Degradation of Indomethacin in Wastewater: Removal with Sodium Hypochlorite and Analysis of Degradation Byproducts
Abstract
1. Introduction
2. Results and Discussion
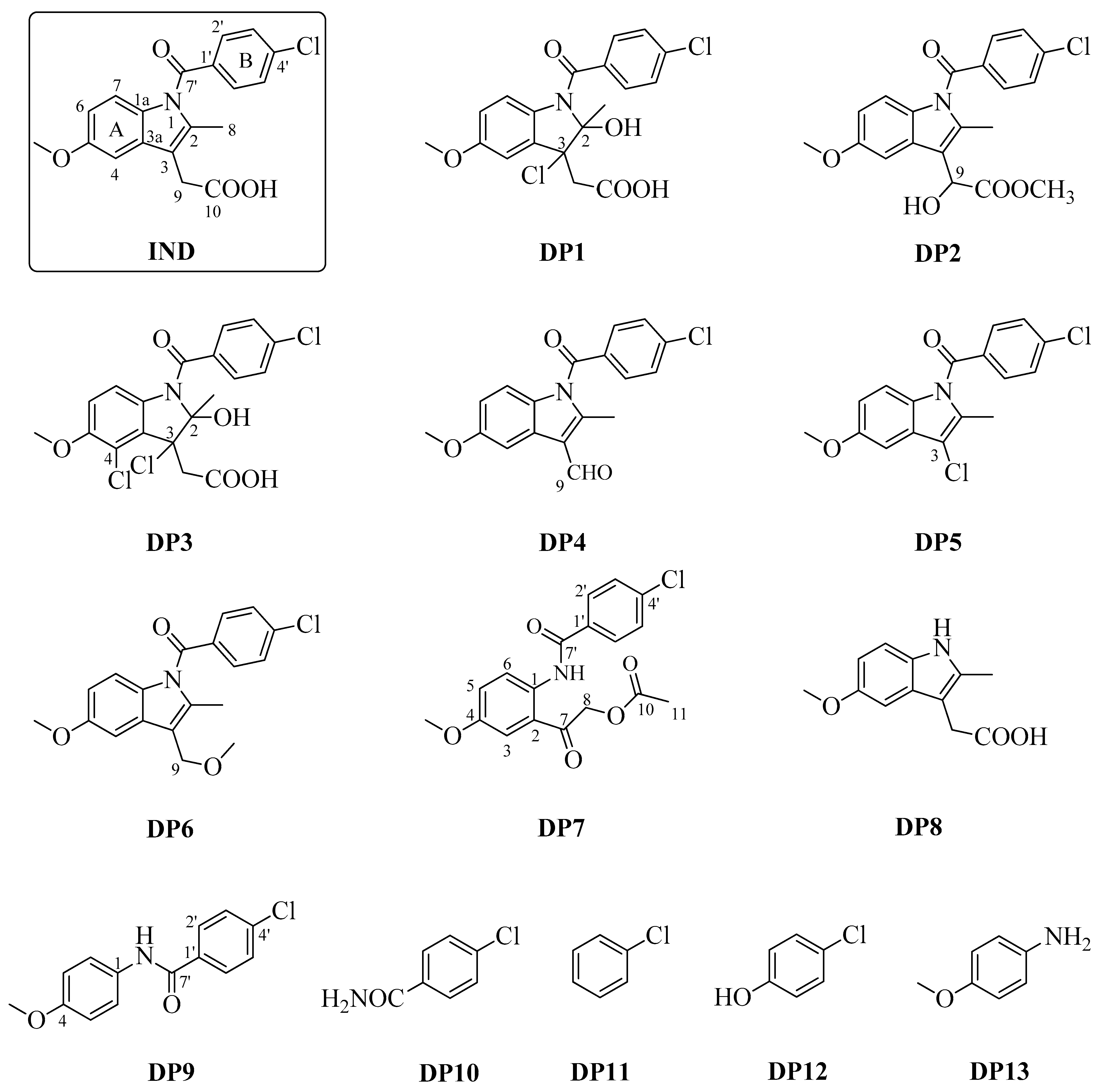
2.1. Degradation Experiments
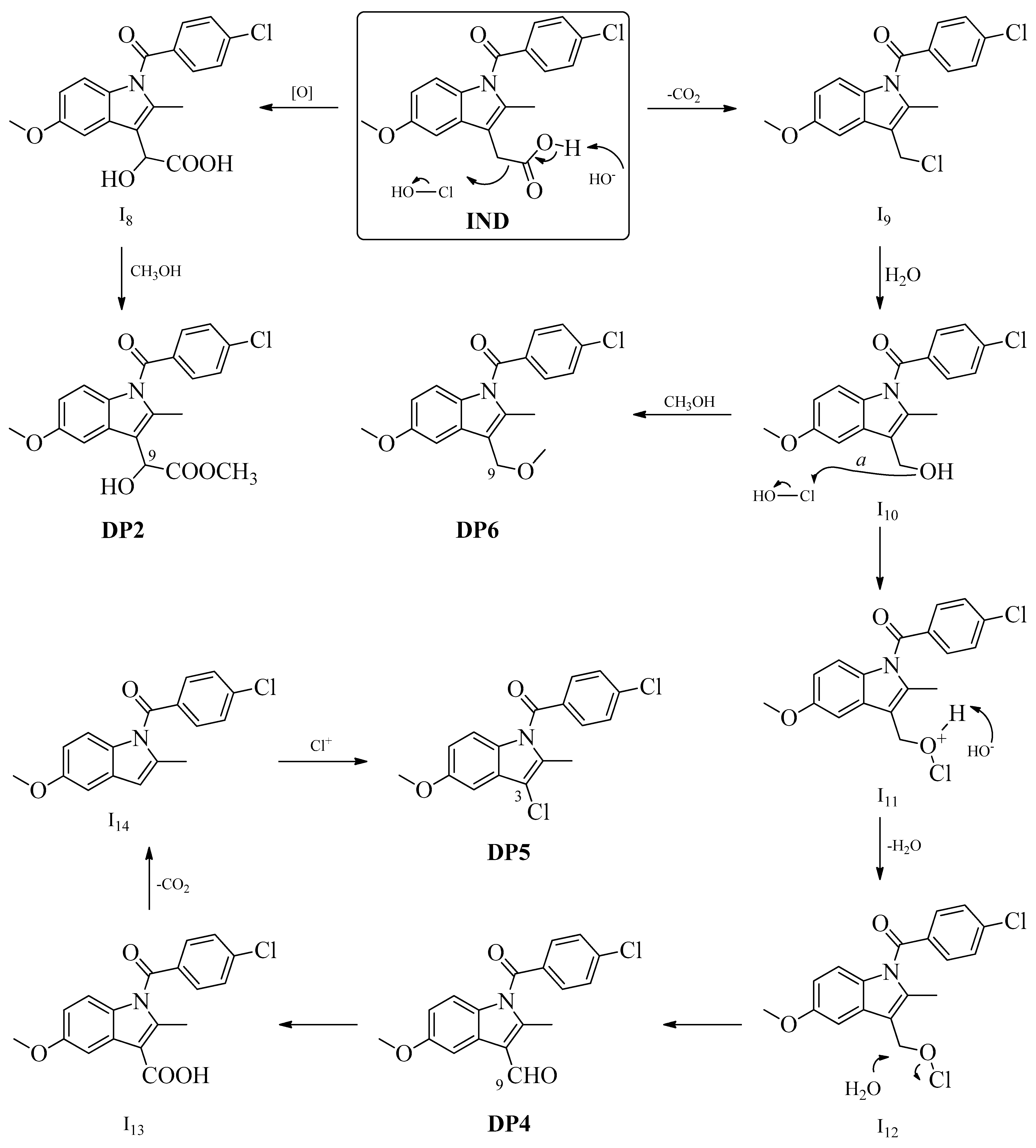
2.2. Mechanistic Interpretation
2.3. Kinetic Study of the Effect of Hypochlorite Concentration
2.4. Environmental Persistence and Toxicological Significance of Degradation Products
3. Materials and Methods
3.1. Drug and Reagents
3.2. Sodium Hypochlorite Reaction
3.2.1. Apparatus and Equipment
3.2.2. Chlorination Reaction
3.2.3. Product Isolation Procedure
3.3. Spectral Data
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bavumiragira, J.P.; Yin, H. Fate and transport of pharmaceuticals in water systems: A processes review. Sci. Total Environ. 2022, 823, 153635. [Google Scholar] [CrossRef]
- Ojoghoro, J.O.; Scrimshaw, M.D.; Sumpter, J. Steroid hormones in the aquatic environment. Sci. Total Environ. 2021, 792, 148306. [Google Scholar] [CrossRef]
- Morin-Crini, N.; Lichtfouse, E.; Liu, G.; Balaram, V.; Ribeiro, A.R.L.; Lu, Z.; Stock, F.; Carmona, E.; Ribau Teixeira, M.; Picos-Corrales, L.A.; et al. Worldwide cases of water pollution by emerging contaminants: A review. Environ. Chem. Lett. 2022, 20, 2311–2338. [Google Scholar] [CrossRef]
- Puri, M.; Gandhi, K.; Kumar, M.S. Emerging environmental contaminants: A global perspective on policies and regulations. J. Environ. Manag. 2023, 332, 117344. [Google Scholar] [CrossRef] [PubMed]
- Mishra, R.K.; Mentha, S.S.; Misra, Y.; Dwivedi, N. Emerging pollutants of severe environmental concern in water and wastewater: A comprehensive review on current developments and future research. Water-Energy Nexus 2023, 6, 74–95. [Google Scholar] [CrossRef]
- Feng, G.; Huang, H.; Chen, Y. Effects of emerging pollutants on the occurrence and transfer of antibiotic resistance genes: A review. J. Hazard. Mater. 2021, 420, 126602. [Google Scholar] [CrossRef]
- Ahmed, S.F.; Mofijur, M.; Nuzhat, S.; Chowdhury, A.T.; Rafa, N.; Uddin, M.A.; Abrar, I.; Mahlia, T.; Hwai, C.; Wen, Y.C.; et al. Recent developments in physical, biological, chemical, and hybrid treatment techniques for removing emerging contaminants from wastewater. J. Hazard. Mater. 2021, 416, 125912. [Google Scholar] [CrossRef]
- Luongo, G.; Previtera, L.; Ladhari, A.; Di Fabio, G.; Zarrelli, A. Peracetic acid vs. sodium hypochlorite: Degradation and transformation of drugs in wastewater. Molecules 2020, 25, 2294. [Google Scholar] [CrossRef]
- Rathi, B.S.; Kumar, P.S.; Show, P.L. A review on effective removal of emerging contaminants from aquatic systems: Current trends and scope for further research. J. Hazard. Mater. 2021, 409, 124413. [Google Scholar] [CrossRef]
- Wu, S.; Yang, Z.; Zhou, Z.; Li, X.; Lin, Y.; Cheng, J.J.; Yang, C. Catalytic activity and reaction mechanisms of single-atom metals anchored on nitrogen-doped carbons for peroxymonosulfate activation. J. Hazard. Mater. 2023, 459, 132133. [Google Scholar] [CrossRef]
- Xie, J.; Wu, S.; Luo, C.; Zou, J.; Lin, Y.; He, S.; Yang, C. Modulating electronic structure of active sites on iron-based nanoparticles enhances peroxymonosulfate activation. Appl. Catal. B-Environ. Energy 2024, 354, 124138. [Google Scholar] [CrossRef]
- Wang, Y.; Lin, Y.; He, S.; Wu, S.; Yang, C. Singlet oxygen: Properties, generation, detection, and environmental applications. J. Hazard. Mater. 2024, 461, 132538. [Google Scholar] [CrossRef]
- Romanucci, V.; Siciliano, A.; Guida, M.; Libralato, G.; Saviano, L.; Luongo, G.; Previtera, L.; Di Fabio, G.; Zarrelli, A. Disinfection by-products and ecotoxic risk associated with hypochlorite treatment of irbesartan. Sci. Total Environ. 2020, 712, 135625. [Google Scholar] [CrossRef]
- Zarrelli, A.; DellaGreca, M.; Parolisi, A.; Iesce, M.R.; Cermola, F.; Temussi, F.; Isidori, M.; Lavorgna, M.; Passananti, M.; Previtera, L. Chemical fate and genotoxic risk associated with hypochlorite treatment of nicotine. Sci. Total Environ. 2012, 426, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Ladhari, A.; La Mura, G.; Di Marino, C.; Di Fabio, G.; Zarrelli, A. Sartans: What they are for, how they degrade, where they are found and how they transform. Sustain. Chem. Pharm. 2021, 20, 100409. [Google Scholar] [CrossRef]
- Ziylan, A.; Ince, N.H. The occurrence and fate of anti-inflammatory and analgesic pharmaceuticals in sewage and fresh water: Treatability by conventional and non-conventional processes. J. Hazard. Mater. 2011, 187, 24–36. [Google Scholar] [CrossRef] [PubMed]
- Guerra, P.; Kim, M.; Shah, A.; Alaee, M.; Smyth, S.A. Occurrence and fate of antibiotic, analgesic/anti-inflammatory, and antifungal compounds in five wastewater treatment processes. Sci. Total Environ. 2014, 473, 235–243. [Google Scholar] [CrossRef]
- Khumalo, S.M.; Makhathini, T.P.; Bwapwa, J.K.; Bakare, B.F.; Rathilal, S. The occurrence and fate of antibiotics and nonsteroidal anti-inflammatory drugs in water treatment processes: A review. J. Hazard. Mater. Adv. 2023, 10, 100330. [Google Scholar] [CrossRef]
- Izadi, P.; Izadi, P.; Salem, R.; Papry, S.A.; Magdouli, S.; Pulicharla, R.; Brar, S.K. Non-steroidal anti-inflammatory drugs in the environment: Where were we and how far we have come? Environ. Pollut. 2020, 267, 115370. [Google Scholar] [CrossRef]
- Rastogi, A.; Tiwari, M.K.; Ghangrekar, M.M. A review on environmental occurrence, toxicity and microbial degradation of Non-Steroidal Anti-Inflammatory Drugs (NSAIDs). J. Environ. Manag. 2021, 300, 113694. [Google Scholar] [CrossRef]
- Valcárcel, Y.; Alonso, S.G.; Rodríguez-Gil, J.L.; Maroto, R.R.; Gil, A.; Catalá, M. Analysis of the presence of cardiovascular and analgesic/anti-inflammatory/antipyretic pharmaceuticals in river-and drinking-water of the Madrid Region in Spain. Chemosphere 2011, 82, 1062–1071. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Xi, H.; Xu, L.; Jin, M.; Zhao, W.; Liu, H. Ecotoxicological effects, environmental fate and risks of pharmaceutical and personal care products in the water environment: A review. Sci. Total Environ. 2021, 788, 147819. [Google Scholar] [CrossRef] [PubMed]
- Samal, K.; Mahapatra, S.; Ali, M.H. Pharmaceutical wastewater as Emerging Contaminants (EC): Treatment technologies, impact on environment and human health. Energy Nexus 2022, 6, 100076. [Google Scholar] [CrossRef]
- Chaturvedi, P.; Shukla, P.; Giri, B.S.; Chowdhary, P.; Chandra, R.; Gupta, P.; Pandey, A. Prevalence and hazardous impact of pharmaceutical and personal care products and antibiotics in environment: A review on emerging contaminants. Environ. Res. 2021, 194, 110664. [Google Scholar] [CrossRef]
- Tran, N.H.; Urase, T.; Ta, T.T. A preliminary study on the occurrence of pharmaceutically active compounds in hospital wastewater and surface water in Hanoi, Vietnam. CLEAN–Soil Air Water 2014, 42, 267–275. [Google Scholar] [CrossRef]
- Kalgutkar, A.S.; Crews, B.C.; Saleh, S.; Prudhomme, D.; Marnett, L.J. Indolyl esters and amides related to indomethacin are selective COX-2 inhibitors. Bioorg. Med. Chem. 2005, 13, 6810–6822. [Google Scholar] [CrossRef]
- Shen, T.Y.; Winter, C.A. Chemical and biological studies on indomethacin, sulindac and their analogs. Adv. Drug Res. 1977, 12, 89–245. [Google Scholar]
- Available online: https://clincalc.com/DrugStats/Drugs/Indomethacin (accessed on 20 January 2025).
- Available online: https://www.ceicdata.com/en/china/pharmaceutical-production-ytd-antipyretics-and-analgesics/cn-production-ytd-indomethacin (accessed on 20 January 2025).
- Nalamachu, S.; Wortmann, R. Role of indomethacin in acute pain and inflammation management: A review of the literature. Postgrad. Med. 2014, 126, 92–97. [Google Scholar] [CrossRef]
- Munjal, A.; Allam, A.E. Indomethacin [Updated 2024 May 28]. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK555936/ (accessed on 20 January 2025).
- Gliszczyńska, A.; Nowaczyk, M. Lipid formulations and bioconjugation strategies for indomethacin therapeutic advances. Molecules 2021, 26, 1576. [Google Scholar] [CrossRef]
- Hua, W.Y.; Bennett, E.R.; Maio, X.S.; Metcalfe, C.D.; Letcher, R.J. Seasonality effects on pharmaceuticals and s-triazine herbicides in wastewater effluent and surface water from the Canadian side of the upper Detroit River. Environ. Toxicol. Chem. 2006, 25, 2356–2365. [Google Scholar] [CrossRef]
- Quintana, J.B.; Reemtsma, T. Sensitive determination of acidic drugs and triclosan in surface and wastewater by ion-pair reverse-phase liquid chromatography/tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2004, 18, 765–774. [Google Scholar] [CrossRef] [PubMed]
- Ternes, T.A. Analytical methods for the determination of pharmaceuticals in aqueous environmental samples. TrAC Trends Anal. Chem. 2001, 20, 419–434. [Google Scholar] [CrossRef]
- Huang, J.; Li, D.; Liu, Y.; Li, R.; Chen, P.; Liu, H.; Lv, W.; Liu, G.; Feng, Y. Ultrathin Ag2WO4-coated P-doped g-C3N4 nanosheets with remarkable photocatalytic performance for indomethacin degradation. J. Hazard. Mater. 2020, 392, 122355. [Google Scholar] [CrossRef]
- Coppola, F.; Cimino, P.; Petrone, A.; Rega, N. Evidence of excited-state vibrational mode governing the photorelaxation of a charge-transfer complex. J. Phys. Chem. A 2024, 128, 1620–1633. [Google Scholar] [CrossRef]
- Hussain, M.; Zhao, J.; Coppola, F.; El-Zohry, A.M.; Pastore, M. Key role of electronic and structural properties in regulating intersystem crossing: An in-depth investigation on naphthalene-diimide triads for thermally activated delayed fluorescence applications. J. Phys. Chem. A 2024, 128, 11998–12009. [Google Scholar]
- Li, R.; Kong, J.; Liu, H.; Chen, P.; Liu, G.; Li, F.; Lv, W. A sulfate radical based ferrous–peroxydisulfate oxidative system for indomethacin degradation in aqueous solutions. RSC Adv. 2017, 7, 22802–22809. [Google Scholar] [CrossRef]
- Li, R.; Kong, J.; Liu, H.; Chen, P.; Su, Y.; Liu, G.; Lv, W. Removal of indomethacin using UV–vis/peroxydisulfate: Kinetics, toxicity, and transformation pathways. Chem. Eng. J. 2018, 331, 809–817. [Google Scholar] [CrossRef]
- Boltze, K.H.; Brendler, O.; Jacobi, H.; Opitz, W.; Raddatz, S.; Seidel, P.R.; Vollbrecht, D. Chemical structure and anti-inflammatory activity in the group of substituted indole-3-acetic acids. Arzneimittelforschung 1980, 30, 1314–1325. [Google Scholar] [PubMed]
- Weedon, A.C.; Wong, D.F. The photochemistry of indomethacin. J. Photochem. Photobiol. 1991, 61, 27–33. [Google Scholar] [CrossRef]
- Temussi, F.; Cermola, F.; DellaGreca, M.; Iesce, M.R.; Passananti, M.; Previtera, L.; Zarrelli, A. Determination of photostability and photodegradation products of indomethacin in aqueous media. J. Pharm. Biomed. Anal. 2011, 56, 678–683. [Google Scholar] [CrossRef]
- Luo, Y.; Feng, L.; Liu, Y.; Zhang, L. Disinfection by-products formation and acute toxicity variation of hospital wastewater under different disinfection processes. Sep. Purif. Technol. 2020, 238, 116405. [Google Scholar] [CrossRef]
- Srivastav, A.L.; Patel, N.; Chaudhary, V.K. Disinfection by-products in drinking water: Occurrence, toxicity and abatement. Environ. Pollut. 2020, 267, 115474. [Google Scholar] [CrossRef] [PubMed]
- Pandian, A.M.K.; Rajamehala, M.; Singh, M.V.P.; Sarojini, G.; Rajamohan, N. Potential risks and approaches to reduce the toxicity of disinfection by-product–A review. Sci. Total Environ. 2022, 822, 153323. [Google Scholar] [CrossRef]
- Erdem, C.U.; Liu, C.; Karanfil, T. Photodegradation of halogenated organic disinfection by-products: Decomposition and reformation. Water Res. 2023, 245, 120565. [Google Scholar] [CrossRef]
- Richardson, S.D.; Plewa, M.J.; Wagner, E.D.; Schoeny, R.; DeMarini, D.M. Occurrence, genotoxicity, and carcinogenicity of regulated and emerging disinfection by-products in drinking water: A review and roadmap for research. Mutat. Res.-Rev. Mutat. 2007, 636, 178–242. [Google Scholar] [CrossRef]
- Plewa, M.J.; Wagner, E.D.; Richardson, S.D.; Thruston, A.D.; Woo, Y.T.; McKague, A.B. Chemical and biological characterization of newly discovered iodoacid drinking water disinfection byproducts. Environ. Sci. Technol. 2004, 38, 4713–4722. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, P.J.; Siraki, A.G.; Shangari, N. Aldehyde sources, metabolism, molecular toxicity mechanisms, and possible effects on human health. Crit. Rev. Chem. 2005, 35, 609–662. [Google Scholar] [CrossRef]
- Ali, K.F.; Albakaa, A.R.M.; Ali, Z.H. New assay method UV spectroscopy for determination of Indomethacin in pharmaceutical formulation. J. Chem. Pharm. Res. 2015, 7, 1591–1596. [Google Scholar]



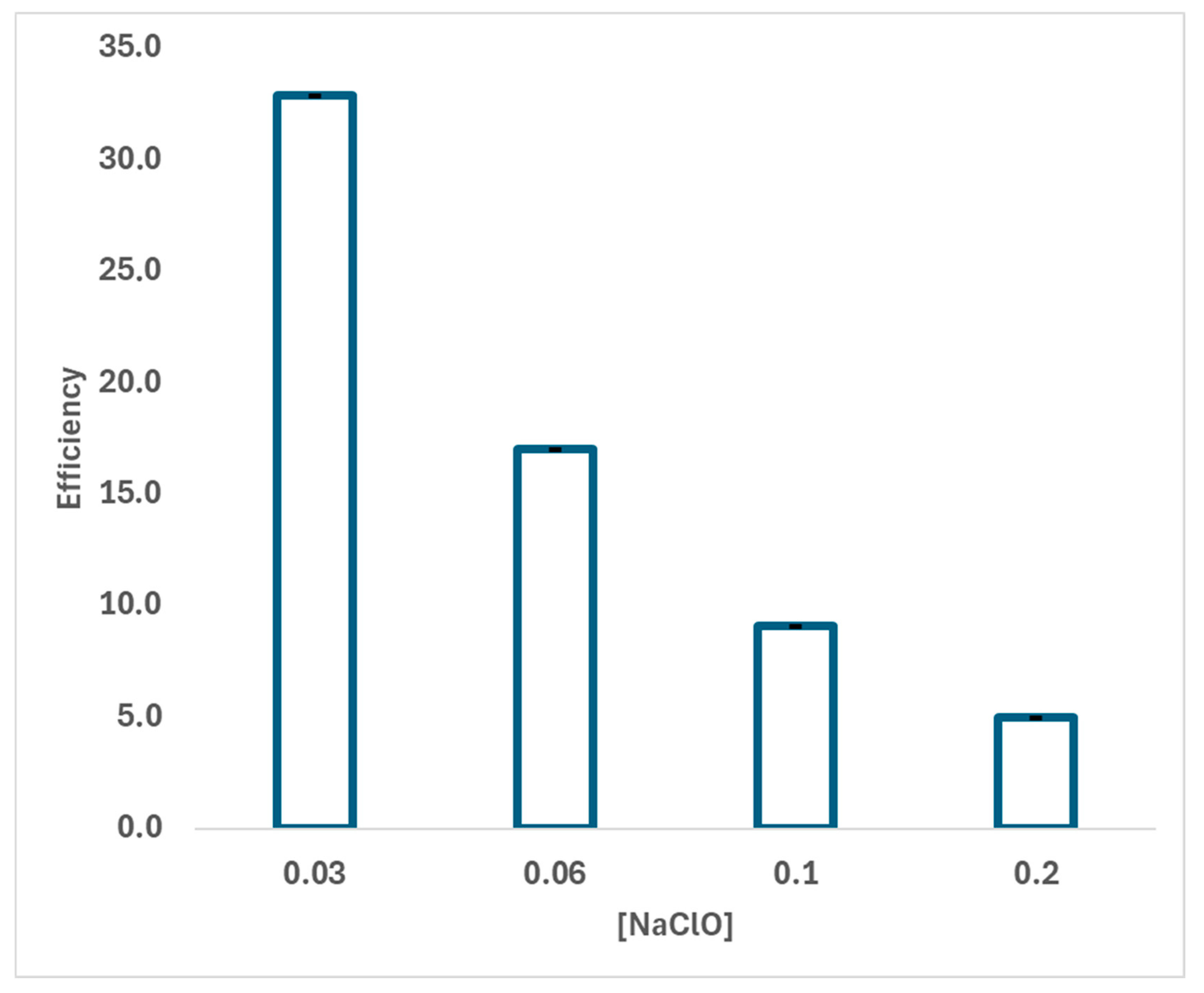
| Initial Concentration (mM) | Final Concentration (mM) | NaClO Concentration (M) | Efficiency (M−1) |
|---|---|---|---|
| 3.00 | 1.50 ± 0.22 ∙ 10−3 | 0.06 | 16.6583 ± 0.0013 |
| 3.00 | 1.61 ± 0.24 ∙ 10−3 | 0.03 | 33.3155 ± 0.0026 |
| 3.00 | 1.91 ± 0.29 ∙ 10−3 | 0.1 | 9.9936 ± 0.0010 |
| 3.00 | 1.11 ± 0.17 ∙ 10−3 | 0.2 | 4.9981 ± 0.0003 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Medici, A.; Luongo, G.; Previtera, L.; Naviglio, D.; Di Fabio, G.; Zarrelli, A. Degradation of Indomethacin in Wastewater: Removal with Sodium Hypochlorite and Analysis of Degradation Byproducts. Molecules 2025, 30, 2180. https://doi.org/10.3390/molecules30102180
Medici A, Luongo G, Previtera L, Naviglio D, Di Fabio G, Zarrelli A. Degradation of Indomethacin in Wastewater: Removal with Sodium Hypochlorite and Analysis of Degradation Byproducts. Molecules. 2025; 30(10):2180. https://doi.org/10.3390/molecules30102180
Chicago/Turabian StyleMedici, Antonio, Giovanni Luongo, Lucio Previtera, Daniele Naviglio, Giovanni Di Fabio, and Armando Zarrelli. 2025. "Degradation of Indomethacin in Wastewater: Removal with Sodium Hypochlorite and Analysis of Degradation Byproducts" Molecules 30, no. 10: 2180. https://doi.org/10.3390/molecules30102180
APA StyleMedici, A., Luongo, G., Previtera, L., Naviglio, D., Di Fabio, G., & Zarrelli, A. (2025). Degradation of Indomethacin in Wastewater: Removal with Sodium Hypochlorite and Analysis of Degradation Byproducts. Molecules, 30(10), 2180. https://doi.org/10.3390/molecules30102180








