Controllable Nitrogen-Doped Hollow Carbon Nano-Cage Structures as Supercapacitor Electrode Materials
Abstract
1. Introduction
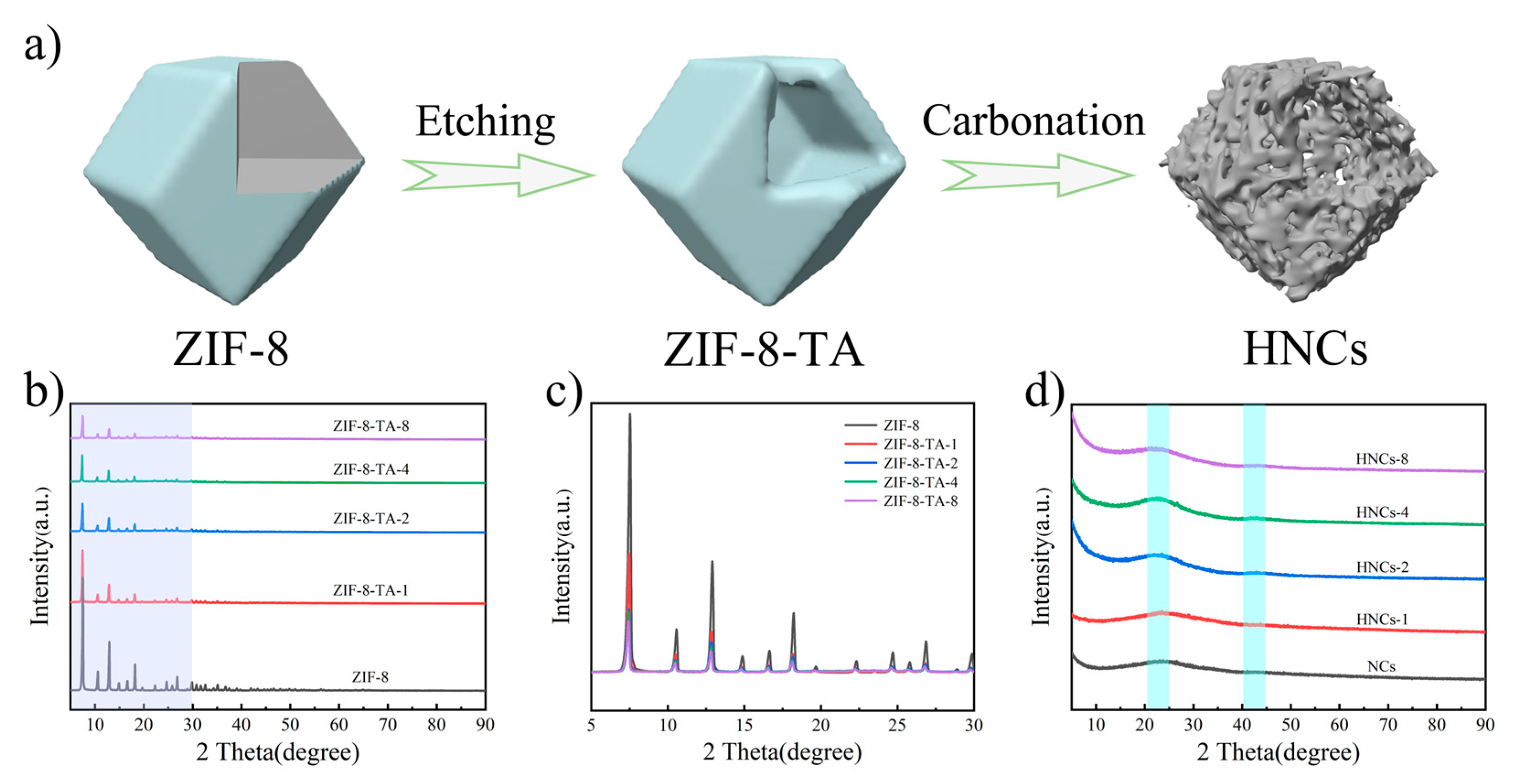
2. Results and Discussion
3. Experiment
3.1. Materials and Chemicals
3.2. Synthesis of Materials
3.3. Characterization of Materials
3.4. Electrochemical Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, X.; Lu, X.; Liu, B.; Chen, D.; Tong, Y.; Shen, G. Flexible Energy-Storage Devices: Design Consideration and Recent Progress. Adv. Mater. 2014, 26, 4763–4782. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Jiang, C.; Liang, J.; Wu, W. Electrode materials and device architecture strategies for flexible supercapacitors in wearable energy storage. J. Mater. Chem. A 2021, 9, 8099–8128. [Google Scholar] [CrossRef]
- Ni, C.; Xue, X.; Mei, S.; Zhang, X.-P.; Chen, X. Technological Research of a Clean Energy Router Based on Advanced Adiabatic Compressed Air Energy Storage System. Entropy 2020, 22, 1440. [Google Scholar] [CrossRef]
- Sharma, S.; Ghoshal, S.K. Hydrogen the future transportation fuel: From production to applications. Renew. Sustain. Energy Rev. 2015, 43, 1151–1158. [Google Scholar] [CrossRef]
- Zheng, Q.; Jiang, L.; Xu, Y.; Gao, S.; Liu, T.; Qu, C.; Chen, H.; Li, X. Research Progress and Development Suggestions of Energy Storage Technology under Background of Carbon Peak and Carbon Neutrality. Bull. Chin. Acad. Sci. 2022, 37, 529–540. [Google Scholar]
- Gupta, A.; Zuk, P.J.; Stone, H.A. Charging Dynamics of Overlapping Double Layers in a Cylindrical Nanopore. Phys. Rev. Lett. 2020, 125, 076001. [Google Scholar] [CrossRef]
- Schiffer, J.; Linzen, D.; Sauer, D.U. Heat generation in double layer capacitors. J. Power Sources 2006, 160, 765–772. [Google Scholar] [CrossRef]
- Volfkovich, Y.M. Electric Double Layer Capacitors: A Review. Russ. J. Electrochem. 2024, 60, 761–794. [Google Scholar] [CrossRef]
- Chen, W.; Rakhi, R.B.; Alshareef, H.N. Facile synthesis of polyaniline nanotubes using reactive oxide templates for high energy density pseudocapacitors. J. Mater. Chem. A 2013, 1, 3315–3324. [Google Scholar] [CrossRef]
- Mahala, S.; Khosravinia, K.; Kiani, A. Unwanted degradation in pseudocapacitors: Challenges and opportunities. J. Energy Storage 2023, 67, 107558. [Google Scholar] [CrossRef]
- Gu, T.; Wei, B. Fast and stable redox reactions of MnO2/CNT hybrid electrodes for dynamically stretchable pseudocapacitors. Nanoscale 2015, 7, 11626–11632. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Liu, D.; Shen, Z.; Bao, B.; Zhao, S.; Wu, L. Functional Biomass Carbons with Hierarchical Porous Structure for Supercapacitor Electrode Materials. Electrochim. Acta 2015, 180, 241–251. [Google Scholar] [CrossRef]
- Yuan, D.; Hu, X.; Liu, Y.; Yang, C. Research progress in carbon materials for supercapacitors. Battery 2007, 37, 466–468. [Google Scholar]
- Wang, D.-W.; Li, F.; Cheng, H.-M. Hierarchical porous nickel oxide and carbon as electrode materials for asymmetric supercapacitor. J. Power Sources 2008, 185, 1563–1568. [Google Scholar] [CrossRef]
- Yan, D.; Liu, L.; Wang, X.; Xu, K.; Zhong, J. Biomass-Derived Activated Carbon Nanoarchitectonics with Hibiscus Flowers for High-Performance Supercapacitor Electrode Applications. Chem. Eng. Technol. 2022, 45, 649–657. [Google Scholar] [CrossRef]
- Buller, S.; Thele, M.; De Doncker, R.; Karden, E. Impedance-based simulation models of supercapacitors and Li-ion batteries for power electronic applications. IEEE Trans. Ind. Appl. 2005, 41, 742–747. [Google Scholar] [CrossRef]
- Cherusseri, J.; Pandey, D.; Thomas, J. Symmetric, Asymmetric, and Battery-Type Supercapacitors Using Two-Dimensional Nanomaterials and Composites. Batter. Supercaps 2020, 3, 860–875. [Google Scholar] [CrossRef]
- Libich, J.; Sedlarikova, M.; Maca, J.; Cudek, P.; Kazda, T.; Fafilek, G.; Rodriguez, J.J.S. Supercapacitors vs. Lithium-ion Batteries: Properties and Applications. Chem. Ing. Tech. 2024, 96, 279–285. [Google Scholar] [CrossRef]
- Yan, J.; Li, S.; Lan, B.; Wu, Y.; Lee, P.S. Rational Design of Nanostructured Electrode Materials toward Multifunctional Supercapacitors. Adv. Funct. Mater. 2020, 30, 1902564. [Google Scholar] [CrossRef]
- Kumar, S.; Saeed, G.; Zhu, L.; Hui, K.N.; Kim, N.H.; Lee, J.H. 0D to 3D carbon-based networks combined with pseudocapacitive electrode material for high energy density supercapacitor: A review. Chem. Eng. J. 2021, 403, 126352. [Google Scholar] [CrossRef]
- Kadam, V.A.; Patil, V.L.; Mujawar, S.H.; Torane, A.P.; Kadam, L.D. Recent advances of spinel CuCo2O4 in different Structural dimensions (0D-3D) for an electrochemical supercapacitor device: A short research review. J. Alloys Compd. 2025, 1010, 177581. [Google Scholar] [CrossRef]
- Ouyang, T.; Cheng, K.; Yang, F.; Zhou, L.; Zhu, K.; Ye, K.; Wang, G.; Cao, D. From biomass with irregular structures to 1D carbon nanobelts: A stripping and cutting strategy to fabricate high performance supercapacitor materials. J. Mater. Chem. A 2017, 5, 14551–14561. [Google Scholar] [CrossRef]
- Sankar, K.V.; Lee, S.C.; Seo, Y.; Ray, C.; Liu, S.; Kundu, A.; Jun, S.C. Binder-free cobalt phosphate one-dimensional nanograsses as ultrahigh-performance cathode material for hybrid supercapacitor applications. J. Power Sources 2018, 373, 211–219. [Google Scholar] [CrossRef]
- Sandhu, A.; Chini, M.K. 2D and 3D Halide Perovskite-Based Supercapacitors. Chemistryselect 2024, 9, e202304441. [Google Scholar] [CrossRef]
- Tian, X. Direct ink writing of 2D material-based supercapacitors. 2D Mater. 2022, 9, 012001. [Google Scholar] [CrossRef]
- Chang, J.; Yue, H.; Zhang, H.; Gao, X.; Lin, X. Three-dimensional graphene:preparation and application in supercapacitor. New Chem. Mater. 2016, 44, 9–11. [Google Scholar]
- Chen, K.; Huang, X.; Wan, C.; Liu, H. Efficient oxygen reduction catalysts formed of cobalt phosphide nanoparticle decorated heteroatom-doped mesoporous carbon nanotubes. Chem. Commun. 2015, 51, 7891–7894. [Google Scholar] [CrossRef]
- Li, W.; Zhang, W.; Xu, Y.; Wang, G.; Sui, W.; Xu, T.; Yuan, Z.; Si, C. Heteroatom-doped lignin derived carbon materials with improved electrochemical performance for advanced supercapacitors. Chem. Eng. J. 2024, 497, 154829. [Google Scholar] [CrossRef]
- Zhang, P.; Mu, J.; Kong, X.; Wang, X.; Wong, S.I.; Sunarso, J.; Xing, W.; Zhou, J.; Zhao, Y.; Zhuo, S. Novel Electrode Materials and Redox-Active Electrolyte for High-Performance Supercapacitor. Chemelectrochem 2022, 9, e202101646. [Google Scholar] [CrossRef]
- Calik, F.D.; Erdogan, B.; Yilmaz, E.; Saygi, G.; Cakicioglu-Ozkan, F. Photocatalytic degradation of aquatic organic pollutants with Zn- and Zr-based metalorganic frameworks: ZIF-8 and UiO-66. Turk. J. Chem. 2022, 46, 1358–1375. [Google Scholar] [CrossRef]
- Saint Remi, J.C.; Remy, T.; Van Hunskerken, V.; van de Perre, S.; Duerinck, T.; Maes, M.; De Vos, D.; Gobechiya, E.; Kirschhock, C.E.A.; Baron, G.V.; et al. Biobutanol Separation with the Metal-Organic Framework ZIF-8. Chemsuschem 2011, 4, 1074–1077. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, M.; Cao, C.; Liu, C.; Liu, J.; Zhu, Y.; Zhang, S.; Song, W. Surfactant-Free Palladium Nanoparticles Encapsulated in ZIF-8 Hollow Nanospheres for Size-Selective Catalysis in Liquid-Phase Solution. Chemcatchem 2016, 8, 3224–3228. [Google Scholar] [CrossRef]
- Wei, C.; Sun, J.; Zhang, Y.; Liu, Y.; Guo, Z.; Du, W.; Liu, L.; Zhang, Y. Hierarchical Ni(OH)2-MnO2 hollow spheres as an electrode material for high-performance supercapacitors. Inorg. Chem. Front. 2022, 9, 3542–3551. [Google Scholar] [CrossRef]
- Long, Y.; An, X.; Zhang, H.; Yang, J.; Liu, L.; Tian, Z.; Yang, G.; Cheng, Z.; Cao, H.; Liu, H.; et al. Highly graphitized lignin-derived porous carbon with hierarchical N/O co-doping “core-shell” superstructure supported by metal-organic frameworks for advanced supercapacitor performance. Chem. Eng. J. 2023, 451, 138877. [Google Scholar] [CrossRef]
- Zhang, H.; Fang, L.; Guo, Y.; Wang, Z.; Hu, H.; He, W.; Wang, P. Nitrogen-sulfur co-doped ZIF-8-derived carbon materials for supercapacitors with low self-discharge. J. Energy Storage 2024, 80, 110138. [Google Scholar] [CrossRef]
- Du, J.; Chen, A.; Gao, X.; Zhang, Y.; Lv, H. Reasonable Construction of Hollow Carbon Spheres with an Adjustable Shell Surface for Supercapacitors. ACS Appl. Mater. Interfaces 2022, 14, 11750–11757. [Google Scholar] [CrossRef]
- Tsai, C.-T.; Chen, J.-P.; Wu, M.-S. Hollow-porous carbon microspheres with hierarchically micromesoporous shells and superhydrophilic surfaces for high-performance aqueous supercapacitors. J. Alloys Compd. 2024, 1009, 176872. [Google Scholar] [CrossRef]
- Chen, G.; Jin, B.; Sun, S.; Jiang, Q. Surface-functionalized metal-organic framework with high specific surface area optimizing composite solid electrolyte for high-performance solid-state lithium metal batteries. Chem. Eng. J. 2024, 501, 157704. [Google Scholar] [CrossRef]
- Cai, C.; Zou, Y.; Xiang, C.; Chu, H.; Qiu, S.; Sui, Q.; Xu, F.; Sun, L.; Shah, A. Broccoli-like porous carbon nitride from ZIF-8 and melamine for high performance supercapacitors. Appl. Surf. Sci. 2018, 440, 47–54. [Google Scholar] [CrossRef]
- Dang, H.; Wang, L.; Ran, F. Polydopamine supporting method fabricating vanadium nitride nanoparticle enclosed into hierarchical hollow carbon nanospheres for supercapacitors. J. Power Sources 2024, 606, 234546. [Google Scholar] [CrossRef]
- Wang, D.; Wang, Y.; Liu, H.; Xu, W.; Xu, L. Unusual carbon nanomesh constructed by interconnected carbon nanocages for ionic liquid-based supercapacitor with superior rate capability. Chem. Eng. J. 2018, 342, 474–483. [Google Scholar] [CrossRef]
- Chen, A.; Wang, Y.; Yu, Y.; Sun, H.; Li, Y.; Xia, K.; Li, S. Nitrogen-doped hollow carbon spheres for supercapacitors. J. Mater. Sci. 2017, 52, 3153–3161. [Google Scholar] [CrossRef]
- Han, J.; Xu, G.; Ding, B.; Pan, J.; Dou, H.; MacFarlane, D.R. Porous nitrogen-doped hollow carbon spheres derived from polyaniline for high performance supercapacitors. J. Mater. Chem. A 2014, 2, 5352–5357. [Google Scholar] [CrossRef]
- Chen, A.; Li, Y.; Yu, Y.; Ren, S.; Wang, Y.; Xia, K.; Li, S. Nitrogen-doped hollow carbon spheres for supercapacitors application. J. Alloys Compd. 2016, 688, 878–884. [Google Scholar] [CrossRef]
- Li, M.; Zhang, W.; Liu, T.; Mou, J.; Xu, Y.; Huang, J.; Liu, M. Hierarchical hollow microspheres of carbon nanorods with enhanced supercapacitor performance. Mater. Today Commun. 2021, 28, 102500. [Google Scholar] [CrossRef]
- You, M.; Zhao, X.; Lu, R.; Ma, J.; Zhang, X.; Yuejing, C.; Yang, X. Raspberry-like hollow carbon spheres: A promising electrode material for high-performance zinc-ion hybrid supercapacitors. Synth. Met. 2025, 311, 117821. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Y.; Niu, X.; Yang, L.; Mi, N.; Zhao, L. Controllable Nitrogen-Doped Hollow Carbon Nano-Cage Structures as Supercapacitor Electrode Materials. Molecules 2025, 30, 2130. https://doi.org/10.3390/molecules30102130
Sun Y, Niu X, Yang L, Mi N, Zhao L. Controllable Nitrogen-Doped Hollow Carbon Nano-Cage Structures as Supercapacitor Electrode Materials. Molecules. 2025; 30(10):2130. https://doi.org/10.3390/molecules30102130
Chicago/Turabian StyleSun, Yitong, Xiaoqin Niu, Laidong Yang, Ning Mi, and Lei Zhao. 2025. "Controllable Nitrogen-Doped Hollow Carbon Nano-Cage Structures as Supercapacitor Electrode Materials" Molecules 30, no. 10: 2130. https://doi.org/10.3390/molecules30102130
APA StyleSun, Y., Niu, X., Yang, L., Mi, N., & Zhao, L. (2025). Controllable Nitrogen-Doped Hollow Carbon Nano-Cage Structures as Supercapacitor Electrode Materials. Molecules, 30(10), 2130. https://doi.org/10.3390/molecules30102130




