Aging and Discoloration of Red Lead (Pb3O4) Caused by Reactive Oxygen Species Under Alkaline Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Four Kinds of ROS
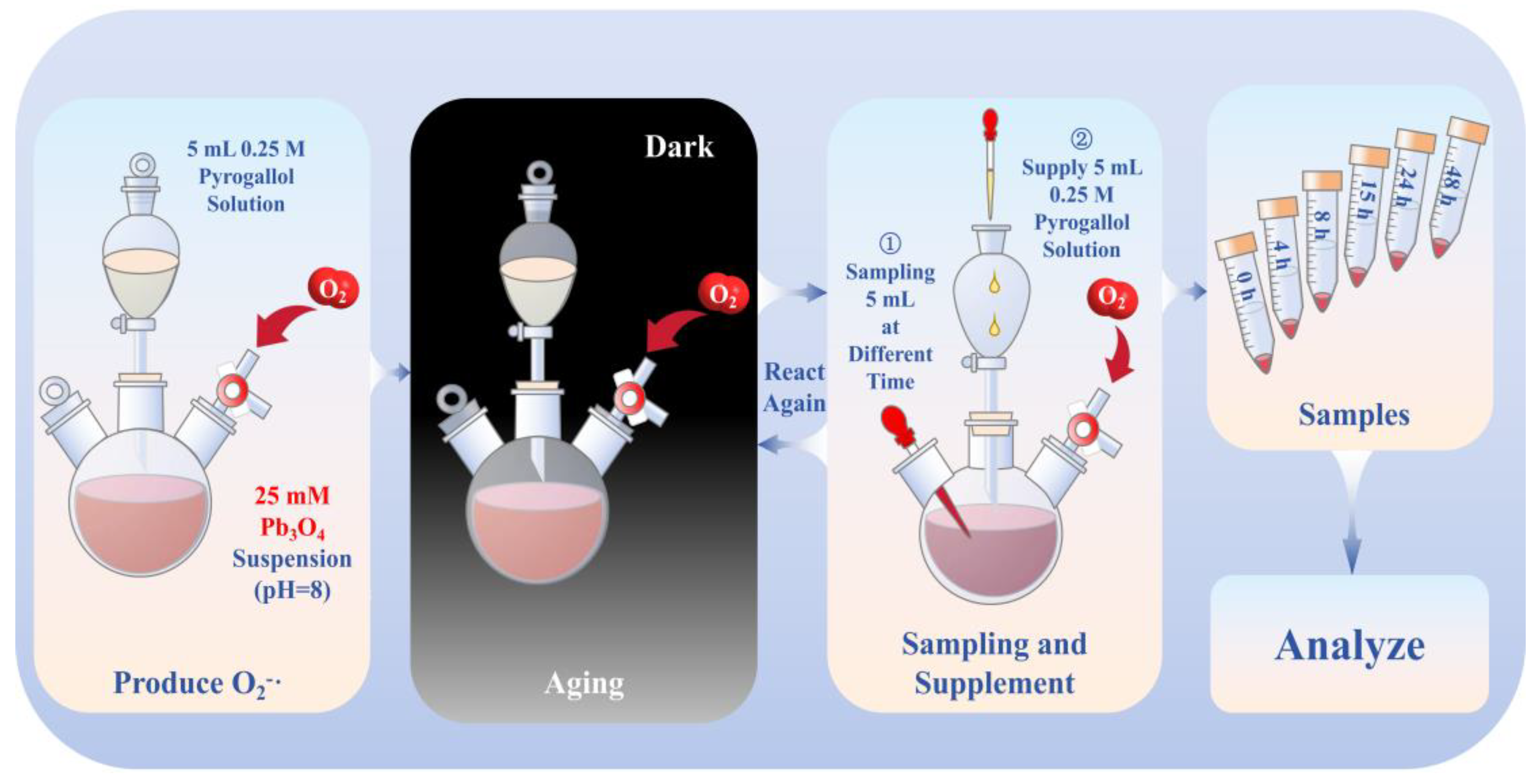
2.2. Aging of Pb3O4 Using Superoxide Radical Under Alkaline Conditions
2.3. Aging Effects of Three Other Different ROS on Pb3O4
2.4. Analytical Techniques
3. Results and Discussion
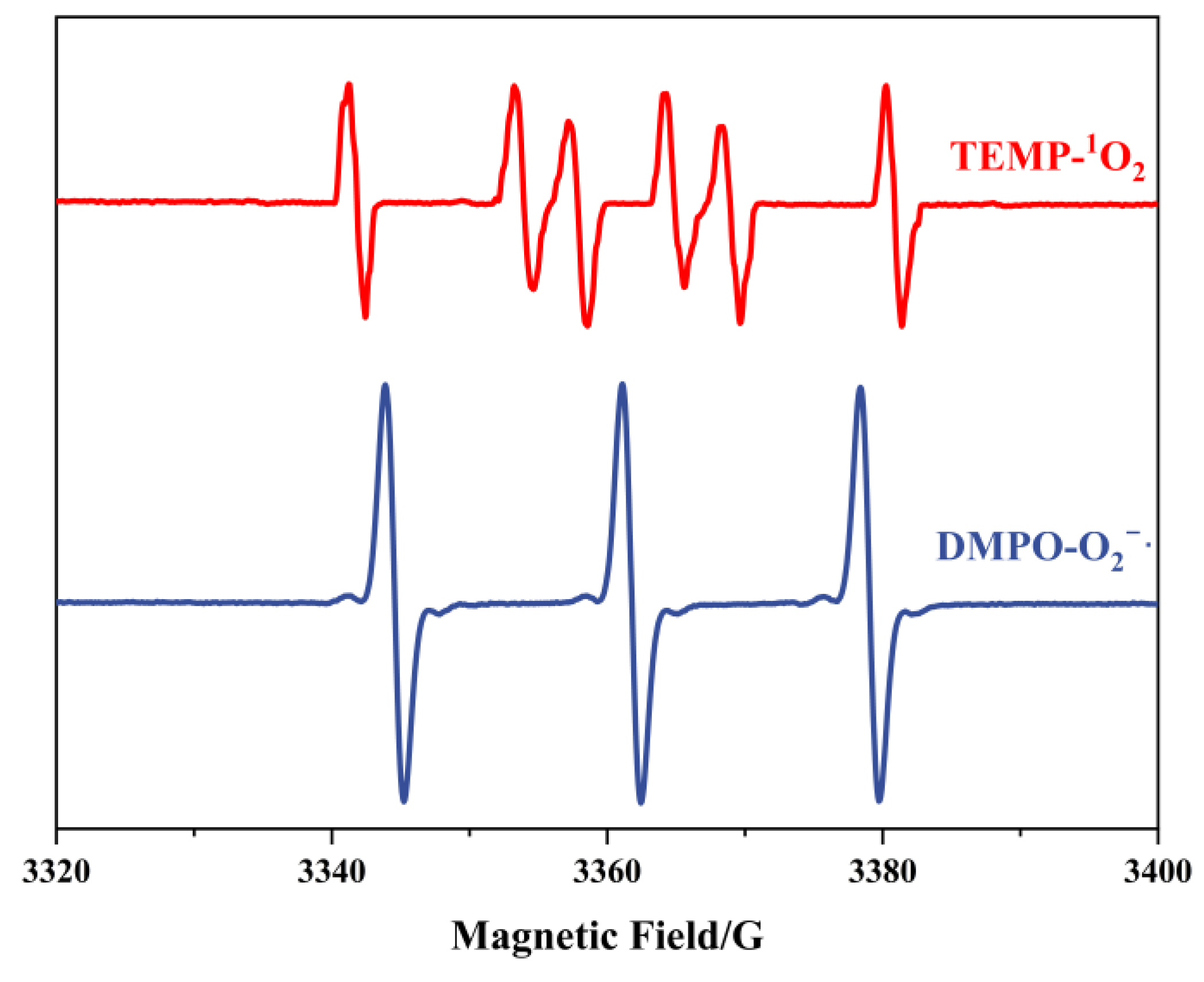
3.1. Confirmation of the ROS Production
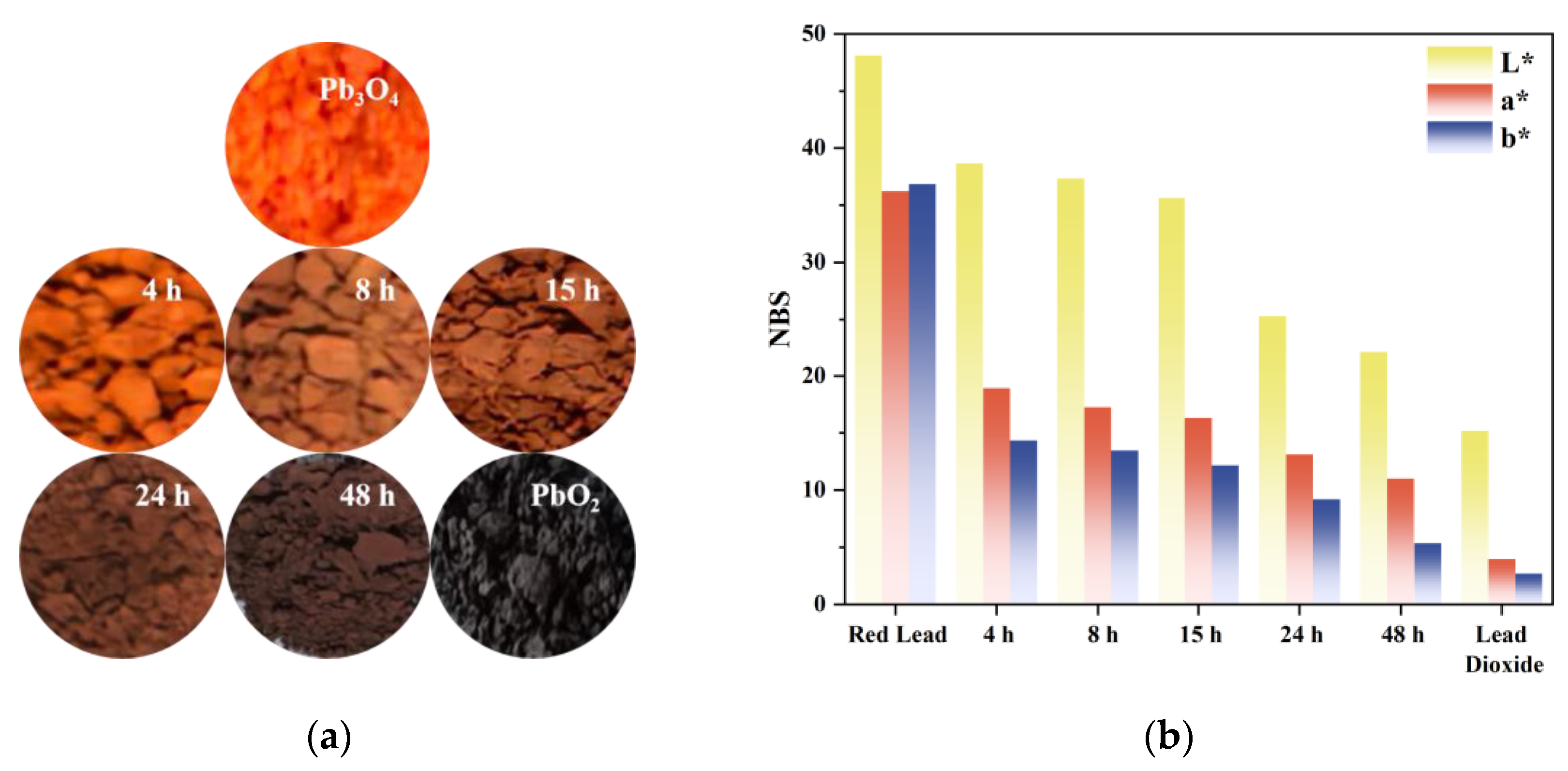
3.2. Chromaticity Coordinates
3.3. Crystal Structure
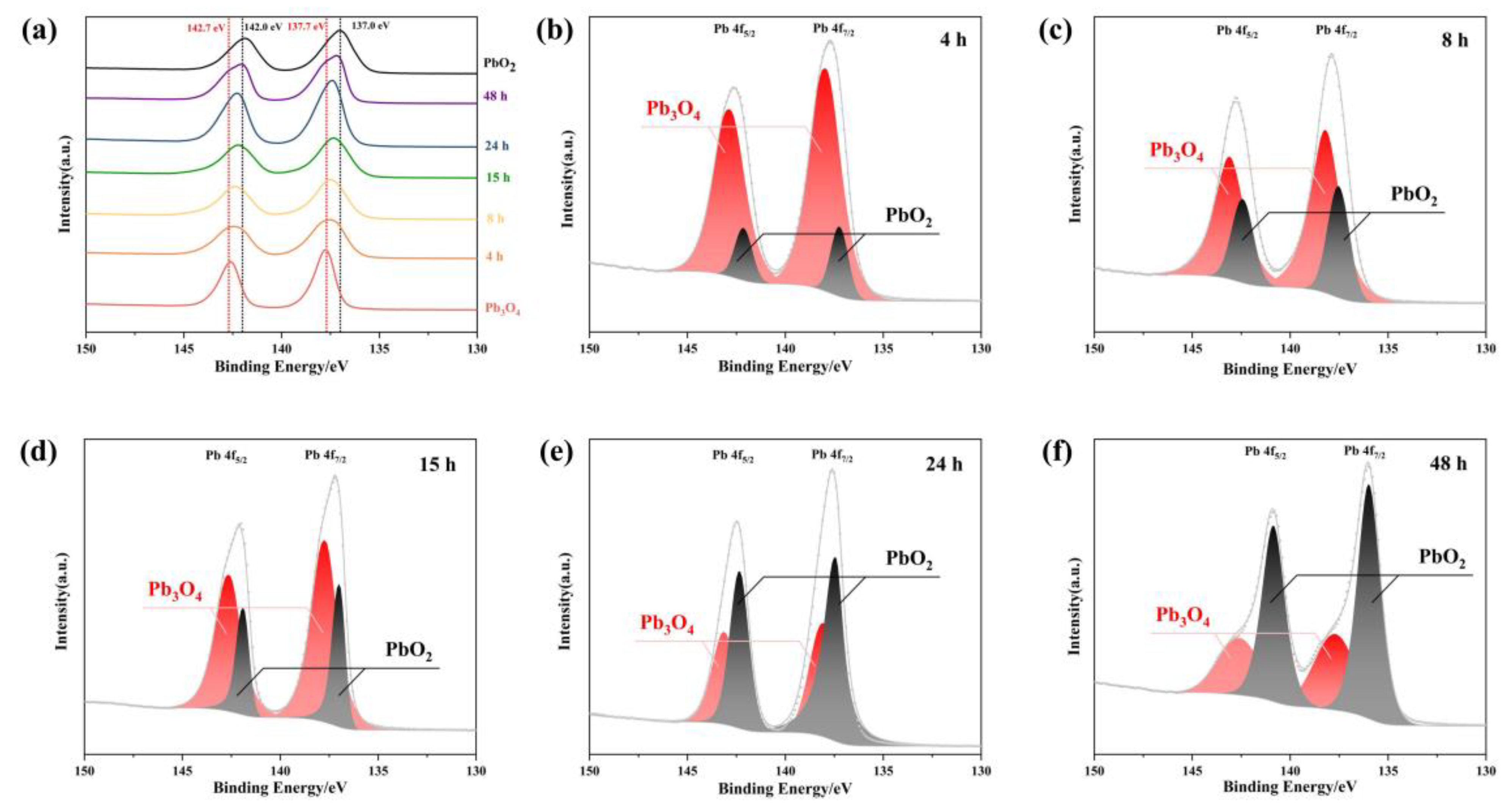
3.4. XPS Analysis
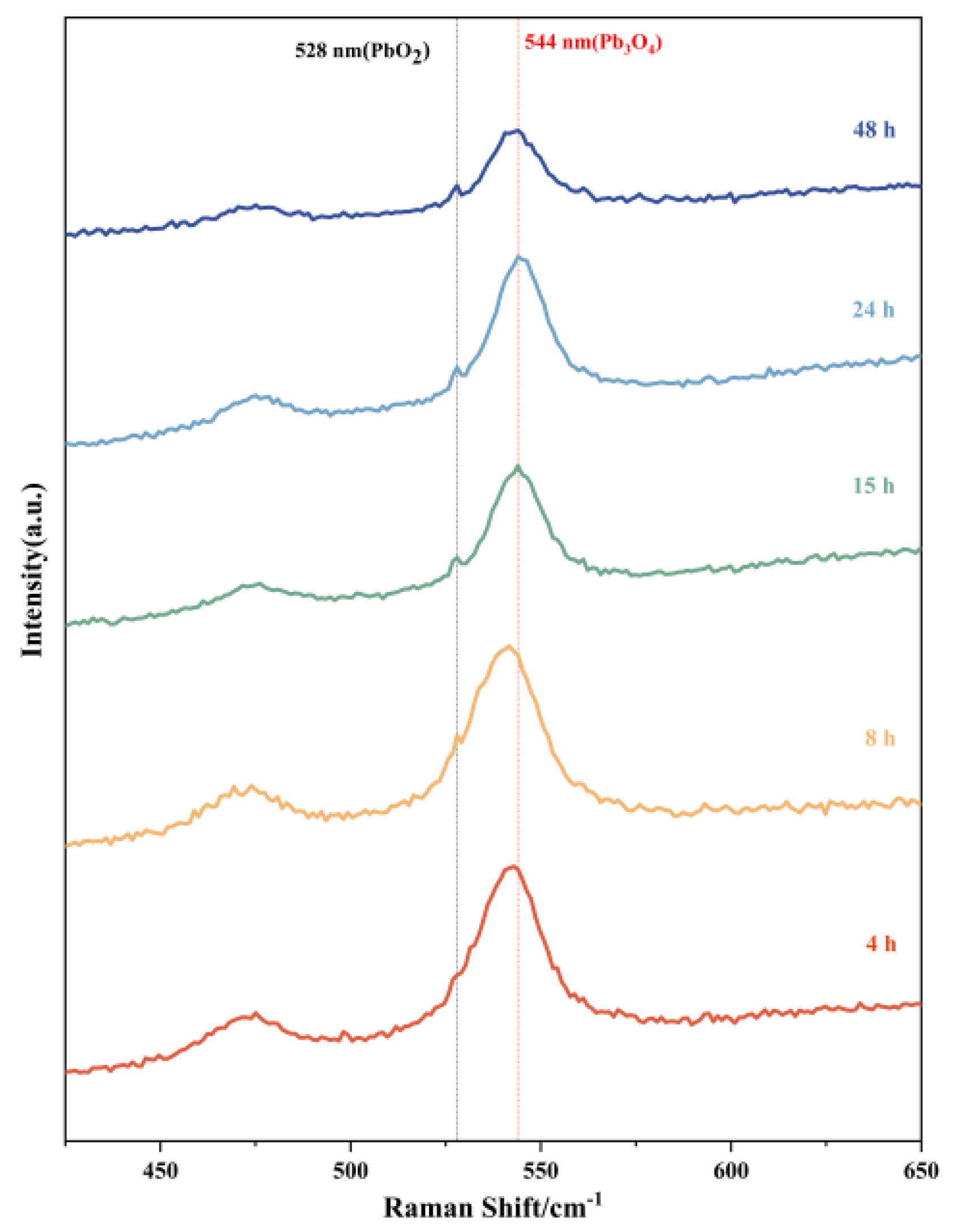
3.5. Raman Spectroscopy
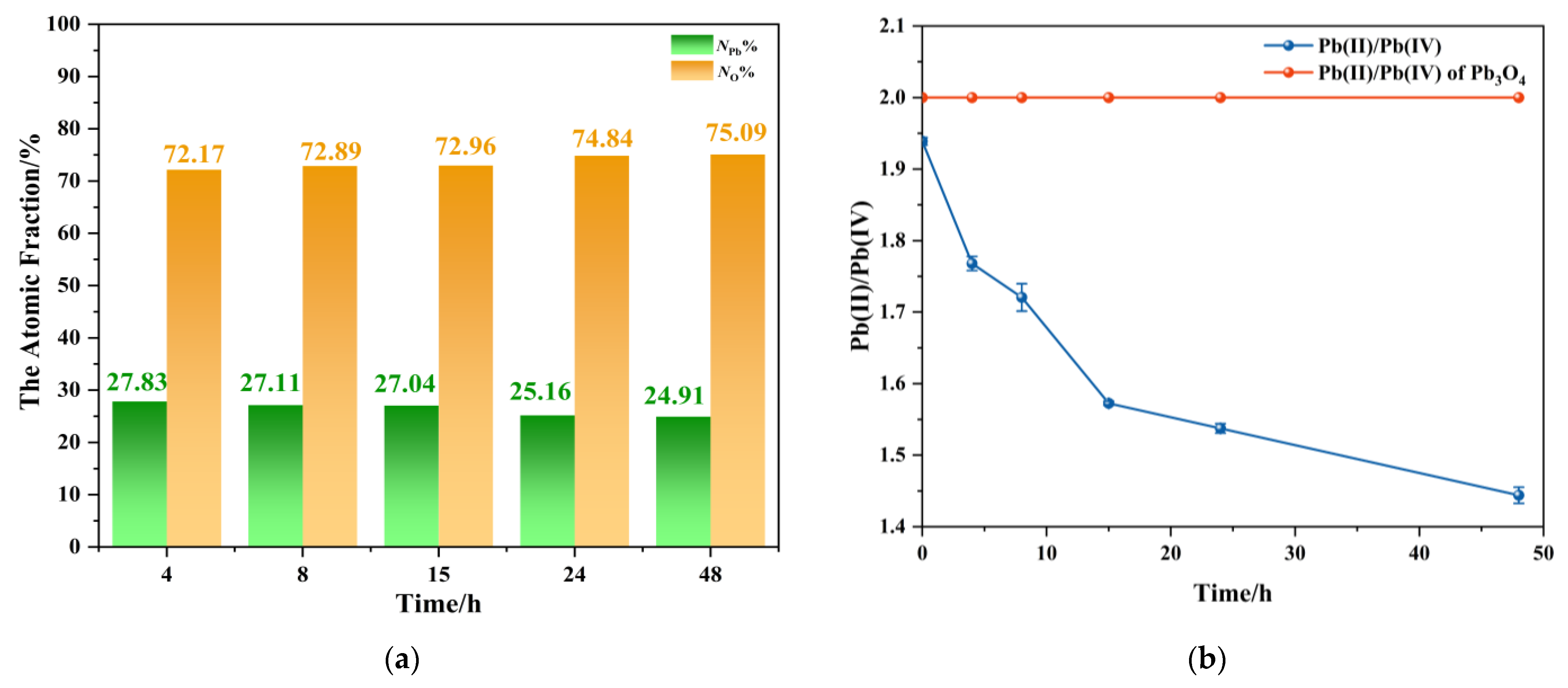
3.6. Ratio of Lead and Oxygen Elements in Aging Products
3.7. Comparison of Aging Effects of Different ROS
3.8. Gibbs Free Energy Calculation
3.9. Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sun, D.; Li, X.; Yin, Y.; Zhang, Y.; Han, W.; Wang, Y.; Su, M.; Dong, C.; Yu, Z.; Su, B. Effect of buffer gas on the analysis of Dunhuang murals by laser-induced breakdown spectroscopy technology. J. Cult. Herit. 2022, 55, 399–408. [Google Scholar] [CrossRef]
- Costantini, I.; Lottici, P.P.; Bersani, D.; Pontiroli, D.; Casoli, A.; Castro, K.; Madariaga, J.M. Darkening of lead-and iron-based pigments on late Gothic Italian wall paintings: Energy dispersive X-ray fluorescence, μ-Raman, and powder X-ray diffraction analyses for diagnosis: Presence of β-PbO2(plattnerite) and α-PbO2(scrutinyite). J. Raman Spectrosc. 2020, 51, 680–692. [Google Scholar] [CrossRef]
- Aze, S.; Vallet, J.M.; Pomey, M.; Baronnet, A.; Grauby, O. Red lead darkening in wall paintings: Natural ageing of experimental wall paintings versus artificial ageing tests. Eur. J. Mineral. 2007, 19, 883–890. [Google Scholar] [CrossRef]
- Aze, S.; Vallet, J.M.; Detalle, V.; Grauby, O.; Baronnet, A. Chromatic alterations of red lead pigments in artworks: A review. Phase Transit. 2008, 81, 145–154. [Google Scholar] [CrossRef]
- Young, J.A. Trilead tetroxide. J. Chem. Educ. 2007, 84, 226. [Google Scholar] [CrossRef]
- Zhao, Y.; Tang, Y.; Tong, T.; Sun, Z.; Yu, Z.; Zhu, Y.; Tong, H. Red lead degradation: Monitoring of color change over time. New J. Chem. 2016, 40, 3686–3692. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, J.; Pan, A.; He, L.; Simon, S. Degradation of red lead pigment in the oil painting during UV aging. Color Res. Appl. 2019, 44, 790–797. [Google Scholar] [CrossRef]
- Athanassiou, A.; Hill, A.E.; Fourrier, T.; Burgio, L.; Clark, R.J. The effects of UV laser light radiation on artists’ pigments. J. Cult. Herit. 2000, 1, S209–S213. [Google Scholar] [CrossRef]
- Gonzalez, V.; Cotte, M.; Vanmeert, F.; de Nolf, W.; Janssens, K. X-ray Diffraction Mapping for Cultural Heritage Science: A Review of Experimental Configurations and Applications. Chem.—Eur. J. 2020, 26, 1703–1719. [Google Scholar] [CrossRef]
- Janssens, K.; Van der Snickt, G.; Vanmeert, F.; Legrand, S.; Nuyts, G.; Alfeld, M.; Monico, L.; Anaf, W.; De Nolf, W.; Vermeulen, M.; et al. Non-invasive and non-destructive examination of artistic pigments, paints, and paintings by means of X-ray methods. In Analytical Chemistry for Cultural Heritage; Springer: Cham, Switzerland, 2017; pp. 77–128. [Google Scholar]
- Vanmeert, F.; Van der Snickt, G.; Janssens, K. Plumbonacrite identified by X-ray powder diffraction tomography as a missing link during degradation of red lead in a Van Gogh painting. Angew. Chem. Int. Ed. 2015, 54, 3607–3610. [Google Scholar] [CrossRef]
- Ingo, G.M.; Riccucci, C.; Pascucci, M.; Messina, E.; Giuliani, C.; Biocca, P.; Tortora, L.; Fierro, G.; Di Carlo, G. Combined use of FE-SEM+ EDS, ToF-SIMS, XPS, XRD and OM for the study of ancient gilded artefacts. Appl. Surf. Sci. 2018, 446, 168–176. [Google Scholar] [CrossRef]
- Bottaini, C.; Brunetti, A.; Bordalo, R.; Valera, A.; Schiavon, N. Non-destructive characterization of archeological Cu-based artifacts from the early metallurgy of southern Portugal. Archaeol. Anthropol. Sci. 2018, 10, 1903–1912. [Google Scholar] [CrossRef]
- Niederschlag, E.; Pernicka, E.; Seifert, T.; Bartelheim, M. The determination of lead isotope ratios by multiple collector ICP-MS: A case study of Early Bronze Age artefacts and their possible relation with ore deposits of the Erzgebirge. Archaeometry 2003, 45, 61–100. [Google Scholar] [CrossRef]
- Macchioni, N.; Pizzo, B.; Bernabei, M.; Visintin, G. Dating trials of wooden historic artefacts through FT-IR spectroscopy. J. Cult. Herit. 2020, 43, 303–310. [Google Scholar] [CrossRef]
- Xia, J.; Zhang, J.; Zhao, Y.; Huang, Y.; Xiong, Y.; Min, S. Fourier transform infrared spectroscopy and chemometrics for the discrimination of paper relic types. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 219, 8–14. [Google Scholar] [CrossRef]
- Vermeulen, M.; Saverwyns, S.; Coudray, A.; Janssens, K.; Sanyova, J. Identification by Raman spectroscopy of pararealgar as a starting material in the synthesis of amorphous arsenic sulfide pigments. Dyes Pigments 2018, 149, 290–297. [Google Scholar] [CrossRef]
- Bersani, D.; Lottici, P.P. Raman spectroscopy of minerals and mineral pigments in archaeometry. J. Raman Spectrosc. 2016, 47, 499–530. [Google Scholar] [CrossRef]
- Casadio, F.; Daher, C.; Bellot-Gurlet, L. Raman spectroscopy of cultural heritage materials: Overview of applications and new frontiers in instrumentation, sampling modalities, and data processing. In Analytical Chemistry for Cultural Heritage; Springer: Cham, Switzerland, 2017; pp. 161–211. [Google Scholar]
- Marušić, K.; Pucić, I.; Desnica, V. Ornaments in radiation treatment of cultural heritage: Color and UV–vis spectral changes in irradiated nacres. Radiat. Phys. Chem. 2016, 124, 62–67. [Google Scholar] [CrossRef]
- Neves, A.; Ramos, A.M.; Callapez, M.E.; Friedel, R.; Réfrégiers, M.; Thoury, M.; Melo, M.J. Novel markers to early detect degradation on cellulose nitrate-based heritage at the submicrometer level using synchrotron UV–VIS multispectral luminescence. Sci. Rep. 2021, 11, 20208. [Google Scholar] [CrossRef]
- Bi, Z.; Wang, W.; Zhao, L.; Wang, X.; Xing, D.; Zhou, Y.; Lee, D.-J.; Ren, N.; Chen, C. The generation and transformation mechanisms of reactive oxygen species in the environment and their implications for pollution control processes: A review. Environ. Res. 2024, 260, 119592. [Google Scholar] [CrossRef]
- Han, R.; Lv, J.; Zhang, S.; Zhang, S. Hematite facet-mediated microbial dissimilatory iron reduction and production of reactive oxygen species during aerobic oxidation. Water Res. 2021, 195, 116988. [Google Scholar] [CrossRef] [PubMed]
- Song, N.; Wu, D.; Xu, H.; Jiang, H. Integrated evaluation of the reactive oxygen species (ROS) production characteristics in one large lake under alternating flood and drought conditions. Water Res. 2022, 225, 119136. [Google Scholar] [CrossRef] [PubMed]
- Wolf, R.; Thrane, J.E.; Hessen, D.O.; Andersen, T. Modelling ROS formation in boreal lakes from interactions between dissolved organic matter and absorbed solar photon flux. Water Res. 2018, 132, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Ingrosso, G. Free radical chemistry and its concern with indoor air quality: An open problem. Microchem. J. 2002, 73, 221–236. [Google Scholar] [CrossRef]
- Jiang, B.; Dai, D.; Yao, Y.; Xu, T.; Li, R.; Xie, R.; Chen, L.; Chen, W. The coupling of hemin with persistent free radicals induces a nonradical mechanism for oxidation of pollutants. Chem. Commun. 2016, 52, 9566–9569. [Google Scholar] [CrossRef]
- Payne, D.J.; Egdell, R.G.; Hao, W.; Foord, J.S.; Walsh, A.; Watson, G.W. Why is lead dioxide metallic? Chem. Phys. Lett. 2005, 411, 181–185. [Google Scholar] [CrossRef]
- Burgio, L.; Clark, R.J.; Firth, S. Raman spectroscopy as a means for the identification of plattnerite (PbO2), of lead pigments and of their degradation products. Analyst 2001, 126, 222–227. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, J.; Han, L.; Zhou, X. Raman Spectra of some mineral pigments used in ancient Chinese artworks. Chin. J. Light Scatt. 2012, 24, 6. [Google Scholar]
- Liu, Z.; Wang, J.; Han, L.; Zhou, X. Raman Spectra of some mineral pigments used in ancient Chinese artworks (II). Chin. J. Light Scatt. 2012, 25, 6. [Google Scholar]
- Choucair, K.; Morand, S.; Stanbery, L.; Edelman, G.; Dworkin, L.; Nemunaitis, J. TMB: A promising immune-response biomarker, and potential spearhead in advancing targeted therapy trials. Cancer Gene Ther. 2020, 27, 841–853. [Google Scholar] [CrossRef]
- Sarwar, G.; Corsi, R.; Kimura, Y.; Allen, D.; Weschler, C.J. Hydroxyl radicals in indoor environments. Atmos. Environ. 2002, 36, 3973–3988. [Google Scholar] [CrossRef]
- Feng, Q.; Zhang, X.; Ma, X. Effects of microbes on color changes of red lead in murals. J. Gen. Appl. Microbiol. 1999, 45, 85–88. [Google Scholar]
- Garg, S.; Rose, A.L.; Waite, T.D. Photochemical production of superoxide and hydrogen peroxide from natural organic matter. Geochim. Cosmochim. Acta 2011, 75, 4310–4320. [Google Scholar] [CrossRef]
- Guo, H.; Wang, Y.; Yao, K.; Zheng, H.; Zhang, X.; Li, R.; Wang, N.; Fu, H. The overlooked formation of environmentally persistent free radicals on particulate matter collected from biomass burning under light irradiation. Environ. Int. 2023, 171, 107668. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Huang, Q.; Sun, J.; Hao, Q.; Zhang, W.; Yu, Z.; Su, B.; Zhang, H. Aging and Discoloration of Red Lead (Pb3O4) Caused by Reactive Oxygen Species Under Alkaline Conditions. Molecules 2025, 30, 2136. https://doi.org/10.3390/molecules30102136
Zhang Z, Huang Q, Sun J, Hao Q, Zhang W, Yu Z, Su B, Zhang H. Aging and Discoloration of Red Lead (Pb3O4) Caused by Reactive Oxygen Species Under Alkaline Conditions. Molecules. 2025; 30(10):2136. https://doi.org/10.3390/molecules30102136
Chicago/Turabian StyleZhang, Zhehan, Qin Huang, Jiaxing Sun, Qilong Hao, Wenyuan Zhang, Zongren Yu, Bomin Su, and Haixia Zhang. 2025. "Aging and Discoloration of Red Lead (Pb3O4) Caused by Reactive Oxygen Species Under Alkaline Conditions" Molecules 30, no. 10: 2136. https://doi.org/10.3390/molecules30102136
APA StyleZhang, Z., Huang, Q., Sun, J., Hao, Q., Zhang, W., Yu, Z., Su, B., & Zhang, H. (2025). Aging and Discoloration of Red Lead (Pb3O4) Caused by Reactive Oxygen Species Under Alkaline Conditions. Molecules, 30(10), 2136. https://doi.org/10.3390/molecules30102136





