Theoretical Insights into the Impact of Pyrrole and Imidazole Substituents on the BODIPY Chromophore
Abstract
1. Introduction
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BODIPY | 4,4-difluoro-4-bora-3a,4a-diaza-s-indacene |
| DFT | Density functional theory |
| TD-DFT | Time-dependent density functional theory |
| NCI | Non-covalent interaction |
| RDG | Reduced density gradient |
| GS | Ground state |
| ES | Excited state |
| SM | Supplementary Materials |
| HOMO | Highest occupied molecular orbital |
| LUMO | Lowest unoccupied molecular orbital |
| LR | Linear-response |
| SS | State-specific |
| PCM | Polarizable continuum model |
| BD1R1 | 1-(2-(1H-pyrrol-2-yl)vinyl)-3,5,7-trimethylphenyl BODIPY |
| BD1R2 | 1-(2-(1H-pyrrol-3-yl)vinyl)-3,5,7-trimethylphenyl BODIPY |
| BD1R3 | 1-(2-(1H-imidazol-2-yl)vinyl)-3,5,7-trimethylphenyl BODIPY |
| BD1R4 | 1-(2-(1H-imidazol-5-yl)vinyl)-3,5,7-trimethylphenyl BODIPY |
| BD2R1 | 2-(2-(1H-pyrrol-2-yl)vinyl)-1,3,5,7-tetramethylphenyl BODIPY |
| BD2R2 | 2-(2-(1H-pyrrol-3-yl)vinyl)-1,3,5,7-tetramethylphenyl BODIPY |
| BD2R3 | 2-(2-(1H-imidazol-2-yl)vinyl)-1,3,5,7-tetramethylphenyl BODIPY |
| BD2R4 | 2-(2-(1H-imidazol-5-yl)vinyl)-1,3,5,7-tetramethylphenyl BODIPY |
| BD3R1 | 3-(2-(1H-pyrrol-2-yl)vinyl)-1,5,7-trimethylphenyl BODIPY |
| BD3R2 | 3-(2-(1H-pyrrol-3-yl)vinyl)-1,5,7-trimethylphenyl BODIPY |
| BD3R3 | 3-(2-(1H-imidazol-2-yl)vinyl)-1,5,7-trimethylphenyl BODIPY |
| BD3R4 | 3-(2-(1H-imidazol-5-yl)vinyl)-1,5,7-trimethylphenyl BODIPY |
References
- Ulrich, G.; Ziessel, R.; Harriman, A. The chemistry of fluorescent bodipy dyes: Versatility unsurpassed. Ang. Chem. Int. Ed. 2008, 47, 1184–1201. [Google Scholar] [CrossRef] [PubMed]
- Boens, N.; Leen, V.; Dehaen, W. Fluorescent indicators based on BODIPY. Chem. Soc. Rev. 2012, 41, 1130–1172. [Google Scholar] [CrossRef]
- Loudet, A.; Burgess, K. BODIPY Dyes and Their Derivatives: Syntheses and Spectroscopic Properties. Chem. Rev. 2007, 107, 4891–4932. [Google Scholar] [CrossRef]
- Ziessel, R.; Ulrich, G.; Harriman, A. The chemistry of Bodipy: A new El Dorado for fluorescence tools. New J. Chem. 2007, 31, 496–501. [Google Scholar] [CrossRef]
- Boens, N.; Verbelen, B.; Dehaen, W. Postfunctionalization of the BODIPY Core: Synthesis and Spectroscopy. Eur. J. Org. Chem. 2015, 2015, 6577–6595. [Google Scholar] [CrossRef]
- Bogomolec, M.; Glavaš, M.; Škorić, I. BODIPY Compounds Substituted on Boron. Molecules 2024, 29, 5157. [Google Scholar] [CrossRef] [PubMed]
- Sekar, N.N. Fluorophores in Fluorescence Spectroscopy, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2022. [Google Scholar]
- Treibs, A.; Kreuzer, F.-H. Difluorboryl-Komplexe von Di- und Tripyrrylmethenen. Justus Liebigs Ann. Chem. 1968, 718, 208–223. [Google Scholar] [CrossRef]
- Kobayashi, H.; Ogawa, M.; Alford, R.; Choyke, P.L.; Urano, Y. New Strategies for Fluorescent Probe Design in Medical Diagnostic Imaging. Chem. Rev. 2010, 110, 2620–2640. [Google Scholar] [CrossRef] [PubMed]
- Marfin, Y.S.; Aleksakhina, E.L.; Merkushev, D.A.; Rumyantsev, E.V.; Tomilova, I.K. Interaction of BODIPY Dyes with the Blood Plasma Proteins. J. Fluoresc. 2016, 26, 255–261. [Google Scholar] [CrossRef]
- Xia, W.; Low, P.S. Folate-Targeted Therapies for Cancer. J. Med. Chem. 2010, 53, 6811–6824. [Google Scholar] [CrossRef]
- Porolnik, W.; Ratajczak, M.; Mackowiak, A.; Murias, M.; Kucinska, M.; Piskorz, J. Liposomal Formulations of Novel BODIPY Dimers as Promising Photosensitizers for Antibacterial and Anticancer Treatment. Molecules 2024, 29, 5304. [Google Scholar] [CrossRef]
- Liu, Y.; Zhuang, D.; Wang, J.; Huang, H.; Li, R.; Wu, C.; Deng, Y.; Hu, G.; Guo, B. Recent advances in small molecular near-infrared fluorescence probes for a targeted diagnosis of the Alzheimer disease. Analyst 2022, 147, 4701. [Google Scholar] [CrossRef]
- Farber, S.A.; Pack, M.; Ho, S.-Y.; Johnson, I.D.; Wagner, D.S.; Dosch, R.; Mullins, M.C.; Hendrickson, H.S.; Hendrickson, E.K.; Halpern, M.E. Genetic Analysis of Digestive Physiology Using Fluorescent Phospholipid Reporters. Science 2001, 292, 1385. [Google Scholar] [CrossRef]
- Luo, S.; Zhang, E.; Su, Y.; Cheng, T.; Shi, C. A review of NIR dyes in cancer targeting and imaging. Biomaterials 2011, 32, 7127–7138. [Google Scholar] [CrossRef]
- Yang, Z.; Cao, J.; He, Y.; Yang, J.H.; Kim, T.; Peng, X.; Kim, J.S. Macro-/micro-environment-sensitive chemosensing and biological imaging. Chem. Soc. Rev. 2014, 43, 4563–4602. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kim, K.; Son, S.-H.; Choi, J.Y.; Lee, K.-H.; Kim, B.-T.; Byun, Y.; Choe, Y.S. 18F-Labeled BODIPY Dye: A Potential Prosthetic Group for Brain Hybrid PET/Optical Imaging Agents. ACS Chem. Neurosci. 2019, 10, 1445–1451. [Google Scholar] [CrossRef] [PubMed]
- Merkushev, D.; Vodyanova, O.; Telegin, F.; Melnikov, P.; Yashtulov, N.; Marfin, Y. Design of Promising aza-BODIPYs for Bioimaging and Sensing. Designs 2022, 6, 21. [Google Scholar] [CrossRef]
- Gurubasavaraj, P.M.; Sajjan, V.P.; Muñoz-Flores, B.M.; Jiménez Pérez, V.M.; Hosmane, N.S. Recent Advances in BODIPY Compounds: Synthetic Methods, Optical and Nonlinear Optical Properties, and Their Medical Applications. Molecules 2022, 27, 1877. [Google Scholar] [CrossRef] [PubMed]
- Marfin, Y.S.; Solomonov, A.V.; Timin, A.S.; Rumyantsev, E.V. Recent advances of individual BODIPY and BODIPY-based functional materials in medical diagnostics and treatment. Curr. Med. Chem. 2017, 24, 2745–2772. [Google Scholar] [CrossRef]
- Guan, Y.; Yu, B.; Ding, J.; Sun, T.; Xie, Z. BODIPY photosensitizers for antibacterial photodynamic therapy. Chin. Chem. Lett. 2024, 110645, in press. [Google Scholar] [CrossRef]
- Ozlem, S.; Akkaya, E.U. Thinking Outside the Silicon Box: Molecular AND Logic As an Additional Layer of Selectivity in Singlet Oxygen Generation for Photodynamic Therapy. J. Am. Chem. Soc. 2009, 131, 48–49. [Google Scholar] [CrossRef]
- Guseva, G.B.; Eremeeva, Y.V.; Ksenofontov, A.A.; Antina, E.V.; Gilfanov, I.R.; Lisovskaya, S.A.; Trizna, E.Y.; Kayumov, A.R.; Babaeva, O.B.; Boichuk, S.V.; et al. A novel terpene-BODIPY conjugates based fluorescent probes: Synthesis, spectral properties, stability, penetration efficiency into bacterial, fungal and mammalian cells. Spectrochim. Acta A 2025, 327, 125387. [Google Scholar] [CrossRef]
- Ilhan, H.; Şeker, M.; Gülseren, G.; Bakırcı, M.E.; Boyacı, A.İ.; Cakmak, Y. Nitric Oxide Activatable Photodynamic Therapy Agents Based on BODIPY–Copper Complexes. ACS Pharmacol. Transl. Sci. 2025, 8, 679–689. [Google Scholar] [CrossRef] [PubMed]
- Piękoś, P.; Maliszewska, I.H.; Tursynova, N.; Sokolnicki, J.; Jerzykiewicz, M.; Bartkiewicz, S.; Filarowski, A. Solvatochromic and biological studies of new meso-benzodioxole-BODIPY-2-Schiff dye. J. Mol. Liq. 2024, 413, 126008. [Google Scholar] [CrossRef]
- Li, X.; Kolemen, S.; Yoon, J.; Akkaya, E.U. Activatable Photosensitizers: Agents for Selective Photodynamic Therapy. Adv. Funct. Mater. 2017, 27, 1604053. [Google Scholar] [CrossRef]
- Sun, Q.; Jia, A.; Zhao, M.; Wang, K.; Sun, T.; Xie, Z. A BODIPY derivative for PDT/PTT synergistic treatment of bacterial infections. J. Photochem. Photobiol. B 2024, 261, 113049. [Google Scholar] [CrossRef]
- Kuehne, A.J.C.; Gather, M.C. Organic Lasers: Recent Developments on Materials, Device Geometries, and Fabrication Techniques. Chem. Rev. 2016, 116, 12823–12864. [Google Scholar] [CrossRef]
- Yadav, I.S.; Misra, R. Design, synthesis and functionalization of BODIPY dyes: Applications in dye-sensitized solar cells (DSSCs) and photodynamic therapy (PDT). J. Mater. Chem. C 2023, 11, 8688–8723. [Google Scholar] [CrossRef]
- Bessette, A.; Hanan, G.S. Design, synthesis and photophysical studies of dipyrromethene-based materials: Insights into their applications in organic photovoltaic devices. Chem. Soc. Rev. 2014, 43, 3342. [Google Scholar] [CrossRef]
- Merkushev, D.A.; Usoltsev, S.D.; Marfin, Y.S.; Pushkarev, A.P.; Volyniuk, D.; Grazulevicius, J.V.; Rumyantsev, E.V. BODIPY associates in organic matrices: Spectral properties, photostability and evaluation as OLED emitters. Mater. Chem. Phys. 2016, 187, 104–111. [Google Scholar] [CrossRef]
- Ge, Y.; O’Shea, D.F. Azadipyrromethenes: From traditional dye chemistry to leading edge applications. Chem. Soc. Rev. 2016, 45, 3846. [Google Scholar] [CrossRef] [PubMed]
- Boodts, S.; Fron, E.; Hofkens, J.; Dehaen, W. The BOPHY fluorophore with double boron chelation: Synthesis and spectroscopy. Coord. Chem. Rev. 2018, 371, 1–10. [Google Scholar] [CrossRef]
- Vodyanova, O.S.; Kochergin, B.A.; Usoltsev, S.D.; Marfin, Y.S.; Rumyantsev, E.V.; Aleksakhina, E.L.; Tomilova, I.K. BODIPY dyes in bio environment: Spectral characteristics and possibilities for practical application. J. Photochem. Photobiol. A 2018, 350, 44–51. [Google Scholar] [CrossRef]
- Boens, N.; Verbelen, B.; Ortiz, M.J.; Jiao, L.; Dehaen, W. Synthesis of BODIPY dyes through postfunctionalization of the boron dipyrromethene core. Coord. Chem. Rev. 2019, 399, 213024. [Google Scholar] [CrossRef]
- Jiang, G.; Tang, Z.; Han, H.; Ding, J.; Zhou, P. Effects of Intermolecular Hydrogen Bonding and Solvation on Enol–Keto Tautomerism and Photophysics of Azomethine–BODIPY Dyads. J. Phys. Chem. B 2021, 125, 9296–9303. [Google Scholar] [CrossRef] [PubMed]
- Filarowski, A.; Lopatkova, M.; Lipkowski, P.; Van der Auweraer, M.; Leen, V.; Dehaen, W. Solvatochromism of BODIPY-Schiff Dye. J. Phys. Chem. B 2015, 119, 2576–2584. [Google Scholar] [CrossRef]
- Filarowski, A.; Kluba, M.; Cieślik-Boczula, K.; Koll, A.; Kochel, A.; Pandey, L.; De Borggraeve, W.M.; Van der Auweraer, M.; Catalán, J.; Boens, N. Generalized solvent scales as a tool for investigating solvent dependence of spectroscopic and kinetic parameters. Application to fluorescent BODIPY dyes. Photochem. Photobiol. Sci. 2010, 9, 996–1008. [Google Scholar] [CrossRef]
- Qin, W.; Baruah, M.; De Borggraeve, W.M.; Boens, N. Photophysical properties of an on/off fluorescent pH indicator excitable with visible light based on a borondipyrromethene-linked phenol. J. Photochem. Photobiol. A 2006, 183, 190–197. [Google Scholar] [CrossRef]
- Strobl, M.; Rappitsch, T.; Borisov, S.M.; Mayr, T.; Klimant, I. NIR-emitting aza-BODIPY dyes-new building blocks for broad-range optical pH sensors. Analyst 2015, 140, 7150–7153. [Google Scholar] [CrossRef]
- Deng, M.; Yang, C.; Gong, D.; Iqbal, A.; Tang, X.; Liu, W.; Qin, W.W. BODIPY-derived piperazidine fluorescent near-neutral pH indicator and its bioimaging. Sens. Actuators B 2016, 232, 492–498. [Google Scholar] [CrossRef]
- Radunz, S.; Tschiche, H.R.; Moldenhauer, D.; Resch-Genger, U. Broad range ON/OFF pH sensors based on pKa tunable fluorescent BODIPYs. Sens. Actuators B 2017, 251, 490–494. [Google Scholar] [CrossRef]
- Glavaš, M.; Zlatić, K.; Jadreško, D.; Ljubić, I.; Basarić, N. Fluorescent pH sensors based on BODIPY structure sensitive in acidic media. Dyes Pigm. 2023, 220, 111660. [Google Scholar] [CrossRef]
- Wu, D.; Sedgwick, A.C.; Gunnlaugsson, T.; Akkaya, E.U.; Yoon, J.; James, T.D. Fluorescent chemosensors: The past, present and future. Chem. Soc. Rev. 2017, 46, 7105–7123. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Jiang, M.; Yan, M.; Ye, J.; Li, Y.; Dehaen, W.; Yin, S. Near-infrared boron–dipyrrin (BODIPY) nanomaterials: Molecular design and anti-tumor therapeutics. Coord. Chem. Rev. 2024, 506, 215718. [Google Scholar] [CrossRef]
- Chibani, S.; Le Guennic, B.; Charaf-Eddin, A.; Laurent, A.D.; Jacquemin, D. Revisiting the optical signatures of BODIPY dyes with theoretical tools. Chem. Sci. 2013, 4, 1950–1963. [Google Scholar] [CrossRef]
- Adamo, C.; Jacquemin, D. The calculations of excited-state properties with time-dependent density functional theory. Chem. Soc. Rev. 2013, 42, 845–856. [Google Scholar] [CrossRef]
- Laurent, A.D.; Adamo, C.; Jacquemin, D. Dye chemistry with time-dependent density functional theory. Phys. Chem. Chem. Phys. 2014, 16, 14334–14356. [Google Scholar] [CrossRef]
- Mallah, R.; Sreenath, M.C.; Chitrambalam, S.; Joe, I.H.; Sekar, N. Excitation energy transfer processes in BODIPY based donor-acceptor system—Synthesis, photophysics, NLO and DFT study. Opt. Mat. 2018, 84, 795–806. [Google Scholar] [CrossRef]
- Charaf-Eddin, A.; Le Guennic, B.; Jacquemin, D. Optical Signatures of Borico Dyes: A TD-DFT Analysis. Theor. Chem. Acc. 2014, 133, 1456. [Google Scholar] [CrossRef]
- Laine, M.; Barbosa, N.A.; Wieczorek, R.; Melnikov, M.Y.; Filarowski, A. Calculations of BODIPY dyes in the ground and excited states using the M06-2X and PBE0 functionals. J. Mol. Model. 2016, 22, 260. [Google Scholar] [CrossRef]
- Baron, T.; Maffeis, V.; Bucher, C.; Le Guennic, B.; Banyasz, A.; Jacquemin, D.; Berginc, G.; Maury, O.; Andraud, C. Tuning the Photophysical Properties of Aza-BODIPYs in the Near-Infrared Region by Introducing Electron-Donating Thiophene Substituents. Chem. Eur. J. 2023, 29, e202301357. [Google Scholar] [CrossRef] [PubMed]
- Ośmiałowski, B.; Petrusevich, E.F.; Nawrot, K.C.; Paszkiewicz, B.K.; Nyk, M.; Zielak, J.; Jȩdrzejewska, B.; Luis, J.M.; Jacquemin, D.; Zaleśny, R. Tailoring the nonlinear absorption of fluorescent dyes by substitution at a boron center. J. Mat. Chem. C 2021, 9, 6225–6233. [Google Scholar] [CrossRef]
- Rybczyński, P.; Bousquet, M.H.E.; Kaczmarek-Kędziera, A.; Jędrzejewska, B.; Jacquemin, D.; Ośmiałowski, B. Controlling the fluorescence quantum yields of benzothiazole-difluoroborates by optimal substitution. Chem. Sci. 2022, 13, 13347–13360. [Google Scholar] [CrossRef]
- Ji, S.; Ge, J.; Escudero, D.; Wang, Z.; Zhao, J.; Jacquemin, D. Molecular structure–intersystem crossing relationship of heavy-atomfree BODIPY triplet photosensitizers. J. Org. Chem. 2015, 80, 5958–5963. [Google Scholar] [CrossRef]
- Thorat, K.G.; Bhakhoa, H.; Ramasami, P.; Bhakhoa, H.; Ramasami, P.; Sekar, N.; Ramasami, P. NIR-Emitting Boradiazaindacene Fluorophores—TD-DFT Studies on Electronic Structure and Photophysical Properties. J. Fluoresc. 2015, 25, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Frey, P.A.; Whitt, S.A.; Tobin, J.B. A Low-Barrier Hydrogen Bond in the Catalytic Triad of Serine Proteases. Science 1994, 264, 1927–1930. [Google Scholar] [CrossRef] [PubMed]
- Cleland, W.W.; Kreevoy, M.M. Low-barrier hydrogen-bonds and enzymatic catalysis. Science 1994, 264, 1887–1890. [Google Scholar] [CrossRef]
- Boens, N.; Qin, W.; Baruah, M.; De Borggraeve, W.M.; Filarowski, A.; Smisdom, N.; Ameloot, M.; Crovetto, L.; Talavera, E.M.; Alvarez-Pez, J.M. Rational design, synthesis and spectroscopic and photophysical properties of a visible-light-excitable, ratiometric, fluorescent near-neutral pH indicator based on BODIPY. Chem.–Eur. J. 2011, 17, 10924–10934. [Google Scholar] [CrossRef]
- Deniz, E.; Isbasar, G.C.; Bozdemir, O.A.; Yildirim, L.T.; Siemiarczuk, A.; Akkaya, E.U. Bidirectional Switching of Near IR Emitting Boradiazaindacene Fluorophores. Org. Lett. 2008, 10, 3401–3403. [Google Scholar] [CrossRef]
- Ekmekci, X.Z.; Yilmaz, M.D.; Akkaya, E.U. A Monostyryl-boradiazaindacene (BODIPY) Derivative as Colorimetric and Fluorescent Probe for Cyanide Ions. Org. Lett. 2008, 10, 461–464. [Google Scholar] [CrossRef]
- Coskun, K.; Deniz, E.; Akkaya, E.U. Effective PET and ICT Switching of Boradiazaindacene Emission: A Unimolecular, Emission-Mode, Molecular Half-Subtractor with Reconfigurable Logic Gates. Org. Lett. 2005, 7, 5187–5189. [Google Scholar] [CrossRef] [PubMed]
- Reichardt, C. Solvatochromic dyes as solvent polarity indicators. Chem. Rev. 1994, 94, 2319–2358. [Google Scholar] [CrossRef]
- Catalan, J. Toward a generalized treatment of the solvent effect based on four empirical scales: Dipolarity (SdP, a New Scale), Polarizability (SP), Acidity (SA), and Basicity (SB) of the medium. J. Phys. Chem. B 2009, 113, 5951–5960. [Google Scholar] [CrossRef]
- de la Cerda-Pedro, J.E.; Hernández-Ortiz, O.J.; Vázquez-García, R.A.; García-Báez, E.V.; Gómez-Aguilar, R.; Espinosa-Roa, A.; Farfán, N.; Padilla-Martínez, I.I. Highly crystalline and fluorescent BODIPY-labelled phenyl-triazole-coumarins as n-type semiconducting materials for OFET devices. Heliyon 2024, 10, e23517. [Google Scholar] [CrossRef]
- Clemens, O.; Basters, M.; Wild, M.; Wilbrand, S.; Reichert, C.; Bauer, M.; Springborg, M.; Jung, G. Solvent effects on the absorption/emission spectra of an organic chromophore: A theoretical study. J. Mol. Struct. THEOCHEM 2008, 866, 15–20. [Google Scholar] [CrossRef]
- Parambil, S.P.; de Jong, F.; Veys, K.; Huang, J.; Veettil, S.P.; Verhaeghe, D.; Meervelt, L.V.; Escudero, D.; Van der Auweraer, M.; Dehaen, W. BOPAHY: A doubly chelated highly fluorescent pyrrole–acyl hydrazone –BF2 chromophore. Chem. Commun. 2020, 56, 5791. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, H.; Wan, L.; Bian, Y.; Jiang, J. 8-Hydroxyquinoline-Substituted Boron–Dipyrromethene Compounds: Synthesis, Structure, and OFF–ON–OFF Type of pH-Sensing Properties. J. Org. Chem. 2011, 76, 3774–3781. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Ditchfield, R.; Hehre, W.J.; Pople, J.A. Self-Consistent Molecular-Orbital Methods. IX. An Extended Gaussian-Type Basis for Molecular-Orbital Studies of Organic Molecules. J. Chem. Phys. 1971, 54, 724–728. [Google Scholar] [CrossRef]
- Zhao, Y.; Truhlar, D.G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: Two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Acc. 2008, 120, 215–241. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional thermochemistry. III. the role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti Correlation Energy Formula into a Functional of the Electron Density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef] [PubMed]
- Petersilka, M.; Gossmann, U.J.; Gross, E.K.U. Excitation Energies from Time-Dependent Density-Functional Theory. Phys. Rev. Lett. 1996, 76, 1212–1215. [Google Scholar] [CrossRef]
- Matulis, V.E.; Ragoyja, E.G.; Ivashkevich, O.A. Accurate theoretical prediction of optical properties of BODIPY dyes. Int. J. Quantum Chem. 2020, 120, e26159. [Google Scholar] [CrossRef]
- Miertuš, S.; Scrocco, E.; Tomasi, J. Electrostatic interaction of a solute with a continuum. A direct utilizaion of AB initio molecular potentials for the prevision of solvent effects. Chem. Phys. 1981, 55, 117–129. [Google Scholar] [CrossRef]
- Tomasi, J.; Mennucci, B.; Cammi, R. Quantum Mechanical Continuum Solvation Models. Chem. Rev. 2005, 105, 2999–3093. [Google Scholar] [CrossRef] [PubMed]
- Cammi, R.; Mennucci, B. Linear response theory for the polarizable continuum model. J. Chem. Phys. 1999, 110, 9877–9886. [Google Scholar] [CrossRef]
- Cossi, M.; Barone, V. Time-dependent density functional theory for molecules in liquid solutions. J. Chem. Phys. 2001, 115, 4708–4717. [Google Scholar] [CrossRef]
- Cossi, M.; Barone, V. Solvent Effect on Vertical Electronic Transitions by the Polarizable Continuum Model. J. Chem. Phys. 2000, 112, 2427–2435. [Google Scholar] [CrossRef]
- Improta, R.; Barone, V.; Santoro, F. Ab Initio Calculations of Absorption Spectra of Large Molecules in Solution: Coumarin C153. Angew. Chem. Int. Ed. 2007, 46, 405–408. [Google Scholar] [CrossRef]
- Grimme, S. Semiempirical GGA-type density functional constructed with a long-range dispersion correction. J. Comput. Chem. 2006, 27, 1787–1799. [Google Scholar] [CrossRef]
- Schaftenaar, G.; Noordik, J.H. Molden: A pre- and post-processing program for molecular and electronic structures. J. Comput.-Aided Mol. Design 2000, 14, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Lu, T. A comprehensive electron wavefunction analysis toolbox for chemists, Multiwfn. J. Chem. Phys. 2024, 161, 082503. [Google Scholar] [CrossRef] [PubMed]
- Hanwell, M.D.; Curtis, D.E.; Lonie, D.C.; Vandermeersch, T.; Zurek, E.; Hutchison, G.R. Avogadro: An advanced semantic chemical editor, visualization, and analysis platform. J. Chem. Inform. 2012, 4, 17. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD—Visual Molecular Dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Dennington, R.; Keith, T.A.; Millam, J.M. GaussView, Version 6.1.1; Semichem Inc.: Shawnee Mission, KS, USA, 2016.


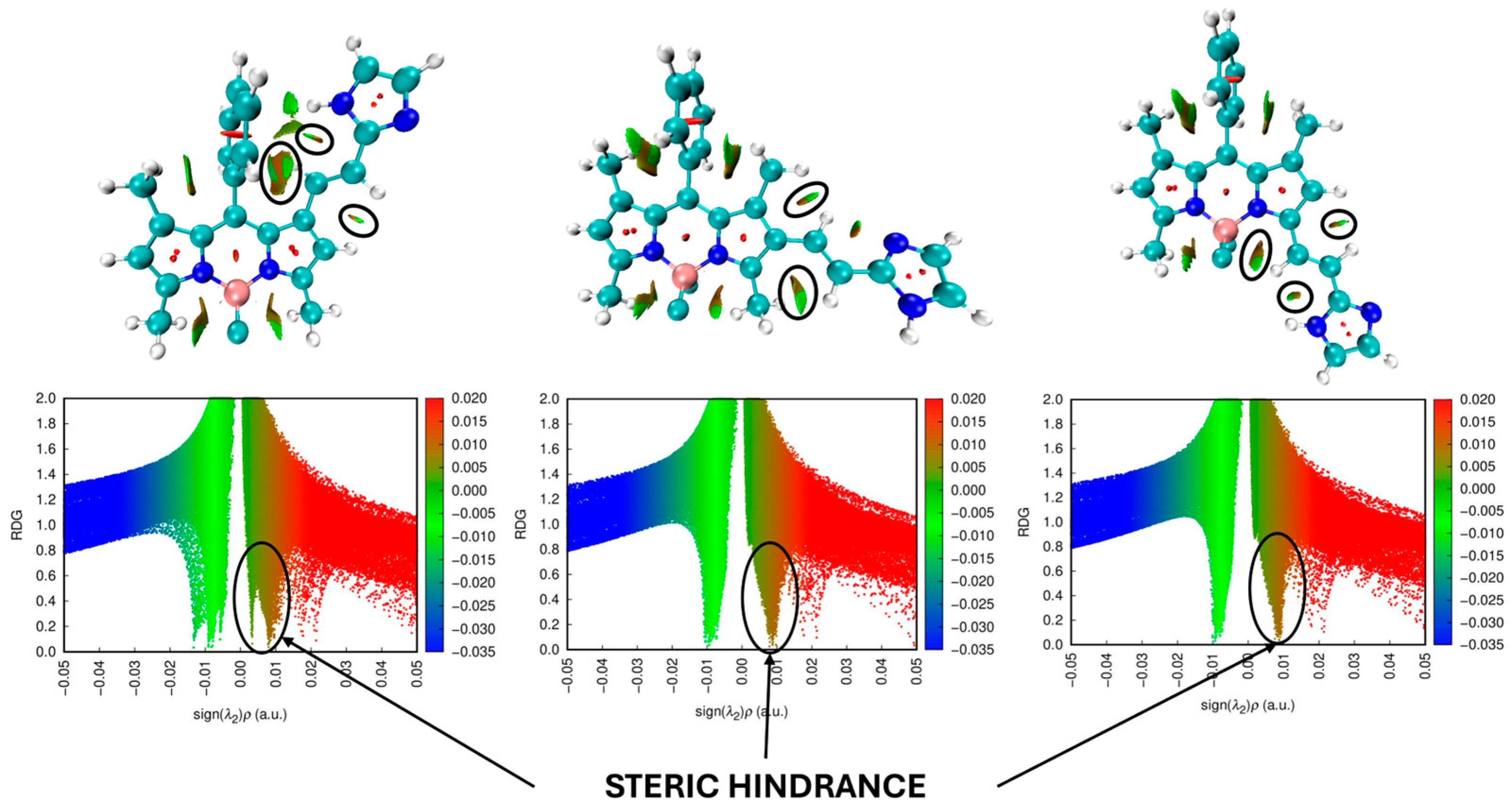

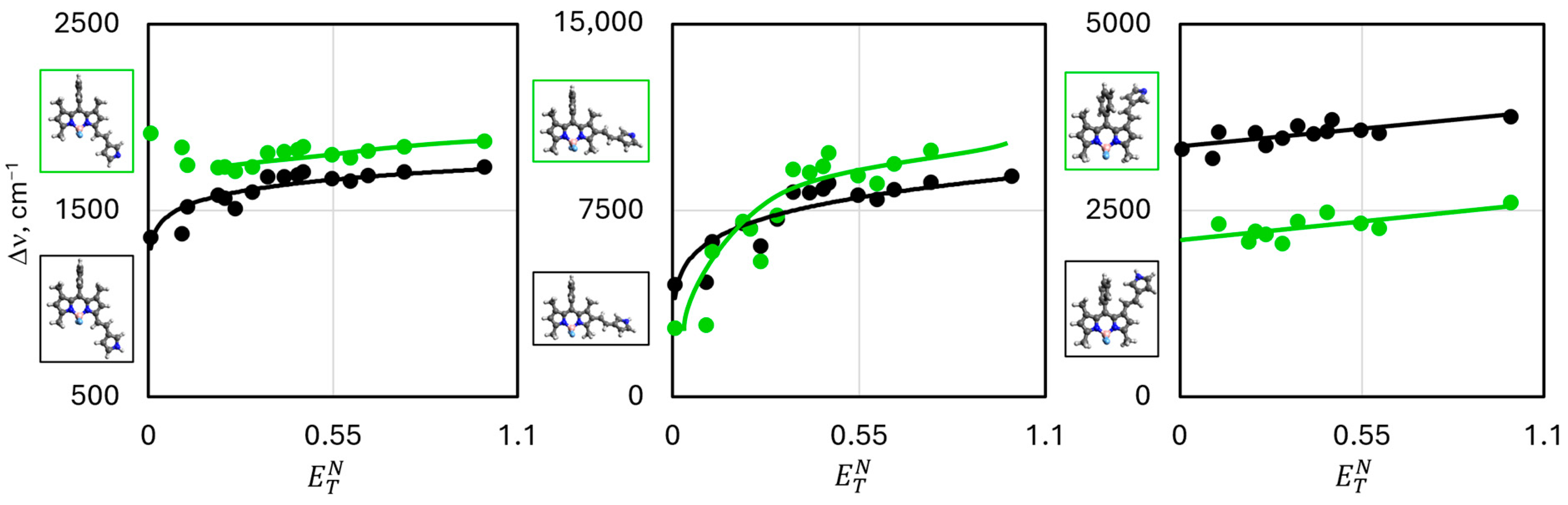
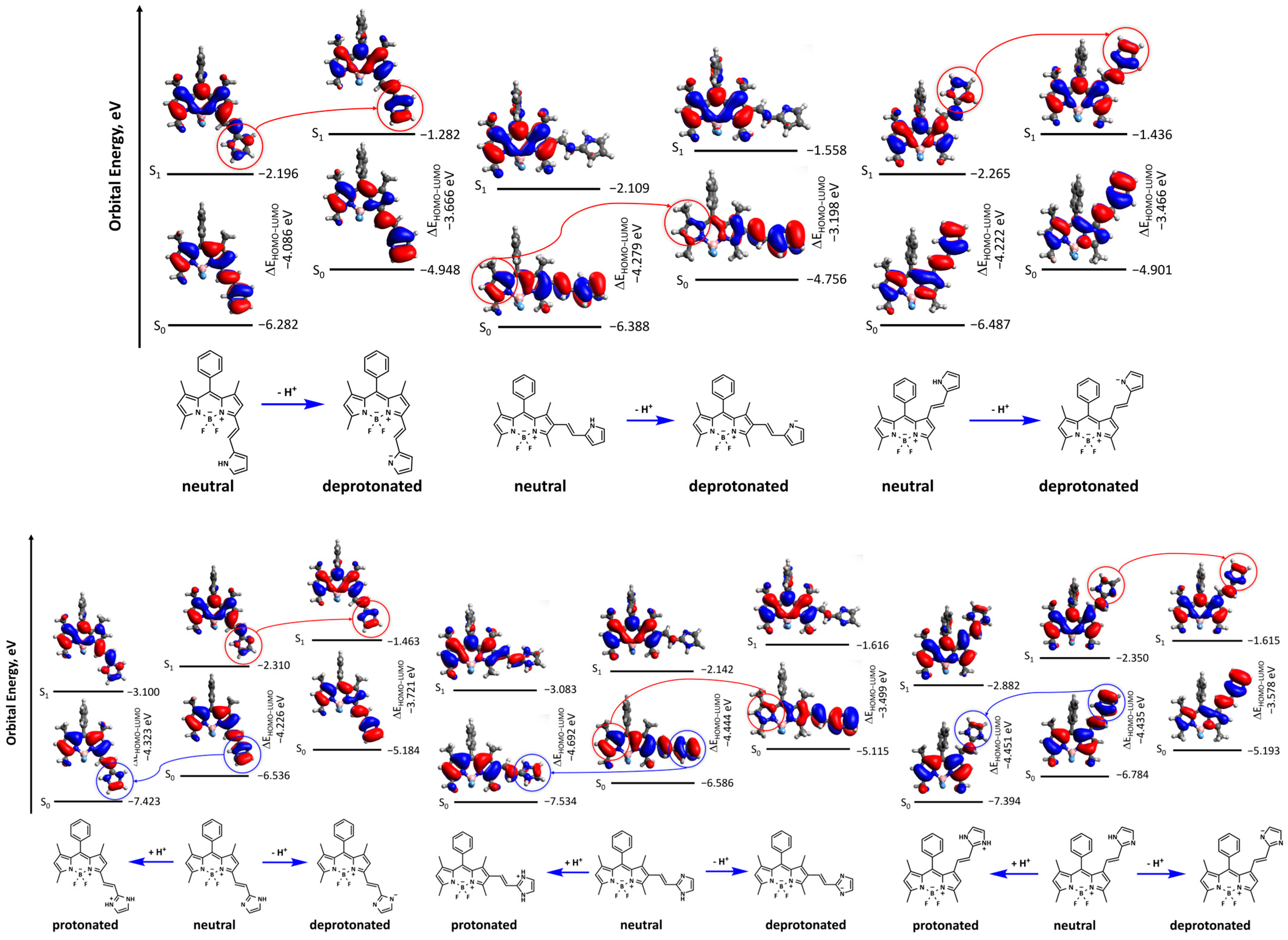
| λabs | λem | |||||
|---|---|---|---|---|---|---|
| Substituent/Position | 3 | 2 | 1 | 3 | 2 | 1 |
| Neutral form | ||||||
| R1 | 484.70 | 496.27 | 491.49 | 525.07 | 704.23 | 575.29 |
| R2 | 473.07 | 479.68 | 474.73 | 509.44 | 668.12 | 559.15 |
| R3 | 467.69 | 453.90 | 445.39 | 504.96 | 581.83 | 532.58 |
| R4 | 468.83 | 455.55 | 450.28 | 503.32 | 583.81 | 531.44 |
| Deprotonated form | ||||||
| R1 | 514.98 | 631.07 | 575.73 | 563.17 | 922.76 | 639.65 |
| R2 | 501.43 | 593.24 | 562.58 | 548.48 | 877.08 | 641.19 |
| R3 | 499.89 | 547.79 | 541.60 | 548.5 | 820.45 | 618.86 |
| R4 | 495.52 | 534.92 | 529.57 | 542.08 | 790.40 | 604.67 |
| Protonated form | ||||||
| R3 | 459.50 | 436.45 | 438.76 | 499.92 | 454.54 | 481.98 |
| R4 | 456.87 | 443.04 | 432.91 | 486.92 | 464.74 | 466.32 |
| 1,3,5,7-Tetramethyl-8-phenyl-4,4-difluoroboradiazaindacene | ||||||
| H | 413.85 | 429.4 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piękoś, P.; Lipkowski, P.; Dehaen, W.; Wieczorek, R.; Filarowski, A. Theoretical Insights into the Impact of Pyrrole and Imidazole Substituents on the BODIPY Chromophore. Molecules 2025, 30, 2209. https://doi.org/10.3390/molecules30102209
Piękoś P, Lipkowski P, Dehaen W, Wieczorek R, Filarowski A. Theoretical Insights into the Impact of Pyrrole and Imidazole Substituents on the BODIPY Chromophore. Molecules. 2025; 30(10):2209. https://doi.org/10.3390/molecules30102209
Chicago/Turabian StylePiękoś, Patrycja, Paweł Lipkowski, Wim Dehaen, Robert Wieczorek, and Aleksander Filarowski. 2025. "Theoretical Insights into the Impact of Pyrrole and Imidazole Substituents on the BODIPY Chromophore" Molecules 30, no. 10: 2209. https://doi.org/10.3390/molecules30102209
APA StylePiękoś, P., Lipkowski, P., Dehaen, W., Wieczorek, R., & Filarowski, A. (2025). Theoretical Insights into the Impact of Pyrrole and Imidazole Substituents on the BODIPY Chromophore. Molecules, 30(10), 2209. https://doi.org/10.3390/molecules30102209










