Strawberry Tree Fruit Residue as Carbon Source Towards Sustainable Fuel Biodesulfurization by Gordonia alkanivorans Strain 1B
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Strawberry Tree Fruit Residue Liquor Preparation
Detoxification
2.3. Microorganism and Culture Conditions
2.4. BDS Assays with Growing Cells
2.5. Chemostat Assays
2.6. BDS Assays Using Resting Cells
2.7. Screening of Added-Value Bioproducts
2.7.1. Carotenoid Extraction from Biomass
2.7.2. Biosurfactant/Bioemulsifer Compounds in Culture Broth
2.8. Analytical Methods
2.8.1. Optical Density and Dry Cell Weight
2.8.2. DBT Desulfurization Evaluation
2.8.3. Sugar Consumption Evaluation
2.8.4. Carotenoid Analysis
2.8.5. Gordofactin Screening: Emulsifying Activity Quantification
2.8.6. Total Phenolic Quantification
3. Results and Discussion
3.1. Production of Sugar-Rich Liquor
3.2. Biodesulfurization Assays
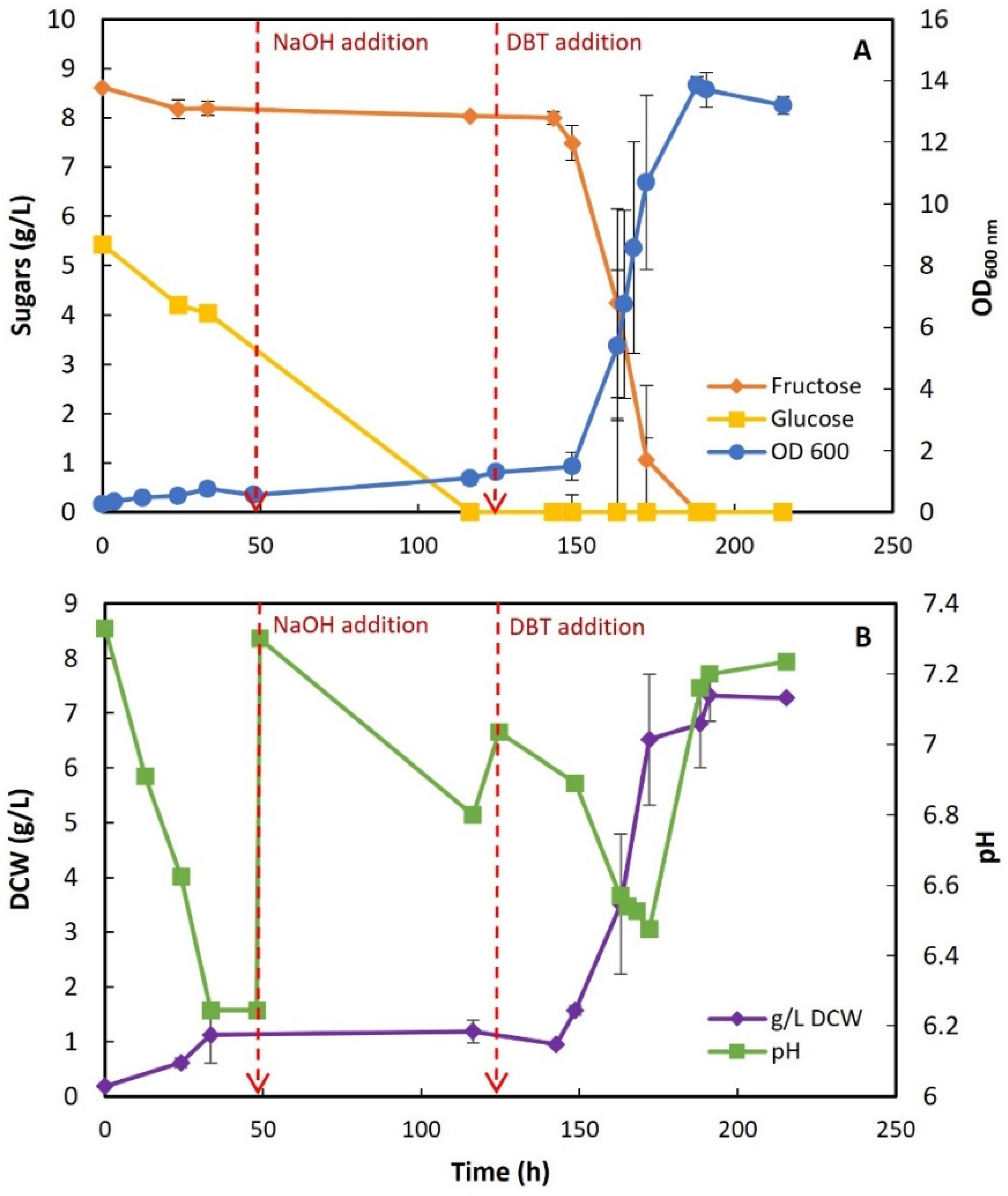
3.2.1. Preliminary Growth Assays with STFr-Liquor
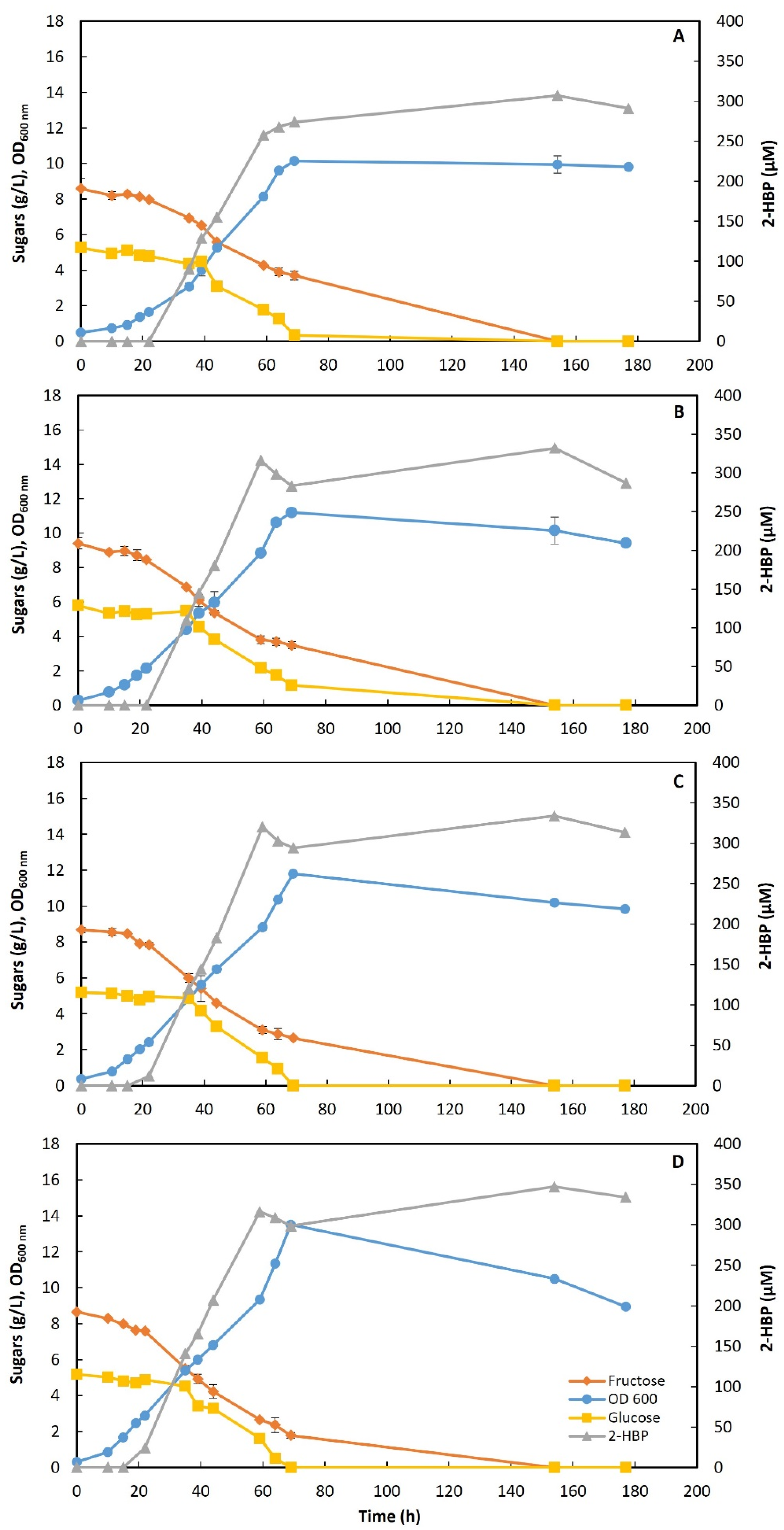
3.2.2. STFr-Liquor Detoxification
3.3. Chemostat Assays
3.3.1. Preliminary Assays
3.3.2. Assays with STFr-Liquor
3.3.3. BDS Assays Using Resting Cells
3.4. Production of Added-Value Bioproducts
3.4.1. Carotenoids
3.4.2. Gordofactin BS/BE
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ahmad, A.; Zamzami, M.A.; Ahmad, V.; Al-Thawadi, S.; Akhtar, M.S.; Khan, M.J. Bacterial biological factories intended for the desulfurization of petroleum products in refineries. Fermentation 2023, 9, 211. [Google Scholar] [CrossRef]
- Prasoulas, G.; Dimos, K.; Glekas, P.; Kalantzi, S.; Sarris, S.; Templis, C.; Vavitsas, K.; Hatzinikolaou, D.G.; Papayannakos, N.; Kekos, D.; et al. Biodesulfurization of dibenzothiophene and its alkylated derivatives in a two-phase bubble column bioreactor by resting cells of Rhodococcus erythropolis IGTS8. Processes 2021, 9, 2064. [Google Scholar] [CrossRef]
- Auersvald, M.; Kejla, L.; Eschenbacher, A.; Thi, H.D.; Van Geem, K.M.; Šimáček, P. Detailed characterization of sulfur compounds in fast pyrolysis bio-oils using GC × GC-SCD and GC–MS. J. Anal. Appl. Pyrolysis 2021, 159, 105288. [Google Scholar] [CrossRef]
- Mamuad, R.Y.; Choi, A.E.S. Biodesulfurization processes for the removal of sulfur from diesel oil: A perspective report. Energies 2023, 16, 2738. [Google Scholar] [CrossRef]
- Villegas-Méndez, M.Á.; Montañez, J.; Contreras-Esquivel, J.C.; Salmerón, I.; Koutinas, A.; Morales-Oyervides, L. Coproduction of microbial oil and carotenoids within the circular bioeconomy concept: A sequential solid-state and submerged fermentation approach. Fermentation 2022, 8, 258. [Google Scholar] [CrossRef]
- Alves, L.; Salgueiro, R.; Rodrigues, C.; Mesquita, E.; Matos, J.; Gírio, F.M. Desulfurization of dibenzothiophene, benzothiophene, and other thiophene analogs by a newly isolated bacterium, Gordonia alkanivorans strain 1B. Appl. Biochem. Biotechnol. 2005, 120, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Alves, L.; Paixão, S.M.; Pacheco, R.; Ferreira, A.F.; Silva, C.M. Biodesulphurization of fossil fuels: Energy, emissions and cost analysis. RSC Adv. 2015, 5, 34047–34057. [Google Scholar] [CrossRef]
- Pacheco, M.; Paixão, S.M.; Silva, T.P.; Alves, L. On the road to cost-effective fossil fuel desulfurization by Gordonia alkanivorans strain 1B. RSC Adv. 2019, 9, 25405–25413. [Google Scholar] [CrossRef]
- Paixão, S.M.; Silva, T.P.; Arez, B.F.; Alves, L. Advances in the reduction of the costs inherent to fossil fuels biodesulfurization towards its potential industrial application. In Nanocomposites for the Desulfurization of Fuels; Saleh, T., Ed.; IGI Global: Hershey, PA, USA, 2020; Chapter 7; pp. 235–283. [Google Scholar] [CrossRef]
- Silva, T.P.; Paixão, S.M.; Alves, L. A new impetus for biodesulfurization: Bypassing sulfate inhibition in biocatalyst production. Green Chem. 2023, 25, 6416–6431. [Google Scholar] [CrossRef]
- Silva, T.P.; Paixão, S.M.; Tavares, J.; Paradela, F.; Crujeira, T.; Roseiro, J.C.; Alves, L. Streamlining the biodesulfurization process: Development of an integrated continuous system prototype using Gordonia alkanivorans strain 1B. RSC Adv. 2024, 14, 725–742. [Google Scholar] [CrossRef]
- Silva, T.P.; Paixão, S.M.; Alves, L. Ability of Gordonia alkanivorans strain 1B for high added value carotenoids production. RSC Adv. 2016, 6, 58055–58063. [Google Scholar] [CrossRef]
- Fernandes, A.S.; Paixão, S.M.; Silva, T.P.; Roseiro, J.C.; Alves, L. Influence of culture conditions towards optimal carotenoid production by Gordonia alkanivorans strain 1B. Bioprocess Biosyst. Eng. 2018, 41, 143–155. [Google Scholar] [CrossRef] [PubMed]
- Silva, T.P.; Alves, L.; Paixão, S.M. Effect of dibenzothiophene and its alkylated derivatives on coupled desulfurization and carotenoid production by Gordonia alkanivorans strain 1B. J. Environ. Manag. 2020, 270, 110825. [Google Scholar] [CrossRef]
- Silva, T.P.; Paixão, S.M.; Fernandes, A.S.; Roseiro, J.C.; Alves, L. New insights on carotenoid production by Gordonia alkanivorans strain 1B. In Carotenoids—New Perspectives and Application; Martinez-Espinosa, R.M., Ed.; IntechOpen: London, UK, 2022; Chapter 2; pp. 11–35. ISBN 978-1-80355-424-2. [Google Scholar] [CrossRef]
- Tavares, J.; Alves, L.; Silva, T.P.; Paixão, S.M. Design and validation of an expeditious analytical method to quantify the emulsifying activity during biosurfactants/bioemulsifiers production. Colloids Surf. B Biointerfaces 2021, 208, 112111. [Google Scholar] [CrossRef] [PubMed]
- Silva, T.P.; Paixão, S.M.; Tavares, J.; Gil, C.V.; Torres, C.A.V.; Freitas, F.; Alves, L. A new biosurfactant/bioemulsifier from Gordonia alkanivorans strain 1B: Production and characterization. Processes 2022, 10, 845. [Google Scholar] [CrossRef]
- Tavares, J.; Paixão, S.M.; Silva, T.P.; Alves, L. New insights on Gordonia alkanivorans strain 1B surface-active biomolecules: Gordofactin properties. Molecules 2025, 30, 1. [Google Scholar] [CrossRef]
- Silva, T.P.; Paixão, S.M.; Teixeira, A.V.; Roseiro, J.C.; Alves, L. Optimization of low sulphur carob pulp liquor as carbon source for fossil fuels biodesulfurization. J. Chem. Technol. Biotechnol. 2013, 88, 919–923. [Google Scholar] [CrossRef]
- Teixeira, A.V.; Paixão, S.M.; Lopes da Silva, T.; Alves, L. Influence of the carbon source on Gordonia alkanivorans strain 1B resistance to 2-hydroxybiphenyl toxicity. Appl. Biochem. Biotechnol. 2014, 173, 870–882. [Google Scholar] [CrossRef]
- Alves, L.; Paixão, S.M. Enhancement of dibenzothiophene desulfurization by Gordonia alkanivorans strain 1B using sugar beet molasses as alternative carbon source. Appl. Biochem. Biotechnol. 2014, 172, 3297–3305. [Google Scholar] [CrossRef]
- Silva, T.P.; Paixão, S.M.; Roseiro, J.C.; Alves, L. Jerusalem artichoke as low-cost fructose-rich feedstock for fossil fuels desulfurization by a fructophilic bacterium. J. Appl. Microbiol. 2015, 118, 609–618. [Google Scholar] [CrossRef]
- Paixão, S.M.; Arez, B.F.; Roseiro, J.C.; Alves, L. Simultaneously saccharification and fermentation approach as a tool for enhanced fossil fuels biodesulfurization. J. Environ. Manag. 2016, 182, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Sadare, O.O.; Obazu, F.; Daramola, M.O. Biodesulfurization of petroleum distillates—Current status, opportunities and future challenges. Environments 2017, 4, 85. [Google Scholar] [CrossRef]
- Gouda, M.K.; Omar, S.H.; Aouad, L.M. Single cell oil production by Gordonia sp. DG using agro-industrial wastes. World J. Microbiol. Biotechnol. 2008, 24, 1703–1711. [Google Scholar] [CrossRef]
- Patthawaro, S.; Lomthaisong, K.; Saejung, C. Bioconversion of agro-industrial waste to value-added product lycopene by photosynthetic bacterium Rhodopseudomonas faecalis and its carotenoid composition. Waste Biomass Valor. 2020, 11, 2375–2386. [Google Scholar] [CrossRef]
- Miao, Y.; To, M.H.; Siddiqui, M.A.; Wang, H.; Lodens, S.; Chopra, S.S.; Kaur, G.; Roelants, S.L.K.W.; Lin, C.S.K. Sustainable biosurfactant production from secondary feedstock—Recent advances, process optimization and perspectives. Front. Chem. 2024, 12, 1327113. [Google Scholar] [CrossRef] [PubMed]
- Alves, L.; Paixão, S.M. Fructophilic behaviour of Gordonia alkanivorans strain 1B during dibenzothiophene desulfurization process. New Biotechnol. 2014, 31, 73–79. [Google Scholar] [CrossRef]
- Alves, L.; Marques, S.; Matos, J.; Tenreiro, R.; Gírio, F.M. Dibenzothiophene desulfurization by Gordonia alkanivorans strain 1B using recycled paper sludge hydrolyzate. Chemosphere 2008, 70, 967–973. [Google Scholar] [CrossRef] [PubMed]
- Bebek Markovinović, A.; Brčić Karačonji, I.; Jurica, K.; Lasić, D.; Skendrović Babojelić, M.; Duralija, B.; Šic Žlabur, J.; Putnik, P.; Bursać Kovačević, D. Strawberry tree fruits and leaves (Arbutus unedo L.) as raw material for sustainable functional food processing: A review. Horticulturae 2022, 8, 881. [Google Scholar] [CrossRef]
- Veiga-Crespo, P.; Vinuesa, T.; Viñas, M.; Villa, T.G. Analysis of canthaxanthin production by Gordonia jacobaea. Methods Mol. Biol. 2012, 892, 159–172. [Google Scholar]
- Celikel, G.; Demirsoy, L.; Demirsoy, H. The strawberry tree (Arbutus unedo L.) selection in Turkey. Sci. Hortic. 2008, 118, 115–119. [Google Scholar] [CrossRef]
- Oliveira, I.; Baptista, P.; Bento, A.; Pereira, J.A. Arbutus unedo L. and its benefits on human health. J. Food Nutr. Res. 2011, 50, 73–85. [Google Scholar]
- Miguel, M.G.; Faleiro, M.L.; Guerreiro, A.C.; Antunes, M.D. Arbutus unedo L.: Chemical and biological properties. Molecules 2014, 19, 15799–15823. [Google Scholar] [CrossRef] [PubMed]
- Cavaco, T.; Longuinho, C.; Quintas, C.; Carvalho, I.S. Chemical and microbial changes during the natural fermentation of strawberry tree (Arbutus unedo L.) fruits. J. Food Biochem. 2007, 31, 715–725. [Google Scholar] [CrossRef]
- Brčić Karačonji, I.; Jurica, K.; Gašić, U.; Dramićanin, A.; Tešić, Ž.; Milojković Opsenica, D. Comparative study on the phenolic fingerprint and antioxidant activity of strawberry tree (Arbutus unedo L.) leaves and fruits. Plants 2022, 11, 25. [Google Scholar] [CrossRef] [PubMed]
- Scarano, P.; Guida, R.; Zuzolo, D.; Tartaglia, M.; Prigioniero, A.; Postiglione, A.; Pinto, G.; Illiano, A.; Amoresano, A.; Schicchi, R.; et al. An endemic plant of the Mediterranean area: Phytochemical characterization of strawberry tree (Arbutus unedo L.) fruits extracts at different ripening stages. Front Nutr. 2022, 9, 915994. [Google Scholar] [CrossRef]
- NERA. Atividades Económicas Fileira do Medronho. Relatório da NERA—Associação Empresarial da Região do Algarve. 2023. Available online: https://www.nera.pt/wp-content/uploads/2023/12/007-RELATORIO-Fileira-MEDRONHO.pdf (accessed on 5 March 2025).
- Florestal, F. Estudo Económico do Desenvolvimento da Fileira do Medronho. Fórum Florestal (FF)—Estrutura Federativa da Floresta Portuguesa. 2022. Available online: https://www.compete2020.gov.pt/admin/images/Medronho_SumarioExecutivo.pdf (accessed on 5 March 2025).
- Santo, D.E.; Galego, L.; Gonçalves, T.; Quintas, C. Yeast diversity in the Mediterranean strawberry tree (Arbutus unedo L.) fruits’ fermentations. Food Res. Int. 2012, 47, 45–50. [Google Scholar] [CrossRef]
- Vallejo, M.; Cordeiro, R.; Dias, P.A.N.; Moura, C.; Henriques, M.; Seabra, I.J.; Malça, C.M.; Morouço, P. Recovery and evaluation of cellulose from agroindustrial residues of corn, grape, pomegranate, strawberry-tree fruit and fava. Bioresour. Bioprocess. 2021, 8, 25. [Google Scholar] [CrossRef]
- Roseiro, J.C.; Partidário, P.; Lobo, N.; Marçal, M.J. Physiology and kinetics of trimethylamine conversion by two methylotrophic strains in continuous cultivation systems. Appl. Microbiol. Biotechnol. 1999, 52, 546–552. [Google Scholar] [CrossRef]
- Silva, T.P.; Alves, L.; Salgado, F.; Roseiro, J.C.; Lukasik, R.M.; Paixão, S.M. Ionic liquids toward enhanced carotenoid extraction from bacterial biomass. Molecules 2024, 29, 4132. [Google Scholar] [CrossRef]
- Nobre, B.; Marcelo, F.; Passos, R.; Beirão, L.; Palavra, A.; Gouveia, L.; Mendes, R. Supercritical carbon dioxide extraction of astaxanthin and other carotenoids from the microalga Haematococcus pluvialis. Eur. Food Res. Technol. 2006, 223, 787–790. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Silva, T.P. Utilization of Agroindustrial Materials as Alternative Carbon Sources for the Biodesulfurization of Fossil Fuels by Gordonia alkanivorans Strain 1B. Master’s Thesis, Faculty of Sciences of the University of Lisbon, Lisboa, Portugal, 2012; p. 50. Available online: http://hdl.handle.net/10451/7954 (accessed on 2 January 2025).
- Hoberg, E.; Marschner, P.; Lieberei, R. Organic acid exudation and pH changes by Gordonia sp. and Pseudomonas fluorescens grown with P adsorbed to goethite. Microbiol. Res. 2005, 160, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Alves, L.; Matos, J.; Tenreiro, R.; Gírio, F.M. Evidence for the role of zinc on the performance of dibenzothiophene desulfurization by Gordonia alkanivorans strain 1B. J. Ind. Microbiol. Biotechnol. 2008, 35, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Alves, L.; Paixão, S.M. Toxicity evaluation of 2-hydroxybiphenyl and other compounds involved in studies of fossil fuels biodesulphurisation. Bioresour. Technol. 2011, 102, 9162–9166. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, M. Production of Gordonia alkanivorans Strain 1B Biomass in Bioreactor and Further Application Towards Fossil Fuels Desulfurization. Master’s Thesis, Faculty of Sciences of the University of Lisbon, Lisboa, Portugal, 2015; p. 50. Available online: http://hdl.handle.net/10451/22590 (accessed on 2 January 2025).
- de Miguel, T.; Sieiro, C.; Poza, M.; Villa, T.G. Isolation and taxonomic study of a new canthaxanthin-containing bacterium, Gordonia jacobaea MV-1 sp. nov. Int. Microbiol. 2000, 3, 107–111. [Google Scholar]
- de Miguel, T.; Sieiro, C.; Poza, M.; Villa, T.G. Analysis of canthaxanthin and related pigments from Gordonia jacobaea mutants. J. Agric. Food Chem. 2001, 49, 1200–1202. [Google Scholar] [CrossRef]
- Veiga-Crespo, P.; Blasco, L.; Rosa-Dos-Santos, F.; Poza, M.; Villa, T.G. Influence of culture conditions of Gordonia jacobaea MV-26 on canthaxanthin production. Int. Microbiol. 2005, 8, 55–58. [Google Scholar]
- Tavares, J.; Silva, T.P.; Paixão, S.M.; Alves, L. Development of a bench-scale photobioreactor with a novel recirculation system for continuous cultivation of microalgae. J. Environ. Manag. 2023, 332, 117418. [Google Scholar] [CrossRef]
| STFr-Liquor Lot (#) | Fructose (g/L) | Glucose (g/L) | Total Sugars (g/L) | Phenolics (g/L) |
|---|---|---|---|---|
| 1 | 66.22 | 43.40 | 109.62 | 3.72 |
| 2 | 41.35 | 24.65 | 66.00 | 4.12 |
| 3 | 63.72 | 43.38 | 107.10 | 3.15 |
| Metabolic Parameters | |
|---|---|
| Maximum OD600 nm | 13.86 |
| Maximum growth rate (h−1) | 0.091 |
| Maximum biomass concentration (g/L) | 7.28 |
| Maximum fructose consumption rate (g/L/h) | 0.270 |
| Maximum biomass production rate (g(DCW)/L/h) | 0.236 |
| AC (%) | Detoxified STFr-Liquor | |||
|---|---|---|---|---|
| Fructose (g/L) | Glucose (g/L) | Total Sugars (g/L) | Phenolics (g/L) | |
| 1% | 60.09 | 36.86 | 96.96 | 1.02 |
| 2% | 65.80 | 40.61 | 106.41 | 0.32 |
| 3% | 60.72 | 36.31 | 97.03 | 0.16 |
| 4% | 60.59 | 36.32 | 96.90 | 0.21 |
| Metabolic Parameters | Detoxified STFr-Liquor | |||
|---|---|---|---|---|
| 1% AC 1 | 2% AC 2 | 3% AC 3 | 4% AC 4 | |
| Maximum OD600nm | 10.15 | 11.20 | 11.80 | 13.50 |
| Maximum growth rate (h−1) | 0.086 | 0.097 | 0.105 | 0.119 |
| Total sugars consumption rate (g/L/h) | 0.142 | 0.153 | 0.162 | 0.175 |
| Maximum biomass (g/L) | 4.55 | 4.52 | 4.37 | 4.43 |
| Maximum 2-HBP production rate (µM/h) | 7.19 | 8.60 | 8.88 | 8.83 |
| Maximum 2-HBP produced (µM) | 307 | 332 | 334 | 347 |
| SFM Culture Medium | SFMM Culture Medium with STFr-Liquor | |||
|---|---|---|---|---|
| Fru + Glu | STFr L1 | STFr L2 | DTX STFr L | |
| Dilution rate (h−1) | 0.04 | 0.04 | 0.05 | 0.04 |
| Initial carbon (g/L) | 5.4 | 5.4 | 5.4 | 4.72 |
| Initial sulfate (mg/L) | 10.14 | 10.14 | 10.14 | 10.14 |
| Accumulated C-source (g/L) | 0.11 | 0 | 1.0 | 0 |
| OD600nm | 5.34 ± 0.3 | 6.88 ± 0.1 | 6.75 ± 0.1 | 6.72 ± 0.08 |
| Biomass (g/L) | 1.89 ± 0.13 | 2.43 ± 0.04 | 2.79 ± 0.07 | 2.40 ± 0.07 |
| Biomass production rate (g/L/h) | 0.076 | 0.097 | 0.140 | 0.096 |
| C-source consumption rate (g/L/h) | 0.212 | 0.216 | 0.220 | 0.191 |
| qC-source (mmol/g(DCW)/h) | 0.62 ± 0.04 | 0.49 ± 0.01 | 0.44 ± 0.01 | 0.38 ± 0.01 |
| qCO2 (mmol/g(DCW)/h) | 0.42 ± 0.09 | 0.20 ± 0.05 | 0.31 ± 0.007 | 0.35 ± 0.01 |
| qO2 (mmol/g(DCW)/h) | 4.93 ± 1.04 | 3.50 ± 0.07 | 2.29 ± 0.05 | 2.66 ± 0.07 |
| CR (%) | 43.29 ± 1.7 | 47.46 ± 1.4 | 68.69 ± 1.34 | 58.62 ± 1.28 |
| CCE (%) | 32.21 ± 2.3 | 40.56 ± 0.8 | 57.07 ± 1.34 | 45.25 ± 1.28 |
| Maximum 2-HBP (µM) | 199.69 ± 4.44 | 212.68 ± 1.55 | 51.87 ± 1.44 | 66.97 ± 2.15 |
| q2-HBP (µmol/g(DCW)/h) | 4.32 ± 0.03 | 6.50 ± 0.14 | 1.84 ± 0.11 | 6.98 ± 0.04 |
| Carbon Source | Sugars (g/L) | Pretreatment | BDS Activity (µmol/g(DCW)/h) | References |
|---|---|---|---|---|
| Recycled paper sludge hydrolysate | 53.9 | Enzymatic hydrolysis and dialysis | 1.1 | [29] |
| Carob pulp liquor | 300 | Water extraction, acidic hydrolysis and sulfate precipitation | 1.56 | [19,46] |
| Jerusalem artichoke juice | 145 | Acidic hydrolysis and sulfate precipitation/enzymatic hydrolysis and sulfate precipitation | 5.06/8.33 | [22,23] |
| Strawberry tree fruit liquor | 173 | Direct extraction | 5.0 | [46] |
| Sugar beet molasses | 500 | Acidic hydrolysis and sulfate precipitation | 3.12 | [21] |
| Strawberry tree fruit residue liquor | 109.62/ 66.0 | Untreated/AC treatment | 6.50/6.98 | Present work |
| SFM Culture Medium | SFMM Culture Medium with STFr-Liquor | ||
|---|---|---|---|
| Fru + Glu | STFr L1 | DTX STFr L | |
| Total carotenoids (μg/g(DCW)) | 125.0 ± 0.3 | 338.0 ± 15.0 | 106.0 ± 2.0 |
| EA (U/mL) | 6.25 | 8.0 | 7.69 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paixão, S.M.; Silva, T.P.; Salgado, F.; Alves, L. Strawberry Tree Fruit Residue as Carbon Source Towards Sustainable Fuel Biodesulfurization by Gordonia alkanivorans Strain 1B. Molecules 2025, 30, 2137. https://doi.org/10.3390/molecules30102137
Paixão SM, Silva TP, Salgado F, Alves L. Strawberry Tree Fruit Residue as Carbon Source Towards Sustainable Fuel Biodesulfurization by Gordonia alkanivorans Strain 1B. Molecules. 2025; 30(10):2137. https://doi.org/10.3390/molecules30102137
Chicago/Turabian StylePaixão, Susana M., Tiago P. Silva, Francisco Salgado, and Luís Alves. 2025. "Strawberry Tree Fruit Residue as Carbon Source Towards Sustainable Fuel Biodesulfurization by Gordonia alkanivorans Strain 1B" Molecules 30, no. 10: 2137. https://doi.org/10.3390/molecules30102137
APA StylePaixão, S. M., Silva, T. P., Salgado, F., & Alves, L. (2025). Strawberry Tree Fruit Residue as Carbon Source Towards Sustainable Fuel Biodesulfurization by Gordonia alkanivorans Strain 1B. Molecules, 30(10), 2137. https://doi.org/10.3390/molecules30102137







