Tetracycline Alleviates Cadmium Toxicity in Rice Seedlings by Altering Pollutant Accumulation, Nutrient Absorption, Osmoregulation and Antioxidant Metabolism
Abstract
1. Introduction
2. Results
2.1. Growth Indexes
2.2. Cd Content
2.3. Photosynthetic Pigments
2.4. Mineral Nutrients
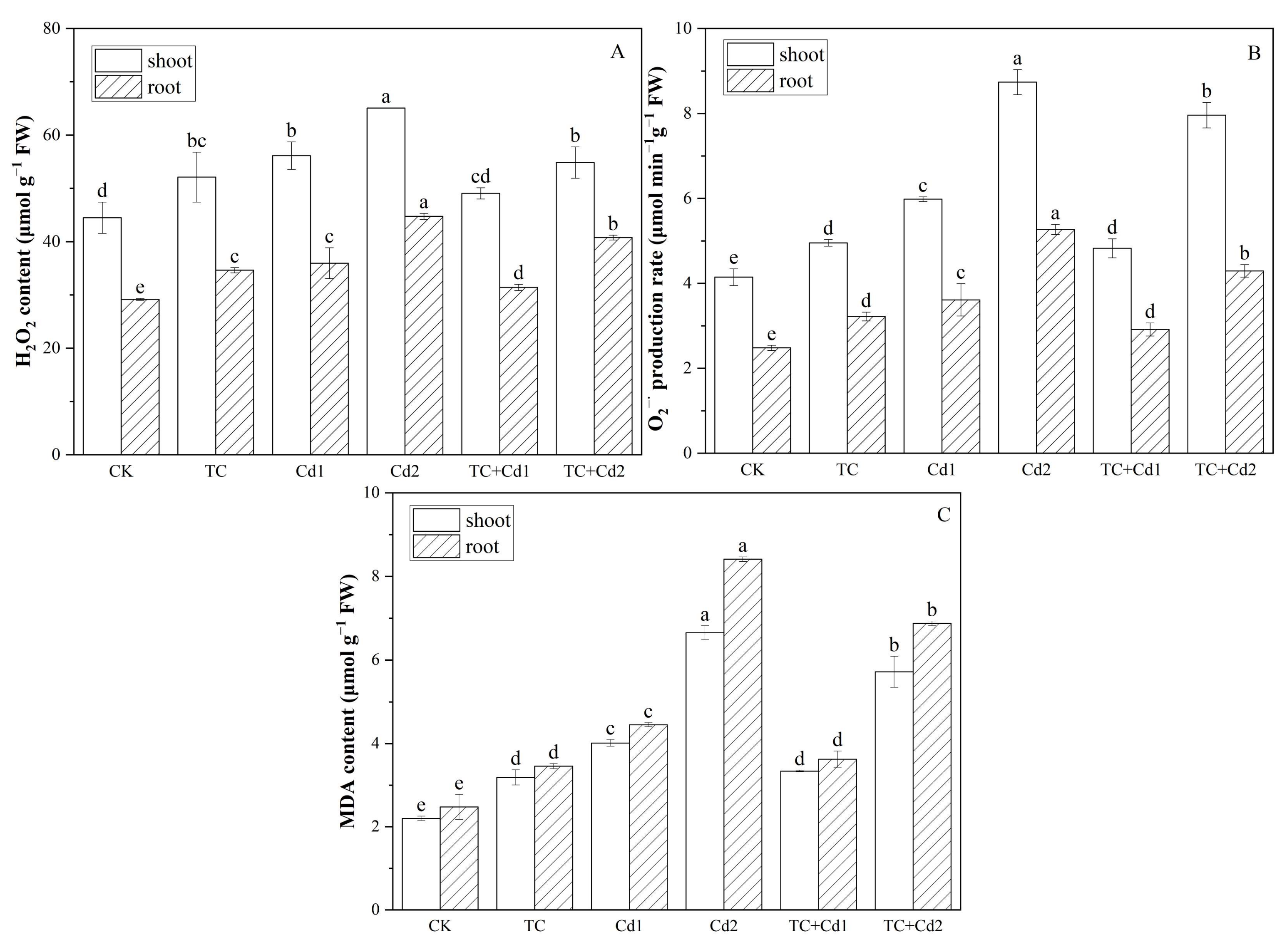
2.5. ROS
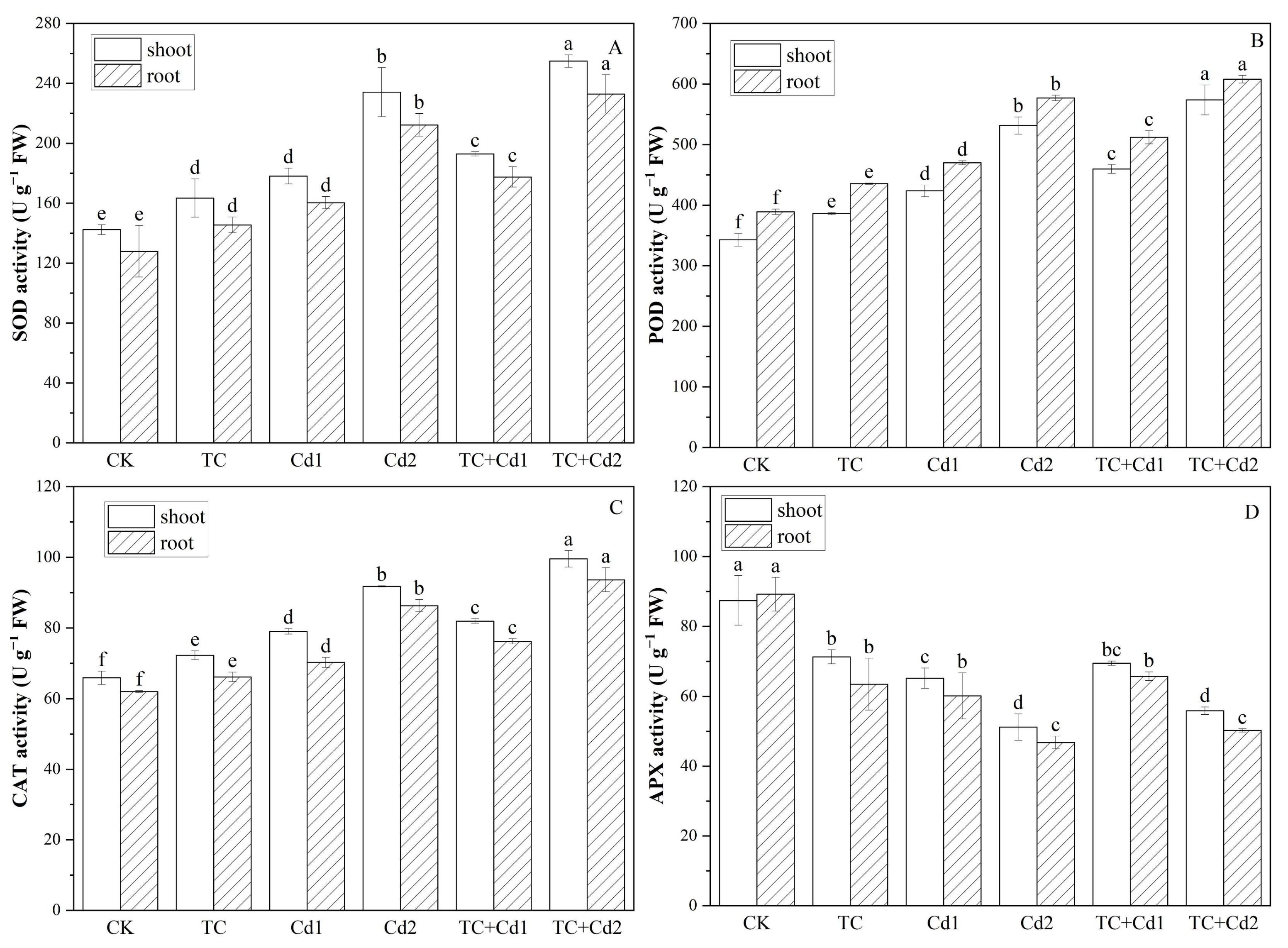
2.6. Antioxidant Enzymes
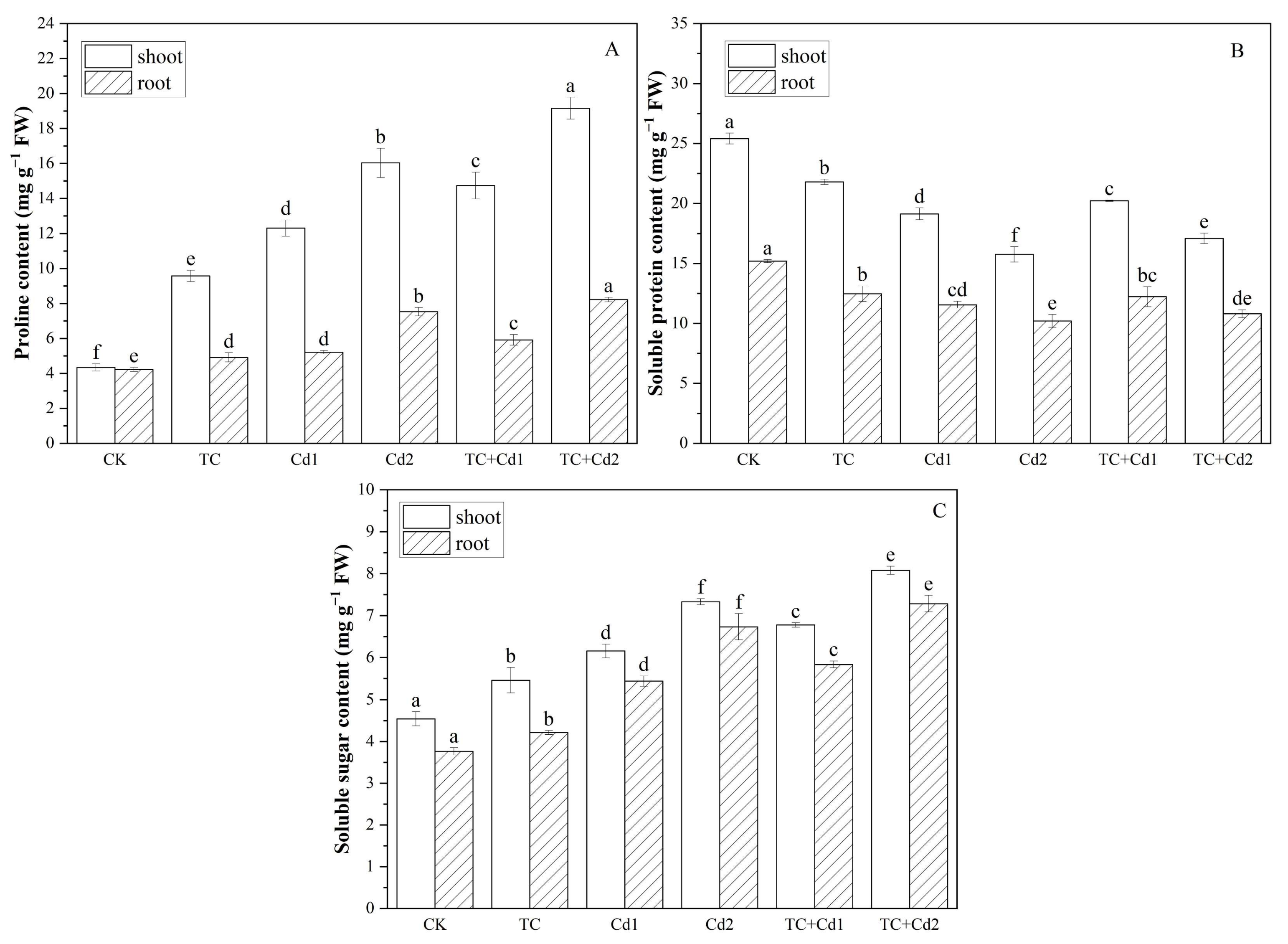
2.7. Osmotic Regulatory Substances
2.8. Secondary Metabolism
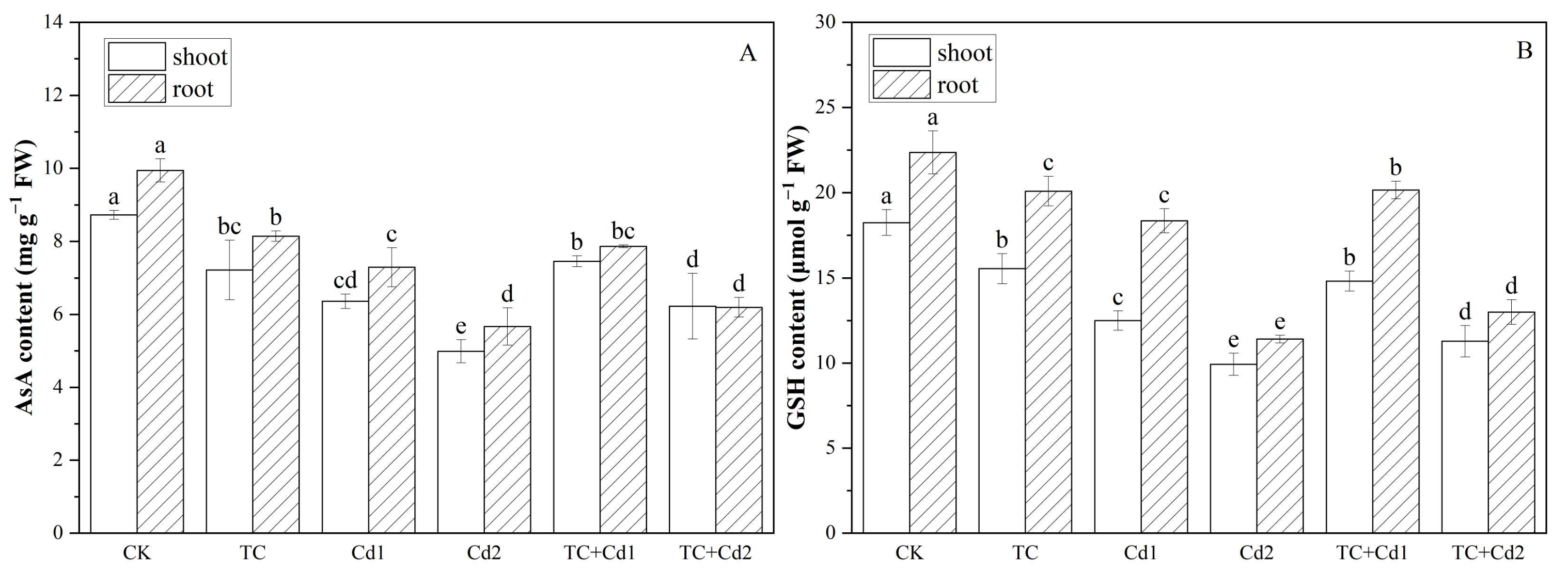
2.9. AsA and GSH
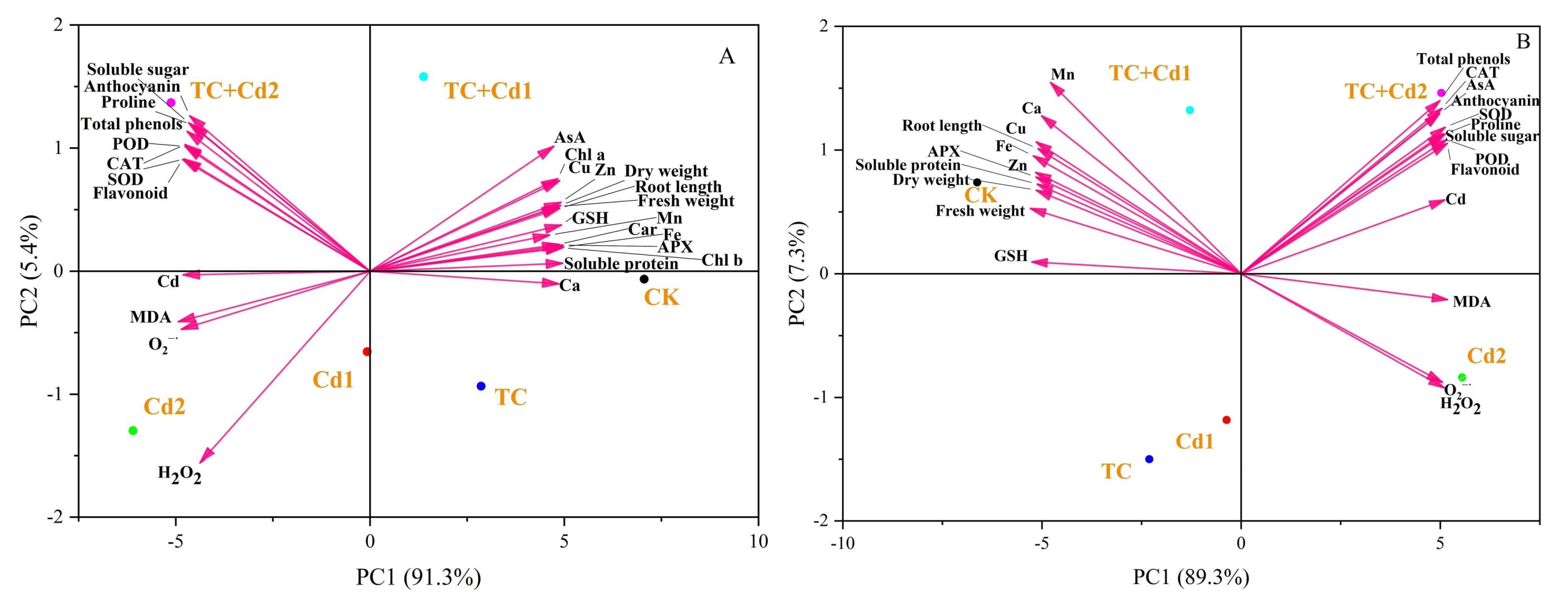
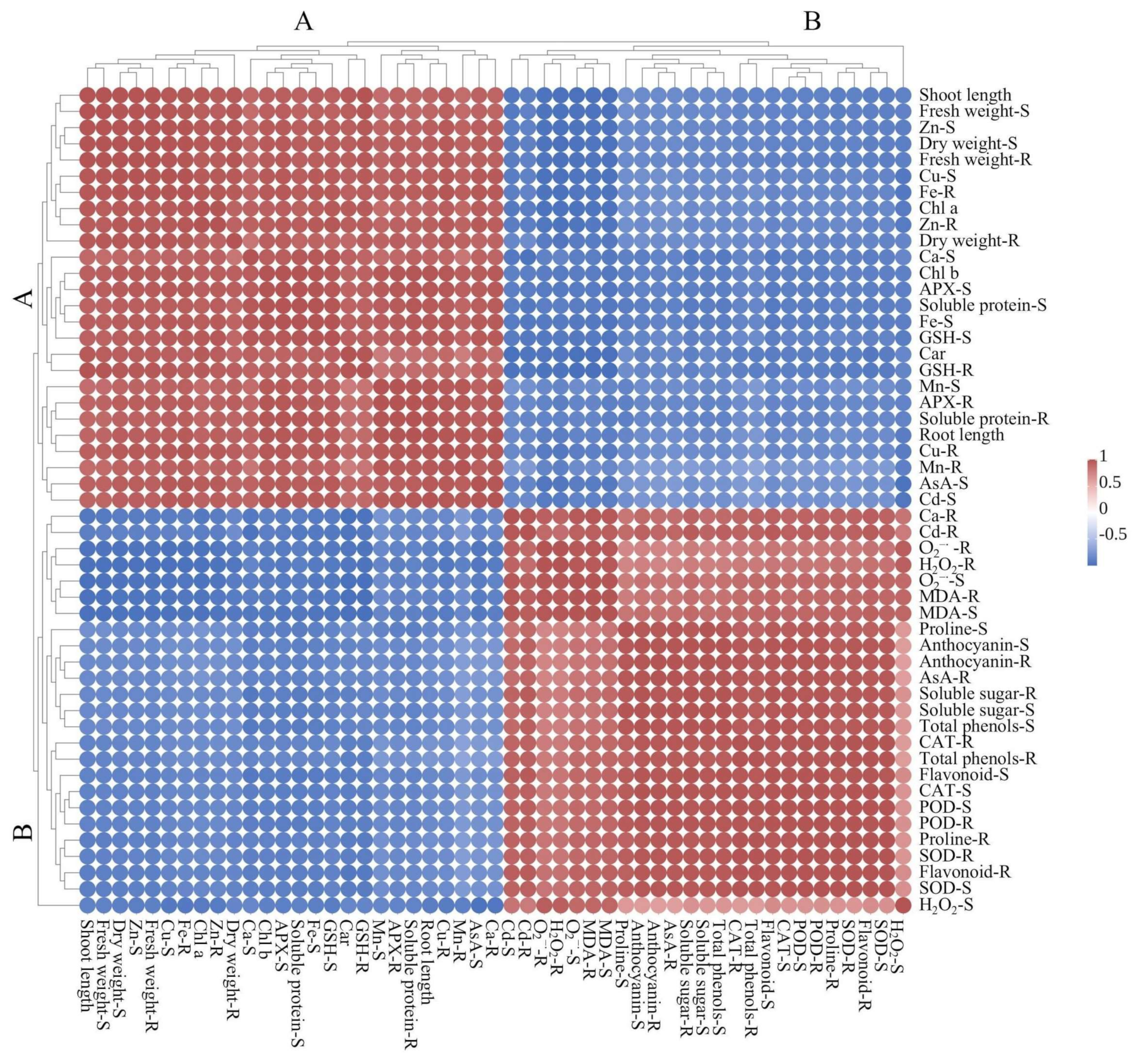
2.10. Principal Component Analysis (PCA) and Heat Map
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Reagents
4.2. Plant Culture and Treatments
4.3. Determination of Rice Growth Indexes
4.4. Determination of the Contents of Cd, Nutrient Elements, and Transport Factor
4.5. Photosynthetic Pigments
4.6. Reactive Oxygen Species (ROS), Malondialdehyde (MDA) Contents, and Histochemical Detection
4.7. Antioxidant Enzyme Activity
4.8. Determination of Osmolytes
4.9. Determination of Secondary Metabolites
4.10. Determination of Ascorbic Acid (AsA) and Glutathione (GSH)
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gu, J.; Hu, C.; Jia, X.; Ren, Y.; Su, D.; He, J. Physiological and biochemical bases of spermidine-induced alleviation of cadmium and lead combined stress in rice. Plant Physiol. Bioch. 2022, 189, 104–114. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Yang, X.; Chi, H.; Liu, X.; Lu, N.; Liu, Y.; Yang, S.; Wen, X. Transport, pollution, and health risk of heavy metals in “soil-medicinal and edible plant-human” system: A case study of farmland around the Beiya mining area in Yunnan, China. Microchem. J. 2024, 207, 111958. [Google Scholar] [CrossRef]
- Xu, H.; Zhou, Q.; Yan, T.; Jia, X.; Lu, D.; Ren, Y.; He, J. Enhanced removal efficiency of Cd2+ and Pb2+ from aqueous solution by H3PO4–modified tea branch biochar: Characterization, adsorption performance and mechanism. J. Environ. Chem. Eng. 2024, 12, 112183. [Google Scholar] [CrossRef]
- Wu, J.; Wang, J.Y.; Li, Z.T.; Guo, S.M.; Li, K.J.; Xu, P.S.; Ok, Y.S.; Jones, D.L.; Zou, J.W. Antibiotics and antibiotic resistance genes in agricultural soils: A systematic analysis. Crit. Rev. Environ. Sci. Technol. 2023, 53, 847–864. [Google Scholar] [CrossRef]
- Lu, J.; Wu, J.; Zhang, C.; Zhang, Y.; Lin, Y.; Luo, Y. Occurrence, distribution, and ecological-health risks of selected antibiotics in coastal waters along the coastline of China. Sci. Total Environ. 2018, 644, 1469–1476. [Google Scholar] [CrossRef]
- Li, C.; Li, A.; Hui, X.; Wang, A.; Wang, L.; Chang, S. Concentrations, probabilistic human and ecological risks assessment attribute to antibiotics residues in river water in China: Systematic review and meta-analysis. Ecotox. Environ. Saf. 2024, 285, 117022. [Google Scholar] [CrossRef]
- Wen, X.; Xu, J.; Wang, Y.; Yang, X.; Peng, G.; Li, S.; Ma, B.; Zou, Y.; Liao, X.; Wang, Y.; et al. Community coalescence and plant host filtering determine the spread of tetracycline resistance genes from pig manure into the microbiome continuum of the soil–plant system. Microbiol. Res. 2024, 284, 127734. [Google Scholar] [CrossRef]
- Cui, E.; Zhou, Z.; Cui, B.; Fan, X.; Ali Abid, A.; Chen, T.; Gao, F.; Du, Z. Effects of nitrogen fertilization on the fate of high-risk antibiotic resistance genes in reclaimed water-irrigated soil and plants. Environ. Int. 2024, 190, 108834. [Google Scholar] [CrossRef]
- Guo, X.; Zhu, L.; Zhong, H.; Li, P.; Zhang, C.; Wei, D. Response of antibiotic and heavy metal resistance genes to tetracyclines and copper in substrate-free hydroponic microcosms with Myriophyllum aquaticum. J. Hazad. Mater. 2021, 413, 125444. [Google Scholar] [CrossRef]
- Shu, Y.; Li, D.; Xie, T.; Zhao, K.; Zhou, L.; Li, F. Antibiotics-heavy metals combined pollution in agricultural soils: Sources, fate, risks, and countermeasures. Green Energy Environ. 2024, in press. [Google Scholar] [CrossRef]
- Zhang, L.; Ren, Y.; Xue, Y.; Cui, Z.; Wei, Q.; Han, C.; He, J. Preparation of biochar by mango peel and its adsorption characteristics of Cd(Ⅱ) in solution. RSC Adv. 2020, 10, 35878–35888. [Google Scholar] [CrossRef] [PubMed]
- Su, D.; Wang, M.; Xu, H.; Zhang, Y.; Hu, Q.; Ren, Y.; He, J. Performance and mechanism of Ficus carica branch waste based biochar in removing Cd2+ from aqueous solution. Biomass Convers. Bior. 2023, 14, 24137–24150. [Google Scholar] [CrossRef]
- Tan, Y.; Liu, X.; Shen, Z.; Xiao, Y.; Zhang, Y.; Du, H.; Wu, Z.; Zhi, D.; Núñez-Delgado, A.; Yang, Y. Effects of seed priming with different concentrations and forms of silicon on germination and growth of rice under cadmium stress. Appl. Soil Ecol. 2025, 207, 105947. [Google Scholar] [CrossRef]
- Saqib, M.; Shahzad, U.; Zulfiqar, F.; Tiwari, R.K.; Lal, M.K.; Naz, S.; Jahan, M.S.; Awan, Z.A.; El-Sheikh, M.A.; Altaf, M.A. Exogenous melatonin alleviates cadmium-induced inhibition of growth and photosynthesis through upregulating antioxidant defense system in strawberry. S. Afr. J. Bot. 2023, 157, 10–18. [Google Scholar] [CrossRef]
- Jamil Abbasi, A.; Anas, M.; Elahi, M.; Khan, A.; Khattak, W.A.; Saleem, M.H.; Fahad, S.; Elansary, H.O.; Mahmoud, E.A.; Ahmed, T.; et al. Restoring wheat productivity and nutrient balance under cadmium stress through reducing toxicity, metal uptake, and oxidative damage using selenium nanoparticles. J. Trace Elem. Med. Biol. 2025, 89, 127644. [Google Scholar] [CrossRef]
- Omuferen, L.O.; Maseko, B.; Olowoyo, J.O. Occurrence of antibiotics in wastewater from hospital and convectional wastewater treatment plants and their impact on the effluent receiving rivers: Current knowledge between 2010 and 2019. Environ. Monit. Assess. 2022, 194, 306. [Google Scholar] [CrossRef]
- Han, T.; Wang, B.; Wu, Z.; Dai, C.; Zhao, J.; Mi, Z.; Lv, Y.; Zhang, C.; Miao, X.; Zhou, J.; et al. Providing a view for toxicity mechanism of tetracycline by analysis of the connections between metabolites and biologic endpoints of wheat. Ecotox. Environ. Saf. 2021, 212, 111998. [Google Scholar] [CrossRef]
- Minden, V.; Deloy, A.; Volkert, A.M.; Leonhardt, S.D.; Pufal, G. Antibiotics impact plant traits, even at small concentrations. AoB Plants 2017, 9, plx010. [Google Scholar] [CrossRef]
- Li, J.; Zheng, X.; Liu, K.; Sun, S.; Li, X. Effect of tetracycline on the growth and nutrient removal capacity of Chlamydomonas reinhardtii in simulated effluent from wastewater treatment plants. Bioresour. Technol. 2016, 218, 1163–1169. [Google Scholar] [CrossRef]
- Yagoubi, A.; Mahjoubi, Y.; Giannakis, S.; Rzigui, T.; Djebali, W.; Chouari, R. The silver lining of antibiotic resistance: Bacterial-mediated reduction of tetracycline plant stress via antibiotrophy. Plant Physiol. Biochem. 2023, 204, 108093. [Google Scholar] [CrossRef]
- Nie, J.; Wang, Q.-M.; Han, L.-J.; Li, J.-S. Synergistic remediation strategies for soil contaminated with compound heavy metals and organic pollutants. J. Environ. Chem. Eng. 2024, 12, 113145. [Google Scholar] [CrossRef]
- Li, Y.; Tang, H.; Hu, Y.; Wang, X.; Ai, X.; Tang, L.; Matthew, C.; Cavanagh, J.; Qiu, J. Enrofloxacin at environmentally relevant concentrations enhances uptake and toxicity of cadmium in the earthworm Eisenia fetida in farm soils. J. Hazard. Mater. 2016, 308, 312–320. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Ma, Q.; Li, S.; Zhu, M.; Xia, Z.; Yu, W. Toxicological effects of single and joint sulfamethazine and cadmium stress in soil on pakchoi (Brassica chinensis L.). Chemosphere 2021, 263, 128296. [Google Scholar] [CrossRef] [PubMed]
- Nafees, M.; Sehrish, A.K.; Alomrani, S.O.; Qiu, L.; Saeed, A.; Ahmad, S.; Ali, S.; Guo, H. Mechanism and synergistic effect of sulfadiazine (SDZ) and cadmium toxicity in spinach (Spinacia oleracea L.) and its alleviation through zinc fortification. J. Hazard. Mater. 2024, 464, 132903. [Google Scholar] [CrossRef]
- Ren, X.; Wang, H.; Wang, W.; Zhang, W.; Li, Q. Co-exposure to organophosphate esters (OPEs) and Cadmium alleviated their accumulation in rice (Oryza sativa L.) by inhibiting transports’ transcription. S. Afr. J. Bot. 2024, 173, 137–146. [Google Scholar] [CrossRef]
- He, C.; Zhou, J.; Yang, C.; Song, Z.; He, J.; Huang, Z.; Deng, Y.; Wang, J.; Xiong, Y.; Dang, Z. Accumulation, transportation, and distribution of tetracycline and cadmium in rice. J. Environ. Sci. 2023, 126, 58–69. [Google Scholar] [CrossRef]
- Rahman, S.U.; Han, J.-C.; Ahmad, M.; Gao, S.; Khan, K.A.; Li, B.; Zhou, Y.; Zhao, X.; Huang, Y. Toxic effects of lead (Pb), cadmium (Cd) and tetracycline (TC) on the growth and development of Triticum aestivum: A meta-analysis. Sci. Total Environ. 2023, 904, 166677. [Google Scholar] [CrossRef]
- Guo, X.; Liu, M.; Zhong, H.; Li, P.; Zhang, C.; Wei, D.; Zhao, T. Potential of Myriophyllum aquaticum for phytoremediation of water contaminated with tetracycline antibiotics and copper. J. Environ. Manag. 2020, 270, 110867. [Google Scholar] [CrossRef]
- Feng, Y.; Xu, S.; Xu, J.; Li, X.; Jiang, J.; Wu, C.; Chen, Y. Arsenic behavior in soil-plant system under the manure application with the combination of antibiotic and roxarsone. Sci. Total Environ. 2024, 946, 174274. [Google Scholar] [CrossRef]
- Ji, Y.; Ren, Y.; Han, C.; Zhu, W.; Gu, J.; He, J. Application of exogenous glycinebetaine alleviates lead toxicity in pakchoi (Brassica chinensis L.) by promoting antioxidant enzymes and suppressing Pb accumulation. Environ. Sci. Pollut. Res. 2022, 29, 25568–25580. [Google Scholar] [CrossRef]
- Wang, M.; Chen, Z.; Chen, D.; Liu, L.; Hamid, Y.; Zhang, S.; Shan, A.; Kang, K.J.; Feng, Y.; Yang, X. Combined cadmium and fluorine inhibit lettuce growth through reducing root elongation, photosynthesis, and nutrient absorption. Environ. Sci. Pollut. Res. 2022, 29, 91255–91267. [Google Scholar] [CrossRef] [PubMed]
- Minden, V.; Schnetger, B.; Pufal, G.; Leonhardt, S.D. Antibiotic-induced effects on scaling relationships and on plant element contents in herbs and grasses. Ecol. Evol. 2018, 8, 6699–6713. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Du, X.; Zhou, R.; Lian, J.; Guo, X.; Tang, Z. Effect of cadmium and polystyrene nanoplastics on the growth, antioxidant content, ionome, and metabolism of dandelion seedlings. Environ. Pollut. 2024, 354, 124188. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Wang, W.; He, J.; Zhang, L.; Wei, Y.; Yang, M. Nitric oxide alleviates salt stress in seed germination and early seedling growth of pakchoi (Brassica chinensis L.) by enhancing physiological and biochemical parameters. Ecotox. Environ. Saf. 2020, 187, 109785. [Google Scholar] [CrossRef]
- Zong, X.; Zhang, J.; Zhu, J.; Zhang, L.; Jiang, L.; Yin, Y.; Guo, H. Effects of polystyrene microplastic on uptake and toxicity of copper and cadmium in hydroponic wheat seedlings (Triticum aestivum L.). Ecotox. Environ. Saf. 2021, 217, 112217. [Google Scholar] [CrossRef]
- Wang, W.; Ren, Y.; He, J.; Zhang, L.; Wang, X.; Cui, Z. Impact of copper oxide nanoparticles on the germination, seedling growth, and physiological responses in Brassica pekinensis L. Environ. Sci. Pollut. Res. 2020, 27, 31505–31515. [Google Scholar] [CrossRef]
- Ren, Y.; Xue, Y.; Tian, D.; Zhang, L.; Xiao, G.; He, J. Improvement of postharvest anthracnose resistance in mango fruit by nitric oxide and the possible mechanisms involved. J. Agric. Food Chem. 2020, 68, 15460–15467. [Google Scholar] [CrossRef]
- Qiao, Z.; Luo, K.; Zhou, S.; Fu, M.; Shao, X.; Gong, K.; Peng, C.; Zhang, W. Response mechanism of lettuce (Lactuca sativa L.) under combined stress of Cd and DBDPE: An integrated physiological and metabolomics analysis. Sci. Total Environ. 2023, 887, 164204. [Google Scholar] [CrossRef]
- Ben, Y.; Cheng, M.; Liu, Y.; Wang, X.; Wang, L.; Yang, Q.; Huang, X.; Zhou, Q. Biomarker changes and oxidative damage in living plant cells as new biomonitoring indicators for combined heavy metal stress assessment. Ecol. Indic. 2023, 154, 110784. [Google Scholar] [CrossRef]
- Wang, W.; Liu, J.; Ren, Y.; Zhang, L.; Xue, Y.; Zhang, L.; He, J. Phytotoxicity assessment of copper oxide nanoparticles on the germination, early seedling growth, and physiological responses in Oryza sativa L. Bull. Environ. Contam. Toxicol. 2020, 104, 770–777. [Google Scholar] [CrossRef]
- Abuelsoud, W.; Madany, M.M.Y.; Sheteiwy, M.S.; Korany, S.M.; Alsharef, E.; AbdElgawad, H. Alleviation of gadolinium stress on Medicago by elevated atmospheric CO2 is mediated by changes in carbohydrates, anthocyanin, and proline metabolism. Plant Physiol. Biochem. 2023, 202, 107925. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, X.; Sun, H.; Li, Y.; Ma, L.; Li, Y.; Wang, L.; Zhang, L.; Li, X. Integrated transcriptomics and metabolomics reveal the role of soluble sugars and GABA in rice leaf response to Pb stress. Plant Physiol. Biochem. 2025, 221, 109595. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wang, S.; Zhao, C.; Jiang, X.; Gao, D. Responses of non-structural carbohydrates and biomass in plant to heavy metal treatment. Sci. Total Environ. 2024, 909, 168559. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Ren, Y.; Xue, Y.; Lu, D.; Yan, T.; He, J. Putrescine (1,4-Diaminobutane) enhances antifungal activity in postharvest mango fruit against Colletotrichum gloeosporioides through direct fungicidal and induced resistance mechanisms. Pestic. Biochem. Phys. 2023, 195, 105581. [Google Scholar] [CrossRef]
- Wu, C.; Men, C.; Yan, L.; Zhang, J.; Wang, Y.; Chen, M.; Liu, C.; Zheng, L. The ERF transcription factor SlERF7 promotes UV-C-induced biosynthesis of phenolic compounds in tomato. Sci. Hortic. 2024, 338, 113643. [Google Scholar] [CrossRef]
- Muhammad, A.; Khan, M.H.U.; Kong, X.; Zheng, S.; Bai, N.; Li, L.; Zhang, N.; Muhammad, S.; Li, Z.; Zhang, X.; et al. Rhizospheric crosstalk: A mechanistic overview of how plant secondary metabolites alleviate abiotic stresses. Plant Sci. 2025, 354, 112431. [Google Scholar] [CrossRef]
- Kang, Y.; Lin, Y.-J.; Abid, U.; Zhang, F.-F.; Yu, X.-Z. Trivalent chromium stress trigger accumulation of secondary metabolites in rice plants: Integration of biochemical and transcriptomic analysis. Environ. Technol. Innov. 2024, 36, 103802. [Google Scholar] [CrossRef]
- Sharma, D.; Shree, B.; Kumar, S.; Kumar, V.; Sharma, S.; Sharma, S. Stress induced production of plant secondary metabolites in vegetables: Functional approach for designing next generation super foods. Plant Physiol. Biochem. 2022, 192, 252–272. [Google Scholar] [CrossRef]
- Jia, X.; He, J.; Yan, T.; Lu, D.; Xu, H.; Li, K.; Ren, Y. Copper oxide nanoparticles mitigate cadmium toxicity in rice seedlings through multiple physiological mechanisms. Environ. Sci. Pollut. Res. 2024, 31, 49026–49039. [Google Scholar] [CrossRef]
- Eichberg, C.; Leiß, A.; Stothut, M.; Bernheine, J.; Jurczyk, K.; Paulus, L.; Thiele-Bruhn, S.; Thomas, F.M.; Donath, T.W. Tetracycline but not sulfamethazine inhibits early root growth of wild grassland species, while seed germination is hardly affected by either antibiotic. Environ. Pollut. 2024, 363, 125178. [Google Scholar] [CrossRef]
- Hu, C.; Zhu, W.; Lu, Y.; Ren, Y.; Gu, J.; Song, Y.; He, J. Correction to: Alpinia officinarum mediated copper oxide nanoparticles: Synthesis and its antifungal activity against Colletotrichum gloeosporioides. Environ. Sci. Pollut. Res. 2023, 30, 28830. [Google Scholar] [CrossRef]
- Kaya, C.; Ugurlar, F.; Ashraf, M.; Alam, P.; Ahmad, P. Nitric oxide and hydrogen sulfide work together to improve tolerance to salinity stress in wheat plants by upraising the AsA-GSH cycle. Plant Physiol. Biochem. 2023, 194, 651–663. [Google Scholar] [CrossRef]


| Treatments | Plant Height (cm) | Root Length (cm) | Fresh Weight (mg Plant−1) | Dry Weight (mg Plant−1) | ||
|---|---|---|---|---|---|---|
| Shoot | Root | Shoot | Root | |||
| CK | 54.25 ± 2.20 a | 23.54 ± 0.79 a | 1128.46 ± 92.30 a | 1170.53 ± 60.46 a | 172.93 ± 10.54 a | 78.75 ± 4.71 a |
| TC | 45.89 ± 1.23 c | 18.52 ± 0.12 b | 927.81 ± 20.69 c | 943.57 ± 35.94 c | 136.84 ± 1.80 c | 52.70 ± 1.51 c |
| Cd1 | 44.05 ± 3.55 c | 17.18 ± 0.48 c | 925.52 ± 9.99 c | 929.71 ± 15.44 c | 130.51 ± 2.25 c | 59.00 ± 1.28 d |
| Cd2 | 28.81 ± 0.68 e | 14.41 ± 0.34 e | 588.12 ± 21.51 e | 639.45 ± 9.33 e | 87.29 ± 1.99 e | 32.99 ± 0.70 f |
| TC + Cd1 | 49.02 ± 0.59 b | 18.82 ± 0.52 b | 1009.04 ± 18.03 b | 1016.42 ± 2.63 b | 149.40 ± 3.05 b | 64.06 ± 3.07 b |
| TC + Cd2 | 32.37 ± 1.08 d | 16.07 ± 0.61 d | 666.36 ± 5.98 d | 703.06 ± 1.40 d | 97.15 ± 3.22 d | 36.98 ± 0.67 e |
| Treatments | Cd Content (mg kg−1) | TF | |
|---|---|---|---|
| Shoot | Root | ||
| Cd1 | 71.95 ± 1.84 c | 667.74 ± 14.28 c | 0.11 |
| Cd2 | 208.25 ± 4.83 a | 1350.73 ± 23.94 a | 0.15 |
| TC + Cd1 | 52.81 ± 2.10 d | 573.62 ± 13.58 d | 0.09 |
| TC + Cd2 | 167.17 ± 1.29 b | 1208.03 ± 26.67 b | 0.14 |
| Treatments | Chl a (mg g−1) | Chl b (mg g−1) | Total Chl (mg g−1) | Car (mg g−1) |
|---|---|---|---|---|
| CK | 1.85 ± 0.01 a | 1.06 ± 0.03 a | 2.91 ± 0.03 a | 0.52 ± 0.02 a |
| TC | 1.74 ± 0.03 b | 0.92 ± 0.04 ab | 2.66 ± 1.01 b | 0.51 ± 0.01 ab |
| Cd1 | 1.61 ± 0.02 c | 0.87 ± 0.01 ab | 2.48 ± 0.02 c | 0.45 ± 0.02 c |
| Cd2 | 1.44 ± 0.09 e | 0.77 ± 0.23 b | 2.21 ± 2.01 e | 0.34 ± 0.01 e |
| TC1 + Cd1 | 1.78 ± 0.02 b | 0.91 ± 0.01 ab | 2.69 ± 0.03 b | 0.49 ± 0.02 b |
| TC1 + Cd2 | 1.52 ± 0.02 d | 0.80 ± 0.02 b | 2.32 ± 0.32 d | 0.36 ± 0.01 d |
| Treatments | Ca (mg kg−1) | Mn (mg kg−1) | Zn (mg kg−1) | Cu (mg kg−1) | Fe (mg kg−1) | |
|---|---|---|---|---|---|---|
| CK | Shoot | 454.65 ± 5.00 a | 187.34 ± 7.58 a | 147.18 ± 6.54 a | 29.57 ± 1.29 a | 211.25 ± 12.19 a |
| Root | 128.60 ± 9.80 a | 299.01 ± 5.14 a | 107.63 ± 4.78 a | 36.27 ± 1.13 a | 331.59 ± 12.61 a | |
| TC | Shoot | 420.63 ± 7.62 b | 115.67 ± 3.23 b | 123.65 ± 4.37 c | 26.43 ± 0.69 b | 172.46 ± 6.34 b |
| Root | 99.42 ± 2.68 b | 193.64 ± 6.25 c | 91.08 ± 4.55 b | 26.38 ± 1.10 c | 249.83 ± 4.67 c | |
| Cd1 | Shoot | 323.05 ± 5.18 d | 106.48 ± 7.04 c | 119.28 ± 3.21 c | 25.90 ± 1.39 b | 144.51 ± 7.12 d |
| Root | 90.03 ± 3.03 c | 187.83 ± 8.32 c | 75.53 ± 1.72 c | 23.56 ± 0.64 d | 228.96 ± 13.90 d | |
| Cd2 | Shoot | 255.36 ± 9.43 f | 80.55 ± 1.33 e | 90.39 ± 1.28 e | 21.95 ± 1.56 d | 104.29 ± 6.75 f |
| Root | 70.62 ± 0.99 d | 151.73 ± 2.49 e | 59.08 ± 8.63 e | 18.05 ± 1.05 f | 147.30 ± 6.17 f | |
| TC + Cd1 | Shoot | 352.22 ± 5.36 c | 121.57 ± 1.21 b | 131.29 ± 1.86 b | 27.28 ± 0.39 b | 160.13 ± 2.16 c |
| Root | 102.87 ± 2.63 b | 232.24 ± 2.57 b | 95.88 ± 2.75 b | 28.88 ± 0.15 b | 276.13 ± 2.90 b | |
| TC + Cd2 | Shoot | 287.48 ± 2.41 e | 89.54 ± 1.17 d | 98.19 ± 2.38 d | 22.37 ± 0.17 c | 120.69 ± 7.56 e |
| Root | 87.16 ± 4.09 c | 174.23 ± 1.29 d | 65.88 ± 4.68 d | 20.43 ± 0.30 e | 184.20 ± 2.69 e | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, K.; Ren, Y.; Dong, X.; Ping, X.; He, J. Tetracycline Alleviates Cadmium Toxicity in Rice Seedlings by Altering Pollutant Accumulation, Nutrient Absorption, Osmoregulation and Antioxidant Metabolism. Molecules 2025, 30, 2160. https://doi.org/10.3390/molecules30102160
Li K, Ren Y, Dong X, Ping X, He J. Tetracycline Alleviates Cadmium Toxicity in Rice Seedlings by Altering Pollutant Accumulation, Nutrient Absorption, Osmoregulation and Antioxidant Metabolism. Molecules. 2025; 30(10):2160. https://doi.org/10.3390/molecules30102160
Chicago/Turabian StyleLi, Ke, Yanfang Ren, Xuejie Dong, Xianyi Ping, and Junyu He. 2025. "Tetracycline Alleviates Cadmium Toxicity in Rice Seedlings by Altering Pollutant Accumulation, Nutrient Absorption, Osmoregulation and Antioxidant Metabolism" Molecules 30, no. 10: 2160. https://doi.org/10.3390/molecules30102160
APA StyleLi, K., Ren, Y., Dong, X., Ping, X., & He, J. (2025). Tetracycline Alleviates Cadmium Toxicity in Rice Seedlings by Altering Pollutant Accumulation, Nutrient Absorption, Osmoregulation and Antioxidant Metabolism. Molecules, 30(10), 2160. https://doi.org/10.3390/molecules30102160







