Polysaccharide Composition of Dietary Fiber During Raspberry and Blackberry Juice Production
Abstract
1. Introduction
2. Results and Discussion
2.1. Alcohol-Insoluble Solids Composition
2.2. Water-Binding Capacity, Swelling, and Oil-Holding Capacity
2.3. Sequential Polysaccharide Extraction
3. Materials and Methods
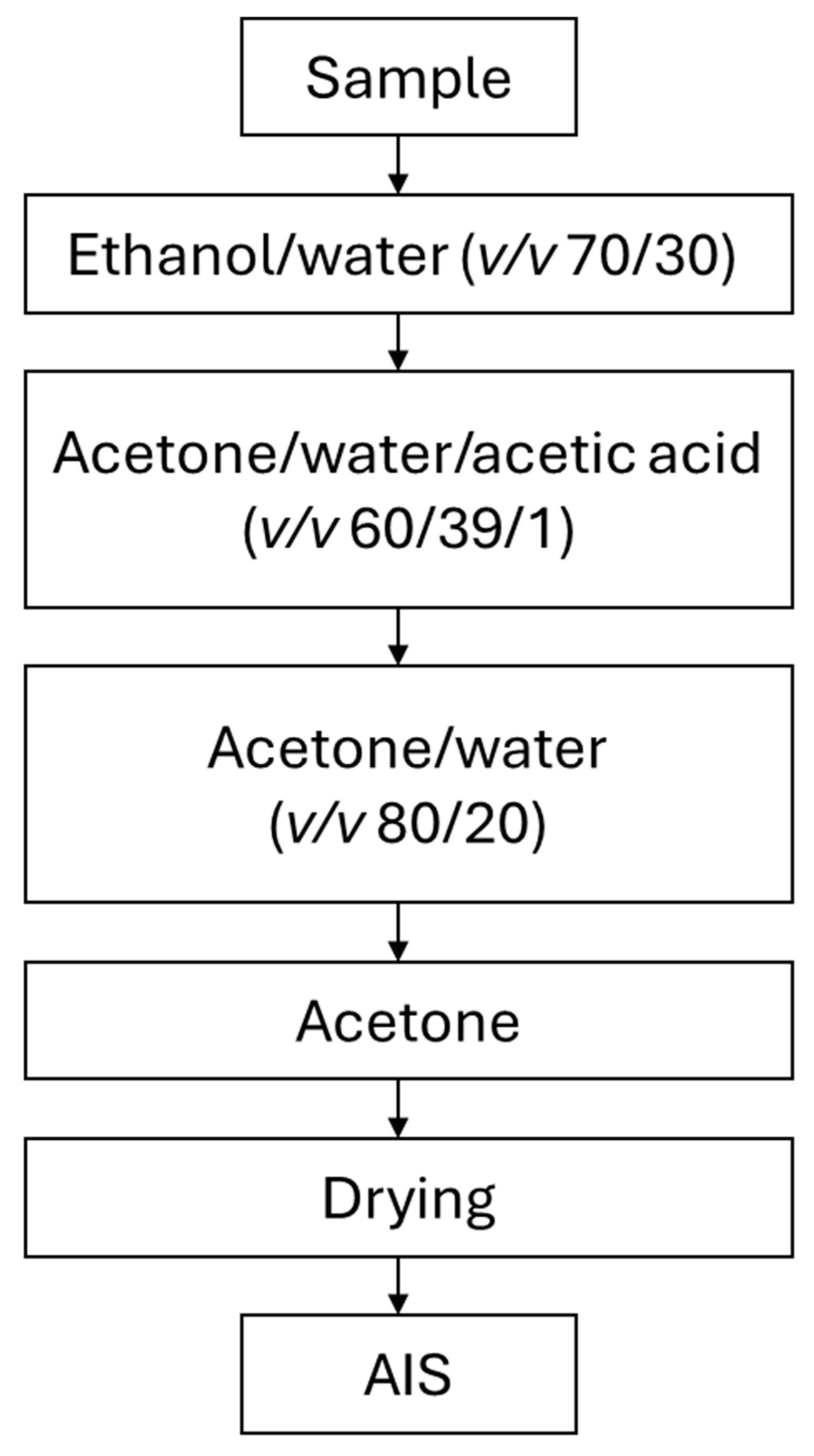
3.1. Plant Material and Method of Preparing the Material for Further Analysis
3.2. Alcohol-Insoluble Solid (AIS) and Sequential Polysaccharide Extraction
3.3. Cell Wall Hydrolysis
3.4. Pectin Hydrolysis
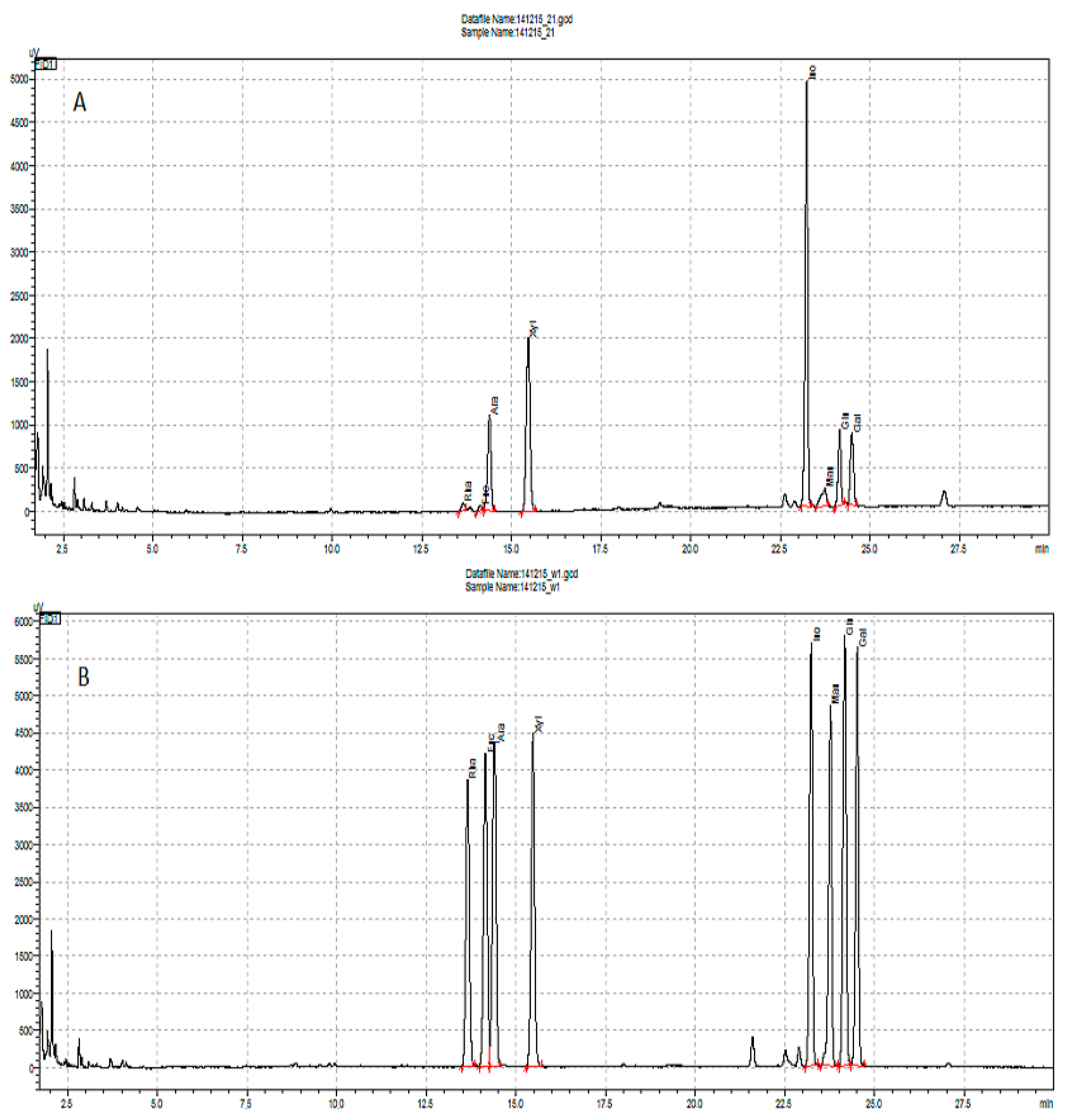
3.5. Derivatization and Gas Chromatography
3.6. Galacturonic Acid
3.7. Water-Binding Capacity
3.8. Swelling
3.9. Oil-Holding Capacity
3.10. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Ara | Arabinose |
| CASP | Concentrated alkali soluble polysaccharides |
| CDTA | Trans-1,2-diaminocyclohexane-N,N,N′,N′-tetraacetic acid |
| ChSP | Chelating agent soluble pectin |
| DASP | Diluted alkali-soluble pectin |
| Fuc | Fucose |
| Gal | Galactose |
| GalA | Galacturonic acid |
| Glc | Glucose |
| Man | Mannose |
| MHDP | m-hydroxydiphenyl |
| OHC | Oil-holding capacity |
| Rha | Rhamnose |
| WBC | Water-binding capacity |
| WR | Water residue |
| WSP | Water soluble pectin |
| Xyl | Xylose |
References
- Kotuła, M.; Kapusta-Duch, J.; Smoleń, S.; Doskocil, I. Phytochemical composition of the fruits and leaves of raspberries (Rubus idaeus L.)—Conventional vs. organic and those wild grown. Appl. Sci. 2022, 12, 11783. [Google Scholar] [CrossRef]
- Jiang, H.; Zhang, W.; Li, X.; Xu, Y.; Cao, J.; Jiang, W. The anti-obesogenic effects of dietary berry fruits: A review. Food Res. Int. 2021, 147, 110539. [Google Scholar] [CrossRef] [PubMed]
- Krivokapić, S.; Vlaović, M.; Damjanović, V.B.; Perović, A.; Perović, S. Biowaste as a potential source of bioactive compounds—A case study of raspberry fruit pomace. Foods 2021, 10, 706. [Google Scholar] [CrossRef]
- Sójka, M.; Macierzyński, J.; Zaweracz, W.; Buczek, M. Transfer and mass balance of ellagitannins, anthocyanins, flavan-3- ols, and flavonols during the processing of red raspberries (Rubus idaeus L.) to juice. J. Agric. Food Chem. 2016, 64, 5549–5563. [Google Scholar] [CrossRef] [PubMed]
- Cechoviciene, I.; Šlepetiene, A.; Gumbyte, M.; Paulauskiene, A.; Taraseviciene, Ž. Composition and physicochemical properties of pomace of various cultivars of blackberry (Rubus fruticosus L.). Horticulturae 2024, 10, 38. [Google Scholar] [CrossRef]
- Álvarez, E.E.; Sánchez, P.G. La fibra dietética. Nutr. Hosp. 2006, 21, 61–72. [Google Scholar]
- Rivas, M.Á.; Casquete, R.; Córdoba, M.D.G.; Ruíz-Moyano, S.; Benito, M.J.; Pérez-Nevado, F.; Martín, A. Chemical composition and functional properties of dietary fibre concentrates from winemaking by-products: Skins, stems and lees. Foods 2021, 10, 1510. [Google Scholar] [CrossRef]
- Tana, C.; Wei, H.; Zhao, X.; Xub, C.; Peng, J. Effects of dietary fibers with high water-binding capacity and swelling capacity on gastrointestinal functions, food intake and body weight in male rats. Food Nutr. Res. 2017, 61, 1308118. [Google Scholar] [CrossRef]
- Gorlov, I.F.; Giro, T.M.; Pryanishnikov, V.V.; Slozhenkina, M.I.; Randelin, A.V.; Mosolova, N.I.; Zlobina, E.Y.; Kulikovskiy, A.V. Using the fiber preparations in meat processing. Mod. Appl. Sci. 2015, 9, 54–64. [Google Scholar] [CrossRef]
- Milala, J.; Grzelak- Błaszczyk, K.; Sójka, M.; Kosmala, M.; Dobrzyńska-Inger, A.; Rój, E. Changes of bioactive components in berry seed oils during supercritical CO2 extraction. J. Food Process. Preserv. 2018, 42, e13368. [Google Scholar] [CrossRef]
- Fotschki, B.; Juśkiewicz, J.; Jurgoński, A.; Rigby, N.; Sójka, M.; Kołodziejczyk, K.; Mackie, A.; Zduńczyk, Z. Raspberry pomace alters cecal microbial activity and reduces secondary bile acids in rats fed a high-fat diet. J. Nutr. Biochem. 2017, 46, 13–20. [Google Scholar] [CrossRef]
- Demigné, C.; Morand, C.; Levrat, M.A.; Besson, C.; Moundras, C.; Rémésy, C. Effect of propionate on fatty acid and cholesterol synthesis and on acetate metabolism in isolated rat hepatocytes. Br. J. Nutr. 1995, 74, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Kosmala, M.; Jurgoński, A.; Juśkiewicz, J.; Karlińska, E.; Macierzyński, J.; Rój, E.; Zduńczyk, Z. Chemical composition of blackberry press cake, polyphenolic extract, and defatted seeds, and their effects on cecal fermentation, bacterial metabolites, and blood lipid profile in rats. J. Agric. Food Chem. 2017, 65, 5470–5479. [Google Scholar] [CrossRef]
- Cui, G.; Zhang, W.; Wang, Q.; Zhang, A.; Mu, H.; Bai, H.; Duan, J. Extraction optimization, characterization and immunity activity of polysaccharides from Fructus Jujubae. Carbohydr. Polym. 2014, 111, 245–255. [Google Scholar] [CrossRef]
- Dou, J.; Meng, Y.H.; Liu, L.; Li, J.; Ren, D.Y.; Guo, Y.R. Purification, characterization and antioxidant activities of polysaccharides from thinned-young apple. Int. J. Biol. Macromol. 2015, 72, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Liu, L.; Xu, Y.; Wang, L.; Tenga, X.; Li, X.; Dai, J. Characterization and biological activities of a novel polysaccharide isolated from raspberry (Rubus idaeus L.) fruits. Carbohydr. Polym. 2015, 132, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Yang, Y.; Hong, E.-K.; Yin, X.; Wang, X.; Wang, Y.; Zhang, D. Analyzing the structure-activity relationship of raspberry polysaccharides using interpretable artificial neural network model. Int. J. Biol. Macromol. 2024, 264, 130354. [Google Scholar] [CrossRef]
- Yang, Y.; Yin, X.; Zhang, D.; Lu, J.; Wang, X. Isolation, structural characterization and macrophage activation activity of an acidic polysaccharide from raspberry pulp. Molecules 2022, 27, 1674. [Google Scholar] [CrossRef]
- Su, J.-P.; Fang, J.-Q.; Wang, P.-P.; Liu, C.; Peng, Y.-P.; Liu, S.-P.; Fu, X. Advances in structure-hypoglycemic activity relationship and mechanisms of berry polysaccharides. Food Biosci. 2024, 62, 105472. [Google Scholar] [CrossRef]
- Li, G.; He, Y.; Liew, A.; Huang, C.; Song, B.; Jia, X.; Sathuvan, M.; Zhong, S.; Cheong, K.-L. Dietary polysaccharides from dragon fruit pomace, a co-product of the fruit processing industry, exhibit therapeutic potential in high-fat diet-induced metabolic disorders. Food Res. Int. 2025, 203, 115818. [Google Scholar] [CrossRef]
- Chen, F.; Ran, L.; Mi, J.; Yan, Y.; Lu, L.; Jin, B.; Li, X.; Cao, Y. Isolation, Characterization and antitumor effect on DU145 cells of a main polysaccharide in pollen of Chinese wolfberry. Molecules 2018, 23, 2430. [Google Scholar] [CrossRef] [PubMed]
- Khan, T.; Date, A.; Chawda, K.; Patel, K. Polysaccharides as potential anticancer agents—A review of their progress. Carbohydr. Polym. 2019, 210, 412–428. [Google Scholar] [CrossRef] [PubMed]
- Teng, H.; He, Y.; Fu, Y.; Xiong, H.; Lu, M.; Zhang, C.; Ai, C.; Cao, H.; Zhong, S.; Chen, L. Effects of blackberry (Rubus spp.) polysaccharide on the structure and thermal behavior of the myofibrillar protein of chicken breast meat. Food Chem. X 2023, 20, 100914. [Google Scholar] [CrossRef]
- Rivas, M.A.; Casquete, R.; de Guía Córdoba, M.; Benito, M.J.; Hernandez, A.; Ruiz Moyano, S.; Martin, A. Functional properties of extracts and residual dietary fibre from pomegranate (Punica granatum L.) peel obtained with different supercritical fluid conditions. LWT- Food Sci. Technol. 2021, 145, 111305. [Google Scholar] [CrossRef]
- Sójka, M.; Nowakowska, A.; Hejduk, A. Influence of enzymatic clarification, filtration, and pasteurization on ellagitannin and anthocyanin content in raspberry juices. Eur. Food Res. Technol. 2024, 250, 351–359. [Google Scholar] [CrossRef]
- Renard, C.M.G.C.; Ginies, C. Comparison of the cell wall composition for flesh and skin from five different plums. Food Chem. 2009, 114, 1042–1049. [Google Scholar] [CrossRef]
- He, Y.; Zhang, C.; Zheng, Y.; Xiong, H.; Ai, C.; Cao, H.; Xiao, J.; El-Seedi, H.; Chen, L.; Teng, H. Effects of blackberry polysaccharide on the quality improvement of boiled chicken breast. Food Chem. X 2023, 18, 100623. [Google Scholar] [CrossRef]
- Elleuch, M.; Bedigian, D.; Roiseux, O.; Besbes, S.; Blecker, C.; Attia, H. Dietary fibre and fibre-rich by-products of food processing: Characterisation, technological functionality and commercial applications: A review. Food Chem. 2011, 124, 411–421. [Google Scholar] [CrossRef]
- Kosmala, M.; Milala, J.; Kołodziejczyk, K.; Markowski, J.; Mieszczakowska, M.; Ginies, C.; Renard, C.M.G.C. Characterization of cell wall polysaccharides of cherry (Prunus cerasus var. Schattenmorelle) fruit and pomace. Plant Foods Hum. Nutr. 2009, 64, 279–285. [Google Scholar] [CrossRef]
- Hotchkiss, A.T.; Chau, H.K.; Strahan, G.D.; Nunez, A.; Harron, A.; Simon, S.; White, A.K.; Dieng, S.; Heuberger, E.R.; Black, I.; et al. Structural characterization of strawberry pomace. Heliyon 2024, 10, e29787. [Google Scholar] [CrossRef]
- Kosmala, M.; Kołodziejczyk, K.; Markowski, J.; Mieszczakowska, M.; Ginies, C.; Renard, C.M.G.C. Co-products of black-currant and apple juice production: Hydration properties and polysaccharide composition. LWT-Food Sci. Technol. 2010, 43, 173–180. [Google Scholar] [CrossRef]
- Renard, C.M.G.C. Variability in cell wall preparations: Quantification and comparison of common methods. Carbohydr. Polym. 2005, 60, 515–522. [Google Scholar] [CrossRef]

| Yield of AIS [mg/g] | Rha [mg/g] | Fuc [mg/g] | Ara [mg/g] | Xyl [mg/g] | Man [mg/g] | Gal [mg/g] | Noncell Glc [mg/g] | Cell Glc [mg/g] | GalA [mg/g] | |
|---|---|---|---|---|---|---|---|---|---|---|
| Raspberry | ||||||||||
| Fruit | 394 ± 4 f | 1 ± 0 c | 1 ± 0 cd | 16 ± 5 c | 39 ± 9 c | 6 ± 1 b | 12 ± 4 d | 12 ± 0 c | 64 ± 6 b | 305 ± 21 b |
| Pomace total | 812 ± 10 a | 1 ± 0 c | 1 ± 0 cd | 9 ± 2 e | 52 ± 10 ab | 6 ± 3 b | 9 ± 1 e | 5 ± 2 d | 66 ± 13 b | 219 ± 52 c |
| Pomace seedless fraction | 717 ± 1 d | 1 ± 0 d | 2 ± 1 b | 11 ± 2 de | 35 ± 10 c | 8 ± 5 ab | 18 ± 3 cd | 12 ± 2 c | 90 ± 29 ab | 177 ± 32 e |
| Pomace seed fraction | 775 ± 11 ab | 1 ± 0 cd | 1 ± 0 cd | 9 ± 2 e | 54 ± 19 ab | 4 ± 1 c | 7 ± 2 e | 3 ± 0 d | 49 ± 3 b | 172 ± 14 e |
| Juice | 38 ± 0 h | 9 ± 1 a | ND | 34 ± 2 b | 15 ± 1 e | 7 ± 2 b | 41 ± 2 a | 20 ± 8 b | ND | 278 ± 16 bc |
| Blackberry | ||||||||||
| Fruit | 333 ± 11 fg | 1 ± 0 c | 1 ± 0 cd | 20 ± 3 c | 46 ± 14 ab | 5 ± 1 bc | 10 ± 1 de | 3 ± 0 d | 59 ± 17 b | 247 ± 20 bc |
| Pomace total | 731 ± 24 cd | 1 ± 0 cd | 1 ± 0 c | 15 ± 3 c | 47 ± 9 ab | 5 ± 2 bc | 9 ± 2 e | 4 ± 1 d | 72 ± 8 ab | 176 ± 22 e |
| Pomace seedless fraction | 631 ± 7 e | 1 ± 0 cd | 3 ± 0 a | 31 ± 4 b | 25 ± 9 d | 8 ± 5 ab | 16 ± 5 cd | 12 ± 7 c | 102 ± 12 a | 236 ± 30 bc |
| Pomace seed fraction | 766 ± 2 bc | 1 ± 0 c | ND | 8 ± 2 e | 58 ± 18 a | 4 ± 2 c | 5 ± 2 e | 2 ± 0 d | 62 ± 6 b | 184 ± 48 e |
| Juice | 49 ± 0 h | 6 ± 0 b | ND | 67 ± 2 a | 3 ± 1 f | 10 ± 2 ab | 27 ± 2 b | 47 ± 9 a | ND | 363 ± 59 a |
| p | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.001 | 0.000 | 0.000 | 0.000 | 0.000 |
| Water-Binding Capacity [g Water/g] | Swelling [ml/g] | Oil-Holding Capacity [g Oil/g] | |
|---|---|---|---|
| Raspberry | |||
| Fruit | 6.5 ± 0.9 c | 4.8 ± 0.2 d | 16.5 ± 0.3 a |
| Pomace total | 8.5 ± 0.2 b | 3.7 ± 0.0 ef | 8.8 ± 0.1 ef |
| Pomace seedless fraction | 14.9 ± 0.4 a | 11.1 ± 0.1 a | 11.7 ± 0.1 c |
| Pomace seed fraction | 4.2 ± 1.1 d | 3.8 ± 0.2 e | 8.0 ± 0.0 g |
| Blackberry | |||
| Fruit | 6.7 ± 0.9 bc | 5.6 ± 0.2 c | 12.2 ± 0.0 b |
| Pomace total | 4.5 ± 1.0 d | 3.8 ± 0.0 e | 8.5 ± 0.1 f |
| Pomace seedless fraction | 13.0 ± 1.7 a | 8.5 ± 0.3 b | 9.5 ± 0.1 d |
| Pomace seed fraction | 2.7 ± 0.3 d | 3.3 ± 0.1 f | 9.0 ± 0.0 e |
| p | 0.000 | 0.000 | 0.000 |
| Yield [mg/g] | Rha [mg/g] | Fuc [mg/g] | Ara [mg/g] | Xyl [mg/g] | Man [mg/g] | Gal [mg/g] | Glc [mg/g] | GalA [mg/g] | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Raspberry fruit | WSP | 74 | 2 ± 0 c | 1 ± 0 d | 47 ± 5 a | 24 ± 2 h | 15 ± 1 c | 25 ± 2 cd | 6 ± 2 fc | 436 ± 38 a |
| ChSP | 220 | 1 ± 0 d | ND | 10 ± 3 f | 5 ± 2 i | 6 ± 5 d | 3 ± 1 h | 2 ± 4 gf | 177 ± 0 ef | |
| DASP | 224 | 1 ± 0 d | ND | 18 ± 5 d | 6 ± 0 i | 2 ± 1 f | 6 ± 1 h | 3 ± 0 gg | 111 ± 24 fgh | |
| CASP | 182 | 2 ± 0 c | 3 ± 0 c | 26 ± 1 d | 59 ± 4 f | 8 ± 0 d | 17 ± 1 e | 52 ± 3 ef | 7 ± 0 i | |
| WR | 58 | 2 ± 0 c | ND | 8 ± 0 f | 59 ± 7 f | 1 ± 1 g | 4 ± 0 h | 12 ± 2 f | 27 ± 2 i | |
| Residue | 304 | 1 ± 0 d | ND | 3 ± 0 g | 118 ± 2 b | 6 ± 1 d | 5 ± 0 h | 253 ± 3 c | 67 ± 5 ghi | |
| Raspberry pomace total | WSP | 57 | 1 ± 0 d | 1 ± 0 d | 20 ± 3 d | 34 ± 5 g | 8 ± 2 d | 18 ± 3 e | 16 ± 2 f | 317 ± 0 bc |
| ChSP | 49 | 1 ± 0 d | ND | 7 ± 1 f | 4 ± 0 i | 3 ± 3 f | 1 ± 1 i | 2 ± 1 g | 150 ± 23 f | |
| DASP | 83 | 1 ± 0 d | ND | 18 ± 1 d | 7 ± 1 i | 1 ± 1 g | 7 ± 1 h | 6 ± 0 f | 222 ± 115 de | |
| CASP | 168 | 2 ± 0 c | 5 ± 0 b | 31 ± 1 c | 88 ± 2 e | 22 ± 0 b | 28 ± 1 b | 64 ± 13 e | 68 ± 0 ghi | |
| WR | 56 | 2 ± 0 c | ND | 13 ± 2 e | 52 ± 4 f | 1 ± 0 g | 4 ± 1 h | 13 ± 0 f | 33 ± 2 hi | |
| Residue | 463 | 1 ± 0 d | ND | 6 ± 0 g | 104 ± 10 cd | 5 ± 1 e | 5 ± 0 h | 345 ± 25 b | 63 ± 6 ghi | |
| Raspberry pomace seedless | WSP | 64 | 1 ± 0 d | 2 ± 0 d | 18 ± 2 d | 40 ± 4 g | 8 ± 1 d | 23 ± 2 d | 18 ± 1 f | 252 ± 18 cd |
| ChSP | 70 | 1 ± 1 d | ND | 15 ± 4 d | 6 ± 2 i | 2 ± 2 f | 5 ± 2 h | 0 ± 1 g | 335 ± 150 b | |
| DASP | 99 | 1 ± 0 d | ND | 19 ± 2 d | 4 ± 0 i | 1 ± 0 g | 8 ± 0 g | 3 ± 0 g | 422 ± 20 a | |
| CASP | 243 | 1 ± 0 d | 7 ± 0 a | 16 ± 0 d | 106 ± 1 c | 38 ± 0 a | 41 ± 1 a | 103 ± 1 d | 65 ± 10 ghi | |
| WR | 39 | 3 ± 1 bc | 1 ± 0 d | 19 ± 6 d | 61 ± 3 f | 2 ± 1 f | 11 ± 4 f | 51 ± 8 e | 13 ± 7 i | |
| Residue | 223 | 1 ± 0 d | ND | 5 ± 0 g | 30 ± 5 gh | 5 ± 0 e | 4 ± 0 h | 605 ± 14 a | 43 ± 5 hi | |
| Raspberry pomace seed | WSP | 13 | 1 ± 0 d | 1 ± 0 d | 13 ± 0 e | 23 ± 3 h | 9 ± 1 d | 11 ± 1 f | 17 ± 1 f | 263 ± 10 cd |
| ChSP | 58 | 0 ± 1 d | 1 ± 0 d | 2 ± 1 g | 4 ± 2 i | 5 ± 1 e | 1 ± 1 i | ND | 122 ± 1 fg | |
| DASP | 69 | 1 ± 0 d | ND | 12 ± 3 e | 6 ± 1 i | 1 ± 1 g | 5 ± 1 h | 5 ± 1 f | 378 ± 0 ab | |
| CASP | 110 | 4 ± 0 b | 4 ± 0 c | 47 ± 2 a | 97 ± 1 de | 16 ± 1 c | 27 ± 1 bc | 48 ± 7 e | 52 ± 3 ghi | |
| WR | 30 | 6 ± 3 a | 0 ± 1 d | 37 ± 6 b | 145 ± 21 a | 3 ± 0 f | 11 ± 4 f | 9 ± 1 f | 78 ± 5 ghi | |
| Residue | 601 | 1 ± 0 d | ND | 3 ± 0 g | 111 ± 6 bc | 6 ± 1 d | 4 ± 0 h | 246 ± 10 c | 44 ± 10 ghi | |
| p | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| Yield [mg/g] | Rha [mg/g] | Fuc [mg/g] | Ara [mg/g] | Xyl [mg/g] | Man [mg/g] | Gal [mg/g] | Glc [mg/g] | GalA [mg/g] | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Blackberry fruit | WSP | 50 | 3 ± 1 cde | 1 ± 0 efgh | 67 ± 2 abcd | 10 ± 1 ijk | 5 ± 1 fg | 28 ± 2 bc | 18 ± 0 ef | 585 ± 50 c |
| ChSP | 82 | 1 ± 0 def | ND | 24 ± 8 hijk | 4 ± 1 k | ND | 6 ± 2 ghij | ND | 247 ± 39 efg | |
| DASP | 67 | 4 ± 0 cd | ND | 73 ± 10 abc | 5 ± 0 k | 2 ± 0 jk | 15 ± 3 de | 5 ± 1 f | 465 ± 27 cd | |
| CASP | 131 | 2 ± 0 def | 6 ± 0 e | 46 ± 1 efg | 73 ± 2 de | 18 ± 1 c | 26 ± 1 c | 73 ± 3 cde | 50 ± 12 hi | |
| WR | 44 | 8 ± 2 ab | ND | 44 ± 7 efg | 24 ± 3 i | 1 ± 1 jk | 12 ± 2 defg | 3 ± 0 f | 12 ± 0 i | |
| Residue | 502 | 1 ± 0 def | ND | 4 ± 0 kl | 107 ± 8 ab | 4 ± 0 fghij | 4 ± 1 ij | 266 ± 15 b | 64 ± 7 hi | |
| Blackberry pomace total | WSP | 23 | 1 ± 0 ef | 1 ± 0 e | 26 ± 0 ghij | 25 ± 0 ih | 9 ± 1 de | 15 ± 0 de | 23 ± 1 def | 355 ± 41 de |
| ChSP | 60 | 1 ± 0 ef | ND | 10 ± 2 jkl | 5 ± 1 k | 2 ± 1 ghijk | 2 ± 0 j | ND | 240 ± 78 efg | |
| DASP | 85 | 1 ± 1 def | ND | 12 ± 5 ijkl | 3 ± 1 k | 1 ± 0 jk | 4 ± 2 hij | 6 ± 2 f | 154 ± 5 fgh | |
| CASP | 114 | 3 ± 0 cd | 7 ± 0 b | 57 ± 1 bcde | 92 ± 1 bc | 25 ± 1 b | 33 ± 1 ab | 80 ± 14 cd | 45 ± 13 hi | |
| WR | 44 | 6 ± 2 b | ND | 32 ± 1 fghi | 21 ± 2 ij | 1 ± 0 jk | 17 ± 2 d | 15 ± 1 ef | 15 ± 3 i | |
| Residue | 577 | 1 ± 0 def | ND | 4 ± 0 kl | 115 ± 5 a | 4 ± 0 fghij | 4 ± 0 hij | 277 ± 3 b | 60 ± 3 hi | |
| Blackberry pomace seedless | WSP | 30 | 1 ± 0 def | 3 ± 0 d | 70 ± 12 abc | 49 ± 7 fg | 10 ± 3 e | 30 ± 7 bc | 14 ± 2 f | 793 ± 154 b |
| ChSP | 102 | 3 ± 1 cdef | ND | 76 ± 20 ab | 5 ± 2 jk | 2 ± 1 ghijk | 13 ± 4 def | 1 ± 0 f | 264 ± 2 ef | |
| DASP | 128 | 2 ± 1 def | ND | 48 ± 8 def | 3 ± 0 k | 2 ± 1 jk | 11 ± 2 defg | 7 ± 2 f | 927 ± 0 a | |
| CASP | 248 | 2 ± 0 def | 9 ± 0 a | 37 ± 1 efgh | 93 ± 5 bc | 40 ± 1 a | 38 ± 2 a | 110 ± 5 c | 42 ± 19 hi | |
| WR | 35 | 9 ± 1 a | 1 ± 0 efg | 81 ± 9 a | 23 ± 3 i | 2 ± 0 ijk | 32 ± 3 abc | 18 ± 2 ef | 11 ± 5 i | |
| Residue | 257 | 1 ± 0 def | ND | 13 ± 2 ijkl | 40 ± 19 gh | 6 ± 1 ef | 6 ± 1 fghij | 612 ± 87 a | 47 ± 4 hi | |
| Blackberry pomace seed | WSP | 28 | 1 ± 0 def | 1 ± 0 ef | 19 ± 2 hijkl | 17 ± 1 ijk | 12 ± 2 e | 9 ± 0 efghi | 26 ± 2 def | 241 ± 6 efg |
| ChSP | 65 | ND | ND | 3 ± 0 l | 5 ± 1 k | 1 ± 1 ijk | 1 ± 0 j | ND | 126 ± 6 ghi | |
| DASP | 60 | ND | ND | 3 ± 0 l | 3 ± 0 k | 1 ± 0 jk | 1 ± 0 j | 4 ± 1 f | 139 ± 63 fghi | |
| CASP | 49 | 4 ± 0 cd | 3 ± 0 d | 55 ± 1 cde | 62 ± 2 ef | 5 ± 0 fgh | 16 ± 0 d | 28 ± 7 def | 62 ± 4 hi | |
| WR | 25 | 5 ± 2 bc | ND | 30 ± 5 fghi | 81 ± 5 cd | 2 ± 0 ijk | 11 ± 2 defgh | 8 ± 1 f | 18 ± 13 i | |
| Residue | 503 | 1 ± 0 def | ND | 2 ± 0 l | 122 ± 3 a | 5 ± 0 fghi | 3 ± 0 ij | 263 ± 6 b | 45 ± 4 hi | |
| p | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kosmala, M.; Milala, J.; Karlińska, E. Polysaccharide Composition of Dietary Fiber During Raspberry and Blackberry Juice Production. Molecules 2025, 30, 2098. https://doi.org/10.3390/molecules30102098
Kosmala M, Milala J, Karlińska E. Polysaccharide Composition of Dietary Fiber During Raspberry and Blackberry Juice Production. Molecules. 2025; 30(10):2098. https://doi.org/10.3390/molecules30102098
Chicago/Turabian StyleKosmala, Monika, Joanna Milala, and Elżbieta Karlińska. 2025. "Polysaccharide Composition of Dietary Fiber During Raspberry and Blackberry Juice Production" Molecules 30, no. 10: 2098. https://doi.org/10.3390/molecules30102098
APA StyleKosmala, M., Milala, J., & Karlińska, E. (2025). Polysaccharide Composition of Dietary Fiber During Raspberry and Blackberry Juice Production. Molecules, 30(10), 2098. https://doi.org/10.3390/molecules30102098







