Development of High-Pressure Extraction and Automatic Steam Distillation Methods for Aronia mitschurinii, Juvenile Ginger, and Holy Basil Plants
Abstract
1. Introduction
2. Results and Discussion
2.1. Distillation Results
2.2. Pressure Extraction Results
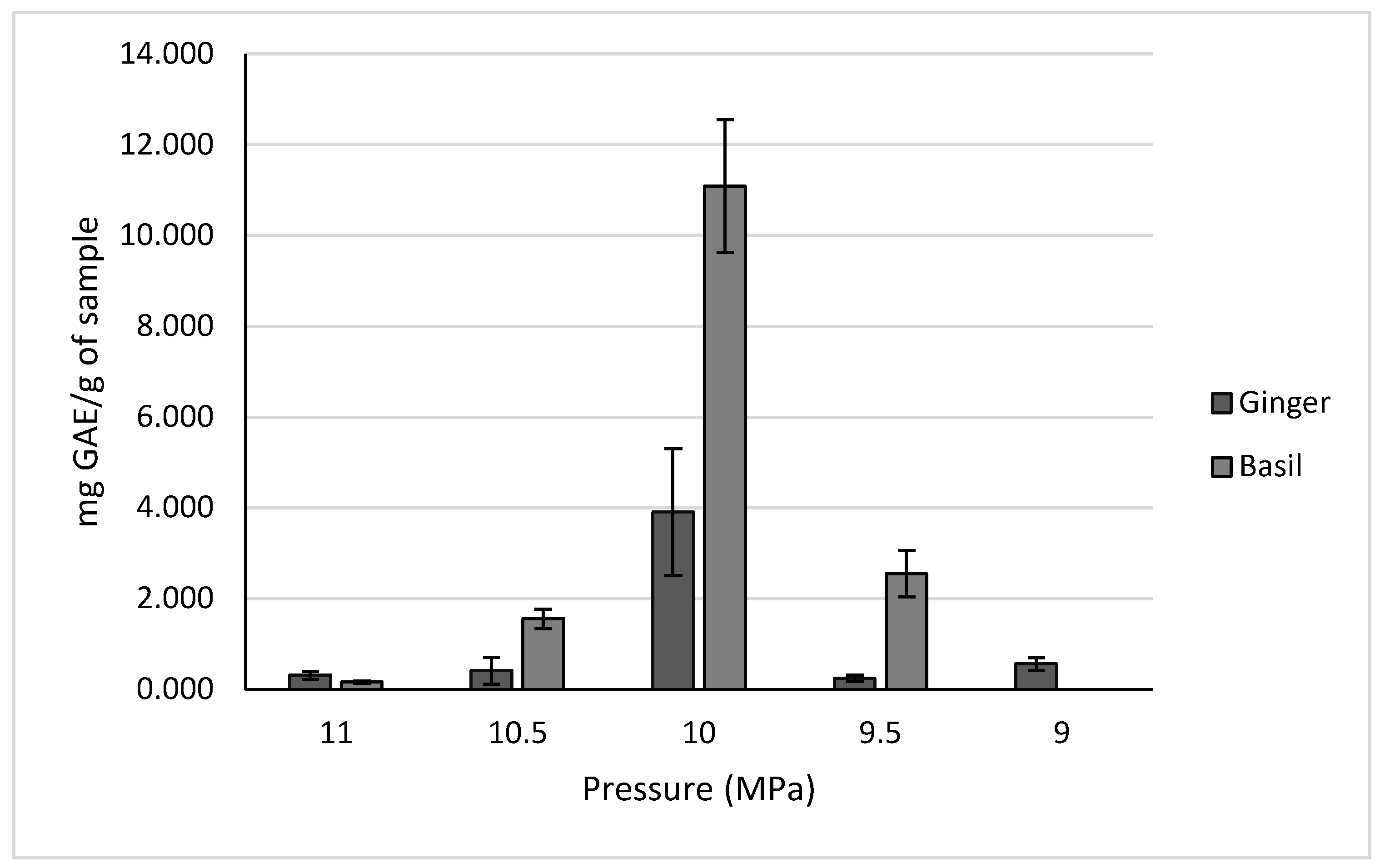
2.2.1. Polyphenols of Ginger and Holy Basil
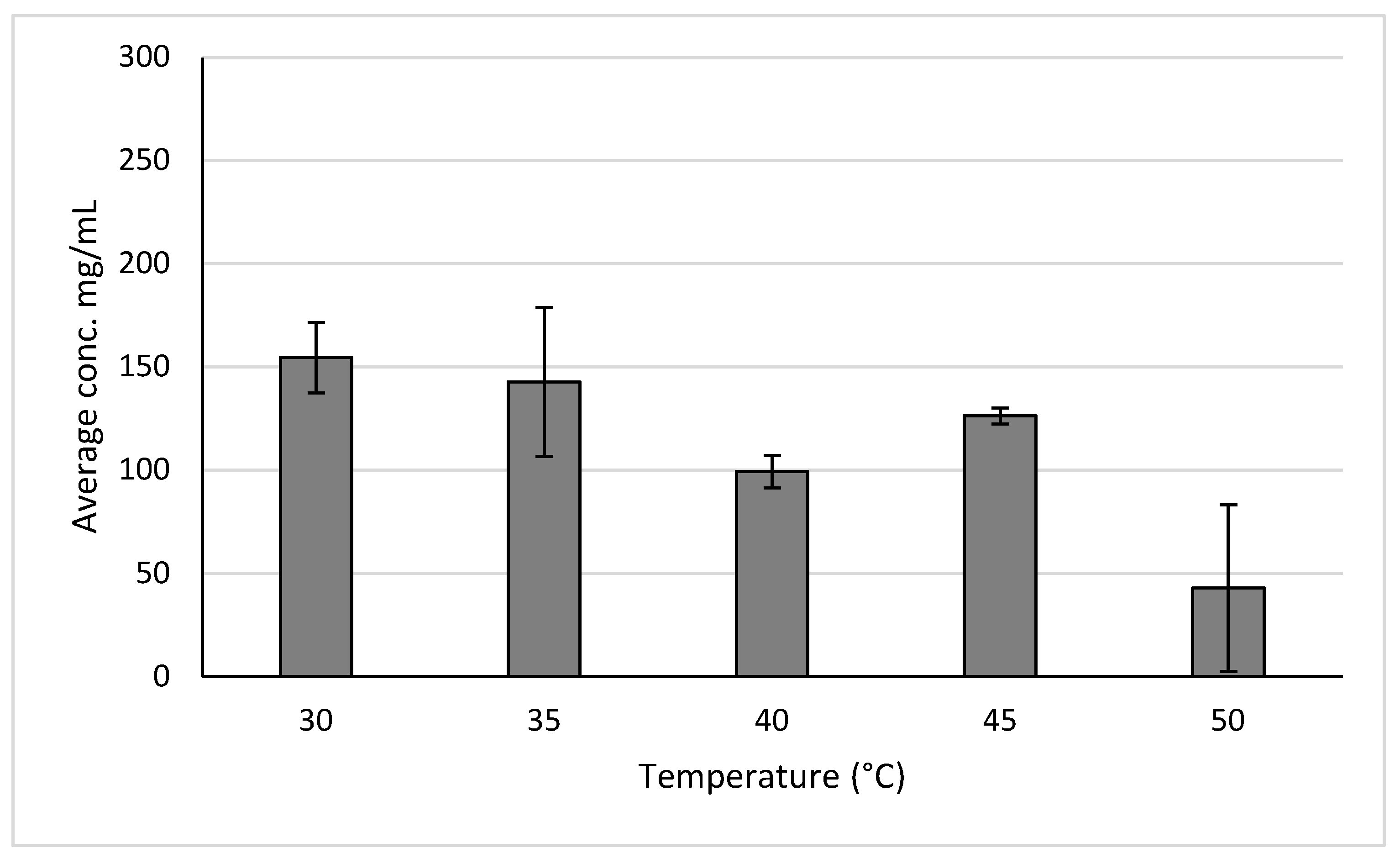
2.2.2. Anthocyanins of Aronia
2.2.3. Optimal Extraction Conditions and Comparison to Literature Data
3. Materials and Methods
3.1. Sample Sourcing and Processing
3.2. Automatic Steam Distillation Methods
3.2.1. Steam Time Trials
3.2.2. % Steam Power Trials
3.3. Traditional Extraction Methods
3.4. Pressure Extractor Method
3.4.1. Temperature Trials
3.4.2. Pressure Trials
3.5. Phytochemical Screening
3.5.1. Polyphenols
3.5.2. Anthocyanins
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| UV/Vis | ultraviolet–visible spectrophotometry |
| nm | Nanometer |
| ml | Milliliter |
| μL | microliter |
| M | molar |
| g | gram |
| mg | milligram |
| MPa | Megapascal |
| DF | dilution factor |
| DI water | de-ionized water |
| °C | degree Celsius |
| GCMS | gas chromatography–mass spectroscopy |
| C3G | cyanidin-3-glucoside |
| DW | Dry Weight |
| FW | Fresh Weight |
| GAE | gallic acid equivalents |
| QE | Quercetin Equivalents |
References
- López-Lorente, Á.I.; Pena-Pereira, F.; Pedersen-Bjergaard, S.; Zuin, V.G.; Ozkan, S.A.; Psillakis, E. The Ten Principles of Green Sample Preparation. TrAC Trends Anal. Chem. 2022, 148, 116530. [Google Scholar] [CrossRef]
- Chemat, F.L.; Boutekedjiret, C. Extraction//Steam Distilation. In Reference Module in Chemistry, Molecular Sciences and Chemical Engineering; Elsevier: Amsterdam, The Netherlands, 2015. [Google Scholar] [CrossRef]
- Machado, C.A.; Oliveira, F.O.; de Andrade, M.A.; Hodel, K.V.S.; Lepikson, H.; Machado, B.A.S. Steam Distillation for Essential Oil Extraction: An Evaluation of Technological Advances Based on an Analysis of Patent Documents. Sustainability 2022, 14, 7119. [Google Scholar] [CrossRef]
- Lachenmeier, D.W.; Plato, L.; Suessmann, M.; Carmine, M.D.; Krueger, B.; Kukuck, A.; Kranz, M. Improved automatic steam distillation combined with oscillation-type densimetry for determining alcoholic strength in spirits and liqueurs. SpringerPlus 2015, 4, 783. [Google Scholar] [CrossRef]
- Lachenmeier, D.W. Application of steam distillation for the rapid determination of alcoholic strength of spirit drinks. Int. Lab. News 2004, 34, 10–13. [Google Scholar]
- Kasuan, N.; Yusoff, Z.M.; Muhammad, Z.; Nordin, N.M.; Rahiman, M.H.; Taib, M.N. Essential Oil Extraction with Automated Steam Distillation: FMRLC for steam temperature regulation. In Proceedings of the 2012 IEEE International Conference on Control System, Computing and Engineering, Penang, Malaysia, 23–25 November 2012. [Google Scholar] [CrossRef]
- Sylla, B.; Volkis, V. Phytochemical Properties, Processing, and Applications of Juvenile Zingiber Officinale. ACS Omega, 2024; submitted. [Google Scholar]
- Chanioti, S.; Liadakis, G.; Tzia, C. Solid-Liquid Extraction. In Food Engineering Handbook, 1st ed.; Taylor & Francis Group: Abingdon-on-Thames, UK, 2014; pp. 253–286. [Google Scholar] [CrossRef]
- Mustafa, A.; Turner, C. Pressurized liquid extraction as a green approach in food and herbal plants extraction: A review. Anal. Chim. Acta 2011, 703, 8–18. [Google Scholar] [CrossRef] [PubMed]
- BÜCHI Labortechnik AG. SpeedExtractor E-916/E-916XL/E-914 Operation Manual. BÜCHI Labortechnik AG. 2019. Available online: https://assets.buchi.com/image/upload/v1605799602/pdf/Operation-Manuals/OM_093218_E-914_E-916_E-916XL_en.pdf (accessed on 22 October 2024).
- Dionex Corporation. ASE 200 Accelerated Solvent Extractor. Dionex Corporation. 2006. Available online: https://assets.thermofisher.com/TFS-Assets/CMD/Specification-Sheets/PS-LPN-0981-ASE-200-Accelerated-Solvent-Extractor-LPN0981-EN.pdf (accessed on 22 October 2024).
- Huang, H.W.; Cheng, M.C.; Chen, B.Y.; Wang, C.Y. Effects of High-Pressure Extraction on the Extraction Yield, Phenolic Compounds, Antioxidant and Anti-Tyrosinase Activity of Djulis Hull. J. Food Sci. Technol. 2019, 56, 4016–4024. [Google Scholar] [CrossRef]
- Briones-Labarca, V.; Giovagnoli-Vicuña, C.; Cañas-Sarazúa, R. Optimization of Extraction Yield, Flavonoids and Lycopene from Tomato Pulp by High Hydrostatic Pressure-Assisted Extraction. Food Chem. 2019, 278, 751–759. [Google Scholar] [CrossRef] [PubMed]
- Ghasemzadeh, A.; Jaafar, H.Z.E.; Rahmat, A. Optimization Protocol for the Extraction of 6-Gingerol and 6-Shogaol from Zingiber officinale var. rubrum Theilade and Improving Antioxidant and Anticancer Activity Using Response Surface Methodology. BMC Complement. Altern. Med. 2015, 15, 258. [Google Scholar] [CrossRef]
- Rafie, R.; Nartea, T.; Mullins, C. Growing High Tunnel Ginger in High Tunnels: A Niche Crop with Market Potential. Proc. Fla. State Hortic. Soc. 2012, 125, 142–143. [Google Scholar]
- Sharifi-Rad, M.; Varoni, E.M.; Salehi, B.; Sharifi-Rad, J.; Matthews, K.R.; Ayatollahi, S.A.; Kobarfard, F.; Ibrahim, S.A.; Mnayer, D.; Zakaria, Z.A.; et al. Plants of the Genus Zingiber as a Source of Bioactive Phytochemicals: From Tradition to Pharmacy. Molecules 2017, 22, 2145. [Google Scholar] [CrossRef]
- Sen, K.; Goyal, M.; Mukopadayay, S. Review on phytochemical and pharmacological, medicinal properties of Holy Basil (Ocimum sanctum L.). Int. J. Health Sci. 2022, 6, 7276–7286. [Google Scholar] [CrossRef]
- Hasan, M.R.; Alotaibi, B.S.; Althafar, Z.M.; Mujamammi, A.H.; Jameela, J. An Update on the Therapeutic Anticancer Potential of Ocimum sanctum L.:“Elixir of Life”. Molecules 2023, 28, 1193. [Google Scholar] [CrossRef]
- Shiwakoti, S.; Saleh, O.; Poudyal, S.; Barka, A.; Qian, Y.; Zheljazkov, V.D. Yield, Composition and Antioxidant Capacity of the Essential Oil of Sweet Basil and Holy Basil as Influenced by Distillation Methods. Biochem. Biodiv. 2017, 14, e1600417. [Google Scholar] [CrossRef] [PubMed]
- Leonard, P.J.; Brand, M.H.; Connolly, B.A.; Obae, S.G. Investigation of the Origin of Aronia mitschurinii using Amplified Fragment Length Polymorphism Analysis. HortScience 2013, 48, 520–524. [Google Scholar] [CrossRef]
- Taheri, R.; Connolly, B.A.; Brand, M.H.; Bolling, B.W. Underutilized Chokeberry (Aronia melanocarpa, Aronia arbutifolia, Aronia prunifolia) Accessions Are Rich Sources of Anthocyanins, Flavonoids, Hydroxycinnamic Acids, and Proanthocyanidins. J. Agric. Food Chem. 2013, 61, 8581–8588. [Google Scholar] [CrossRef]
- Bolling, B.W.; Taheri, R.; Pei, R.; Kranz, S.; Yu, M.; Durocher, S.N.; Brand, M.H. Harvest Date Affects Aronia Juice Polyphenols, Sugars, and Antioxidant Activity, but not Anthocyanin Stability. Food Chem. 2015, 187, 189–196. [Google Scholar] [CrossRef]
- Fuller, N.J.; Pegg, R.B.; Affolter, J.; Berle, D. Variation in Growth and Development, and Essential Oil Yield between Two Ocimum Species (O. tenuiflorum and O. gratissimum) Grown in Georgia. HortScience 2018, 53, 1275–1282. [Google Scholar] [CrossRef]
- Brophy, J.J.; Goldsack, R.J.; Clarkson, J.R. The Essential Oil of Ocimum tenuiflorum L. (Lamiaceae) Growing in Northern Australia. J. Essent. Oil Res. 1993, 5, 459–461. [Google Scholar] [CrossRef]
- Sharma, S.; Rolta, R.; Salaria, D.; Dev, K. In vitro antibacterial and antifungal potentials of Ocimum tenuiflorum and Ocimum gratissimum essential oil. Pharm. Res. Nat. Prod. 2024, 4, 100065. [Google Scholar] [CrossRef]
- Onyenekwe, P.C.; Hashimoto, S. The composition of the essential oil of dried Nigerian ginger (Zingiber officinale Roscoe). Eur. Food Res. Technol. 1999, 209, 407–410. [Google Scholar] [CrossRef]
- Kiran, C.R.; Chakka, A.K.; Amma, K.P.P.; Menon, A.N.; Kumar, M.M.S.; Venugopalan, V.V. Essential Oil Composition of Fresh Ginger Cultivars from North-East India. J. Ess. Oil Res. 2013, 25, 380–387. [Google Scholar] [CrossRef]
- Racoti, A.; Buttress, A.J.; Binner, E.; Dodds, C.; Trifan, A.; Calinescu, I. Microwave assisted hydro-distillation of essential oils from fresh ginger root (Zingiber officinale Roscoe). J. Essent. Oil Res. 2016, 29, 471–480. [Google Scholar] [CrossRef]
- Kalhoro, M.T.; Zhang, H.; Kalhoro, G.M.; Wang, F.; Chen, T.; Faqir, Y.; Nabi, F. Fungicidal properties of ginger (Zingiber officinale) essential oils against Phytophthora colocasiae. Sci. Rep. 2022, 12, 2191. [Google Scholar] [CrossRef]
- Gajula, D.; Verghese, M.; Boateng, J.; Walker, L.T.; Shackelford, L.; Mentreddy, S.R.; Cedric, S. Determination of Total Phenolics, Flavonoids and Antioxidant and Chemopreventive Potential of Basil (Ocimum basilicum L. and Ocimum tenuiflorum L.). Int. J. Cancer Res. 2009, 5, 130–143. [Google Scholar] [CrossRef]
- Saelao, T.; Chutimanukul, P.; Suratanee, A.; Plaimas, K. Analysis of Antioxidant Capacity Variation among Thai Holy Basil Cultivars (Ocimum tenuiflorum L.) Using Density-Based Clustering Algorithm. Horticulturae 2023, 9, 1094. [Google Scholar] [CrossRef]
- Sankhalkar, S.; Vernekar, V. Quantitative and Qualitative analysis of Phenolic and Flavonoid content in Moringa oleifera Lam and Ocimum tenuiflorum L. Pharmacogn. Res. 2016, 8, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Yousfi, F.; Abrigach, F.; Petrovic, J.D.; Sokovic, M.; Ramdani, M. Phytochemical Screening and Evaluation of the Antioxidant and Antibacterial Potential of Zingiber officinale extracts. S. Afr. J. Bot. 2021, 142, 433–440. [Google Scholar] [CrossRef]
- Abuelgassim, A.O. Antioxidant Potential of Date Palm Leaves and Acacia nilotica Fruit in Comparison with Other Four Common Arabian Medicinal Plants. Life Sci. J. 2013, 10, 3405–3410. [Google Scholar] [CrossRef]
- Sharif, M.F.; Bennett, M.T. The Effect of Different Methods and Solvents on the Extraction of Polyphenols in Ginger (Zingiber officinale). J. Teknol. 2016, 78, 49–52. [Google Scholar] [CrossRef][Green Version]
- Hellström, J.K.; Shikov, A.N.; Makarova, M.N.; Pihlanto, A.N.; Pozharitskaya, O.N.; Ryhänen, E.L.; Kivijärvi, P.; Makarov, V.G.; Mattila, P.H. Blood pressure-lowering properties of chokeberry (Aronia mitchurinii, var. Viking). J. Funct. Foods 2010, 2, 163–169. [Google Scholar] [CrossRef]
- Nowak, D.; Gośliński, M.; Wojtowicz, E. Comparative Analysis of the Antioxidant Capacity of Selected Fruit Juices and Nectars: Chokeberry Juice as a Rich Source of Polyphenols. Int. J. Food Prop. 2015, 19, 1317–1324. [Google Scholar] [CrossRef]
- Guisti, M.M.; Worlstad, R.E. Characterization and Measurement of Anthocyanins by UV-Visible Spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, F1.2.1–F1.2.13. [Google Scholar] [CrossRef]
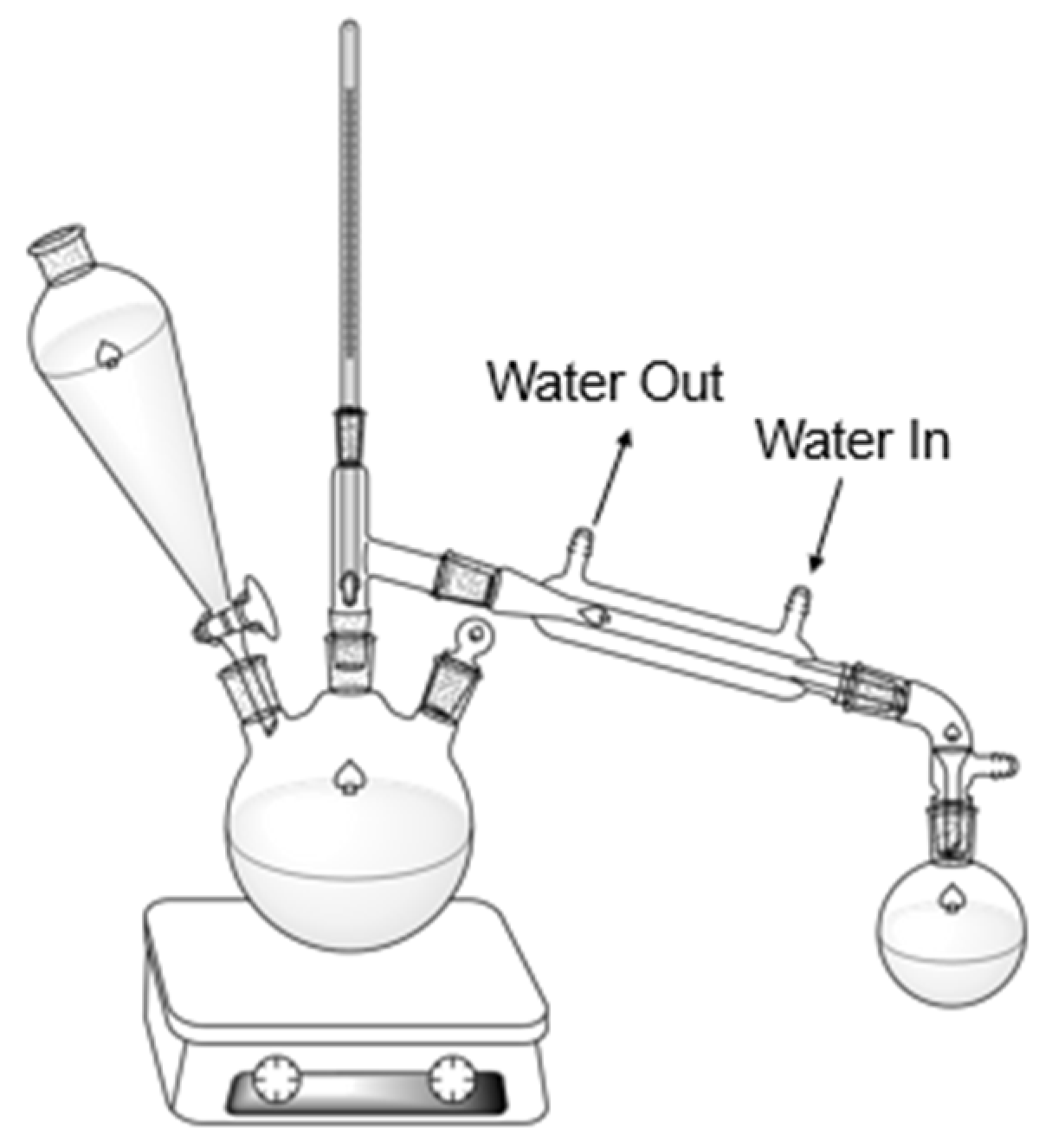

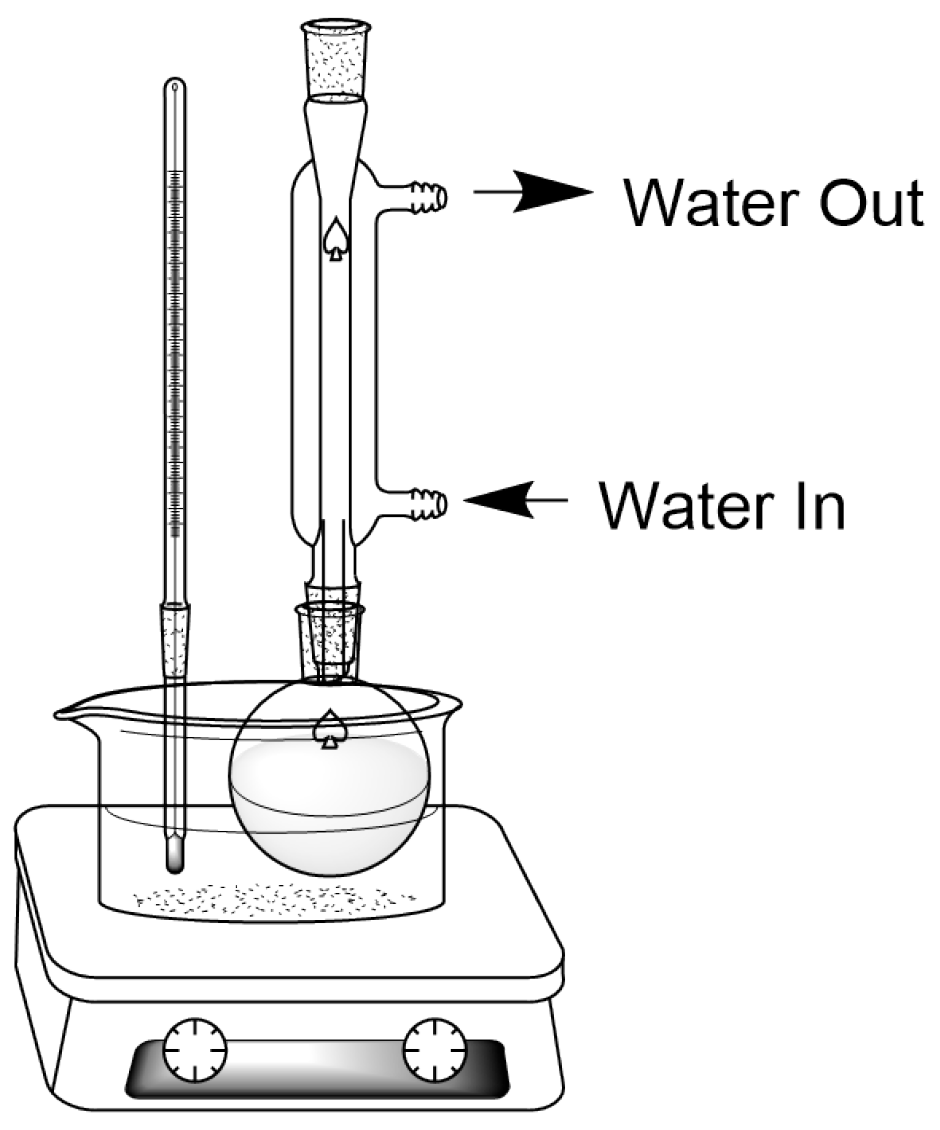





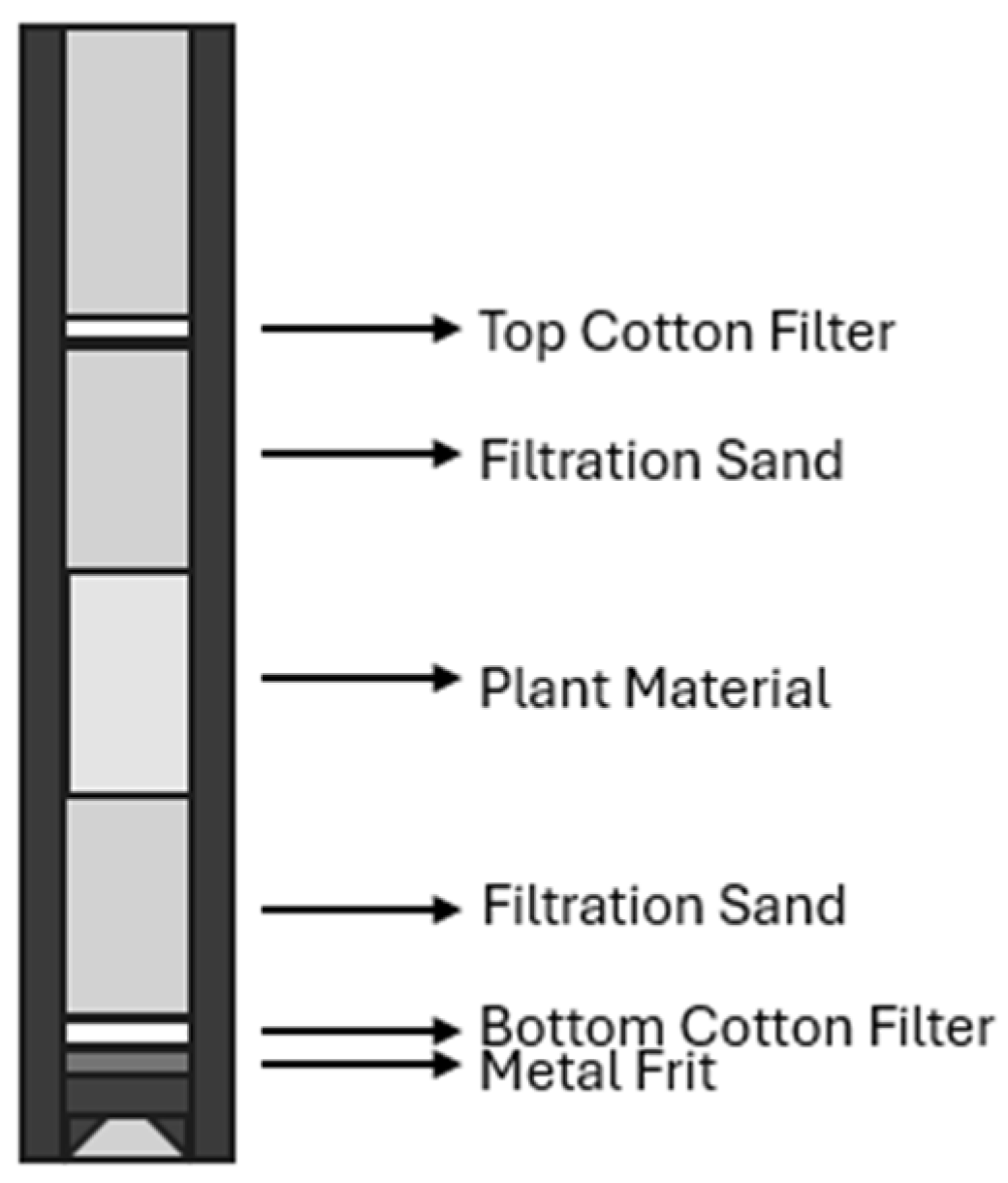
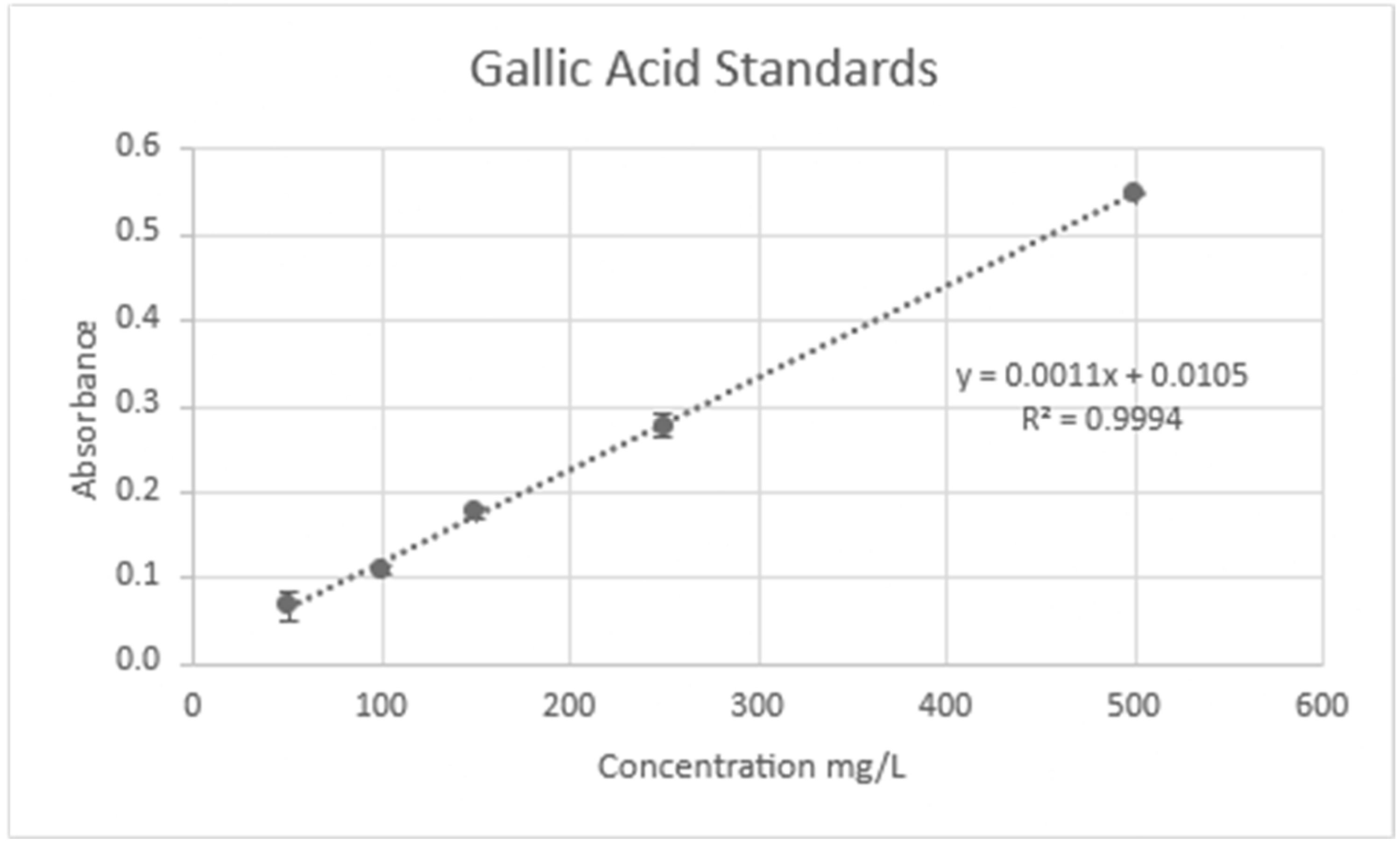
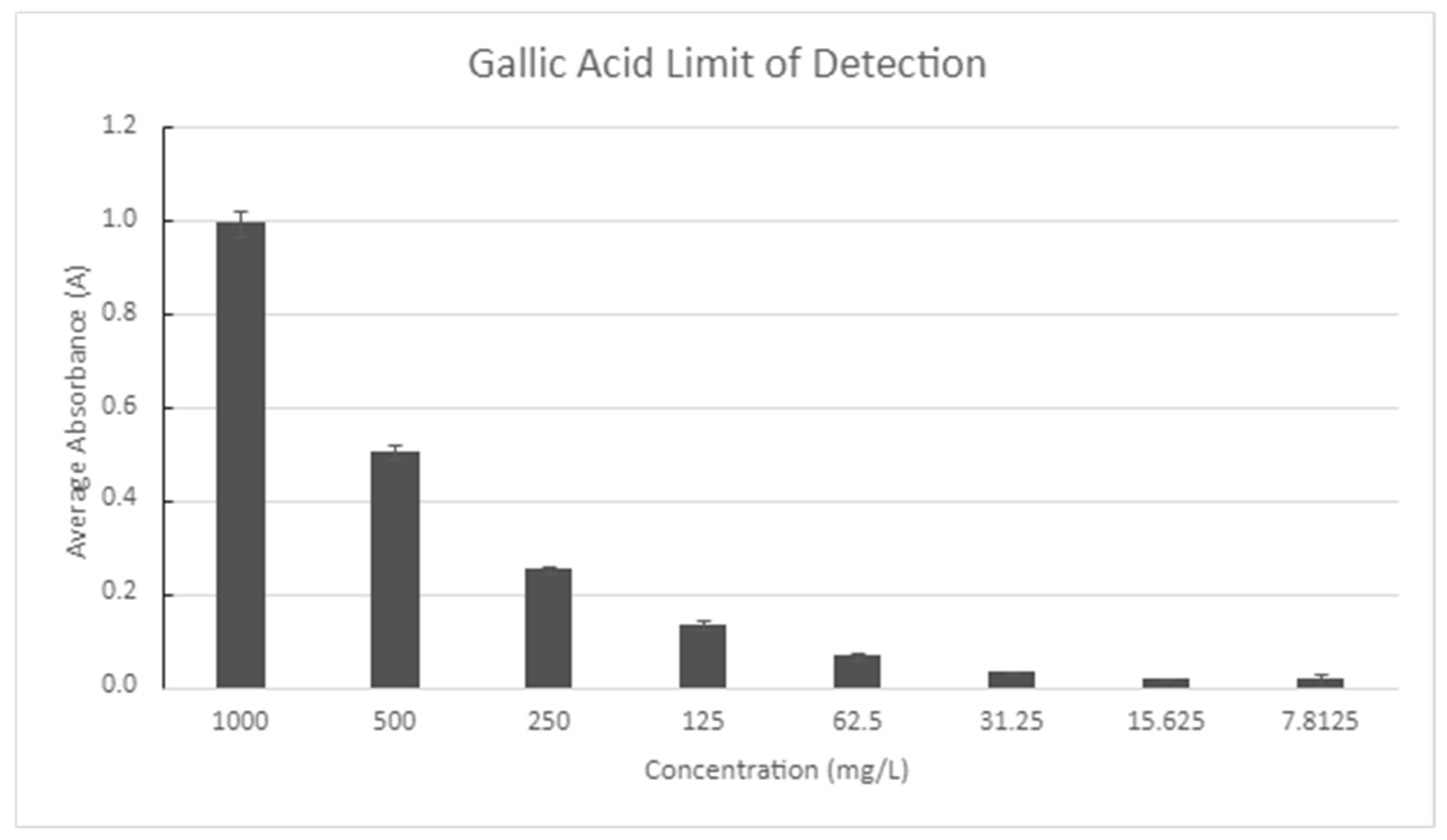

| Crop | Place of Growth | Oil Concentration | Conditions | Reference |
|---|---|---|---|---|
| Holy Basil | Maryland, USA | 55.81 ± 1.97 mg/g of sample | Automatic steam distillation 90% steam power 210 s steam time | |
| Holy Basil | Wyoming, USA | 0.68 ± 0.05 g oil/100 g leaves | 60 min of steam distillation | [19] |
| Holy Basil | Georiga (lat. 33°53′55.5″ N; long. 83°22′09.2″ W), USA | 0.65 (w/w) | Hydrodistillation | [23] |
| Holy Basil | Australia | 4.1% w/v | Hydrodistillation | [24] |
| Holy Basil | Himachal Pradesh, India | 2.74 ± 0.57% w/v | 3 h hydrodistillation | [25] |
| Ginger | Virginia, USA | 61.52 ± 0.61 mg/g of sample | Automatic steam distillation 90% steam power 210 s steam time | |
| Ginger | Zaria, Nigeria | 2.4% | 6 h distillation | [26] |
| Ginger | Assan, India | 4.17 ± 0.05% | Hydrodistillation | [27] |
| Ginger | Nottingham, UK | 0.35% (w/w) | Microwave-assisted hydrodistillation | [28] |
| Ginger | Sichuan Province, China | 2.5% | Microwave-assisted hydrodistillation | [29] |
| Crop | Place of Growth | Phytochemical Concentration | Conditions | Reference |
|---|---|---|---|---|
| Holy Basil | Maryland, USA | 11.086 ± 1.463 mg GAE/g of sample | High-pressure extraction 45 °C, 10 MPa | |
| Holy Basil | Maryland, USA | 32.709 ± 5.222 mg GAE/g of sample | 48 h reflux in 50% ethanol at 30 °C | |
| Holy Basil | Alabama | 31.37 ± 1.29 | 80% methanol and a dry sample | [30] |
| Holy Basil | Nakhon Pathom, Thailand | 23.19 mg GAE/gDW | 25 °C absolute methanol, 3 h | [31] |
| Holy Basil | India | 2.18 ± 0.015 mg/mL GAE | Overnight solid–liquid extraction by emersion in methanol | [32] |
| Ginger | Virginia, USA | 6.600 ± 0.549 mg GAE/g of sample | High-pressure extraction 55 °C, 10 MPa | |
| Ginger | Virginia, USA | 3.273 ± 0.248 mg GAE/g of sample | High-pressure extraction 50 °C, 10 MPa | |
| Ginger | Virginia, USA | 3.341 ± 2.066 mg GAE/g of sample | 48 h reflux in 50% ethanol at 30 °C | |
| Ginger | Morocco | 322.11 µg GAE/mg Ext | 40 °C ethanol extraction | [33] |
| Ginger | Riyadh, Saudi Arabia | 2.4 mg GAE/g | 25 °C methanol extraction | [34] |
| Ginger | Malaysia | 2.63 mg GAE/g | ethanol maceration | [35] |
| Aronia | Maryland, USA | 154.5 ± 17.1 mg of anthocyanins/mL | High-pressure extraction 30 °C, 10 MPa | |
| Aronia | Maryland, USA | 253.0 ± 39.56 mg of anthocyanins/mL | 48 h reflux in 50% ethanol at 30 °C | |
| Aronia | Connecticut, USA | 0.469 ± 0.008 mg/g Cy3Glu 9.00 ± 0.05 mg/g Cy3Gal | 2 g of berry powder was diluted in 40 mL of 70% acetone, 29.5% water, and 0.5% acetic acid; sonicated for 5 min; and centrifuged at 950 g for 10 min | [21] |
| Aronia | Finland | 38% Cyanidin-3-galactoside | Extracted with 4% acetic acid in 65% aqueous methanol | [36] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lahoff, S.; Cable, E.E.; Buzzetto-More, R.; Volkis, V.V. Development of High-Pressure Extraction and Automatic Steam Distillation Methods for Aronia mitschurinii, Juvenile Ginger, and Holy Basil Plants. Molecules 2025, 30, 2199. https://doi.org/10.3390/molecules30102199
Lahoff S, Cable EE, Buzzetto-More R, Volkis VV. Development of High-Pressure Extraction and Automatic Steam Distillation Methods for Aronia mitschurinii, Juvenile Ginger, and Holy Basil Plants. Molecules. 2025; 30(10):2199. https://doi.org/10.3390/molecules30102199
Chicago/Turabian StyleLahoff, Sara, Ezra E. Cable, Ryan Buzzetto-More, and Victoria V. Volkis. 2025. "Development of High-Pressure Extraction and Automatic Steam Distillation Methods for Aronia mitschurinii, Juvenile Ginger, and Holy Basil Plants" Molecules 30, no. 10: 2199. https://doi.org/10.3390/molecules30102199
APA StyleLahoff, S., Cable, E. E., Buzzetto-More, R., & Volkis, V. V. (2025). Development of High-Pressure Extraction and Automatic Steam Distillation Methods for Aronia mitschurinii, Juvenile Ginger, and Holy Basil Plants. Molecules, 30(10), 2199. https://doi.org/10.3390/molecules30102199








