Active Packaging Based on a PET/PP Food-Grade Film Coated with Pullulan and Clove Essential Oil: Physicochemical and Antimicrobial Properties
Abstract
1. Introduction
2. Result and Discussion
2.1. Physical Characteristics of Films
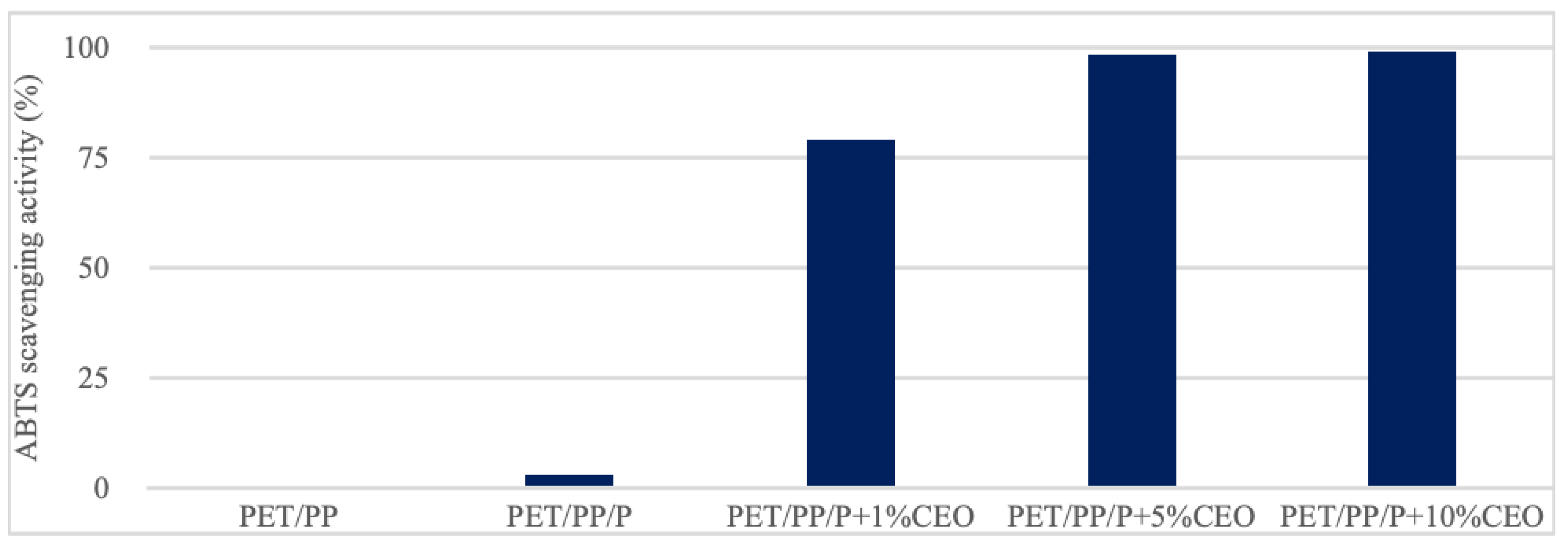
2.2. Antibacterial and Antioxidant Activity of Films
2.3. Antimicrobial Activity of Model Active Package Bags
2.4. Visual Appearance
3. Materials and Methods
3.1. Materials
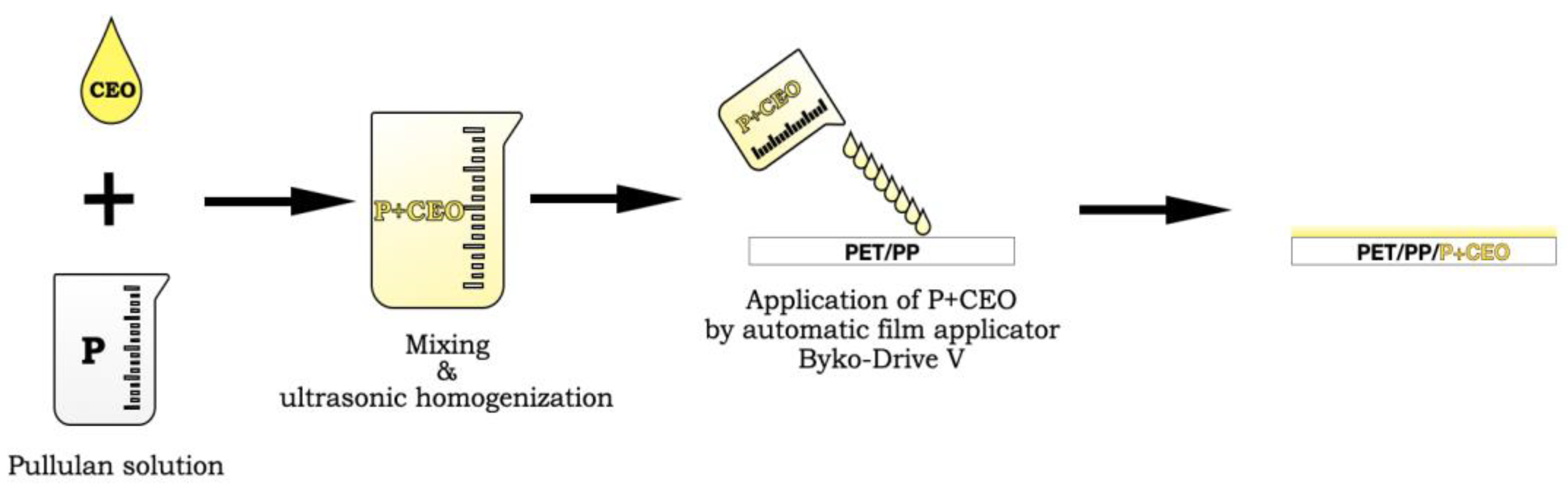
3.2. Preparation of Pullulan Film-Forming Solution
3.3. Application of Pullulan Film-Forming Solution onto PET/PP Films
3.4. Physical Characteristics of Film
3.4.1. Thickness
3.4.2. Optical Properties (Opacity and Light Transmittance)
3.4.3. Color Analysis
3.5. Antibacterial Activity of Films
3.6. ABTS Radical Scavenging Activity of Films
3.7. Preparation of a Model Packaging Bags
3.7.1. Preparation and Inoculation of Spinach Leaves
3.7.2. Packaging and Microbial Enumeration
3.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Farrell, R.; Cortese, Y.J.; Devine, D.M.; Gately, N.; Rueda, M.; Rodriguez, L.; Pezzoli, R. The function and properties of common food packaging materials and their suitability for reusable packaging: The transition from a linear to circular economy. Curr. Res. Green Sustain. Chem. 2024, 9, 100429. [Google Scholar] [CrossRef]
- Alamri, M.S.; Qasem, A.A.A.; Mohamed, A.A.; Hussain, S.; Ibraheem, M.A.; Shamlan, G.; Alqah, H.A.; Qasha, A.S. Food packaging’s materials: A food safety perspective. Saudi J. Biol. Sci. 2021, 28, 4490–4499. [Google Scholar] [CrossRef]
- Pereira de Abreu, D.A.; Cruz, J.M.; Paseiro Losada, P. Active and intelligent packaging for the food industry. Food Rev. Int. 2012, 28, 146–187. [Google Scholar] [CrossRef]
- Otoni, C.G.; Espitia, P.J.P.; Avena-Bustillos, R.J.; McHugh, T.H. Trends in antimicrobial food packaging systems: Emitting sachets and absorbent pads. Food Res. Int. 2016, 83, 60–73. [Google Scholar] [CrossRef]
- Appendini, P.; Hotchkiss, J.H. Review of antimicrobial food packaging. Innov. Food Sci. Emerg. Technol. 2002, 3, 113–126. [Google Scholar] [CrossRef]
- Vasile, C.; Baican, M. Progresses in Food Packaging, Food Quality, and Safety—Controlled-Release Antioxidant and/or Antimicrobial Packaging. Molecules 2021, 26, 1263. [Google Scholar] [CrossRef]
- Niu, B.; Yan, Z.; Shao, P.; Kang, J.; Chen, H. Encapsulation of Cinnamon Essential Oil for Active Food Packaging Film with Synergistic Antimicrobial Activity. Nanomaterials 2018, 8, 598. [Google Scholar] [CrossRef]
- Alves Medeiros, E.A.; Ferreira Soares, N.D.F.; Sales Polito, T.D.O.; De Sousa, M.M.; Pereira Silva, D.F. Antimicrobial sachets post-harvest mango fruits. Rev. Bras. De Frutic. 2011, 33, 363–370. [Google Scholar]
- Chang, Y.; Choi, I.; Cho, A.R.; Han, J. Reduction of Dickeya chrysanthemi on fresh-cut iceberg lettuce using antimicrobial sachet containing microencapsulated oregano essential oil. LWT-Food Sci. Technol. 2017, 82, 361–368. [Google Scholar] [CrossRef]
- Fasihnia, S.H.; Peighambardoust, S.H.; Peighambardoust, S.J.; Oromiehie, A.; Soltanzadeh, M.; Pateiro, M.; Lorenzo, J.M. Properties and application of multifunctional composite polypropylene-based films incorporating a combination of BHT, BHA and sorbic acid in extending donut shelf-life. Molecules 2020, 25, 5197. [Google Scholar] [CrossRef]
- Lopresti, F.; Botta, L.; La Carrubba, V.; Di Pasquale, L.; Settanni, L.; Gaglio, R. Combining carvacrol and nisin in biodegradable films for antibacterial packaging applications. Int. J. Biol. Macromol. 2021, 193, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Jin, T.Z.; Yang, R. Antimicrobial polylactic acid packaging films against Listeria and Salmonella in culture medium and on ready-to-eat meat. Food Bioprocess Technol. 2014, 7, 3293–3307. [Google Scholar] [CrossRef]
- Torlak, E.; Sert, D. Antibacterial effectiveness of chitosan–propolis coated polypropylene films against foodborne pathogens. Int. J. Biol. Macromol. 2013, 60, 52–55. [Google Scholar] [CrossRef]
- Ha, J.U.; Kim, Y.M.; Lee, D.S. Multilayered antimicrobial polyethylene films applied to the packaging of ground beef. Packag. Technol. Sci. 2001, 14, 55–62. [Google Scholar] [CrossRef]
- Natrajan, N.; Sheldon, B.W. Efficacy of nisin-coated polymer films to inactivate Salmonella typhimurium on fresh broiler skin. J. Food Prot. 2000, 63, 1189–1196. [Google Scholar] [CrossRef]
- Fasihnia, S.H.; Peighambardoust, S.H.; Peighambardoust, S.J.; Oromiehie, A. Development of novel active polypropylene based packaging films containing different concentrations of sorbic acid. Food Packag. Shelf Life 2018, 18, 87–94. [Google Scholar] [CrossRef]
- Kraśniewska, K.; Galus, S.; Gniewosz, M. Biopolymers-Based Materials Containing Silver Nanoparticles as Active Packaging for Food Applications–A Review. Int. J. Mol. Sci. 2020, 21, 698. [Google Scholar] [CrossRef] [PubMed]
- Konfo, T.R.C.; Djouhou, F.M.C.; Koudoro, Y.A.; Dahouenon-Ahoussi, E.; Avlessi, F.; Sohounhloue, C.K.D.; Simal-Gandara, J. Essential oils as natural antioxidants for the control of food preservation. Food Chem. Adv. 2023, 2, 100312. [Google Scholar] [CrossRef]
- Hyldgaard, M.; Mygind, T.; Meyer, R.L. Essential oils in food preservation: Mode of action, synergies, and interactions with food matrix components. Front. Microbiol. 2012, 3, 12. [Google Scholar] [CrossRef]
- Abdul Aziz, A.H.; Rizkiyah, D.N.; Qomariyah, L.; Irianto, I.; Che Yunus, M.A.; Putra, N.R. Unlocking the Full Potential of Clove (Syzygium aromaticum) Spice: An Overview of Extraction Techniques, Bioactivity, and Future Opportunities in the Food and Beverage Industry. Processes 2023, 11, 2453. [Google Scholar] [CrossRef]
- Pandey, V.K.; Srivastava, S.; Ashish; Dash, K.K.; Singh, R.; Dar, A.H.; Singh, T.; Farooqui, A.; Shaikh, A.M.; Kovacs, B. Bioactive properties of clove (Syzygium aromaticum) essential oil nanoemulsion: A comprehensive review. Heliyon 2023, 10, e22437. [Google Scholar] [CrossRef]
- Shahbazi, Y.; Shahbazi, Y. Antioxidant antibacterial antifungal properties of nanoemulsion of clove essential oil Nanomed. Res. J. 2019, 4, 204–208. [Google Scholar]
- Haro-González, J.N.; Castillo-Herrera, G.A.; Martínez-Velázquez, M.; Espinosa-Andrews, H. Clove Essential Oil (Syzygium aromaticum L. Myrtaceae): Extraction, Chemical Composition, Food Applications, and Essential Bioactivity for Human Health. Molecules 2021, 26, 6387. [Google Scholar] [CrossRef] [PubMed]
- Razafimamonjison, G.; Jahiel, M.; Duclos, T.; Ramanoelina, P.; Fawbush, F.; Danthu, P. Bud, leaf and stem essential oil composition of Syzygium aromaticum from Madagascar, Indonesia and Zanzibar. Int. J. Basic Appl. Sci. 2014, 3, 224–233. [Google Scholar]
- Papadopoulou, C.P.; Kalfoglou, N.K. Comparison of compatibilizer effectiveness for PET/PP blends: Their mechanical, thermal and morphology characterization. Polymer 2000, 41, 2543–2555. [Google Scholar] [CrossRef]
- Franco, P.; Incarnato, L.; De Marco, I. Supercritical CO2 impregnation of a-tocopherol into PET/PPfilms for active packaging application. J. CO2 Util. 2019, 34, 266–273. [Google Scholar] [CrossRef]
- Gabrić, D.; Kurek, M.; Ščetar, M.; Brnčić, M.; Galić, K. Characterization of Synthetic Polymer Coated with Biopolymer Layer with Natural Orange Peel Extract Aimed for Food Packaging. Polymers 2023, 15, 2569. [Google Scholar] [CrossRef]
- Cejudo Bastante, C.; Cran, M.J.; Casas Cardoso, L.; Serrano, C.M.; Bigger, S.W. Mass Transfer and Optical Properties of Active PET/PP Food-Grade Films Impregnated with Olive Leaf Extract. Polymers 2022, 14, 84. [Google Scholar] [CrossRef]
- Belizón, M.; Fernández-Ponce, M.T.; Casas, L.; Mantell, C.; Martínez de la Ossa-Fernández, E.J. Supercritical impregnation of antioxidant mango polyphenols into a multilayer PET/PP food-grade film. J. CO2 Util. 2018, 25, 56–67. [Google Scholar] [CrossRef]
- Gniewosz, M.; Duszkiewicz-Reinhard, W. Comparative studies on pullulan synthesis, melanin synthesis and morphology of white mutant Aureobasidium pullulans B-1 and parent strain A.p.-3. Carbohydr. Polym. 2008, 72, 431–438. [Google Scholar] [CrossRef]
- Kraśniewska, K.; Pobiega, K.; Gniewosz, M. Pullulan—Biopolymer with potential for use as food packaging. Int. J. Food Eng. 2019, 15, 20190030. [Google Scholar] [CrossRef]
- Yuen, S. Pullulan and its applications. Process Biochem. 1974, 22, 7–9. [Google Scholar]
- Diab, T.; Biliaderis, C.G.; Gerasopoulos, D.; Sfakiotakis, E. Physicochemical properties and application of pullulan edible films and coatings in fruit preservation. J. Sci. Food Agric. 2001, 81, 988–1000. [Google Scholar] [CrossRef]
- Kraśniewska, K.; Gniewosz, M.; Kosakowska, O.; Cis, A. Preservation of brussels sprouts by pullulan coating containing oregano essential oil. J. Food Prot. 2016, 79, 493–500. [Google Scholar] [CrossRef]
- Kraśniewska, K.; Ścibisz, I.; Gniewosz, M.; Mitek, M.; Pobiega, K.; Cendrowski, A. Effect of Pullulan Coating on Postharvest Quality and Shelf-Life of Highbush Blueberry (Vaccinium corymbosum L.). Materials 2017, 10, 965. [Google Scholar] [CrossRef]
- Kharel, K.; Kraśniewska, K.; Gniewosz, M.; Prinyawiwatkul, W.; Fontenot, K.; Adhikari, A. Antimicrobial screening of pecan shell extract and efficacy of pecan shell extract-pullulan coating against Listeria monocytogenes, Salmonella enterica, and Staphylococcus aureus on blueberries. Heliyon 2024, 10, e29610. [Google Scholar] [CrossRef] [PubMed]
- Rashid, A.; Qayum, A.; Liang, Q.; Kang, L.; Ekumah, J.-N.; Han, X.; Ren, X.; Ma, X. Exploring the potential of pullulan-based films and coatings for effective food preservation: A comprehensive analysis of properties, activation strategies and applications. Int. J. Biol. Macromol. 2024, 260, 129479. [Google Scholar] [CrossRef]
- Gasti, T.; Dixit, S.; Hiremani, V.D.; Chougale, R.B.; Masti, S.P.; Kumar Vootla, S.; Midigoudra, B.S. Chitosan/pullulan based films incorporated with clove essential oil loaded chitosan-ZnO hybrid nanoparticles for active food packaging. Carbohydr. Polym. 2022, 277, 118866. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Ni, Z.-J.; Thakur, K.; Zhang, J.-G.; Hu, F.; Wei, Z.-J. Preparation and characterization of clove essential oil loaded nanoemulsion and pickering emulsion activated pullulan-gelatin based edible film. Int. J. Biol. Macromol. 2021, 181, 528–539. [Google Scholar] [CrossRef]
- Artés, F.; Gómez, P.; Aguayo, E.; Escalona, V.; Artés-Hernández, F. Sustainable sanitation techniques for keeping quality and safety of fresh-cut plant commodities. Postharvest Biol. Technol. 2020, 51, 287–296. [Google Scholar] [CrossRef]
- Abadias, M.; Usar, J.; Anguera, M.; Solsona, C.; Viñas, I. Microbiological quality of fresh, minimally-processed fruit and vegetables, and sprouts from retail establishments. Int. J. Food Microbiol. 2008, 123, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Silva, B.N.; Cadavez, V.; Teixeira, J.A.; Gonzales-Barron, U. Meta-analysis of the incidence of food borne pathogens in vegetables and fruits from retail establishments in Europe. Curr. Opin. Food Sci. 2017, 18, 21–28. [Google Scholar] [CrossRef]
- Kwon, S.J.; Chang, Y.; Han, J. Oregano essential oil-based natural antimicrobial packaging film to inactivate Salmonella enterica and yeasts/molds in the atmosphere surrounding cherry tomatoes. Food Microbiol. 2017, 65, 114–121. [Google Scholar] [CrossRef]
- Salmieri, S.; Islam, F.; Khan, R.A.; Hossain, F.M.; Ibrahim, H.M.; Miao, C.; Hamad, W.Y.; Lacroix, M. Antimicrobial nanocomposite films made of poly (lactic acid)–cellulose nanocrystals (PLA–CNC) in food applications—Part B: Effect of oregano essential oil release on the inactivation of Listeria monocytogenes in mixed vegetables. Cellulose 2014, 21, 4271–4285. [Google Scholar] [CrossRef]
- Chu, Y.; Xu, T.; Gao, C.C.; Liu, X.; Zhang, N.; Feng, X.; Liu, X.; Shen, X.; Tang, X. Evaluations of physicochemical and biological properties of pullulan-based films incorporated with cinnamon essential oil and Tween 80. Int. J. Biol. Macromol. 2019, 122, 388–394. [Google Scholar] [CrossRef]
- Luís, Â.; Ramos, A.; Domingues, F. Pullulan Films Containing Rockrose Essential Oil for Potential Food Packaging Applications. Antibiotics 2020, 9, 681. [Google Scholar] [CrossRef]
- Lei, Y.; Mao, L.; Yao, J.; Zhu, H. Improved mechanical, antibacterial and UV barrier properties of catechol-functionalized chitosan/polyvinyl alcohol biodegradable composites for active food packaging. Carbohydr. Polym. 2021, 264, 117997. [Google Scholar] [CrossRef]
- He, Y.; Zhang, X.; Zhang, Z.; Lin, B.; Yu, H. Preparation and Improved Properties of Vanillin-Crosslinked Polyvinyl Alcohol/Chitosan Active Packaging Films. Molecules 2025, 30, 1334. [Google Scholar] [CrossRef]
- Sharma, S.; Barkauskaite, S.; Duffy, B.; Jaiswal, A.K.; Jaiswal, S. Characterization and Antimicrobial Activity of Biodegradable Active Packaging Enriched with Clove and Thyme Essential Oil for Food Packaging Application. Foods 2020, 9, 1117. [Google Scholar] [CrossRef]
- Barbosa, M.H.R.; Gonçalves, S.D.A.; Marangoni Júnior, L.; Alves, R.M.V.; Vieira, R.P. Physicochemical properties of chitosan-based films incorporated with limonene. Food Measure 2022, 16, 2011–2023. [Google Scholar] [CrossRef]
- Ket-on, A.; Pongmongkol, N.; Somwangthanaroj, A.; Janjarasskul, T.; Tananuwong, K. Properties and storage stability of whey protein edible film with spice powders. J Food Sci. Technol. 2016, 53, 2933–2942. [Google Scholar] [CrossRef] [PubMed]
- Liñán-Atero, R.; Aghababaei, F.; García, S.R.; Hasiri, Z.; Ziogkas, D.; Moreno, A.; Hadidi, M. Clove Essential Oil: Chemical Profile, Biological Activities, Encapsulation Strategies, and Food Applications. Antioxidants 2024, 13, 488. [Google Scholar] [CrossRef] [PubMed]
- Carpena, M.; Nuñez-Estevez, B.; Soria-Lopez, A.; Garcia-Oliveira, P.; Prieto, M.A. Essential Oils and Their Application on Active Packaging Systems: A Review. Resources 2021, 10, 7. [Google Scholar] [CrossRef]
- Al-Hashimi, A.G.; Ammar, A.B.; Cacciola, F.; Lakhssassi, N. Development of a Millet Starch Edible Film Containing Clove Essential Oil. Foods 2020, 9, 184. [Google Scholar] [CrossRef] [PubMed]
- Gniewosz, M.; Kraśniewska, K.; Woreta, M.; Kosakowska, O. Antimicrobial Activity of a Pullulan–Caraway Essential Oil Coating on Reduction of Food Microorganisms and Quality in Fresh Baby Carrot. J. Food Sci. 2013, 78, M1242–M1248. [Google Scholar] [CrossRef]
- Kwiatkowski, P.; Giedrys-Kalemba, S.; Mizielińska, M.; Bartkowiak, A. Modification of pla foil surface by ethylcellulose and essential oils. J. Microbiol. Biotechnol. Food Sci. 2016, 5, 440–444. [Google Scholar] [CrossRef]
- Devecioglu, D.; Turker, M.; Karbancioglu-Guler, F. Antifungal Activities of Different Essential Oils and Their Electrospun Nanofibers against Aspergillus and Penicillium Species Isolated from Bread. ACS Omega 2022, 7, 37943–37953. [Google Scholar] [CrossRef]
- Rai, S.; Wai, P.P.; Koirala, P.; Bromage, S.; Nirmal, N.P.; Pandiselvam, R.; Nor-Khaizura, M.A.R.; Mehta, N.K. Food product quality, environmental and personal characteristics affecting consumer perception toward food. Front. Sustain. Food Syst. 2023, 7, 1222760. [Google Scholar] [CrossRef]
- Ramos, B.; Miller, F.A.; Brandão, T.R.S.; Teixeira, P.; Silva, C.L.M. Fresh fruits and vegetables—An overview on applied methodologies to improve its quality and safety. Innov. Food Sci. Emerg. Technol. 2013, 20, 1–15. [Google Scholar] [CrossRef]
- Pakulska, A.; Bartosiewicz, E.; Galus, S. The Potential of Apple and Blackcurrant Pomace Powders as the Components of Pectin Packaging Films. Coatings 2023, 13, 1409. [Google Scholar] [CrossRef]
- ISO 22196—2011; Measurement of Antibacterial Activity on Plastics and Other Non-Porous Surfaces. ISO—International Organization for Standardization: Geneva, Switzerland, 2011.
- Scuri, S.; Petrelli, F.; Grappasonni, I.; Idemudia, L.; Marchetti, F.; Nicola, C.D. Evaluation of the antimicrobial activity of novel composite plastics containing two silver (I) additives, acyl pyrazolonate and acyl pyrazolone. Acta Biomed. 2019, 90, 370–377. [Google Scholar]
- Ma, Y.; Chen, S.; Liu, P.; He, Y.; Chen, F.; Cai, Y.; Yang, X. Gelatin Improves the Performance of Oregano Essential Oil Nanoparticle Composite Films—Application to the Preservation of Mullet. Foods 2023, 12, 2542. [Google Scholar] [CrossRef] [PubMed]



| Film Sample | Thickness [mm] | Opacity [a.u./mm] | Transmittance (%) | Color | ||||
|---|---|---|---|---|---|---|---|---|
| 280 nm | 600 nm | L* | a* | b* | ΔE | |||
| PET/PP | 0.052 ± 0.00 a ** | 1.21 ± 0.04 a | 0.121 ± 0.003 c | 86.14 ± 0.46 c | 91.61 ± 0.65 c | −0.46 ± 0.03 a | 0.53 ± 0.05 a | - |
| PET/PP/P | 0.064 ± 0.005 b | 2.20 ± 0.08 b | 0.093 ± 0.005 b | 72.25 ± 1.42 b | 91.18 ± 068 b | −0.66 ± 0.15 bc | 0.63 ± 0.05 b | 0.49 |
| PET/PP/P + 1%CEO | 0.065 ± 0.004 b | 2.30 ± 0.13 c | 0.095 ± 0.005 b | 70.93 ± 1.63 b | 90.62 ± 0.35 a | −0.64 ± 0.18 b | 0.64 ± 0.08 b | 1.02 |
| PET/PP/P + 5%CEO | 0.069 ± 0.006 c | 2.61 ± 0.28 d | 0.091 ± 0.009 b | 68.2 ± 05.76 b | 90.56 ± 0.20 a | −0.74 ± 0.08 cd | 0.93 ± 0.19 c | 1.16 |
| PET/PP/P + 10%CEO | 0.073 ± 0.007 d | 3.29 ± 0.38 e | 0.080 ± 0.007 a | 58.57 ± 5.41 a | 90.75 ± 0.51 a | −0.78 ± 0.11 d | 1.12 ± 0.15 d | 1.10 |
| Bacteria Strains | Film Sample | Number of Bacteria (log CFU/cm2) | R (Log Reduction) | % Reduction |
|---|---|---|---|---|
| E. coli | PET/PP | 4.03 | - | |
| PET/PP/P | 3.83 | 0.14 | ||
| PET/PP/P + 1% CEO | n/d * | 4.03 | >99.9% | |
| PET/PP/P + 5% CEO | n/d | 4.03 | >99.9% | |
| PET/PP/P + 10% CEO | n/d | 4.03 | >99.9% | |
| Salmonella Enteritidis | PET/PP | 3.72 | - | - |
| PET/PP/P | 3.91 | 0 | ||
| PET/PP/P + 1% CEO | n/d | 3.72 | >99.9% | |
| PET/PP/P + 5% CEO | n/d | 3.72 | >99.9% | |
| PET/PP/P + 10% CEO | n/d | 3.72 | >99.9% | |
| S. aureus | PET/PP | 5.55 | - | |
| PET/PP/P | 5.80 | 0 | ||
| PET/PP/P + 1% CEO | 3.77 | 1.78 | 98.95% | |
| PET/PP/P + 5% CEO | n/d | 5.55 | >99.9% | |
| PET/PP/P + 10% CEO | n/d | 5.55 | >99.9% | |
| L. monocytogenes | PET/PP | 3.34 | - | |
| PET/PP/P | 3.26 | 0.08 | ||
| PET/PP/P + 1% CEO | 2.17 | 1.17 | 93.2% | |
| PET/PP/P + 5% CEO | n/d | 3.34 | >99.9% | |
| PET/PP/P + 10% CEO | n/d | 3.34 | >99.9% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kraśniewska, K.; Gniewosz, M. Active Packaging Based on a PET/PP Food-Grade Film Coated with Pullulan and Clove Essential Oil: Physicochemical and Antimicrobial Properties. Molecules 2025, 30, 2118. https://doi.org/10.3390/molecules30102118
Kraśniewska K, Gniewosz M. Active Packaging Based on a PET/PP Food-Grade Film Coated with Pullulan and Clove Essential Oil: Physicochemical and Antimicrobial Properties. Molecules. 2025; 30(10):2118. https://doi.org/10.3390/molecules30102118
Chicago/Turabian StyleKraśniewska, Karolina, and Małgorzata Gniewosz. 2025. "Active Packaging Based on a PET/PP Food-Grade Film Coated with Pullulan and Clove Essential Oil: Physicochemical and Antimicrobial Properties" Molecules 30, no. 10: 2118. https://doi.org/10.3390/molecules30102118
APA StyleKraśniewska, K., & Gniewosz, M. (2025). Active Packaging Based on a PET/PP Food-Grade Film Coated with Pullulan and Clove Essential Oil: Physicochemical and Antimicrobial Properties. Molecules, 30(10), 2118. https://doi.org/10.3390/molecules30102118








