Nuclear Magnetic Resonance Analysis Seeking for Metabolic Markers of Hypertension in Human Serum
Abstract
1. Introduction
2. Results
2.1. Sociodemographic and Clinical Data
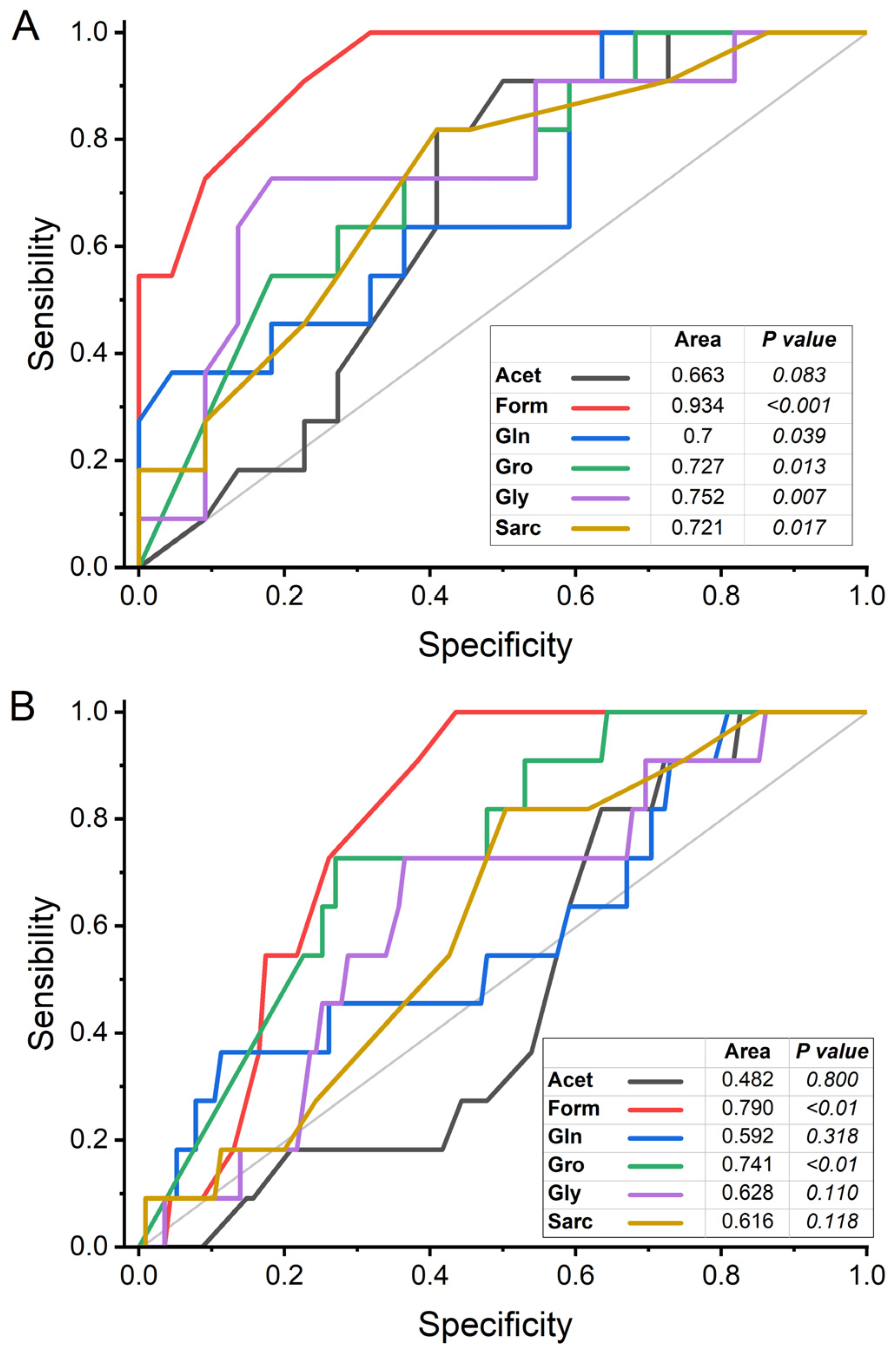
2.2. Univariate Analysis of Metabolites
2.3. Analysis of Possible Influence of Drug Treatments
3. Discussion
4. Materials and Methods
4.1. Population, Data, and Groups
4.2. Data and Sample Collection and Processing
4.3. NMR Experiment, Analysis, and Quantification
4.4. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACEi | Angiotensin-Converting Enzyme inhibitors |
| ARA | Angiotensin-Receptor Antagonists |
| BAA | Beta-Adrenergic Antagonists |
| BP | Blood Pressure |
| CCB | Calcium Channel Blockers |
| WHO | World Health Organization |
References
- World Health Organization. Global Report on Hypertension: The Race Against a Silent Killer; World Health Organization: Geneva, Switzerland, 2023. [Google Scholar]
- Louca, P.; Nogal, A.; Moskal, A.; Goulding, N.J.; Shipley, M.J.; Alkis, T.; Lindbohm, J.V.; Hu, J.; Kifer, D.; Wang, N.; et al. Cross-sectional blood metabolite markers of hypertension: A multicohort analysis of 44,306 Individuals from the Consortium of Metabolomics Studies. Metabolites 2022, 12, 601. [Google Scholar] [CrossRef] [PubMed]
- Ameta, K.; Gupta, A.; Kumar, S.; Sethi, R.; Kumar, D.; Mahdi, A.A. Essential hypertension: A filtered serum based metabolomics study. Sci. Rep. 2017, 7, 2153. [Google Scholar] [CrossRef]
- Bai, Q.; Peng, B.; Wu, X.; Cao, Y.; Sun, X.; Hong, M.; Na, R.; Liu, B.; Li, Q.; Li, Z.; et al. Metabolomic study for essential hypertension patients based on dried blood spot mass spectrometry approach. IUBMB Life 2018, 70, 777–785. [Google Scholar] [CrossRef] [PubMed]
- Ke, C.; Zhu, X.; Zhang, Y.; Shen, Y. Metabolomic characterization of hypertension and dyslipidemia. Metabolomics 2018, 14, 117. [Google Scholar] [CrossRef]
- Arnett, D.K.; Claas, S.A. Omics of blood pressure and hypertension. Circ. Res. 2018, 122, 1409–1419. [Google Scholar] [CrossRef]
- Au, A.; Cheng, K.-K.; Wei, L.K. Metabolomics, lipidomics and pharmacometabolomics of human hypertension. In Hypertension: From Basic Research to Clinical Practice; Islam, M.S., Ed.; Springer International Publishing: Cham, Switzerland, 2017; pp. 599–613. [Google Scholar]
- Tsiropoulou, S.; McBride, M.; Padmanabhan, S. Urine metabolomics in hypertension research. In Hypertension: Methods and Protocols; Touyz, R.M., Schiffrin, E.L., Eds.; Springer: New York, NY, USA, 2017; pp. 61–68. [Google Scholar]
- Tzoulaki, I.; Iliou, A.; Mikros, E.; Elliott, P. An Overview of metabolic phenotyping in blood pressure research. Curr. Hypertens. Rep. 2018, 20, 78. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Wang, J.; Yu, Y.; Zhao, M.; Yang, W.; Liu, J.; Zhao, Y.; Yang, Y.; Wang, G.; Guo, L.; et al. Alterations of gut microbiota are associated with blood pressure: A cross-sectional clinical trial in Northwestern China. J. Transl. Med. 2023, 21, 429. [Google Scholar] [CrossRef]
- Yang, T.; Santisteban, M.M.; Rodriguez, V.; Li, E.; Ahmari, N.; Carvajal, J.M.; Zadeh, M.; Gong, M.; Qi, Y.; Zubcevic, J.; et al. Gut dysbiosis is linked to hypertension. Hypertension 2015, 65, 1331–1340. [Google Scholar] [CrossRef]
- Li, J.; Zhao, F.; Wang, Y.; Chen, J.; Tao, J.; Tian, G.; Wu, S.; Liu, W.; Cui, Q.; Geng, B.; et al. Gut microbiota dysbiosis contributes to the development of hypertension. Microbiome 2017, 5, 14. [Google Scholar] [CrossRef]
- Holmes, E.; Loo, R.L.; Stamler, J.; Bictash, M.; Yap, I.K.; Chan, Q.; Ebbels, T.; De Iorio, M.; Brown, I.J.; Veselkov, K.A.; et al. Human metabolic phenotype diversity and its association with diet and blood pressure. Nature 2008, 453, 396–400. [Google Scholar] [CrossRef]
- Zheng, Y.; Yu, B.; Alexander, D.; Mosley, T.H.; Heiss, G.; Nettleton, J.A.; Boerwinkle, E. Metabolomics and incident hypertension among blacks. Hypertension 2013, 62, 398–403. [Google Scholar] [CrossRef]
- Hao, Y.; Wang, Y.; Xi, L.; Li, G.; Zhao, F.; Qi, Y.; Liu, J.; Zhao, D. A nested case-control study of association between metabolome and hypertension risk. Biomed Res. Int. 2016, 2016, 7646979. [Google Scholar] [CrossRef] [PubMed]
- Krzemińska, J.; Wronka, M.; Młynarska, E.; Franczyk, B.; Rysz, J. Arterial hypertension-oxidative stress and inflammation. Antioxidants 2022, 11, 172. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, S.; Floegel, A.; Weikert, C.; Prehn, C.; Adamski, J.; Pischon, T.; Boeing, H.; Drogan, D. Identification of serum metabolites associated with incident hypertension in the european prospective investigation into cancer and nutrition–potsdam study. Hypertension 2016, 68, 471–477. [Google Scholar] [CrossRef]
- Wang, L.; Hou, E.; Wang, L.; Wang, Y.; Yang, L.; Zheng, X.; Xie, G.; Sun, Q.; Liang, M.; Tian, Z. Reconstruction and analysis of correlation networks based on GC-MS metabolomics data for young hypertensive men. Anal. Chim. Acta 2015, 854, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Mels, C.M.; Delles, C.; Louw, R.; Schutte, A.E. Central systolic pressure and a nonessential amino acid metabolomics profile: The African Prospective study on the Early Detection and Identification of Cardiovascular disease and Hypertension. J. Hypertens. 2019, 37, 1157–1166. [Google Scholar] [CrossRef]
- García-Puig, J.; Ruilope, L.M.; Luque, M.; Fernández, J.; Ortega, R.; Dal-Ré, R. Glucose metabolism in patients with essential hypertension. Am. J. Med. 2006, 119, 318–326. [Google Scholar] [CrossRef]
- Palmu, J.; Tikkanen, E.; Havulinna, A.S.; Vartiainen, E.; Lundqvist, A.; Ruuskanen, M.O.; Perola, M.; Ala-Korpela, M.; Jousilahti, P.; Würtz, P.; et al. Comprehensive biomarker profiling of hypertension in 36985 Finnish individuals. J. Hypertens. 2022, 40, 579–587. [Google Scholar] [CrossRef]
- Pinto, E. Blood pressure and ageing. Postgrad. Med. J. 2007, 83, 109–114. [Google Scholar] [CrossRef]
- Lima, R.; Wofford, M.; Reckelhoff, J.F. Hypertension in postmenopausal women. Curr. Hypertens. Rep. 2012, 14, 254–260. [Google Scholar] [CrossRef]
- Marques da Silva, P.; Lima, M.J.; Neves, P.M.; Espiga de Macedo, M. Prevalence of cardiovascular risk factors and other comorbidities in patients with hypertension in Portuguese primary health care populations: The PRECISE study. Rev. Port. Cardiol. (Engl. Ed.) 2019, 38, 427–437. [Google Scholar] [CrossRef] [PubMed]
- Lauder, L.; Mahfoud, F.; Azizi, M.; Bhatt, D.L.; Ewen, S.; Kario, K.; Parati, G.; Rossignol, P.; Schlaich, M.P.; Teo, K.K.; et al. Hypertension management in patients with cardiovascular comorbidities. Eur. Heart J. 2022, 44, 2066–2077. [Google Scholar] [CrossRef]
- Rodrigues, A.P.; Gaio, V.; Kislaya, I.; Graff-Iversen, S.; Cordeiro, E.; Silva, A.C.; Namorado, S.; Barreto, M.; Gil, A.P.; Antunes, L.; et al. Sociodemographic disparities in hypertension prevalence: Results from the first Portuguese National Health Examination Survey. Rev. Port. Cardiol. (Engl. Ed.) 2019, 38, 547–555. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Yu, Z.; Deng, S.; Chen, X.; Chen, L.; Guo, Z.; Zheng, H.; Chen, L.; Cai, D.; Wen, B.; et al. A targeted metabolomics mrm-ms study on identifying potential hypertension biomarkers in human plasma and evaluating acupuncture effects. Sci. Rep. 2016, 6, 25871. [Google Scholar] [CrossRef]
- Zhao, H.; Liu, Y.; Li, Z.; Song, Y.; Cai, X.; Liu, Y.; Zhang, T.; Yang, L.; Li, L.; Gao, S.; et al. Identification of essential hypertension biomarkers in human urine by non-targeted metabolomics based on UPLC-Q-TOF/MS. Clin. Chim. Acta 2018, 486, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.; Zhang, J.P.; Nuermaimaiti, A.G.; Yunusi, K.X. Study on plasmatic metabolomics of Uygur patients with essential hypertension based on nuclear magnetic resonance technique. Eur. Rev. Med. Pharmacol. Sci. 2014, 18, 3673–3680. [Google Scholar]
- Liu, Y.; Chen, T.; Qiu, Y.; Cheng, Y.; Cao, Y.; Zhao, A.; Jia, W. An ultrasonication-assisted extraction and derivatization protocol for GC/TOFMS-based metabolite profiling. Anal. Bioanal. Chem. 2011, 400, 1405–1417. [Google Scholar] [CrossRef][Green Version]
- Linderman, G.C.; Lu, J.; Lu, Y.; Sun, X.; Xu, W.; Nasir, K.; Schulz, W.; Jiang, L.; Krumholz, H.M. Association of body mass index with blood pressure among 1.7 million chinese adults. JAMA Netw. Open 2018, 1, e181271. [Google Scholar] [CrossRef]
- van Deventer, C.A.; Lindeque, J.Z.; van Rensburg, P.J.; Malan, L.; van der Westhuizen, F.H.; Louw, R. Use of metabolomics to elucidate the metabolic perturbation associated with hypertension in a black South African male cohort: The SABPA study. J. Am. Soc. Hypertens. 2015, 9, 104–114. [Google Scholar] [CrossRef]
- Ríos-Covián, D.; Ruas-Madiedo, P.; Margolles, A.; Gueimonde, M.; de Los Reyes-Gavilán, C.G.; Salazar, N. Intestinal short chain fatty acids and their link with diet and human health. Front. Microbiol. 2016, 7, 185. [Google Scholar] [CrossRef]
- Jama, H.A.; Marques, F.Z. Gut microbial metabolites lower blood pressure in patients with hypertension. Nat. Cardiovasc. Res. 2023, 2, 18–19. [Google Scholar]
- Marques, F.Z.; Nelson, E.; Chu, P.Y.; Horlock, D.; Fiedler, A.; Ziemann, M.; Tan, J.K.; Kuruppu, S.; Rajapakse, N.W.; El-Osta, A.; et al. High-fiber diet and acetate supplementation change the gut microbiota and prevent the development of hypertension and heart failure in hypertensive mice. Circulation 2017, 135, 964–977. [Google Scholar] [CrossRef] [PubMed]
- Lamarre, S.G.; Morrow, G.; Macmillan, L.; Brosnan, M.E.; Brosnan, J.T. Formate: An essential metabolite, a biomarker, or more? Clin Chem Lab Med 2013, 51, 571–578. [Google Scholar] [CrossRef] [PubMed]
- Gil-Redondo, R.; Conde, R.; Bruzzone, C.; Seco, M.L.; Bizkarguenaga, M.; González-Valle, B.; de Diego, A.; Laín, A.; Habisch, H.; Haudum, C.; et al. MetSCORE: A molecular metric to evaluate the risk of metabolic syndrome based on serum NMR metabolomics. Cardiovasc. Diabetol. 2024, 23, 272. [Google Scholar] [CrossRef]
- Oliveira, N.; Sousa, A.; Amaral, A.; Graça, G.; Verde, I. Searching for metabolic markers of stroke in human plasma via NMR analysis. Int. J. Mol. Sci. 2023, 24, 16173. [Google Scholar] [CrossRef]
- Mahendran, Y.; Cederberg, H.; Vangipurapu, J.; Kangas, A.J.; Soininen, P.; Kuusisto, J.; Uusitupa, M.; Ala-Korpela, M.; Laakso, M. Glycerol and fatty acids in serum predict the development of hyperglycemia and type 2 diabetes in Finnish men. Diabetes Care 2013, 36, 3732–3738. [Google Scholar] [CrossRef]
- Rybka, J.; Kupczyk, D.; Kędziora-Kornatowska, K.; Motyl, J.; Czuczejko, J.; Szewczyk-Golec, K.; Kozakiewicz, M.; Pawluk, H.; Carvalho, L.A.; Kędziora, J. Glutathione-related antioxidant defense system in elderly patients treated for hypertension. Cardiovasc. Toxicol. 2011, 11, 1–9. [Google Scholar] [CrossRef]
- Lin, C.; Sun, Z.; Mei, Z.; Zeng, H.; Zhao, M.; Hu, J.; Xia, M.; Huang, T.; Wang, C.; Gao, X.; et al. The causal associations of circulating amino acids with blood pressure: A Mendelian randomization study. BMC Med. 2022, 20, 414. [Google Scholar] [CrossRef] [PubMed]
- Dziedzic, M.; Józefczuk, E.; Guzik, T.J.; Siedlinski, M. Interplay between plasma glycine and branched-chain amino acids contributes to the development of hypertension and coronary heart disease. Hypertension 2024, 81, 1320–1331. [Google Scholar] [CrossRef]
- Díaz-Flores, M.; Cruz, M.; Duran-Reyes, G.; Munguia-Miranda, C.; Loza-Rodríguez, H.; Pulido-Casas, E.; Torres-Ramírez, N.; Gaja-Rodriguez, O.; Kumate, J.; Baiza-Gutman, L.A.; et al. Oral supplementation with glycine reduces oxidative stress in patients with metabolic syndrome, improving their systolic blood pressure. Can. J. Physiol. Pharmacol. 2013, 91, 855–860. [Google Scholar] [CrossRef]
- Ding, Y.; Svingen, G.F.; Pedersen, E.R.; Gregory, J.F.; Ueland, P.M.; Tell, G.S.; Nygård, O.K. Plasma glycine and risk of acute myocardial infarction in patients with suspected stable angina pectoris. J. Am. Heart Assoc. 2015, 5, e002621. [Google Scholar] [CrossRef]
- Merino, J.; Leong, A.; Liu, C.T.; Porneala, B.; Walford, G.A.; von Grotthuss, M.; Wang, T.J.; Flannick, J.; Dupuis, J.; Levy, D.; et al. Metabolomics insights into early type 2 diabetes pathogenesis and detection in individuals with normal fasting glucose. Diabetologia 2018, 61, 1315–1324. [Google Scholar] [CrossRef]
- Wang, H.; Ni, X.; Dong, W.; Qin, W.; Xu, L.; Jiang, Y. Accurately quantified plasma free glycine concentration as a biomarker in patients with acute ischemic stroke. Amino Acids 2023, 55, 385–402. [Google Scholar] [CrossRef]
- Durante, W. The emerging role of L-glutamine in cardiovascular health and disease. Nutrients 2019, 11, 2092. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Xu, L.; Li, J.; Wang, H.; Sun, J.; Yu, Z.; Zhao, X.; Zhao, M.; Xi, B. The association of dietary glutamine supplementation with the development of high salt-induced hypertension in rats. Front. Nutr. 2022, 9, 1011739. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Rhee, E.P.; Larson, M.G.; Lewis, G.D.; McCabe, E.L.; Shen, D.; Palma, M.J.; Roberts, L.D.; Dejam, A.; Souza, A.L.; et al. Metabolite profiling identifies pathways associated with metabolic risk in humans. Circulation 2012, 125, 2222–2231. [Google Scholar] [CrossRef] [PubMed]
- Goïta, Y.; Chao de la Barca, J.M.; Keïta, A.; Diarra, M.B.; Dembélé, K.C.; Chabrun, F.; Dramé, B.S.I.; Kassogué, Y.; Diakité, M.; Mirebeau-Prunier, D.; et al. Sexual dimorphism of metabolomic profile in arterial hypertension. Sci. Rep. 2020, 10, 7517. [Google Scholar] [CrossRef]
- Yuan, C.; Chen, H.X.; Hou, H.T.; Wang, J.; Yang, Q.; He, G.W. Protein biomarkers and risk scores in pulmonary arterial hypertension associated with ventricular septal defect: Integration of multi-omics and validation. Am. J. Physiol. Lung Cell Mol. Physiol. 2020, 319, L810–L822. [Google Scholar] [CrossRef]
- Yousefi, M.; Qujeq, D.; Shafi, H.; Tilaki, K.H. Serum and urine levels of sarcosine in benign prostatic hyperplasia and newly diagnosed prostate cancer patients. J. Kermanshah Univ. Med. Sci. 2020, 24, e97000. [Google Scholar] [CrossRef]
- Sreekumar, A.; Poisson, L.M.; Rajendiran, T.M.; Khan, A.P.; Cao, Q.; Yu, J.; Laxman, B.; Mehra, R.; Lonigro, R.J.; Li, Y.; et al. Metabolomic profiles delineate potential role for sarcosine in prostate cancer progression. Nature 2009, 457, 910–914. [Google Scholar] [CrossRef]
- Reisberg, B.; Ferris, S.H.; de Leon, M.J.; Crook, T. The global deterioration scale for assessment of primary degenerative dementia. Am. J. Psychiatry 1982, 139, 1136–1139. [Google Scholar] [PubMed]
- Mathuranath, P.S.; Nestor, P.J.; Berrios, G.E.; Rakowicz, W.; Hodges, J.R. A brief cognitive test battery to differentiate Alzheimer’s disease and frontotemporal dementia. Neurology 2000, 55, 1613–1620. [Google Scholar] [CrossRef] [PubMed]
- Peixoto, B.; Machado, M.; Rocha, P.; Macedo, C.; Machado, A.; Baeta, E.; Goncalves, G.; Pimentel, P.; Lopes, E.; Monteiro, L. Validation of the Portuguese version of Addenbrooke’s Cognitive Examination III in mild cognitive impairment and dementia. Adv. Clin. Exp. Med. Off. Organ. Wroc. Med. Univ. 2018, 27, 781–786. [Google Scholar] [CrossRef] [PubMed]
- Bizkarguenaga, M.; Bruzzone, C.; Gil-Redondo, R.; SanJuan, I.; Martin-Ruiz, I.; Barriales, D.; Palacios, A.; Pasco, S.T.; González-Valle, B.; Laín, A.; et al. Uneven metabolic and lipidomic profiles in recovered COVID-19 patients as investigated by plasma NMR metabolomics. NMR Biomed. 2022, 35, e4637. [Google Scholar] [CrossRef]
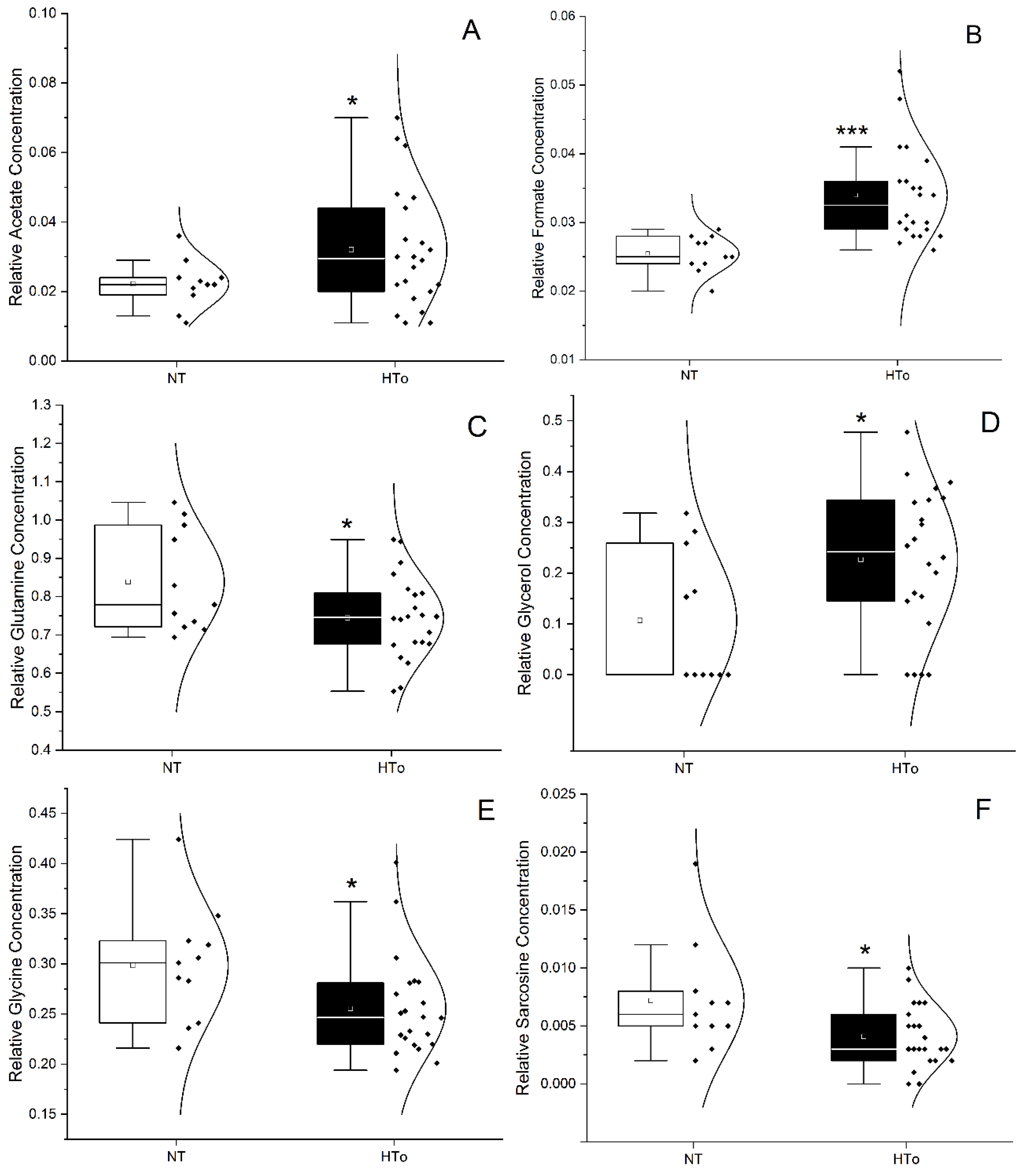
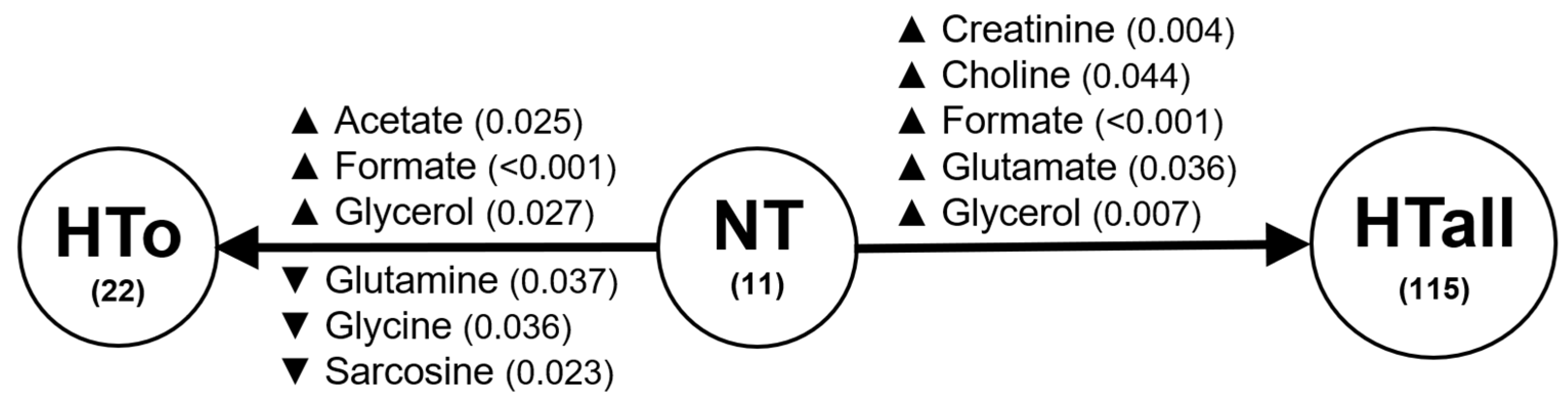

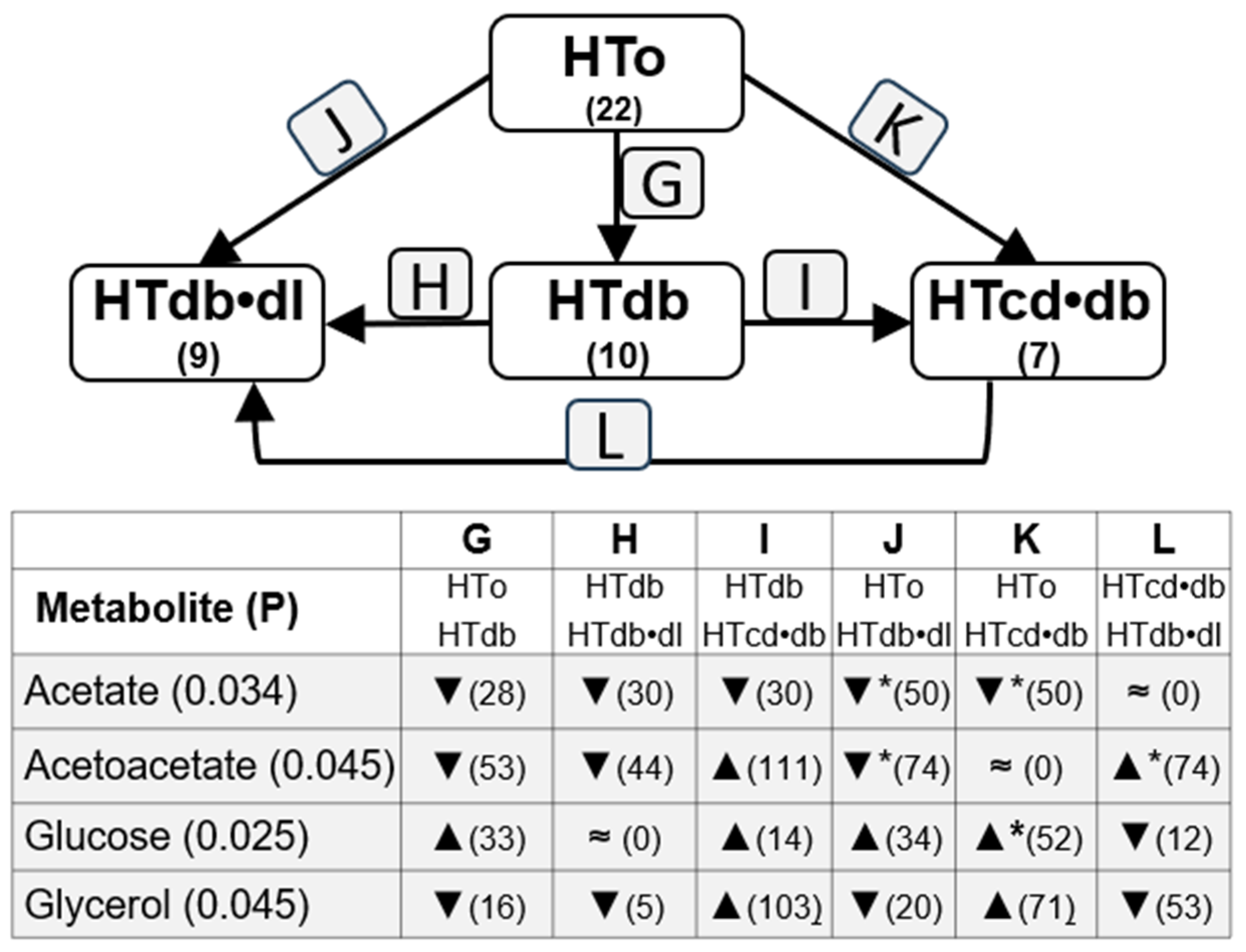
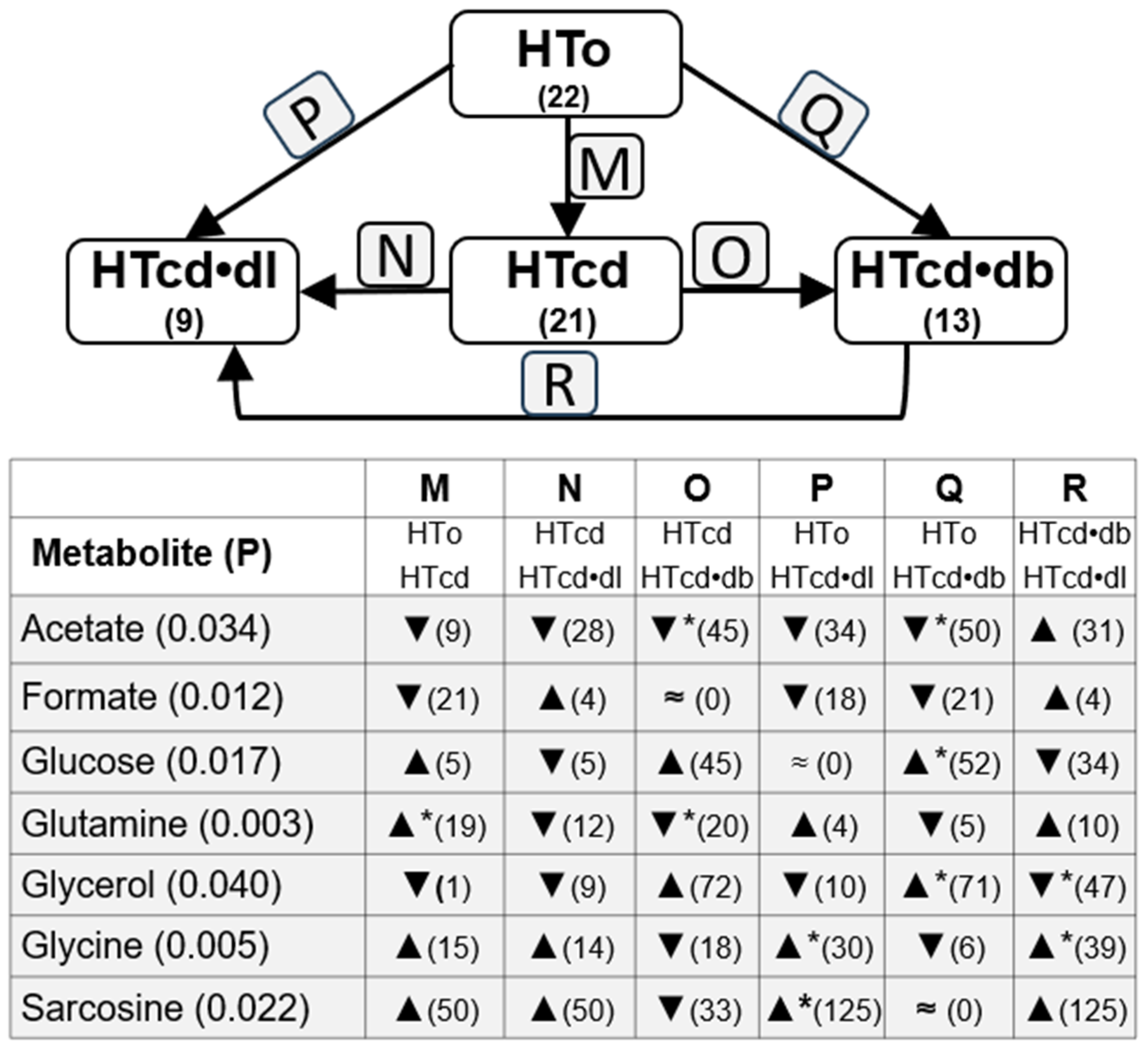

| PARAMETERS | NT | HTall | p Value | HTo | p Value |
|---|---|---|---|---|---|
| SOCIODEMOGRAPHIC DATA | |||||
| Number (n) | 11 | 115 | 22 | ||
| Age (years) | 77.82 ± 3.45 | 84.23 ± 0.69 | 0.048 | 81.32 ± 2.15 | 0.376 |
| Sex, female (% (n)) | 45.50 (5) | 71.30 (82) | 0.080 | 54.50 (12) | 0.645 |
| Body mass index (kg/m2) | 25.09 ± 1.51 | 27.58 ± 0.51 | 0.057 | 27.29 ± 1.76 | 0.394 |
| BLOOD PRESSURE | |||||
| Systolic blood pressure (mmHg) | 125.64 ± 3.14 | 126.32 ± 2.10 | 0.429 | 126.32 ± 4.27 | 0.898 |
| Diastolic blood pressure (mmHg) | 69.73 ± 3.71 | 69.08 ± 0.99 | 0.423 | 72.43 ± 2.12 | 0.501 |
| BIOCHEMICAL TEST RESULTS | |||||
| Serum glucose (mg/dL) | 94.65 ± 5.12 | 99.20 ± 2.62 | 0.298 | 94.32 ± 2.72 | 0.950 |
| Serum triglycerides (mg/dL) | 112.03 ± 12.15 | 115.64 ± 5.38 | 0.419 | 108.27 ± 2.02 | 0.821 |
| Serum total cholesterol (mg/dL) | 187.63 ± 8.65 | 164.74 ± 4.58 | 0.064 | 190.37 ± 8.96 | 0.845 |
| Serum HDL cholesterol (mg/dL) | 59.56 ± 3.96 | 54.51 ± 1.15 | 0.099 | 55.32 ± 2.39 | 0.340 |
| Serum LDL cholesterol (mg/dL) | 105.67 ± 7.05 | 87.11 ± 3.98 | 0.078 | 113.39 ± 9.34 | 0.584 |
| COGNITIVE TESTS RESULTS | |||||
| Revised Addenbrooke’s Cognitive Test (ACE-R) | 48.20 ± 5.34 | 48.22 ± 2.00 | 0.998 | 46.70 ± 4.75 | 0.847 |
| Mini-Mental State Examination (MMSE) | 19.45 ± 1.42 | 17.60 ± 0.58 | 0.322 | 17.10 ± 1.58 | 0.332 |
| Global Deterioration Scale (GDS) | 3.18 ± 0.33 | 3.11 ± 0.15 | 0.877 | 3.24 ± 0.34 | 0.917 |
| TREATMENTS | NT | HTall | p Value NT vs. HTall | HTo | p Value NT vs. HTo |
|---|---|---|---|---|---|
| Number (n) | 11 | 115 | 22 | ||
| Antiarrhythmics | 0.0 (0) | 4.3 (5) | 0.628 | 0.0 (0) | - |
| Antianginal | 0.0 (0) | 14.8 (17) | 0.189 | 4.5 (1) | 0.667 |
| ACEi | 0.0 (0) | 23.5 (27) | 0.062 | 27.3 (6) | 0.067 |
| ARA | 0.0 (0) | 53.0 (61) | <0.001 * | 45.5 (10) | 0.007 * |
| BAA | 0.0 (0) | 20.9 (24) | 0.088 | 13.6 (3) | 0.282 |
| CCB | 0.0 (0) | 26.1 (30) | 0.043 * | 13.6 (3) | 0.282 |
| Diuretics | 0.0 (0) | 69.6 (80) | <0.001 * | 50.0 (11) | 0.004 * |
| Venotropics | 18.2 (2) | 15.7 (18) | 0.550 | 13.6 (3) | 0.550 |
| Anticoagulants | 18.2 (2) | 59.1 (68) | 0.010 * | 54.5 (12) | 0.051 |
| Sulfonylureas | 0.0 (0) | 4.3 (5) | 0.628 | 0.0 (0) | - |
| Biguanides | 0.0 (0) | 17.4 (20) | 0.137 | 0.0 (0) | - |
| DPP-4 inhibitors | 0.0 (0) | 17.4 (20) | 0.137 | 0.0 (0) | - |
| Insulin | 0.0 (0) | 7.8 (9) | 0.427 | 0.0 (0) | - |
| Statins | 18.2 (2) | 46.1 (53) | 0.068 | 0.0 (0) | - |
| Bronchodilators | 0.0 (0) | 17.4 (20) | 0.137 | 0.0 (0) | - |
| AChE inhibitors | 0.0 (0) | 8.7 (10) | 0.598 | 13.6 (3) | 0.282 |
| MAO inhibitors | 0.0 (0) | 0.9 (1) | 1.000 | 0.0 (0) | - |
| NMDA antagonist | 0.0 (0) | 8.7 (10) | 0.598 | 4.5 (1) | 0.667 |
| Antiepileptics | 18.2 (2) | 4.3 (5) | 0.114 | 4.5 (1) | 0.252 |
| Antipsychotics | 36.4 (4) | 27.8 (32) | 0.509 | 36.4 (8) | 0.645 |
| Antidepressants | 27.3 (3) | 45.2 (52) | 0.346 | 40.9 (9) | 0.355 |
| THERAPIES (n) | Mean ± s.e.m. | Percent of Change | Mean ± s.e.m. | Percent of Change |
|---|---|---|---|---|
| ACETATE | ACETOACETATE | |||
| Without drugs (4) | 0.0295 ± 0.0063 | 0.0195 ± 0.0100 | ||
| ACEi (3) | 0.0380 ± 0.0120 | ▲28.8% | 0.0120 ± 0.0031 | ▼38.5% |
| ACEi + AnCoa (4) | 0.0155 ± 0.0045 | ▼47.5% | 0.0110 ± 0.0052 | ▼43.6% |
| ACEi + AnCoa + DIU (7) | 0.0249 ± 0.0040 | ▼15.6% | 0.0269 ± 0.0202 | ▲37.9% |
| ACEi + AnCoa + CCB + DIU (5) | 0.0342 ± 0.0068 | ▲15.9% | 0.0148 ± 0.0074 | ▼24.1% |
| ARA (8) | 0.0205 ± 0.0033 | ▼30.5% | 0.0184 ± 0.0106 | ▼5.6% |
| ARA + DIU (13) | 0.0257 ± 0.0044 | ▼12.9% | 0.0140 ± 0.0038 | ▼28.2% |
| ARA + AnCoa + DIU (13) | 0.0295 ± 0.0057 | 0% | 0.0173 ± 0.0045 | ▼11.3% |
| ARA + AnCoa + CCB (4) | 0.0323 ± 0.0112 | ▲9.5% | 0.0150 ± 0.0061 | ▼23.1% |
| ARA + AnCoa + DIU + CCB (7) | 0.0243 ± 0.0041 | ▼17.6% | 0.0209 ± 0.0061 | ▲7.2% |
| DIU (6) | 0.0275 ± 0.0072 | ▼6.8% | 0.0068 ± 0.0021 | ▼65.1% |
| DIU + AnCoa (5) | 0.0344 ± 0.0103 | ▲16.6% | 0.0152 ± 0.0045 | ▼22.1% |
| DIU + AnCoa +BAA (4) | 0.0245 ± 0.0032 | ▼16.9% | 0.0150 ± 0.0044 | ▼23.1% |
| FORMATE | GLUCOSE | |||
| Without drugs | 0.0373 ± 0.0071 | 5.311 ± 0.5468 | ||
| ACEi | 0.0323 ± 0.0034 | ▼13.4% | 5.1093 ± 0.7319 | ▼3.8% |
| ACEi + AnCoa | 0.0258 ± 0.0030 | ▼30.8% | 5.0998 ± 0.4259 | ▼4.0% |
| ACEi + AnCoa + DIU | 0.0329 ± 0.0019 | ▼11.8% | 5.2799 ± 0.3706 | ▼0.6% |
| ACEi + AnCoa + CCB + DIU | 0.0319 ± 0.0018 | ▼14.5% | 5.6760 ± 0.6433 | ▲6.9% |
| ARA | 0.0305 ± 0.0030 | ▼18.2% | 4.9129 ± 0.1766 | ▼7.5% |
| ARA + DIU | 0.0305 ± 0.0015 | ▼18.2% | 5.6579 ± 0.4689 | ▲6.5% |
| ARA + AnCoa + DIU | 0.0305 ± 0.0018 | ▼18.2% | 6.1078 ± 0.3766 | ▲15.0% |
| ARA + AnCoa + CCB | 0.0290 ± 0.0030 | ▼22.3% | 7.0460 ± 1.4553 | ▲32.7% |
| ARA + AnCoa + DIU + CCB | 0.0320 ± 0.0031 | ▼14.2% | 7.4503 ± 1.4988 | ▲40.3% |
| DIU | 0.0332 ± 0.0041 | ▼11.0% | 5.9345 ± 0.4818 | ▲11.7% |
| DIU + AnCoa | 0.0358 ± 0.0048 | ▼4.0% | 5.7498 ± 0.8535 | ▲8.3% |
| DIU + AnCoa +BAA | 0.0275 ± 0.0026 | ▼26.3% | 5.331 ± 0.7177 | ▲0.4% |
| THERAPIES | Mean ± s.e.m. | Percent of Change | Mean ± s.e.m. | Percent of Change |
|---|---|---|---|---|
| GLUTAMINE | GLYCEROL | |||
| Without drugs | 0.7973 ± 0.0315 | 0.2273 ± 0.0904 | ||
| ACEi | 0.7660 ± 0.1131 | ▼3.9% | 0.2147 ± 0.0618 | ▼5.5% |
| ACEi + AnCoa | 0.7408 ± 0.0308 | ▼7.1% | 0.2378 ± 0.1149 | ▲4.6% |
| ACEi + AnCoa + DIU | 0.8200 ± 0.0484 | ▲2.8% | 0.2577 ± 0.0706 | ▲13.4% |
| ACEi + AnCoa + CCB + DIU | 0.7490 ± 0.0333 | ▼6.1% | 0.1120 ± 0.0713 | ▼50.7% |
| ARA | 0.7973 ± 0.0315 | ▲9.8% | 0.2090 ± 0.0503 | ▼8.1% |
| ARA + DIU | 0.8758 ± 0.0444 | ▼3.2% | 0.3042 ± 0.0361 | ▲33.8% |
| ARA + AnCoa + DIU | 0.7717 ± 0.024 | ▼0.4% | 0.2142 ± 0.0451 | ▼5.8% |
| ARA + AnCoa + CCB | 0.7945 ± 0.0349 | ▼3.2% | 0.1223 ± 0.0727 | ▼46.2% |
| ARA + AnCoa + DIU + CCB | 0.7720 ± 0.0232 | ▼4.7% | 0.3059 ± 0.0806 | ▲34.6% |
| DIU | 0.8030 ± 0.0257 | ▲0.7% | 0.1487 ± 0.0722 | ▼34.6% |
| DIU + AnCoa | 0.8162 ± 0.0826 | ▲2.4% | 0.2228 ± 0.1068 | ▼2.0% |
| DIU + AnCoa +BAA | 0.7168 ± 0.0281 | ▼10.1% | 0.3155 ± 0.0582 | ▲38.8% |
| GLYCINE | SARCOSINE | |||
| Without drugs | 0.271 ± 0.0276 | 0.0040 ± 0.0011 | ||
| ACEi | 0.284 ± 0.034 | ▲4.8% | 0.0037 ± 0.0009 | ▼7.5% |
| ACEi + AnCoa | 0.2468 ± 0.0423 | ▼8.9% | 0.0063 ± 0.002 | ▲57.5% |
| ACEi + AnCoa + DIU | 0.3049 ± 0.0139 | ▲12.5% | 0.0056 ± 0.0018 | ▲40.0% |
| ACEi + AnCoa + CCB + DIU | 0.2668 ± 0.0713 | ▼1.5% | 0.0098 ± 0.0024 | ▲145.0% * |
| ARA | 0.3236 ± 0.0388 | ▲19.4% | 0.0073 ± 0.0023 | ▲82.5% |
| ARA + DIU | 0.2725 ± 0.0123 | ▲0.6% | 0.0049 ± 0.001 | ▲22.5% |
| ARA + AnCoa + DIU | 0.2502 ± 0.0106 | ▼7.7% | 0.0036 ± 0.001 | ▼10.0% |
| ARA + AnCoa + CCB | 0.2505 ± 0.0298 | ▼7.6% | 0.0095 ± 0.0029 | ▲137.5% |
| ARA + AnCoa + DIU + CCB | 0.2443 ± 0.0208 | ▼9.9% | 0.0056 ± 0.0011 | ▲40.0% |
| DIU | 0.2818 ± 0.0326 | ▲4.0% | 0.0032 ± 0.0012 | ▼20.0% |
| DIU + AnCoa | 0.3048 ± 0.0488 | ▲12.5% | 0.0068 ± 0.0047 | ▲70.0% |
| DIU + AnCoa +BAA | 0.3238 ± 0.0579 | ▲19.5% | 0.0063 ± 0.0015 | ▲57.5% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sousa, A.; Oliveira, N.; Conde, R.; Morais, E.; Amaral, A.P.; Embade, N.; Millet, O.; Verde, I. Nuclear Magnetic Resonance Analysis Seeking for Metabolic Markers of Hypertension in Human Serum. Molecules 2025, 30, 2145. https://doi.org/10.3390/molecules30102145
Sousa A, Oliveira N, Conde R, Morais E, Amaral AP, Embade N, Millet O, Verde I. Nuclear Magnetic Resonance Analysis Seeking for Metabolic Markers of Hypertension in Human Serum. Molecules. 2025; 30(10):2145. https://doi.org/10.3390/molecules30102145
Chicago/Turabian StyleSousa, Adriana, Nádia Oliveira, Ricardo Conde, Elisabete Morais, Ana Paula Amaral, Nieves Embade, Oscar Millet, and Ignacio Verde. 2025. "Nuclear Magnetic Resonance Analysis Seeking for Metabolic Markers of Hypertension in Human Serum" Molecules 30, no. 10: 2145. https://doi.org/10.3390/molecules30102145
APA StyleSousa, A., Oliveira, N., Conde, R., Morais, E., Amaral, A. P., Embade, N., Millet, O., & Verde, I. (2025). Nuclear Magnetic Resonance Analysis Seeking for Metabolic Markers of Hypertension in Human Serum. Molecules, 30(10), 2145. https://doi.org/10.3390/molecules30102145







