, interfacial and emulsifying properties of coconut protein isolate. Food Res. Int. 2022, 156, 111363. [Google Scholar] [CrossRef] [PubMed]
- Xie, F.; Zhang, W.; Lan, X.; Gong, S.X.; Wu, J.H.; Wang, Z.W. Effects of high hydrostatic pressure and high-pressure homogenization processing on characteristics of potato peel waste pectin. Carbohydr. Polym. 2018, 196, 474–482. [Google Scholar] [CrossRef]
- Wu, X.Z.; Zhang, H.Y.; He, H.Y.; Pan, L.H.; Luo, S.Z.; Zheng, Z. Study on the amino acid modification of low-ester pectin and the gelling properties of the modification. Bull. Ferment. Sci. Technol. 2019, 48, 197–203. [Google Scholar] [CrossRef]
- Gao, R.P.; Tang, W.; Liang, M.Y.; Zhou, W.B.; Wu, L.; Wu, Q.Y.; Wang, R.R.; Liu, J. Structure, physicochemical properties and antioxidant activity of pectin from different varieties of potato residues. Sci. Technol. Food Ind. 2025, 1–15. [Google Scholar] [CrossRef]
- Palomino, H.G.R.; Ramos M., P.; Oliveira, G.; Kock, F.V.C.; Venâncio, T.; Córdova, B.M. Structural elucidation of pectin extracted from cocoa pod husk (Theobroma Cacao L.): Evaluation of the degree of esterification using FT-IR and 1H NMR. Biomass Convers. Biorefinery 2023, 15, 2047–2061. [Google Scholar] [CrossRef]
- Jiang, Y.; Du, J.; Zhang, L.; Li, W. Properties of pectin extracted from fermented and steeped hawthorn wine pomace: A comparison. Carbohydr. Polym. 2018, 197, 174–182. [Google Scholar] [CrossRef]
- Yang, J.S.; Mu, T.H.; Ma, M.M. Extraction, structure, and emulsifying properties of pectin from potato pulp. Food Chem. 2018, 244, 197–205. [Google Scholar] [CrossRef]
- Yang, Y.J.; Ma, Z.L.; Bai, Y. Effect of extraction methods on the structural characteristics and properties of pectin from potato residues. Food Ferment. Ind. 2021, 47, 146–152. [Google Scholar] [CrossRef]
- Sanandiya, N.D.; Siddhanta, A.K. Chemical studies on the polysaccharides of Salicornia brachiata. Carbohydr. Polym. 2014, 112, 300–307. [Google Scholar] [CrossRef]
- Zhang, C.; Zhao, F.; Li, R.; Wu, Y.; Liu, S.; Liang, Q. Purification, characterization, antioxidant and moisture-preserving activities of polysaccharides from Rosa rugosa petals. Int. J. Biol. Macromol. 2019, 124, 938–945. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zhu, P.; Zhao, S.; Nie, C.Z.; Wang, N.F.; Du, X.F.; Zhou, Y.B. Characterization, antioxidant activity and immunomodulatory activity of polysaccharides from the swollen culms of Zizania latifolia. Int. J. Biol. Macromol. 2017, 95, 809–817. [Google Scholar] [CrossRef] [PubMed]
- Zhu, R.; Zhang, X.; Wang, Y.; Zhang, L.J.; Wang, C.Y.; Hu, F.Q.; Ning, C.; Chen, G. Pectin oligosaccharides from hawthorn (Crataegus pinnatifida Bunge. Var. major): Molecular characterization and potential antiglycation activities. Food Chem. 2019, 286, 129–135. [Google Scholar] [CrossRef]
- Liu, N.; Yang, W.; Li, X.; Zhao, P.; Liu, Y.; Guo, L.P.; Huang, L.Q.; Gao, W.Y. Comparison of characterization and antioxidant activity of different citrus peel pectins. Food Chem. 2022, 386, 132683. [Google Scholar] [CrossRef]
- Zhang, Z.; Lin, R.; Chen, M.; Wang, X.; Ni, Q.X.; Zhang, Y.Z.; Xu, G.Z. Valorization of pectic polysaccharides from Gardenia jasminoides Ellis flower: Physicochemical, structural, rheological, and functional properties. Arab. J. Chem. 2023, 16, 105116. [Google Scholar] [CrossRef]
- Adeli, M.; Samavati, V. Studies on the steady shear flow behavior and chemical properties of water-soluble polysaccharide from Ziziphus lotus fruit. Int. J. Biol. Macromol. 2015, 72, 580–587. [Google Scholar] [CrossRef]
- Li, L.; Liao, B.-Y.; Thakur, K.; Zhang, J.G.; Wei, Z.J. The rheological behavior of polysaccharides sequential extracted from Polygonatum cyrtonema Hua. Int. J. Biol. Macromol. 2018, 109, 761–771. [Google Scholar] [CrossRef] [PubMed]
- Sengkhamparn, N.; Sagis, L.M.C.; de Vries, R.; Schols, H.A.; Sajjaanantakul, T.; Voragen, A.G.J. Physicochemical properties of pectins from okra (Abelmoschus esculentus (L.) Moench). Food Hydrocoll. 2010, 24, 35–41. [Google Scholar] [CrossRef]
- Lu, Y.; He, S.; Zhao, Z.; Liu, C.X.; Lei, Y.; Liu, M.Y.; Zhang, Q.; Lin, D.R.; Liu, Y.W.; Lin, S.; et al. Structural Characteristics, Gelling Properties, In Vitro Antioxidant Activity and Immunomodulatory Effects of Rhamnogalacturonan-I Rich Pectic Polysaccharides Alkaline-Extracted from Wax Apple (Syzygium samarangense). Foods 2025, 14, 1227. [Google Scholar] [CrossRef]
- Zhu, K.X.; Zhao, S.F.; Zhu, H.Y.; Tang, B.; He, S.Z.; Tan, L.H. Study on rheological properties of ilex cornuta hooker polysaccharides. Sci. Technol. Food Ind. 2017, 38, 61–65+70. [Google Scholar] [CrossRef]
- Vardhanabhuti, B.; Ikeda, S. Isolation and characterization of hydrocolloids from monoi (Cissampelos pareira) leaves. Food Hydrocoll. 2006, 20, 885–891. [Google Scholar] [CrossRef]
- Hu, S.; Wang, J.; Nie, S.; Wang, Q.; Xu, X.J. Chain conformations and steady-shear viscosity properties of pectic polysaccharides from apple and tomato. Food Chem. 2022, 14, 100296. [Google Scholar] [CrossRef]
- Šurlan, J.; Šereš, Z.; Dokić, L.; Krstonošić, V.; Maravić, N. Evaluation of sugar beet pectin viscosity, surface activity, conductivity and zeta potential in sodium chloride aqueous solutions. Food Hydrocoll. 2023, 139, 108490. [Google Scholar] [CrossRef]
- Wang, X.J.; Luo, J.L.; Yang, C.X.; Zhao, Q.; Xiong, H. Structure and Rheological Properties of Akebia trifoliata Fruit Pectin. J. Chin. Inst. Food Sci. Technol. 2020, 20, 48–56. [Google Scholar] [CrossRef]
- Yang, X.D.; Guo, C.; Yuan, K.; Yang, X.; Wang, X.Y.; Meng, Y.H.; Guo, Y.R. Research Progress on Gelling Properties of Low-Ester Pectin and Its Application. Food Ferment. Ind. 2022, 48, 299–308. [Google Scholar] [CrossRef]
- Yasser, Y.Z.; Kyong, R.Y. Experimental data and modeling of storage and loss moduli for a biosensor based on polymer nanocomposites. Results Phys. 2020, 19, 103537. [Google Scholar] [CrossRef]
- Torralbo, D.F.; Batista, K.A.; Di-Medeiros, M.C.B.; Fernandes, K.F. Extraction and partial characterization of Solanum lycocarpum pectin. Food Hydrocoll. 2012, 27, 378–383. [Google Scholar] [CrossRef]
- Wu, Y.; Cui, S.W.; Wu, J.; Ai, L.Z.; Wang, Q.; Tang, J. Structure characteristics and rheological properties of acidic polysaccharide from boat-fruited sterculia seeds. Carbohydr. Polym. 2012, 88, 926–930. [Google Scholar] [CrossRef]
- Hosseini, S.S.; Khodaiyan, F.; Kazemi, M.; Najari, Z. Optimization and characterization of pectin extracted from sour orange peel by ultrasound assisted method. Int. J. Biol. Macromol. 2019, 125, 621–629. [Google Scholar] [CrossRef]
- Zhu, R.; Zhang, X.; Wang, Y.; Zhang, L.J.; Zhao, J.; Chen, G.; Fan, J.G.; Jia, Y.F.; Yan, F.W.; Ning, C. Characterization of polysaccharide fractions from fruit of Actinidia arguta and assessment of their antioxidant and antiglycated activities. Carbohydr. Polym. 2019, 210, 73–84. [Google Scholar] [CrossRef]
- Fang, C.; Chen, G.; Kan, J. Comparison on characterization and biological activities of Mentha haplocalyx polysaccharides at different solvent extractions. Int. J. Biol. Macromol. 2020, 154, 916–928. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Guo, C.; Wang, S.; Li, W.X.; Zhang, X.; Lu, S.; Ning, C.; Tan, C. The mechanism of pectin in improving anthocyanin stability and the application progress of their complexes: A review. Food Chem. X 2024, 24, 101955. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Yuan, Q.; Fu, Y.; Liu, W.; Su, Y.H.; Liu, H.; Wu, C.Y.; Zhao, L.; Zhang, Q.; Lin, D.R.; et al. Extraction Optimization and Effects of Extraction Methods on the Chemical Structures and Antioxidant Activities of Polysaccharides from Snow Chrysanthemum (Coreopsis tinctoria). Polymers 2019, 11, 215. [Google Scholar] [CrossRef] [PubMed]
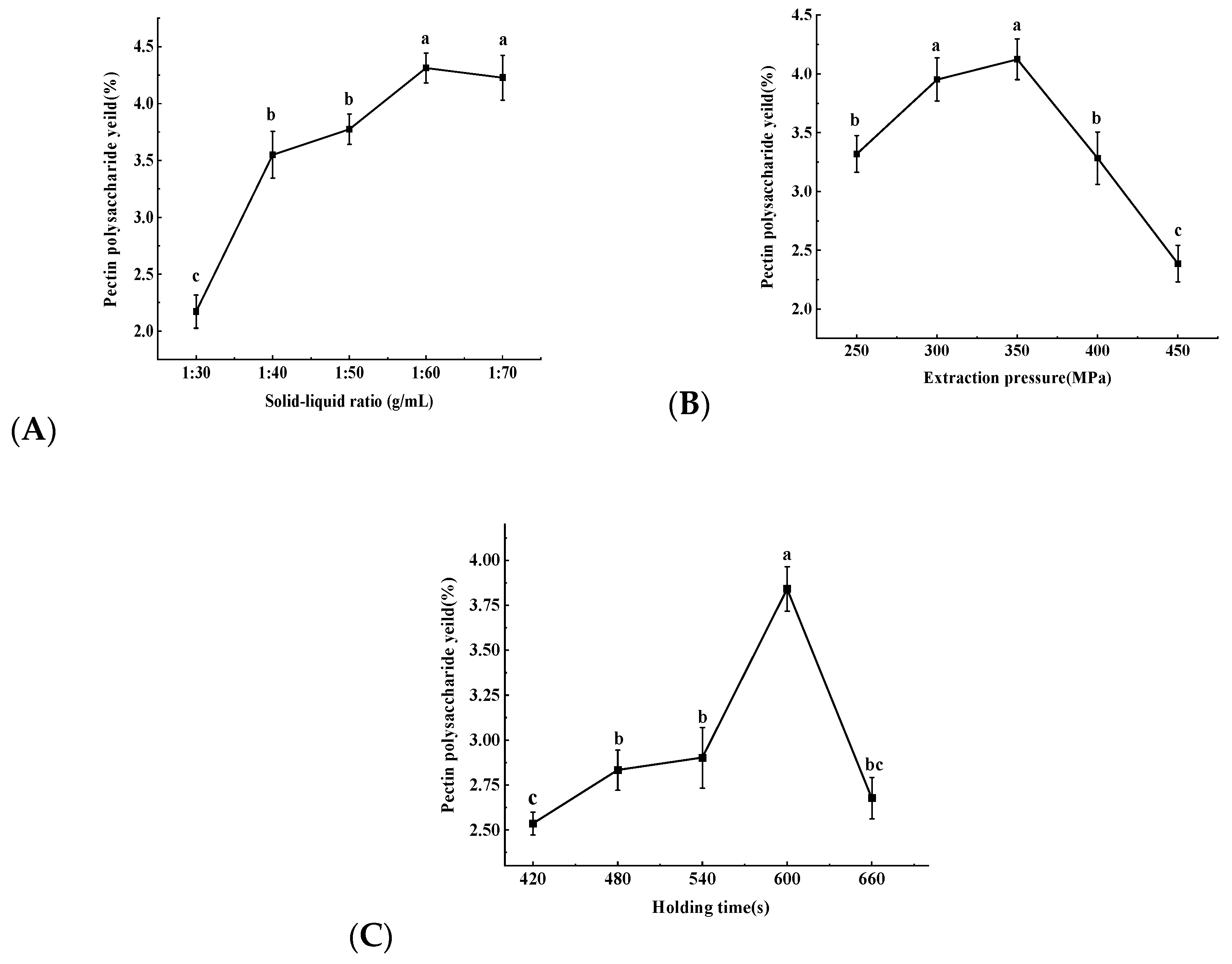

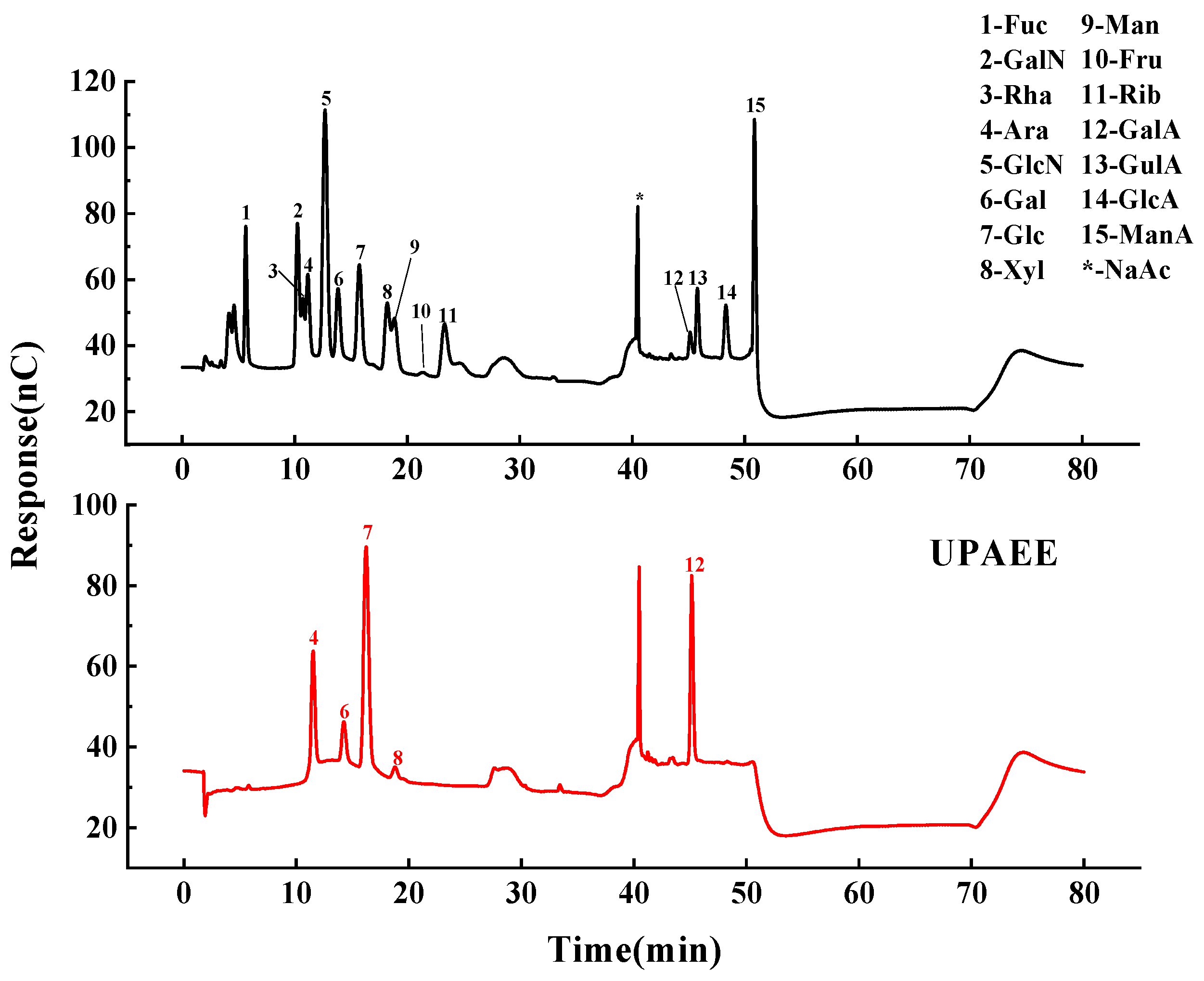
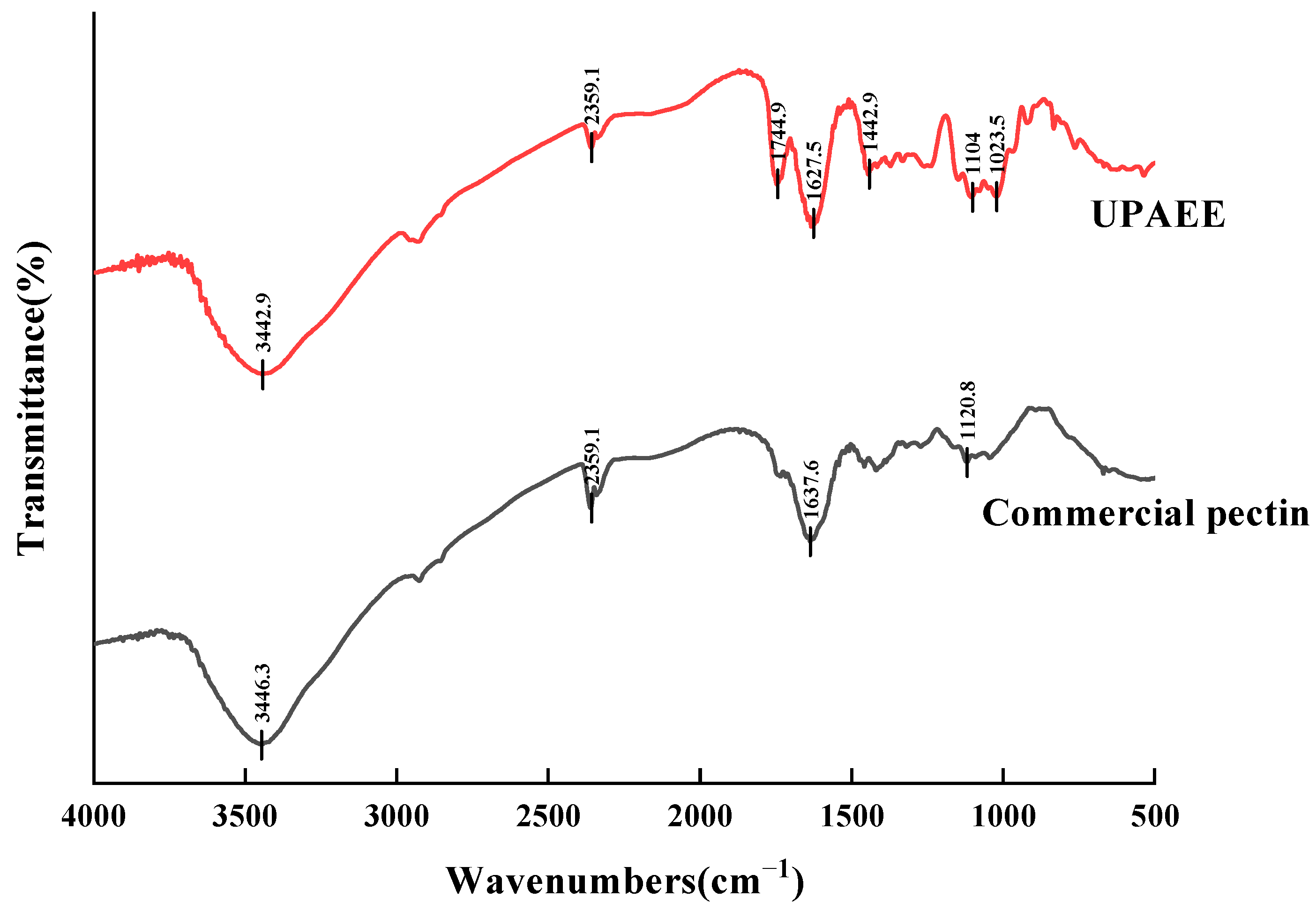

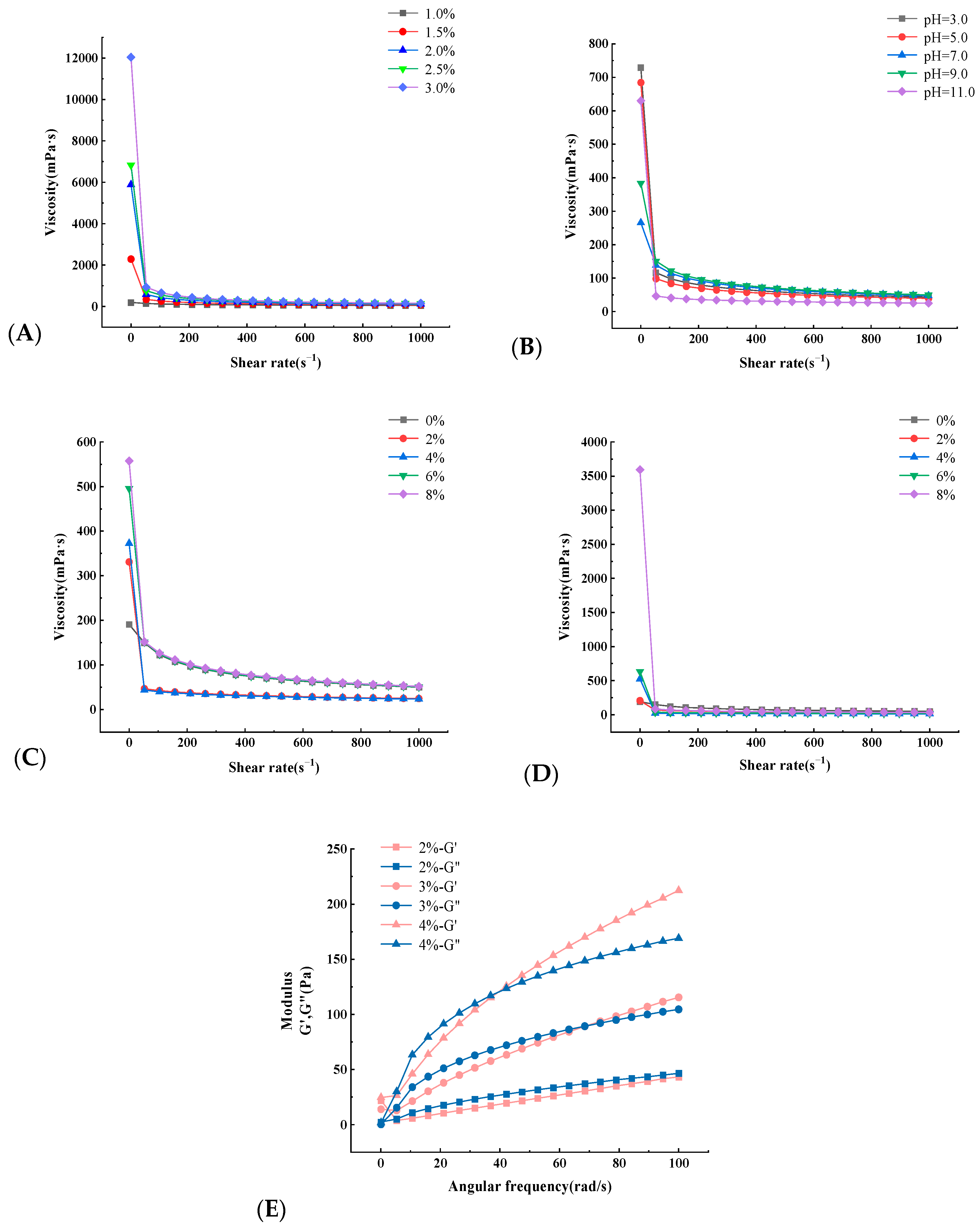
| Number | A Solid–Liquid Ratio/(g/mL) | B Extraction Pressure/(MPa) | C Holding Time/(s) | Pectin Polysaccharide Yield/(%) |
|---|---|---|---|---|
| 1 | −1 | 0 | −1 | 2.67 ± 0.10 |
| 2 | −1 | 1 | 0 | 2.39 ± 0.11 |
| 3 | −1 | −1 | 0 | 3.20 ± 0.18 |
| 4 | −1 | 0 | 1 | 2.96 ± 0.06 |
| 5 | 0 | −1 | 1 | 3.41 ± 0.02 |
| 6 | 0 | 1 | −1 | 1.95 ± 0.17 |
| 7 | 0 | 0 | 0 | 2.97 ± 0.08 |
| 8 | 0 | 0 | 0 | 2.76 ± 0.11 |
| 9 | 0 | 0 | 0 | 2.83 ± 0.12 |
| 10 | 0 | 0 | 0 | 2.84 ± 0.10 |
| 11 | 0 | 0 | 0 | 2.89 ± 0.15 |
| 12 | 0 | −1 | −1 | 3.11 ± 0.26 |
| 13 | 0 | 1 | 1 | 2.59 ± 0.13 |
| 14 | 1 | 0 | 1 | 3.90 ± 0.11 |
| 15 | 1 | −1 | 0 | 3.98 ± 0.07 |
| 16 | 1 | 1 | 0 | 3.04 ± 0.22 |
| 17 | 1 | 0 | −1 | 3.06 ± 0.19 |
| Source of Variance | Sum of Squares | Degrees of Freedom | Mean Square | F-Value | p-Value | Significance |
|---|---|---|---|---|---|---|
| Model | 3.82 | 9 | 0.4247 | 83.48 | <0.0001 | ** |
| A (Solid–Liquid Ratio) | 0.9534 | 1 | 0.9534 | 187.39 | <0.0001 | ** |
| B (Extraction Pressure) | 1.73 | 1 | 1.73 | 339.95 | <0.0001 | ** |
| C (Holding time) | 0.5434 | 1 | 0.5434 | 106.8 | <0.0001 | ** |
| AB | 0.0041 | 1 | 0.0041 | 0.7998 | 0.4009 | |
| AC | 0.0746 | 1 | 0.0746 | 14.67 | 0.0065 | ** |
| BC | 0.0293 | 1 | 0.0293 | 5.76 | 0.0474 | * |
| A2 | 0.4805 | 1 | 0.4805 | 94.43 | <0.0001 | ** |
| B2 | 0.0078 | 1 | 0.0078 | 1.54 | 0.2544 | |
| C2 | 0.0109 | 1 | 0.0109 | 2.13 | 0.1874 | |
| Residual | 0.0356 | 7 | 0.0051 | |||
| Lack of Fit | 0.0126 | 3 | 0.0042 | 0.7257 | 0.5875 | |
| Pure Error | 0.0231 | 4 | 0.0058 | |||
| sum | 3.86 | 16 |
| Level | A Solid–Liquid Ratio (g/mL) | B Extraction Pressure (MPa) | C Holding Time (s) |
|---|---|---|---|
| −1 | 1:50 | 300 | 540 |
| 0 | 1:60 | 350 | 600 |
| 1 | 1:70 | 400 | 660 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ye, Z.; Lu, Q.; Lv, D.; Yang, C. Enzymatic Extraction of Hawthorn Pectin Under Ultrahigh Pressure: Optimization and Characterization. Molecules 2025, 30, 2210. https://doi.org/10.3390/molecules30102210
Ye Z, Lu Q, Lv D, Yang C. Enzymatic Extraction of Hawthorn Pectin Under Ultrahigh Pressure: Optimization and Characterization. Molecules. 2025; 30(10):2210. https://doi.org/10.3390/molecules30102210
Chicago/Turabian StyleYe, Zheng, Qiaoshuang Lu, Dihu Lv, and Chun Yang. 2025. "Enzymatic Extraction of Hawthorn Pectin Under Ultrahigh Pressure: Optimization and Characterization" Molecules 30, no. 10: 2210. https://doi.org/10.3390/molecules30102210
APA StyleYe, Z., Lu, Q., Lv, D., & Yang, C. (2025). Enzymatic Extraction of Hawthorn Pectin Under Ultrahigh Pressure: Optimization and Characterization. Molecules, 30(10), 2210. https://doi.org/10.3390/molecules30102210





