A Comprehensive Study of the Synthesis, Spectral Characteristics, Quantum–Chemical Molecular Electron Density Theory, and In Silico Future Perspective of Novel CBr3-Functionalyzed Nitro-2-Isoxazolines Obtained via (3 + 2) Cycloaddition of (E)-3,3,3-Tribromo-1-Nitroprop-1-ene
Abstract
1. Introduction
2. Results and Discussion
2.1. MEDT Study of Electronic Properties of ANOs (1a-e) and TBNP (2)
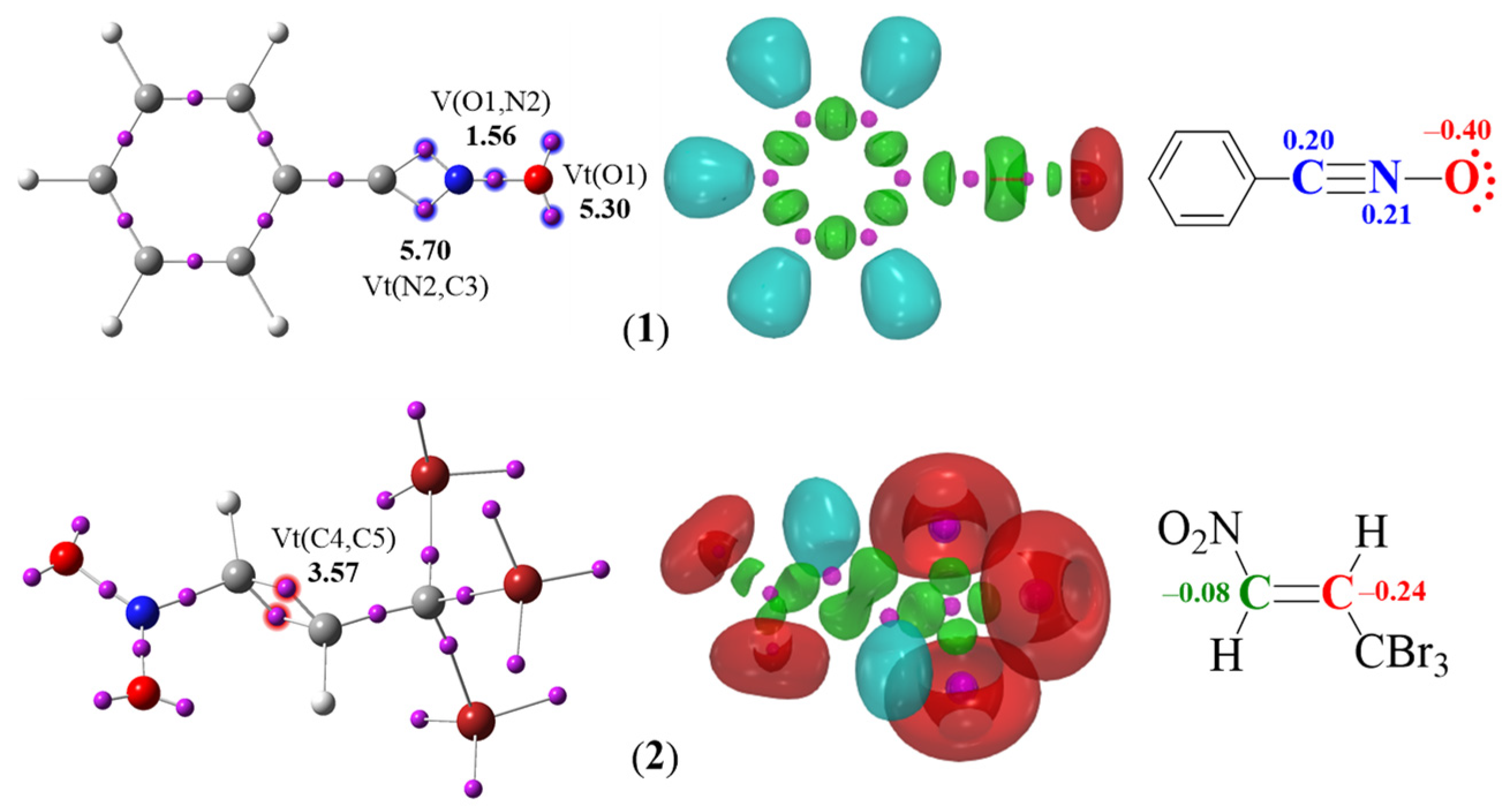
2.1.1. Study of the Electronic Properties of Used Reagents Based on the ELF and NPA
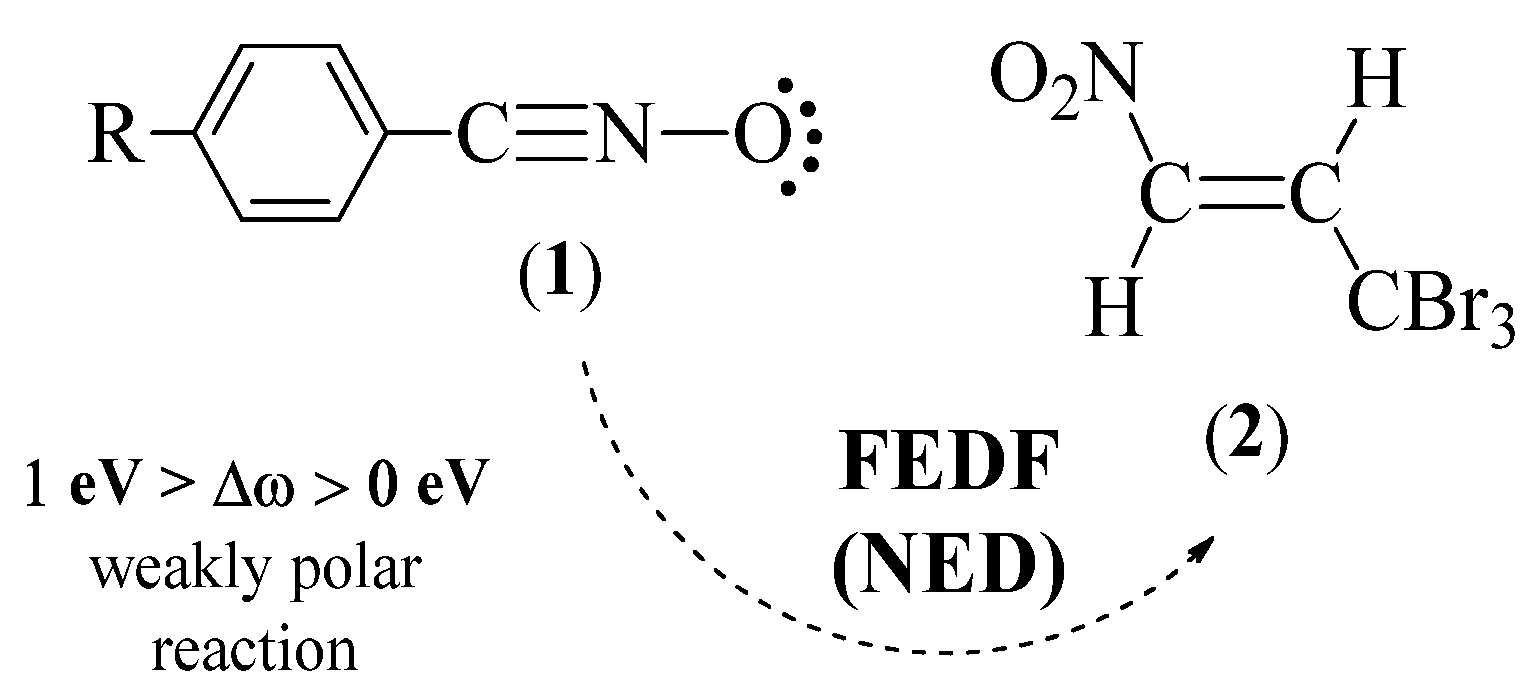
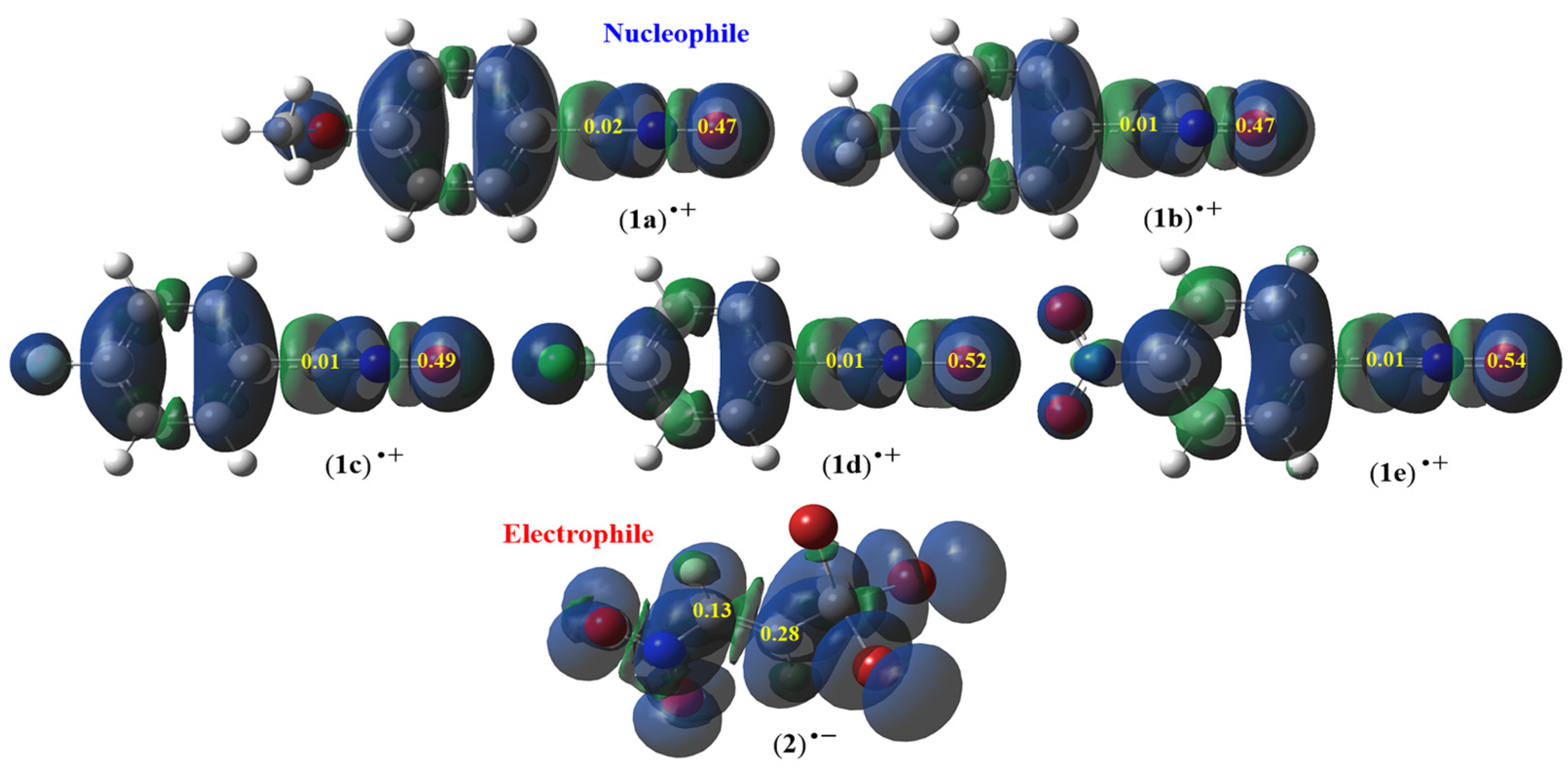
2.1.2. CDFT View of Global and Local Reactivity
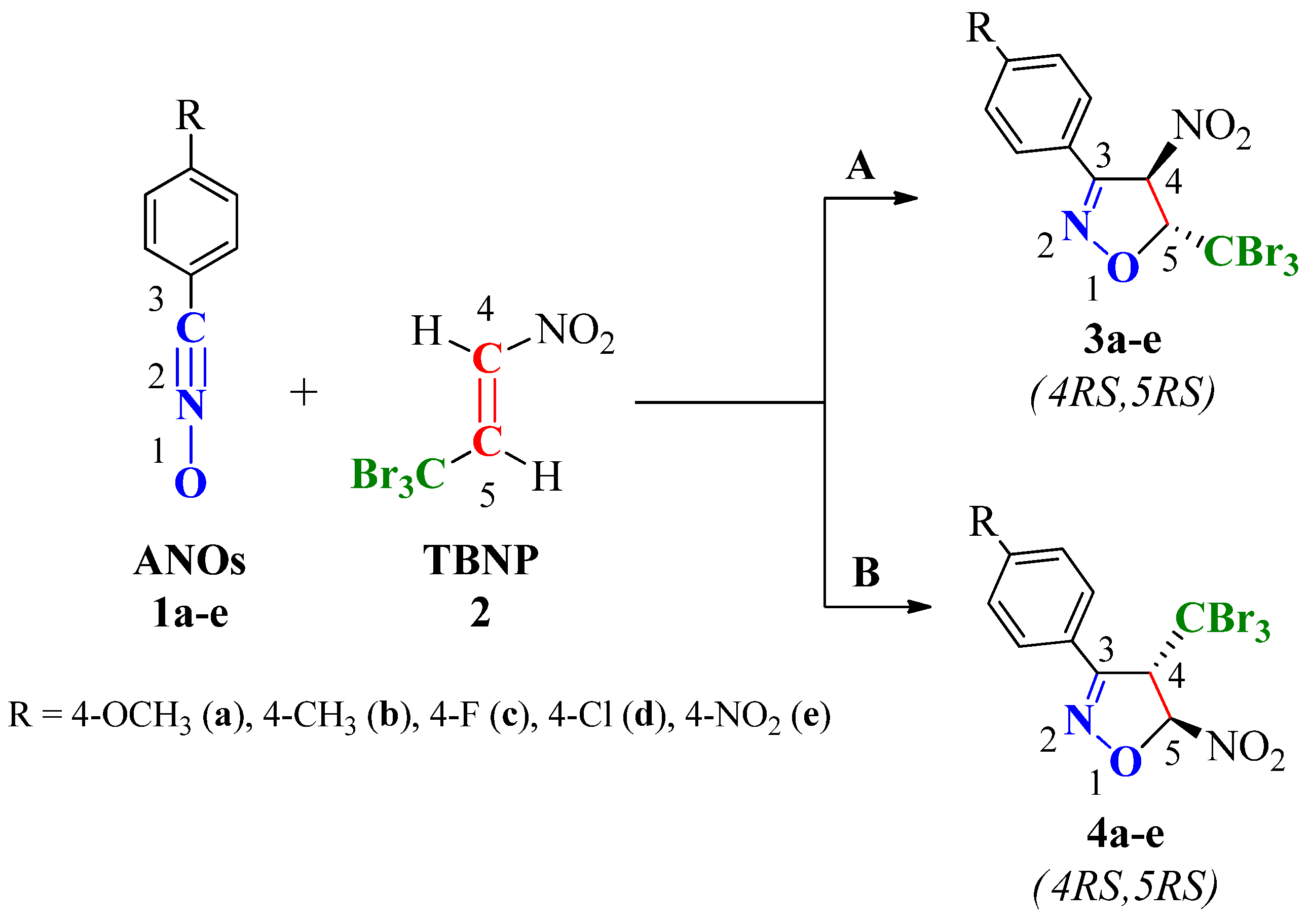
2.2. Experimental Study of (3 + 2) Cycloaddition Reactions Between ANOs (1a-e) and TBNP (2)
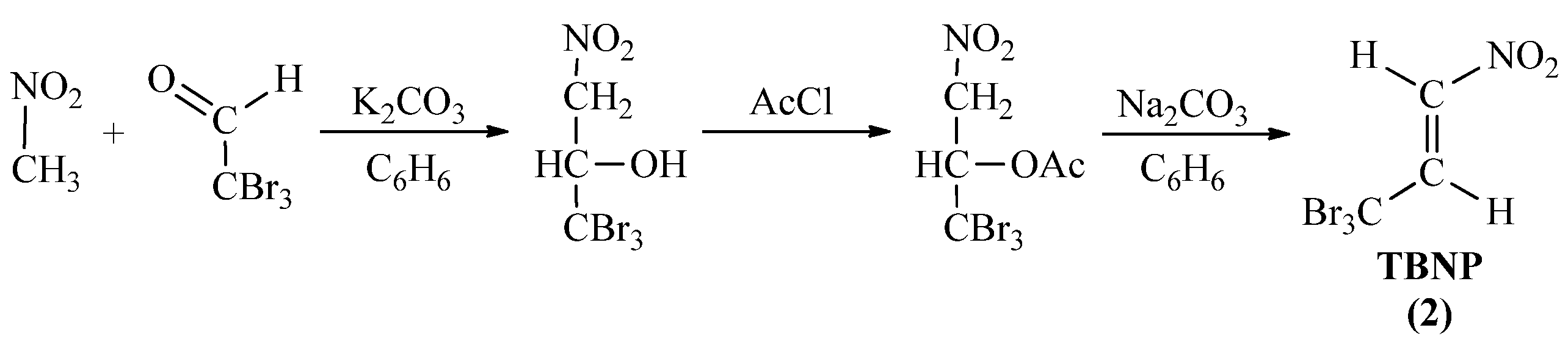
2.2.1. Protocol Synthesis Details of Cycloaddition Components (1a-e) and (2)
2.2.2. Regio- and Stereochemistry of the Reaction Between ANOs (1a-e) and TBNP (2)
2.3. Quantum–Chemical Study of the Reaction Course
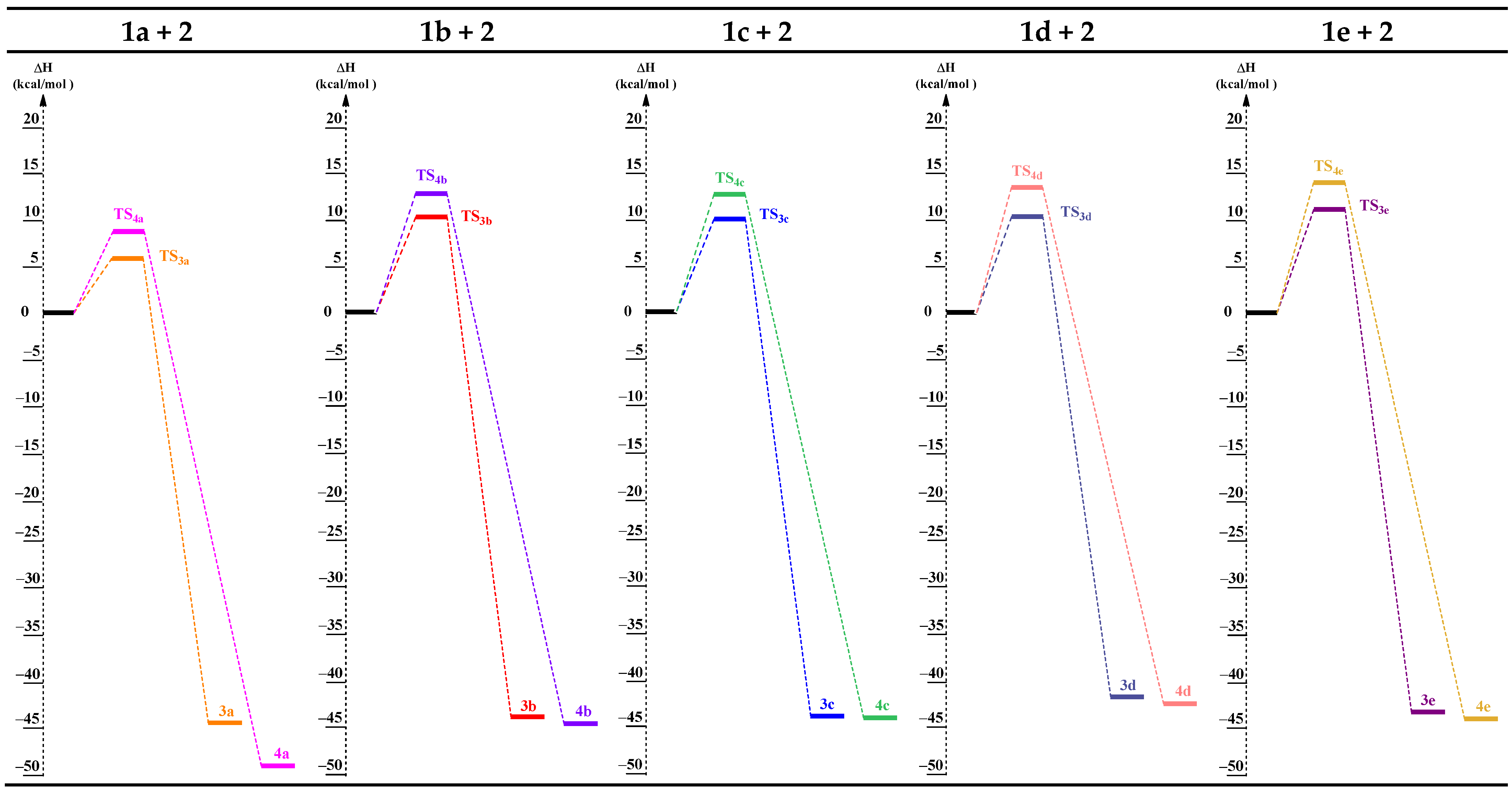
2.3.1. Exploration of the PES of Cycloadditions of ANOs (1a-e) with TBNP (2)
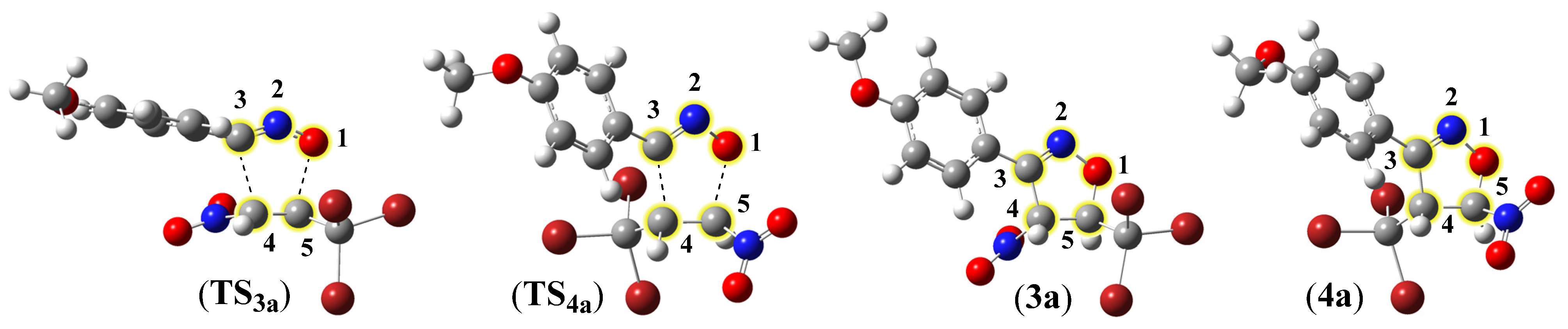
2.3.2. Analysis of Critical Structures for the Studied Reaction of ANOs (1a-e) with TBNP (2)
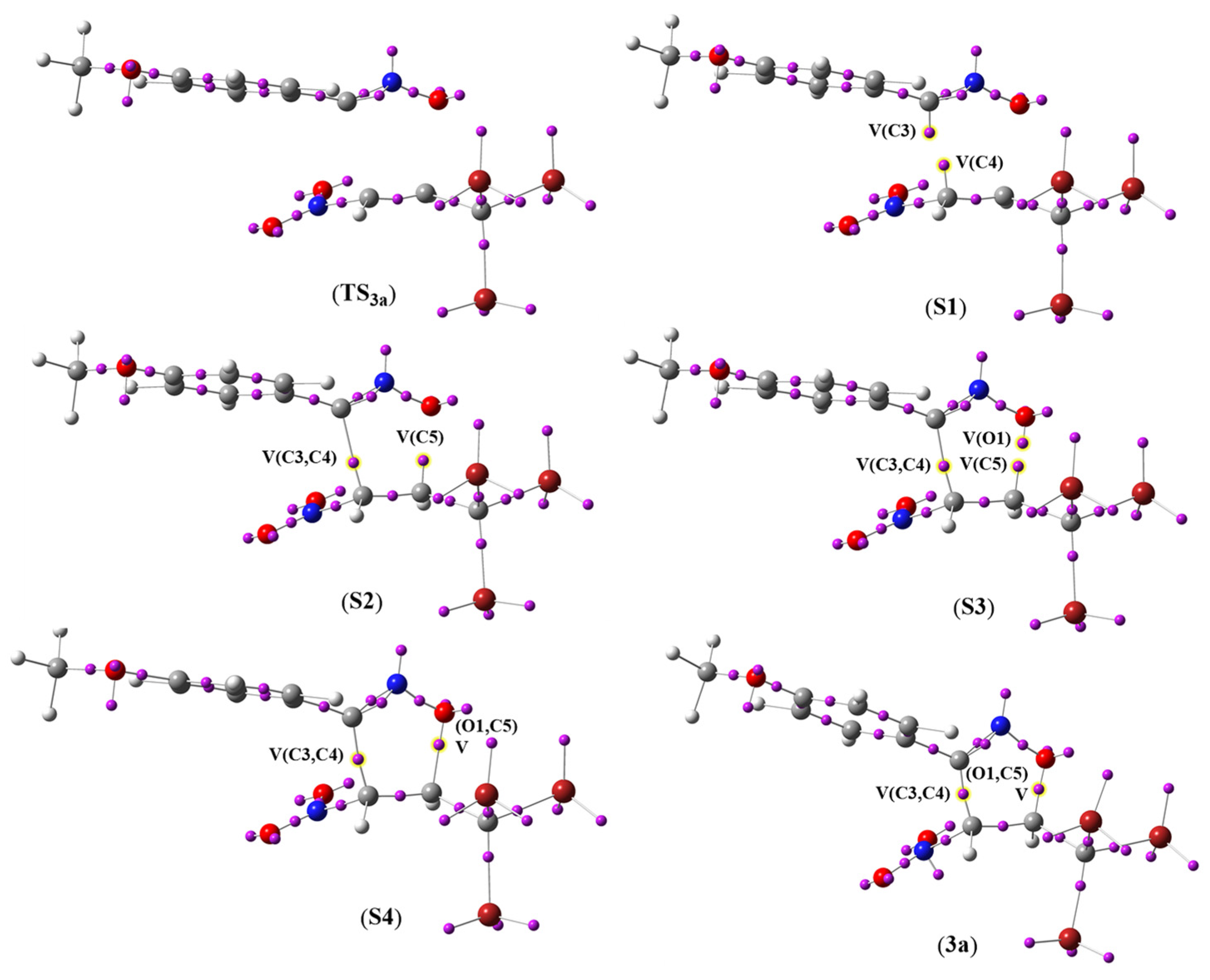
2.3.3. BET Analysis for the Studied Reaction of ANOs (1a-e) with TBNP (2)
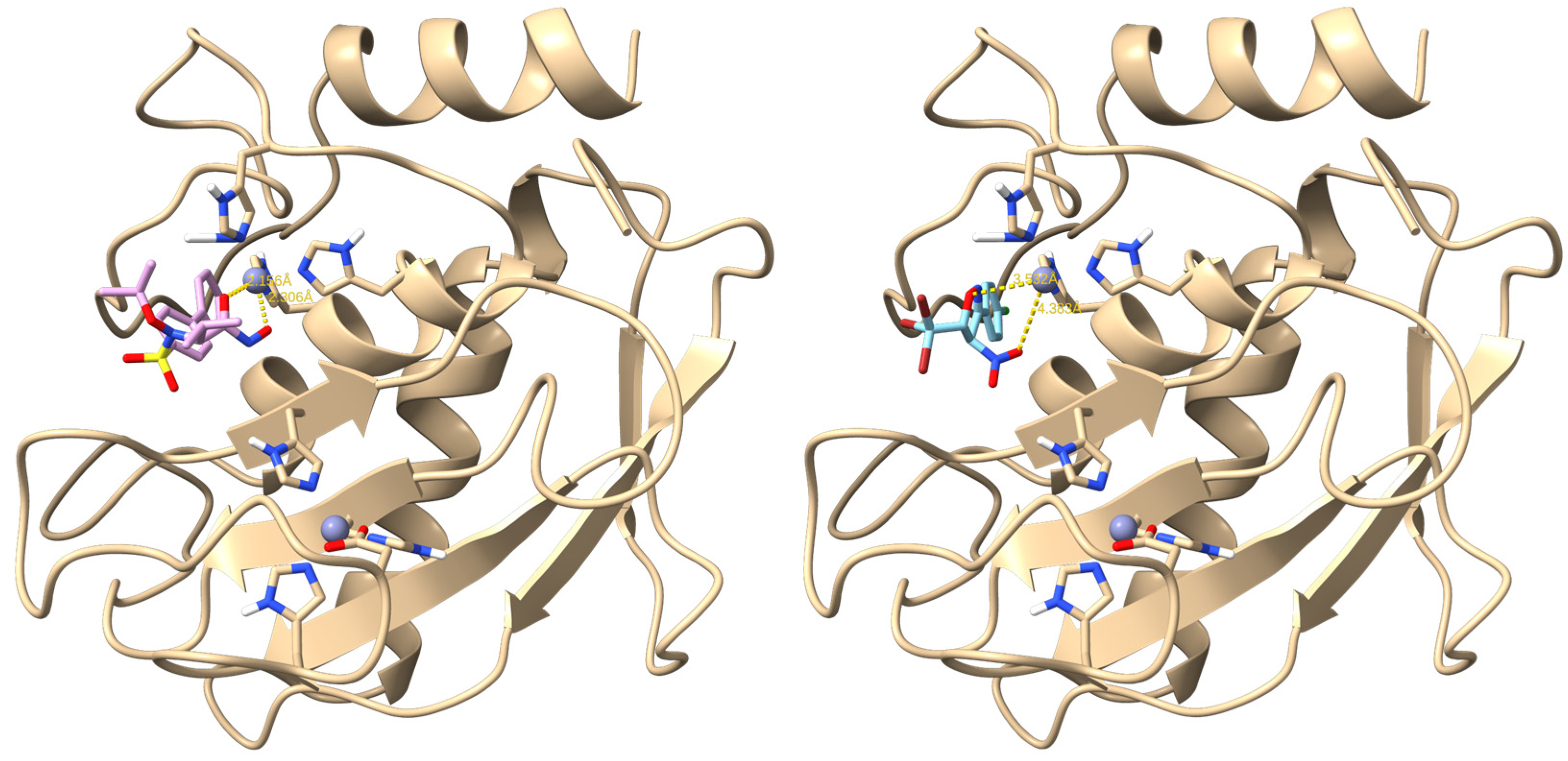
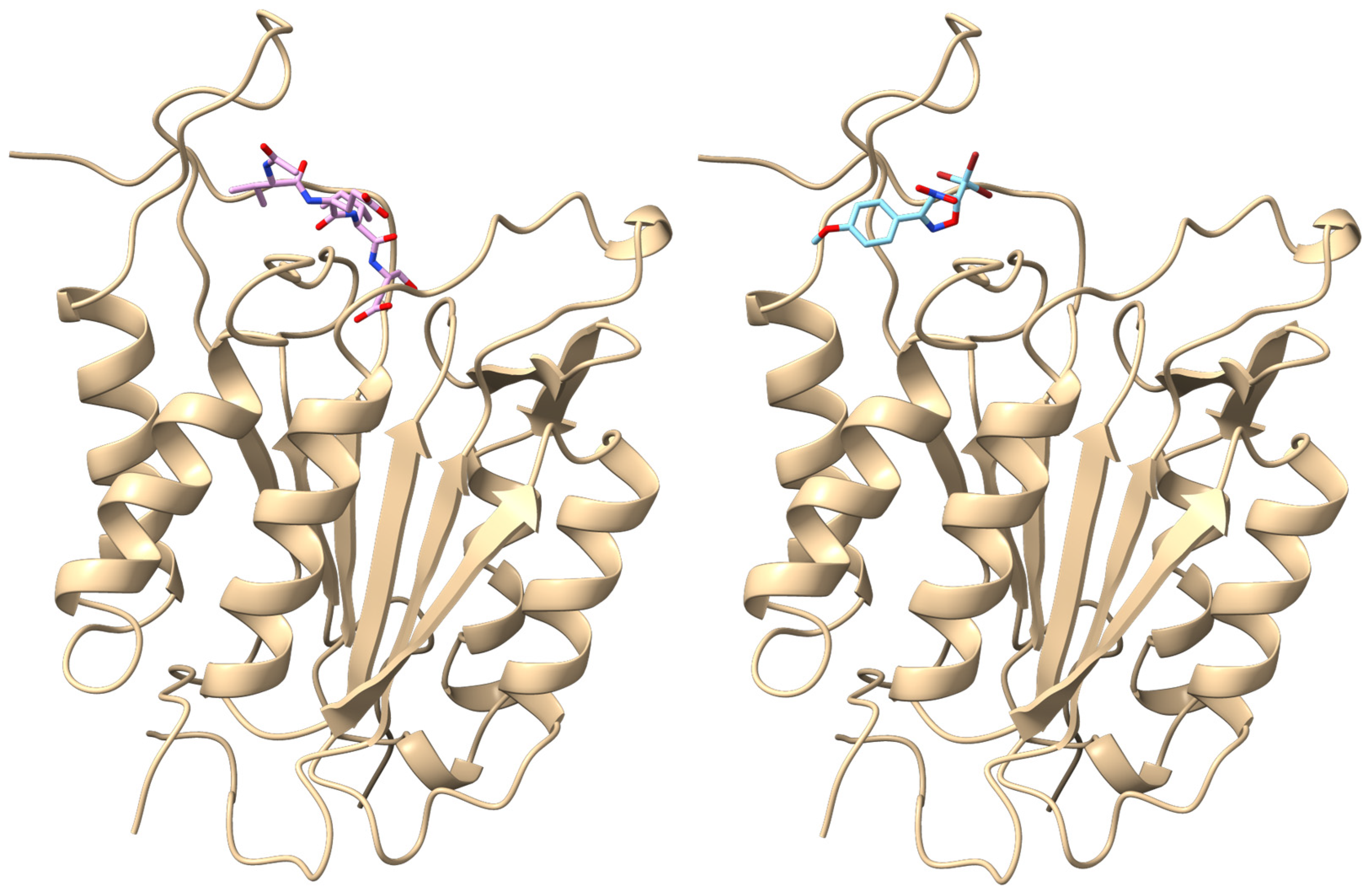
2.4. Molecular Docking Consideration of the Obtained (4RS,5RS)-3-aryl-4-Nitro-5-Tribromomethyl-2-Isoxazolines (3a-e)
3. Materials and Methods
3.1. Materials
3.2. Cycloaddition Between ANOs (1a-e) and TBNP (2)—General Procedure
3.3. Analytical Techniques
3.4. Computational Details
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nishiwaki, N. A Walk Through Recent Nitro Chemistry Advances. Molecules 2020, 25, 3680. [Google Scholar] [CrossRef] [PubMed]
- Halimehjani, A.Z.; Namboothiri, I.N.N.; Hooshmand, S.E. Part I: Nitroalkenes in the synthesis of heterocyclic compounds. RSC Adv. 2014, 4, 48022–48084. [Google Scholar] [CrossRef]
- Halimehjani, A.Z.; Namboothiri, I.N.N.; Hooshmand, S.E. Part II: Nitroalkenes in the synthesis of heterocyclic compounds. RSC Adv. 2014, 4, 51794–51829. [Google Scholar] [CrossRef]
- Zawadzińska, K.; Gaurav, G.K.; Jasiński, R. Preparation of Conjugated Nitroalkenes: Short Review. Sci. Radices 2022, 1, 69–83. [Google Scholar] [CrossRef]
- Sadowski, M.; Synkiewicz-Musialska, B.; Kula, K. (1E,3E)-1,4-Dinitro-1,3-butadiene—Synthesis, Spectral Characteristics and Computational Study Based on MEDT, ADME and PASS Simulation. Molecules 2024, 29, 542. [Google Scholar] [CrossRef]
- Sadowski, M.; Kula, K. Unexpected Course of Reaction Between (1E,3E)-1,4-Dinitro-1,3-butadiene and N-Methyl Azomethine Ylide—A Comprehensive Experimental and Quantum-Chemical Study. Molecules 2024, 29, 5066. [Google Scholar] [CrossRef]
- Durden, J.A.; Heywood, D.L.; Sousa, A.A.; Spurr, H.W. Synthesis and Microbial Toxicity of Dinitrobutadienes and Related Compounds. J. Agric. Food Chem. 1970, 18, 50–56. [Google Scholar] [CrossRef]
- Boguszewska-Czubara, A.; Kula, K.; Wnorowski, A.; Biernasiuk, A.; Popiolek, Ł.; Miodowski, D.; Demchuk, O.M.; Jasiński, R. Novel Functionalized β-nitrostyrenes: Promising Candidates for New Antibacterial Drugs. Saudi Pharm. J. 2019, 27, 593–601. [Google Scholar] [CrossRef]
- Latif, N.; Girgis, N.S.; Assad, F.M.; Grant, N. (Nitroethenyl) Salicylic Acid Anilides and Related Substances. A New Group of Molluscicidal and Microbicidal Compounds. Liebigs Ann. 1985, 6, 1202–1209. [Google Scholar] [CrossRef]
- Łapczuk-Krygier, A.; Kącka-Zych, A.; Kula, K. Recent Progress in the Field of Cycloaddition Reactions Involving Conjugated Nitroalkenes. Curr. Chem. Lett. 2019, 8, 13–38. [Google Scholar] [CrossRef]
- Sadowski, M.; Kula, K. Nitro-functionalized Analogues of 1,3-Butadiene: An Overview of Characteristic, Synthesis, Chemical Transformations and Biological Activity. Curr. Chem. Lett. 2024, 13, 15–30. [Google Scholar] [CrossRef]
- Dresler, E. The participation of oleic acid and its esters in [3 + 2] cycloaddition reactions: A mini-review. Sci. Radices 2024, 3, 53–61. [Google Scholar] [CrossRef]
- Woliński, P.; Kącka-Zych, A.; Wróblewska, A.; Wielgus, E.; Dolot, R.; Jasiński, R. Fully Selective Synthesis of Spirocyclic-1,2-oxazine N-Oxides via Non-Catalysed Hetero Diels-Alder Reactions with the Participation of Cyanofunctionalysed Conjugated Nitroalkenes. Molecules 2023, 28, 4586. [Google Scholar] [CrossRef] [PubMed]
- Dresler, E.; Wróblewska, A.; Jasiński, R. Understanding the Molecular Mechanism of Thermal and LA-Catalysed Diels—Alder Reactions Between Cyclopentadiene and Isopropyl 3-Nitroprop-2-Enate. Molecules 2023, 28, 5289. [Google Scholar] [CrossRef]
- Kula, K.; Łapczuk, A.; Sadowski, M.; Kras, J.; Zawadzińska, K.; Demchuk, O.M.; Gaurav, G.K.; Wróblewska, A.; Jasiński, R. On the Question of the Formation of Nitro-Functionalized 2,4-Pyrazole Analogs on the Basis of Nitrylimine Molecular Systems and 3,3,3-Trichloro-1-Nitroprop-1-Ene. Molecules 2022, 27, 8409. [Google Scholar] [CrossRef]
- Kula, K.; Kącka-Zych, A.; Łapczuk-Krygier, A.; Wzorek, Z.; Nowak, A.K.; Jasiński, R. Experimental and Theoretical Mechanistic Study on the Thermal Decomposition of 3,3-Diphenyl-4-(Trichloromethyl)-5-Nitropyrazoline. Molecules 2021, 26, 1364. [Google Scholar] [CrossRef]
- Żmigrodzka, M.; Sadowski, M.; Kras, J.; Dresler, E.; Demchuk, O.M.; Kula, K. Polar [3 + 2] Cycloaddition Between N-Methyl Azomethine Ylide and Trans-3,3,3-trichloro-1-nitroprop-1-ene. Sci. Radices 2022, 1, 26–35. [Google Scholar] [CrossRef]
- García-Mingüens, E.; Ferrándiz-Saperas, M.; de Gracia Retamosa, M.; Nájera, C.; Yus, M.; Sansano, J.M. Enantioselective 1,3-Dipolar Cycloaddition Using (Z)-α-Amidonitroalkenes as a Key Step to the Access to Chiral cis-3,4-Diaminopyrrolidines. Molecules 2022, 27, 4579. [Google Scholar] [CrossRef]
- Jasiński, R. Recent Progress in the Synthesis of Nitroisoxazoles and Their Hydrogenated Analogs via [3 + 2] Cycloaddition Reactions (Microreview). Chem. Heterocycl. Compd. 2023, 59, 730–732. [Google Scholar] [CrossRef]
- Dresler, E.; Sadowski, M.; Demchuk, O.M. Reactivity of the ethyl oleate in the [3 + 2] cycloaddition to arylonitrile N-oxide: A reexamination. Sci. Radices 2024, 3, 108–121. [Google Scholar] [CrossRef]
- Mirosław, B.; Babyuk, D.; Łapczuk-Krygier, A.; Kącka-Zych, A.; Demchuk, O.M.; Jasinski, R. Regiospecific formation of the nitromethyl-substituted 3-phenyl-4,5-dihydroisoxazole via [3 + 2] cycloaddition. Monatsh. Chem. 2018, 149, 1877–1884. [Google Scholar] [CrossRef] [PubMed]
- Fryźlewicz, A.; Łapczuk-Krygier, A.; Kula, K.; Demchuk, O.M.; Dresler, E.; Jasiński, R. Regio- and Stereoselective Synthesis of Nitrofunctionalized 1,2-Oxazolidine Analogs of Nicotine. Chem. Heterocycl. Comp. 2020, 56, 120–122. [Google Scholar] [CrossRef]
- Jasiński, R.; Żmigrodzka, M.; Dresler, E.; Kula, K. A full regio- and stereoselective synthesis of 4-nitroisoxazolidines via stepwise [3 + 2] cycloaddition reactions between (Z)-C-(9-anthryl)-N-arylnitrones and (E)-3,3,3-trichloro-1-nitroprop-1-ene: Comprehensive experimental and theoretical study. J. Heterocyclic. Chem. 2017, 54, 3314–3320. [Google Scholar] [CrossRef]
- Zawadzińska, K.; Gostyński, B. Nitrosubstituted analogs of isoxazolines and isoxazolidines: A surprising estimation of their biological activity via molecular docking. Sci. Radices 2023, 2, 25–46. [Google Scholar] [CrossRef]
- Zawadzińska, K.; Zavecz, I.; Hirka, S. The recent progress in the field of the applications of isoxazoles and their hydrogenated analogs: Mini review. Sci. Radices 2024, 3, 228–247. [Google Scholar] [CrossRef]
- Zawadzińska, K.; Ríos-Gutiérrez, M.; Kula, K.; Woliński, P.; Mirosław, B.; Krawczyk, T.; Jasiński, R. The Participation of 3,3,3-Trichloro-1-nitroprop-1-ene in the [3 + 2] Cycloaddition Reaction with Selected Nitrile N-Oxides in the Light of the Experimental and MEDT Quantum Chemical Study. Molecules 2021, 26, 6774. [Google Scholar] [CrossRef]
- Ríos-Gutiérrez, M.; Domingo, L.R. Unravelling the Mysteries of the [3 + 2] Cycloaddition Reactions. Eur. J. Org. Chem. 2019, 2019, 267–282. [Google Scholar] [CrossRef]
- Ríos-Gutiérrez, M.; Domingo, L.R.; Ghodsi, F. Unveiling the Different Reactivity of Bent and Linear Three-Atom-Components Participating in [3 + 2] Cycloaddition Reactions. Organics 2021, 2, 274–286. [Google Scholar] [CrossRef]
- Maľučká, L.U.; Vilková, M. Spectral Assignment in the [3 + 2] Cycloadditions of Methyl (2E)-3-(Acridin-4-yl)-prop-2-enoate and 4-[(E)-2-Phenylethenyl]acridin with Unstable Nitrile N-Oxides. Molecules 2024, 29, 2756. [Google Scholar] [CrossRef]
- Abdoul-Hakim, M.; Idrissi, K.E.; Zeroual, A.; Garmes, H. Investigation of the solvent effect, regioselectivity, and the mechanism of the cycloaddition reaction between 2-chlorobenzimidazole and benzonitrile oxide. Chem. Heterocycl. Compd. 2023, 59, 155–164. [Google Scholar] [CrossRef]
- Ríos-Gutiérrez, M.; Chafaa, F.; Khorief Nacereddine, A.; Djerourou, A.; Domingo, L.R. A DFT study of [3 + 2] cycloaddition reactions of an azomethine imine with N-vinyl pyrrole and N-vinyl tetrahydroindole. J. Mol. Graph. Model. 2016, 70, 296–304. [Google Scholar] [CrossRef] [PubMed]
- Ríos-Gutiérrez, M.; Domingo, L.R.; Esseffar, M.; Oubella, A.; Ait Itto, M.Y. Unveiling the Different Chemical Reactivity of Diphenyl Nitrilimine and Phenyl Nitrile Oxide in [3 + 2] Cycloaddition Reactions with (R)-Carvone through the Molecular Electron Density Theory. Molecules 2020, 25, 1085. [Google Scholar] [CrossRef] [PubMed]
- Zeroual, A.; Ríos-Gutiérrez, M.; El Idrissi, M.; El Alaoui El Abdallaoui, H.; Domingo, L.R. An MEDT study of the mechanism and selectivities of the [3 + 2] cycloaddition reaction of tomentosin with benzonitrile oxide. Int. J. Quantum. Chem. 2019, 119, 25980. [Google Scholar] [CrossRef]
- Mondal, A.; Mohammad-Salim, H.A.; Acharjee, N. Unveiling substituent effects in [3 + 2] cycloaddition reactions of benzonitrile N-oxide and benzylideneanilines from the molecular electron density theory perspective. Sci. Radices 2023, 2, 75–92. [Google Scholar] [CrossRef]
- Yavari, I.; Malekafzali, A.; Eivazzadeh-Keihan, R.; Skoulika, S.; Alivaisi, R. A one-pot synthesis of trichloromethylated pyrimidines from trichloroacetimidamides and acetylenic esters. Tetrahedron Lett. 2016, 57, 1733–1735. [Google Scholar] [CrossRef]
- Furuya, T.; Kamlet, A.S.; Ritter, T. Catalysis for fluorination and trifluoromethylation. Nature 2011, 473, 470–477. [Google Scholar] [CrossRef]
- Jasiński, R.; Mróz, K. Kinetic aspects of [3 + 2] cycloaddition reactions between (E)-3,3,3-trichloro-1-nitroprop-1-ene and ketonitrones. React. Kinet. Mech. Catal. 2015, 116, 35–41. [Google Scholar] [CrossRef]
- Kula, K.; Dobosz, J.; Jasiński, R.; Kącka-Zych, A.; Łapczuk-Krygier, A.; Mirosław, B.; Demchuk, O.M. [3+2] Cycloaddition of diaryldiazomethanes with (E)-3,3,3-trichloro-1-nitroprop-1-ene: An experimental, theoretical and structural study. J. Mol. Struct. 2020, 1203, 127473. [Google Scholar] [CrossRef]
- Kras, J.; Sadowski, M.; Zawadzińska, K.; Nagatsky, R.; Woliński, P.; Kula, K.; Łapczuk, A. Thermal [3 + 2] Cycloaddition Reactions as Most Universal Way for the Effective Preparation of Five-Membered Nitrogen Containing Heterocycles. Sci. Radices 2023, 2, 247–267. [Google Scholar] [CrossRef]
- Anisimova, N.A.; Slobodchikova, E.K.; Kuzhaeva, A.A.; Rybalova, T.V.; Stukan, E.V.; Berestovitskaya, V.M. 3,3,3-Tribromo-1-nitropropene: Synthesis and structure. Russ. J. Gen. Chem. 2014, 84, 834–838. [Google Scholar] [CrossRef]
- Anisimova, N.A.; Slobodchikova, E.K.; Ivanova, M.E.; Rybalova, T.V. Reaction of 3,3,3-tribromo- and 1,3,3,3-tetrabromo-1-nitroprop-1-enes with aliphatic dienes. Russ. J. Gen. Chem. 2020, 90, 1388–1397. [Google Scholar] [CrossRef]
- Kras, J.; Woliński, P.; Nagatsky, R.; Demchuk, O.M.; Jasiński, R. Full Regio- and Stereoselective Protocol for the Synthesis of New Nicotinoids via Cycloaddition Processes with the Participation of Trans-Substituted Nitroethenes: Comprehensive Experimental and MEDT Study. Molecules 2023, 28, 3535. [Google Scholar] [CrossRef] [PubMed]
- Zawadzińska, K.; Gadocha, Z.; Pabian, K.; Wróblewska, A.; Wielgus, E.; Jasiński, R. The First Examples of [3 + 2] Cycloadditions with the Participation of (E)-3,3,3-Tribromo-1-Nitroprop-1-Ene. Materials 2022, 15, 7584. [Google Scholar] [CrossRef] [PubMed]
- Domingo, L.R. Molecular Electron Density Theory: A Modern View of Reactivity in Organic Chemistry. Molecules 2016, 21, 1319. [Google Scholar] [CrossRef]
- Domingo, L.R.; Ríos-Gutiérrez, M. Revealing the Critical Role of Global Electron Density Transfer in the Reaction Rate of Polar Organic Reactions within Molecular Electron Density Theory. Molecules 2024, 29, 1870. [Google Scholar] [CrossRef]
- Krokidis, X.; Noury, S.; Silvi, B. Characterization of Elementary Chemical Processes by Catastrophe Theory. J. Phys. Chem. A 1997, 101, 7277–7282. [Google Scholar] [CrossRef]
- Polo, V.; Andres, J.; Berski, S.; Domingo, L.R.; Silvi, B. Understanding Reaction Mechanisms in Organic Chemistry from Catastrophe Theory Applied to the Electron Localization Function Topology. J. Phys. Chem. A 2008, 112, 7128–7136. [Google Scholar] [CrossRef]
- Morris, G.M.; Lim-Wilby, M. Molecular docking. Methods Mol. Biol. 2008, 443, 365–382. [Google Scholar] [CrossRef]
- Fan, J.; Fu, A.; Zhang, L. Progress in molecular docking. Quant. Biol. 2019, 7, 83–89. [Google Scholar] [CrossRef]
- Domingo, L.R.; Ríos-Gutiérrez, M. A Useful Classification of Organic Reactions Based on the Flux of the Electron Density. Sci. Radices 2023, 2, 1–24. [Google Scholar] [CrossRef]
- Kula, K.; Sadowski, M. Regio- and stereoselectivity of [3 + 2] cycloaddition reactions Between (Z)-C-(9-anthryl)-N-methylnitrone and analogues of trans-β-nitrostyrene in the light of MEDT computational study. Chem. Heterocycl. Comp. 2023, 59, 138–144. [Google Scholar] [CrossRef]
- Becke, A.D.; Edgecombe, K.E. A Simple Measure of Electron Localization in Atomic and Molecular Systems. J. Chem. Phys. 1990, 92, 5397–5403. [Google Scholar] [CrossRef]
- Reed, A.E.; Weinstock, R.B.; Weinhold, F. Natural population analysis. J. Chem. Phys. 1985, 83, 735–746. [Google Scholar] [CrossRef]
- Reed, A.E.; Curtiss, L.A.; Weinhold, F. Intermolecular interactions from a natural bond orbital, donor-acceptor viewpoint. Chem. Rev. 1988, 88, 899–926. [Google Scholar] [CrossRef]
- Breugst, M.; Reissig, H. The Huisgen Reaction: Milestones of the 1, 3-Dipolar Cycloaddition. Angew. Chem. Int. Ed. 2020, 115, 12293–12307. [Google Scholar] [CrossRef]
- Sadowski, M.; Dresler, E.; Jasiński, R. The Puzzle of the Regioselectivity and Molecular Mechanism of the (3 + 2) Cycloaddition Reaction Between E-2-(Trimethylsilyl)-1-Nitroethene and Arylonitrile N-Oxides: Molecular Electron Density Theory (MEDT) Quantumchemical Study. Molecules 2025, 30, 974. [Google Scholar] [CrossRef]
- Wróblewska, A.; Sadowski, M.; Jasiński, R. Selectivity and molecular mechanism of the Au(III)-catalyzed [3 + 2] cycloaddition reaction between (Z)-C,N-diphenylnitrone and nitroethene in the light of the molecular electron density theory computational study. Chem. Heterocycl. Comp. 2024, 60, 639–645. [Google Scholar] [CrossRef]
- Domingo, L.R.; Ríos-Gutiérrez, M.; Pérez, P. Applications of the Conceptual Density Functional Theory Indices to Organic Chemistry Reactivity. Molecules 2016, 21, 748. [Google Scholar] [CrossRef]
- Domingo, L.R. 1999–2024, a quarter century of the Parr’s electrophilicity ω index. Sci. Radices 2024, 3, 157–186. [Google Scholar] [CrossRef]
- Domingo, L.R.; Ríos-Gutiérrez, M.; Pérez, P. Electrophilicity w and Nucleophilicity N Scales for Cationic and Anionic Species. Sci. Radices 2025, 4, 1–17. [Google Scholar] [CrossRef]
- Aurell, M.J.; Domingo, L.R.; Pérez, P.; Contreras, R. A Theoretical Study on the Regioselectivity Of 1,3-Dipolar Cycloadditions Using DFT-based Reactivity Indexes. Tetrahedron 2004, 60, 11503–11509. [Google Scholar] [CrossRef]
- Domingo, L.R.; Pérez, P.; Sáez, J.A. Understanding the Local Reactivity in Polar Organic Reactions Through Electrophilic and Nucleophilic Parr Functions. RSC Adv. 2013, 3, 1486–1494. [Google Scholar] [CrossRef]
- Domingo, L.R.; Aurell, M.J.; Pérez, P.; Contreras, R. Quantitative characterization of the global electrophilicity power of common diene/dienophile pairs in Diels–Alder reactions. Tetrahedron 2002, 58, 4417–4423. [Google Scholar] [CrossRef]
- Ríos-Gutiérrez, M.; Saz Sousa, A.; Domingo, L.R. Electrophilicity and nucleophilicity scales at different DFT computational levels. J. Phys. Org. Chem. 2023, 36, 4503. [Google Scholar] [CrossRef]
- Parr, R.G.; von Szentpaly, L.; Liu, S. Electrophilicity Index. J. Am. Chem. Soc. 1999, 121, 1922–1924. [Google Scholar] [CrossRef]
- Domingo, L.R.; Pérez, P. The Nucleophilicity N Index in Organic Chemistry. Org. Biomol. Chem. 2011, 9, 7168–7175. [Google Scholar] [CrossRef]
- Liu, K.-C.; Shelton, B.R.; Howe, R.K. A particularly convenient preparation of benzohydroximinoyl chlorides (nitrile oxide precursors). J. Org. Chem. 1980, 45, 3916–3918. [Google Scholar] [CrossRef]
- Ghaemi, M.; Pordel, M. Isoxazolo [4,3-e]indazole as a new heterocyclic system: Design, synthesis, spectroscopic characterization, and antibacterial activity. Chem. Heterocycl. Comp. 2016, 52, 52–57. [Google Scholar] [CrossRef]
- Saber, A.; Driowya, M.; Alaoui, S.; Marzag, H.; Demange, L.; Álvarez, E.; Benhida, R.; Bougrin, K. Solvent-Free Regioselective Synthesis of Novel Isoxazoline and Pyrazoline N-Substituted Saccharin Derivatives Under Microwave Irradiation. Chem. Heterocycl. Comp. 2016, 52, 31–40. [Google Scholar] [CrossRef]
- Tomasi, J.; Persico, M. Molecular Interactions in Solution: An Overview of Methods Based on Continuous Distributions of the Solvent. Chem. Rev. 1994, 94, 2027–2094. [Google Scholar] [CrossRef]
- Cossi, M.; Barone, V.; Cammi, R.; Tomasi, J. Ab initio study of solvated molecules: A new implementation of the polarizable continuum model. Chem. Phys. Chem. 1996, 225, 327–335. [Google Scholar] [CrossRef]
- Barone, V.; Cossi, M.; Tomasi, J. Geometry optimization of molecular structures in solution by the polarizable continuum model. J. Comput. Chem. 1998, 19, 404–417. [Google Scholar] [CrossRef]
- Jasiński, R. A stepwise, zwitterionic mechanism for the 1,3-dipolar cycloaddition between (Z)-C-4-methoxyphenyl-N-phenylnitrone and gem-chloronitroethene catalysed by 1-butyl-3-methylimidazolium ionic liquid cations. Tetrahedron Lett. 2015, 56, 532–535. [Google Scholar] [CrossRef]
- Dresler, E.; Allnajar, R.; Jasiński, R. Sterical index: A novel, simple tool for the interpretation of organic reaction mechanisms. Sci. Radices 2023, 2, 69–74. [Google Scholar] [CrossRef]
- Domingo, L.R. A new C–C bond formation model based on the quantum chemical topology of electron density. RSC Adv. 2014, 4, 32415–32428. [Google Scholar] [CrossRef]
- Kula, K.; Jasiński, R. Synthesis of bis(het)aryl systems via domino reaction involving (2E,4E)-2,5-dinitrohexa-2,4-diene: DFT mechanistic considerations. Chem. Heterocycl. Comp. 2024, 60, 600–610. [Google Scholar] [CrossRef]
- Berski, S.; Andrés, J.; Silvi, B.; Domingo, L.R. The Joint Use of Catastrophe Theory and Electron Localization Function to Characterize Molecular Mechanisms. A Density Functional Study of the Diels−Alder Reaction between Ethylene and 1,3-Butadiene. J. Phys. Chem. A 2003, 107, 6014–6024. [Google Scholar] [CrossRef]
- Domingo, L.R.; Sáez, J.A. Understanding the Electronic Reorganization along the Nonpolar [3 + 2] Cycloaddition Reactions of Carbonyl Ylides. J. Org. Chem. 2011, 76, 373–379. [Google Scholar] [CrossRef]
- Zawadzińska, K.; Kula, K. Application of β-phosphorylated nitroethenes in [3 + 2] cycloaddition reactions involving benzonitrile N-oxide in the light of DFT computational study. Organics 2021, 2, 26–37. [Google Scholar] [CrossRef]
- Domingo, L.R.; Sáez, J.A.; Zaragozá, R.J.; Arnó, M. Understanding the Participation of Quadricyclane as Nucleophile in Polar Cycloadditions toward Electrophilic Molecules. J. Org. Chem. 2008, 73, 8791–8799. [Google Scholar] [CrossRef]
- Ouzounthanasis, K.A.; Glamočlija, J.; Ćirić, A.; Koumbis, A.E. Studies of the Synthesis of Fused Isoxazoline/Isoquinolinones and Evaluation of the Antifungal Activity of Isoxazole-like Benzamide and Isoquinolinone Hybrids. Molecules 2025, 30, 589. [Google Scholar] [CrossRef] [PubMed]
- Kosylo, N.; Hotynchan, A.; Skrypska, O.; Horak, Y.; Obushak, M. Synthesis and prediction of toxicological and pharmacological properties of Schiff bases containing arylfuran and pyrazole moiety. Sci. Radices 2024, 3, 62–73. [Google Scholar] [CrossRef]
- Lambert, M.P.; Neuhaus, F.C. Mechanism of D-cycloserine action: Alanine racemase from Escherichia coli W. J. Bacteriol. Res. 1972, 110, 978–987. [Google Scholar] [CrossRef] [PubMed]
- Mtiraou, H.; Ghabi, A.; Al-Ghulikah, H.; Habib, M.A.; Hajji, M. Structural and chemical reactivity insights of a benzimidazolidinone-based N-heterocycle: A multiapproach quantum-chemical study. Chem. Heterocycl. Comp. 2024, 60, 611–616. [Google Scholar] [CrossRef]
- Li, F.; Jiang, B.; Luo, Y.; He, S.; Feng, D.; Hu, D.; Song, R. Discovery of a Novel Class of Acylthiourea-Containing Isoxazoline Insecticides against Plutella xylostella. Molecules 2023, 28, 3300. [Google Scholar] [CrossRef]
- Shybanov, D.E.; Kukushkin, M.E.; Grishin, Y.K.; Roznyatovsky, V.A.; Tafeenko, V.A.; Abo Qoura, L.; Pokrovsky, V.S.; Yarovaya, O.I.; Belyaevskaya, S.V.; Volobueva, A.S.; et al. 1,3-Dipolar Cycloaddition of Nitrile Oxides and Nitrilimines to (−)-β-Caryophyllene: Stereoselective Synthesis of Polycyclic Derivatives and Their Biological Testing. Int. J. Mol. Sci. 2024, 25, 11435. [Google Scholar] [CrossRef]
- Master, J.; Sydney, S.; Rajapaske, H.; Saffiddine, M.; Reyes, V.; Denton, R.W. A Facile Synthesis of Some Bioactive Isoxazoline Dicarboxylic Acids via Microwave-Assisted 1,3-Dipolar Cycloaddition Reaction. Reactions 2024, 5, 1080–1088. [Google Scholar] [CrossRef]
- Nuti, E.; Cantelmo, A.R.; Gallo, C.; Bruno, A.; Bassani, B.; Camodeca, C.; Tuccinardi, T.; Vera, L.; Orlandini, E.; Nencetti, S.; et al. N-O-Isopropyl Sulfonamido-based hydroxamates as matrix metalloproteinase inhibitors: Hit selection and in vivo antiangiogenic activity. J. Med. Chem. 2015, 58, 7224–7240. [Google Scholar] [CrossRef]
- Smith, W.L.; Urade, Y.; Jakobsson, P.J. Enzymes of the cyclooxygenase pathways of prostanoid biosynthesis. Chem. Rev. 2011, 111, 5821–5865. [Google Scholar] [CrossRef]
- Lamkanfi, M.; Kanneganti, T.D. Caspase-7: A protease involved in apoptosis and inflammation. Int. J. Biochem. Cell Biol. 2010, 42, 21–24. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Chai, J.-D.; Head-Gordon, M. Long-range corrected hybrid density functionals with damped atom-atom dispersion corrections. Phys. Chem. Chem. Phys. 2008, 10, 6615–6620. [Google Scholar] [CrossRef] [PubMed]
- Hehre, W.J.; Radom, L.; Schleyer, P.v.R.; Pople, J.A. Ab Initio Molecular Orbital Theory; Wiley: New York, NY, USA, 1986. [Google Scholar] [CrossRef]
- Abbasi, M.; Farnia, S.M.F.; Tahghighi, A. The possibility of applying some heteroatom-decorated g-C3N4 heterocyclic nanosheets for delivering 5-aminosalicylic acid anti-inflammatory agent. Chem. Heterocycl. Comp. 2024, 60, 655–662. [Google Scholar] [CrossRef]
- Łapczuk-Krygier, A.; Kazimierczuk, K.; Pikies, J.; Ríos-Gutiérrez, M. A Comprehensive Experimental and Theoretical Study on the [{(η5-C5H5)2Zr[P(µ-PNEt2)2P(NEt2)2P]}]2O Crystalline System. Molecules 2021, 26, 7282. [Google Scholar] [CrossRef]
- Demchuk, O.M.; Jasiński, R.; Strzelecka, D.; Dziuba, K.; Kula, K.; Chrzanowski, J.; Krasowska, D. A clean and simple method for deprotection of phosphines from borane complexes. Pure Appl. Chem. 2018, 90, 49–62. [Google Scholar] [CrossRef]
- Ryachi, K.; Mohammad-Salim, H.; Al-Sadoon, M.K.; Zeroual, A.; de Julián-Ortiz, J.V.; Idrissi, M.E.; Tounsi, A. Quantum study of the [3 + 2] cycloaddition of nitrile oxide and carvone oxime: Insights into toxicity, pharmacokinetics, and mechanism. Chem. Heterocycl. Comp. 2024, 60, 646–654. [Google Scholar] [CrossRef]
- Abdoul-Hakim, M.; Kenzy, C.; Subramaniam, M.; Zeroual, A.; Syed, A.; Bahkali, A.H.; Garmes, H.; Verma, M.; Wang, S. Elucidating chemoselectivity and unraveling the mechanism of 1,3-dipolar cycloaddition between diphenyl nitrilimine and (isoxazol-3-yl)methylbenzimidazole through molecular electron density theory. Chem. Heterocycl. Comp. 2024, 60, 617–626. [Google Scholar] [CrossRef]
- Fryźlewicz, A.; Olszewska, A.; Zawadzińska, K.; Woliński, P.; Kula, K.; Kącka-Zych, A.; Łapczuk-Krygier, A.; Jasiński, R. On the Mechanism of the Synthesis of Nitrofunctionalised Δ2-Pyrazolines via [3 + 2] Cycloaddition Reactions between α-EWG-Activated Nitroethenes and Nitrylimine TAC Systems. Organics 2022, 3, 59–76. [Google Scholar] [CrossRef]
- Schlegel, H.B. Optimization of equilibrium geometries and transition structures. J. Comput. Chem. 1982, 3, 214–218. [Google Scholar] [CrossRef]
- Schlegel, H.B. Geometry Optimization on Potential Energy Surfaces. In Modern Electronic Structure Theory; Yarkony, D.R., Ed.; World Scientific Publishing: Singapore, 1994; pp. 459–501. [Google Scholar] [CrossRef]
- Fukui, K. Formulation of the reaction coordinate. J. Phys. Chem. 1970, 74, 4161–4163. [Google Scholar] [CrossRef]
- Gonzalez, C.; Schlegel, H.B. Reaction path following in mass-weighted internal coordinates. J. Phys. Chem. 1990, 94, 5523–5527. [Google Scholar] [CrossRef]
- Gonzalez, C.; Schlegel, H.B. Improved algorithms for reaction path following: Higher order implicit algorithms. J. Chem. Phys. 1991, 95, 5853–5860. [Google Scholar] [CrossRef]
- Noury, S.; Krokidis, X.; Fuster, F.; Silvi, B. Computational tools for the electron localization function topological analysis. Comput. Chem. 1999, 23, 597–604. [Google Scholar] [CrossRef]
- Dennington, R.; Keith, T.A.; Millam, J.M. GaussView, Version 6.0; Semichem Inc.: Shawnee, KS, USA, 2016. [Google Scholar]
- Ayachit, U. The ParaView Guide: A Parallel Visualization Application; Kitware Inc.: New York, NY, USA, 2015. [Google Scholar]
- McNutt, A.T.; Francoeur, P.; Aggarwal, R.; Masuda, T.; Meli, R.; Ragoza, M.; Sunseri, J.; Koes, D.R. GNINA 1.0: Molecular docking with deep learning. J. Cheminform. 2021, 13, 43. [Google Scholar] [CrossRef] [PubMed]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- RCSB PDB Protein Data Bank. Available online: https://www.rcsb.org/ (accessed on 15 February 2025).


| [eV] | 1a | 1b | 1c | 1d | 1e | 2 |
|---|---|---|---|---|---|---|
| Electronic chemical potential, μ | −4.02 | −3.99 | −4.09 | −4.35 | −4.78 | −5.71 |
| Chemical hardness, η | 8.84 | 8.82 | 8.91 | 8.81 | 8.19 | 8.89 |
| Global electrophilicity, ω | 0.91 | 0.90 | 0.94 | 1.07 | 1.40 | 1.83 |
| Global nucleophilicity, N | 2.39 | 2.43 | 2.28 | 2.07 | 1.94 | 0.67 |
| Reaction | Structure | ΔH | ΔS | ΔG |
|---|---|---|---|---|
| 1a + 2 | TS3a | 6.3 | −44.7 | 17.2 |
| TS4a | 8.0 | −52.5 | 19.7 | |
| 3a | −46.5 | −49.2 | −31.8 | |
| 4a | −48.0 | −50.9 | −30.8 | |
| 1b + 2 | TS3b | 10.6 | −40.5 | 22.7 |
| TS4b | 13.1 | −42.7 | 25.8 | |
| 3b | −43.2 | −45.8 | −29.7 | |
| 4b | −44.3 | −52.2 | −27.6 | |
| 1c + 2 | TS3c | 10.3 | −46.1 | 24.1 |
| TS4c | 12.7 | −48.0 | 27.0 | |
| 3c | −43.1 | −51.7 | −27.6 | |
| 4c | −43.4 | −52.2 | −28.9 | |
| 1d + 2 | TS3d | 11.0 | −47.2 | 25.0 |
| TS4d | 13.1 | −47.7 | 27.3 | |
| 3d | −42.5 | −52.2 | −27.9 | |
| 4d | −43.4 | −57.2 | −28.3 | |
| 1e + 2 | TS3e | 11.8 | −45.4 | 25.4 |
| TS4e | 13.6 | −47.1 | 27.7 | |
| 3e | −43.4 | −51.2 | −28.2 | |
| 4e | −44.1 | −52.4 | −28.5 |
| Reaction | Path | Structure | O1-C5 | C3-C4 | Δl | GEDT | ||
|---|---|---|---|---|---|---|---|---|
| r | lO1-C5 | r | lC3-C4 | |||||
| 1a + 2 | A | TS3a | 2.067 | 0.513 | 2.246 | 0.550 | 0.04 | 0.13 |
| TS4a | 2.072 | 0.616 | 2.228 | 0.546 | 0.07 | 0.13 | ||
| B | 3a | 1.426 | 1.510 | |||||
| 4a | 1.497 | 1.532 | ||||||
| 1b + 2 | A | TS3b | 2.081 | 0.520 | 2.235 | 0.541 | 0.02 | 0.12 |
| TS4b | 2.113 | 0.466 | 2.191 | 0.560 | 0.09 | 0.12 | ||
| B | 3b | 1.427 | 1.510 | |||||
| 4b | 1.378 | 1.521 | ||||||
| 1c + 2 | A | TS3c | 2.093 | 0.523 | 2.230 | 0.534 | 0.01 | 0.11 |
| TS4c | 2.120 | 0.462 | 2.192 | 0.559 | 0.10 | 0.11 | ||
| B | 3c | 1.428 | 1.510 | |||||
| 4c | 1.378 | 1.521 | ||||||
| 1d + 2 | A | TS3d | 2.113 | 0.530 | 2.217 | 0.522 | 0.01 | 0.12 |
| TS4d | 2.135 | 0.475 | 2.187 | 0.560 | 0.09 | 0.11 | ||
| B | 3d | 1.429 | 1.509 | |||||
| 4d | 1.400 | 1.519 | ||||||
| 1e + 2 | A | TS3e | 2.127 | 0.537 | 2.207 | 0.514 | 0.02 | 0.10 |
| TS4e | 2.145 | 0.447 | 2.183 | 0.563 | 0.12 | 0.09 | ||
| B | 3e | 1.431 | 1.509 | |||||
| 4e | 1.381 | 1.519 | ||||||
| TS3a | S1 | S2 | S3 | S4 | 3a | |
|---|---|---|---|---|---|---|
| d(O1-C5) | 2.067 | 2.039 | 1.912 | 1.817 | 1.725 | 1.426 |
| d(C3-C4) | 2.246 | 2.177 | 1.995 | 1.868 | 1.759 | 1.510 |
| V(O1,N2) | 1.42 | 1.40 | 1.32 | 1.26 | 1.21 | 1.08 |
| V(N2) | 2.06 | 2.16 | 2.40 | 2.54 | 2.64 | 2.79 |
| V(N2,C3) | 2.21 | 2.01 | 1.83 | 1.77 | 1.72 | 1.66 |
| V′(N2,C3) | 2.13 | 2.07 | 1.91 | 1.83 | 1.77 | 1.61 |
| V(C4,C5) | 3.44 | 2.96 | 2.50 | 2.31 | 2.21 | 2.04 |
| V(O1) | 2.83 | 2.81 | 2.85 | 2.57 | 2.53 | 2.38 |
| V′(O1) | 2.83 | 2.81 | 2.85 | 2.57 | 2.53 | 2.38 |
| V″(O1) | 0.37 | |||||
| V(C3) | 0.50 | |||||
| V(C4) | 0.24 | |||||
| V(C5) | 0.23 | 0.32 | ||||
| V(C3,C4) | 1.29 | 1.53 | 1.69 | 2.02 | ||
| V(O1,C5) | 0.86 | 1.64 |
| Compound | 4XCT [5 Å] | 3KK6 [5 Å] | 4ZVT [5 Å] | |||
|---|---|---|---|---|---|---|
| CNN Pose Score | CNN Affinity | CNN Pose Score | CNN Affinity | CNN Pose Score | CNN Affinity | |
| 3a | 0.8792 | 4.949 | 0.9258 | 6.255 | 0.8800 | 4.682 |
| 3b | 0.8909 | 4.935 | 0.9098 | 6.215 | 0.7703 | 4.038 |
| 3c | 0.8657 | 4.824 | 0.8412 | 6.046 | 0.7438 | 4.522 |
| 3d | 0.9036 | 5.691 | 0.9031 | 6.252 | 0.7681 | 4.671 |
| 3e | 0.8511 | 5.318 | 0.7835 | 6.016 | 0.8679 | 4.779 |
| Compound | 4XCT [3.5 Å] | 3KK6 [3.5 Å] | 4ZVT [3.5 Å] | |||
| CNN Pose Score | CNN Affinity | CNN Pose Score | CNN Affinity | CNN Pose Score | CNN Affinity | |
| (4R,5R)-3a | 0.9242 | 5.688 | 0.9322 | 6.382 | 0.8904 | 4.635 |
| (4S,5S)-3a | 0.8631 | 5.810 | 0.9189 | 6.321 | 0.8368 | 4.445 |
| (4R,5R)-3b | 0.9084 | 5.531 | 0.9098 | 6.329 | 0.8297 | 4.5800 |
| (4S,5S)-3b | 0.8627 | 5.808 | 0.9428 | 6.159 | 0.8231 | 4.353 |
| (4R,5R)-3c | 0.9101 | 5.469 | 0.8629 | 6.172 | 0.8145 | 4.5800 |
| (4S,5S)-3c | 0.8421 | 5.298 | 0.9041 | 6.028 | 0.8125 | 4.365 |
| (4R,5R)-3d | 0.9335 | 5.687 | 0.9113 | 6.453 | 0.7800 | 4.584 |
| (4S,5S)-3d | 0.8761 | 5.593 | 0.9402 | 6.285 | 0.8392 | 4.520 |
| (4R,5R)-3e | 0.8603 | 5.326 | 0.8152 | 6.354 | 0.8144 | 4.775 |
| (4S,5S)-3e | 0.8405 | 5.313 | 0.8242 | 6.076 | 0.7911 | 4.691 |
| Compound | 4XCT [3.5 Å] | 3KK6 [3.5 Å] | 4ZVT [3.5 Å] |
|---|---|---|---|
| Affinity | |||
| (4R,5R)-3a | −9.36 | −7.84 | −5.82 |
| (4S,5S)-3a | −8.66 | −7.47 | −5.19 |
| (4R,5R)-3b | −9.62 | −8.11 | −6.00 |
| (4S,5S)-3b | −8.92 | −8.41 | −5.74 |
| (4R,5R)-3c | −9.43 | −8.19 | −5.99 |
| (4S,5S)-3c | −7.38 | −8.20 | −5.67 |
| (4R,5R)-3d | −9.25 | −8.01 | −5.94 |
| (4S,5S)-3d | −7.69 | −8.17 | −6.09 |
| (4R,5R)-3e | −6.15 | −7.77 | −6.33 |
| (4S,5S)-3e | −6.41 | −8.71 | −6.70 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zawadzińska-Wrochniak, K.; Kula, K.; Ríos-Gutiérrez, M.; Gostyński, B.; Krawczyk, T.; Jasiński, R. A Comprehensive Study of the Synthesis, Spectral Characteristics, Quantum–Chemical Molecular Electron Density Theory, and In Silico Future Perspective of Novel CBr3-Functionalyzed Nitro-2-Isoxazolines Obtained via (3 + 2) Cycloaddition of (E)-3,3,3-Tribromo-1-Nitroprop-1-ene. Molecules 2025, 30, 2149. https://doi.org/10.3390/molecules30102149
Zawadzińska-Wrochniak K, Kula K, Ríos-Gutiérrez M, Gostyński B, Krawczyk T, Jasiński R. A Comprehensive Study of the Synthesis, Spectral Characteristics, Quantum–Chemical Molecular Electron Density Theory, and In Silico Future Perspective of Novel CBr3-Functionalyzed Nitro-2-Isoxazolines Obtained via (3 + 2) Cycloaddition of (E)-3,3,3-Tribromo-1-Nitroprop-1-ene. Molecules. 2025; 30(10):2149. https://doi.org/10.3390/molecules30102149
Chicago/Turabian StyleZawadzińska-Wrochniak, Karolina, Karolina Kula, Mar Ríos-Gutiérrez, Bartłomiej Gostyński, Tomasz Krawczyk, and Radomir Jasiński. 2025. "A Comprehensive Study of the Synthesis, Spectral Characteristics, Quantum–Chemical Molecular Electron Density Theory, and In Silico Future Perspective of Novel CBr3-Functionalyzed Nitro-2-Isoxazolines Obtained via (3 + 2) Cycloaddition of (E)-3,3,3-Tribromo-1-Nitroprop-1-ene" Molecules 30, no. 10: 2149. https://doi.org/10.3390/molecules30102149
APA StyleZawadzińska-Wrochniak, K., Kula, K., Ríos-Gutiérrez, M., Gostyński, B., Krawczyk, T., & Jasiński, R. (2025). A Comprehensive Study of the Synthesis, Spectral Characteristics, Quantum–Chemical Molecular Electron Density Theory, and In Silico Future Perspective of Novel CBr3-Functionalyzed Nitro-2-Isoxazolines Obtained via (3 + 2) Cycloaddition of (E)-3,3,3-Tribromo-1-Nitroprop-1-ene. Molecules, 30(10), 2149. https://doi.org/10.3390/molecules30102149









