Modified Silica Particles Coated with Cu-Al Layered Double Hydroxide for Phosphate and Arsenate Removal in Water Treatment
Abstract
1. Introduction
2. Results and Discussion
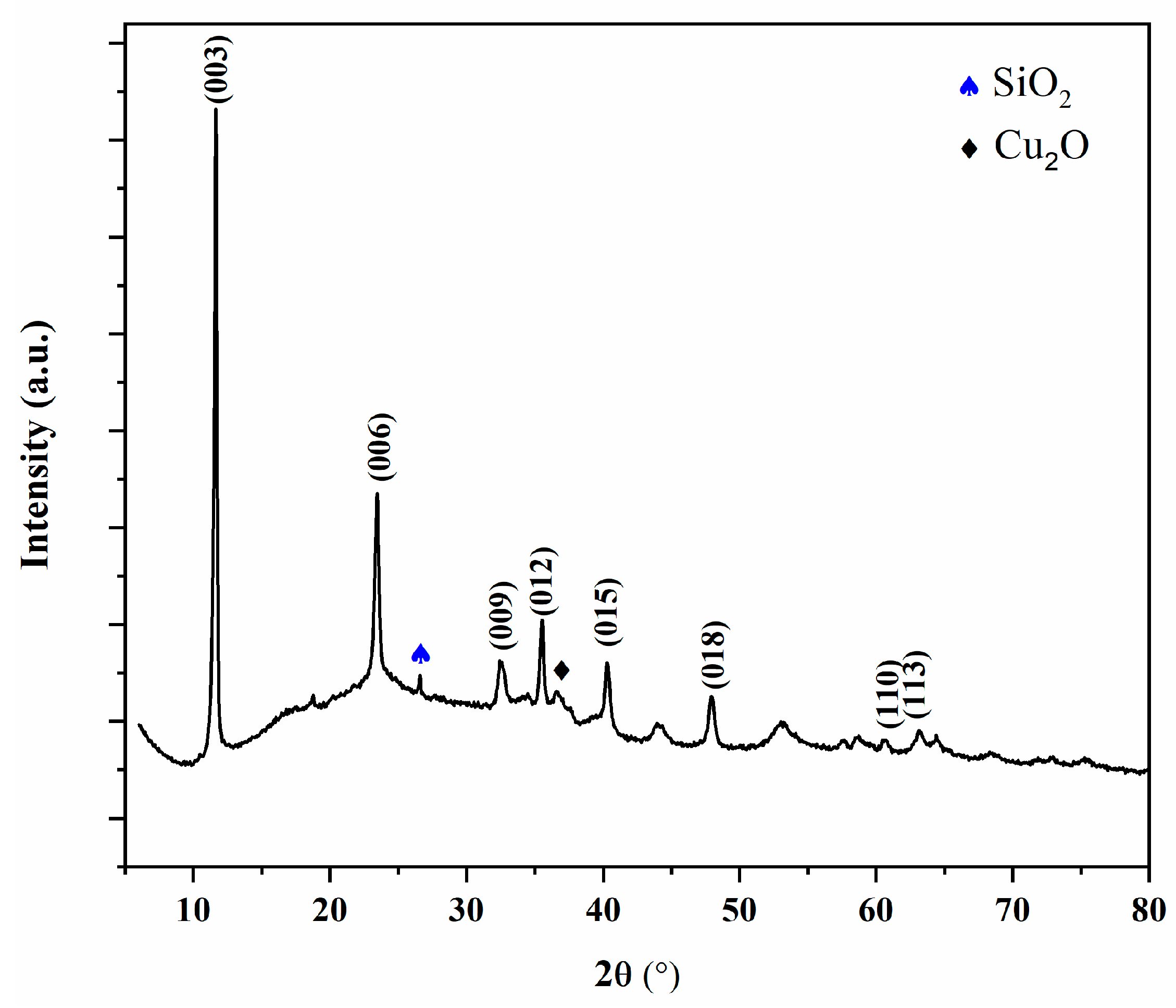
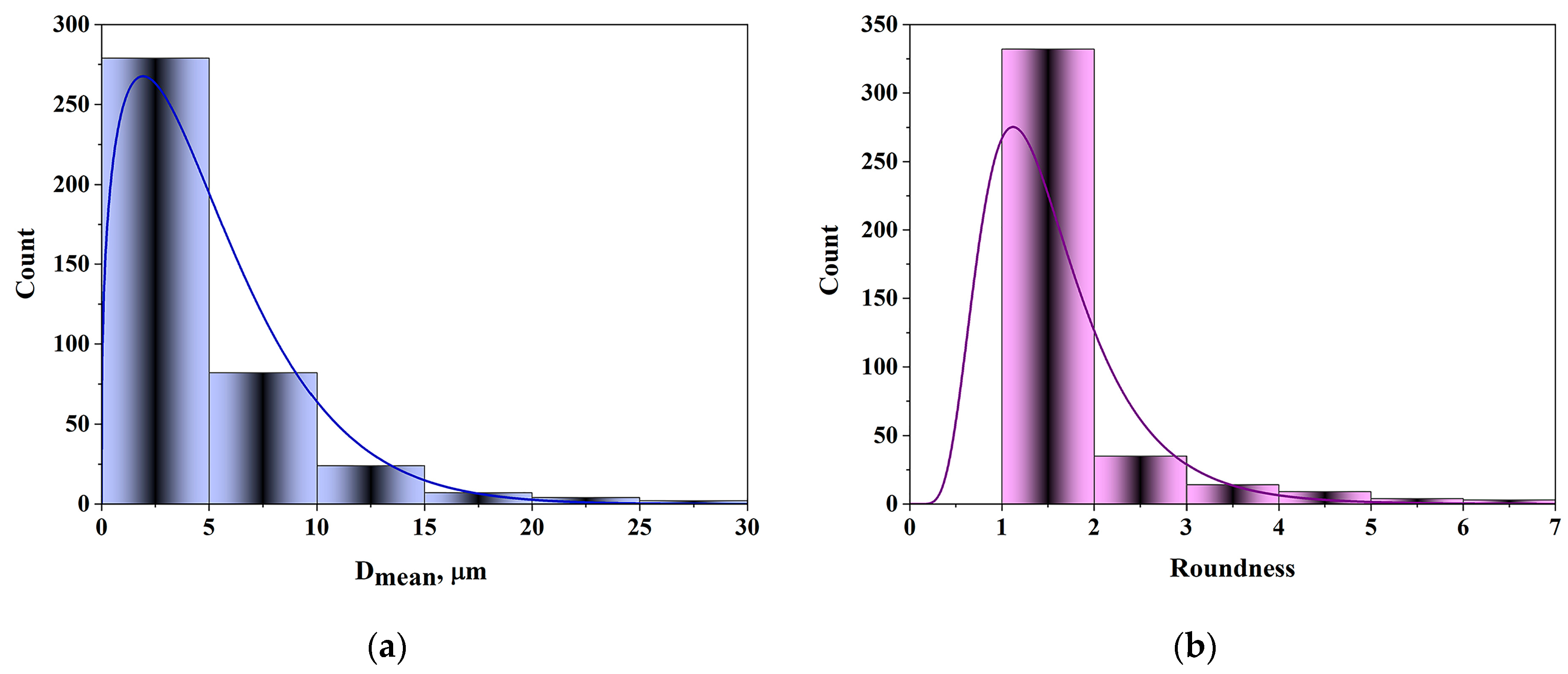
2.1. Characterization of CuAl-LDH@SiO2
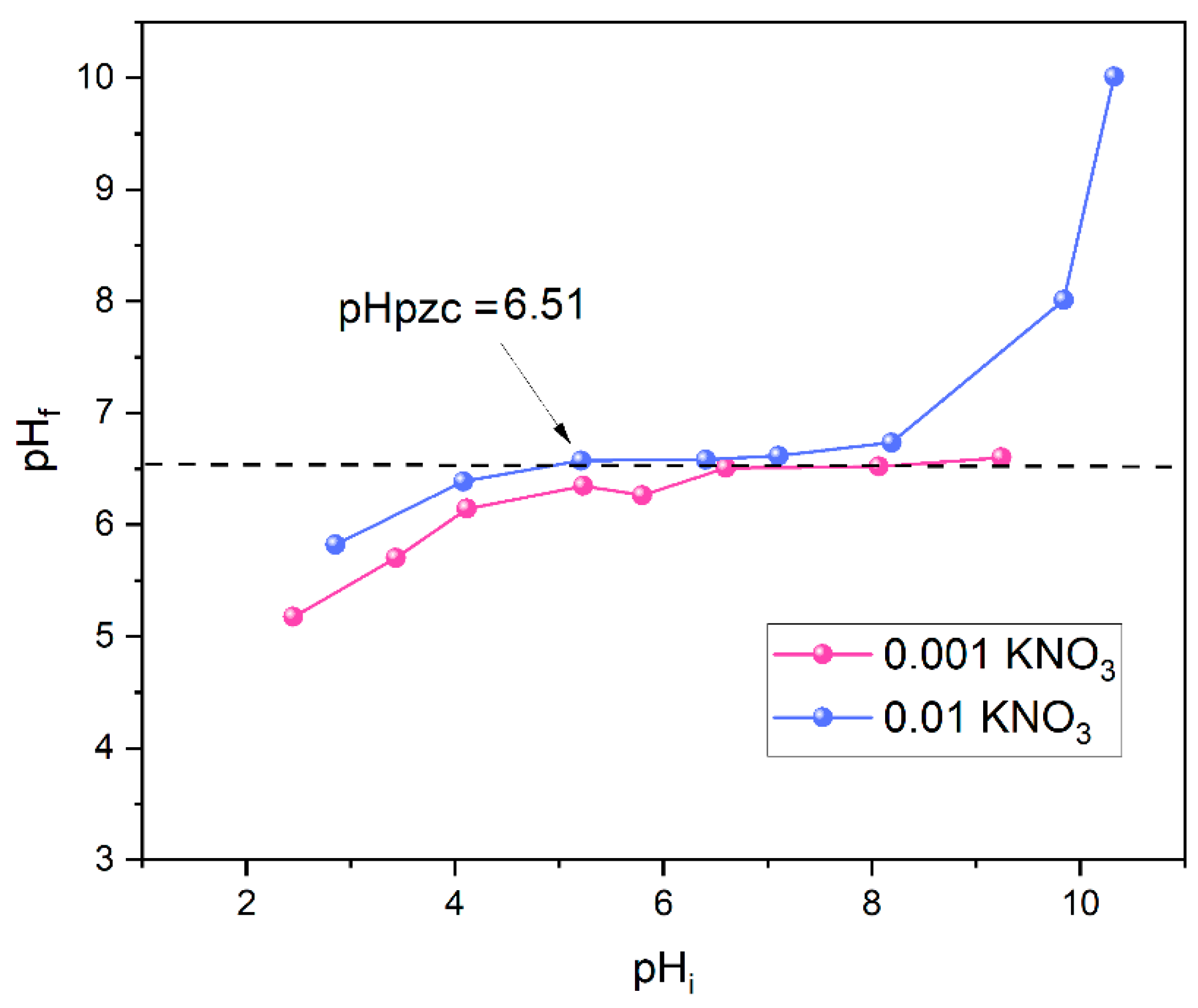
2.2. Determination of Zero Point Charge and BET Analysis
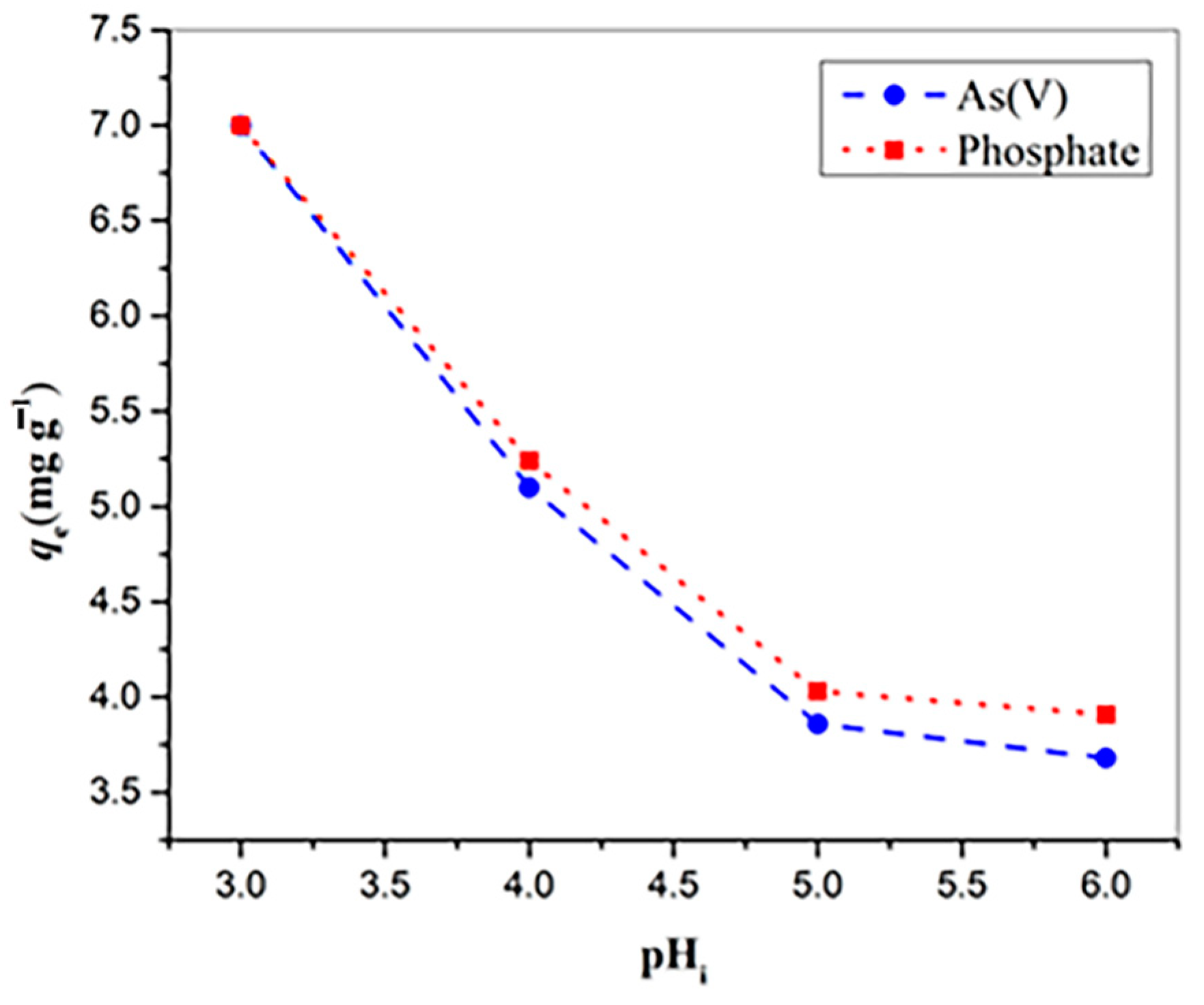
2.3. Sorption Experiments
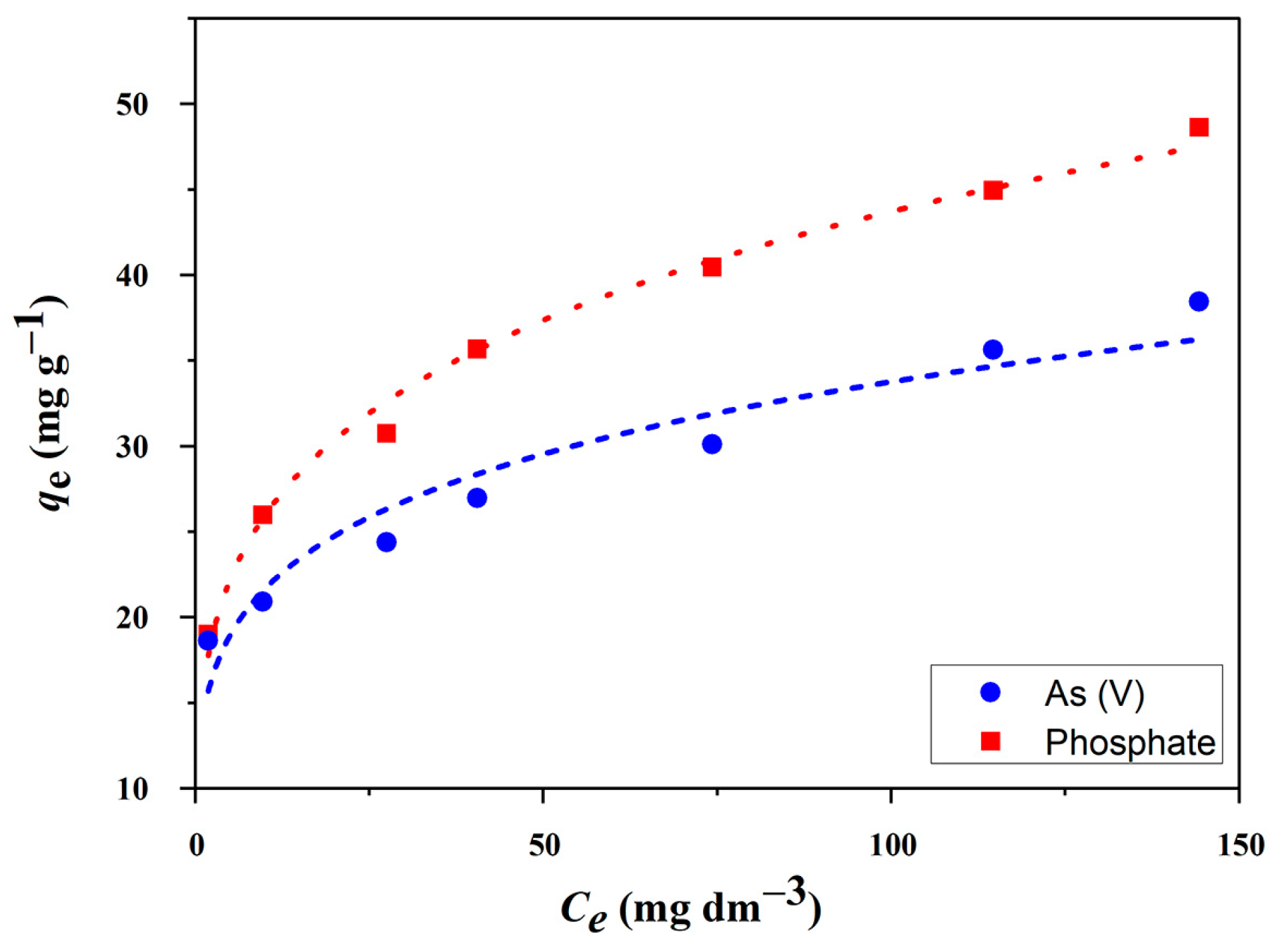
2.3.1. Adsorption Isotherms
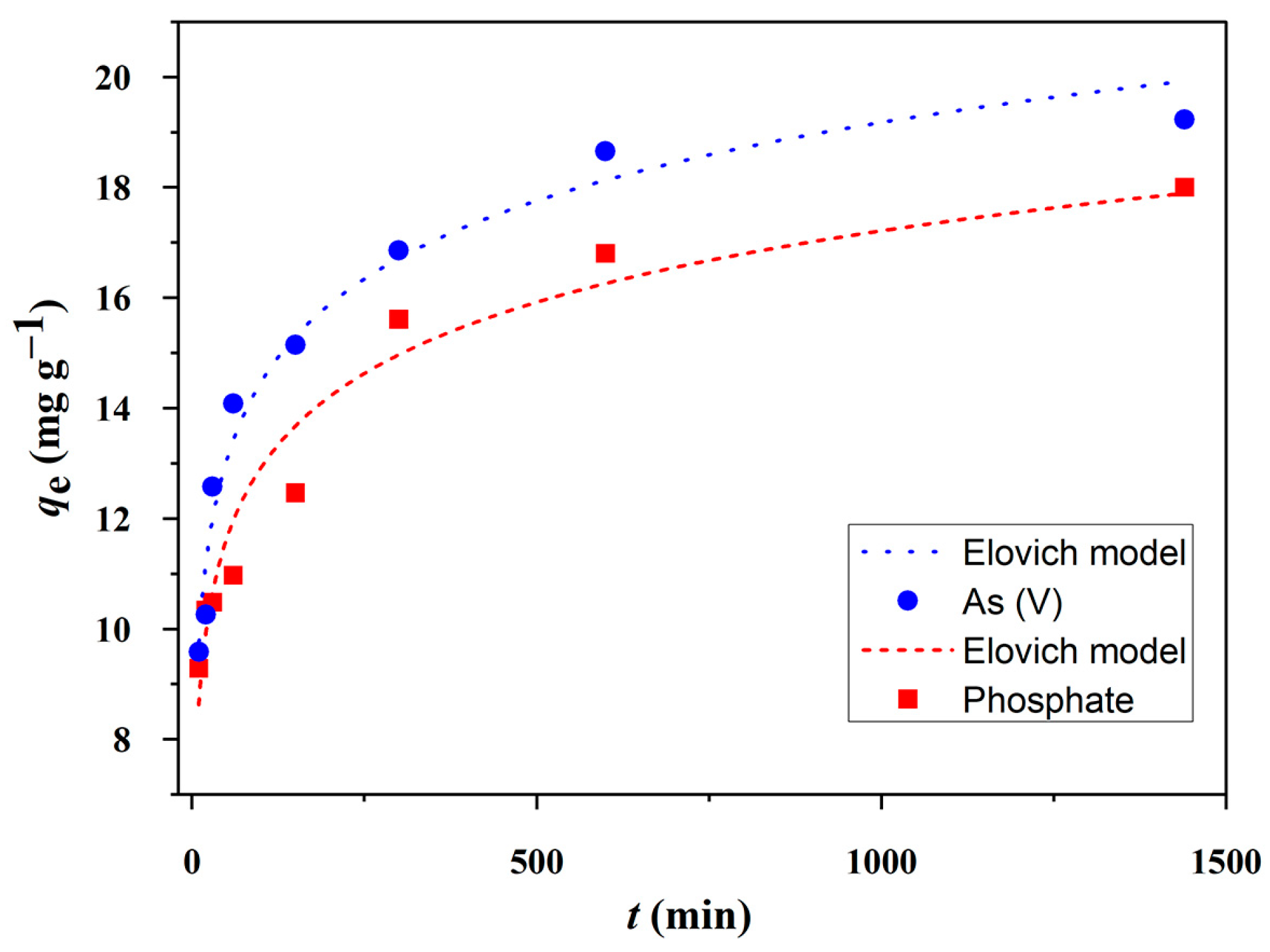
2.3.2. Adsorption Kinetic Studies
2.3.3. Real Water Matrix Studies
2.3.4. Comparative Discussion of Adsorption Results Using LDH-Based Adsorbents
3. Materials and Methods
3.1. Materials
3.2. Modification of Silica with GLYMO Silane (SiO2/GLYMO)
3.3. Synthesis of CuAl-LDH and Preparation of CuAl-LDH@SiO2 Composites
3.4. Material Characterization
3.5. Adsorption Experiments
3.6. pHPZC Determination and Texture Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| LDHs | Layered double hydroxides |
| PEI | Polyethyleneimine |
| XRD | X-ray diffraction |
| SEM | Scanning electron microscopy |
| EDS | Electronic dispersive spectroscopy |
| BET | Brunauer–Emmett–Teller |
| GLYMO | 3-glycidyloxypropyltrimethoxysilane |
| FESEM | Field-emission scanning electron microscopy |
| FAAS | Flame atomic absorption spectrometry |
| PFO | Pseudo-first-order |
| PSO | Pseudo-second-order |
References
- Rahman, S.; Navarathna, C.M.; Krishna Das, N.; Alchouron, J.; Reneau, P.; Stokes, S.; Thirumalai, R.V.; Perez, F.; Barbary Hassan, E.; Mohan, D.; et al. High Capacity Aqueous Phosphate Reclamation Using Fe/Mg-Layered Double Hydroxide (LDH) Dispersed on Biochar. J. Colloid Interface Sci. 2021, 597, 182–195. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zhang, M.; Pan, G.; Lundehøj, L.; Nielsen, U.G.; Shi, Y.; Hansen, H.C.B. Phosphate Capture by Ultrathin MgAl Layered Double Hydroxide Nanoparticles. Appl. Clay Sci. 2019, 177, 82–90. [Google Scholar] [CrossRef]
- Savić, A.B.; Čokeša, D.; Lazarević, S.; Jokić, B.; Janaćković, D.; Petrović, R.; Živković, L.S. Tailoring of Magnetite Powder Properties for Enhanced Phosphate Removal: Effect of PEG Addition in the Synthesis Process. Powder Technol. 2016, 301, 511–519. [Google Scholar] [CrossRef]
- Yang, K.; Yan, L.; Yang, Y.; Yu, S.; Shan, R.; Yu, H.; Zhu, B.; Du, B. Adsorptive Removal of Phosphate by Mg–Al and Zn–Al Layered Double Hydroxides: Kinetics, Isotherms and Mechanisms. Sep. Purif. Technol. 2014, 124, 36–42. [Google Scholar] [CrossRef]
- Zhang, Q.; Ji, F.; Zhao, T.; Shen, Q.; Fang, D.; Kuang, L.; Jiang, L.; Ding, S. Systematic Screening of Layered Double Hydroxides for Phosphate Removal and Mechanism Insight. Appl. Clay Sci. 2019, 174, 159–169. [Google Scholar] [CrossRef]
- Chitrakar, R.; Tezuka, S.; Sonoda, A.; Sakane, K.; Ooi, K.; Hirotsu, T. Adsorption of Phosphate from Seawater on Calcined MgMn-Layered Double Hydroxides. J. Colloid Interface Sci. 2005, 290, 45–51. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, T.; Li, M.; Yang, Y.; Lu, P.; Ning, P.; Wang, Q. Arsenic Removal from Water/Wastewater Using Layered Double Hydroxide Derived Adsorbents, a Critical Review. RSC Adv. 2018, 8, 22694–22709. [Google Scholar] [CrossRef]
- Shankar, S.; Shanker, U. Shikha Arsenic Contamination of Groundwater: A Review of Sources, Prevalence, Health Risks, and Strategies for Mitigation. Sci. World J. 2014, 2014, 304524. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Tran, H.N.; Nguyen, T.V.; Vigneswaran, S.; Trinh, V.T.; Nguyen, T.D.; Ha Nguyen, T.H.; Mai, T.N.; Chao, H.-P. Single-Step Removal of Arsenite Ions from Water through Oxidation-Coupled Adsorption Using Mn/Mg/Fe Layered Double Hydroxide as Catalyst and Adsorbent. Chemosphere 2022, 295, 133370. [Google Scholar] [CrossRef]
- Yadav, M.K.; Gupta, A.K.; Ghosal, P.S.; Mukherjee, A. Effect of Coexisting Ions on Adsorptive Removal of Arsenate by Mg-Fe-(CO3) LDH: Multi-Component Adsorption and ANN-Based Multivariate Modeling. J. Environ. Sci. Health Part A 2021, 56, 572–584. [Google Scholar] [CrossRef]
- Raheem, S.A.; Mohammed, A.A. Synthesis, Characterization, and Applications of Layered Double Hydroxides Nanocomposites for the Adsorption of Organic and Inorganic Contaminants from an Aqueous Solution: An Overview. Results Surf. Interfaces 2025, 18, 100386. [Google Scholar] [CrossRef]
- Chubar, N.I.; Kanibolotskyy, V.A.; Strelko, V.V.; Gallios, G.G.; Samanidou, V.F.; Shaposhnikova, T.O.; Milgrandt, V.G.; Zhuravlev, I.Z. Adsorption of Phosphate Ions on Novel Inorganic Ion Exchangers. Colloids Surf. A Physicochem. Eng. Asp. 2005, 255, 55–63. [Google Scholar] [CrossRef]
- Feng, Q.; Zhang, Y.; Zhang, G.; Han, G.; Zhao, W. A Novel Sulfidization System for Enhancing Hemimorphite Flotation through Cu/Pb Binary Metal Ions. Int. J. Min. Sci. Technol. 2024, 34, 1741–1752. [Google Scholar] [CrossRef]
- Wen, S.; Miao, Y.; Tang, Y.; Song, Z.; Feng, Q. Theoretical and Experimental Study on High-Entropy Flotation of Micro-Fine Cassiterite. Int. J. Min. Sci. Technol. 2025, 35, 19–39. [Google Scholar] [CrossRef]
- Egelja, A.; Savic, A.; Savic, M.; Kokunesoski, M.; Pantic, K.; Rancic, M.; Vuksanovic, M. Application of Fe-Al Layered Double Hydroxides on Silica for Phosphate and Arsenate Removal from Water. Sci. Sinter. 2024, 56, 309–326. [Google Scholar] [CrossRef]
- Savić, A.B.; Čokeša, D.; Savić Biserčić, M.; Častvan-Janković, I.; Petrović, R.; Živković, L.S. Multifunctional Use of Magnetite-Coated Tuff Grains in Water Treatment: Removal of Arsenates and Phosphates. Adv. Powder Technol. 2019, 30, 1687–1695. [Google Scholar] [CrossRef]
- Abduarahman, M.A.; Vuksanović, M.M.; Knežević, N.; Banjanac, K.; Milošević, M.; Veličković, Z.; Marinković, A. MgAl-Layered Double Hydroxide-Coated Bio-Silica as an Adsorbent for Anionic Pollutants Removal: A Case Study of the Implementation of Sustainable Technologies. Int. J. Mol. Sci. 2024, 25, 11837. [Google Scholar] [CrossRef]
- Zubair, M.; Jarrah, N.; Ihsanullah; Khalid, A.; Manzar, M.S.; Kazeem, T.S.; Al-Harthi, M.A. Starch-NiFe-Layered Double Hydroxide Composites: Efficient Removal of Methyl Orange from Aqueous Phase. J. Mol. Liq. 2018, 249, 254–264. [Google Scholar] [CrossRef]
- Kumari, S.; Soni, S.; Sharma, A.; Kumar, S.; Sharma, V.; Jaswal, V.S.; Bhatia, S.K.; Sharma, A.K. Layered Double Hydroxides Based Composite Materials and Their Applications in Food Packaging. Appl. Clay Sci. 2024, 247, 107216. [Google Scholar] [CrossRef]
- Kameliya, J.; Verma, A.; Dutta, P.; Arora, C.; Vyas, S.; Varma, R.S. Layered Double Hydroxide Materials: A Review on Their Preparation, Characterization, and Applications. Inorganics 2023, 11, 121. [Google Scholar] [CrossRef]
- Mohapi, M.; Sefadi, J.S.; Mochane, M.J.; Magagula, S.I.; Lebelo, K. Effect of LDHs and Other Clays on Polymer Composite in Adsorptive Removal of Contaminants: A Review. Crystals 2020, 10, 957. [Google Scholar] [CrossRef]
- Wu, M.J.; Wu, J.Z.; Zhang, J.; Chen, H.; Zhou, J.Z.; Qian, G.R.; Xu, Z.P.; Du, Z.; Rao, Q.L. A Review on Fabricating Heterostructures from Layered Double Hydroxides for Enhanced Photocatalytic Activities. Catal. Sci. Technol. 2018, 8, 1207–1228. [Google Scholar] [CrossRef]
- Ahmed, A.A.A.; Talib, Z.A.; bin Hussein, M.Z.; Zakaria, A. Zn–Al Layered Double Hydroxide Prepared at Different Molar Ratios: Preparation, Characterization, Optical and Dielectric Properties. J. Solid State Chem. 2012, 191, 271–278. [Google Scholar] [CrossRef]
- Constantino, V.R.L.; Pinnavaia, T.J. Basic Properties of Mg2+1-XAl3+x Layered Double Hydroxides Intercalated by Carbonate, Hydroxide, Chloride, and Sulfate Anions. Inorg. Chem. 1995, 34, 883–892. [Google Scholar] [CrossRef]
- Keyikoglu, R.; Khataee, A.; Yoon, Y. Layered Double Hydroxides for Removing and Recovering Phosphate: Recent Advances and Future Directions. Adv. Colloid Interface Sci. 2022, 300, 102598. [Google Scholar] [CrossRef]
- Meili, L.; Lins, P.V.; Zanta, C.L.P.S.; Soletti, J.I.; Ribeiro, L.M.O.; Dornelas, C.B.; Silva, T.L.; Vieira, M.G.A. MgAl-LDH/Biochar Composites for Methylene Blue Removal by Adsorption. Appl. Clay Sci. 2019, 168, 11–20. [Google Scholar] [CrossRef]
- Li, R.; Wang, J.J.; Zhou, B.; Awasthi, M.K.; Ali, A.; Zhang, Z.; Gaston, L.A.; Lahori, A.H.; Mahar, A. Enhancing Phosphate Adsorption by Mg/Al Layered Double Hydroxide Functionalized Biochar with Different Mg/Al Ratios. Sci. Total Environ. 2016, 559, 121–129. [Google Scholar] [CrossRef]
- Lartey-Young, G.; Ma, L. Optimization, Equilibrium, Adsorption Behaviour of Cu/Zn/Fe LDH and LDHBC Composites towards Atrazine Reclamation in an Aqueous Environment. Chemosphere 2022, 293, 133526. [Google Scholar] [CrossRef]
- Palapa, N.R.; Taher, T.; Rahayu, B.R.; Mohadi, R.; Rachmat, A.; Lesbani, A. CuAl LDH/Rice Husk Biochar Composite for Enhanced Adsorptive Removal of Cationic Dye from Aqueous Solution. Bull. Chem. React. Eng. Catal. 2020, 15, 525–537. [Google Scholar] [CrossRef]
- Normah, N.; Juleanti, N.; Siregar, P.M.S.B.N.; Wijaya, A.; Palapa, N.R.; Taher, T.; Lesbani, A. Size Selectivity of Anionic and Cationic Dyes Using LDH Modified Adsorbent with Low-Cost Rambutan Peel to Hydrochar. Bull. Chem. React. Eng. Catal. 2021, 16, 869–880. [Google Scholar] [CrossRef]
- Han, W.; Hao, H.; Zhang, Q.; Shao, Z. Activated Biochar Loaded CuAl-Layered Double Hydroxide Composite for the Removal of Aniline Aerofloat in Wastewater: Synthesis, Characterization, and Adsorption Mechanism. J. Environ. Chem. Eng. 2023, 11, 109293. [Google Scholar] [CrossRef]
- Ayalew, Z.M.; Guo, X.; Zhang, X. Synthesis and Application of Polyethyleneimine (PEI)-based Composite/Nanocomposite Material for Heavy Metals Removal from Wastewater: A Critical Review. J. Hazard. Mater. Adv. 2022, 8, 100158. [Google Scholar] [CrossRef]
- Cohen, S.; Chejanovsky, I.; Suckeveriene, R.Y. Grafting of Poly(Ethylene Imine) to Silica Nanoparticles for Odor Removal from Recycled Materials. Nanomaterials 2022, 12, 2237. [Google Scholar] [CrossRef] [PubMed]
- Palapa, N.R.; Mohadi, R.; Rachmat, A.; Lesbani, A. Adsorption Study of Malachite Green Removal from Aqueous Solution Using Cu/M3+ (M3+=Al, Cr) Layered Double Hydroxide. Mediterr. J. Chem. 2020, 10, 33–45. [Google Scholar] [CrossRef]
- Li, J.; Zhang, S.; Chen, Y.; Liu, T.; Liu, C.; Zhang, X.; Yi, M.; Chu, Z.; Han, X. A Novel Three-Dimensional Hierarchical CuAl Layered Double Hydroxide with Excellent Catalytic Activity for Degradation of Methyl Orange. RSC Adv. 2017, 7, 29051–29057. [Google Scholar] [CrossRef]
- Zayyoun, N.; Bahmad, L.; Laânab, L.; Jaber, B. The Effect of PH on the Synthesis of Stable Cu2O/CuO Nanoparticles by Sol–Gel Method in a Glycolic Medium. Appl. Phys. A 2016, 122, 488. [Google Scholar] [CrossRef]
- Knežević, N.; Vuksanović, M.M.; Banjanac, K.; Pantić, K.; Veličković, Z.; Cvijetić, I.; Marinković, A.; Milošević, M. Cationic Waste Hemp Fibers-Based Membrane: Case Study of Anionic Pollutants Removal through Environmentally Friendly Processes. J. Environ. Manag. 2024, 371, 123174. [Google Scholar] [CrossRef]
- Hegedűs, V.; Kerényi, F.; Boda, R.; Horváth, D.; Lázár, I.; Tóth-Győri, E.; Dezső, B.; Hegedus, C. β-Tricalcium Phosphate-Silica Aerogel as an Alternative Bioactive Ceramic for the Potential Use in Dentistry. Adv. Appl. Ceram. 2020, 119, 364–371. [Google Scholar] [CrossRef]
- Iakunkov, A.; Skrypnychuk, V.; Nordenström, A.; Shilayeva, E.A.; Korobov, M.; Prodana, M.; Enachescu, M.; Larsson, S.H.; V.Talyzin, A. Activated Graphene as a Material for Supercapacitor Electrodes: Effects of Surface Area, Pore Size Distribution and Hydrophilicity. Phys. Chem. Chem. Phys. 2019, 21, 17901–17912. [Google Scholar] [CrossRef]
- Farouq, R.; Yousef, N.S. Equilibrium and Kinetics Studies of Adsorption of Copper (II) Ions on Natural Biosorbent. Int. J. Chem. Eng. Appl. 2015, 6, 319–324. [Google Scholar] [CrossRef]
- Wu, F.-C.; Tseng, R.-L.; Juang, R.-S. Characteristics of Elovich Equation Used for the Analysis of Adsorption Kinetics in Dye-Chitosan Systems. Chem. Eng. J. 2009, 150, 366–373. [Google Scholar] [CrossRef]
- Boyer, T.H.; Briese, E.; Crane, L.; Bhadha, J.; Call, D.F.; McLamore, E.S.; Rittmann, B.; Tuberty, S.; Westerhoff, P.; Duckworth, O.W. Guidance on Aqueous Matrices for Evaluating Novel Precipitants and Adsorbents for Phosphorus Removal and Recovery. Chemosphere 2024, 367, 143648. [Google Scholar] [CrossRef] [PubMed]
- da Gama, B.M.V.; Selvasembian, R.; Giannakoudakis, D.A.; Triantafyllidis, K.S.; McKay, G.; Meili, L. Layered Double Hydroxides as Rising-Star Adsorbents for Water Purification: A Brief Discussion. Molecules 2022, 27, 4900. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Gao, Y.; Zhu, Z.; Zhang, L.; Zhao, N.; Fang, Y.; Zhu, Y.; Liu, G. Enhanced Arsenic Removal from Aqueous Solution by Fe/Mn-C Layered Double Hydroxide Composite. Adsorpt. Sci. Technol. 2021, 2021, 1–12. [Google Scholar] [CrossRef]
- Shiriazar, M.A.; Sepehr, E.; Maleki, R.; Khodaverdiloo, H.; Asadzadeh, F.; Dovlati, B.; Rengel, Z. Arsenate Removal from Aqueous Solutions by Mg/Fe-LDH-Modified Biochar Derived from Apple Tree Residues. Earth Environ. Sci. Trans. R. Soc. Edinb. 2022, 113, 1–10. [Google Scholar] [CrossRef]
- Feng, L.; Zhang, Q.; Ji, F.; Jiang, L.; Liu, C.; Shen, Q.; Liu, Q. Phosphate Removal Performances of Layered Double Hydroxides (LDH) Embedded Polyvinyl Alcohol/Lanthanum Alginate Hydrogels. Chem. Eng. J. 2022, 430, 132754. [Google Scholar] [CrossRef]
- Ding, W.; Wan, X.; Zheng, H.; Wu, Y.; Muhammad, S. Sulfite-Assisted Oxidation/Adsorption Coupled with a TiO2 Supported CuO Composite for Rapid Arsenic Removal: Performance and Mechanistic Studies. J. Hazard. Mater. 2021, 413, 125449. [Google Scholar] [CrossRef]
- Sürmeli, M.; Yazıcı, H. Comprehensive Insight Into Removal and Recovery of Phosphate by a Magnetic Nanocomposite Microparticle Modified With MgFe-Zr Layered Double Hydroxide. Water Air Soil Pollut. 2025, 236, 15. [Google Scholar] [CrossRef]
- Isidoro Ribeiro, N.; Barreto Pessanha, O.; Luiza Gomes Soares Pessanha, M.; Guimarães, D. Efficient Phosphate Adsorption by a Composite Composed of Mg6Al2(CO3)(OH)16·4H2O LDH and Chitosan: Kinetic, Thermodynamic, Desorption, and Characterization Studies. Sep. Purif. Technol. 2023, 307, 122717. [Google Scholar] [CrossRef]
- Hokkanen, S.; Repo, E.; Lou, S.; Sillanpää, M. Removal of Arsenic(V) by Magnetic Nanoparticle Activated Microfibrillated Cellulose. Chem. Eng. J. 2015, 260, 886–894. [Google Scholar] [CrossRef]
- Seftel, E.M.; Ciocarlan, R.G.; Michielsen, B.; Meynen, V.; Mullens, S.; Cool, P. Insights into Phosphate Adsorption Behavior on Structurally Modified ZnAl Layered Double Hydroxides. Appl. Clay Sci. 2018, 165, 234–246. [Google Scholar] [CrossRef]
- Khitous, M.; Salem, Z.; Halliche, D. Removal of Phosphate from Industrial Wastewater Using Uncalcined MgAl-NO3 Layered Double Hydroxide: Batch Study and Modeling. Desalination Water Treat. 2016, 57, 15920–15931. [Google Scholar] [CrossRef]
- Wu, X.; Tan, X.; Yang, S.; Wen, T.; Guo, H.; Wang, X.; Xu, A. Coexistence of Adsorption and Coagulation Processes of Both Arsenate and NOM from Contaminated Groundwater by Nanocrystallined Mg/Al Layered Double Hydroxides. Water Res. 2013, 47, 4159–4168. [Google Scholar] [CrossRef]
- Johnston, A.-L.; Lester, E.; Williams, O.; Gomes, R.L. Understanding Layered Double Hydroxide Properties as Sorbent Materials for Removing Organic Pollutants from Environmental Waters. J. Environ. Chem. Eng. 2021, 9, 105197. [Google Scholar] [CrossRef]
- Mahjoubi, F.Z.; Khalidi, A.; Cherkaoui, O.; Elmoubarki, R.; Abdennouri, M.; Barka, N. Treatment of Textile Effluents by Chloride-Intercalated Zn-, Mg- and Ni-Al Layered Double Hydroxides. J. Water Reuse Desalination 2017, 7, 307–318. [Google Scholar] [CrossRef]
- Zubair, M.; Ihsanullah, I.; Abdul Aziz, H.; Azmier Ahmad, M.; Al-Harthi, M.A. Sustainable Wastewater Treatment by Biochar/Layered Double Hydroxide Composites: Progress, Challenges, and Outlook. Bioresour. Technol. 2021, 319, 124128. [Google Scholar] [CrossRef]
- Knežević, N.; Milanović, J.; Veličković, Z.; Milošević, M.; Vuksanović, M.M.; Onjia, A.; Marinković, A. A Closed Cycle of Sustainable Development: Effective Removal and Desorption of Lead and Dyes Using an Oxidized Cellulose Membrane. J. Ind. Eng. Chem. 2023, 126, 520–536. [Google Scholar] [CrossRef]
- Wang, J.; Guo, X. Rethinking of the Intraparticle Diffusion Adsorption Kinetics Model: Interpretation, Solving Methods and Applications. Chemosphere 2022, 309, 136732. [Google Scholar] [CrossRef]




| Sample | Sp, m2/g | Vtotal, cm3/g | Vmeso, cm3/g | Dsr, nm | Dmax, nm |
|---|---|---|---|---|---|
| SiO2 | 3.5–9.5 | – | – | – | – |
| CuAl-LDH@SiO2 | 19.1 | 0.0823 | 0.0812 | 13.5 | 11.0 |
| Model | Parameter | Pollutants | |
|---|---|---|---|
| PO43− | As(V) | ||
| Langmuir | Qm (mg g−1) | 44.6 | 32.3 |
| KL (L mg−1) | 0.183 | 0.396 | |
| χ2 | 31.5 | 29.4 | |
| R2 | 0.798 | 0.611 | |
| Freundlich | KF (L mg−1) | 15.4 | 13.9 |
| n | 4.41 | 5.18 | |
| χ2 | 1.33 | 4.74 | |
| R2 | 0.992 | 0.937 | |
| Model | Parameter | Pollutants | |
|---|---|---|---|
| PO43− | As(V) | ||
| Pseudo-first-order | qe (mg g−1) | 14.8 | 17.0 |
| k1 (min−1) | 0.0591 | 0.0521 | |
| χ2 | 7.06 | 4.05 | |
| R2 | 0.524 | 0.768 | |
| Pseudo-second-order | qe (mg g−1) | 16.0 | 18.06 |
| k2 (g mg−1 min−1) | 0.00512 | 0.00433 | |
| χ2 | 3.66 | 1.52 | |
| R2 | 0.753 | 0.913 | |
| Intraparticle diffusion | kint (mg g−1 min−0.5) | 0.264 | 0.271 |
| C | 9.27 | 10.7 | |
| χ2 | 1.13 | 2.77 | |
| R2 | 0.924 | 0.841 | |
| Elovich | α (mg g−1 min−1) | 19.0 | 24.0 |
| β (g mg−1) | 0.536 | 0.489 | |
| χ2 | 0.637 | 0.404 | |
| R2 | 0.957 | 0.978 | |
| Adsorbent | Pollutant | Ci, mg dm−3 | qm, mg g−1 | k, g mg−1 min−1 | Reference |
|---|---|---|---|---|---|
| Mg/Mn/Fe-LDH | As(V) | 4–270 | 32.2 | 0.019 | [9] |
| Fe/Mn-C-LDH | As(V) | 100 | 22.2 | 0.0077 | [44] |
| Mg/Fe-modified biochar | As(V) | 1 | 3.6 | 0.02 | [45] |
| PS-La-LDH | Phosphate | 5–50 | 34.2 | 0.001 | [46] |
| Cu-Zn-Fe LDH | Atrazine | 5–30 | 21.85 | - | [28] |
| Cu-TiO2 | As(V) | 3 | 19.72 | 0.056 | [47] |
| NCMP@MgFe-Zr | Phosphate | 25 | 9.6 | 0.017 | [48] |
| Mg-Al-CO3 LDH | Phosphate | 10–120 | 54.9 | 0.006 | [49] |
| FeNP/MFC | As(V) | 0.14–10.68 | 2.46 | 0.0375 | [50] |
| Zn-Al LDH | Phosphate | 5–200 | 35.9 | 0.055 | [51] |
| MgAl-NO3 LDH | Phosphate | 100 | 64.1 | 0.005 | [52] |
| LDH-CO3 LDH-Cl | As(V) | 0.2 | 44.7 88.3 | - - | [53] |
| FeAl-LDH@SiO2 | As(V) Phosphate | 100 100 | 46.0 58.9 | - - | [15] |
| CuAl-LDH@SiO2 | As(V) Phosphate | 2–200 2–200 | 32.3 44.6 | 0.00433 0.00512 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Savić, A.; Vuksanović, M.M.; Savić, M.; Knežević, N.; Šaponjić, A.; Ilić, S.; Egelja, A. Modified Silica Particles Coated with Cu-Al Layered Double Hydroxide for Phosphate and Arsenate Removal in Water Treatment. Molecules 2025, 30, 2138. https://doi.org/10.3390/molecules30102138
Savić A, Vuksanović MM, Savić M, Knežević N, Šaponjić A, Ilić S, Egelja A. Modified Silica Particles Coated with Cu-Al Layered Double Hydroxide for Phosphate and Arsenate Removal in Water Treatment. Molecules. 2025; 30(10):2138. https://doi.org/10.3390/molecules30102138
Chicago/Turabian StyleSavić, Andrija, Marija M. Vuksanović, Marjetka Savić, Nataša Knežević, Aleksandra Šaponjić, Svetlana Ilić, and Adela Egelja. 2025. "Modified Silica Particles Coated with Cu-Al Layered Double Hydroxide for Phosphate and Arsenate Removal in Water Treatment" Molecules 30, no. 10: 2138. https://doi.org/10.3390/molecules30102138
APA StyleSavić, A., Vuksanović, M. M., Savić, M., Knežević, N., Šaponjić, A., Ilić, S., & Egelja, A. (2025). Modified Silica Particles Coated with Cu-Al Layered Double Hydroxide for Phosphate and Arsenate Removal in Water Treatment. Molecules, 30(10), 2138. https://doi.org/10.3390/molecules30102138









