Tuning Solvation Dynamics of Electrolytes at Their Eutectic Point Through Halide Identity
Abstract
1. Introduction
2. Results
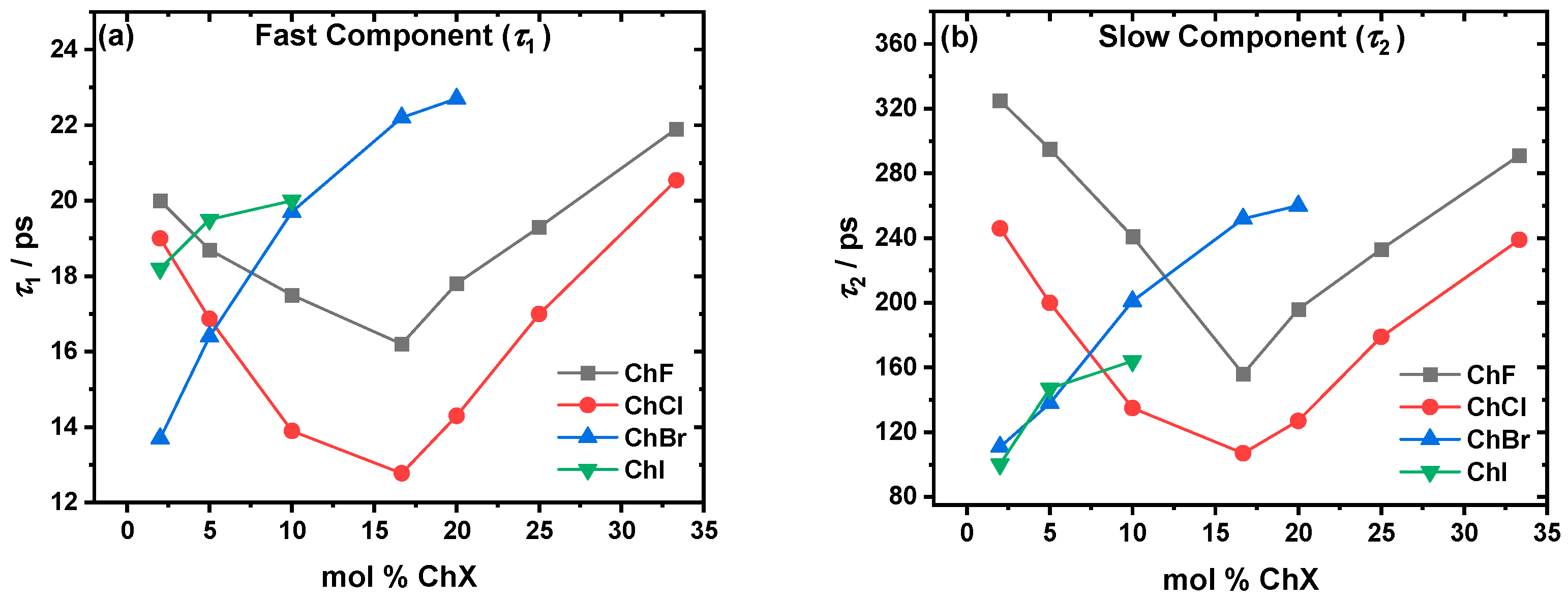
2.1. Solvent Relaxation Dynamics
2.2. Liquidus Temperatures
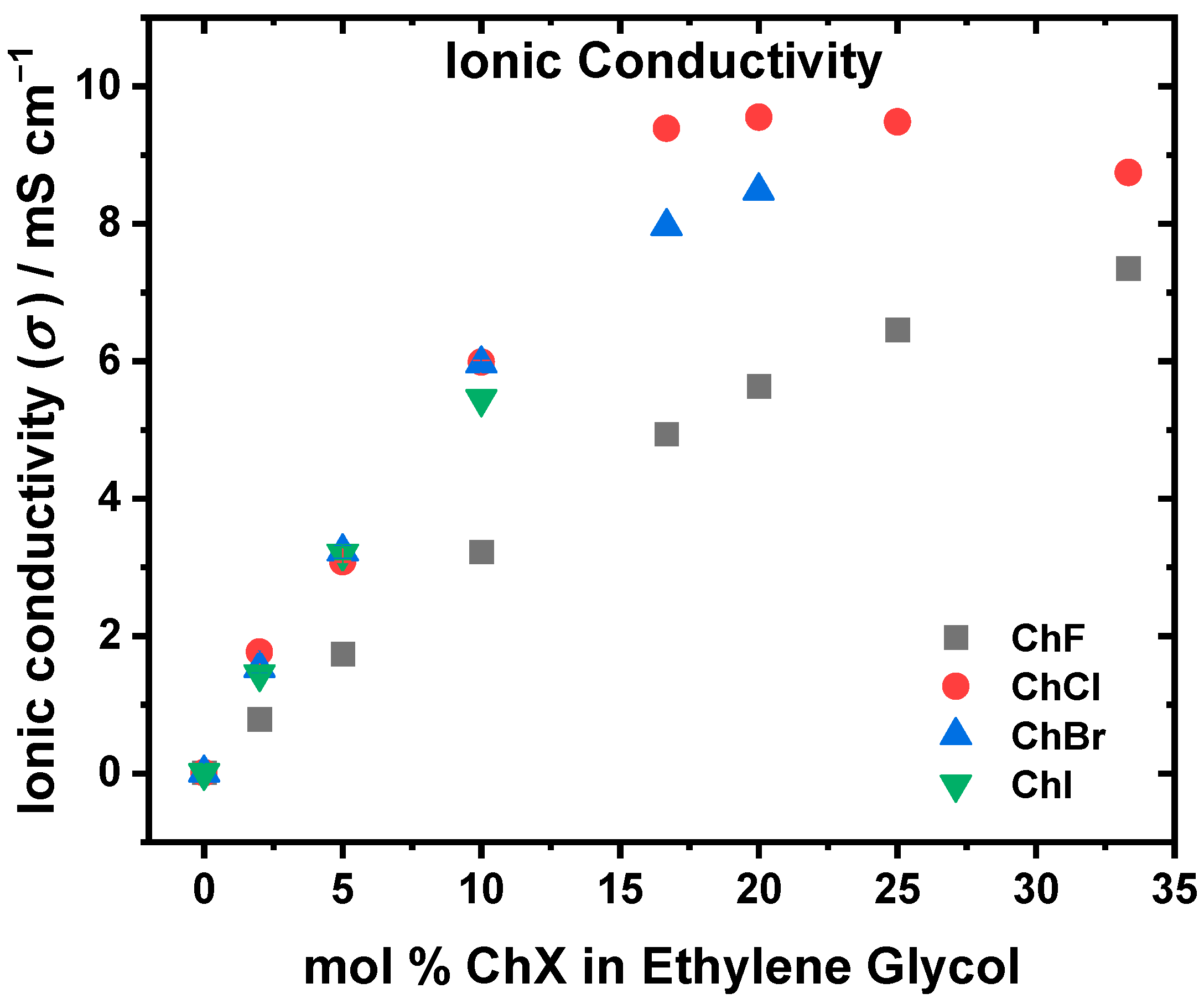
2.3. Viscosity, Ionic Conductivity, and Density
2.4. Polarity
2.5. Self-Diffusion Coefficients
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Synthesis of ChF
4.3. Preparation of Solvent Mixtures
4.4. Differential Scanning Calorimetry
4.5. Femtosecond Transient Absorption Spectroscopy
4.6. Steady-State Absorption Spectroscopy and Solvent Polarity
4.7. Viscosity and Density
4.8. Ionic Conductivity
4.9. NMR Spectroscopy and Pulsed-Field Gradient (PFG) NMR
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Armaroli, N.; Balzani, V. The Legacy of Fossil Fuels. Chem. Asian J. 2011, 6, 768–784. [Google Scholar] [CrossRef]
- Weber, A.Z.; Mench, M.M.; Meyers, J.P.; Ross, P.N.; Gostick, J.T.; Liu, Q. Redox Flow Batteries: A Review. J. Appl. Electrochem. 2011, 41, 1137–1164. [Google Scholar] [CrossRef]
- Alotto, P.; Guarnieri, M.; Moro, F. Redox Flow Batteries for the Storage of Renewable Energy: A Review. Renew. Sustain. Energy Rev. 2014, 29, 325–335. [Google Scholar] [CrossRef]
- Mohamed, M.R.; Sharkh, S.M.; Walsh, F.C. Redox Flow Batteries for Hybrid Electric Vehicles: Progress and Challenges. In Proceedings of the 5th IEEE Vehicle Power and Propulsion Conference (VPPC ‘09), Dearborn, MI, USA, 7–10 September 2009; pp. 551–557. [Google Scholar]
- Winsberg, J.; Hagemann, T.; Janoschka, T.; Hager, M.D.; Schubert, U.S. Redox-Flow Batteries: From Metals to Organic Redox-Active Materials. Angew. Chem. Int. Ed. 2017, 129, 702–729. [Google Scholar] [CrossRef]
- Tang, L.; Leung, P.; Xu, Q.; Mohamed, M.R.; Dai, S.; Zhu, X.; Flox, C.; Shah, A.A. Future Perspective on Redox Flow Batteries: Aqueous versus Nonaqueous Electrolytes. Curr. Opin. Chem. Eng. 2022, 37, 100833. [Google Scholar] [CrossRef]
- Smith, E.L.; Abbott, A.P.; Ryder, K.S. Deep Eutectic Solvents (DESs) and Their Applications. Chem. Rev. 2014, 114, 11060–11082. [Google Scholar] [CrossRef]
- Abbott, A.P.; Boothby, D.; Capper, G.; Davies, D.L.; Rasheed, R.K. Deep Eutectic Solvents Formed between Choline Chloride and Carboxylic Acids: Versatile Alternatives to Ionic Liquids. J. Am. Chem. Soc. 2004, 126, 9142–9147. [Google Scholar] [CrossRef]
- Abbott, A.P.; Harris, R.C.; Ryder, K.S.; D’Agostino, C.; Gladden, L.F.; Mantle, M.D. Glycerol Eutectics as Sustainable Solvent Systems. Phys. Chem. Chem. Phys. 2011, 13, 82–90. [Google Scholar] [CrossRef]
- Abbott, A.P.; Capper, G.; Davies, D.L.; Rasheed, R.K.; Tambyrajah, V. Novel Solvent Properties of Choline Chloride/Urea Mixtures. Chem. Commun. 2003, 1, 70–71. [Google Scholar] [CrossRef]
- Hansen, B.B.; Spittle, S.; Chen, B.; Poe, D.; Zhang, Y.; Klein, J.M.; Horton, A.; Adhikari, L.; Zelovich, T.; Doherty, B.W.; et al. Deep Eutectic Solvents: A Review of Fundamentals and Applications. Chem. Rev. 2021, 121, 1232–1285. [Google Scholar] [CrossRef]
- García, G.; Aparicio, S.; Ullah, R.; Atilhan, M. Deep Eutectic Solvents: Physicochemical Properties and Gas Separation Applications. Energy Fuels 2015, 29, 2616–2644. [Google Scholar] [CrossRef]
- Zhang, Q.; De Oliveira Vigier, K.; Royer, S.; Jérôme, F. Deep Eutectic Solvents: Syntheses, Properties and Applications. Chem. Soc. Rev. 2012, 41, 7108–7146. [Google Scholar] [CrossRef]
- Cao, X.; Wang, S.; Xue, X. A Zn–Ce Redox Flow Battery with Ethaline Deep Eutectic Solvent. ChemSusChem 2021, 14, 1747–1755. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Sinclair, N.; Kellamis, C.; Gurkan, B.; Wainright, J.; Savinell, R. Effects of Alkyl Chain Length and Halide Anion on Hydrogen Bonding, Electrochemical Transport Properties and Double Layer Capacitance in Eutectic Solvents. J. Mol. Liq. 2023, 391, 123314. [Google Scholar] [CrossRef]
- Brammer, L.; Bruton, E.A.; Sherwood, P. Understanding the Behavior of Halogens as Hydrogen Bond Acceptors. Cryst. Growth Des. 2001, 1, 277–290. [Google Scholar] [CrossRef]
- Peris, E.; Lee, J.C.J.; Rambo, J.R.; Eisenstein, O.; Crabtree, R.H. Factors Affecting the Strength of X–H···H–M Hydrogen Bonds. J. Am. Chem. Soc. 1995, 117, 3485–3491. [Google Scholar] [CrossRef]
- Prado, D.M.; Burda, C. Untapped Potential of Fluoride Ions in Maximizing the Electrochemical Stability of Deep Eutectic Solvents. J. Phys. Chem. Lett. 2024, 15, 6343–6346. [Google Scholar] [CrossRef]
- Boogaart, D.J.; Essner, J.B.; Baker, G.A. Halide effects on the performance of equimolar choline halide: Guanidinium thiocyanate deep eutectic solvents as dye-sensitized solar cell electrolytes. Green Chem. Lett. Rev. 2022, 15, 615–626. [Google Scholar] [CrossRef]
- Lane, J.N.; Klimkowski, V.J.; Hopkins, T.A. Molecular dynamics investigation of deep eutectic solvent structure and properties based on hydrogen bond acceptor variation. J. Mol. Liq. 2025, 423, 127009. [Google Scholar] [CrossRef]
- Alfurayj, I.; Pandian, R.; Springer, S.; Burda, C. Choline Fluoride–Ethylene Glycol Deep Eutectic Solvent Mixture—Synthesis and Physicochemical Properties. J. Mol. Liq. 2023, 386, 122454. [Google Scholar] [CrossRef]
- Alfurayj, I.; Fraenza, C.; Pandian, R.; Greenbaum, S.; Burda, C. Solvation Dynamics of Choline Fluoride in Ethylene Glycol–Water Mixtures. J. Mol. Liq. 2023, 392, 123448. [Google Scholar] [CrossRef]
- Wang, H.; Liu, S.; Zhao, Y.; Wang, J.; Yu, Z. Insights into the Hydrogen Bond Interactions in Deep Eutectic Solvents Composed of Choline Chloride and Polyols. ACS Sustain. Chem. Eng. 2019, 7, 7760–7767. [Google Scholar] [CrossRef]
- D’Agostino, C.H.; Harris, R.C.; Abbott, A.P.; Gladden, L.F.; Mantle, M.D. Molecular Motion and Ion Diffusion in Choline Chloride-Based Deep Eutectic Solvents Studied by ¹H Pulsed Field Gradient NMR Spectroscopy. Phys. Chem. Chem. Phys. 2011, 13, 21383–21391. [Google Scholar] [CrossRef] [PubMed]
- Dimroth, K.; Reichardt, C.; Siepmann, T.; Bohlmann, F. Über Pyridinium-N-Phenol-Betaine und Ihre Verwendung zur Charakterisierung der Polarität von Lösungsmitteln. Justus Liebigs Ann. Chem. 1963, 661, 1–37. [Google Scholar] [CrossRef]
- Beard, M.C.; Turner, G.M.; Schmuttenmaer, C.A. Measuring Intramolecular Charge Transfer via Coherent Generation of THz Radiation. J. Phys. Chem. A 2002, 106, 878–883. [Google Scholar] [CrossRef]
- Pandian, R.; Burda, H.; Alfurayj, I.; Reichardt, C.; Burda, C. 60 Years of Betaine 30─From Solvatochromic Discovery to Future Frontiers. J. Phys. Chem. B 2024, 128, 6990–7001. [Google Scholar] [CrossRef]
- Reichardt, C. Empirische Parameter der Lösungsmittelpolarität. Angew. Chem. 1965, 77, 30–40. [Google Scholar] [CrossRef]
- Reichardt, C.; Harbusch-Gornert, E.; Schafer, G. Herstellung und UV/Vis-Spektroskopische Eigenschaften Eines Wasserlöslichen Carboxylat-Substituierten Pyridinium-N-Phenolat-Betainfarbstoffs. Liebigs Ann. Chem. 1988, 1988, 839–844. [Google Scholar] [CrossRef]
- Reichardt, C. Polarity of Ionic Liquids Determined Empirically by Means of Solvatochromic Pyridinium N-Phenolate Betaine Dyes. Green Chem. 2005, 7, 339–351. [Google Scholar] [CrossRef]
- Reichardt, C. Pyridinium N-Phenolate Betaine Dyes as Empirical Indicators of Solvent Polarity: Some New Findings. Pure Appl. Chem. 2004, 76, 1903–1919. [Google Scholar] [CrossRef]
- Reichardt, C. Solvation Effects in Organic Chemistry: A Short Historical Overview. J. Org. Chem. 2022, 87, 1616–1629. [Google Scholar] [CrossRef] [PubMed]
- Reichardt, C. Solvatochromic Dyes as Solvent Polarity Indicators. Chem. Rev. 1994, 94, 2319–2358. [Google Scholar] [CrossRef]
- Reichardt, C. Solvatochromism, Thermochromism, Piezochromism, Halochromism, and Chiro-Solvatochromism of Pyridinium N-Phenoxide Betaine Dyes. Chem. Soc. Rev. 1992, 21, 147–153. [Google Scholar] [CrossRef]
- Reichardt, C.; Welton, T. Solvents and Solvent Effects in Organic Chemistry, 4th ed.; Wiley-VCH: Weinheim, Germany, 2011. [Google Scholar]
- Reichardt, C.; Che, D.; Heckenkemper, G.; Schafer, G. Syntheses and UV/Vis-Spectroscopic Properties of Hydrophilic 2-, 3-, and 4-Pyridyl-Substituted Solvatochromic and Halochromic Pyridinium N-Phenolate Betaine Dyes as New Empirical Solvent Polarity Indicators. Eur. J. Org. Chem. 2001, 2001, 2343–2361. [Google Scholar] [CrossRef]
- Reichardt, C.; Harbusch-Gornert, E. Über Pyridinium-N-Phenolat-Betaine und Ihre Verwendung zur Charakterisierung der Polarität von Lösungsmitteln, X. Erweiterung, Korrektur und Neudefinition der ET-Lösungsmittelpolaritätsskala mit Hilfe Eines Lipophilen Penta-Tert-Butyl-Substituierten Pyridinium-N-Phenolat-Betainfarbstoffs. Liebigs Ann. Chem. 1983, 1983, 721–743. [Google Scholar]
- Mente, S.R.; Maroncelli, M. Computer Simulations of the Solvatochromism of Betaine-30. J. Phys. Chem. B 1999, 103, 7704–7719. [Google Scholar] [CrossRef]
- Reid, P.J.; Barbara, P.F. Dynamic Solvent Effect on Betaine-30 Electron-Transfer Kinetics in Alcohols. J. Phys. Chem. 1995, 99, 3554–3565. [Google Scholar] [CrossRef]
- Alfurayj, I.; Fraenza, C.C.; Zhang, Y.; Pandian, R.; Spittle, S.; Hansen, B.; Dean, W.; Gurkan, B.; Savinell, R.; Greenbaum, S.; et al. Solvation Dynamics of Wet Ethaline: Water Is the Magic Component. J. Phys. Chem. B 2021, 125, 8888–8901. [Google Scholar] [CrossRef]
- Pandian, R.; Kim, D.; Zhang, Y.; Alfurayj, I.; Prado, D.M.; Maginn, E.; Burda, C. Chain Length and OH-Spacing Effects on Diol-Based Deep Eutectic Solvents. J. Mol. Liq. 2024, 393, 123534. [Google Scholar] [CrossRef]
- Kovalenko, S.A.; Eilers-König, N.; Senyushkina, T.A.; Ernsting, N.P. Charge Transfer and Solvation of Betaine-30 in Polar Solvents—A Femtosecond Broadband Transient Absorption Study. J. Phys. Chem. A 2001, 105, 4834–4843. [Google Scholar] [CrossRef]
- Gajardo-Parra, N.F.; Cotroneo-Figueroa, V.P.; Aravena, P.; Vesovic, V.; Canales, R.I. Viscosity of Choline Chloride-Based Deep Eutectic Solvents: Experiments and Modeling. J. Chem. Eng. Data 2020, 65, 5581–5592. [Google Scholar] [CrossRef]
- Spittle, S.; Alfurayj, I.; Hansen, B.B.; Glynn, K.; Brackett, W.; Pandian, R.; Burda, C.; Sangoro, J. Enhanced Dynamics and Charge Transport at the Eutectic Point: A New Paradigm for the Use of Deep Eutectic Solvent Systems. JACS Au 2023, 3, 3024–3030. [Google Scholar] [CrossRef]
- Reichardt, C. Empirical Parameters of the Polarity of Solvents. Angew. Chem. Int. Ed. Engl. 1965, 4, 29–40. [Google Scholar] [CrossRef]
- Migliorati, V.; D’Angelo, P. Deep Eutectic Solvents: A Structural Point of View on the Role of the Anion. Chem. Phys. Lett. 2021, 777, 138702. [Google Scholar] [CrossRef]
- Harmon, K.M.; Madeura, S.L.; Jacks, M.J.; Avci, G.F.; Thiel, A.C. Part 18. The Nature of the CHF Hydrogen Bond in Choline Fluoride. J. Mol. Struct. 1885, 128, 305–314. [Google Scholar] [CrossRef]
- Alfurayj, I.; Prado, D.M.; Prado, R.C.; Samia, A.C.; Burda, C. Unusual Hydration Properties of Choline Fluoride-Based Deep Eutectic Solvents. J. Phys. Chem. B 2024, 128, 2762–2772. [Google Scholar] [CrossRef]
- Gaur, A.; Avula, N.V.S.; Balasubramanian, S. Insights into the Stabilization of Fluoride Ions in Ionic Liquids: Pointers to Better Fluorinating Agents. J. Phys. Chem. B 2020, 124, 8844–8856. [Google Scholar] [CrossRef]
- Das, L.; Mukherjee, S.; Kumar Maity, D.; Adhikari, S. Synthesis and Characterization of a Computationally Predicted Redox and Radiation Stable Deep Eutectic Solvent. J. Mol. Liq. 2022, 360, 119377. [Google Scholar] [CrossRef]
- van den Bruinhorst, A.; Kollau, L.J.B.M.; Vis, M.; Hendrix, M.M.R.M.; Meuldijk, J.; Tuinier, R.; Esteves, A.C.C. From a eutectic mixture to a deep eutectic system via anion selection: Glutaric acid + tetraethylammonium halides. J. Chem. Phys. 2021, 155, 014502. [Google Scholar] [CrossRef]
- Agieienko, V.; Neklyudov, V.; Buchner, R. Why Does Ethaline Apparently Behave as an Ideal Binary Mixture? J. Phys. Chem. Lett. 2022, 13, 10805–10809. [Google Scholar] [CrossRef]
- Kadhim, M.J.; Gamaj, M.I. Estimation of the Diffusion Coefficient and Hydrodynamic Radius (Stokes Radius) for Inorganic Ions in Solution Depending on Molar Conductivity as Electro-Analytical Technique—A Review. J. Chem. Rev. 2020, 2, 182–188. [Google Scholar]
- Curnow, O.J.; MacFarlene, D.R.; Walst, K.J. Fluoride Ionic Liquids in Salts of Ethylmethylimidazolium and Substituted Cyclopropenium Cation Families. Front. Chem. 2018, 6, 603. [Google Scholar] [CrossRef]
- Radomski, R.; Radomska, M. Determination of Solidus and Liquidus Temperatures by Means of a Perkin-Elmer 1B Differential Scanning Calorimeter. J. Therm. Anal. 1982, 24, 101–109. [Google Scholar] [CrossRef]
- Crichton, S.N.; Moynihan, C.T. Dependence of the Glass Transition Temperature on Heating Rate. J. Non-Cryst. Solids 1988, 99, 413–417. [Google Scholar] [CrossRef]
- Kremer, F.; Schönhals, A. Broadband Dielectric Spectroscopy; Springer Science & Business Media: Berlin, Germany, 2002. [Google Scholar]
- Reid, P.J.; Alex, S.; Jarzeba, W.; Schlief, R.E.; Johnson, A.E.; Barbara, P.F. Evidence for Intermolecular Hydrogen-Bond Rearrangement in the Electron Transfer Dynamics of Betaine-30 in n-Butanol. Chem. Phys. Lett. 1994, 229, 93–100. [Google Scholar] [CrossRef]
- Åkesson, E.; Walker, G.C.; Barbara, P.F. Dynamic Solvent Effects on Electron Transfer Rates in the Inverted Regime: Ultrafast Studies on the Betaines. J. Chem. Phys. 1991, 95, 4188–4194. [Google Scholar] [CrossRef]
- Stejskal, E.O.; Tanner, J.E. Spin Diffusion Measurements: Spin Echoes in the Presence of a Time-Dependent Field Gradient. J. Chem. Phys. 1965, 42, 288–292. [Google Scholar] [CrossRef]





| System | Self-Diffusion Coefficient (×10−11 m2 s−1) | ||
|---|---|---|---|
| Ch+ | EG | 19F | |
| ChF:EG (10 mol %) | 4.87 | 7.71 | 3.90 (downfield peak); 7.75 (upfield peak) |
| ChCl:EG (10 mol %) | 5.77 | 8.59 | - |
| ChBr:EG (10 mol %) | 5.86 | 9.07 | - |
| ChI:EG (10 mol %) | 6.15 | 9.42 | - |
| ChCl:EG (20 mol %) | 4.39 | 7.14 | - |
| ChBr:EG (20 mol %) | 4.58 | 6.04 | - |
| ChF:EG (33.33 mol %) [22] | 3.09 | 4.64 | 2.20 (downfield peak); 5.29 (upfield peak) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pandian, R.; Hansen, B.B.; de Araujo Lima e Souza, G.; Sangoro, J.R.; Greenbaum, S.; Burda, C. Tuning Solvation Dynamics of Electrolytes at Their Eutectic Point Through Halide Identity. Molecules 2025, 30, 2113. https://doi.org/10.3390/molecules30102113
Pandian R, Hansen BB, de Araujo Lima e Souza G, Sangoro JR, Greenbaum S, Burda C. Tuning Solvation Dynamics of Electrolytes at Their Eutectic Point Through Halide Identity. Molecules. 2025; 30(10):2113. https://doi.org/10.3390/molecules30102113
Chicago/Turabian StylePandian, Rathiesh, Benworth B. Hansen, Giselle de Araujo Lima e Souza, Joshua R. Sangoro, Steven Greenbaum, and Clemens Burda. 2025. "Tuning Solvation Dynamics of Electrolytes at Their Eutectic Point Through Halide Identity" Molecules 30, no. 10: 2113. https://doi.org/10.3390/molecules30102113
APA StylePandian, R., Hansen, B. B., de Araujo Lima e Souza, G., Sangoro, J. R., Greenbaum, S., & Burda, C. (2025). Tuning Solvation Dynamics of Electrolytes at Their Eutectic Point Through Halide Identity. Molecules, 30(10), 2113. https://doi.org/10.3390/molecules30102113








