Biomedical Application of Nanogels: From Cancer to Wound Healing
Abstract
1. Background
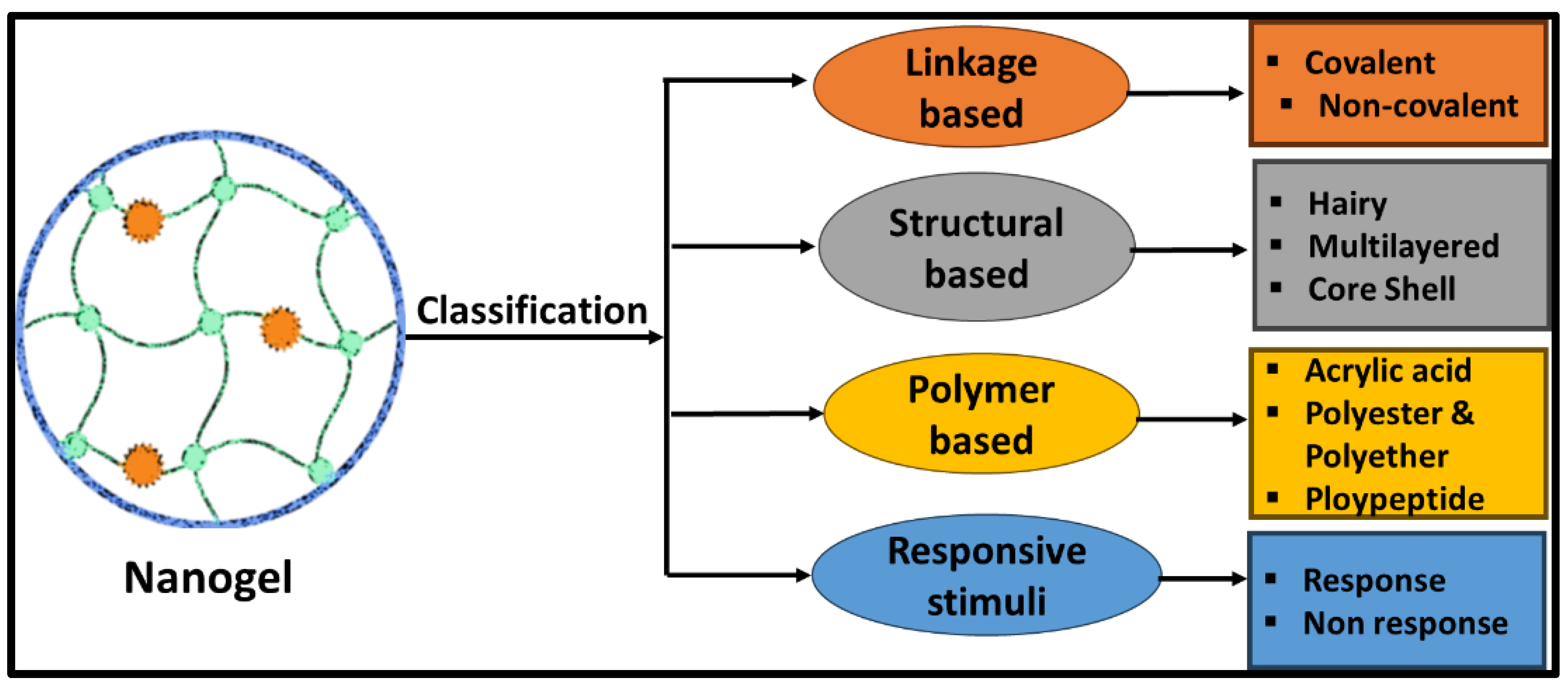
2. Classification of Nanogels
2.1. Linkage-Based Nanogel
2.2. Structural-Based Nanogels
2.2.1. Hollow Nanogels
2.2.2. Multilayered Nanogels
2.2.3. Hairy Nanogels
2.2.4. Core–Shell Nanogels
2.3. Polymer-Based Nanogels
2.4. Stimuli-Based Nanogels
3. Advantages and Shortcomings of Nanogels
4. Advantages of Biodegradable Nanogels
5. Nanogels for Bioimaging
6. Nanogels as Carrier for Drug Delivery
6.1. Diffusion
6.2. Erosion of the Nanogels Matrices
6.3. Ionic Exchange with the Environment
6.4. Stimuli Responsiveness
6.4.1. pH-Sensitive Release
6.4.2. Thermo-Sensitive Triggered Release
6.4.3. Magnetic Field-Responsive Release
6.4.4. Photo-Sensitive Release
6.4.5. Redox-Responsive Release
7. Nanogels as Carrier of Drug Delivery for Cancer Therapy
7.1. Nanogel Formulation for Breast Cancer
7.2. Nanogel Formulation for Skin Cancer
7.3. Nanogel Formulation for Colorectal Cancer
7.4. Nanogel Formulation for Prostate Cancer
7.5. Nanogel Formulation for Lung Cancer
7.6. Nanogel Formulation for Glioma Cancer
7.7. Nanogel Formulation for Ovarian Cancer
8. Nanogels as Carrier for Drug Delivery for Skin Dermal and Transdermal
9. Conclusions and Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Soni, K.S.; Desale, S.S.; Bronich, T.K. Nanogels: An overview of properties, biomedical applications and obstacles to clinical translation. J. Control Release 2016, 240, 109–126. [Google Scholar] [CrossRef] [PubMed]
- Anooj, E.S.; Charumathy, M.; Sharma, V.; Vibala, B.V.; Gopukumar, S.T.; Jainab, S.B.; Vallinayagam, S. Nanogels: An overview of properties, biomedical applications, future research trends and developments. J. Mol. Struct. 2021, 1239, 130446. [Google Scholar] [CrossRef]
- Rolland, J.P.; Maynor, B.W.; Euliss, L.E.; Exner, A.E.; Denison, G.M.; DeSimone, J.M. Direct fabrication and harvesting of monodisperse, shape-specific nanobiomaterials. J. Am. Chem. Soc. 2005, 127, 10096–10100. [Google Scholar] [CrossRef]
- Kersey, F.R.; Merkel, T.J.; Perry, J.L.; Napier, M.E.; DeSimone, J.M. Effect of aspect ratio and deformability on nanoparticle extravasation through nanopores. Langmuir 2012, 28, 8773–8781. [Google Scholar] [CrossRef]
- Sivaram, A.J.; Rajitha, P.; Maya, S.; Jayakumar, R.; Sabitha, M. Nanogels for delivery, imaging and therapy. Nanobiotechnology 2015, 7, 509–533. [Google Scholar] [CrossRef]
- Kabanov, A.V.; Vinogradov, S.V. Nanogels as pharmaceutical carriers: Finite networks of infinite capabilities. Angew. Chem. Int. Ed. 2009, 48, 5418–5429. [Google Scholar] [CrossRef]
- Oh, J.K.; Drumright, R.; Siegwart, D.J.; Matyjaszewski, K. The development of microgels/nanogels for drug delivery applications. Prog. Polym. Sci. 2008, 33, 448–477. [Google Scholar] [CrossRef]
- Ayame, H.; Morimoto, N.; Akiyoshi, K. Self-assembled cationic nanogels for intracellular protein delivery. Bioconjug. Chem. 2008, 19, 882–890. [Google Scholar] [CrossRef]
- McAllister, K.; Sazani, P.; Adam, M.; Cho, M.J.; Rubinstein, M.; Samulski, R.J. Polymeric nanogels produced via inverse microemulsion polymerization as potential gene and antisense delivery agents. J. Am. Chem. Soc. 2002, 124, 15198–15207. [Google Scholar] [CrossRef]
- Ferozekhan, S.; Umashankar, M.S.; Narayanasamy, D.A. Comprehensive Review of Nanogel-Based Drug Delivery Systems. Cureus 2024, 4, 68633. [Google Scholar]
- Sasaki, Y.; Akiyoshi, K. Nanogel engineering for new nanobiomaterials: From chaperoning engineering to biomedical applications. Chem. Rec. 2010, 10, 366–376. [Google Scholar] [CrossRef] [PubMed]
- Yallapu, M.M.; Jaggi, M.; Chauhan, S.C. Design and engineering of nanogels for cancer treatment. Drug Discov. Today 2011, 16, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Tao, Q.; Zhong, J.; Wang, R.; Huang, Y. Ionic and Enzymatic Multiple-Crosslinked Nanogels for Drug Delivery. Polymers 2021, 13, 3565. [Google Scholar] [CrossRef] [PubMed]
- Mastella, P.; Todaro, B.; Luin, S. Nanogels: Recent Advances in Synthesis and Biomedical Applications. Nanomaterials 2024, 14, 1300. [Google Scholar] [CrossRef]
- Mura, S.; Nicolas, J.; Couvreur, P. Stimuli-responsive nanocarriers for drug delivery. Nat. Mater. 2013, 12, 991–1003. [Google Scholar] [CrossRef]
- Karg, M.; Pich, A.; Hellweg, T.; Hoare, T.; Lyon, L.A.; Crassous, J.J.; Suzuki, D.; Gumerov, R.A.; Schneider, S.; Potemkin, I.I.; et al. Nanogels and Microgels: From Model Colloids to Applications, Recent Developments, and Future Trends. Langmuir. 2019, 35, 6231–6255. [Google Scholar] [CrossRef]
- Shah, S.; Rangaraj, N.; Laxmikeshav, K.; Sampathi, S. Nanogels as drug carriers—Introduction, chemical aspects, release mechanisms and potential applications. Int. J. Pharm. 2020, 581, 119268. [Google Scholar] [CrossRef]
- Kumar, N.; Singh, S.; Sharma, P.; Kumar, B.; Kumar, A. Single-, Dual-, and Multi-Stimuli-Responsive Nanogels for Biomedical Applications. Gels 2024, 10, 61. [Google Scholar] [CrossRef]
- Zha, L.; Zhang, Y.; Yang, W. Monodisperse temperature-sensitive microcontainers. Adv. Mater. 2002, 14, 1090–1092. [Google Scholar] [CrossRef]
- Xing, Z.; Wang, C.; Yan, J. Dual stimuli responsive hollow nanogels with IPN structure for temperature controlling drug loading and pH triggering drug release. Soft Matter 2011, 7, 7992–7997. [Google Scholar] [CrossRef]
- Averick, S.; Magenau, A.; Simakova, A. Covalently incorporated protein-nanogels using AGET ATRP in an inverse miniemulsion. Polym. Chem. 2011, 2, 1476–1478. [Google Scholar] [CrossRef]
- Mandal, A.; Patel, P.; Pal, D.; Mitra, A.K. Multi-Layered Nanomicelles as Self-Assembled Nanocarrier Systems for Ocular Peptide Delivery. AAPS PharmSciTech. 2019, 20, 66. [Google Scholar] [CrossRef] [PubMed]
- Fu, W.; Luo, C.; Morin, E.A.; He, W.; Li, Z.; Zhao, B. UCST-type thermosensitive hairy nanogels synthesized by RAFT polymerization-induced self-assembly. ACS Macro Lett. 2017, 6, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yang, B.; Zhang, S. Facile synthesis of hairy microparticle-/nanoparticle-supported MacMillan and its application to Diels-Alder reaction in water. Colloid. Polym. Sci. 2017, 295, 573–582. [Google Scholar] [CrossRef]
- Carrot, G.; Rutot-Houzé, D.; Pottier, A. Surface-initiated ring-opening polymerization: A versatile method for nanoparticle ordering. Macromolecules 2002, 35, 8400–8404. [Google Scholar] [CrossRef]
- Gao, L.; Zabihi, F.; Ehrmann, S.; Hedtrich, S.; Haag, R. Supramolecular nanogels fabricated via host–guest molecular recognition as penetration enhancer for dermal drug delivery. J. Control Release 2019, 300, 64–72. [Google Scholar] [CrossRef]
- Neamtu, I.; Rusu, A.G.; Diaconu, A.; Nita, L.E.; Chiriac, A.P. Basic concepts and recent advances in nanogels as carriers for medical applications. Drug Deliv. 2017, 24, 539–557. [Google Scholar] [CrossRef]
- Zhang, S.; Zhao, B.; Jiang, H.; Wang, B.; Ma, B. Cationic lipids and polymers mediated vectors for delivery of siRNA. J. Control Release 2007, 18, 1–10. [Google Scholar] [CrossRef]
- Ballarín-González, B.; Howard, K.A. Polycation-based nanoparticle delivery of RNAi therapeutics: Adverse effects and solutions. Adv. Drug Deliv. Rev. 2012, 64, 1717–1729. [Google Scholar] [CrossRef]
- Jiang, Y.; Chen, J.; Deng, C.; Suuronen, E.J.; Zhong, Z. Click hydrogels, microgels and nanogels: Emerging platforms for drug delivery and tissue engineering. Biomaterials 2014, 35, 4969–4985. [Google Scholar] [CrossRef]
- Elmowafy, E.M.; Tiboni, M.; Soliman, M.E. Biocompatibility, biodegradation and biomedical applications of poly(lactic acid)/poly(lactic-co-glycolic acid) micro and nanoparticles. J. Pharm. Investig. 2019, 49, 347–380. [Google Scholar] [CrossRef]
- Zhao, C.; Zhou, B. Polyethyleneimine-Based Drug Delivery Systems for Cancer Theranostics. J. Funct. Biomater. 2022, 14, 12. [Google Scholar] [CrossRef]
- Li, Z.; Huang, J.; Wu, J. pH-Sensitive nanogels for drug delivery in cancer therapy. Biomater. Sci. 2021, 9, 574–589. [Google Scholar] [CrossRef]
- Pinelli, F.; Sacchetti, A.; Perale, G.; Rossi, F. Is nanoparticle functionalization a versatile approach to meet the challenges of drug and gene delivery? Ther. Deliv. 2020, 11, 401–404. [Google Scholar] [CrossRef]
- Wang, Y.; Lou, X.; Yang, L.; Hou, Y. Application of Chitosan-based Nanogel in Cancer Nanomedicine. Curr. Pharm. Des. 2025, 31, e13816128347060. [Google Scholar] [CrossRef]
- Loo, H.L.; Goh, B.H.; Lee, L.H.; Chuah, L.H. Application of chitosan-based nanoparticles in skin wound healing. Asian J. Pharm. Sci. 2022, 17, 299–332. [Google Scholar] [CrossRef]
- Wang, M.; Muhammad, T.; Gao, H.; Liu, J.; Liang, H. Targeted pH-responsive chitosan nanogels with Tanshinone IIA for enhancing the antibacterial/anti-biofilm efficacy. Int. J. Biol. Macromol. 2023, 237, 124177. [Google Scholar] [CrossRef]
- Wang, Z.; Ye, Q.; Yu, S.; Akhavan, B. Poly Ethylene Glycol (PEG)-Based Hydrogels for Drug Delivery in Cancer Therapy: A Comprehensive Review. Adv. Health. Mater. 2023, 12, 2300105. [Google Scholar] [CrossRef]
- Bai, Z.; Yang, Y.; Cui, Z.; Liang, W.; Zhang, X.; Zhang, Z.; Sun, J.; Liu, Z.; Li, K.; Shi, M.; et al. Double-targeted liposomes coated with matrix metallopeptidase-2-responsive polypeptide nanogel for chemotherapy and enhanced immunotherapy against cervical cancer. Mater. Today Bio. 2024, 30, 01412. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, F.; Chen, Y.; Liu, J.; Wang, X.; Chen, A.T.; Deng, G.; Zhang, H.; Liu, J.; Hong, Z.; et al. Targeted Delivery of CRISPR/Cas9-Mediated Cancer Gene Therapy via Liposome-Templated Hydrogel Nanoparticles. Adv. Funct. Mater. 2017, 27, 1703036. [Google Scholar] [CrossRef]
- Xu, X.; Liu, Y.; Fu, W.; Yao, M.; Ding, Z.; Xuan, J.; Li, D.; Wang, S.; Xia, Y.; Cao, M. Poly(N-isopropylacrylamide)-Based Thermoresponsive Composite Hydrogels for Biomedical Applications. Polymers 2020, 12, 580. [Google Scholar] [CrossRef]
- Ansari, M.J.; Rajendran, R.R.; Mohanto, S.; Agarwal, U.; Panda, K.; Dhotre, K.; Manne, R.; Deepak, A.; Zafar, A.; Yasir, M.; et al. Poly(N-isopropylacrylamide)-Based Hydrogels for Biomedical Applications: A Review of the State-of-the-Art. Gels 2022, 8, 454. [Google Scholar] [CrossRef]
- Kazakov, S. Liposome-Nanogel Structures for Future Pharmaceutical Applications: An Updated Review. Curr Pharm Des. 2016, 22, 1391-413. [Google Scholar] [CrossRef]
- Li, H.; Zhou, Z.; Zhang, F. A networked swellable dextrin nanogels loading Bcl2 siRNA for melanoma tumor therapy. Nano Res. 2018, 11, 4627–4642. [Google Scholar] [CrossRef]
- Islam, P.; Water, J.J.; Bohr, A.; Rantanen, J. Chitosan-Based Nano-Embedded Microparticles: Impact of Nanogel Composition on Physicochemical Properties. Pharmaceutics 2016, 9, 1. [Google Scholar] [CrossRef]
- Knop, K.; Hoogenboom, R.; Fischer, D.; Schubert, U.S. Poly(ethylene glycol) in drug delivery: Pros and cons as well as potential alternatives. Angew. Chem. Int. Ed. Engl. 2010, 9, 6288–6308. [Google Scholar] [CrossRef]
- Daniels, T.R.; Bernabeu, E.; Rodríguez, J.A.; Patel, S.; Kozman, M.; Chiappetta, D.A.; Holler, E.; Ljubimova, J.Y.; Helguera, G.; Penichet, M.L. The transferrin receptor and the targeted delivery of therapeutic agents against cancer. Biochim. Biophys. Acta. 2012, 1820, 291–317. [Google Scholar] [CrossRef]
- Gao, D.; Xu, H.; Philbert, M.A.; Kopelman, R. Bioeliminable nanohydrogels for drug delivery. Nano Lett. 2008, 8, 3320–3324. [Google Scholar] [CrossRef]
- Luanda, A.; Badalamoole, V. Past, present and future of biomedical applications of dextran-based hydrogels: A review. Int. J. Biol. Macromol. 2023, 228, 794–807. [Google Scholar] [CrossRef]
- Li, Y.L.; Rodrigues, J.; Tomás, H. Injectable and biodegradable hydrogels: Gelation, biodegradation and biomedical applications. Chem. Soc. Rev. 2012, 41, 2193–2221. [Google Scholar] [CrossRef]
- Balakrishnan, B.; Banerjee, R. Biopolymer-Based Hydrogels for Cartilage Tissue Engineering. Chem. Rev. 2011, 111, 4453–4474. [Google Scholar] [CrossRef]
- Pawar, S.N.; Edgar, K.J. Alginate derivatization: A review of chemistry, properties and applications. Biomaterials 2012, 33, 3279–3305. [Google Scholar] [CrossRef]
- Ma, D.; Tu, K.; Zhang, L.M. Bioactive Supramolecular Hydrogel with Controlled Dual Drug Release Characteristics. Biomacromolecules 2010, 11, 2204–2212. [Google Scholar] [CrossRef]
- Tully, S.E.; Mabon, R.; Gama, C.I.; Tsai, S.M.; Liu, X.W.; Hsieh-Wilson, L.C. A chondroitin sulfate small molecule that stimulates neuronal growth. J. Am. Chem. Soc. 2004, 126, 7736–7737. [Google Scholar] [CrossRef]
- Kumar, M.N.V.R.; Muzzarelli, R.A.A.; Muzzarelli, C.; Sashiwa, H.; Domb, A.J. Chitosan chemistry and pharmaceutical perspectives. Chem. Rev. 2004, 104, 6017–6084. [Google Scholar] [CrossRef]
- Mizrahy, S.; Peer, D. Polysaccharides as building blocks for nanotherapeutics. Chem. Soc. Rev. 2012, 41, 2623–2640. [Google Scholar] [CrossRef]
- Delair, T. Colloidal polyelectrolyte complexes of chitosan and dextran sulfate towards versatile nanocarriers of bioactive molecules. Eur. J. Pharm. Biopharm. 2011, 78, 10–18. [Google Scholar] [CrossRef]
- Higuchi, A.; Ling, Q.D.; Hsu, S.T.; Umezawa, A. Biomimetic Cell Culture Proteins as Extracellular Matrices for Stem Cell Differentiation. Chem. Rev. 2012, 112, 4507–4540. [Google Scholar] [CrossRef]
- Zhu, J.M. Bioactive modification of poly(ethylene glycol) hydrogels for tissue engineering. Biomaterials 2010, 31, 4639–4656. [Google Scholar] [CrossRef]
- Garcia-Fuentes, M.; Alonso, M.J. Chitosan-based drug nanocarriers: Where do we stand? J. Control. Release 2012, 161, 496–504. [Google Scholar] [CrossRef]
- Asadi, H.; Rostamizadeh, K.; Salari, D.; Hamidi, M. Preparation and characterization of tri-block poly(lactide)-poly(ethylene glycol)-poly(lactide) nanogels for controlled release of naltrexone. Int. J. Pharm. 2011, 416, 356–364. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.H.; Holzwarth, J.M.; Ma, P.X. Functionalized Synthetic Biodegradable Polymer Scaffolds for Tissue Engineering. Macromol. Biosci. 2012, 12, 911–919. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Maciel, D.; Rodrigues, J.; Shi, X.; Tomás, H. Biodegradable Polymer Nanogels for Drug/Nucleic Acid Delivery. Chem. Rev. 2015, 115, 8564–8608. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Li, F.; Wang, X.; Yu, J.; Wu, D. Hyaluronic acid and polyethylene glycol hybrid hydrogel encapsulating Nanogel with Hemostasis and sustainable antibacterial property for wound healing. ACS Appl. Mater. Interfaces 2018, 10, 13304–13316. [Google Scholar] [CrossRef]
- Molina, M.; Asadian-Birjand, M.; Balach, J.; Bergueiro, J.; Miceliac, E.; Calderón, M. Stimuli-responsive nanogel composites and their application in nanomedicine. Chem. Soc. Rev. 2015, 44, 6161–6186. [Google Scholar] [CrossRef]
- Sierra-Martin, B.; Fernandez-Barbero, A. Multifunctional hybrid nanogels for theranostic applications. Soft Matter 2015, 11, 8205–8216. [Google Scholar] [CrossRef]
- Wu, W.; Zhou, S. Hybrid micro-/nanogels for optical sensing and intracellular imaging. Nano Rev. 2010, 1, 1. [Google Scholar] [CrossRef]
- Wu, W.; Shen, J.; Banerjee, P.; Zhou, S. Core-shell hybrid nanogels for integration of optical temperature-sensing, targeted tumor cell imaging, and combined chemo-photothermal treatment. Biomaterials 2010, 31, 7555–7566. [Google Scholar] [CrossRef]
- Zhang, Q.M.; Xu, W.; Serpe, M.J. Optical devices constructed from multiresponsive microgels. Angew. Chem. Int. Ed. Engl. 2014, 53, 4827–4831. [Google Scholar] [CrossRef]
- Peng, H.S.; Stolwijk, J.A.; Sun, L.N.; Wegener, J.; Wolfbeis, O.S. A nanogel for ratiometric fluorescent sensing of intracellular pH values. Angew. Chem. Int. Ed. Engl. 2010, 49, 4246–4249. [Google Scholar] [CrossRef]
- Song, X.R.; Wang, X.; Yu, S.X.; Cao, J.; Li, S.H.; Li, J.; Liu, G.; Yang, H.H.; Chen, X. Co9 Se8 nanoplates as a new theranostic platform for photoacoustic/magnetic resonance dual-modal-imaging-guided chemo-photothermal combination therapy. Adv. Mater. 2015, 27, 3285–3291. [Google Scholar] [CrossRef] [PubMed]
- Gianella, A.; Jarzyna, P.A.; Mani, V.; Ramachandran, S.; Calcagno, C.; Tang, J.; Kann, B.; Dijk, W.J.; Thijssen, V.L.; Griffioen, A.W.; et al. Multifunctional nanoemulsion platform for imaging guided therapy evaluated in experimental cancer. ACS Nano. 2011, 5, 4422–4433. [Google Scholar] [CrossRef] [PubMed]
- Haigron, P.; Dillenseger, J.L.; Luo, L.; Coatrieux, J.L. Image-guided therapy: Evolution and breakthrough. IEEE Eng. Med. Biol. Mag. 2010, 29, 100–104. [Google Scholar] [CrossRef] [PubMed]
- Kolouchova, K.; Sedlacek, O.; Jirak, D.; Babuka, D.; Blahut, J.; Kotek, J.; Vit, M.; Trousil, J.; Konefał, R.; Janouskova, O.; et al. Self-Assembled Thermoresponsive Polymeric Nanogels for 19F MR Imaging. Biomacromolecules 2018, 19, 3515–3524. [Google Scholar] [CrossRef]
- Rejinold, N.S.; Ranjusha, R.; Balakrishnan, A.; Mohammed, N.; Jayakumar, R. Gold–chitin–manganese dioxide ternary composite nanogels for radio frequency assisted cancer therapy. RSC Adv. 2014, 4, 5819–5825. [Google Scholar] [CrossRef]
- Zan, M.; Li, J.; Huang, M.; Lin, S.; Luo, D.; Luo, S.; Ge, Z. Near-infrared light-triggered drug release nanogels for combined photothermal-chemotherapy of cancer. Biomater. Sci. 2015, 3, 147–1156. [Google Scholar] [CrossRef]
- Kimura, A.; Jo, J.I.; Yoshida, F.; Hong, Z.; Tabata, Y.; Sumiyoshi, A.; Taguchi, M.; Aoki, I. Ultra-small size gelatin nanogel as a blood brain barrier impermeable contrast agent for magnetic resonance imaging. Acta Biomater. 2021, 125, 290–299. [Google Scholar] [CrossRef]
- Jia, X.; Han, Y.; Pei, M.; Zhao, X.; Tian, K.; Zhou, T.; Liu, P. Multi-functionalized hyaluronic acid nanogels crosslinked with carbon dots as dual receptor-mediated targeting tumor theranostics. Carbohydr. Polym. 2016, 152, 391–397. [Google Scholar] [CrossRef]
- Das, S.; Mondal, S.; Ghosh, D. Carbon quantum dots in bioimaging and biomedicines. Front. Bioeng. Biotechnol. 2024, 11, 1333752. [Google Scholar] [CrossRef]
- Khatun, Z.; Nurunnabi, M.; Nafiujjaman, M.; Reeck, G.R.; Khan, H.A.; Cho, K.J.; Lee, Y.K. A hyaluronic acid nanogel for photo-chemo theranostics of lung cancer with simultaneous light-responsive controlled release of doxorubicin. Nanoscale 2015, 7, 10680–10689. [Google Scholar] [CrossRef]
- Peng, N.; Ding, X.; Wang, Z.; Cheng, Y.; Gong, Z.; Xu, X.; Gao, X.; Cai, Q.; Huang, S.; Liu, Y. Novel dual responsive alginate-based magnetic nanogels for onco-theranostics. Carbohydr. Polym. 2019, 204, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhai, Y.; Wang, J.; Zhai, G. New progress and prospects: The application of nanogel in drug delivery. Mater. Sci. Eng. C 2016, 60, 560–568. [Google Scholar] [CrossRef] [PubMed]
- Vashist, A.; Kaushik, A.; Vashist, A.; Bala, J.; Nikkhah-Moshaie, R.; Sagar, V.; Nair, M. Nanogels as potential drug nanocarriers for CNS drug delivery. Drug Discov. Today 2018, 23, 1436–1443. [Google Scholar] [CrossRef]
- Ashrafizadeh, M.; Tam, K.; Javadi, A. Synthesis and physicochemical properties of dual-responsive acrylic acid/butyl acrylate cross-linked nanogel systems. J. Colloid. Interface Sci. 2019, 556, 313–323. [Google Scholar] [CrossRef]
- Bae, K.; Mok, H.; Park, T. Synthesis, characterization, and intracellular delivery of reducible heparin nanogels for apoptotic cell death. Biomaterials 2008, 29, 3376–3383. [Google Scholar] [CrossRef]
- Morimoto, N.; Nomura, S.; Miyazawa, N. Nanogel engineered designs for polymeric drug delivery. Polym. Matrices Drug Part. Eng. 2006, 924, 88–101. [Google Scholar]
- Setia, A.; Ahuja, P. Nanohydrogels: Emerging trend for drug delivery. In Organic Materials as Smart Nanocarriers for Drug Delivery; William Andrew: Norwich, NY, USA, 2018; pp. 293–368. [Google Scholar]
- Ahmed, S.; Alhareth, K.; Mignet, N. Advancement in nanogel formulations provides controlled drug release. Int. J. Pharm. 2020, 584, 119435. [Google Scholar] [CrossRef]
- Li, J.; Mooney, D.J. Designing hydrogels for controlled drug delivery. Nat. Rev. Mater. 2016, 1, 16071. [Google Scholar] [CrossRef]
- Oerlemans, C.; Bult, W.; Bos, M.; Storm, G.; Nijsen, J.F.W.; Hennink, W.E. Polymeric micelles in anticancer therapy: Targeting, imaging and triggered release. Pharm. Res. 2010, 27, 2569–2589. [Google Scholar] [CrossRef]
- Soni, G.; Yadav, K.S. Nanogels as potential nanomedicine carrier for treatment of cancer: A mini review of the state of the art. Saudi Pharm. J. 2016, 24, 133–139. [Google Scholar] [CrossRef]
- GhavamiNejad, A.; SamariKhalaj, M.; Aguilar, L. pH/NIR Light-Controlled Multidrug Release via a Mussel-Inspired Nanocomposite Hydrogel for Chemo-Photothermal Cancer Therapy. Sci. Rep. 2016, 6, 33594. [Google Scholar] [CrossRef] [PubMed]
- Fleige, E.; Quadir, M.A.; Haag, R. Stimuli-responsive polymeric nanocarriers for the controlled transport of active compounds: Concepts and applications. Adv. Drug Deliv. Rev. 2012, 64, 866–884. [Google Scholar] [CrossRef] [PubMed]
- Sood, N.; Bhardwaj, A.; Mehta, S.; Mehta, A. Stimuli-responsive hydrogels in drug delivery and tissue engineering. Drug Deliv. 2016, 23, 748–770. [Google Scholar] [CrossRef]
- Klouda, L.; Mikos, A.G. Thermoresponsive hydrogels in biomedical applications. Eur. J. Pharm. Biopharm. 2008, 68, 34–45. [Google Scholar] [CrossRef]
- Bergueiro, J.; Calderón, M. Thermoresponsive nanodevices in biomedical applications. Macromol. Biosci. 2015, 15, 183–199. [Google Scholar] [CrossRef]
- Lu, X.; Sun, M.; Barron, A.E. Non-ionic, thermo-responsive DEA/DMA nanogels: Synthesis, characterization, and use for DNA separations by microchip electrophoresis. J. Colloid. Interface Sci. 2011, 357, 345–353. [Google Scholar] [CrossRef]
- Medeiros, S.; Santos, A.; Fessi, H.; Elaissari, A. Stimuli-responsive magnetic particles for biomedical applications. Int. J. Pharm. 2011, 403, 139–161. [Google Scholar] [CrossRef]
- Zha, L.; Banik, B.; Alexis, F. Stimulus responsive nanogels for drug delivery. Soft Matter 2011, 7, 5908–5916. [Google Scholar] [CrossRef]
- Murakami, Y.; Maeda, M. DNA-responsive hydrogels that can shrink or swell. Biomacromolecules 2005, 6, 2927–2929. [Google Scholar] [CrossRef]
- Satarkar, N.S.; Biswal, D.; Hilt, J.Z. Hydrogel nanocomposites: A review of applications as remote controlled biomaterials. Soft Matter 2010, 6, 2364–2371. [Google Scholar] [CrossRef]
- Ghaeini-Hesaroeiye, S.; Boddohi, S.; Vasheghani-Farahani, E. Dual responsive chondroitin sulfate based nanogel for antimicrobial peptide delivery. Int. J. Biol. Macromol. 2020, 143, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Chung, S.J.; Cho, H.J.; Kim, D.D. Bile acid-conjugated chondroitin sulfate A-based nanoparticles for tumor-targeted anticancer drug delivery. Eur. J. Pharm. Biopharm. 2015, 94, 532–541. [Google Scholar] [CrossRef] [PubMed]
- Tayeferad, M.; Boddohi, S.; Bakhshi, B. Dual-responsive nisin loaded chondroitin sulfate nanogel for treatment of bacterial infection in soft tissues. Int. J. Biol. Macromol. 2021, 193, 166–172. [Google Scholar] [CrossRef]
- Setayesh, A.; Bagheri, F.; Boddohi, S. Self-assembled formation of chondroitin sulfate-based micellar nanogel for curcumin delivery to breast cancer cells. Int. J. Biol. Macromol. 2020, 161, 771–778. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Cheng, Y.; Zhao, X.; Luo, Y.; Chen, J.; Yuan, W.E. Advances in redox-responsive drug delivery systems of tumor microenvironment. J. Nanobiotechnol. 2018, 16, 74. [Google Scholar] [CrossRef]
- Zhang, F.; Gong, S.; Wu, J.; Li, H.; Oupicky, D.; Sun, M. CXCR4-targeted and redox responsive dextrin nanogel for metastatic breast cancer therapy. Biomacromolecules 2017, 18, 1793–1802. [Google Scholar] [CrossRef]
- Tian, Y.; Lei, M.; Yan, L.; An, F. Diselenide-crosslinked zwitterionic nanogels with dual redox-labile properties for controlled drug release. Polym. Chem. 2020, 11, 2360–2369. [Google Scholar] [CrossRef]
- Meng, F.; Hennink, W.E.; Zhong, Z. Reduction-sensitive polymers and bioconjugates for biomedical applications. Biomaterials 2009, 30, 2180–2198. [Google Scholar] [CrossRef]
- Meng, F.; Cheng, R.; Deng, C.; Zhong, Z. Intracellular drug release nanosystems. Mater. Today 2012, 15, 436–442. [Google Scholar] [CrossRef]
- Lv, Q.; He, C.L.; Quan, F.L.; Yu, S.J. DOX/IL-2/IFN-gamma co-loaded thermo-sensitive polypeptide hydrogel for efficient melanoma treatment. Bioact. Mat. 2018, 3, 118–128. [Google Scholar]
- Ye, F.; Wen, J.; Yang, A.; Wang, Y.; Li, N.; Yu, P. The influence of hormone therapy on secondary diabetes mellitus in breast cancer: A meta-analysis. Clin. Breast Cancer 2021, 22, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Heit, J.A.; Silverstein, M.D.; Mohr, D.N.; Petterson, T.M.; O’Fallon, W.M.; Melton, L.J. Risk factors for deep vein thrombosis and pulmonary embolism: A population-based case-control study. Arch. Intern. Med. 2002, 160, 809–815. [Google Scholar] [CrossRef] [PubMed]
- Kaur, M.; Garg, T.; Rath, G. Current nanotechnological strategies for effective delivery of bioactive drug molecules in the treatment of tuberculosis. Crit. Rev. Ther. Drug Carr. Syst. 2014, 31, 49–88. [Google Scholar] [CrossRef] [PubMed]
- DePeaux, K.; Delgoffe, G.M. Metabolic barriers to cancer immunotherapy. Nat. Rev. Immunol. 2021, 21, 785–797. [Google Scholar] [CrossRef]
- Tang, L.; Zheng, Y.; Melo, M.B.; Mabardi, L.; Castano, A.P.; Xie, Y. Enhancing T cell therapy through TCR-signaling-responsive nanoparticle drug delivery. Nat. Biotechnol. 2018, 36, 707–716. [Google Scholar] [CrossRef]
- Saman, H.; Raza, S.S.; Uddin, S.; Rasul, K. Inducing angiogenesis, a key step in cancer vascularization, and treatment approaches. Cancers 2020, 12, 1172. [Google Scholar] [CrossRef]
- Su, S.; Wang, H.; Liu, X.; Wu, Y.; Nie, G. iRGD-coupled responsive fluorescent nanogel for targeted drug delivery. Biomaterials 2013, 34, 3523–3533. [Google Scholar] [CrossRef]
- Wang, K.; Tepper, J.E. Radiation therapy-associated toxicity: Etiology, management, and prevention. CA Cancer J. Clin. 2021, 71, 437–454. [Google Scholar] [CrossRef]
- Liu, K.; Zheng, D.; Zhao, J.; Tao, Y.; Wang, Y.; He, J. pH-Sensitive nanogels based on the electrostatic self-assembly of radionuclide 131I labeled albumin and carboxymethyl cellulose for synergistic combined chemo-radioisotope therapy of cancer. J. Mat. Chem. B 2018, 6, 4738–4746. [Google Scholar] [CrossRef]
- Tyler, B.; Fowers, K.D.; Li, K.W.; Recinos, V.R.; Caplan, J.M.; Hdeib, A. A thermal gel depot for local delivery of paclitaxel to treat experimental brain tumors in rats. J. Neurosurg. 2010, 113, 210–217. [Google Scholar] [CrossRef]
- Jiang, L.; Zhou, Q.; Mu, K.; Xie, H.; Zhu, Y.; Zhu, W. pH/temperature sensitive magnetic nanogels conjugated with Cy5.5-labled lactoferrin for MR and fluorescence imaging of glioma in rats. Biomaterials 2013, 34, 7418–7428. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.; Ouyang, B.; Xin, Y. Hypoxia-degradable and long-circulating zwitterionic phosphorylcholine-based nanogel for enhanced tumor drug delivery. Acta Pharm. Sin. B 2021, 11, 560–571. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Kyung Taek, O.h.; Sup Kwag, D.; Yeo Lee, S.; Jin Lee, D.; Seong Lee, E. Photoresponsive hyaluronate nanogel as an anticancer drug carrier. Polym. Adv. Technol. 2013, 24, 9791–9796. [Google Scholar] [CrossRef]
- Bardajee, G.R.; Hosseini, S.S.; Ghavami, S. Embedded of Nanogel into Multi-responsive Hydrogel Nanocomposite for Anticancer Drug Delivery. J. Inorg. Organomet. Polym. Mater. 2018, 28, 2196–2205. [Google Scholar] [CrossRef]
- Godau, B.; Samimi, S.; Seyfoori, A.; Samiei, E.; Khani, T.; Naserzadeh, P.; Najafabadi, A.H.; Lesha, E.; Majidzadeh-A, K.; Ashtari, B. A Drug-Eluting Injectable NanoGel for Localized Delivery of Anticancer Drugs to Solid Tumors. Pharmaceutics 2023, 15, 2255. [Google Scholar] [CrossRef]
- Song, E.; Han, W.; Li, C.; Cheng, D.; Li, L.; Liu, L.; Zhu, G.; Song, Y.; Tan, W. Hyaluronic Acid-Decorated Graphene Oxide Nanohybrids as Nanocarriers for Targeted and pH-Responsive Anticancer Drug Delivery. ACS Appl. Mater. Interfaces 2014, 6, 11882–11890. [Google Scholar] [CrossRef]
- Zhang, B.; Yan, Y.; Shen, Q.; Ma, D.; Huang, L.; Cai, X.; Tan, S. A colon targeted drug delivery system based on alginate modificated graphene oxide for colorectal liver metastasis. Mater. Sci. Eng. C 2017, 79, 185–190. [Google Scholar] [CrossRef]
- Pan, Q.; Lv, Y.; Williams, G.R.; Tao, L.; Yang, H.; Li, H.; Zhu, L. Lactobionic acid and carboxymethyl chitosan functionalized graphene oxide nanocomposites as targeted anticancer drug delivery systems. Carbohydr. Polym. 2016, 151, 812–820. [Google Scholar] [CrossRef]
- Gao, X.; Li, S.; Ding, F.; Liu, X.; Wu, Y.; Li, J.; Feng, J.; Zhu, X.; Zhang, C. A Virus-Mimicking Nucleic Acid Nanogel Reprograms Microglia and Macrophages for Glioblastoma Therapy. Adv. Mater. 2021, 33, 2006116. [Google Scholar] [CrossRef]
- Dinga, F.; Gaob, X.; Huanga, X.; Gea, H.; Xiea, M.; Qiana, J.; Songc, J.; Lid, J.; Zhua, X.; Zhang, C. Polydopamine-coated nucleic acid nanogel for siRNA-mediated lowtemperature photothermal therapy. Biomaterials 2020, 245, 119976. [Google Scholar]
- Zhang, W.; Tun, C.H. Redox-responsive cisplatin nanogels for anticancer drug delivery. Chem. Commun. 2018, 54, 8367–8370. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Yao, Y.; Zhang, J.; Gui, H.; Liu, J.; Liu, J. Tumor-Targeted Injectable Double-Network Hydrogel for Prevention of Breast Cancer Recurrence and Wound Infection via Synergistic Photothermal and Brachytherapy. Adv. Sci. 2022, 9, 24. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Zhang, Q.; Zhu, Q.; Gao, J.; Zhu, X.; Yu, H.; Li, Y.; Zhang, C. Copackaging photosensitizer and PD-L1 siRNA in a nucleic acid nanogel for synergistic cancer photoimmunotherapy. Sci. Adv. 2022, 8, 16. [Google Scholar] [CrossRef] [PubMed]
- Ding, F.; Huang, X.; Gao, X.; Xie, M.; Pan, G.; Li, Q.; Song, J.; Zhu, X.; Zhang, C. A non-cationic nucleic acid nanogel for the delivery of the CRISPR/Cas9 gene editing tool. Nanoscale 2019, 11, 17211–17215. [Google Scholar] [CrossRef]
- Xu, J.; Qiu, W.; Liang, M.; Ye, M.; Hu, J.; Ma, J.; Shi, X.; Xue, P.; Kang, Y.; Xiao, B.; et al. Dual-stimulus phototherapeutic nanogel for triggering pyroptosis to promote cancer immunotherapy. J. Control Release 2023, 358, 219–231. [Google Scholar] [CrossRef]
- Song, C.; Phuengkham, H.; Kim, Y.S. Syringeable immunotherapeutic nanogel reshapes tumor microenvironment and prevents tumor metastasis and recurrence. Nat. Commun. 2019, 10, 3745. [Google Scholar] [CrossRef]
- Zhang, D.; Li, Q.; Chen, X.; Nie, X.; Xue, F.; Xu, W.; Luan, Y. An Injectable Hydrogel to Modulate T Cells for Cancer Immunotherapy. Small 2022, 18, 2202663. [Google Scholar] [CrossRef]
- Mustafa, A.; Indiran, M.A.; Ramalingam, K. Anticancer potential of thiocolchicoside and lauric acid loaded chitosan nanogel against oral cancer cell lines: A comprehensive study. Sci. Rep. 2024, 14, 9270. [Google Scholar] [CrossRef]
- Si, X.; Ma, S.; Xu, Y.; Zhang, D.; Shen, N.; Yu, H. Hypoxia-sensitive supramolecular nanogels for the cytosolic delivery of ribonuclease A as a breast cancer therapeutic. J. Control. Release 2020, 320, 83–95. [Google Scholar] [CrossRef]
- Chen, J.; He, H.; Deng, C.; Yin, L.; Zhong, Z. Saporin-loaded CD44 and EGFR dual-targeted nanogels for potent inhibition of metastatic breast cancer in vivo. Int. J. Pharm. 2019, 560, 57–64. [Google Scholar] [CrossRef]
- Wild, C.P.; Weiderpass, E.; Stewart, B.W. (Eds.) World Cancer Report: Cancer Research for Cancer Prevention; International Agency for Research on Cancer: Lyon, France, 2020; Available online: http://publications.iarc.fr/586 (accessed on 4 February 2020).
- Sahu, P.; Kashaw, S.K.; Sau, S.; Kushwah, V.; Jain, S.; Agrawal, R.K. pH responsive 5-fluorouracil loaded biocompatible nanogels for topical chemotherapy of aggressive melanoma. Colloids Surf. B Biointerfaces 2019, 174, 232–245. [Google Scholar] [CrossRef] [PubMed]
- Bailly, C. Irinotecan: 25 years of cancer treatment. Pharmacol. Res. 2019, 148, 104398. [Google Scholar] [CrossRef] [PubMed]
- Liwei, X.; Tongqi, S.; Xinyue, X.; Lide, Z.; Li, S. Platelets membrane camouflaged irinotecan-loaded gelatin nanogels for in vivo colorectal carcinoma therapy. J. Drug Deliv. Sci. Technol. 2019, 53, 101190. [Google Scholar]
- Kaur, H.; Bruno, J.G.; Kumar, A.; Sharma, T.K. Aptamers in the Therapeutics and Diagnostics Pipelines. Theranostics 2018, 8, 4016–4032. [Google Scholar] [CrossRef]
- McNamara, J.O.; Andrechek, E.R.; Wang, Y.; Viles, K.D.; Rempel, R.E.; Gilboa, E. Cell type-specific delivery of siRNAs with aptamer-siRNA chimeras. Nat. Biotechnol. 2006, 24, 1005–1015. [Google Scholar] [CrossRef]
- Han, J.; Gao, L.; Wang, J.; Wang, J. Application and development of aptamer in cancer: From clinical diagnosis to cancer therapy. J. Cancer 2020, 11, 6902–6915. [Google Scholar] [CrossRef]
- Atabi, F.; Mousavi Gargari, S.L.; Hashemi, M.; Yaghmaei, P. Doxorubicin loaded DNA aptamer linked myristilated chitosan nanogel for targeted drug delivery to prostate cancer. Iran. J. Pharm. Res. 2017, 16, 35–49. [Google Scholar]
- Giacomini, I.; Ragazzi, E.; Pasut, G.; Montopoli, M. The pentose phosphate pathway and its involvement in cisplatin resistance. Int. J. Mol. Sci. 2020, 21, 937. [Google Scholar] [CrossRef]
- Sun, M.; He, L.; Fan, Z.; Tang, R.; Du, J. Effective treatment of drug-resistant lung cancer via a nanogel capable of reactivating cisplatin and enhancing early apoptosis. Biomaterials 2020, 257, 120252. [Google Scholar] [CrossRef]
- Sharma, A.; Lee, H.J. Ginsenoside Compound K: Insights into Recent Studies on Pharmacokinetics and Health-Promoting Activities. Biomolecules 2020, 10, 1028. [Google Scholar] [CrossRef]
- Xue, Z.; Fu, R.; Duan, Z.; Chi, L.; Zhu, C.; Fan, D. Inhibitory effect of pH-responsive nanogel encapsulating ginsenoside CK against lung cancer. Polymers 2021, 13, 1784. [Google Scholar] [CrossRef] [PubMed]
- Stawicki, B.; Schacher, T.; Cho, H. Nanogels as a versatile drug delivery system for brain cancer. Gels 2021, 7, 63. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Drude, N.; Blank, N.; Desai, P.B.; Königs, H.; Rütten, S.; Langen, K.; Möller Mottaghy, F.M.; Morgenroth, A. Protease Responsive Nanogels for Transcytosis across the Blood–Brain Barrier and Intracellular Delivery of Radiopharmaceuticals to Brain Tumor Cells. Adv. Health. Mater. Mater. 2021, 10, 2100812. [Google Scholar] [CrossRef] [PubMed]
- Song, P.; Song, N.; Li, L.; Wu, M.; Lu, Z.; Zhao, X. Angiopep-2-Modified carboxymethyl chitosan-based pH/reduction dual-stimuli-responsive nanogels for enhanced targeting glioblastoma. Biomacromolecules 2021, 22, 2921–2934. [Google Scholar] [CrossRef]
- Gadhave, D.; Rasal, N.; Sonawane, R.; Sekar, M.; Kokare, C. Nose-to-brain delivery of teriflunomide-loaded lipid-based carbopol-gellan gum nanogel for glioma: Pharmacological and in vitro cytotoxicity studies. Int. J. Biol. Macromol. 2021, 67, 906–920. [Google Scholar] [CrossRef]
- Cho, H.; Jammalamadaka, U.; Tappa, K.; Egbulefu, C.; Prior, J.; Tang, R. 3D printing of poloxamer 407 nanogel discs and their applications in adjuvant ovarian cancer therapy. Mol. Pharm. 2019, 16, 552–560. [Google Scholar] [CrossRef]
- Li, X.; Ouyang, Z.; Li, H.; Hu, C.; Saha, P.; Xing, L. Dendrimer-decorated nanogels: Efficient nanocarriers for bio distribution in vivo and chemotherapy of ovarian carcinoma. Bioact. Mat. 2021, 6, 3244–3253. [Google Scholar] [CrossRef]
- Limiti, E.; Mozetic, P.; Giannitelli, S.M.; Pinelli, F.; Han, X.; Del Rio, D. Hyaluronic acid–polyethyleneimine nanogels for controlled drug delivery in cancer treatment. ACS Appl. Nano Mater. 2022, 5, 5544–5557. [Google Scholar] [CrossRef]
- Yamaguchi, K.; Hiraike, O.; Iwaki, H.; Matsumiya, K.; Nakamura, N.; Sone, K. Intraperitoneal administration of a cisplatin-loaded nanogel through a hybrid system containing an alginic acid-based nanogel and an in situ cross-linkable hydrogel for peritoneal dissemination of ovarian cancer. Mol. Pharm. 2021, 18, 4090–4098. [Google Scholar] [CrossRef]
- Anastasiadis, S.H.; Chrissopoulou, K.; Stratakis, E.; Kavatzikidou, P.; Kaklamani, G.; Ranella, A. How the Physicochemical Properties of Manufactured Nanomaterials Affect Their Performance in Dispersion and Their Applications in Biomedicine: A Review. Nanomaterials 2022, 12, 552. [Google Scholar] [CrossRef]
- Algharib, S.A.; Dawood, A.; Zhou, K.; Chen, D.; Li, C.; Meng, K.; Maa, M.K.; Ahmed, S.; Huang, L.; Xie, S. Designing, structural determination and biological effects of rifaximin loaded chitosan- carboxymethyl chitosan nanogel. Carbohydr. Polym. 2020, 248, 116782. [Google Scholar] [CrossRef] [PubMed]
- Hama, S.; Kimura, Y.; Mikami, A.; Shiota, K.; Toyoda, M.; Tamura, A.; Nagasaki, Y.; Kanamura, K.; Kajimoto, K.; Kogure, K. Electric Stimulus Opens Intercellular Spaces in Skin. J. Biol. Chem. 2014, 289, 2450–2456. [Google Scholar] [CrossRef] [PubMed]
- Sabitha, M.; Sanoj Rejinold, N.; Nair, A.; Lakshmanan, V.K.; Nair, S.V.; Jayakumar, R. Development and evaluation of 5-fluorouracil loaded chitin nanogels for treatment of skin cancer. Carbohydr. Polym. 2013, 91, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Taha, M.; Alhakamy, N.A.; Md, S.; Ahmad, M.Z.; Rizwanullah, M.; Fatima, S.; Ahmed, N.; Alyazedi, F.M.; Karim, S.; Ahmad, J. Nanogels as Potential Delivery Vehicles in Improving the Therapeutic Efficacy of Phytopharmaceuticals. Polymers 2022, 14, 4141. [Google Scholar] [CrossRef]
- Nosrati, H.; Heydari, M.; Tootiaei, Z.; Ganjbar, S.; Khodaei, M.J. Delivery of antibacterial agents for wound healing applications using polysaccharide-based scaffolds. Drug Deliv. Sci. Technol. 2023, 84, 104516. [Google Scholar] [CrossRef]
- Eivazzadeh-Keihan, R.; Pajoum, Z.; Aliabadi, H.A.M.; Mohammadi, A.; Kashtiaray, A.; Bani, M.S.; Pishva, B.; Maleki, A.; Heravi, M.M.; Mahdavi, M.; et al. Magnetized chitosan hydrogel and silk fibroin, reinforced with PVA: A novel nanobiocomposite for biomedical and hyperthermia applications. RSC Adv. 2023, 13, 8540–8550. [Google Scholar] [CrossRef]
- Yuan, N.; Shao, K.; Huang, S.; Chen, C. Chitosan, alginate, hyaluronic acid and other novel multifunctional hydrogel dressings for wound healing: A review. Int. J. Biol. Macromol. 2023, 240, 124321. [Google Scholar] [CrossRef]
- Chen, K.; Liu, Y.; Liu, X. Hyaluronic acid-modified and verteporfin-loaded polylactic acid nanogels promote scarless wound healing by accelerating wound re-epithelialization and controlling scar formation. J. Nanobiotechnol. 2023, 21, 241. [Google Scholar] [CrossRef]
- Pathan, I.B.; Munde, S.J.; Shelke, S.; Ambekar, W.; Mallikarjuna Setty, C. Curcumin loaded fish scale collagen-HPMC nanogel for wound healing application: Ex-vivo and In-vivo evaluation. Int. J. Polym. Mater. Polym. Biomater. 2018, 68, 165–174. [Google Scholar] [CrossRef]
- Sakthiganapathi, M.; Yoganandam, G.P.; Gopal, V. Formulation, Characterization, and Evaluation of Wound Healing Potency of a Novel Mattan tailam Nanogel Based on a Famous Traditional Siddha Formula. Avicenna J. Med. Biotechnol. 2023, 15, 38–47. [Google Scholar] [CrossRef]
- Han, Q.; Wang, X.; Qiu, L.; Zhou, X.; Hui, Z.; Ni, X.; Xuan, Y.; Lei, X.; Wang, J. Gelatinase Responsive Nanogel for Antibacterial Phototherapy and Wound Healing. Gels 2022, 8, 397. [Google Scholar] [CrossRef]
- Ali, A.; Rahman, M.A.; Warsi, M.H.; Yusuf, M.; Alam, P. Development of Nanogel Loaded with Lidocaine for Wound-Healing: Illustration of Improved Drug Deposition and Skin Safety Analysis. Gels 2022, 8, 466. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.; Singh, R.; Badhwar, R.; Gupta, T.; Popli, H. Development and optimization of Clitoria teratea synthesized silver nanoparticles and its application to nanogel systems for wound healing. Drug Dev. Ind. Pharm. 2024, 50, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Yang, Z.; Tao, X.; Ma, C.; Cao, P.; Wei, P.; Jiang, C.; Ren, H.; Li, X. Sprayable chitosan nanogel with nitric oxide to accelerate diabetic wound healing through bacteria inhibition, biofilm eradication and macrophage polarization. Int. J. Biol. Macromol. 2024, 254, 27806. [Google Scholar] [CrossRef] [PubMed]
- Noori, F.; Osanloo, M.; Moradi, H.R. Fabrication, characterization, and in vivo implantation of eugenol-loaded nanogels and PCL/Cs electrospun nanofibers for wound healing applications. J. Bioact. Compat. Pol. 2023, 38, 480–492. [Google Scholar] [CrossRef]
- Chen, R.N.; Lee, L.W.; Chen, L.C.; Ho, H.O.; Lui, S.C.; Sheu, M.T.; Su, C.H. Wound-healing effect of micronized sacchachitin (mSC) nanogel on corneal epithelium. Int. J. Nanomed. 2012, 7, 4697–4706. [Google Scholar]
- Han, X.; Saengow, C.; Ju, L. Exosome-coated oxygen nanobubble-laden hydrogel augments intracellular delivery of exosomes for enhanced wound healing. Nat. Commun. 2024, 15, 3435. [Google Scholar] [CrossRef]
- Chaithanya, K.J.; Mahalaxmi, C.; Patil Banerjee, M.; Ashvini, H.M. Preparation and Characterization of Ficus Lacor Metallic particles Based Nanogel for Wound Healing activity. Int. J. Curr. Pharm. Res. 2024, 16, 0975–7066. [Google Scholar]
- Xu, J.; Xu, J.J.; Lin, Q.; Jiang, L.; Zhang, D.; Li, Z.; Ma, B.; Zhang, C.; Li, L.; Kai, D.; et al. Lignin-Incorporated Nanogel Serving as an Antioxidant Biomaterial for Wound Healing. CS Appl. Bio Mater. 2021, 4, 3–13. [Google Scholar] [CrossRef]
- Barkat, M.A.; Harshita; Ahmad, I.; Ali, R.; Singh, S.P.; Pottoo, F.H.; Beg, S.; Ahmad, F.J. Nanosuspension-Based Aloe vera Gel of Silver Sulfadiazine with Improved Wound Healing Activity. AAPS PharmSciTech. 2017, 18, 3274–3285. [Google Scholar] [CrossRef]
- Banna, A.H.E.; Youssef, F.S.; Elzorba, H.Y.; Soliman, A.M.; Mohamed, G.G.; Ismail, S.H.; Mousa, M.R.; Elbanna, H.A.; Osman, A.S. Evaluation of the wound healing effect of neomycin-silver nano-composite gel in rats. Int. J. Immunopathol. Pharmacol. 2022, 36, 3946320221113486. [Google Scholar] [CrossRef] [PubMed]
- Soriano, J.L.; Calpena, A.C.; Rincón, M.; Pérez, N.; Halbaut, L.; Rodríguez-Lagunas, M.J.; Clares, B. Melatonin nanogel promotes skin healing response in burn wounds of rats. Nanomedicine 2020, 15, 2133–2147. [Google Scholar] [CrossRef] [PubMed]
- Amato, G.; Grimaudo, M.A.; Alvarez-Lorenzo, C.; Concheiro, A.; Carbone, C.; Bonaccorso, A.; Puglisi, G.; Musumeci, T. Hyaluronan/Poly-L-lysine/Berberine Nanogels for Impaired Wound Healing. Pharmaceutics 2020, 13, 34. [Google Scholar] [CrossRef]
- Nnamani, P.; Ogechukwu, N.; Odo, A.; Abimibola, V.; Ugwu, A.; Ibezim, E.; Ogbonna, J.; Onoja, S.; Ajogu, E.; Adikwu, M.; et al. Gentamicin nanogel films based on Carrageenan-Prosopis africana for improved wound healing. Med. Mater. Sci. 2022, 5, 879–896. [Google Scholar] [CrossRef]
- Morsy, M.A.; Abdel-Latif, R.G.; Nair, A.B.; Venugopala, K.N.; Ahmed, A.F.; Elsewedy, H.S.; Shehata, T.M. Preparation and Evaluation of Atorvastatin-Loaded Nanoemulgel on Wound-Healing Efficacy. Pharmaceutics 2019, 11, 609. [Google Scholar] [CrossRef]
- Aslan, C.; Çelebi, N.; Değim, I.T.; Atak, A.; Özer, Ç. Development of Interleukin-2 Loaded Chitosan-Based Nanogels Using Artificial Neural Networks and Investigating the Effects on Wound Healing in Rats. AAPS PharmSciTech. 2017, 18, 1019–1030. [Google Scholar] [CrossRef]
- Asadi, K.; Azarpira, N.; Heidari, R.; Hamidi, M.; Yousefzadeh-Chabok, S.; Nemati, M.M.; Ommati, M.M.; Amini, A.; Gholami, A. Trinitroglycerin-loaded chitosan nanogels accelerate angiogenesis in wound healing process. Int. J. Biol. Macromol. 2024, 278, 134937. [Google Scholar] [CrossRef]
- Ameena, M.; Arumugham, M.; Ramalingam, K.; Rajeshkumar, S.; Perumal, E.; Shanmugam, R. Cytocompatibility and Wound Healing Activity of Chitosan Thiocolchicoside Lauric Acid Nanogel in Human Gingival Fibroblast Cells. Cureus 2023, 15, e43727. [Google Scholar]
- Yang, X.; Lin, X.; Li, J.; Viitala, T.; Zhao, Y.; Zhang, H. Vitamin C encapsulated biomimetic nanogels with macrophage membrane decoration for chronic wound healing. Chem. Eng. J. 2025, 505, 159080. [Google Scholar] [CrossRef]
- Zhou, S.; Xie, M.; Su, J.; Cai, B.; Li, J.; Zhang, K. New insights into balancing wound healing and scarless skin repair. J. Tissue Eng. 2023, 14, 20417314231185848. [Google Scholar] [CrossRef]
- Sakthiganapathi, M.; Yoganandam, G.P.; Gopal, V. Modernization of a Traditional Siddha Medicine Paccai eruvai into a Novel Nanogel Formulation for the Potent Wound Healing Activity-A Phyto- Pharmaceutical Approach. Pharm. Nanotechnol. 2023, 11, 70–81. [Google Scholar] [PubMed]


| Types of Nanogels | Advantages | Shortcomings |
|---|---|---|
| Chitosan-based Nanogel | Cancer: Applied in cancer nanomedicine serving as drug delivery, gene delivery, and bioimaging [35]. Wound Healing: Used to promote wound healing by delivering growth factors and antibacterial agents locally [36]. Antibacterial: Chitosan nanogel loaded with antibacterial drug for infection prevention [37]. | The poor colloidal stability of these nanogels is a major disadvantage; they are prone to aggregation, precipitation, and deterioration over time, particularly in aqueous suspensions, which can impair their functionality in biological systems [38]. |
| Polyethylene Glycol (PEG)-based nanogel | Cancer: PEG-based nanogel enhances the stability and solubility of chemotherapy drugs used in cancer treatment [39] | PEG’s nonbiodegradability is a disadvantage since it may accumulate in the body over time and might result in long-term toxicity [40] |
| Liposome-based nanogel | Cancer: For cancer therapy, Matrix metallopeptidase-2-responsive polypeptide nanogel-coated double-targeted liposomes for chemotherapy and improved immunotherapy against cervical cancer [41]. Gene Delivery: Liposome hydrogel nanoparticles used for the targeted delivery of CRISPR/Cas9-mediated cancer gene therapy [42] | One significant problem is their chemical and physical instability, which can impair the effectiveness of treatment. This includes propensity for phospholipid breakdown, drug leakage, and aggregation during storage [43] |
| Poly (N-isopropylacrylamide) (PNIPAM)-based nanogel | Cancer: Nanogels made of poly(N-isopropylacrylamide) showed thermosensitive self-assembly and GSH-triggered drug release for effective tumor treatment [44]. Tissue engineering: In cardiac tissue engineering, PNIPAM-based hydrogels have been used to promote the growth and differentiation of heart stromal cells, aiding in the restoration of damage caused by myocardial infarction [45]. | The regulated distribution of therapeutic agents may be jeopardized by PNIPAM’s poor drug loading capacity and propensity for instantaneous drug release following temperature changes [45]. |
| Dextron-based nanogel | Cancer: Because dextrin-based nanogels are biocompatible, biodegradable, and responsive to stimuli unique to tumors, they have become intriguing vehicles for targeted cancer therapy [46]. Targeted delivery: Dextron-based nanogel has been used for targeted delivery of siRNA [47]. | Long-term toxicity could result from accumulation of degraded products in the body brought on by the breakdown of dextran nanogels [48] |
| Therapeutic Agents | Nanogels Adopted | Cancer Types | Properties | References |
|---|---|---|---|---|
| Doxorubicin | HPMPC Nanogel | Live cancer | Showing anticancer activity with good biocompatibility both in vitro and in vivo. | [123] |
| Hyaluronate (HA) | Photolabile 4-(4-(1-hydroxyethyl)-2-methoxy-5-nitrophenoxy)butyric acid (HMNB) | Human nasopharyngeal epidermal carcinoma | Significant improvement in KB tumor-cell-killing efficacy | [124] |
| Doxorubicin hydrochloride | poly [(N-isopropylacrylamide)-co-(2-dimethylamino ethyl methacrylate) Nanogel | Breast cancer | A significant decrease in toxicity was observed in the case of doxorubicin embedded in hydrogel. | [125] |
| Doxorubicin | Injectable shear-thinning hydrogel (STH) | Breast cancer and Glioblastoma | Increase overall survival in breast tumor- and glioblastoma-bearing animal models. | [126] |
| Doxorubicin | Nanohybrid of hyaluronic acid (HA)-decorated graphene oxide (GO) | Hepatic cancer | Higher tumor inhibition rate for mice having H22 hepatic cancer cells when compared to the GO-DOX formulation and free DOX. | [127] |
| 5-fluorouracil (5-FU) | Alginate-modified graphene oxide | Liver cancer | Increased the mice life period and markedly reduced tumor growth and liver metastasis. | [128] |
| Doxorubicin | Lactobionic acid and carboxymethyl chitosan functionalized graphene oxide nanocomposites | Liver cancer | Target drug delivery to liver cells and efficiently trigger cell death | [129] |
| miR155 | Nucleic acid Nanogel | Glioblastoma | Strong tumor-targeting ability along with outstanding tumor-inhibition effectiveness against glioblastoma. | [130] |
| siRNA | Polydopamine-coated nucleic acid Nanogel | Human cervical carcinoma | Anticancer activity against cervical cancer tumor induced by Hela cells. | [131] |
| Doxorubicin | Redox-responsive cisplatin Nanogels | Ovarian cancer | Boost anticancer activity against cisplatin resistance ovarian cancer | [132] |
| Iodine-labeled RGDY | 125I-GNR-RGDY hydrogel | Breast cancer | During a 4-week course of NDN hydrogel treatment, the combination of continuous brachytherapy and photothermal effect effectively avoided wound infection and breast cancer recurrence. | [133] |
| siRNA | Nucleic acid Nanogel | Melanoma | Increases antitumor efficacy in a synergistic manner and significantly boosts the anticancer immune response. | [134] |
| CRISPR/Cas9 | Noncationic nucleic acid Nanogel | Cervical cancer | Increased cellular absorption effectiveness and delivery system ability to modify the target genome. | [135] |
| IMS/ICG | GSH/ROS dual response Nanogel | Solid tumor | Developed for endorsing cancer immunotherapy | [136] |
| Gemcitabin, R837 | Immunomodulatory multidomain nanogel (iGel) | Triple negative Breast cancer and Cervical cancer | Anticancer efficacies against TNBC and TC1 cervical cancer cells | [137] |
| Axitinib | Injectable hydrogel | Modulate T cells for immunotherapy against cancer | [138] | |
| Thiocolchicoside and lauric acid | chitosan Nanogel | Oral cancer | Anticancer activity against oral cancer cell lines | [139] |
| Therapeutic Agents | Nanogels Adopted | Wound Types | Properties | References |
|---|---|---|---|---|
| Hyaluronic acid/verteporfin | Polylactic acid nanogels | Wound re-epithelialization | Promote scarless wound healing by controlling scar formation and speeding up wound re-epithelialization. | [170] |
| Curcumin | Fish scale collagen-HPMC nanogel | In vivo murine wound model | FSC-HPMC nanogel showed safe, promising, and more stable material for wound healing applications. | [171] |
| Polyherbal Mattan tailam | Novel Mattan tailam Nanogel | Rats skin wound | Formulation significantly increases collagen synthesis, tensile strength, and wound contraction. | [172] |
| Copper sulfide (CuS) | Gelatinase Responsive Nanogel | Mice wound infected with S. aureus | Improved wound healing and removed the colonized microbes from mice S. aureus infected wounds. | [173] |
| Lidocaine | Lidocaine-loaded nanoemulsion convert into gel using carbopol-940 as a gelling agent | In vivo mice wound | The dermatokinetic profile of nanogel was superior than that of traditional gel. | [174] |
| AgNPs | CLT-AgNPs Nanogel | Acute and chronic wounds | Promotes agranulocytosis and fibroblast growth, resulted efficient and quick wound healing. | [175] |
| Nitric oxide | Sprayable chitosan Nanogel | Diabetic wound | Accelerate diabetic wound healing through bacteria inhibition, biofilm eradication and macrophage polarization | [176] |
| Eugenol | Eugenol-loaded nanogel + PCL/Cs nanofiber | Excision wound model in Wistar rats | Promoting wound healing by reducing inflammation and edema and encouraging angiogenesis, collagen synthesis, and re-epithelialization. | [177] |
| Sacchachitin | Micronized sacchachitin (mSC) Nanogel | Superficial chemical corneal burns | Wound healing against corneal epithelium | [178] |
| Exosomes | Exosome-coated oxygen nanobubble-laden hydrogel | Rat full-thickness wound model | Promoting angiogenesis, boosting exosome distribution, improving healing, lowering hypoxia, and preventing inflammation in a male rat full thickness wound model | [179] |
| Silver nanoparticles | Ficus lacor-silver nanoparticle gel | Rat excision wound model | Demonstrated considerable improvement in the excision wound model | [180] |
| Lignin | Lignin-Incorporated Nanogel | Helping as an antioxidant biomaterial for Wound healing | [181] | |
| Silver Sulfadiazine | Nanosuspension-Based Aloe vera Gel | Burn wounds in mice | Improved burn wound Healing | [182] |
| Silver nanoparticles | Neomycin-silver nanocomposite | Rat excision wound model | Potential wound healing activity | [183] |
| Melatonin | Melatonin Nanogel | Burn wounds of rats | Promoted epidermis growth with evident wound contraction | [184] |
| Berberine | Hyaluronan/Poly-L-lysine/Berberine Nanogels | In vitro on fibroblast cells | In 42 h, berberine-loaded nanogels were able to fully seal the fibroblast gap | [185] |
| Gentamicin | Gentamicin nanogel films | Rat full-thickness excisional model | Developed a more effective and affordable topical therapy for the healing of cutaneous wounds. | [186] |
| Atorvastatin | Atorvastatin-Loaded Nanoemulgel | Ex vivoRat skin model | Enhancing wound healing | [187] |
| Interleukin-2 | Chitosan-Based Nanogel | Rat wound | Enhancing wound healing by raising GSH levels in injured tissues and lowering MDA levels. | [188] |
| Trinitroglycerin | Chitosan Nanogel | Full-thickness skin wounds model | Continued release of trinitroglycerinfrom nanogel improved wound tissue vascularization | [189] |
| Chitosan | Chitosan thiocolichoside lauric acid Nanogel | In vitro scratch wound healing | Cytoprotective and wound healing activity | [190] |
| Vitamin C derivative | Gelatin and Alginate Nanogel | Chronic wound healing | Regulating the inflammatory wound microenvironment. | [191] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zafaryab, M.; Vig, K. Biomedical Application of Nanogels: From Cancer to Wound Healing. Molecules 2025, 30, 2144. https://doi.org/10.3390/molecules30102144
Zafaryab M, Vig K. Biomedical Application of Nanogels: From Cancer to Wound Healing. Molecules. 2025; 30(10):2144. https://doi.org/10.3390/molecules30102144
Chicago/Turabian StyleZafaryab, Mohammad, and Komal Vig. 2025. "Biomedical Application of Nanogels: From Cancer to Wound Healing" Molecules 30, no. 10: 2144. https://doi.org/10.3390/molecules30102144
APA StyleZafaryab, M., & Vig, K. (2025). Biomedical Application of Nanogels: From Cancer to Wound Healing. Molecules, 30(10), 2144. https://doi.org/10.3390/molecules30102144







