Chlorine Dioxide (ClO2)-Releasing Sachet for Preservation of Cherry Tomatoes
Abstract
1. Introduction
2. Results and Discussion
2.1. Characterization of the Composite Films
2.1.1. Fourier Transform Infrared (FT-IR) Spectroscopy
2.1.2. Scanning Electron Microscopy (SEM)
2.1.3. Thermal Properties
2.1.4. Mechanical Properties
2.1.5. Water Sorption
2.2. Release Test of ClO2 from the Sachets
2.3. Storage Test in a Plastic Box
2.4. Storage Test in a Corrugated Box
3. Materials and Methods
3.1. Materials
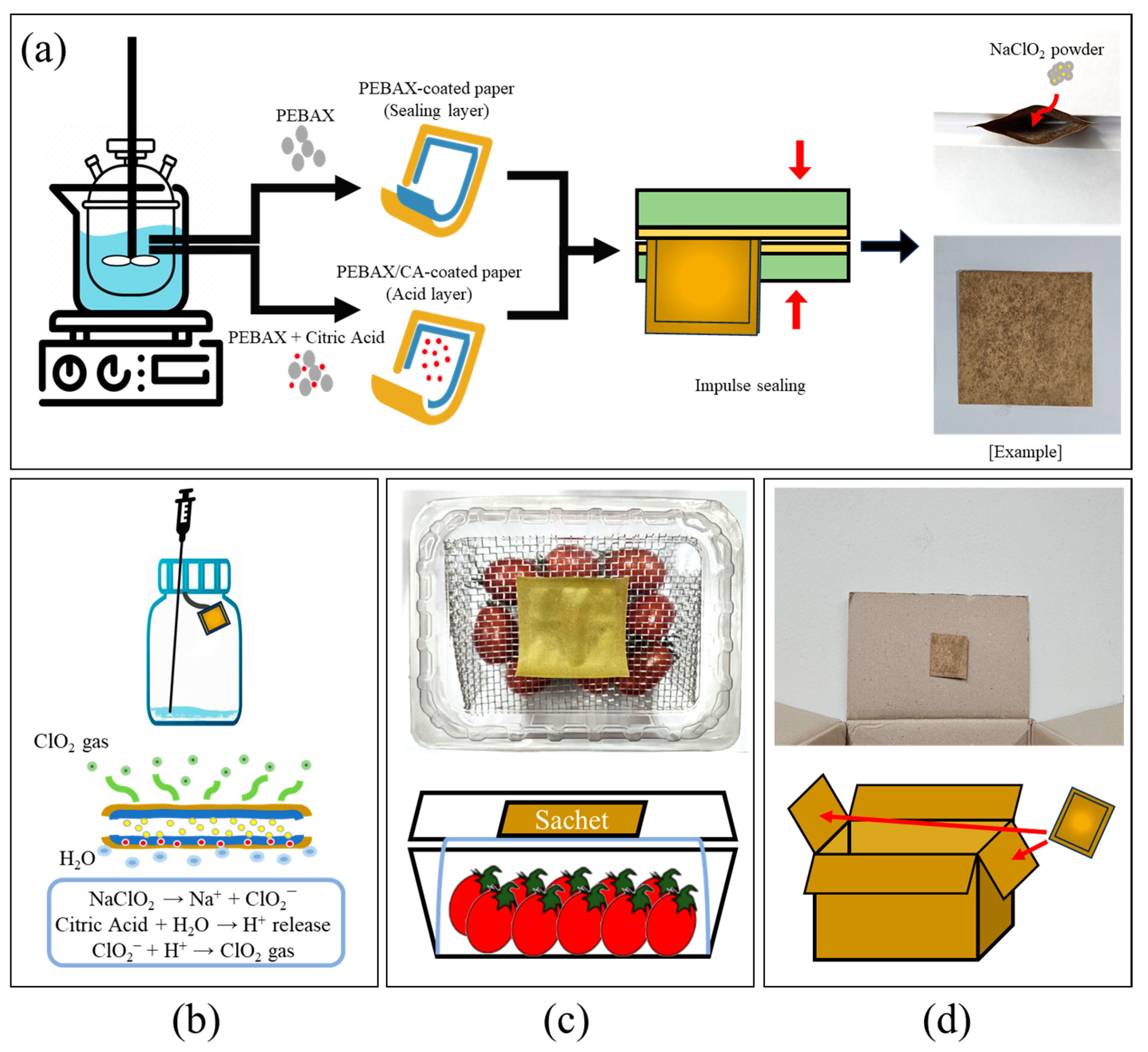
3.2. Preparation of the PEBAX/CA Composite Films and Sachets
3.3. Characterization
3.3.1. Properties of the Composite Films
3.3.2. Gas-Release Tests
3.3.3. Storage Tests in a Plastic Box
3.3.4. Large-Scale Storage Test Using Corrugated Boxes
3.3.5. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CA | citric acid |
| ClO2 | chlorine dioxide |
| FDA | Food and Drug Administration |
| FT-IR | Fourier transform infrared |
| NaClO2 | sodium chlorite |
| PA | polyamide |
| PE | polyether |
| PEBAX | polyether-b-amide |
| PEO | polyethylene oxide |
| RH | relative humidity |
| SEM | scanning electron microscopy |
| TGA | thermogravimetric analysis |
| UTM | universal testing machine |
References
- Cao, K.; Gao, Y. Optimal fresh agricultural products private brand introduction and sourcing strategy considering different power structures. Manag. Decis. Econ. 2023, 44, 3827–3845. [Google Scholar] [CrossRef]
- Qu, P.; Zhang, M.; Fan, K.; Guo, Z. Microporous modified atmosphere packaging to extend shelf life of fresh foods: A review. Crit. Rev. Food Sci. Nutr. 2022, 62, 51–65. [Google Scholar] [CrossRef] [PubMed]
- Alegbeleye, O.; Odeyemi, O.A.; Strateva, M.; Stratev, D. Microbial spoilage of vegetables, fruits and cereals. Appl. Food Res. 2022, 2, 100122. [Google Scholar] [CrossRef]
- Karanth, S.; Feng, S.; Patra, D.; Pradhan, A.K. Linking microbial contamination to food spoilage and food waste: The role of smart packaging, spoilage risk assessments, and date labeling. Front. Microbiol. 2023, 14, 1198124. [Google Scholar] [CrossRef]
- Al-Hamzah, A.; Rahman, M.M.; Kurup, P.; Barnawi, A.; Ghannam, B.; Musharraf, I.; Najjar, F.; Obeidallah, A.; Palmer, N. Use of chlorine dioxide as alternative to chlorination in reverse osmosis product water. Desalinat. Water Treat 2019, 163, 57–66. [Google Scholar] [CrossRef]
- Chen, Z. A focus on chlorine dioxide: The promising food preservative. J. Exp. Food Chem. 2017, 3, e107. [Google Scholar] [CrossRef]
- Wu, V.C.; Kim, B. Effect of a simple chlorine dioxide method for controlling five foodborne pathogens, yeasts and molds on blueberries. Food Microbiol. 2007, 24, 794–800. [Google Scholar] [CrossRef] [PubMed]
- Hua, G.; Reckhow, D.A. Comparison of disinfection byproduct formation from chlorine and alternative disinfectants. Water Res. 2007, 41, 1667–1678. [Google Scholar] [CrossRef]
- Rougé, V.; Allard, S.; Croué, J.-P.; von Gunten, U. In situ formation of free chlorine during ClO2 treatment: Implications on the formation of disinfection byproducts. Environ. Sci. Technol. 2018, 52, 13421–13429. [Google Scholar] [CrossRef]
- EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS). Safety of gaseous chlorine dioxide as a preservative slowly released in cold food storage areas. EFSA J. 2016, 14, 4388. [Google Scholar]
- Malka, S.K.; Park, M.-H. Fresh produce safety and quality: Chlorine dioxide’s role. Front. Plant Sci. 2022, 12, 775629. [Google Scholar] [CrossRef]
- Palmer, N.L. Optimisation of Chlorine Dioxide Generators and Investigation into an Ultra-Pure Chlorine Dioxide Generation Method for Drinking Water Treatment. Ph.D. Thesis, Heriot-Watt University, Edinburgh, Scotland, 2021. [Google Scholar]
- Su, H.; Chen, Z.; Zhao, Y.; An, J.; Huang, H.; Liu, R.; Huang, C. Polyvinyl alcohol film with chlorine dioxide microcapsules can be used for blueberry preservation by slow-release of chlorine dioxide gas. Front. Nutr. 2023, 10, 1177950. [Google Scholar] [CrossRef]
- Trinetta, V.; Vaidya, N.; Linton, R.; Morgan, M. Evaluation of chlorine dioxide gas residues on selected food produce. J. Food Sci. 2011, 76, T11–T15. [Google Scholar] [CrossRef]
- Jin, R.-y.; Hu, S.-q.; Zhang, Y.-g.; Bo, T. Concentration-dependence of the explosion characteristics of chlorine dioxide gas. J. Hazard. Mater. 2009, 166, 842–847. [Google Scholar] [CrossRef]
- Annous, B.A.; Buckley, D.A.; Kingsley, D.H. Efficacy of chlorine dioxide gas against hepatitis A virus on blueberries, blackberries, raspberries, and strawberries. Food Environ. Virol. 2021, 13, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Lee, J.; Sadeghi, K.; Seo, J. Controlled self-release of ClO2 as an encapsulated antimicrobial agent for smart packaging. Innov. Food Sci. Emerg. Technol. 2021, 74, 102802. [Google Scholar] [CrossRef]
- Sadeghi, K.; Kasi, G.; Ketsuk, P.; Thanakkasaranee, S.; Khan, S.B.; Seo, J. A polymeric chlorine dioxide self-releasing sheet to prolong postharvest life of cherry tomatoes. Postharvest Biol. Technol. 2020, 161, 111040. [Google Scholar] [CrossRef]
- Huang, H.; Huang, X.; Zhang, L.; Zhao, H.; Huang, C.; Huang, L.; Chen, Z. Chlorine dioxide/water-borne polyurethane antibacterial film activated by carboxyl group. Polym. Test. 2023, 121, 107980. [Google Scholar] [CrossRef]
- Flagiello, D.; Erto, A.; Lancia, A.; Di Natale, F. Advanced Flue-Gas cleaning by wet oxidative scrubbing (WOS) using NaClO2 aqueous solutions. Chem. Eng. J. 2022, 447, 137585. [Google Scholar] [CrossRef]
- Gamali, P.A.; Kazemi, A.; Zadmard, R.; Anjareghi, M.J.; Rezakhani, A.; Rahighi, R.; Madani, M. Distinguished discriminatory separation of CO2 from its methane-containing gas mixture via PEBAX mixed matrix membrane. Chin. J. Chem. Eng. 2018, 26, 73–80. [Google Scholar] [CrossRef]
- Casadei, R.; Baschetti, M.G.; Rerolle, B.G.; Park, H.B.; Giorgini, L. Synthesis and characterization of a benzoyl modified Pebax materials for gas separation applications. Polymer 2021, 228, 123944. [Google Scholar] [CrossRef]
- Eliuz, E. Antimicrobial activity of citric acid against Escherichia coli, Staphylococcus aureus and Candida albicans as a sanitizer agent. Eurasian J. For. Sci. 2020, 8, 295–301. [Google Scholar] [CrossRef]
- Nangare, S.; Vispute, Y.; Tade, R.; Dugam, S.; Patil, P. Pharmaceutical applications of citric acid. Future J. Pharm. Sci. 2021, 7, 1–23. [Google Scholar] [CrossRef]
- Castro-Cabado, M.; Parra-Ruiz, F.J.; Casado, A.; Roman, J.S. Thermal crosslinking of maltodextrin and citric acid. Methodology to control the polycondensation reaction under processing conditions. Polym. Polym. Compos. 2016, 24, 643–654. [Google Scholar] [CrossRef]
- Pimpang, P.; Sumang, R.; Choopun, S. Effect of concentration of citric acid on size and optical properties of fluorescence graphene quantum dots prepared by tuning carbonization degree. Chiang Mai J. Sci. 2018, 45, 2005. [Google Scholar]
- Shi, R.; Zhang, Z.; Liu, Q.; Han, Y.; Zhang, L.; Chen, D.; Tian, W. Characterization of citric acid/glycerol co-plasticized thermoplastic starch prepared by melt blending. Carbohydr. Polym. 2007, 69, 748–755. [Google Scholar] [CrossRef]
- Zhou, J.; Tong, J.; Su, X.; Ren, L. Hydrophobic starch nanocrystals preparations through crosslinking modification using citric acid. Int. J. Biol. Macromol. 2016, 91, 1186–1193. [Google Scholar] [CrossRef]
- Bernardo, P.; Clarizia, G. Enhancing gas permeation properties of Pebax® 1657 membranes via polysorbate nonionic surfactants doping. Polymers 2020, 12, 253. [Google Scholar] [CrossRef]
- Ebadi, R.; Maghsoudi, H.; Babaluo, A.A. Fabrication and characterization of Pebax-1657 mixed matrix membrane loaded with Si-CHA zeolite for CO2 separation from CH4. J. Nat. Gas Sci. Eng. 2021, 90, 103947. [Google Scholar] [CrossRef]
- Isanejad, M.; Azizi, N.; Mohammadi, T. Pebax membrane for CO2/CH4 separation: Effects of various solvents on morphology and performance. J. Appl. Polym. Sci. 2017, 134, 44531. [Google Scholar] [CrossRef]
- Knozowska, K.; Li, G.; Kujawski, W.; Kujawa, J. Novel heterogeneous membranes for enhanced separation in organic-organic pervaporation. J. Membr. Sci. 2020, 599, 117814. [Google Scholar] [CrossRef]
- Thanakkasaranee, S.; Kim, D.; Seo, J. Preparation and characterization of poly (ether-block-amide)/polyethylene glycol composite films with temperature-dependent permeation. Polymers 2018, 10, 225. [Google Scholar] [CrossRef]
- Khosravi, T.; Omidkhah, M.; Kaliaguine, S.; Rodrigue, D. Amine-functionalized CuBTC/poly (ether-b-amide-6)(Pebax® MH 1657) mixed matrix membranes for CO2/CH4 separation. Can. J. Chem. Eng. 2017, 95, 2024–2033. [Google Scholar] [CrossRef]
- Meshkat, S.; Kaliaguine, S.; Rodrigue, D. Mixed matrix membranes based on amine and non-amine MIL-53 (Al) in Pebax® MH-1657 for CO2 separation. Sep. Purif. Technol. 2018, 200, 177–190. [Google Scholar] [CrossRef]
- Nobakht, D.; Abedini, R. Improved gas separation performance of Pebax® 1657 membrane modified by poly-alcoholic compounds. J. Environ. Chem. Eng. 2022, 10, 107568. [Google Scholar] [CrossRef]
- Huang, S.-M.; Liu, S.-M.; Tseng, H.-Y.; Chen, W.-C. Effect of citric acid on swelling resistance and physicochemical properties of post-crosslinked electrospun polyvinyl alcohol fibrous membrane. Polymers 2023, 15, 1738. [Google Scholar] [CrossRef]
- Seligra, P.G.; Jaramillo, C.M.; Famá, L.; Goyanes, S. Biodegradable and non-retrogradable eco-films based on starch–glycerol with citric acid as crosslinking agent. Carbohydr. Polym. 2016, 138, 66–74. [Google Scholar] [CrossRef]
- Aghili, M.; Yazdi, M.K.; Ranjbar, Z.; Jafari, S.H. Anticorrosion performance of electro-deposited epoxy/amine functionalized graphene oxide nanocomposite coatings. Corros. Sci. 2021, 179, 109143. [Google Scholar] [CrossRef]
- Zhang, Y.Z.; Wang, Y.; Jiang, Q.; El-Demellawi, J.K.; Kim, H.; Alshareef, H.N. MXene printing and patterned coating for device applications. Adv. Mater. 2020, 32, 1908486. [Google Scholar] [CrossRef]
- Akhtar, F.H.; Kumar, M.; Peinemann, K.-V. Pebax® 1657/Graphene oxide composite membranes for improved water vapor separation. J. Membr. Sci. 2017, 525, 187–194. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, S.; Yao, Y.; Wu, N.; Xu, M.; Yin, Z.; Zhao, Y.; Tu, Y. Effects of citric acid crosslinking on the structure and properties of ovotransferrin and chitosan composite films. Int. J. Biol. Macromol. 2023, 229, 268–281. [Google Scholar] [CrossRef]
- Taheri, P.; Maleh, M.S.; Raisi, A. Cross-linking of poly (ether-block-amide) by poly (ethylene glycol) diacrylate to prepare plasticizing-resistant CO2-selective membranes. J. Environ. Chem. Eng. 2021, 9, 105877. [Google Scholar] [CrossRef]
- Radi, B.; Wellard, R.M.; George, G.A. Effect of dangling chains on the structure and physical properties of a tightly crosslinked poly (ethylene glycol) network. Soft Matter 2013, 9, 3262–3271. [Google Scholar] [CrossRef]
- Chaibi, S.; Benachour, D.; Merbah, M.; Esperanza Cagiao, M.; Baltá Calleja, F.J. The role of crosslinking on the physical properties of gelatin based films. Colloid Polym. Sci. 2015, 293, 2741–2752. [Google Scholar] [CrossRef]
- Gomaa, M.M.; Hugenschmidt, C.; Dickmann, M.; Abdel-Hady, E.E.; Mohamed, H.F.; Abdel-Hamed, M.O. Crosslinked PVA/SSA proton exchange membranes: Correlation between physiochemical properties and free volume determined by positron annihilation spectroscopy. Phys. Chem. Chem. Phys. 2018, 20, 28287–28299. [Google Scholar] [CrossRef]
- Tisserat, B.; O’kuru, R.H.; Hwang, H.; Mohamed, A.A.; Holser, R. Glycerol citrate polyesters produced through heating without catalysis. J. Appl. Polym. Sci. 2012, 125, 3429–3437. [Google Scholar] [CrossRef]
- Jose, J.; Al-Harthi, M.A. Citric acid crosslinking of poly (vinyl alcohol)/starch/graphene nanocomposites for superior properties. Iran. Polym. J. 2017, 26, 579–587. [Google Scholar] [CrossRef]
- Reddy, N.; Yang, Y. Citric acid cross-linking of starch films. Food Chem. 2010, 118, 702–711. [Google Scholar] [CrossRef]
- Rahman, M.M.; Filiz, V.; Shishatskiy, S.; Abetz, C.; Neumann, S.; Bolmer, S.; Khan, M.M.; Abetz, V. PEBAX® with PEG functionalized POSS as nanocomposite membranes for CO2 separation. J. Membr. Sci. 2013, 437, 286–297. [Google Scholar] [CrossRef]
- Thanakkasaranee, S.; Sadeghi, K.; Seo, J. Smart steam release of newly developed temperature-responsive nanocomposite films derived from phase change material. Polymer 2021, 219, 123543. [Google Scholar] [CrossRef]
- Abdillahi, H.; Chabrat, E.; Rouilly, A.; Rigal, L. Influence of citric acid on thermoplastic wheat flour/poly (lactic acid) blends. II. Barrier properties and water vapor sorption isotherms. Ind. Crops Prod. 2013, 50, 104–111. [Google Scholar] [CrossRef]
- Olsson, E.; Hedenqvist, M.S.; Johansson, C.; Järnström, L. Influence of citric acid and curing on moisture sorption, diffusion and permeability of starch films. Carbohydr. Polym. 2013, 94, 765–772. [Google Scholar] [CrossRef] [PubMed]
- Hui, Y.H.; Evranuz, E.Ö. Handbook of Vegetable Preservation and Processing; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- Lichter, A.; Dvir, O.; Fallik, E.; Cohen, S.; Golan, R.; Shemer, Z.; Sagi, M. Cracking of cherry tomatoes in solution. Postharvest Biol. Technol. 2002, 26, 305–312. [Google Scholar] [CrossRef]
- Agius, C.; von Tucher, S.; Rozhon, W. The effect of salinity on fruit quality and yield of cherry tomatoes. Horticulturae 2022, 8, 59. [Google Scholar] [CrossRef]
- Tsouvaltzis, P.; Gkountina, S.; Siomos, A.S. Quality Traits and Nutritional Components of Cherry Tomato in Relation to the Harvesting Period, Storage Duration and Fruit Position in the Truss. Plants 2023, 12, 315. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhu, C.; Han, Z. Effects of aqueous chlorine dioxide treatment on nutritional components and shelf-life of mulberry fruit (Morus alba L. ). J. Biosci. Bioeng. 2011, 111, 675–681. [Google Scholar] [CrossRef]
- Han, Y.; Selby, T.; Schultze, K.; Nelson, P.; Linton, R.H. Decontamination of strawberries using batch and continuous chlorine dioxide gas treatments. J. Food Prot. 2004, 67, 2450–2455. [Google Scholar] [CrossRef]
- Sun, X.; Baldwin, E.; Bai, J. Applications of gaseous chlorine dioxide on postharvest handling and storage of fruits and vegetables–A review. Food Control. 2019, 95, 18–26. [Google Scholar] [CrossRef]
- Al-Dairi, M.; Pathare, P.B.; Al-Yahyai, R. Chemical and nutritional quality changes of tomato during postharvest transportation and storage. J. Saudi Soc. Agric. Sci. 2021, 20, 401–408. [Google Scholar] [CrossRef]
- Wang, D.; Wang, Y.; Lv, Z.; Pan, Z.; Wei, Y.; Shu, C.; Zeng, Q.; Chen, Y.; Zhang, W. Analysis of nutrients and volatile compounds in cherry tomatoes stored at different temperatures. Foods 2021, 12, 6. [Google Scholar] [CrossRef]
- Gómez-López, V.M.; Ragaert, P.; Jeyachchandran, V.; Debevere, J.; Devlieghere, F. Shelf-life of minimally processed lettuce and cabbage treated with gaseous chlorine dioxide and cysteine. Int. J. Food Microbiol. 2008, 121, 74–83. [Google Scholar] [CrossRef] [PubMed]
- ASTM Subcommittee D20; 10 on Mechanical Properties. Standard Test Method for Tensile Properties of Plastics. American Society for Testing and Materials: West Conshohocken, PA, USA, 1998.
- Saade, C.; Annous, B.A.; Gualtieri, A.J.; Schaich, K.M.; Liu, L.; Yam, K.L. System feasibility: Designing a chlorine dioxide self-generating package label to improve fresh produce safety part II: Solution casting approach. Innov. Food Sci. Emerg. Technol. 2018, 47, 110–119. [Google Scholar] [CrossRef]
- Tzanavaras, P.; Themelis, D.; Kika, F. Review of analytical methods for the determination of chlorine dioxide. Open Chem. 2007, 5, 1–12. [Google Scholar] [CrossRef]
- KÖrtvÉlyesi, Z.; Gordon, G. Chlorite ion interference in the spectrophotometric measurement of chlorine dioxide. J.-Am. Water Work. Assoc. 2004, 96, 81–87. [Google Scholar] [CrossRef]
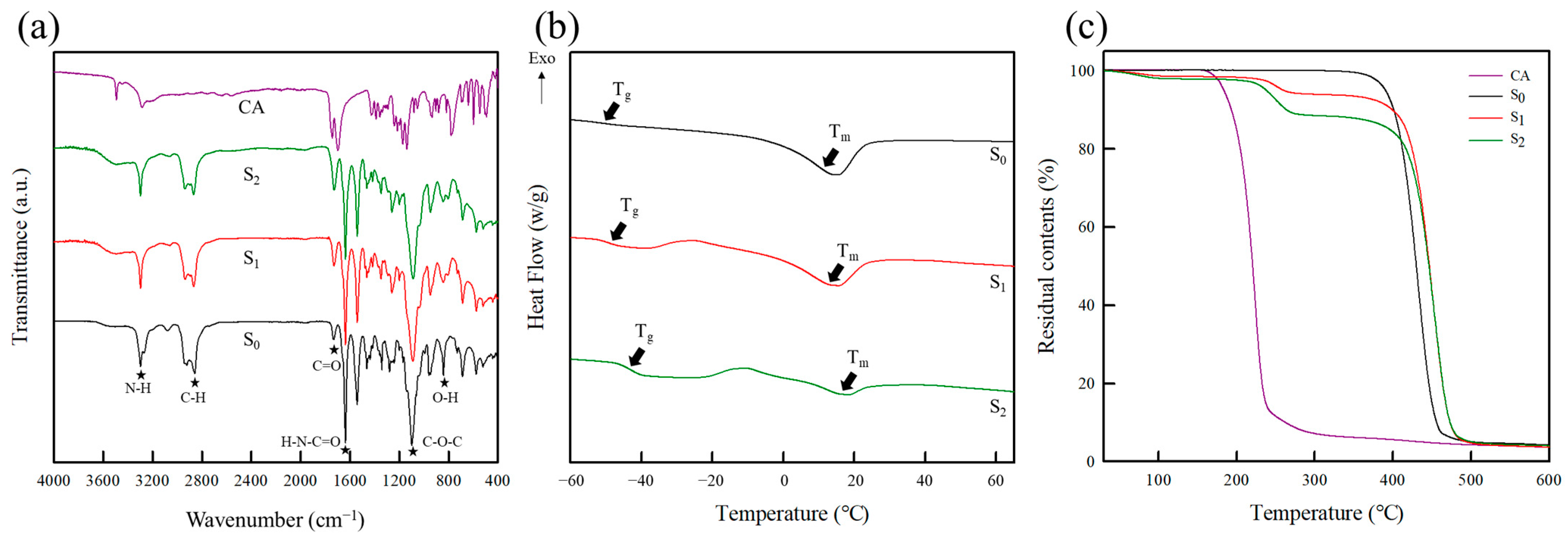
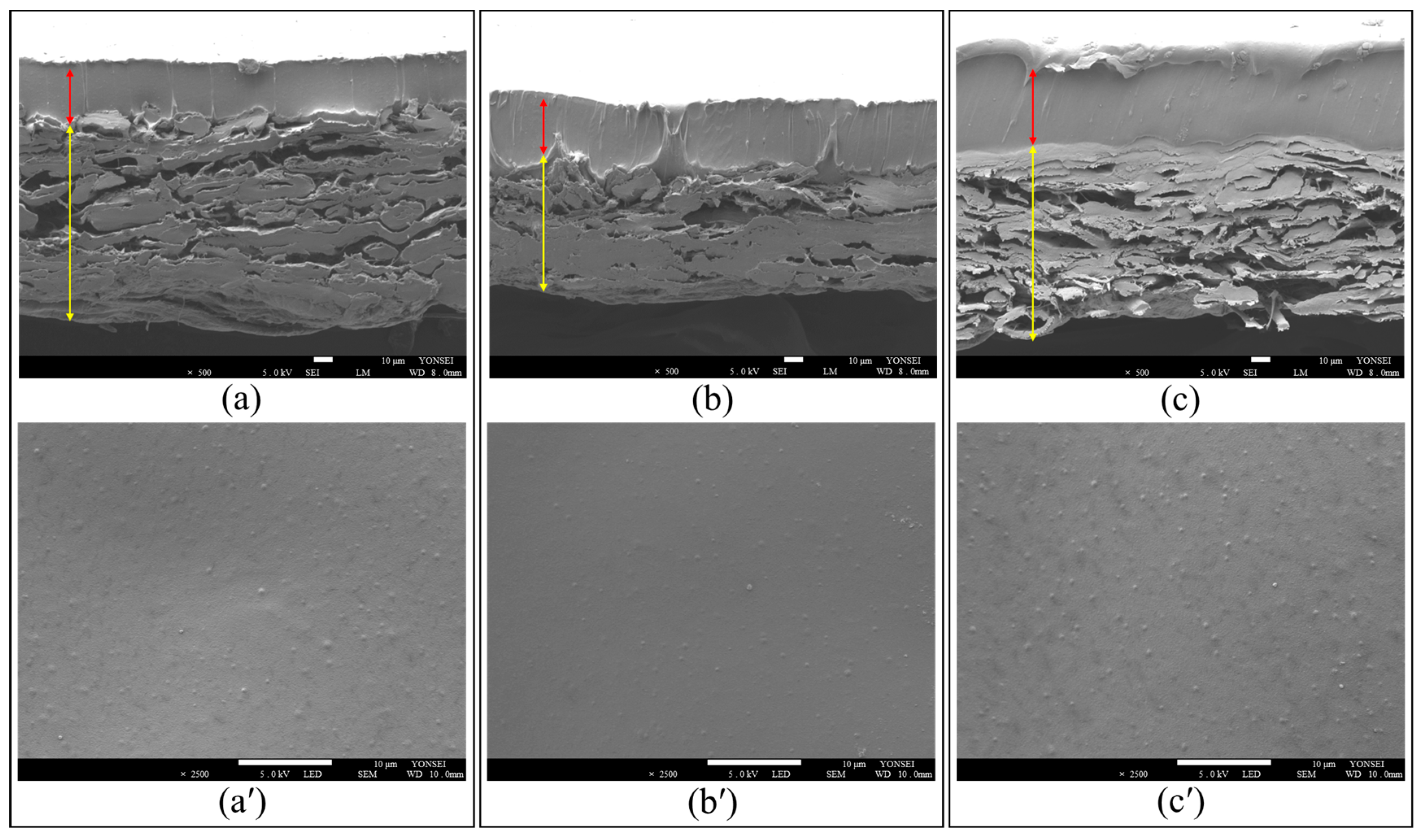

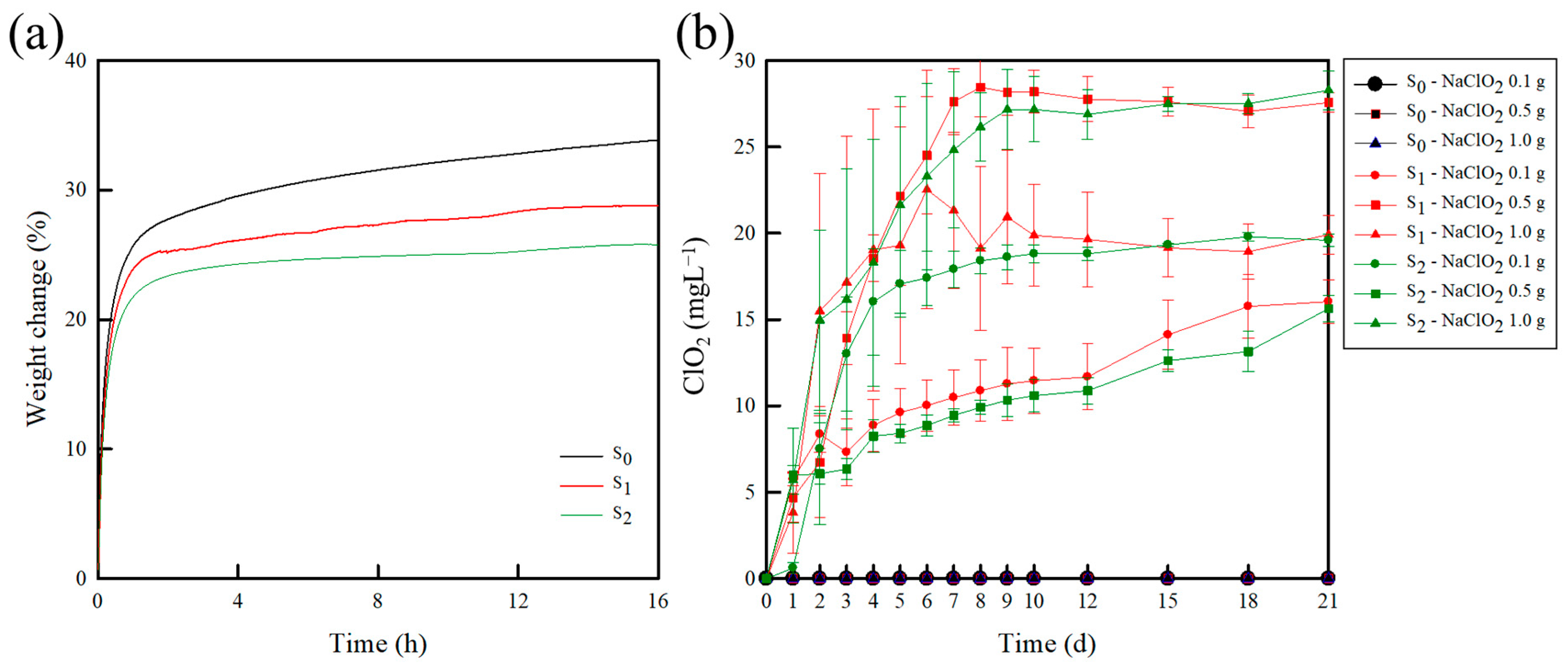

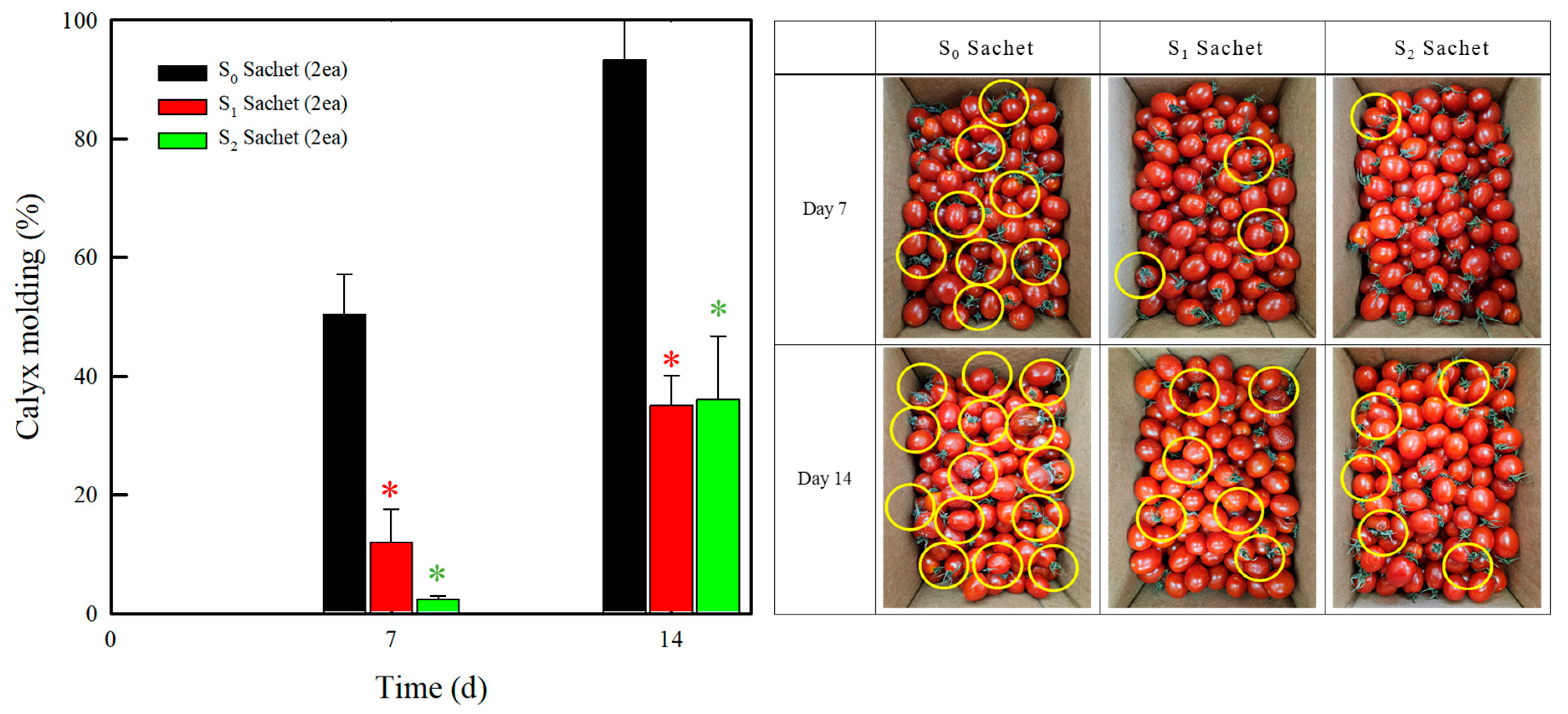
| Assignment | Characteristic Bands (cm−1) | |||
|---|---|---|---|---|
| CA | S0 | S1 | S2 | |
| O–H stretching | 3292, 3495 | 845 | 845 | 845 |
| C–O–C stretching | − | 1100 | 1100 | 1100 |
| C=O stretching | 1699, 1742 | 1735 | 1731 | 1731 |
| H–N–C=O stretching | − | 1638 | 1638 | 1638 |
| N–H stretching | − | 3297 | 3297 | 3297 |
| C–H bending | − | 2856, 2948 | 2856, 2948 | 2856, 2948 |
| Sample | DSC | TGA | |||
|---|---|---|---|---|---|
| Tg (°C) a | Tm (°C) b | ΔHm (J/g) c | T1st (°C) d | T2nd (°C) e | |
| S0 | −50.8 ± 0.2 f | 13.4 ± 0.6 f | 21.3 ± 0.9 f | – | 350–460 |
| S1 | −49.3 ± 0.3 g | 15.4 ± 0.4 g | 15.4 ± 1.5 g | 220–270 | 350–490 |
| S2 | −42.9 ± 0.5 h | 17.7 ± 0.4 h | 4.1 ± 0.8 h | 220–270 | 350–490 |
| Day | S0-0.1 g | S1-0.1 g | S2-0.1 g | S0-0.5 g | S1-0.5 g | S2-0.5 g | S0-1.0 g | S1-1.0 g | S2-1.0 g |
|---|---|---|---|---|---|---|---|---|---|
| 0 | 0.00 ± 0.00 a | 0.00 ± 0.00 b | 0.00 ± 0.00 c | 0.00 ± 0.00 a | 0.00 ± 0.00 b | 0.00 ± 0.00 c | 0.00 ± 0.00 a | 0.00 ± 0.00 b | 0.00 ± 0.00 c |
| 1 | 0.00 ± 0.00 a | 5.75 ± 0.38 b | 0.61 ± 0.30 c | 0.00 ± 0.00 a | 4.69 ± 1.43 b | 5.98 ± 2.75 c | 0.00 ± 0.00 a | 3.83 ± 2.37 b | 5.71 ± 0.85 c |
| 2 | 0.00 ± 0.00 a | 8.37 ± 1.06 b | 7.51 ± 2.06 c | 0.00 ± 0.00 a | 6.73 ± 3.22 b | 6.08 ± 2.95 c | 0.00 ± 0.00 a | 15.48 ± 7.96 b | 14.96 ± 5.22 c |
| 3 | 0.00 ± 0.00 a | 7.31 ± 1.95 b | 13.01 ± 3.30 c | 0.00 ± 0.00 a | 13.93 ± 1.51 b | 6.34 ± 0.61 c | 0.00 ± 0.00 a | 17.16 ± 8.45 b | 16.16 ± 7.56 c |
| 4 | 0.00 ± 0.00 a | 8.87 ± 1.50 b | 16.02 ± 3.09 c | 0.00 ± 0.00 a | 18.53 ± 1.35 b | 8.25 ± 0.94 c | 0.00 ± 0.00 a | 19.04 ± 8.16 b | 18.29 ± 7.15 c |
| 5 | 0.00 ± 0.00 a | 9.62 ± 1.38 b | 17.06 ± 1.95 c | 0.00 ± 0.00 a | 22.15 ± 5.16 b | 8.41 ± 0.53 c | 0.00 ± 0.00 a | 19.29 ± 6.86 b | 21.63 ± 6.28 c |
| 6 | 0.00 ± 0.00 a | 10.02 ± 1.49 b | 17.40 ± 1.57 c | 0.00 ± 0.00 a | 24.51 ± 3.40 b | 8.86 ± 0.61 c | 0.00 ± 0.00 a | 22.53 ± 6.89 b | 23.29 ± 5.39 c |
| 7 | 0.00 ± 0.00 a | 10.48 ± 1.60 b | 17.91 ± 1.06 c | 0.00 ± 0.00 a | 27.61 ± 1.89 b | 9.45 ± 0.37 c | 0.00 ± 0.00 a | 21.32 ± 4.52 b | 24.82 ± 4.51 c |
| 8 | 0.00 ± 0.00 a | 10.88 ± 1.78 b | 18.40 ± 0.75 c | 0.00 ± 0.00 a | 28.45 ± 1.70 b | 9.91 ± 0.42 c | 0.00 ± 0.00 a | 19.11 ± 4.73 b | 26.14 ± 1.99 c |
| 9 | 0.00 ± 0.00 a | 11.27 ± 2.12 b | 18.61 ± 0.72 c | 0.00 ± 0.00 a | 28.16 ± 1.34 b | 10.33 ± 0.95 c | 0.00 ± 0.00 a | 20.94 ± 3.86 b | 27.16 ± 2.32 c |
| 10 | 0.00 ± 0.00 a | 11.45 ± 1.88 b | 18.81 ± 0.50 c | 0.00 ± 0.00 a | 28.20 ± 1.25 b | 10.59 ± 0.93 c | 0.00 ± 0.00 a | 19.87 ± 2.95 b | 27.16 ± 1.89 c |
| 12 | 0.00 ± 0.00 a | 11.68 ± 1.91 b | 18.81 ± 0.39 c | 0.00 ± 0.00 a | 27.76 ± 1.31 b | 10.88 ± 0.76 c | 0.00 ± 0.00 a | 19.64 ± 2.74 b | 26.88 ± 1.45 c |
| 15 | 0.00 ± 0.00 a | 14.11 ± 1.99 b | 19.32 ± 0.09 c | 0.00 ± 0.00 a | 27.61 ± 0.85 b | 12.61 ± 0.64 c | 0.00 ± 0.00 a | 19.15 ± 1.68 b | 27.48 ± 0.41 c |
| 18 | 0.00 ± 0.00 a | 15.76 ± 1.83 b | 19.79 ± 0.25 c | 0.00 ± 0.00 a | 27.05 ± 0.94 b | 13.15 ± 1.16 c | 0.00 ± 0.00 a | 18.93 ± 1.59 b | 27.50 ± 0.59 c |
| 21 | 0.00 ± 0.00 a | 16.03 ± 1.27 b | 19.59 ± 0.35 c | 0.00 ± 0.00 a | 27.56 ± 0.57 b | 15.64 ± 0.76 c | 0.00 ± 0.00 a | 19.92 ± 1.12 b | 28.27 ± 1.13 c |
| TSS | |||
|---|---|---|---|
| Day | S0 | S1 | S2 |
| 0 | 7.57 ± 0.11 f | 7.57 ± 0.11 f | 7.57 ± 0.11 f |
| 3 | 7.05 ± 0.17 d | 7.30 ± 0.26 e | 7.23 ± 0.11 e |
| 6 | 6.90 ± 0.12 c | 7.10 ± 0.08 d | 7.15 ± 0.15 d |
| 9 | 6.78 ± 0.09 c | 7.07 ± 0.05 d | 7.22 ± 0.07 d |
| 12 | 6.48 ± 0.11 b | 6.72 ± 0.07 c | 6.72 ± 0.11 c |
| 16 | 6.27 ± 0.09 a | 6.57 ± 0.14 b | 6.55 ± 0.15 b |
| Firmness | |||
| Day | S0 | S1 | S2 |
| 0 | 8.29 ± 1.06 a | 8.29 ± 1.06 a | 8.29 ± 1.06 a |
| 3 | 7.98 ± 0.99 ab | 8.61 ± 1.14 b | 8.76 ± 1.01 b |
| 6 | 8.32 ± 1.11 b | 9.41 ± 0.79 c | 9.03 ± 1.17 bc |
| 9 | 8.64 ± 0.99 bc | 8.80 ± 0.91 bc | 8.92 ± 1.28 bc |
| 12 | 7.97 ± 1.16 ab | 8.87 ± 0.94 bc | 8.99 ± 1.04 c |
| 16 | 7.48 ± 1.28 a | 9.74 ± 1.22 c | 9.10 ± 1.24 c |
| pH | |||
| Day | S0 | S1 | S2 |
| 0 | 4.30 ± 0.02 a | 4.30 ± 0.02 a | 4.30 ± 0.02 a |
| 3 | 4.29 ± 0.02 a | 4.34 ± 0.02 b | 4.35 ± 0.03 b |
| 6 | 4.37 ± 0.02 b | 4.33 ± 0.02 b | 4.33 ± 0.02 b |
| 9 | 4.41 ± 0.01 c | 4.39 ± 0.01 c | 4.40 ± 0.01 c |
| 12 | 4.42 ± 0.03 cd | 4.40 ± 0.02 c | 4.44 ± 0.01 d |
| 16 | 4.67 ± 0.03 f | 4.52 ± 0.02 e | 4.54 ± 0.01 e |
| Sample Code [Series of PEBAX/CA] | Composition (w/w) | |
|---|---|---|
| PEBAX | CA | |
| S0 (Pure PEBAX) | 100 | 0 |
| S1 | 95 | 5 |
| S2 | 90 | 10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.; Shin, H.; Sadeghi, K.; Seo, J. Chlorine Dioxide (ClO2)-Releasing Sachet for Preservation of Cherry Tomatoes. Molecules 2025, 30, 2041. https://doi.org/10.3390/molecules30092041
Lee J, Shin H, Sadeghi K, Seo J. Chlorine Dioxide (ClO2)-Releasing Sachet for Preservation of Cherry Tomatoes. Molecules. 2025; 30(9):2041. https://doi.org/10.3390/molecules30092041
Chicago/Turabian StyleLee, Junseok, Hojun Shin, Kambiz Sadeghi, and Jongchul Seo. 2025. "Chlorine Dioxide (ClO2)-Releasing Sachet for Preservation of Cherry Tomatoes" Molecules 30, no. 9: 2041. https://doi.org/10.3390/molecules30092041
APA StyleLee, J., Shin, H., Sadeghi, K., & Seo, J. (2025). Chlorine Dioxide (ClO2)-Releasing Sachet for Preservation of Cherry Tomatoes. Molecules, 30(9), 2041. https://doi.org/10.3390/molecules30092041








