Metal Uptake by Birches and Scots Pines Grown on a Porcelain Landfill
Abstract
1. Introduction
2. Results and Discussion
2.1. Figures of Merit of the Analytical Procedure
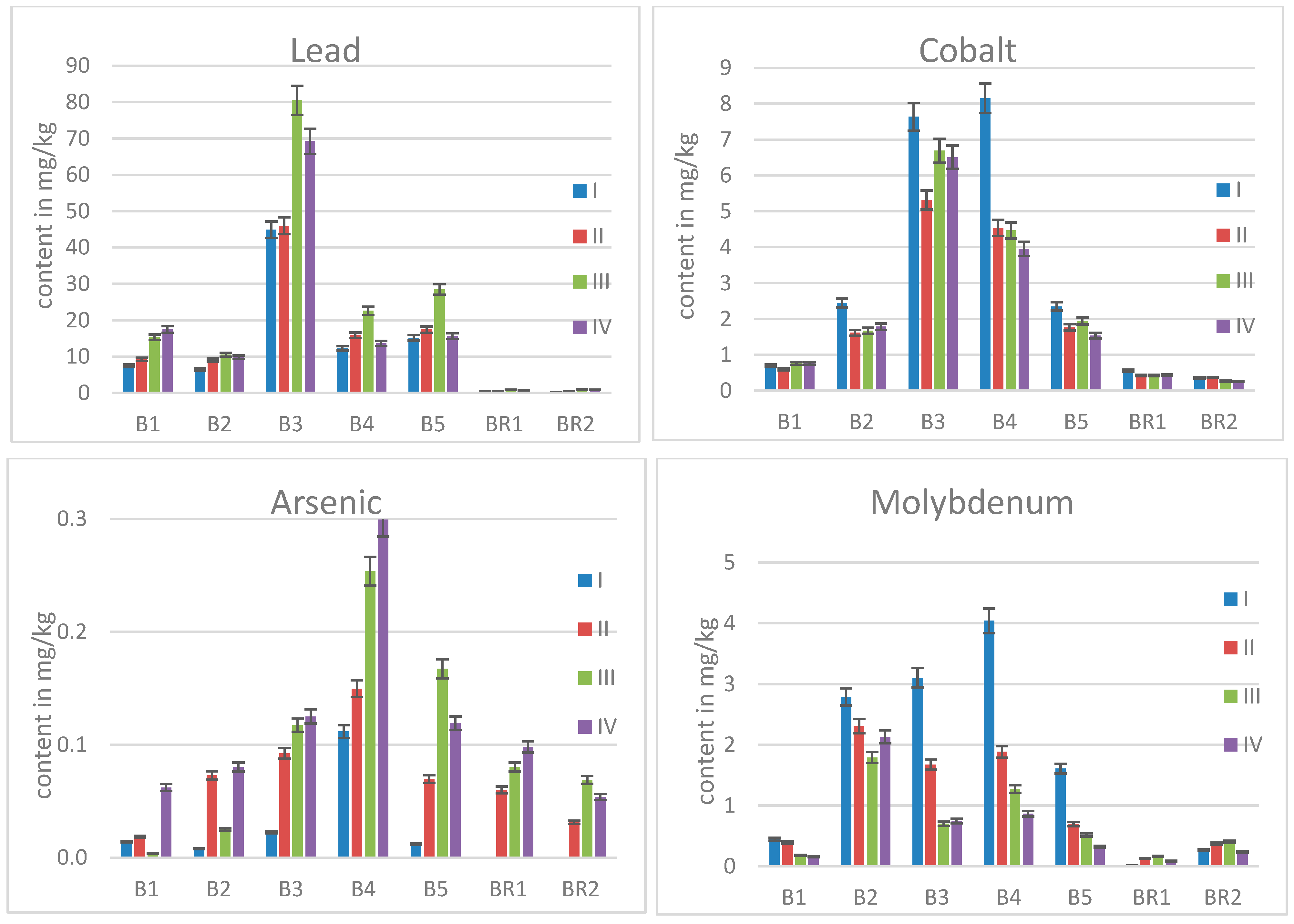
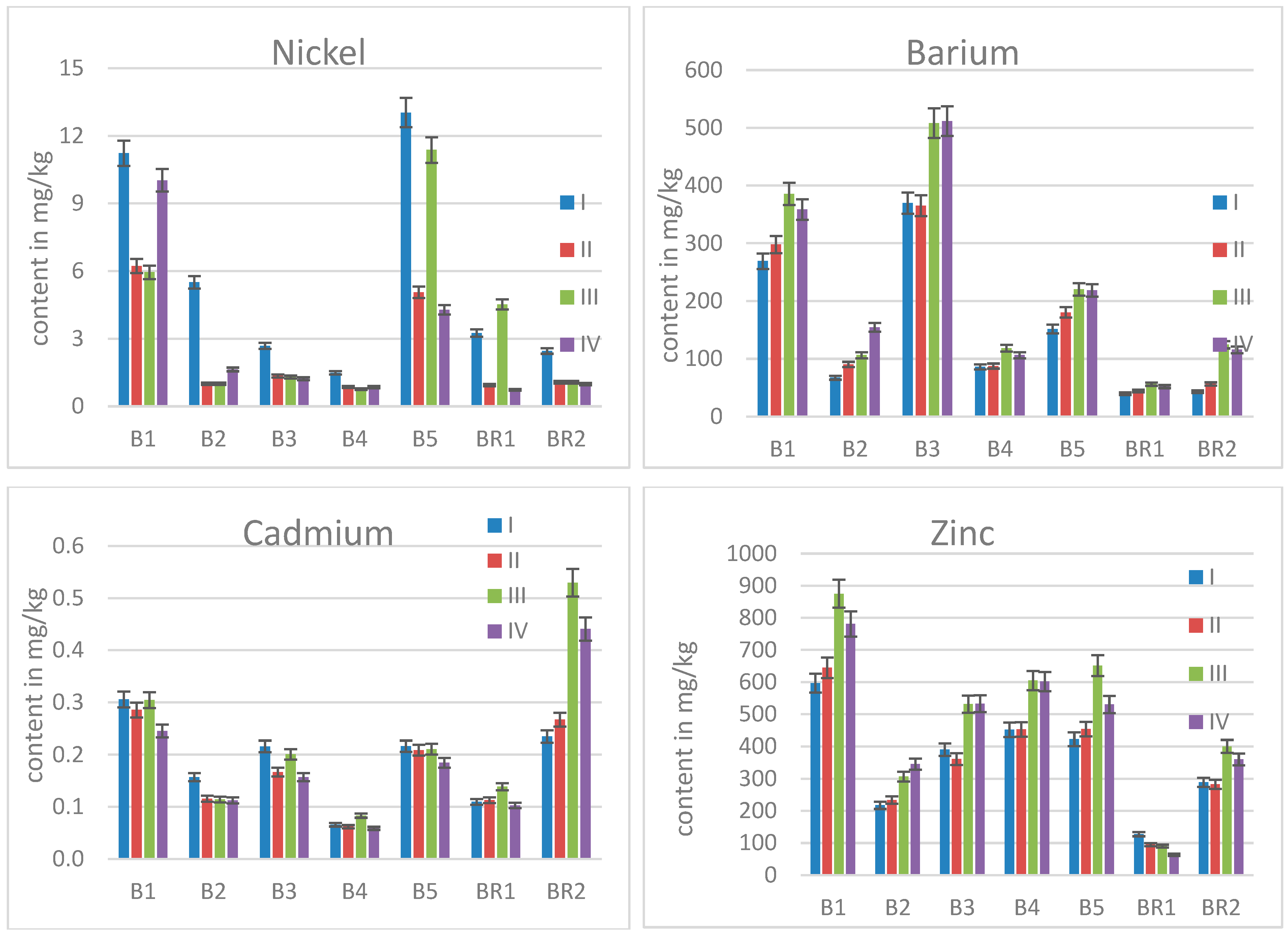
2.2. Elemental Contents in Birch Leaves
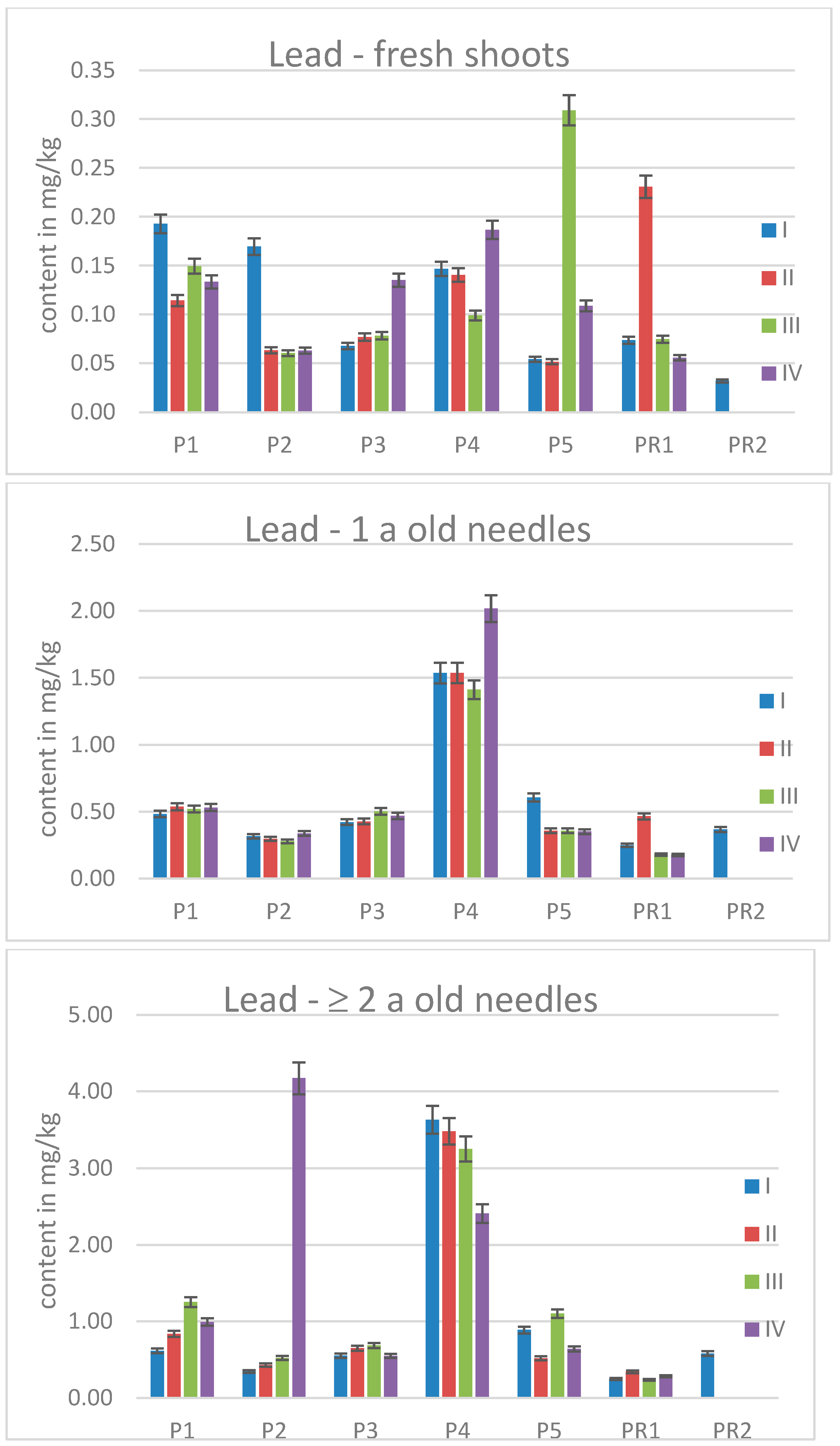
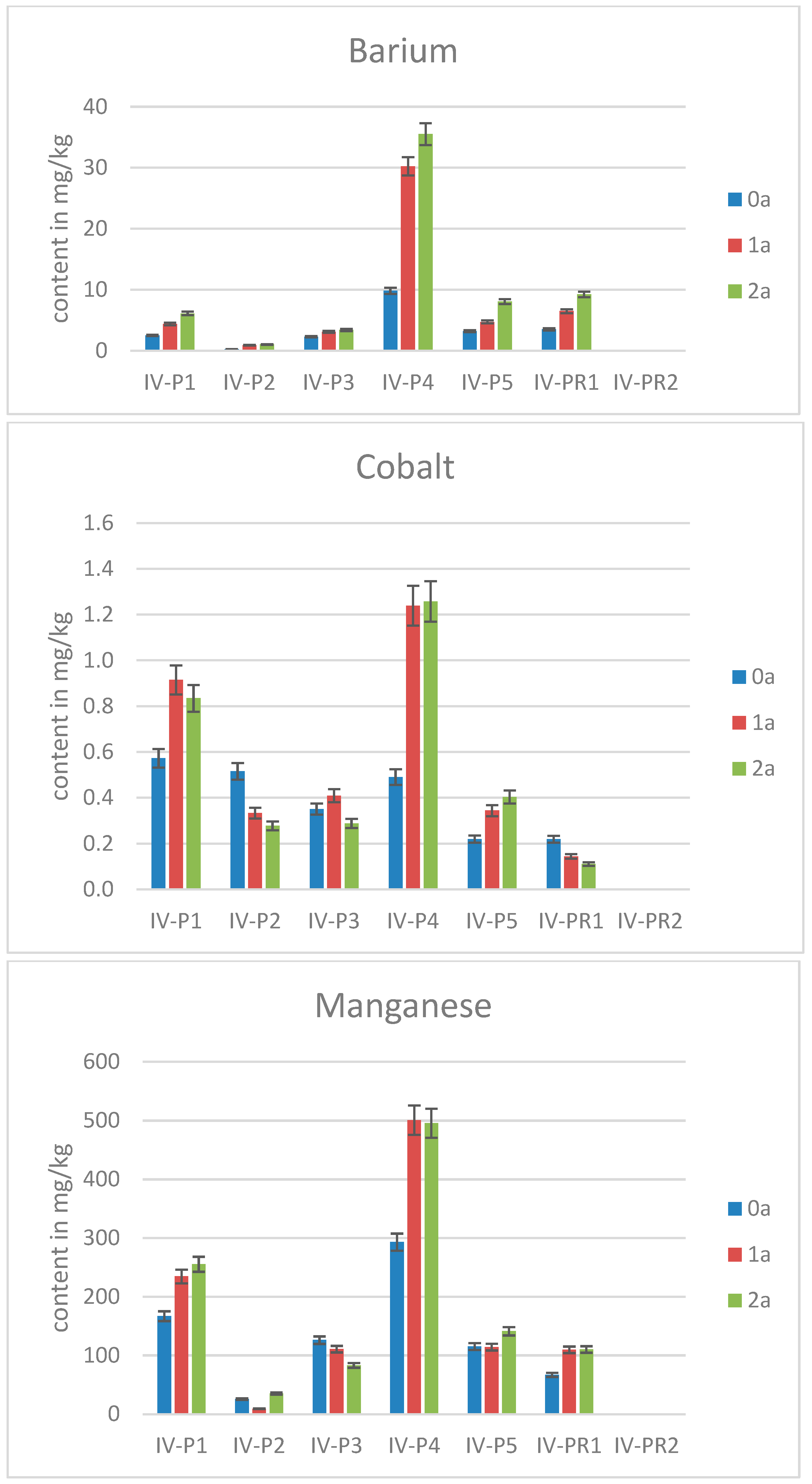
2.3. Elemental Contents in Scots Pine Needles
2.4. Comparison of Birches and Scots Pines
3. Materials and Methods
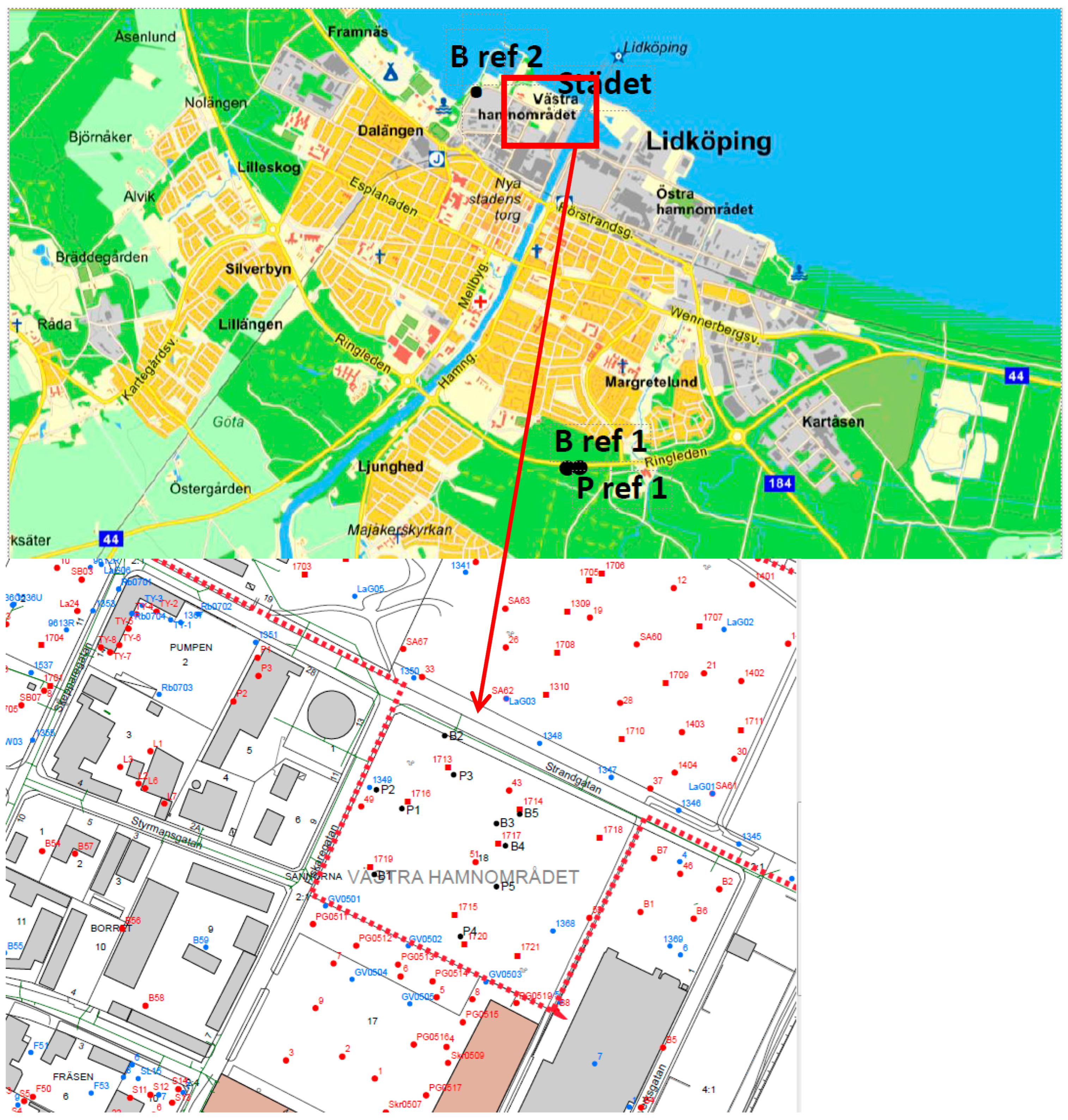
3.1. Sampling
3.2. Sample Preparation and Elemental Analysis
3.3. Data Evaluation and Statistical Tests
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WSP. PM Historik Och Tidigare Verksamheter—Norra Delen av Hamnstaden Lidköping; WSP: Gothenburg, Sweden, 2014. [Google Scholar]
- Jiang, Y.; Sihong, C.; Jianwei, L.; Yue, Y.; Yanjiao, C.; Aichen, Z.; Hongbin, C. Source apportionment and health risk assessment of heavy metals in soil for a township in Jiangsu Province, China. Chemosphere 2017, 168, 1658–1668. [Google Scholar] [CrossRef] [PubMed]
- Rörstrand Museum. n.d. ALP Lidköping. Available online: https://rorstrand-museum.se// (accessed on 1 August 2022).
- Denio, A. Chemistry for potters. J. Chem. Educ. 1980, 57, 272–275. [Google Scholar] [CrossRef]
- Tabata, M.; Yagi, N.; Nishimoto, J.; Ghaffar, A. Estimation of places of production of porcelains of unknown origins excavated at the Mietsu Naval Facility site based on differences in the solubility of trace metals during the elutriation process. J. Archaeol. Sci. Rep. 2021, 36, 102823. [Google Scholar] [CrossRef]
- Tunstall, S.; Amarasiriwardena, D. Characterization of lead and lead leaching properties of lead glazed ceramics from the Solis Valley, Mexico, using inductively coupled plasma-mass spectrometry (ICP-MS) and diffuse reflectance infrared Fourier transform spectroscopy (DRIFT). Microchem. J. 2002, 73, 335–347. [Google Scholar] [CrossRef]
- Mohamed, N.; Chin, Y.; Pok, F. Leaching of lead from local ceramic tableware. Food Chem. 1995, 54, 245–249. [Google Scholar] [CrossRef]
- Frankel, G.; Vienna, J.; Lian, J.; Scully, J.; Gin, S.; Ryan, J.; Wang, J.; Kim, S.; Windl, W.; Du, J. A comparative review of the aqueous corrosion of glasses, crystalline ceramics, and metals. NPJ Mater. Degrad. 2018, 2, 15. [Google Scholar] [CrossRef]
- Schreck, E.; Foucault, Y.; Geret, F.; Pradère, P.; Dumat, C. Influence of soil ageing on bioavailability and ecotoxicity of lead carried by process waste metallic ultrafine particles. Chemosphere 2011, 85, 1555–1562. [Google Scholar] [CrossRef] [PubMed]
- Wijayawardena, M.A.A.; Naidu, R.; Megharaj, M.; Lamb, D.; Thavamani, P.; Kuchel, T. Influence of ageing on lead bioavailability in soils: A swine study. Environ. Sci. Pollut. Res. Int. 2015, 22, 8979–8988. [Google Scholar] [CrossRef] [PubMed]
- Shahid, M.; Dumat, C.; Khalid, S.; Schreck, E.; Xiong, T.; Niazi, N.K. Foliar heavy metal uptake, toxicity and detoxification in plants: A comparison of foliar and root metal uptake. J. Hazard. Mater. 2017, 325, 36–58. [Google Scholar] [CrossRef]
- Maurice, C.; Lagerkvist, A. Using Betula pendula and Telephora caryophyllea for Soil Pollution Assessment. J. Soil. Contam. 2000, 9, 31–50. [Google Scholar] [CrossRef]
- Zeiner, M.; Juranović Cindrić, I. Accumulation of major, minor and trace elements in pine needles (Pinus nigra) in Vienna (Austria). Molecules 2021, 26, 3318. [Google Scholar] [CrossRef] [PubMed]
- Soetan, K.O.; Olaiya, C.O.; Oyewole, O.E. The importance of mineral elements for humans, domestic animals and plants: A review. Afr. J. Food Sci. 2010, 4, 200–222. [Google Scholar] [CrossRef]
- Steinnes, E. Soil and human health. In Sustaining Soil Productivity in Response to Global Climate Change: Science, Policy, and Ethics, 1st ed.; Sauer, T.J., Norman, J.M., Sivakumar, M.V.K., Eds.; Wiley-Blackwell: Oxford, UK, 2011. [Google Scholar]
- Zeiner, M.; Juranović Cindrić, I.; Mihajlov Konanov, D.; Stingeder, G. Determination of selected toxic elements in leaves of White Hawthorn grown in a remote area. E3S Web Conf. 2013, 1, 34003. [Google Scholar] [CrossRef]
- Serbula, S.M.; Kalinovic, T.S.; Ilic, A.A.; Kalinovic, J.V.; Steharnik, M.M. Assessment of Airborne Heavy Metal Pollution Using Pinus spp. and Tilia spp. Aerosol Air Qual. Res. 2013, 13, 563–573. [Google Scholar] [CrossRef]
- Zeiner, M.; Juranović Cindrić, I.; Ivanović, M.; Medunic, G. Availability of Selected (Pollutant) Elements and their Influence on Soil Composition in Urban Area. Croat. Chem. Acta 2015, 88, 23–33. [Google Scholar] [CrossRef]
- Zeiner, M.; Juranović Cindrić, I.; Pozgaj, M.; Pirkl, R.; Šilić, T.; Stingeder, G. Influence of soil composition on the major, minor and trace metal content of Velebit biomedical plants. J. Pharm. Biomed. Anal. 2015, 106, 153. [Google Scholar] [CrossRef]
- Jurić, D.; Puntarić, D.; Gvozdić, V.; Vidosavljević, D.; Lonèarić, Z.; Puntarić, A.; Puntarić, E.; Puntarić, I.; Vidosavljević, M.; Begović, L.; et al. Cabbage (Brassica oleracea var. capitata) as possible indicator of wartime metal and metalloid contamination in eastern Croatia (ICP-MS method). Acta Agric. Scand. B Soil. Plant Sci. 2017, 67, 270–277. [Google Scholar] [CrossRef]
- Juranović Cindrić, I.; Zeiner, M.; Starčević, A.; Liber, Z.; Rusak, G.; Idžojtić, M.; Stingeder, G. Influence of F1 hybridization on the metal uptake behaviour of pine trees (Pinus nigra x Pinus thunbergiana; Pinus thunbergiana x Pinus nigra). J. Trace Elem. Med. Biol. 2018, 48, 190–195. [Google Scholar] [CrossRef]
- Juranović Cindrić, I.; Zeiner, M.; Starčević, A.; Stingeder, G. Metals in pine needles: Characterisation of bio-indicators depending on species. Int. J. Env. Sci. Technol. 2019, 16, 4339–4346. [Google Scholar] [CrossRef]
- Ejaz, U.; Khan, S.; Khalid, N.; Ahmad, Z.; Jehangir, S.; Rizvi, Z.; Lho, L.; Han, H.; Raposo, A. Detoxifying the heavy metals: A multipronged study of tolerance strategies against heavy metals toxicity in plants. Front. Plant Sci. 2003, 14, 1154571. [Google Scholar] [CrossRef]
- Kozlov, M.; Haukioja, E.; Bakhtiarov, A.; Stroganov, D.; Zimina, S. Root versus canopy uptake of heavy metals by birch in an industrially polluted area: Contrasting behaviour of nickel and copper. Environ. Pollut. 2000, 107, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Gatasheh, M.; Abbas, T.; Shaffique, S.; Kang, S.; Lee, I.; Shah, A. Comparative analysis of biodiversity, physiology, and anatomical adaptations in riparian flora exposed to industrial pollution stress. Sci. Rep. 2025, 15, 3006. [Google Scholar] [CrossRef] [PubMed]
- Pająk, M.; Halecki, W.; Gąsiorek, M. Accumulative response of Scots pine (Pinus sylvestris L.) and silver birch (Betula pendula Roth) to heavy metals enhanced by Pb-Zn ore mining and processing plants: Explicitly spatial considerations of ordinary kriging based on a GIS approach. Chemosphere 2017, 168, 851–859. [Google Scholar] [CrossRef] [PubMed]
- Vacek, Z.; Linda, R.; Cukor, J.; Vacek, S.; Šimůnek, V.; Gallo, J.; Vančura, K. Scots pine (Pinus sylvestris L.), the suitable pioneer species for afforestation of reclamation sites? For. Ecol. Manag. 2021, 485, 118951. [Google Scholar] [CrossRef]
- Parzych, A.; Mochnacký, S.; Sobisz, Z.; Kurhaluk, N.; Polláková, N. Accumulation of heavy metals in needles and bark of Pinus species. Folia Pol. Ser. A 2017, 59, 34–44. [Google Scholar] [CrossRef]
- Hederfeld, G. Risk Estimation of Multi-Polluted Soils in Contact with Lacustrine Systems. Master’s Thesis, Örebro University, Örebro, Sweden, 2018. Available online: https://www.diva-portal.org/smash/record.jsf?pid=diva2%3A1266488&dswid=9903 (accessed on 20 March 2025).
- Baker, A.J.M.; Brooks, R.R. Terrestrial Higher Plants which Hyperaccumulate Metallic Elements—A Review of their Distribution. Ecol. Phytochem. Biorecovery 1989, 1, 81–126. [Google Scholar] [CrossRef]
- Khare, D.; Mitsuda, N.; Lee, S.; Song, W.; Hwang, D.; Ohme-Takagi, M.; Martinoia, E.; Lee, Y.; Hwang, J. Root avoidance of toxic metals requires the GeBP-LIKE 4 transcription factor in Arabidopsis thaliana. New Phytol. 2017, 213, 1257–1273. [Google Scholar] [CrossRef]
- Hu, X.; Wei, X.; Ling, J.; Chen, J. Cobalt: An Essential Micronutrient for Plant Growth? Front. Plant Sci. 2021, 12, 768523. [Google Scholar] [CrossRef]
- Chen, Y.; Zhou, Y. The contents and release behavior of heavy metals in construction and demolition waste used in freeway construction. Environ. Sci. Pollut. Res. 2020, 27, 1078–1086. [Google Scholar] [CrossRef]
- Kaiser, B.N.; Gridley, K.L.; Ngaire Brady, J.; Phillips, T.; Tyerman, S.D. The role of molybdenum in agricultural plant production. Ann. Bot. 2005, 96, 745–754. [Google Scholar] [CrossRef]
- Intawongse, M.; Dean, J.R. Uptake of heavy metals by vegetable plants grown on contaminated soil and their bioavailability in the human gastrointestinal tract. Food Addit. Contam. 2006, 23, 36–48. [Google Scholar] [CrossRef] [PubMed]
- Sitko, K.; Opała-Owczarek, M.; Jemioła, G.; Gieroń, Ż.; Szopiński, M.; Owczarek, P.; Rudnicka, M.; Małkowski, E. Effect of Drought and Heavy Metal Contamination on Growth and Photosynthesis of Silver Birch Trees Growing on Post-Industrial Heaps. Cells 2022, 11, 53. [Google Scholar] [CrossRef] [PubMed]
- Dresler, S.; Wójciak, M.; Sowa, I.; Sawicki, J.; Strzemski, M.; Hawrylak-Nowak, B.; Hanaka, A. Accumulation ability of trace metals by silver birch leaves in areas contaminated by Zn-Pb ore processing: Effects of excessive trace metal accumulation on specialized metabolism. Chemosphere 2024, 362, 142719. [Google Scholar] [CrossRef] [PubMed]
- Çomaklı, E.; Bingöl, M. Heavy metal accumulation of urban Scots pine (Pinus sylvestris L.) plantation. Environ. Monit. Assess. 2021, 193, 192. [Google Scholar] [CrossRef] [PubMed]
- Hiller, E.; Faragó, T.; Kolesár, M.; Filová, L.; Mihaljevič, M.; Jurkovič, Ľ.; Demko, R.; Machlica, A.; Štefánek, J.; Vítková, M. Metal(loid)s in urban soil from historical municipal solid waste landfill: Geochemistry, source apportionment, bioaccessibility testing and human health risks. Chemosphere 2024, 362, 142677. [Google Scholar] [CrossRef]
- Tatuśko-Krygier, N.; Diatta, J.; Chudzińska, E.; Waraczewska, Z.; Gawroński, D.; Youssef, N. Bioactive levels of Zn, Pb, Cu, Cd and Mg, Fe in pollution sensitive and tolerant Scots pines needles—Is survival mineral-dependent? Ecol. Indic. 2023, 146, 109751. [Google Scholar] [CrossRef]
- Holiaka, D.; Yoschenko, V.; Cherniaiev, O.; Moskaliuk, A.; Lesnik, O.; Levchuk, S.; Holiaka, M.; Gumenuk, V.; Kovbasa, Y.; Borsuk, O.; et al. Variability of activity concentrations and radial distributions of 137Cs and 90Sr in trunk wood of Scots pine and Silver birch. J. Environ. Radioact. 2023, 263, 107186. [Google Scholar] [CrossRef]
- Baltrėnaitė, E.; Lietuvninkas, A.; Baltrėnas, P. Use of Dynamic Factors to Assess Metal Uptake and Transfer in Plants—Example of Trees. Water Air Soil. Pollut. 2012, 223, 4297–4306. [Google Scholar] [CrossRef]
- Rautio, P.; Fürst, A.; Stefan, K.; Raitio, H.; Bartels, U. Part XII: Sampling and Analysis of Needles and Leaves. In Manual on Methods and Criteria for Harmonized Sampling, Assessment, Monitoring and Analysis of the Effects of Air Pollution on Forests; UNECE ICP Forests Programme Co-ordinating Centre, Ed.; Thünen Institute of Forest Ecosystems: Eberswalde, Germany, 2016; p. 19. Available online: http://icp-forests.net/page/icp-forests-manual (accessed on 1 August 2022).
| Analyte | Recovery (%) | RSD (%) |
|---|---|---|
| As | 101 | 2.9 |
| Ba | 86.2 | 2.3 |
| Cd | 98.9 | 1.4 |
| Co | 85.9 | 2.6 |
| Cu | 96.0 | 1.5 |
| Cr | 105 | 2.9 |
| Mn | 90.9 | 1.8 |
| Mo | n/a * | n/a |
| Ni | 96.1 | 2.5 |
| Pb | 91.2 | 2.8 |
| V | n/a * | n/a |
| Zn | 88.4 | 2.7 |
| Analyte ↓/Tree → | B1 | B2 | B3 | B4 | B5 |
|---|---|---|---|---|---|
| As | 0.030 | 0.33 | 0.30 | 0.0054 | 0.38 |
| Ba | 1.0 × 10−6 | 0.10 | 4.6 × 10−7 | 0.097 | 0.00011 |
| Cd | 0.62 | 0.19 | 0.52 | 0.064 | 0.67 |
| Co | 0.00025 | 7.9 × 10−7 | 3.5 × 10−9 | 2.0 × 10−5 | 2.9 × 10−7 |
| Cu | 0.12 | 0.87 | 0.0022 | 0.039 | 0.85 |
| Cr | 0.29 | 0.61 | 0.23 | 0.61 | 0.32 |
| Mo | 0.34 | 1.3 × 10−7 | 0.0056 | 0.0036 | 0.018 |
| Ni | 0.00021 | 0.70 | 0.76 | 0.25 | 0.0025 |
| Pb | 2.7 × 10−5 | 8.4 × 10−8 | 1.5 × 10−6 | 1.6 × 10−6 | 5.1 × 10−6 |
| V | 0.00058 | 0.68 | 0.77 | 0.13 | 0.32 |
| Zn | 8.7 × 10−5 | 0.41 | 0.0095 | 0.0018 | 0.0029 |
| Analyte ↓/Tree→ | B1 | B2 | B3a | B3b | B4 | B5 | Min | Max | Mean |
|---|---|---|---|---|---|---|---|---|---|
| As | 0.0007 | 0.0027 | 0.0198 | 0.0206 | 0.0428 | 0.0293 | 0.0007 | 0.0428 | 0.0193 |
| Ba | 2.9171 | 6.6390 | 7.8302 | 3.6299 | 1.8243 | 1.5727 | 1.5727 | 7.8302 | 4.0689 |
| Cd | 0.6614 | 0.5701 | 0.2255 | 1.0033 | 0.0936 | 1.0532 | 0.0936 | 1.0532 | 0.6012 |
| Co | 0.0126 | 0.0044 | 0.1574 | 0.0752 | 0.1050 | 0.0219 | 0.0044 | 0.1574 | 0.0627 |
| Cu | 0.0325 | 0.1159 | 0.0419 | 0.0635 | 0.0492 | 0.1147 | 0.0325 | 0.1159 | 0.0696 |
| Cr | 0.0423 | 0.0453 | 0.0135 | 0.0223 | 0.0128 | 0.0094 | 0.0094 | 0.0453 | 0.0243 |
| Mo | 0.3924 | n/a * | 0.3763 | n/a * | 0.6851 | n/a * | 0.3763 | 0.6851 | 0.4846 |
| Ni | 0.2631 | 0.0527 | 0.0466 | 0.0684 | 0.0274 | 0.5987 | 0.0274 | 0.5987 | 0.1762 |
| Pb | 0.0008 | 0.0015 | 0.0160 | 0.0136 | 0.0045 | 0.0048 | 0.0008 | 0.0160 | 0.0069 |
| V | 0.0224 | 0.0222 | 0.0029 | 0.0076 | 0.0019 | 0.0134 | 0.0019 | 0.0224 | 0.0117 |
| Zn | 1.2641 | 0.7482 | 0.0772 | 0.6480 | 0.0879 | 0.7940 | 0.0772 | 1.2641 | 0.6032 |
| Analyte ↓/Tree→ | P1 | P2 | P3 | P4 | P5 |
|---|---|---|---|---|---|
| As | 0.12 | 0.99 | 0.35 | 0.83 | 0.50 |
| Ba | 0.87 | 0.52 | 0.56 | 1.5 × 10−5 | 0.51 |
| Cd | 0.13 | 0.86 | 2.4 × 10−5 | 0.0022 | 0.23 |
| Co | 0.0012 | 0.026 | 0.13 | 0.0030 | 0.13 |
| Cu | 0.96 | 0.27 | 0.44 | 0.66 | 0.30 |
| Cr | 0.094 | 0.95 | 0.76 | 0.67 | 0.14 |
| Mo | 0.010 | 0.0016 | 0.00026 | 0.0030 | 0.0012 |
| Ni | 0.60 | 0.38 | 0.37 | 0.0024 | 0.096 |
| Pb | 0.054 | 0.49 | 0.46 | 0.066 | 0.23 |
| V | 0.61 | 0.51 | 0.49 | 0.50 | 0.85 |
| Zn | 0.56 | 0.18 | 0.16 | 0.69 | 0.11 |
| Parameter | ICP-MS |
|---|---|
| Instrument | Agilent 7500cx ICP-MS (Agilent, Tokyo, Japan) |
| Output power | 1500 W |
| Argon flows | Plasma:15 L min−1 Auxiliary: 0.9 L min−1 Nebulizer: 0.2 L min−1 |
| Sample flow | 0.3 mL min−1 |
| Nebulizer | MicroMist |
| Spray chamber | Scott double pass |
| Isotopes | 51V, 53Cr, 55Mn, 59Co, 60Ni, 63Cu, 66Zn, 75As, 95Mo,111Cd, 137Ba, 204+206+207+208Pb |
| Collison cell | Off: Ba, Pb, Cd, Co, Cu, Mn, Mo, Ni, Zn On (He 5 mL/min): V, Cr, As |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeiner, M.; Sjöberg, V.; Olsman, H. Metal Uptake by Birches and Scots Pines Grown on a Porcelain Landfill. Molecules 2025, 30, 2196. https://doi.org/10.3390/molecules30102196
Zeiner M, Sjöberg V, Olsman H. Metal Uptake by Birches and Scots Pines Grown on a Porcelain Landfill. Molecules. 2025; 30(10):2196. https://doi.org/10.3390/molecules30102196
Chicago/Turabian StyleZeiner, Michaela, Viktor Sjöberg, and Helena Olsman. 2025. "Metal Uptake by Birches and Scots Pines Grown on a Porcelain Landfill" Molecules 30, no. 10: 2196. https://doi.org/10.3390/molecules30102196
APA StyleZeiner, M., Sjöberg, V., & Olsman, H. (2025). Metal Uptake by Birches and Scots Pines Grown on a Porcelain Landfill. Molecules, 30(10), 2196. https://doi.org/10.3390/molecules30102196







