Spectroscopic and Chromatographic Characterization of Two Isomeric Cathinone Derivatives: N-Butyl-Norbutylone and N-Ethylhexylone
Abstract
1. Introduction
- N-Alkylated cathinones: Includes mephedrone, pentedrone, and 4-methylethcathinone (4-MEC), which exhibit stimulant and empathogenic properties.
- Methylenedioxy-substituted cathinones: Includes methylone, butylone, and pentylone, which share structural similarities with MDMA and exert serotonergic effects.
- Pyrrolidine-containing cathinones: Includes α-pyrrolidinovalerophenone (α-PVP) and methylenedioxypyrovalerone (MDPV), which act as potent dopamine reuptake inhibitors and exhibit vigorous psychostimulant activity.
2. Results
3. Discussion
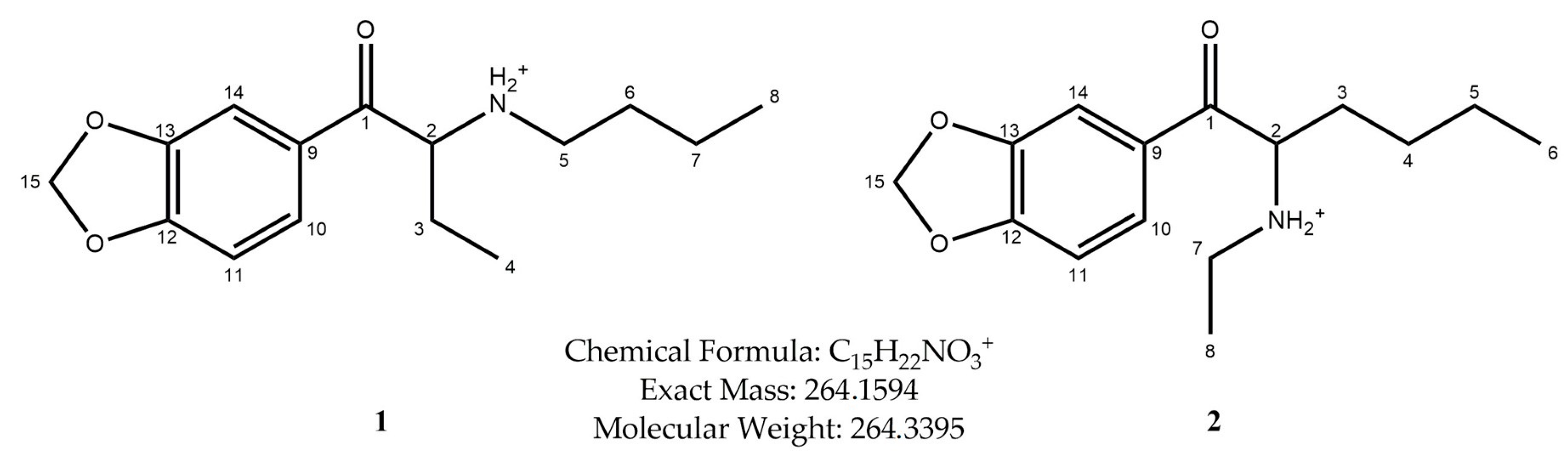
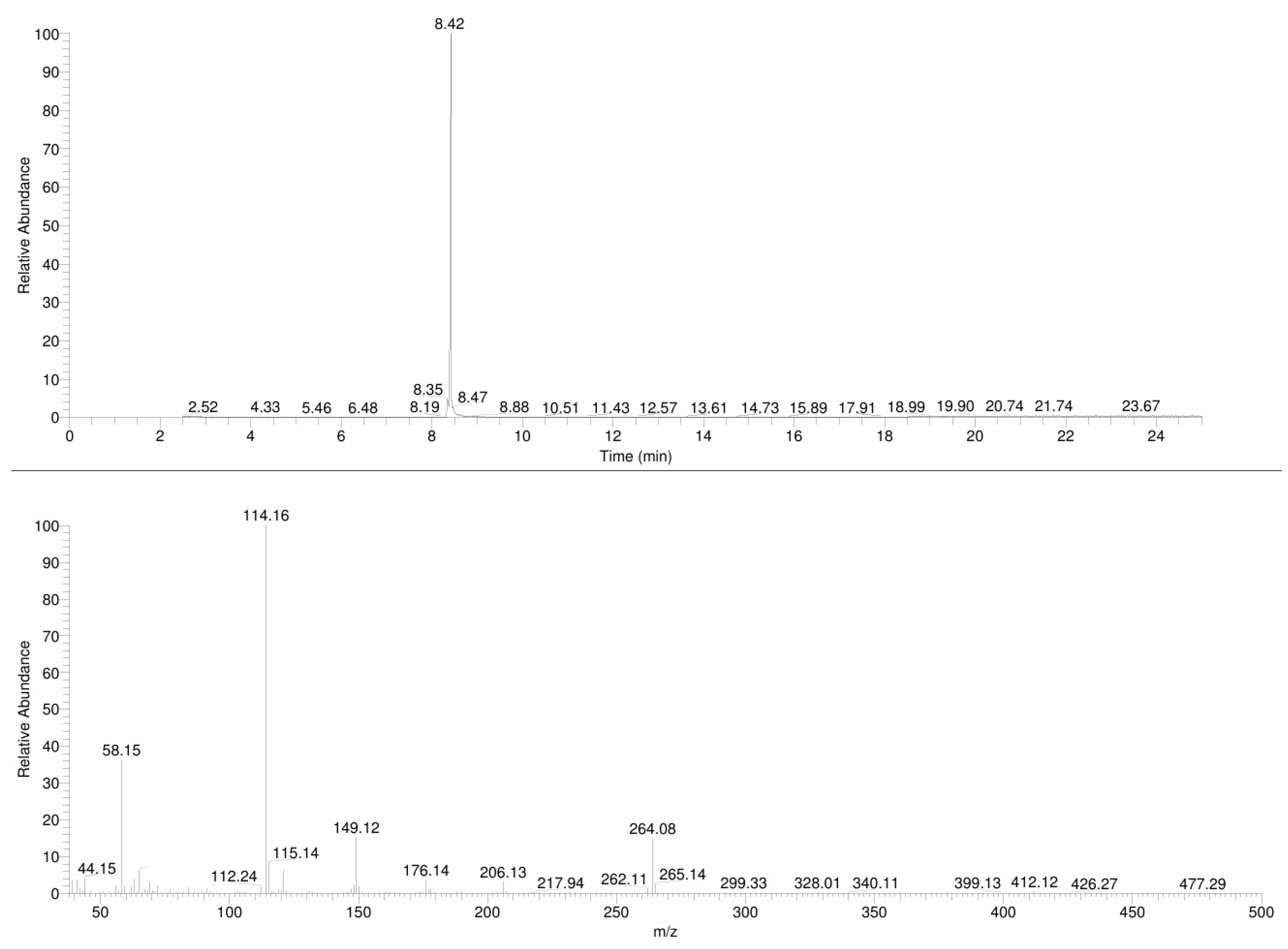
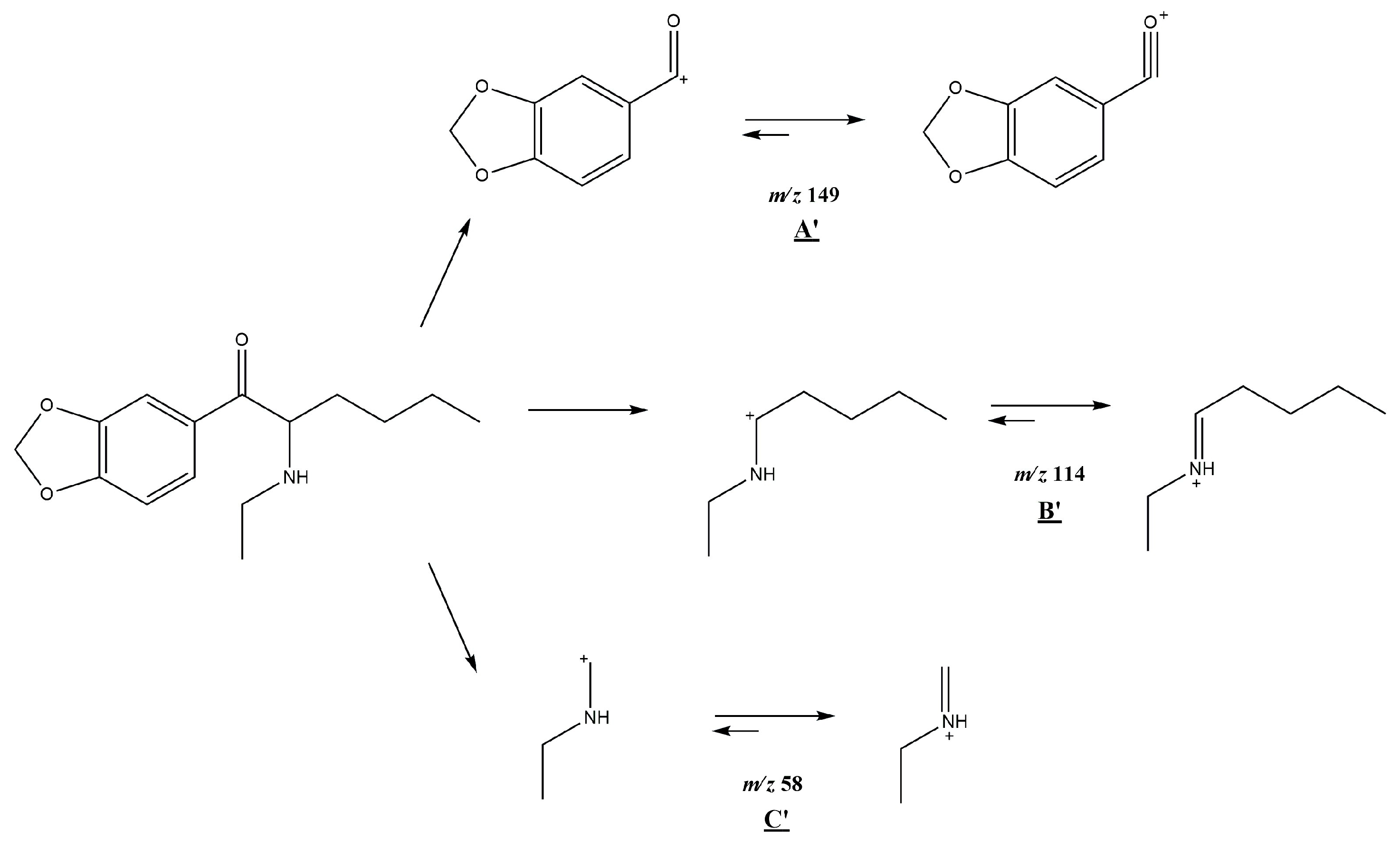
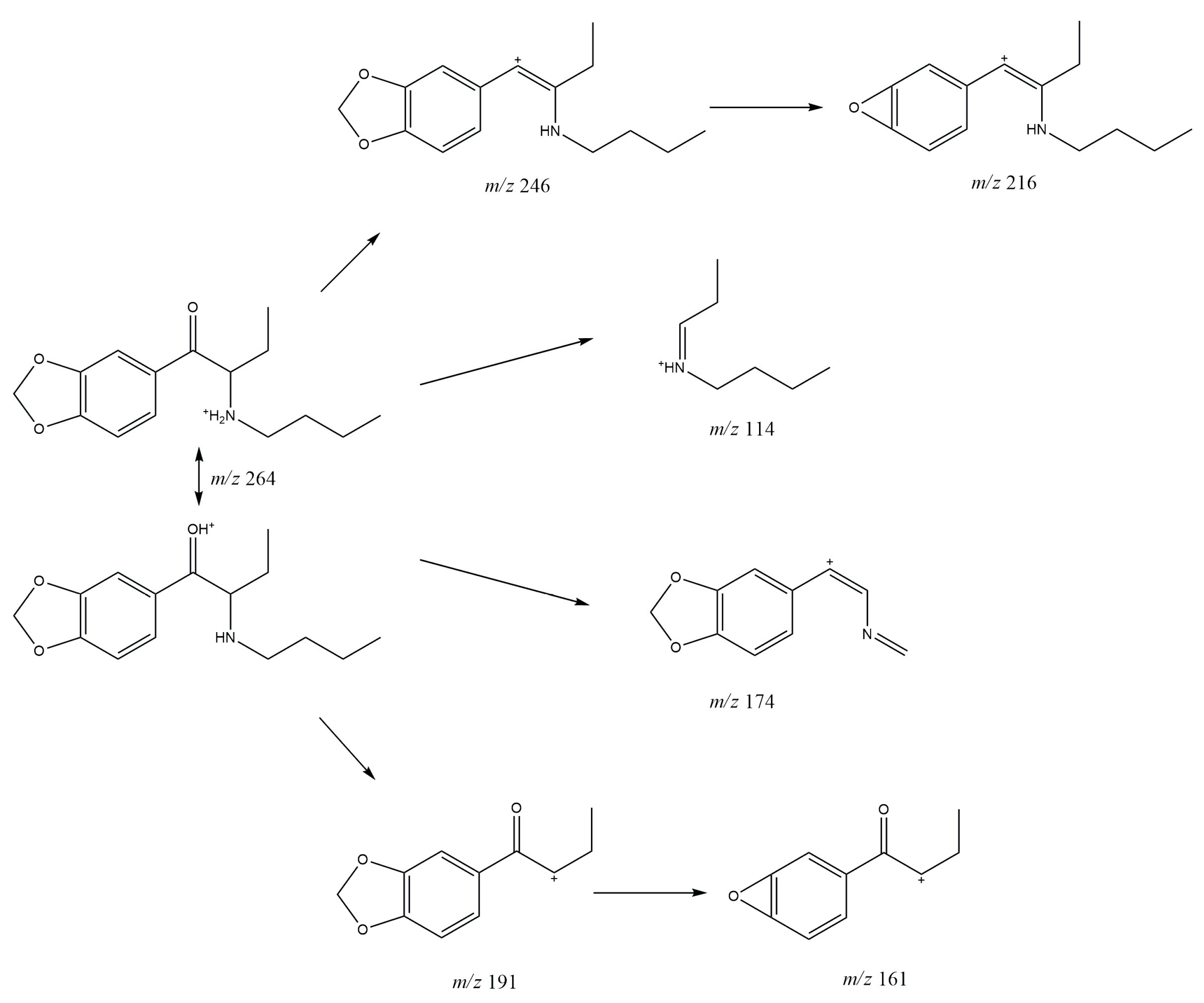
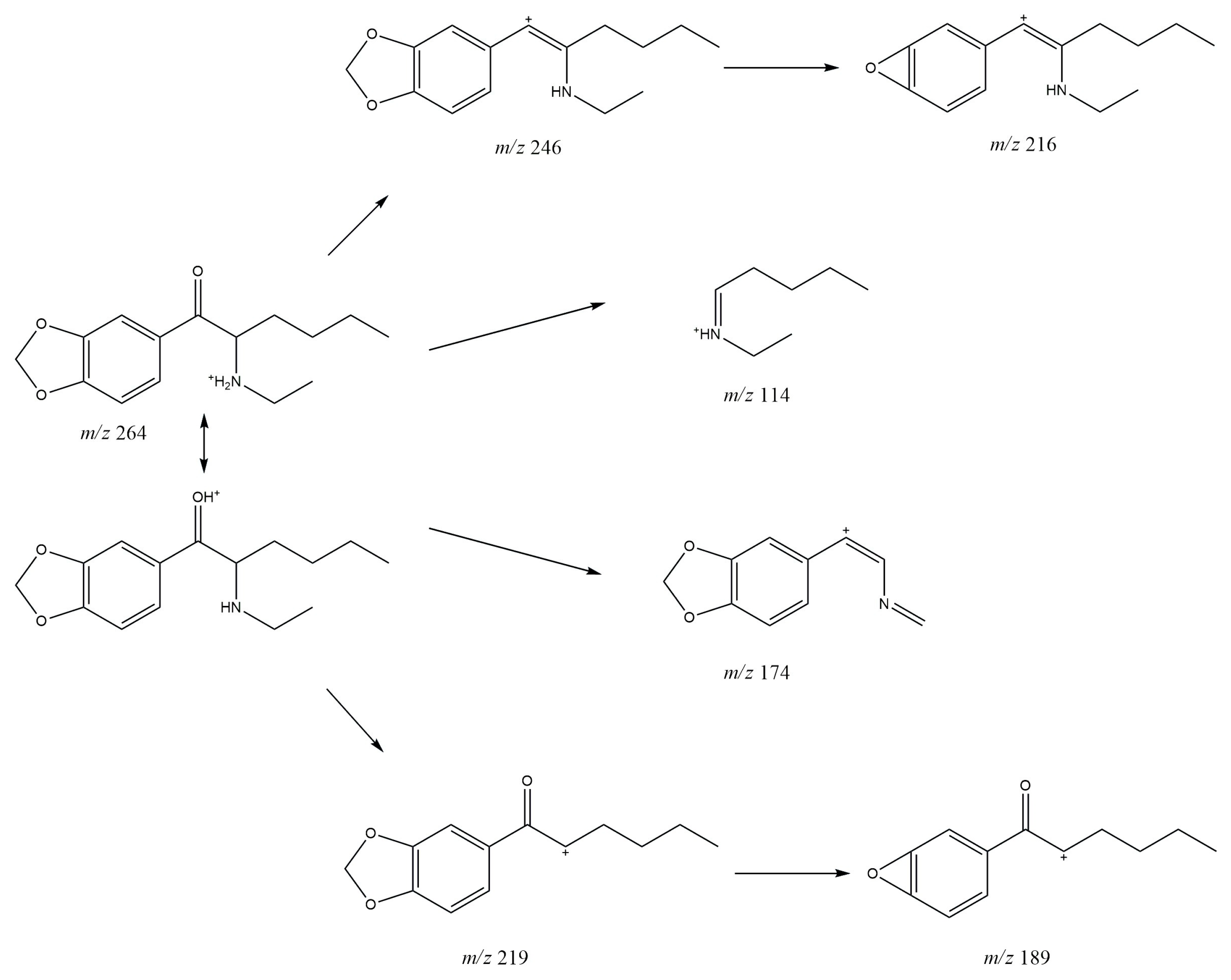
3.1. GC-MS
3.2. ESI-MS
3.3. 1H and 13C NMR
4. Materials and Methods
4.1. Chemicals
4.2. Sample Preparation
4.3. Gas Chromatography Mass Spectrometry (GC-MS) Analysis
4.4. Direct Infusion Electrospray Ionization Mass Spectrometry (ESI-MS)
4.5. High-Resolution Mass Spectrometry (HR-MS)
4.6. NMR Spectroscopy
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- European Monitoring Centre for Drugs and Drug Addiction and Europol. EU Drug Market: New Psychoactive Substances—In-Depth Analysis. 2024. Available online: https://www.emcdda.europa.eu/publications/eu-drug-markets/new-psychoactive-drugs_en (accessed on 8 May 2025).
- Kalix, P. The pharmacology of khat. Gen. Pharmac. 1984, 15, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Simmler, L.D.; Buser, T.A.; Donzelli, M.; Schramm, Y.; Dieu, L.-H.; Huwyler, J.; Chaboz, S.; Hoener, M.C.; Liechti, M.E. Pharmacological characterization of designer cathinones in vitro. Br. J. Pharmacol. 2013, 168, 458–470. [Google Scholar] [CrossRef] [PubMed]
- Zawilska, J.B.; Wojcieszak, J. Designer cathinones—An emerging class of novel recreational drugs. Forensic Sci. Int. 2013, 231, 42–53. [Google Scholar] [CrossRef] [PubMed]
- Valente, M.J.; Guedes de Pinho, P.; de Lourdes Bastos, M.; Carvalho, F.; Carvalho, M. Khat and synthetic cathinones: A review. Arch. Toxicol. 2014, 88, 15–45. [Google Scholar] [CrossRef] [PubMed]
- Prosser, J.M.; Nelson, L.S. The toxicology of bath salts: A review of synthetic cathinones. J. Med. Toxicol. 2012, 8, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Schifano, F.; Napoletano, F.; Arillotta, D.; Zangani, C.; Gilgar, L.; Guirguis, A.; Corkery, J.M.; Vento, A. The clinical challenges of synthetic cathinones. Br. J. Clin. Pharmacol. 2020, 86, 410–419. [Google Scholar] [CrossRef] [PubMed]
- European Monitoring Centre for Drugs and Drug Addiction. European Drug Report 2024: Trends and Developments. 2024. Available online: https://www.emcdda.europa.eu/publications/european-drug-report/2024_en (accessed on 10 April 2025).
- Patel, N.B. “Natural Amphetamine” Khat: A Cultural Tradition or a Drug of Abuse? Int. Rev. Neurobiol. 2015, 120, 235–255. [Google Scholar] [PubMed]
- Brenneisen, R.; Fisch, H.U.; Koelbing, U.; Geisshüsler, S.; Kalix, P. Amphetamine-like effects in humans of the khat alkaloid cathinone. Br. J. Clin. Pharmacol. 1990, 30, 825–828. [Google Scholar] [CrossRef] [PubMed]
- Gannon, B.M.; Baumann, M.H.; Walther, D.; Jimenez-Morigosa, C.; Sulima, A.; Rice, K.C.; Collins, G.T. The abuse-related effects of pyrrolidine-containing cathinones are related to their potency and selectivity to inhibit the dopamine transporter. Neuropsychopharmacology 2018, 43, 2399–2407. [Google Scholar] [CrossRef] [PubMed]
- Banks, M.L.; Worst, T.J.; Rusyniak, D.E.; Sprague, J.E. Synthetic Cathinones (“Bath Salts”). J. Emerg. Med. 2014, 46, 632–642. [Google Scholar] [CrossRef] [PubMed]
- Kaizaki, A.; Tanaka, S.; Numazawa, S. New recreational drug 1-phenyl-2-(1-pyrrolidinyl)-1-pentanone (alpha-PVP) activates central nervous system via dopaminergic neuron. J. Toxicol. Sci. 2014, 39, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Meltzer, P.C.; Butler, D.; Deschamps, J.R.; Madras, B.K. 1-(4-Methylphenyl)-2-pyrrolidin-1-yl-pentan-1-one (pyrovalerone) analogues: A promisingclass of monoamine uptake inhibitors. J. Med. Chem. 2006, 49, 1420–1432. [Google Scholar] [CrossRef] [PubMed]
- Eshleman, A.J.; Wolfrum, K.M.; Reed, J.F.; Kim, S.O.; Swanson, T.; Johnson, R.A.; Janowsky, A.J. Structure-activity relationships of substituted cathinones, with transporter binding, uptake, and release. J. Pharmacol. Exp. Ther. 2017, 360, 33–47. [Google Scholar] [CrossRef] [PubMed]
- Zawilska, J.; Wojcieszak, J. α-Pyrrolidinophenones: A new wave of designer cathinones. Forensic Toxicol. 2017, 35, 201–216. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, W.; Lai, M. Synthetic Cathinones: Epidemiology, Toxicity, Potential for Abuse, and Current Public Health Perspective. Brain Sci. 2024, 14, 334. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.M.; Hua, Z.D.; Song, C.H.; Jia, W. Identification and analytical characterization of N-propyl norbutylone, N-butyl norbutylone, N-benzyl norheptedrone, and N-pyrrolidinyl-3,4-DMA. Drug Test. Anal. 2023, 15, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Matsuta, S.; Katagi, M.; Nishioka, H.; Kamata, H.; Sasaki, K.; Shima, N.; Kamata, T.; Miki, A.; Tatsuno, M.; Zaitsu, K.; et al. Structural characterization of cathinone-type designer drugs by EI mass spectrometry. Jpn. J. Forensic Sci. Technol. 2014, 19, 77–89. [Google Scholar] [CrossRef]
- Fornal, E. Study of collision-induced dissociation of electrospray-generated protonated cathinones. Drug Test. Anal. 2014, 6, 705–715. [Google Scholar] [CrossRef] [PubMed]
- Fornal, E. Formation of odd-electron product ions in collision-induced fragmentation of electrospray-generated protonated cathinone derivatives: Aryl α-primary amino ketones. Rapid Commun. Mass. Spectrom. 2013, 27, 1858–1866. [Google Scholar] [CrossRef] [PubMed]

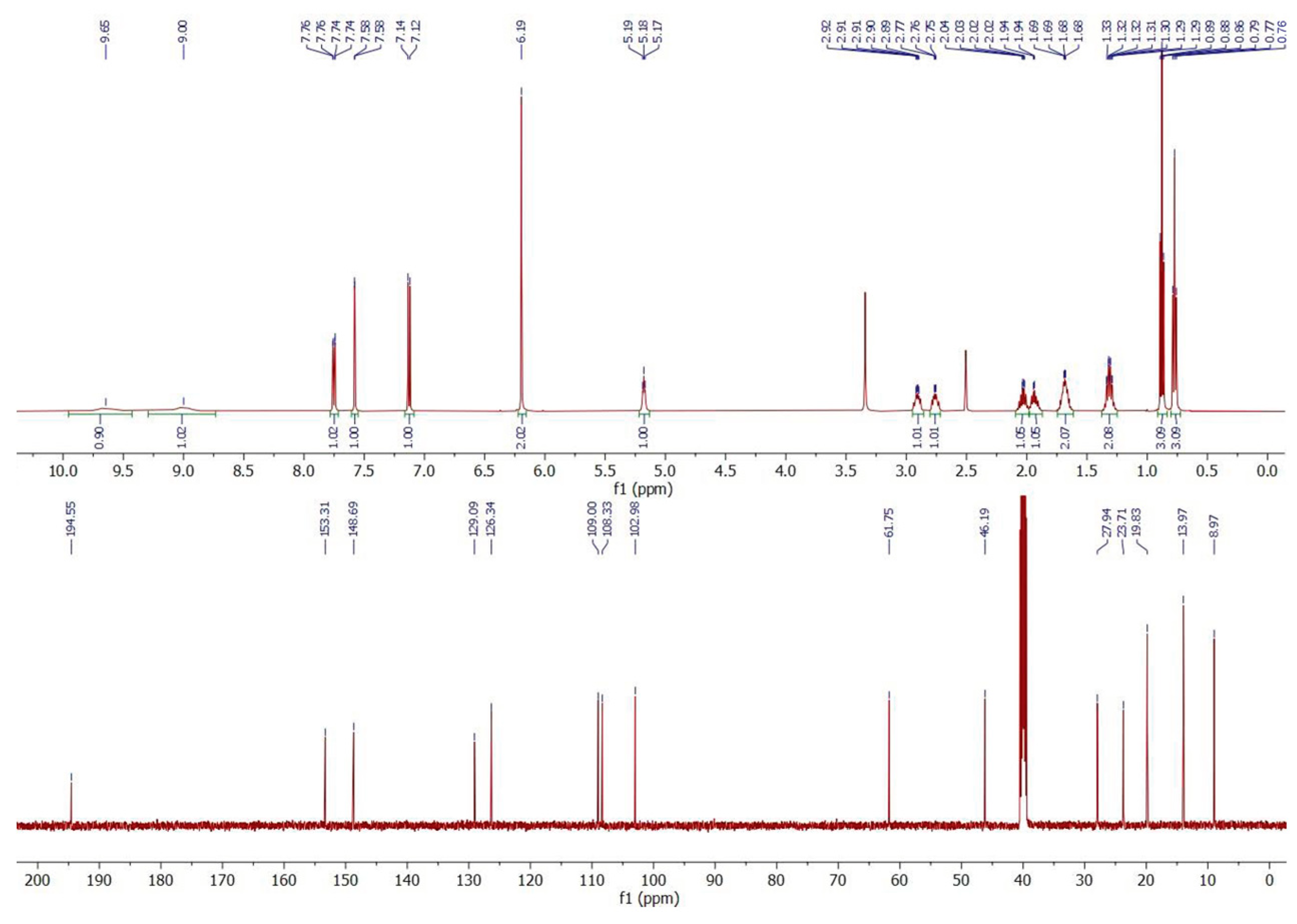


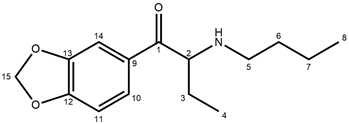 | ||
|---|---|---|
| Atom Position | Carbon Chemical Shifts (ppm) | Proton Chemical Shifts (ppm) |
| 1 | 194.55 | – |
| 2 | 61.75 | 5.18 (t, J = 5.4 Hz, 1H) |
| 3 | 23.71 | 1.94 (m, 1H); 2.02 (m, 1H) |
| 4 | 8.97 | 0.77 (t, J = 7.5 Hz, 3H) |
| 5 | 46.19 | 2.76 (m, 1H); 2.91 (m, 1H) |
| 6 | 27.94 | 1.68 (m, 2H) |
| 7 | 19.83 | 1.31 (m, 2H) |
| 8 | 13.97 | 0.88 (t, J = 7.4 Hz, 3H) |
| 9 | 129.09 | – |
| 10 | 126.34 | 7.75 (dd, J = 8.2; 1.8 Hz, 1H) |
| 11 | 109.00 | 7.13 (d, J = 8.2 Hz, 1H) |
| 12 | 153.31 | – |
| 13 | 148.69 | – |
| 14 | 108.33 | 7.58 (d, J = 1.8 Hz, 1H) |
| 15 | 102.98 | 6.19 (s, 2H) |
| N-H | – | 9.00 (bs, 1H); 9.65 (bs, 1H) |
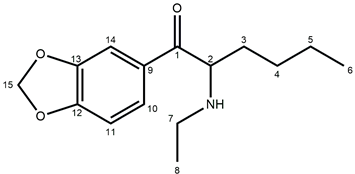 | ||
|---|---|---|
| Atom Position | Carbon Chemical Shifts (ppm) | Proton Chemical Shifts (ppm) |
| 1 | 194.60 | – |
| 2 | 60.62 | 5.20 (t, J = 5.5 Hz, 1H) |
| 3 | 30.22 | 1.89 (m, 2H) |
| 4 | 26.11 | 1,19 (m, 2H) |
| 5 | 22.34 | 1.04 (m, 2H) |
| 6 | 11.68 | 0.76 (t, J = 7.2 Hz, 3H) |
| 7 | 41.65 | 2.88 (m, 1H); 2.99 (m, 1H) |
| 8 | 13.99 | 1.24 (t, J = 7.3 Hz, 3H) |
| 9 | 128.91 | – |
| 10 | 126.41 | 7.76 (dd, J = 8.2; 1.8 Hz, 1H) |
| 11 | 109.03 | 7.13 (d, J = 8.2 Hz, 1H) |
| 12 | 153.40 | – |
| 13 | 148.73 | – |
| 14 | 108.26 | 7.57 (d, J = 1.7 Hz, 1H) |
| 15 | 103.02 | 6.20 (s, 2H) |
| N-H | – | 8.96 (bs, 1H); 9.28 (bs, 1H) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rojkiewicz, M.; Kuś, P.; Jampilek, J.; Bąk, A.; Kozik, V. Spectroscopic and Chromatographic Characterization of Two Isomeric Cathinone Derivatives: N-Butyl-Norbutylone and N-Ethylhexylone. Molecules 2025, 30, 2182. https://doi.org/10.3390/molecules30102182
Rojkiewicz M, Kuś P, Jampilek J, Bąk A, Kozik V. Spectroscopic and Chromatographic Characterization of Two Isomeric Cathinone Derivatives: N-Butyl-Norbutylone and N-Ethylhexylone. Molecules. 2025; 30(10):2182. https://doi.org/10.3390/molecules30102182
Chicago/Turabian StyleRojkiewicz, Marcin, Piotr Kuś, Josef Jampilek, Andrzej Bąk, and Violetta Kozik. 2025. "Spectroscopic and Chromatographic Characterization of Two Isomeric Cathinone Derivatives: N-Butyl-Norbutylone and N-Ethylhexylone" Molecules 30, no. 10: 2182. https://doi.org/10.3390/molecules30102182
APA StyleRojkiewicz, M., Kuś, P., Jampilek, J., Bąk, A., & Kozik, V. (2025). Spectroscopic and Chromatographic Characterization of Two Isomeric Cathinone Derivatives: N-Butyl-Norbutylone and N-Ethylhexylone. Molecules, 30(10), 2182. https://doi.org/10.3390/molecules30102182







