Abstract
The enduring problem of CO2 emissions and their consequent influence on the earth’s atmosphere has captured the attention of researchers. Photocatalytic CO2 reduction holds great significance; however, it is constrained by the effect of carrier recombination. Simultaneously, the structural modification of heterojunction catalysts has emerged as a promising approach to boost the photocatalytic performance. Herein, Zn2GeO4@CeO2 core@shell nanorods were prepared by a simple self-assembly method for photocatalytic CO2 reduction. The thickness of the CeO2 shell can be regulated rapidly and conveniently. The photocatalytic results indicate that the structure regulation could affect the photocatalytic performance by controlling the amount of active sites and the shielding effect. X-ray photoelectron spectroscopy (XPS) and Mott–Schottky analyses reveal that Zn2GeO4 and CeO2 formed Type-I heterojunctions, which prolonged the lifetime of the photogenerated carriers. The CO2 adsorption and activation capacities of CeO2 also exert a beneficial influence on the progress of CO2 photoreduction, thus enabling efficient photocatalytic CO2 reduction. Moreover, the in situ FT-IR spectra show that Zn2GeO4@CeO2 suppresses the formation of byproduct intermediates and shows higher CO selectivity. The best sample of Zn2GeO4@0.07CeO2 can exhibit a CO yield of as high as 1190.9 μmol g−1 h−1.
1. Introduction
In recent decades, the combustion of fossil fuels has led to a substantial increase in the atmospheric concentration of CO2. As a greenhouse gas, an excessive CO2 concentration can trigger a series of environmental problems [1,2,3]. Considerable efforts have been devoted to reducing atmospheric CO2 levels such as photocatalysis, electrocatalysis and thermocatalysis. Among these, photocatalytic CO2 reduction into high-value-added products is regarded as an effective approach to mitigate energy problems and reduce the greenhouse effect [4,5,6]. A variety of semiconductors, including TiO2, CdS, and Zn2GeO4, have been employed as photocatalysts [7,8,9].
For photocatalytic CO2 reduction catalysts, the primary limitation lies in the intense photogenerated carrier recombination, which significantly impacts the lifetime of carriers [10,11,12]. In an attempt to surmount this constraint on carrier lifetime, heterojunction photocatalysts have been extensively investigated [13]. The photogenerated carriers in the heterojunctions can transfer between semiconductors, thus generating highly active charge carriers, which is advantageous for reducing the probability of recombination [14,15,16]. Currently, a wide variety of heterojunction photocatalysts with diverse morphologies and structures have been investigated, and their structure and morphology have emerged as a prevalent research focus [7,14,17,18]. It is generally accepted that the carrier transfer process predominantly takes place at the heterostructure interface [19]. In this case, heterojunction catalysts with a special core@shell structure, which possess a large interfacial area, have been widely studied [16]. Structure regulation of the core@shell heterojunction catalysts enables the components to leverage strengths to offset weaknesses, thereby achieving the optimal effect [17]. Moreover, controllable tuning is of great significance in catalyst design.
Zinc germanate (Zn2GeO4), owing to its good CO2 reduction capability, is widely utilized in the field of photocatalysis [20,21]. However, the wide band gap, low charge separation efficiency, and insufficient active sites of Zn2GeO4 impose limitations on its photocatalytic efficiency [22]. To remove these obstacles, various methods have been employed by researchers such as adjusting the oxygen vacancies concentration [9]. Cerium dioxide (CeO2) is an n-type semiconductor, and there are a substantial number of oxygen vacancies on its surface, which can act as reaction sites for the activation of CO2 [23,24,25]. Inspired by the above analysis, the construction of Zn2GeO4@CeO2 might prove to be an effective way to achieve an ideal photocatalytic effect.
In this work, Zn2GeO4@CeO2 was fabricated by a self-assembly method. The amount of the coated CeO2 shell was carefully controlled to adjust the amount of active sites. The as-obtained Zn2GeO4@CeO2 catalysts were then comprehensively characterized to identify the optimal amount of the CeO2 shell that exhibits the best performance on photocatalytic CO2 reduction. Mott–Schottky analysis and UV–visible spectroscopy (UV-vis) characterizations were conducted to reveal the reaction mechanism. The results suggest that the formation of Zn2GeO4@CeO2 nanorods could extend the carrier lifetime and facilitate the light absorption process, with their catalytic performance being significantly influenced by the coating thickness of the CeO2 layer. It is believed that this work can provide a novel strategy for the design of heterojunction catalysts for photocatalytic CO2 reduction.
2. Results and Discussion
The schematic synthesis process of a series of Zn2GeO4@CeO2 core@shell nanorods is presented in Figure 1a. Initially, the Zn2GeO4 precursor was synthesized via hydrothermal treatment, followed by CeO2 coating to form the core@shell architecture. A series of Zn2GeO4@CeO2 samples have been prepared, which are named as Zn2GeO4@xCeO2, where x represents the amount of Ce added per 50 mg of Zn2GeO4 (Figure 1b–e). Scanning electron microscopy (SEM) was applied to characterize the morphology of the series samples. As shown in Figure 1b and Figure S1, the as-obtained Zn2GeO4 shows a rod-shaped morphology. After coating, the surface of these nanorods became rough, implying the formation of the core@shell structure [26,27]. The high-resolution transmission electron microscopy (HRTEM) images of Zn2GeO4@xCeO2 in Figure 1f show that a layer of CeO2 nanoparticles could be observed clearly on the surface of Zn2GeO4. To further investigate the structure of the samples, Zn2GeO4@0.07CeO2 was chosen as the representative sample. As shown in Figure 1g, it can be observed that the lattice spacings of the core and shell of 0.29 and 0.31 nm correspond well to the characteristic (113) plane of Zn2GeO4 and the (111) plane of CeO2, respectively [20,23,28]. Figure 1h and Figure S4 present the X-ray diffraction (XRD) patterns of the samples, where all diffraction peaks of the as-obtained Zn2GeO4 and CeO2 could be well indexed to the standard patterns of Zn2GeO4 (JCPDS #11-0687) and CeO2 (JCPDS #11-0394). The intensities of the signals corresponding to Zn2GeO4 decrease when compared to those of Zn2GeO4, which should be attributed to the encapsulation of the CeO2 shell. However, the intensities of the characteristic peaks of CeO2 gradually increase as the Ce dosage increases [8]. Therefore, as shown in Figure 1i–l and Figure S5, energy dispersive X-ray spectroscopy (EDS) mapping analysis shows that the elements Zn and Ge are present in the core position; however, Ce is only distributed on the nanorod surface. Furthermore, the thickness of the CeO2 shell increases with the increase in CeO2 (Figures S5 and S6).
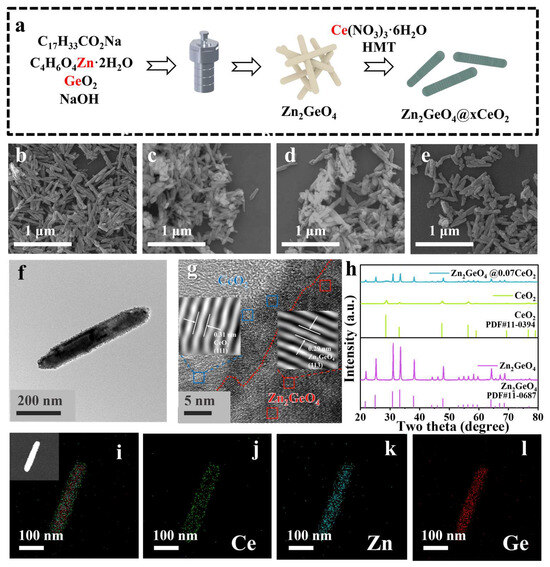
Figure 1.
(a) Schematic synthesis of Zn2GeO4@xCeO2 samples; (b–e) SEM images of the as-obtained samples: (b) Zn2GeO4, (c) Zn2GeO4@0.05CeO2, (d) Zn2GeO4@0.07CeO2, and (e) Zn2GeO4@0.1CeO2; (f,g) HRTEM images of Zn2GeO4@0.07CeO2; (h) XRD patterns of Zn2GeO4, CeO2, and Zn2GeO4@0.07CeO2; (i–l) EDS images of Zn2GeO4@0.07CeO2.
X-ray photoelectron spectroscopy (XPS) was employed to identify the surface chemical environments of the as-obtained samples. As shown in Figure 2a, the peaks centered at 530.7 and 531.6 eV can be assigned to the lattice oxygen and oxygen atoms near oxygen vacancy in Zn2GeO4 [29,30,31]. For CeO2, the O 1s XPS peak at 532.9 eV can be ascribed to the adsorbed (CO2 or H2O) oxygen on the surface of CeO2, indicating the outstanding CO2-storing ability (Figure 2b) [26,32,33]. The significant peak at 530.8 eV can be attributed to the oxygen atoms near oxygen vacancy defects. It is further substantiated by the Electron Paramagnetic Resonance (EPR) tests that the signal of oxygen vacancy at g = 2.002 can be observed (Figure S7). The abundant O vacancies in CeO2 could act as active sites for photocatalytic CO2 reduction [24]. All the peaks displayed in the O 1s signals mentioned above have been included in Zn2GeO4@0.07CeO2 (Figure 2c).
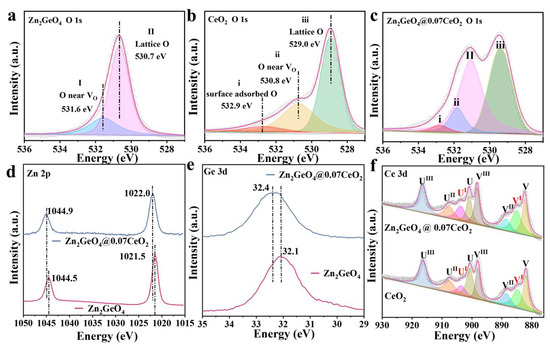
Figure 2.
O 1s XPS spectra of (a) Zn2GeO4, (b) CeO2, and (c) Zn2GeO4@0.07CeO2; (d) Zn 2p XPS spectra of Zn2GeO4@0.07CeO2 and Zn2GeO4; (e) Ge 3d XPS spectra of Zn2GeO4@0.07CeO2 and Zn2GeO4; (f) Ce 3d XPS spectra of Zn2GeO4@0.07CeO2 and CeO2.
Moreover, the electron binding energy of the element originates from the Coulomb attraction between the outer electrons of the atom and the atomic nucleus, in which a chemical shift can directly reflect the changes in electron density [17,34,35]. For the XPS spectra of Zn 2p and Ge 3d, the electron transfer process after the formation of the heterojunction was characterized by comparing Zn2GeO4 with Zn2GeO4@0.07CeO2 (Figure 2d,e). As shown in Figure 2d, the peaks located at 1021.5 and 1044.5 eV can be fitted into Zn 2p3/2 and Zn 2p1/2, respectively. In Figure 2e, the peaks centered at 32.1 eV can be assigned to Ge 3d [20,29]. Compared to Zn2GeO4, Zn 2p3/2 and Zn 2p1/2 in Zn2GeO4@0.07CeO2 shift to 1022.0 and 1044.9 eV, and Ge 3d shifts to 32.4 eV, which could be attributed to the electron transfer from Zn2GeO4 to CeO2 [23]. In Figure 2f, the Ce 3d XPS spectra can be deconvoluted into eight peaks denoted as V and U, ascribed to the spin−orbit coupling of the Ce 3d5/2 and Ce 3d3/2 states, respectively, which also correspond to Ce3+ and Ce4+ [26,36,37]. The presence of Ce3+/Ce4+ redox couples can create oxygen vacancies, which can serve as active sites and enhance the photocatalytic activity [25]. As a result, the Ce3+ content in Zn2GeO4@0.07CeO2 is 35.7%, which is significantly higher than the 25.8% in pure CeO2 (Table S1, Supporting Information). This can be mainly attributed to the electron transfer from Zn2GeO4 to CeO2 [37,38].
Photocatalytic CO2 reduction was then conducted under full-spectrum irradiation and room temperature to evaluate the catalytic activities of the samples. As shown in Figure 3a, CO, H2, CH4, and C2 are the major gas-phase products. The CO yield of Zn2GeO4 is 380.1 μmol g−1 h−1, but the selectivity is only 10.0%. Among the samples, Zn2GeO4@0.07CeO2 has the highest CO yield of 1190.9 μmol g−1 h−1, which is 3.1 times that of Zn2GeO4, while its CO selectivity reaches 51.5% (Table S2). For Zn2GeO4, Zn2GeO4@0.05CeO2, and Zn2GeO4@0.07CeO2, the CO generation rates increase with the increase in CeO2, owing to the fact that the active sites on CeO2 near the interface are crucial for the reaction. The further increase in the thickness of CeO2 would result in a decline in catalytic performance, which can be attributed to the shielding effect, where the excessively thick CeO2 restricts the access of CO2 to the heterojunction interface, thereby impeding its reduction process [39]. 1H nuclear magnetic resonance (NMR) analysis indicates that negligible amounts of liquid-phase products were identified was detected in the system (Figure S8). As shown in Figure S9, the CO yield of Zn2GeO4@0.07CeO2 drops to 10.9 μmol g−1 h−1 in the absence of a sacrificial agent, comparable to that reported in the literature [29]. It is found that only a negligible amount of CO products are detected when no catalysts or external light source is present, further validating the origin of the product. Zn2GeO4@0.07CeO2 exhibits a similar CO yield over four cycles (Figure 3b). Moreover, there is no significant morphological change in Zn2GeO4@0.07CeO2 after reaction (Figure S10). A comparison of the XRD and XPS spectra of Zn2GeO4@0.07CeO2 before and after reaction indicates that there is no significant change, further demonstrating the structural stability of Zn2GeO4 (Figures S11 and S12).
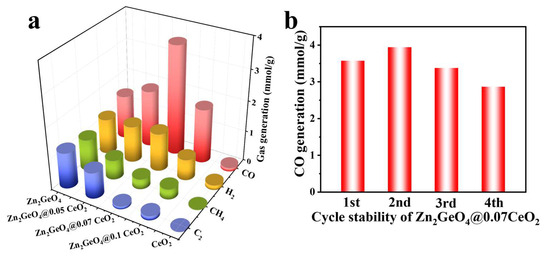
Figure 3.
(a) Yields of products for different samples; (b) CO yields of Zn2GeO4@0.07CeO2 after four cycles.
The UV-vis spectra depict the light absorption capacity of the samples. Among them, Zn2GeO4@0.07CeO2 exhibits two absorption edges, which can be respectively attributed to Zn2GeO4 and CeO2 (Figure 4a). The formation of heterojunctions expands the absorption spectrum, thereby enhancing the light absorption performance of Zn2GeO4. In addition, the results demonstrate that the absorption edge of Zn2GeO4@0.07CeO2 undergoes a slight red shift as the amount of CeO2 increases, which can be attributed to the bandgap narrowing caused by the gradual enhancement of the absorption contribution of CeO2 (Figure S13). As shown in Figure 4b, Zn2GeO4@0.07CeO2 demonstrates a higher photocurrent density compared to Zn2GeO4 and CeO2, further confirming the highly efficient transfer of photogenerated charge and separation of carriers occurring on the Zn2GeO4@0.07CeO2 nanorods. Such efficiency can lead to more effective photogenerated electrons to enhance the ability of photocatalytic CO2 reduction [40]. Figure 4c demonstrates that Zn2GeO4@0.07CeO2 has a smaller electrochemical impedance spectroscopy (EIS) radius than that of Zn2GeO4. This also implies the effective separation and transfer of charge carriers in Zn2GeO4@0.07CeO2. The above results prove that the formation of Zn2GeO4@0.07CeO2 enhances the photocatalytic capability, which is also consistent with the results of the XPS analysis [35,41,42]. The photoluminescence (PL) intensity reveals the defect emission of Zn2GeO4, indicating a strong effect of carrier recombination [43]. In comparison, the decrease in the PL of Zn2GeO4@0.07CeO2 demonstrates the extension of the carrier lifetime [15]. Moreover, the CO2 temperature-programmed desorption (TPD) reflects that the adsorbed HCO3− peak area increases with the increase in CeO2 (Figure 4e). This suggests that CeO2 provides abundant active sites for CO2 adsorption and activation [24,44,45].
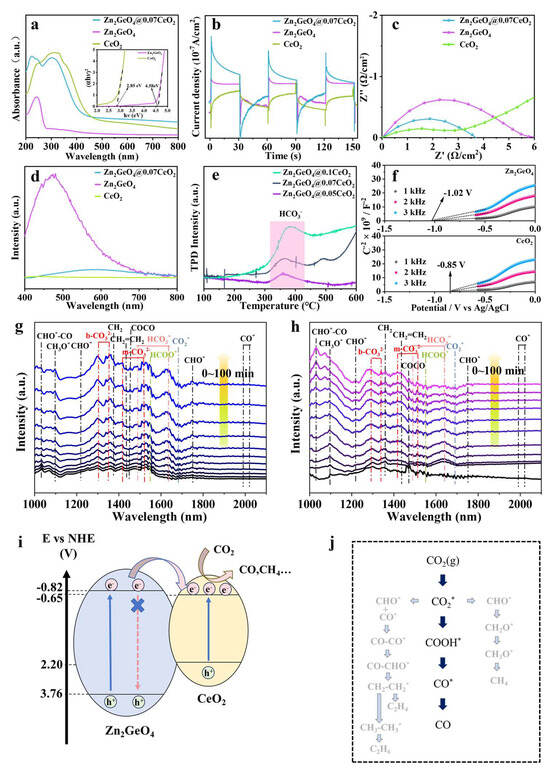
Figure 4.
(a) UV-vis and Tauc spectra of Zn2GeO4 and CeO2; (b) transient photocurrent of Zn2GeO4@0.07CeO2; (c) EIS curves of Zn2GeO4@0.07CeO2; (d) PL spectra of Zn2GeO4@0.07CeO2; (e) CO2-TPD plots of Zn2GeO4@xCeO2; (f) Mott–Schottky curves of Zn2GeO4 and CeO2; in situ FT-IR spectra for CO2 photoreduction on (g) Zn2GeO4@0.07CeO2 and (h) Zn2GeO4; (i) band structures of Zn2GeO4 and CeO2 (pH = 7, vs. NHE); (j) reaction pathway of photocatalytic CO2 reduction.
The band structure of Zn2GeO4 and CeO2 was conducted by Mott–Schottky spectrum measurements. The plots of Zn2GeO4 and CeO2 exhibit positive slopes, suggesting that they are n-type semiconductors (Figure 4f) [7,17,44]. The flat-band potentials (Efb) of Zn2GeO4 and CeO2 are −0.82 and −0.65 V (vs. normal hydrogen electrode (NHE)), respectively [9,23,46,47]. The Efb could roughly be equal to the conduction band (CB) potential of an n-type semiconductor [7,17,18]. Moreover, the band gaps (Eg) of Zn2GeO4 and CeO2 are determined by the UV-vis spectra and Tauc plots (Figure 4a), which are 4.58 eV and 2.85 eV, respectively [47,48]. Therefore, the valence band maximum (VBM) potentials of Zn2GeO4 and CeO2 are 2.20 and 3.76 V (vs. NHE), respectively. Due to the standard reduction potential of CO2/CO being −0.52 V (vs. NHE at pH = 7), the photocatalytic CO2 reduction can thermodynamically proceed under the photocatalysis of Zn2GeO4 and CeO2, and this is consistent with the experimental results [1,4]. The band structures in Figure 4i show that Zn2GeO4 and CeO2 should be ascribed to Type-I heterojunction. Under light excitation, the electrons of Zn2GeO4 and CeO2 will be excited from the valence band to the conduction band, respectively [42]. Due to the different conduction band energies, the photogenerated electrons with higher energy on the conduction band of Zn2GeO4 will migrate to the conduction band of CeO2, thus extending the carrier lifetime. The carriers will accumulate at the heterojunction interface of CeO2. Furthermore, the migration rate of holes is generally about two orders of magnitude slower than that of electrons. This effectively reduces the probability of carrier recombination [11,49]. The formation of heterojunction endows Zn2GeO4@0.07CeO2 with superior catalytic performance (Tables S3 and S4) [50,51,52,53,54,55,56,57,58,59,60].
To further clarify the reaction mechanism, in situ FT-IR measurements were performed on Zn2GeO4@0.07CeO2 and Zn2GeO4 during the CO2 photoreduction process. As shown in Figure 4g, the peak attributed to COOH* was detected at 1550 cm−1 [61]. The COOH* species are considered to be a key intermediate during CO2 reduction to CO. The bands located at 1478 cm−1 and 1637 cm−1 can be assigned to HCO3* species [9]. A negative peak corresponding to CO2* can be observed at 1683 cm−1, which can be attributed to the CO2 consumption by Zn2GeO4@0.07CeO2, demonstrating the high activity and fast reaction rates [62]. Furthermore, the distinct peaks observed at 1302 cm−1 and 1350 cm−1 can be assigned to the bidentate carbonate species (b-CO32−), which indicates the abundant oxygen vacancies on the surface of Zn2GeO4@0.07CeO2 [62,63]. There will be a large amount of CO2 adsorbed near the oxygen vacancy of CeO2, and this adsorption is further enhanced due to the electron accumulation near the interface [24,62]. In contrast, Zn2GeO4 demonstrates more pronounced characteristic peaks indicative of intermediate species diversity, which correlates well with the poor selectivity. Moreover, Zn2GeO4 exhibits a certain capability to catalyze water splitting, thereby providing an abundant source of hydrogen species for the hydrogenation of adsorbed CO2 molecules, leading to the formation of CH4 [9,47]. Furthermore, due to the presence of asymmetric Zn-O-Ge sites, the CO* intermediates adsorbed on the Zn and Ge atoms exhibit different charge distributions, which facilitates C-C coupling [9]. As a result, Zn2GeO4 is more inclined to produce C2 products compared to CeO2. After coating with CeO2, the surface CeO2 serves as the adsorption and activation site for CO2 reduction, with CeO2 favoring the production of CO, thus altering the reaction pathway selectivity and reducing byproduct intermediates. Based on the in situ FT-IR spectra and the above analysis, the specific reaction pathway of Zn2GeO4@0.07CeO2 and Zn2GeO4 is depicted in Figure 4j [61,62,63,64,65].
3. Materials and Methods
3.1. Synthesis of Zn2GeO4
A total of 1100 mg of Zn(CH3COO)2·2H2O was added to 25 mL of deionized water and stirred magnetically for 10 min, and then 450 mg of GeO2 was added to the solution and stirred for 20 min. Next, 300 mg of sodium oleate (NaOA) was added slowly and stirred for another 20 min. Then, 5 mL of 3 mol L−1 NaOH aqueous solution was added dropwise to maintain the pH value at 7.5. After stirring for 20 min, the mixture was transferred to a 50 mL hydrothermal high-pressure reactor, sealed and heated at 140 °C for 24 h, and cooled naturally to room temperature. Then, the mixture was centrifuged and washed twice with 5 mol L−1 NaOH solution and dispersed into ethanol and water for washing. The sample was centrifuged five times and dried in a vacuum. Finally, the product was calcined in a N2 atmosphere at 400 °C for 3 h.
3.2. Synthesis of CeO2
A total of 1 mmol of Ce(NO3)3·6H2O was dissolved in a mixed solution of 20 mL of water and 20 mL of ethanol. Next, 25 mL of 0.02 g L−1 hexamethylenetetramine (HMT) solution was added. The mixture was heated to 70 °C, held for 2 h, and then cooled to room temperature. The product was purified by centrifugation, washed three times with water, and dried at 60 °C. Finally, the product was calcined in a N2 atmosphere at 400 °C for 3 h.
3.3. Synthesis of Zn2GeO4@xCeO2
First, 50 mg of uncalcined Zn2GeO4 crystals was dispersed by ultrasound in a mixed solution of 40 mL of water and 40 mL of ethanol. Next, 0.05 mmol Ce(NO3)3·6H2O and 0.1 mmol HMT were added sequentially. Then, the mixture was heated at 60 °C for 2 h and cooled to room temperature. The product was purified by centrifugation, washed three times with water, and dried at 60 °C. Finally, the product was calcined in a N2 atmosphere at 400 °C for 3 h. The prepared samples are named Zn2GeO4@0.05CeO2 based on the amount of added Ce(NO3)3·6H2O. Using the same method, Zn2GeO4@0.07CeO2 and Zn2GeO4@0.1CeO2 were prepared by controlling the amount of added Ce(NO3)3·6H2O and HMT, where x represents the amount of Ce added per 50 mg of Zn2GeO4. The corresponding amount of HMT is added proportionally to the amount of Ce(NO3)3·6H2O, maintaining a molar ratio of 2:1 between HMT and Ce(NO3)3·6H2O.
3.4. Characterization
The XRD patterns were obtained on an XRD-6000 (Shimadzu Corporation, Kyoto, Japan) X-ray diffractometer. The morphologies of the samples were measured by a field-emission scanning electron microscope on the Quanta 250FEG scanning electron microscope (FEI, Hillsboro, OR, USA). The XPS measurements were performed by a K-Alpha electron spectrometer (Thermo Fisher Scientific, Waltham, MA, USA). The quantification of liquid products was performed using NMR spectroscopy (Bruker Corporation, Billerica, MA, USA) with dimethyl sulfoxide as the internal standard. The UV-vis analysis was carried out on a UV-300 UV Visible Spectrophotometer (Shimadzu Corporation, Kyoto, Japan). The CO2-TPD experiments were conducted in a quartz tube reactor under a flow of 20 vol % of CO2/Ar (20 mL/min) over 100 mg of catalysts, and the desorption was conducted under a flow of N2 (20 mL/min). The photoluminescence (PL) emission spectra of samples were collected using an FLS-1000 luminescence spectrometer (Edinburgh Instruments, Edinburgh, UK). The in situ FT-IR spectra were measured through Fourier transform infrared spectroscopy (Bruker Corporation, MA, USA).
3.5. Photocatalytic Performance Measurements
A total of 25 mg of the photocatalyst samples and 20 mL of deionized water with 10 mL of trolamine were added into a 230 mL reactor (CEL-APR100H, China Education Au-light Technology Co. Ltd, Beijing, China) under ultrasonic dispersion. After that, high-purity CO2 gas was injected for 30 min to achieve a partial pressure of 1.0 atm. The reactor was irradiated by a 300 W Xe lamp (CEL-PUV300-T8, China Education Au-light Technology Co., Ltd., Beijing, China) with circulating cooling water to keep it at room temperature. After the irradiation reaction had occurred for 3 h, 1 mL of the evolved gas was pumped from the reactor and examined by a gas chromatograph.
For the cycle tests, the photocatalyst after reaction is centrifugally separated, washed with deionized water and ethanol sequentially, and then vacuum-dried prior to subsequent testing under the same conditions.
The gas product (CO, CH4, H2, C2) yield (μmol g−1 h−1) and product selectivity of gas product were calculated using the following equations:
Here, (ppm) represents the molar concentration of obtained by gas chromatography (GC), (Pa) is the pressure inside the reaction system, (m3) is the volume of the reactor, is the universal gas constant, (K) is the temperature under light irradiation, (g) is the mass of the catalyst, (h) is the reaction time, and is the mole number of .
3.6. Photoelectrochemical Measurements
For the photoelectrochemical testing, 5 mg of the sample was added to a solution of 1000 μL of isopropanol and 50 μL of naphthol, sonicated for 40 min, and stirred for 5 min. After uniform dispersion, 80 μL of the dispersion solution was applied to an ITO glass slide to form a square area of 1 cm × 1 cm and dried for 15 min. Photoelectrochemical testing was conducted using a CH650E electrochemical workstation in a three-electrode system, with the electrolyte being 0.5 M sodium sulfate solution. A Ag/AgCl electrode and Pt electrode were used as the reference electrode and counter electrode, respectively. The ITO glass sheet coated with the sample was used as the working electrode. The electrochemical impedance test was conducted under a forward bias voltage of the open circuit voltage, amplitude of 0.005 V, and frequency range of 0.001–100,000 Hz. The photocurrent test was conducted under a bias voltage of 0 V, using a 300 W xenon lamp (CEL-PUV300-T8, China Education Au-light Technology Co. Ltd., Beijing, China) to illuminate the working electrode, and turning on/off the light source every 30 s for testing. Mott–Schottky measurements were also tested at frequencies of 1000/2000/3000 Hz.
4. Conclusions
In this work, Zn2GeO4@CeO2 nanorods with a special core@shell structure have been successfully fabricated through a simple self-assembly method. By precisely controlling the amount of Ce(NO3)3·6H2O, a series of rod-shaped Zn2GeO4@CeO2 composites with varying amounts of CeO2 can be obtained. The photocatalytic CO2 reduction experiments demonstrated that an appropriate CeO2 thickness is beneficial for catalytic performance, in which Zn2GeO4@0.07CeO2 exhibited the highest activity with a CO yield of 1190.9 μmol g−1 h−1. Further characterization indicates that Zn2GeO4 and CeO2 formed a Type-I heterojunction. The excited electrons will transfer from Zn2GeO4 to CeO2, which could extend the carrier lifetime. Furthermore, the CO2 TPD results demonstrated the enhanced CO2 adsorption capacity of the CeO2 shell. Finally, the in situ FT-IR spectra confirmed the high CO selectivity of Zn2GeO4@0.07CeO2 and demonstrated that the CeO2 coating modifies the pathway selectivity of CO2 adsorption and reduction, suppresses the formation of byproduct intermediates, and thereby achieves enhanced CO selectivity.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules30102205/s1, Figure S1: SEM images of the as-obtained Zn2GeO4@CeO2 samples: (a,b) Zn2GeO4, (c,d) CeO2 nanoparticles, (e,f) Zn2GeO4@0.05CeO2, (g,h) Zn2GeO4@0.07CeO2, and (i,j) Zn2GeO4@0.1CeO2; Figure S2: SEM images of the as-obtained Zn2GeO4@CeO2 samples: (a) Zn2GeO4@0.05CeO2, (b) Zn2GeO4@0.07CeO2, and (c) Zn2GeO4@0.1CeO2; Figure S3: HRTEM images of the as-obtained Zn2GeO4@0.07CeO2; Figure S4: XRD patterns of Zn2GeO4@0.05CeO2, Zn2GeO4@0.07CeO2, and Zn2GeO4@0.1CeO2; Figure S5: EDS mapping images of Zn2GeO4@xCeO2: (a) Zn2GeO4@0.05CeO2, (b) Zn2GeO4@0.07CeO2, and (c) Zn2GeO4@0.1CeO2; Figure S6: EDS line scan images of Zn2GeO4@xCeO2: (a,d) Zn2GeO4@0.05CeO2, (b,e) Zn2GeO4@0.07CeO2, and (c,f) Zn2GeO4@0.1CeO2; Figure S7: EPR patterns of CeO2; Figure S8: Simulated 1H-NMR spectra for the liquid-phase products of the Zn2GeO4@0.07CeO2 system after reaction; Figure S9: CO yield of Zn2GeO4@0.07CeO2 under different conditions; Figure S10: SEM images of Zn2GeO4@0.07CeO2 (a) before and (b) after reaction; Figure S11: XRD patterns of Zn2GeO4@0.07CeO2 before and after the reaction; Figure S12: XPS spectra of Zn2GeO4@0.07CeO2 before and after the reaction. (a) Zn 2p; (b) Ge 3d; (c) O 1s; (d) Ce 3d; Figure S13: (a) UV-vis spectra, (b) Tauc spectra, and (c) Mott–Schottky curves of Zn2GeO4@xCeO2; Figure S14: Band structures of Zn2GeO4@0.07CeO2 (pH = 7, vs. NHE); Figure S15: PL spectra of Z2GeO4, CeO2, and Zn2GeO4@xCeO2; Table S1: The relative concentrations of Ce3+ and Ce4+ calculated by XPS spectra; Table S2: Yield of products in photocatalytic CO2 reduction; Table S3: Comparison with similar catalysts; Table S4: Comparison with similar catalysts without using hole scavengers.
Author Contributions
Conceptualization, D.L. and Y.Z.; methodology, D.L., X.F., and Y.B.; validation, J.S., Y.B., and X.F.; formal analysis, J.S. and Y.B.; investigation, J.S. and Y.B.; resources, D.L. and Y.Z.; data curation, J.S. and Y.B.; writing—original draft preparation, J.S.; writing—review and editing, D.L. and Y.B.; supervision, D.L., X.F., and Y.B.; project administration, D.L. and Y.Z.; funding acquisition, D.L., Y.Z., and X.F. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Research Funding of Hangzhou International Innovation Institute of Beihang University (Grant No. 2024KQ102 and 2024KQ131), the National Natural Science Foundation of China (Grant No. 51925202, 52432004, and 52472183), and the Central Government Guiding Local Science and Technology Development Funds (2025ZY01029). The authors also acknowledge the facilities and the scientific and technical assistance of the Analysis & Testing Center of Beihang University and the High-Performance Computing Center of Beihang University.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Chang, X.; Wang, T.; Gong, J. CO2 Photo-Reduction: Insights into CO2 Activation and Reaction on Surfaces of Photocatalysts. Energy Environ. Sci. 2016, 9, 2177–2196. [Google Scholar] [CrossRef]
- Huang, H.; Tu, S.; Zeng, C.; Zhang, T.; Reshak, A.H.; Zhang, Y. Macroscopic Polarization Enhancement Promoting Photo- and Piezoelectric-Induced Charge Separation and Molecular Oxygen Activation. Angew. Chem. Int. Ed. 2017, 56, 11860–11864. [Google Scholar] [CrossRef]
- Shangguan, W.; Liu, Q.; Wang, Y.; Sun, N.; Liu, Y.; Zhao, R.; Li, Y.; Wang, C.; Zhao, J. Molecular-Level Insight into Photocatalytic CO2 Reduction with H2O over Au Nanoparticles by Interband Transitions. Nat. Commun. 2022, 13, 3894. [Google Scholar] [CrossRef]
- Yao, S.; He, J.; Gao, F.; Wang, H.; Lin, J.; Bai, Y.; Fang, J.; Zhu, F.; Huang, F.; Wang, M. Highly Selective Semiconductor Photocatalysis for CO2 Reduction. J. Mater. Chem. A 2023, 11, 12539–12558. [Google Scholar] [CrossRef]
- Zhu, X.; Zhou, G.; Yi, J.; Ding, P.; Yang, J.; Zhong, K.; Song, Y.; Hua, Y.; Zhu, X.; Yuan, J.; et al. Accelerated Photoreduction of CO2 to CO over a Stable Heterostructure with a Seamless Interface. ACS Appl. Mater. Interfaces 2021, 13, 39523–39532. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Cheng, K.; Kang, J.; Zhou, C.; Subramanian, V.; Zhang, Q.; Wang, Y. New Horizon in C1 Chemistry: Breaking the Selectivity Limitation in Transformation of Syngas and Hydrogenation of CO2 into Hydrocarbon Chemicals and Fuels. Chem. Soc. Rev. 2019, 48, 3193–3228. [Google Scholar] [CrossRef]
- He, F.; Zhu, B.; Cheng, B.; Yu, J.; Ho, W.; Macyk, W. 2D/2D/0D TiO2/C3N4/Ti3C2 MXene Composite S-Scheme Photocatalyst with Enhanced CO2 Reduction Activity. Appl. Catal. B Environ. 2020, 272, 119006. [Google Scholar] [CrossRef]
- She, H.; Hua, R.; Zhao, J.; Xia, Y.; Wang, L.; Huang, J.; Wang, Q. Synergetic Regulation of Interfacial Electronic Structure of Cu, N Co-Doped Carbon Modified TiO2 for Efficient Photocatalytic CO2 Reduction. Chem. Eng. J. 2024, 496, 153799. [Google Scholar] [CrossRef]
- Zhu, J.; Shao, W.; Li, X.; Jiao, X.; Zhu, J.; Sun, Y.; Xie, Y. Asymmetric Triple-Atom Sites Confined in Ternary Oxide Enabling Selective CO2 Photothermal Reduction to Acetate. J. Am. Chem. Soc. 2021, 143, 18233–18241. [Google Scholar] [CrossRef]
- Jiang, G.; Tang, B.; Li, X.; Wei, Z.; Wang, X.; Chen, W.; Wan, J.; Shen, L. Preparation of Ag-Modified Zn2GeO4 Nanorods for Photo-Degradation of Organic Pollutants. Powder Technol. 2014, 251, 37–40. [Google Scholar] [CrossRef]
- Li, M.; Wu, S.; Liu, D.; Ye, Z.; Wang, L.; Kan, M.; Ye, Z.; Khan, M.; Zhang, J. Engineering Spatially Adjacent Redox Sites with Synergistic Spin Polarization Effect to Boost Photocatalytic CO2 Methanation. J. Am. Chem. Soc. 2024, 146, 15538–15548. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, H.; Nian, Y.; Chen, Y.; Cheng, H.; Yang, C.; Han, Y.; Tan, X.; Ye, J.; Yu, T. Disruption Symmetric Crystal Structure Favoring Photocatalytic CO2 Reduction: Reduced *COOH Formation Energy Barrier on Al Doped CuS/TiO2. Adv. Funct. Mater. 2024, 34, 2406549. [Google Scholar] [CrossRef]
- Low, J.; Yu, J.; Jaroniec, M.; Wageh, S.; Al-Ghamdi, A.A. Heterojunction Photocatalysts. Adv. Mater. 2017, 29, 1601694. [Google Scholar] [CrossRef]
- Lai, K.; Sun, Y.; Li, N.; Gao, Y.; Li, H.; Ge, L.; Ma, T. Photocatalytic CO2-to-CH4 Conversion with Ultrahigh Selectivity of 95.93% on S-Vacancy Modulated Spatial In2S3/In2O3 Heterojunction. Adv. Funct. Mater. 2024, 34, 2409031. [Google Scholar] [CrossRef]
- Cao, S.; Yu, J.; Wageh, S.; Al-Ghamdi, A.A.; Mousavi, M.; Ghasemi, J.B.; Xu, F. H2-Production and Electron-Transfer Mechanism of a Noble-Metal-Free WO3@ZnIn2S4 S-Scheme Heterojunction Photocatalyst. J. Mater. Chem. A 2022, 10, 17174–17184. [Google Scholar] [CrossRef]
- Pan, C.; Xu, J.; Wang, Y.; Li, D.; Zhu, Y. Dramatic Activity of C3N4/BiPO4 Photocatalyst with Core/Shell Structure Formed by Self-Assembly. Adv. Funct. Mater. 2012, 22, 1518–1524. [Google Scholar] [CrossRef]
- Wang, L.; Cheng, B.; Zhang, L.; Yu, J. In Situ Irradiated XPS Investigation on S-Scheme TiO2@ZnIn2S4 Photocatalyst for Efficient Photocatalytic CO2 Reduction. Small 2021, 17, 2103447. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Zhang, J.; Qi, K.; Liang, G.; Xu, F.; Yu, J. Ultrafast Electron Transfer at the In2O3/Nb2O5 S-Scheme Interface for CO2 Photoreduction. Nat. Commun. 2024, 15, 4807. [Google Scholar] [CrossRef]
- Cheng, Z.; Yu, M.; Yang, G.; Kang, L. Fabrication of NiCo2O4@CeO2 Core@shell Nanotubes with Enhanced Catalytic Performances. CrystEngComm 2016, 18, 6331–6335. [Google Scholar] [CrossRef]
- Yan, T.; Liu, H.; Gao, P.; Sun, M.; Wei, Q.; Xu, W.; Wang, X.; Du, B. Facile Synthesized Highly Active BiOI/Zn2GeO4 Composites for the Elimination of Endocrine Disrupter BPA under Visible Light Irradiation. New J. Chem. 2015, 39, 3964–3972. [Google Scholar] [CrossRef]
- Liu, Q.; Zhou, Y.; Kou, J.; Chen, X.; Tian, Z.; Gao, J.; Yan, S.; Zou, Z. High-Yield Synthesis of Ultralong and Ultrathin Zn2GeO4 Nanoribbons toward Improved Photocatalytic Reduction of CO2 into Renewable Hydrocarbon Fuel. J. Am. Chem. Soc. 2010, 132, 14385–14387. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zheng, M.; Zhao, H.; Qin, H.; Fan, W.; Zhao, X. Zn2GeO4−x/ZnS Heterojunctions Fabricated via in Situ Etching Sulfurization for Pt-Free Photocatalytic Hydrogen Evolution: Interface Roughness and Defect Engineering. Phys. Chem. Chem. Phys. 2020, 22, 10265–10277. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, X.; Wei, Q.; He, P.; Cheng, Z.; Wu, J.; Qi, Y.; Sun, Y.; Song, J.; Yang, W. Cu3SnS4/CeO2 Heterojunction Photocatalyst with Dual Redox Pairs Synergistically Promotes the Photocatalytic Reduction of CO2. Energy Fuels 2022, 36, 7763–7774. [Google Scholar] [CrossRef]
- Zhu, C.; Wei, X.; Li, W.; Pu, Y.; Sun, J.; Tang, K.; Wan, H.; Ge, C.; Zou, W.; Dong, L. Crystal-Plane Effects of CeO2 {110} and CeO2 {100} on Photocatalytic CO2 Reduction: Synergistic Interactions of Oxygen Defects and Hydroxyl Groups. ACS Sustain. Chem. Eng. 2020, 8, 14397–14406. [Google Scholar] [CrossRef]
- Cai, J.; Li, D.; Jiang, L.; Yuan, J.; Li, Z.; Li, K. Review on CeO2-Based Photocatalysts for Photocatalytic Reduction of CO2: Progresses and Perspectives. Energy Fuels 2023, 37, 4878–4897. [Google Scholar] [CrossRef]
- Liu, L.; Shi, J.; Wang, R. Facile Construction of Mn2O3@CeO2 Core@shell Cubes with Enhanced Catalytic Activity toward CO Oxidation. J. Solid State Chem. 2019, 269, 419–427. [Google Scholar] [CrossRef]
- Wang, X.; Liu, D.; Li, J.; Zhen, J.; Zhang, H. Clean Synthesis of Cu2O@CeO2 Core@shell Nanocubes with Highly Active Interface. NPG Asia Mater. 2015, 7, e158. [Google Scholar] [CrossRef]
- Huang, J.; Ding, K.; Hou, Y.; Wang, X.; Fu, X. Synthesis and Photocatalytic Activity of Zn2GeO4 Nanorods for the Degradation of Organic Pollutants in Water. ChemSusChem 2008, 1, 1011–1019. [Google Scholar] [CrossRef]
- Ma, Z.; Liu, X.; Wang, X.; Luo, Z.; Li, W.; Nie, Y.; Pei, L.; Mao, Q.; Wen, X.; Zhong, J. Manipulating the D-Band Center Enhances Photoreduction of CO2 to CO in Zn2GeO4 Nanorods. Chem. Eng. J. 2023, 468, 143569. [Google Scholar] [CrossRef]
- Dolado, J.; Martínez-Casado, R.; Hidalgo, P.; Gutierrez, R.; Dianat, A.; Cuniberti, G.; Domínguez-Adame, F.; Díaz, E.; Méndez, B. Understanding the UV Luminescence of Zinc Germanate: The Role of Native Defects. Acta Mater. 2020, 196, 626–634. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, S.; Zhu, J.; Ji, G.; Peng, F. The Study of Oxygen Ion Motion in Zn2GeO4 by Raman Spectroscopy. Solid State Ion. 2015, 274, 12–16. [Google Scholar] [CrossRef]
- Qin, Y.; Liu, X.; Zhu, T.; Zhu, T. Catalytic Oxidation of Ethyl Acetate over Silver Catalysts Supported on CeO2 with Different Morphologies. Mater. Chem. Phys. 2019, 229, 32–38. [Google Scholar] [CrossRef]
- Guo, Y.; Li, H.; Ma, W.; Shi, W.; Zhu, Y.; Choi, W. Photocatalytic Activity Enhanced via Surface Hybridization. Carbon Energy 2020, 2, 308–349. [Google Scholar] [CrossRef]
- Wang, N.; Cheong, S.; Yoon, D.-E.; Lu, P.; Lee, H.; Lee, Y.K.; Park, Y.-S.; Lee, D.C. Efficient, Selective CO2 Photoreduction Enabled by Facet-Resolved Redox-Active Sites on Colloidal CdS Nanosheets. J. Am. Chem. Soc. 2022, 144, 16974–16983. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Ni, H.; Dai, J.; Liu, T.; Wu, Z.; Chen, X.; Dong, Z.; Qian, J.; Wu, Z. Comparison of Highly Active Type-I and Type-II Heterojunction Photocatalytic Composites Synthesized by Electrospinning for Humic Acid Degradation. Chem. Phys. Lett. 2022, 803, 139815. [Google Scholar] [CrossRef]
- Cui, Z.; Wang, W.; Zhao, C.; Chen, C.; Han, M.; Wang, G.; Zhang, Y.; Zhang, H.; Zhao, H. Spontaneous Redox Approach to the Self-Assembly Synthesis of Au/CeO2 Plasmonic Photocatalysts with Rich Oxygen Vacancies for Selective Photocatalytic Conversion of Alcohols. ACS Appl. Mater. Interfaces 2018, 10, 31394–31403. [Google Scholar] [CrossRef] [PubMed]
- Qi, L.; Yu, Q.; Dai, Y.; Tang, C.; Liu, L.; Zhang, H.; Gao, F.; Dong, L.; Chen, Y. Influence of Cerium Precursors on the Structure and Reducibility of Mesoporous CuO-CeO2 Catalysts for CO Oxidation. Appl. Catal. B Environ. 2012, 119–120, 308–320. [Google Scholar] [CrossRef]
- Li, X.; Wang, M.; Wang, R.; Wang, Y.; Zhu, M.; Zhang, L.; Shi, J. Construction of Ru Single-Atoms on Ceria to Reform the Products of CO2 Photoreduction. ACS Nano 2024, 18, 5741–5751. [Google Scholar] [CrossRef]
- Li, X.; Liu, Q.; Wang, J.; Meng, D.; Shu, Y.; Lv, X.; Zhao, B.; Yang, H.; Cheng, T.; Gao, Q.; et al. Enhanced Electroreduction of CO2 to C2+ Products on Heterostructured Cu/Oxide Electrodes. Chem 2022, 8, 2148–2162. [Google Scholar] [CrossRef]
- Wang, S.; Wang, Y.; Zhang, S.L.; Zang, S.; Lou, X.W. Supporting Ultrathin ZnIn2S4 Nanosheets on Co/N-Doped Graphitic Carbon Nanocages for Efficient Photocatalytic H2 Generation. Adv. Mater. 2019, 31, 1903404. [Google Scholar] [CrossRef]
- Kim, D.; Yong, K. Boron Doping Induced Charge Transfer Switching of a C3N4/ZnO Photocatalyst from Z-Scheme to Type II to Enhance Photocatalytic Hydrogen Production. Appl. Catal. B Environ. 2021, 282, 119538. [Google Scholar] [CrossRef]
- Spilarewicz, K.; Urbanek, K.; Jakimińska, A.; Macyk, W. ZnFe2O4/TiO2 Composites with Type-I Heterojunction for Photocatalytic Reduction of CO2. J. CO2 Util. 2023, 75, 102574. [Google Scholar] [CrossRef]
- Tang, H. Vapor Phase Growth and Photoluminescence of Oriented-Attachment Zn2GeO4 Nanorods Array. J. Cryst. Growth 2016, 451, 170–173. [Google Scholar] [CrossRef]
- Zheng, J.; Li, S.; Zhang, Y.; Zheng, P.; Hu, X.; Fang, Y.; Duan, R.; Chen, Q. Ag–O–Ce3+ Atomic Interface and Surface Oxygen Vacancies on CeO2 Synergistically Promoted the Selective Visible Photocatalytic Reduction of Carbon Dioxide. J. Mater. Chem. C 2023, 11, 7320–7330. [Google Scholar] [CrossRef]
- Pu, Y.; Luo, Y.; Wei, X.; Sun, J.; Li, L.; Zou, W.; Dong, L. Synergistic Effects of Cu2O-Decorated CeO2 on Photocatalytic CO2 Reduction: Surface Lewis Acid/Base and Oxygen Defect. Appl. Catal. B Environ. 2019, 254, 580–586. [Google Scholar] [CrossRef]
- Yang, S.; Zhang, W.; Pan, G.; Chen, J.; Deng, J.; Chen, K.; Xie, X.; Han, D.; Dai, M.; Niu, L. Photocatalytic Co-Reduction of N2 and CO2 with CeO2 Catalyst for Urea Synthesis. Angew. Chem. Int. Ed. 2023, 62, e202312076. [Google Scholar] [CrossRef]
- Yuan, Y.; Dai, H.; Chi, H.; Gao, W.; Liu, Q.; Ding, C.; Shen, Y.; Tang, Z.; Zhuang, C.; Yang, Y.; et al. Atomically Thin Zn2GeO4 Nanoribbons: Facile Synthesis and Selective Photocatalytic CO2 Reduction toward CO. ACS Mater. Lett. 2022, 4, 2631–2637. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, A.; Chen, C.; Zhang, C.; Zhang, J.; Jia, G. Controllable Synthesis and Morphology-Dependent Photoluminescence Properties of Well-Defined One-Dimensional Zn2GeO4:Mn2+ Nanostructures. Dyes Pigm. 2018, 150, 267–274. [Google Scholar] [CrossRef]
- Li, X.; Liu, B.; Wen, M.; Gao, Y.; Wu, H.; Huang, M.; Li, Z.; Chen, B.; Tung, C.; Wu, L. Hole-Accepting-Ligand-Modified CdSe QDs for Dramatic Enhancement of Photocatalytic and Photoelectrochemical Hydrogen Evolution by Solar Energy. Adv. Sci. 2016, 3, 1500282. [Google Scholar] [CrossRef]
- Su, B.; Zheng, M.; Lin, W.; Lu, X.F.; Luan, D.; Wang, S.; Lou, X.W. (David). S-Scheme Co9S8@Cd0.8Zn0.2S-DETA Hierarchical Nanocages Bearing Organic CO2 Activators for Photocatalytic Syngas Production. Adv. Energy Mater. 2023, 13, 2203290. [Google Scholar] [CrossRef]
- Zeng, R.; Liu, T.; Qiu, M.; Tan, H.; Gu, Y.; Ye, N.; Dong, Z.; Li, L.; Lin, F.; Sun, Q.; et al. High-Volumetric Density Atomic Cobalt on Multishell ZnxCd1–xS Boosts Photocatalytic CO2 Reduction. J. Am. Chem. Soc. 2024, 146, 9721–9727. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Chen, Z.; Li, M.; Li, J.; Zhuang, W.; Yang, X.; Wu, S.; Zhang, J. Construction of Single Ni Atom-Immobilized ZIF-8 with Ordered Hierarchical Pore Structures for Selective CO2 Photoreduction. ACS Catal. 2023, 13, 6630–6640. [Google Scholar] [CrossRef]
- Zhang, G.; Wang, Z.; He, T.; Wu, J.; Zhang, J.; Wu, J. Rationally Design and In-Situ Fabrication of Ultrasmall Pomegranate-like CdIn2S4/ZnIn2S4 Z-Scheme Heterojunction with Abundant Vacancies for Improving CO2 Reduction and Water Splitting. Chem. Eng. J. 2022, 442, 136309. [Google Scholar] [CrossRef]
- Huang, N.; Li, B.; Wu, D.; Chen, Z.; Shao, B.; Chen, D.; Zheng, Y.; Wang, W.; Yang, C.; Gu, M.; et al. Crystal Engineering of MOF-Derived Bimetallic Oxide Solid Solution Anchored with Au Nanoparticles for Photocatalytic CO2 Reduction to Syngas and C2 Hydrocarbons. Angew. Chem. Int. Ed 2024, 63, e202319177. [Google Scholar] [CrossRef]
- Wang, W.; Deng, C.; Xie, S.; Li, Y.; Zhang, W.; Sheng, H.; Chen, C.; Zhao, J. Photocatalytic C–C Coupling from Carbon Dioxide Reduction on Copper Oxide with Mixed-Valence Copper(I)/Copper(II). J. Am. Chem. Soc. 2021, 143, 2984–2993. [Google Scholar] [CrossRef]
- Wei, J.; Mu, X.; Hu, Y.; Liu, L.; Wu, X.; Liu, Q.; Zhang, T.; Peng, Y.; Cao, J.; Yan, C.; et al. A General Preparation of Solid Solution-Oxide Heterojunction Photocatalysts through Metal–Organic Framework Transformation Induced Pre-nucleation. Angew. Chem. Int. Ed. 2023, 62, e202302986. [Google Scholar] [CrossRef]
- Zhao, H.; Wang, X.; Feng, J.; Chen, Y.; Yang, X.; Gao, S.; Cao, R. Synthesis and Characterization of Zn2GeO4/Mg-MOF-74 Composites with Enhanced Photocatalytic Activity for CO2 Reduction. Catal. Sci. Technol. 2018, 8, 1288. [Google Scholar] [CrossRef]
- Wang, J.; Huang, L.; Sun, B.; Zhang, H.; Hou, D.; Qiao, X.; Ma, H.; Li, D.-S. Efficient Photothermal Catalytic CO2 Reduction over in Situ Construction ZnIn2S4@Ni(OH)2/NiO Z-Scheme Heterojunction. Chem. Eng. J. 2024, 479, 147719. [Google Scholar] [CrossRef]
- Jia, W.; Xiong, R.; Sun, Y.; Xiao, Y.; Cheng, B.; Lei, S. A Well-Designed Hierarchical Bi19S27Br3 nanorods@SnIn4S8 Nanosheet Core–Shell S-Scheme Heterostructure for Robust Photothermal-Assisted Photocatalytic CO2 Reduction. J. Mater. Chem. A 2024, 12, 4513–4524. [Google Scholar] [CrossRef]
- Liu, X.; Wu, Y.; Li, Y.; Yang, X.; Ma, Q.; Luo, J. MOF-on-MOF-Derived CuO@In2O3 S-Scheme Heterojunction with Core–Shell Structure for Efficient Photocatalytic CO2 Reduction. Chem. Eng. J. 2024, 485, 149855. [Google Scholar] [CrossRef]
- Yang, S.; Byun, W.J.; Zhao, F.; Chen, D.; Mao, J.; Zhang, W.; Peng, J.; Liu, C.; Pan, Y.; Hu, J.; et al. CO2 Enrichment Boosts Highly Selective Infrared-Light-Driven CO2 Conversion to CH4 by UiO-66/Co9S8 Photocatalyst. Adv. Mater. 2024, 36, 2312616. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Shen, M.; Jin, X.; Tian, J.; Zhou, Y.; Shao, Y.; Zhang, L.; Li, Y.; Shi, J. Mild Generation of Surface Oxygen Vacancies on CeO2 for Improved CO2 Photoreduction Activity. Nanoscale 2020, 12, 12374–12382. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Shen, M.; Jin, X.; Tian, J.; Shao, Y.; Zhang, L.; Li, Y.; Shi, J. Exploring the Enhancement Effects of Hetero-Metal Doping in CeO2 on CO2 Photocatalytic Reduction Performance. Chem. Eng. J. 2022, 427, 130987. [Google Scholar] [CrossRef]
- Wang, Y.; Zhuang, G.; Zhang, J.; Luo, F.; Cheng, X.; Sun, F.; Fu, S.; Lu, T.; Zhang, Z. Co-Dissolved Isostructural Polyoxovanadates to Construct Single-Atom-Site Catalysts for Efficient CO2 Photoreduction. Angew. Chem. Int. Ed. 2023, 62, e202216592. [Google Scholar] [CrossRef]
- Wang, J.; Yang, C.; Mao, L.; Cai, X.; Geng, Z.; Zhang, H.; Zhang, J.; Tan, X.; Ye, J.; Yu, T. Regulating the Metallic Cu–Ga Bond by S Vacancy for Improved Photocatalytic CO2 Reduction to C2H4. Adv. Funct. Mater. 2023, 33, 2213901. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).