Abstract
Benzo[d]isothiazol-3(2H)-one and benzo[e][1,3]thiazin-4-one derivatives, as significant sulfur- and nitrogen-containing heterocyclic compounds, are prevalent in natural products, pharmaceuticals, and food items. In recent years, a variety of innovative synthetic methodologies for these compounds have been developed. In this review, we will comprehensively introduce the major advances in the synthesis of benzo[d]isothiazol-3(2H)-one and benzo[e][1,3]thiazin-4-one derivatives via both intramolecular and intermolecular pathways from 2012 to the present.
1. Introduction
Heterocyclic compounds are organic molecules that feature at least one ring structure composed of atoms from two or more different elements, typically carbon in combination with N, O, or S. These compounds play a fundamental role in both life processes and modern science, with applications extending across pharmaceuticals, biochemistry, agriculture, and materials science [1,2,3]. Benzo[d]isothiazol-3(2H)-one and benzo[e][1,3]thiazin-4-one derivatives, as important sulfur- and nitrogen-containing heterocyclic compounds with structural similarity, are extensively found in the fields of pharmaceutical chemistry, agriculture, and the food industry [4,5,6,7,8,9,10,11,12,13,14,15]. Consequently, the development of innovative and efficient methodologies for constructing these scaffolds has emerged as a focal point in these fields.
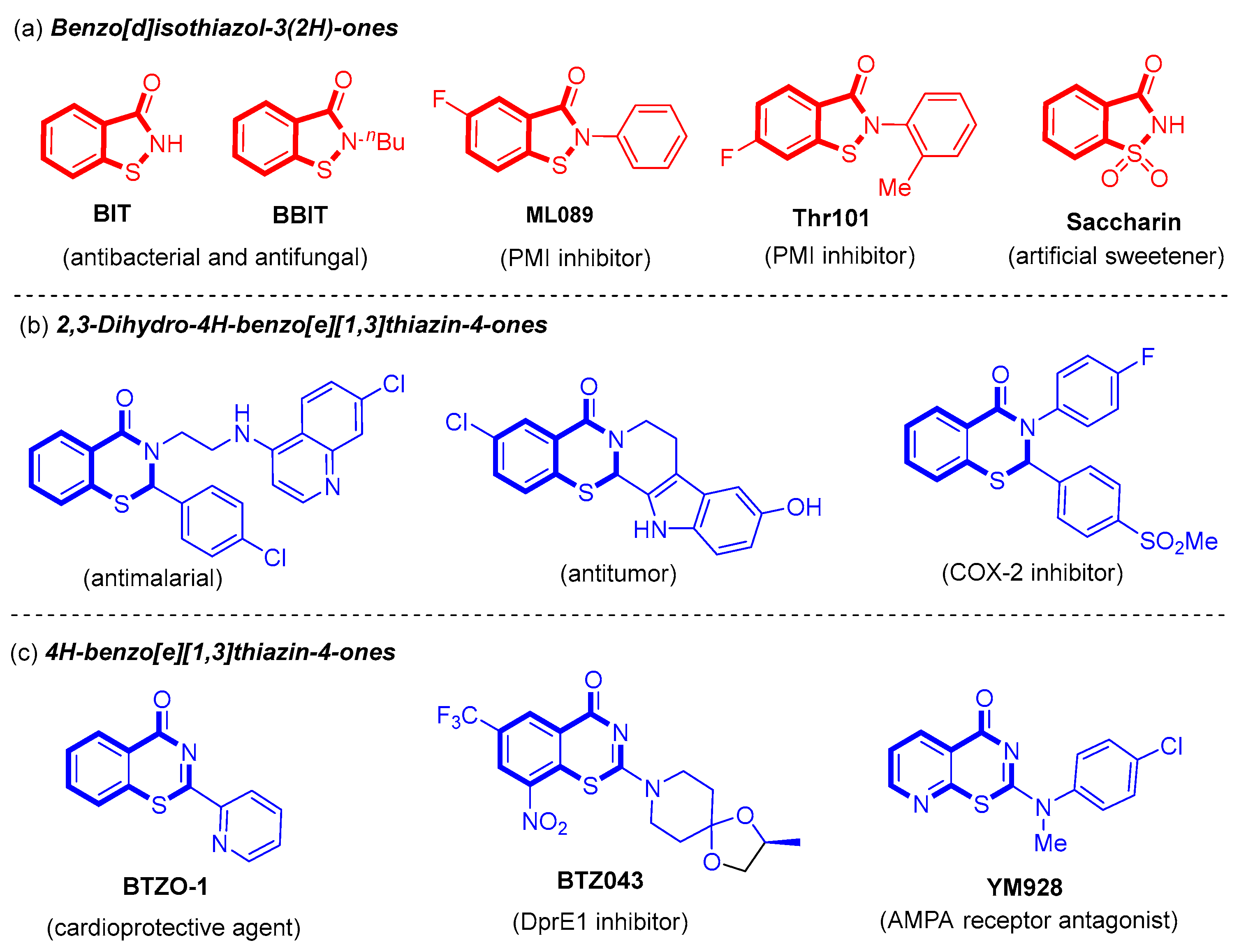
Benzo[d]isothiazol-3(2H)-one derivatives are benzo-fused five-membered N,S-heterocycles (Figure 1a), whereas benzo[e][1,3]thiazin-4-one derivatives are benzo-fused six-membered N,S-heterocycles (Figure 1b,c). The key structural difference lies in the presence of an additional carbon atom between the N and S atoms in benzo[e][1,3]thiazin-4-one derivatives. Benzo[d]isothiazol-3(2H)-one derivatives represent a widely used scaffold in medicinal and agricultural compounds, exhibiting a broad spectrum of biological activities such as antibacterial, antifungal, antineoplastic, and hypoglycemic properties (Figure 1a) [4,5,6,7,8,9]. For example, benzo[d]isothiazol-3(2H)-one (BIT) and 2-butylbenzo[d]isothiazol-3(2H)-one (BBIT) can serve as crucial industrial biocides or cosmetic preservatives with antibacterial and antifungal properties [4,5,6]. 5-Fluoro-2-phenylbenzo[d]isothiazol-3(2H)-one (ML089) and 6-fluoro-2-(o-tolyl)benzo[d]isothiazol-3(2H)-one (Thr101) are potent inhibitors of phosphomannose isomerase (PMI) [7,8]. These compounds have demonstrated efficacy in anti-tumor applications and blood glucose regulation within the human body. Furthermore, saccharin, an oxidized derivative of benzisothiazol-3(2H)-one, is one of the most extensively used artificial sweeteners [9].
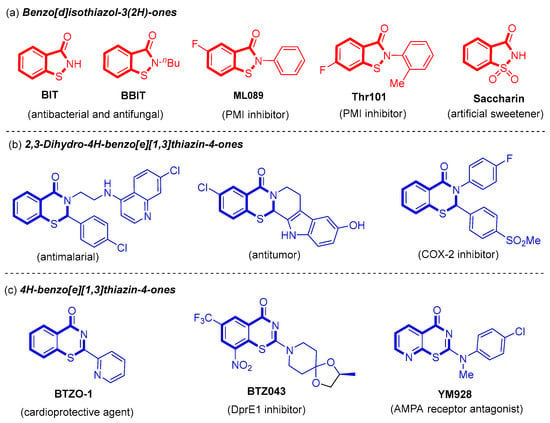
Figure 1.
Some selected important benzo[d]isothiazol-3(2H)-one and benzo[e][1,3]thiazin-4-one derivatives.
Benzo[e][1,3]thiazin-4-one derivatives, including 2,3-dihydro-4H-benzo[e][1,3]thiazin-4-ones (Figure 1b) and 4H-benzo[e][1,3]thiazin-4-ones (Figure 1c), have been identified in various natural products and pharmaceuticals [10,11,12,13,14,15]. Specifically, 2,3-dihydro-4H-benzo[e][1,3]thiazin-4-ones exhibit a diverse range of biological activities, including antimalarial, antitumor, and cyclooxygenase-2 (COX-2) inhibitory effects [10,11,12]. Moreover, 2-(pyridin-2-yl)-4H-benzo[e][1,3]thiazin-4-one (BTZO-1) has been recognized for its cardioprotective properties [13]. Additionally, 2-[(2S)-2-Methyl-1,4-dioxa-8-azaspiro [4.5]dec-8-yl]-8-nitro-6-(trifluoromethyl)-4H-1,3-benzothiazin-4-one (BTZ043) serves as an effective decaprenyl-phosphoribose-epimerase (DprE1) inhibitor that is crucial for tuberculosis treatment [14]. Furthermore, 2-[(4-chlorophenyl)(methyl)amino]-4H-pyrido [3,2-e][1,3]thiazin-4-one (YM928) is an orally active 2-amino-3-(3-hydroxy-5-methyl-4-isoxazolyl)propionic acid (AMPA) receptor antagonist for the treatment of various neurodegenerative diseases [15].
In recent years, various innovative methodologies for the synthesis of benzo[d]isothiazol-3(2H)-one and benzo[e][1,3]thiazin-4-one derivatives have been reported. Notably, in 2012, both the Xi group and the Punniyamurthy group independently pioneered a novel Cu(I)-catalyzed cascade reaction for the synthesis of benzo[d]isothiazol-3(2H)-ones [16,17]. Although considerable efforts have been made, only a few reviews and chapters have focused on the synthesis of these important skeletons [18,19,20,21,22,23,24]. In 2024, the Dehaen group summarized the recent advancements in the synthesis of benzo[d]isothiazol-3(2H)-ones [24]. In this review, we will introduce a comprehensive overview of the recent progress in the synthesis of benzo[d]isothiazol-3(2H)-one and benzo[e][1,3]thiazin-4-one derivatives via intramolecular and intermolecular pathways from 2012 to the present. Additionally, detailed discussions on the proposed mechanisms are included.
2. Synthesis of Benzo[d]isothiazol-3(2H)-Ones via Intramolecular and Intermolecular Pathways
2.1. Synthesis of Benzo[d]isothiazol-3(2H)-Ones via Intramolecular Pathways
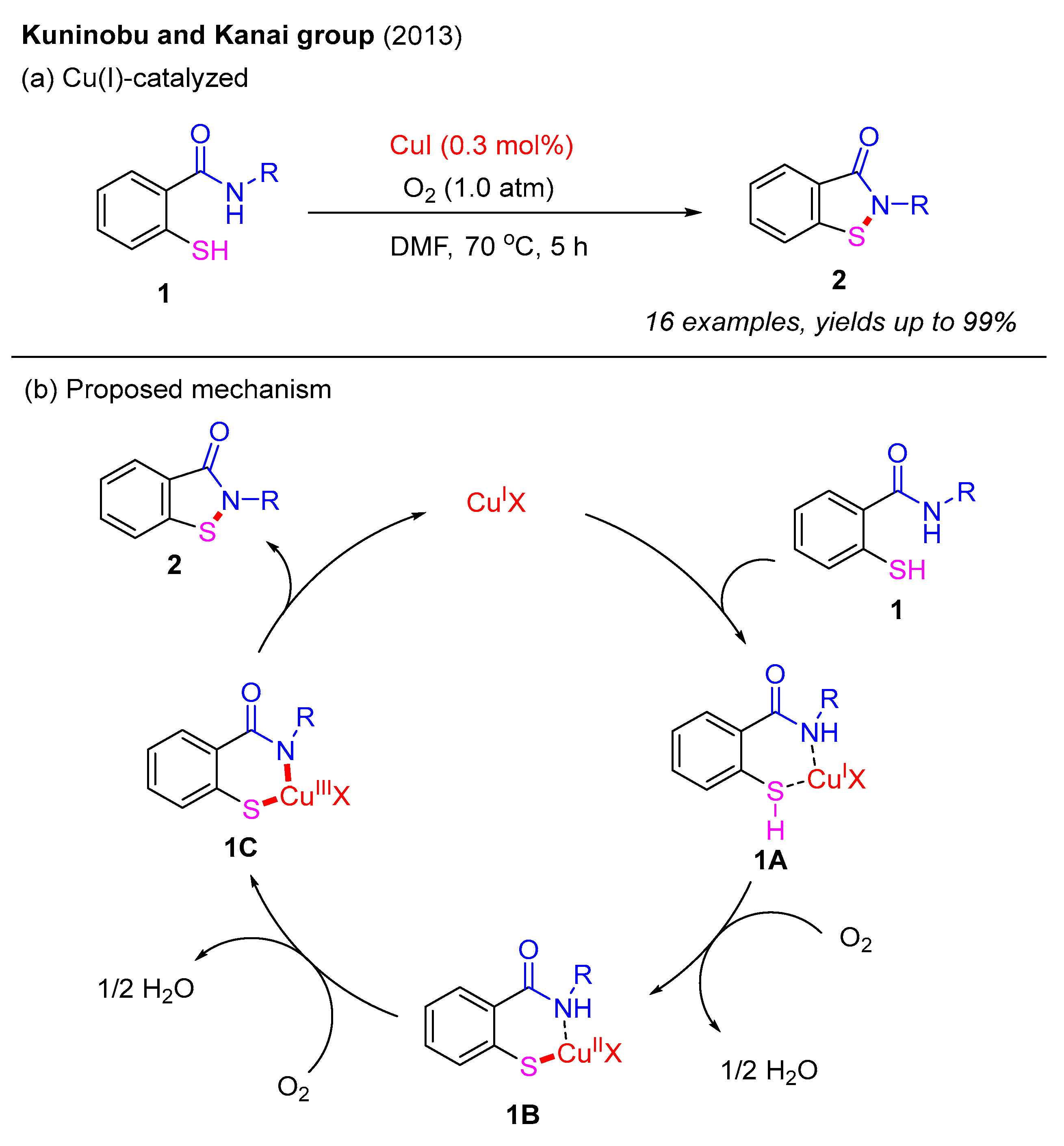
The intramolecular construction of benzo[d]isothiazol-3(2H)-ones frequently employs 2-mercaptobenzamides as the starting materials. In 2013, the Kuninobu and Kanai group developed a Cu(I)-catalyzed intramolecular N–S bond formation method to synthesize benzo[d]isothiazol-3(2H)-ones under an O2 atmosphere (Scheme 1a). In this reaction, 2-mercaptobenzamides undergo intramolecular oxidative dehydrogenative cyclization, providing various benzo[d]isothiazol-3(2H)-ones in excellent yields. This strategy employs O2 as the sole oxidant, facilitating the coupling of N–H and S–H bonds, which led to the formation of a novel N–S bond. Furthermore, corresponding disulfides do not yield benzo[d]isothiazol-3(2H)-ones, suggesting that this process does not proceed via disulfide formation. Based on these observations, a proposed Cu(I)-catalyzed mechanism is illustrated in Scheme 1b. Initially, the coordination of 2-mercaptobenzamide 1 with Cu(I) catalyst generates intermediate 1A, which is further oxidized by O2 to produce the Cu−S bond intermediate 1B. Next, the second oxidation step provides the intermediate 1C. Finally, reductive elimination of 1C affords benzo[d]isothiazol-3(2H)-one 2 and regenerates the Cu(I) catalyst [25].
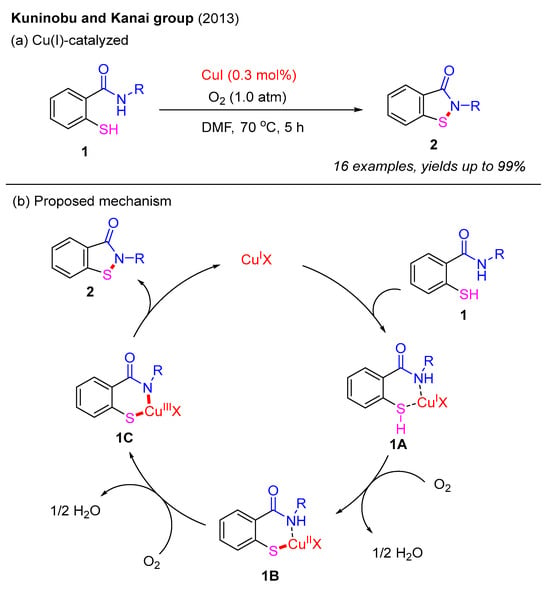
Scheme 1.
Cu(I)-catalyzed intramolecular N–S bond formation method to prepare benzo[d]isothiazol-3(2H)-ones under an O2 atmosphere [25].
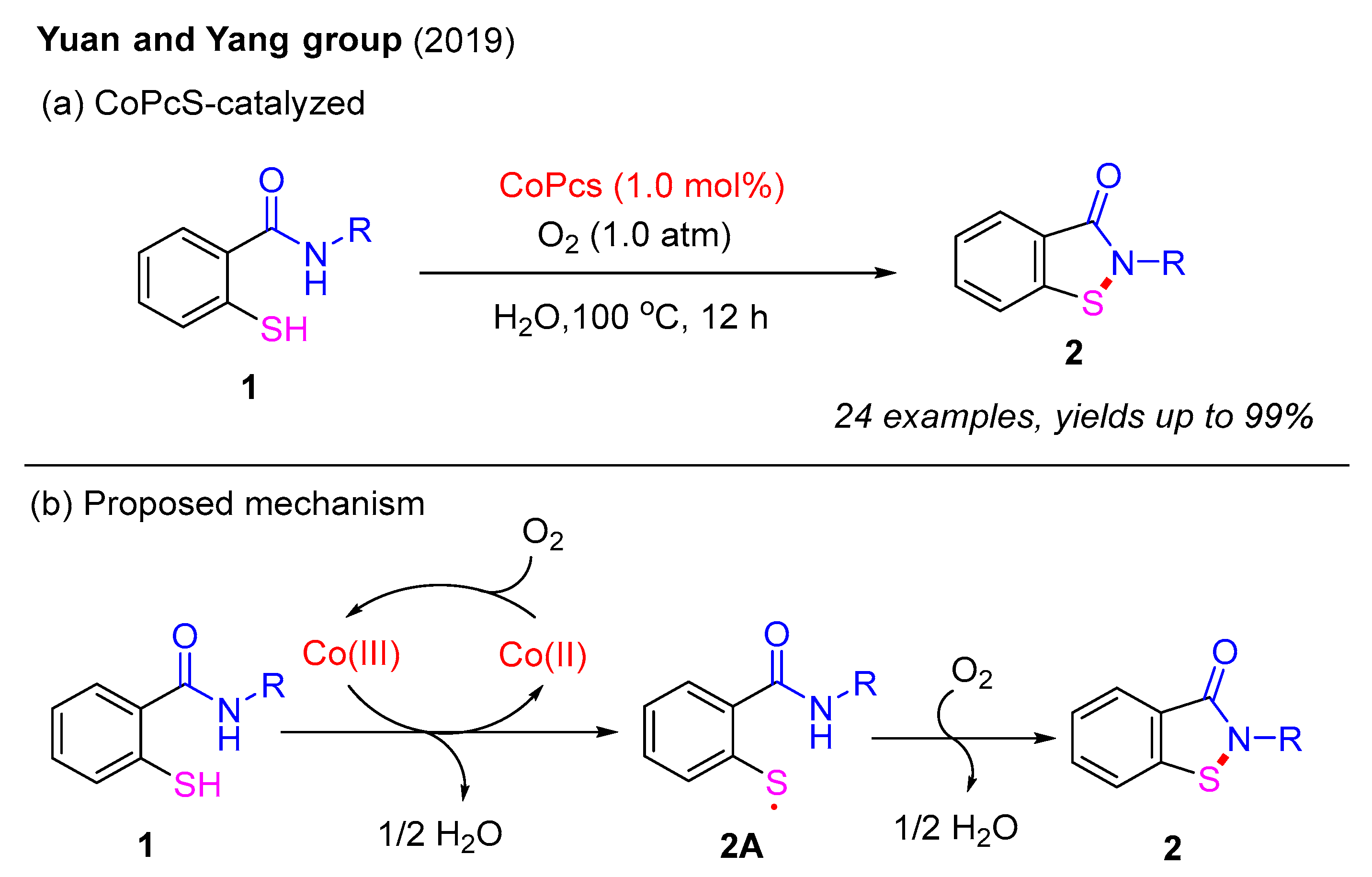
In 2019, the Yuan and Yang group employed a heterogeneous catalyst, tetra-substituted sulfonated cobalt phthalocyanine (CoPcS), to synthesize benzo[d]isothiazol-3(2H)-ones from 2-mercaptobenzamides under an O2 atmosphere in aqueous media (Scheme 2a). A wide range of benzo[d]isothiazol-3(2H)-ones were isolated in good to excellent yields. Notably, the use of H2O as the reaction solvent not only facilitates product purification but also enables the recycling of the mother liquor. A proposed mechanism is outlined in Scheme 2b. The initial oxidation of Co(II) by O2 forms the Co(III) catalyst, which oxidizes 2-mercaptobenzamide 1 to produce the thiyl radical intermediate 2A while regenerating the Co(II) complex. Subsequently, under an O2 atmosphere, intramolecular nucleophilic attack by the N–H bond on the sulfur atom in intermediate 2A leads to the formation of the final product 2 [26].
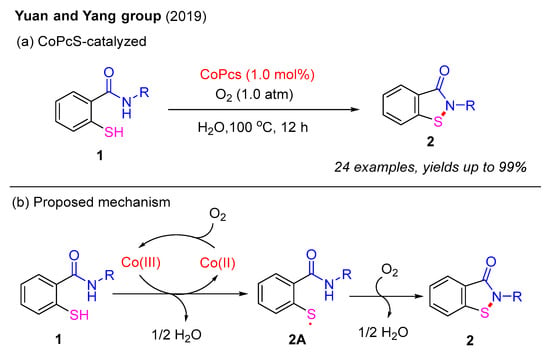
Scheme 2.
CoPcS-catalyzed intramolecular oxidative dehydrogenative cyclization for the synthesis of benzo[d]isothiazol-3(2H)-ones under an O2 atmosphere [26].
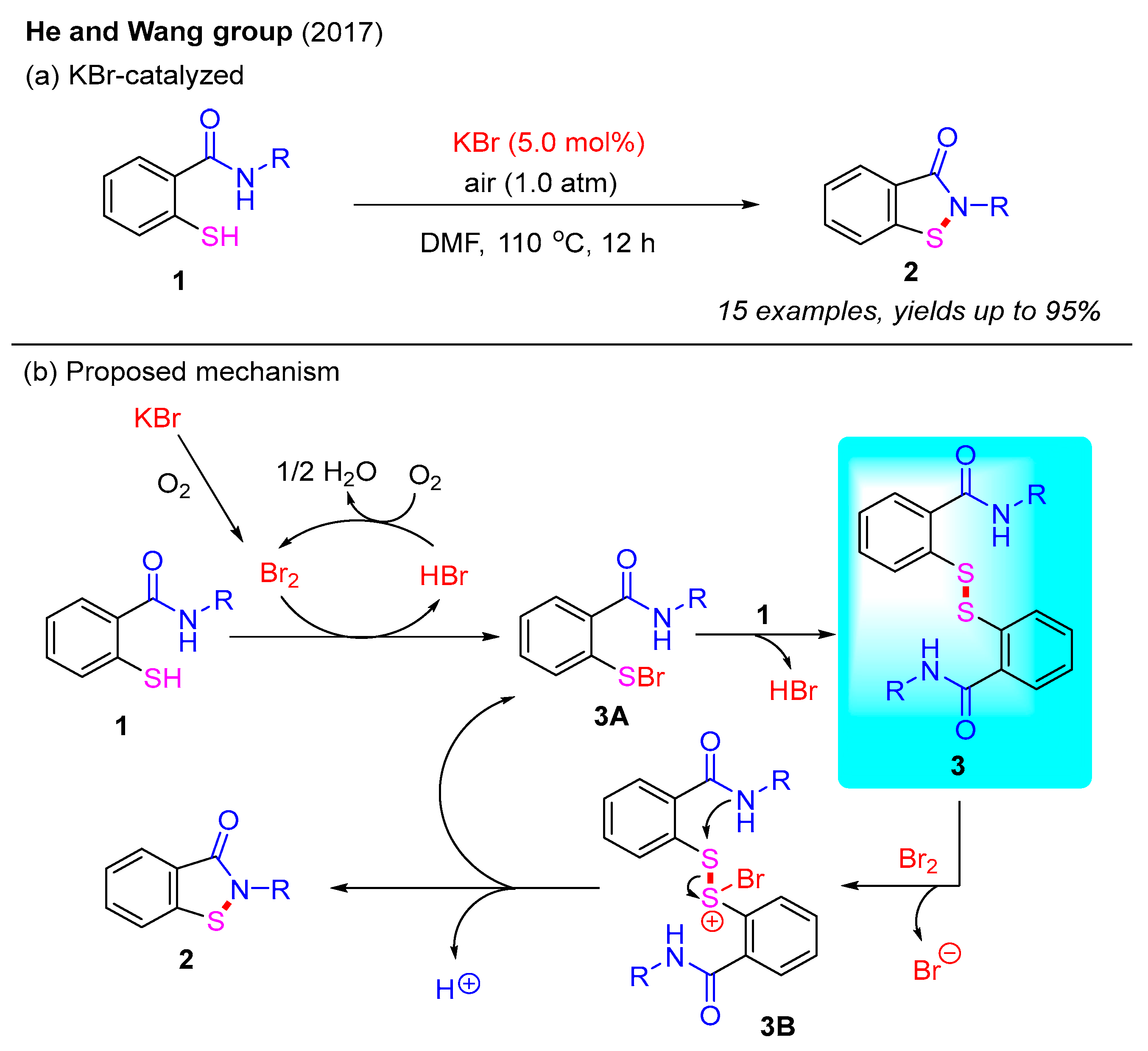
In addition to transition-metal catalysis strategies, metal-free approaches have garnered significant attention due to their green and sustainable properties. In 2017, the He and Wang group reported a KBr-catalyzed intramolecular oxidative dehydrogenative cyclization for constructing benzo[d]isothiazol-3(2H)-ones under an O2 atmosphere (Scheme 3a) [27]. This reaction successfully transformed 2-mercaptobenzamides into the desired benzo[d]isothiazol-3(2H)-ones in excellent yields. The structures of the products were confirmed using X-ray crystallography. Furthermore, mechanistic studies indicate that disulfides may be involved in this process. Based on these findings, a reaction mechanism is proposed in Scheme 3b. First, KBr is oxidized to Br2 under an O2 atmosphere. Next, 2-mercaptobenzamide 1 reacts with Br2 to form intermediate 3A and HBr. Notably, HBr can be re-oxidized by O2 to regenerate Br2, thereby maintaining the catalytic cycle. Meanwhile, the crucial intermediate 3A can react with 2-mercaptobenzamide to form disulfide intermediate 3 via the elimination of HBr. Next, disulfide intermediate 3 is activated by Br2 to generate intermediate 3B, which can further convert into the final product 2 and regenerate intermediate 3A [28].
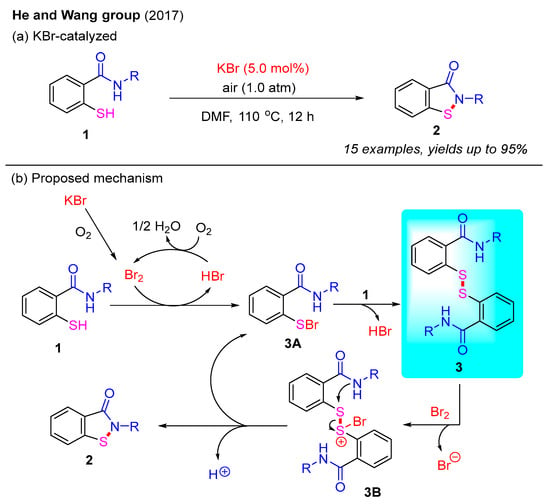
Scheme 3.
KBr-catalyzed intramolecular oxidative dehydrogenative cyclization for the synthesis of benzo[d]isothiazol-3(2H)-ones under an O2 atmosphere [27].
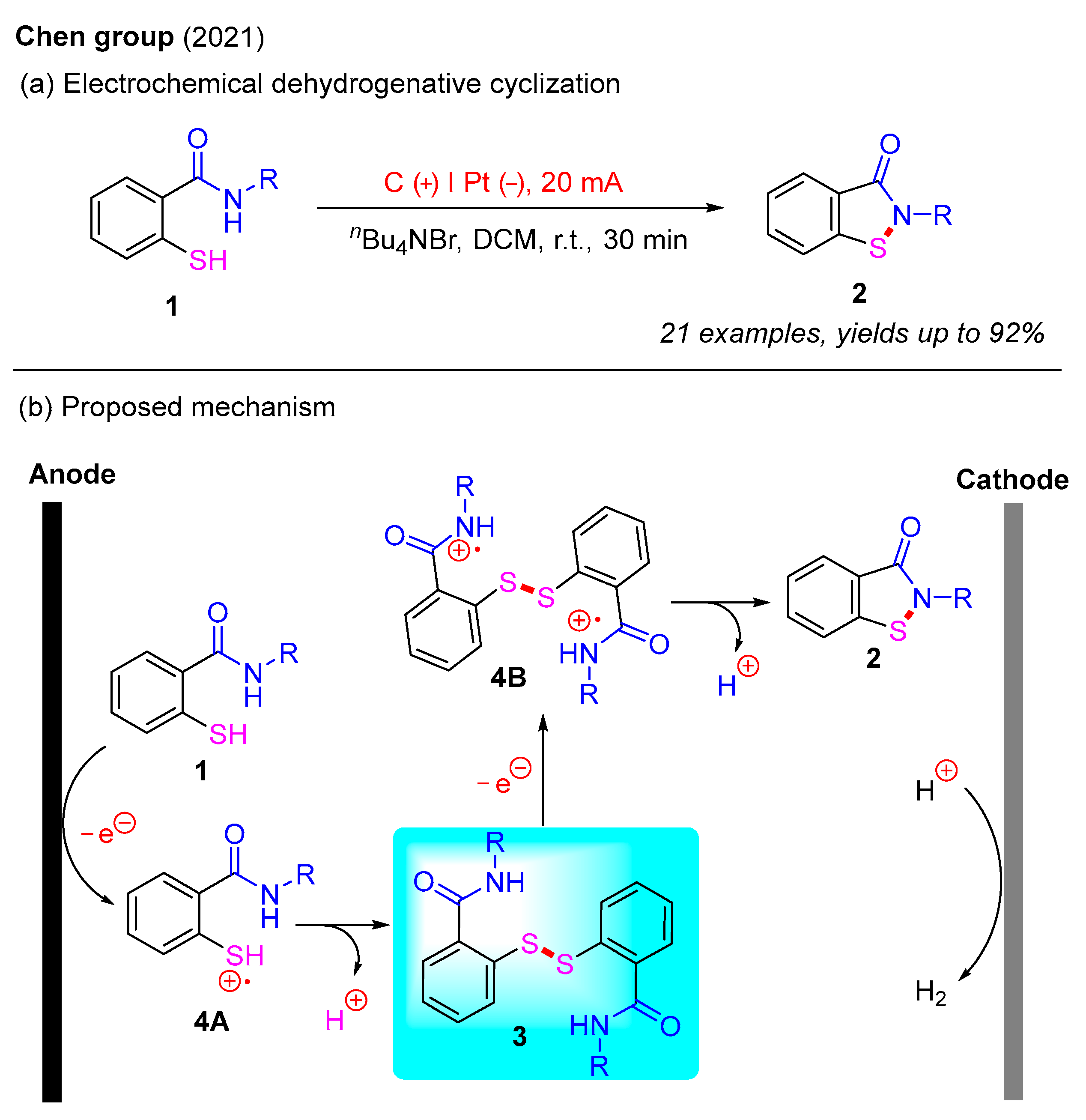
Recently, electrochemistry has emerged as a powerful and versatile tool in the field of organic synthesis. Electricity is gaining recognition as a green and sustainable redox agent because it serves as an ideal alternative to conventional chemical oxidants or reductants [29,30]. In 2021, the Chen group reported an electrochemical dehydrogenative cyclization protocol for the synthesis of benzo[d]isothiazol-3(2H)-ones via intramolecular N–S bond formation (Scheme 4a) [31]. In this study, various benzo[d]isothiazol-3(2H)-ones were isolated in moderate to good yields using 2-mercaptobenzamides as starting materials and tetrabutylammonium bromide [(n-Bu)4NBr] as an additive. Furthermore, this process was carried out through constant-current electrolysis in an undivided cell, with H2 as the nonhazardous byproduct. A proposed electrochemical mechanism is illustrated in Scheme 4b. Initially, the oxidation of 2-mercaptobenzamide 1 at the anode generates intermediate 4A, which subsequently undergoes intermolecular coupling and deprotonation to yield the disulfide intermediate 3. Next, the anodic oxidation of intermediate 3 provides intermediate 4B, which is converted into the final product 2 via intramolecular cyclization and deprotonation [32].
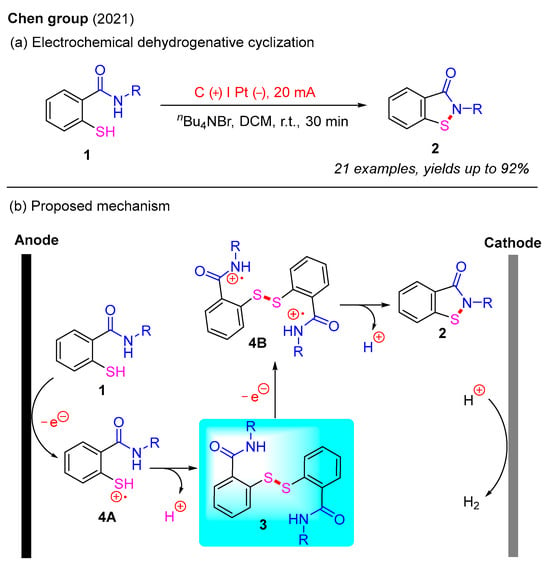
Scheme 4.
Electrochemical intramolecular dehydrogenative cyclization for the synthesis of benzo[d]isothiazol-3(2H)-ones [31].
In recent years, Selectfluor [1-chloromethyl-4-fluoro-1,4-diazoniabicyclo-[2.2.2]octane bis(tetrafluoroborate)] has emerged not only as a crucial electrophilic fluorine reagent but also as a versatile “fluorine-free” reagent in various organic reactions [33,34,35,36,37].
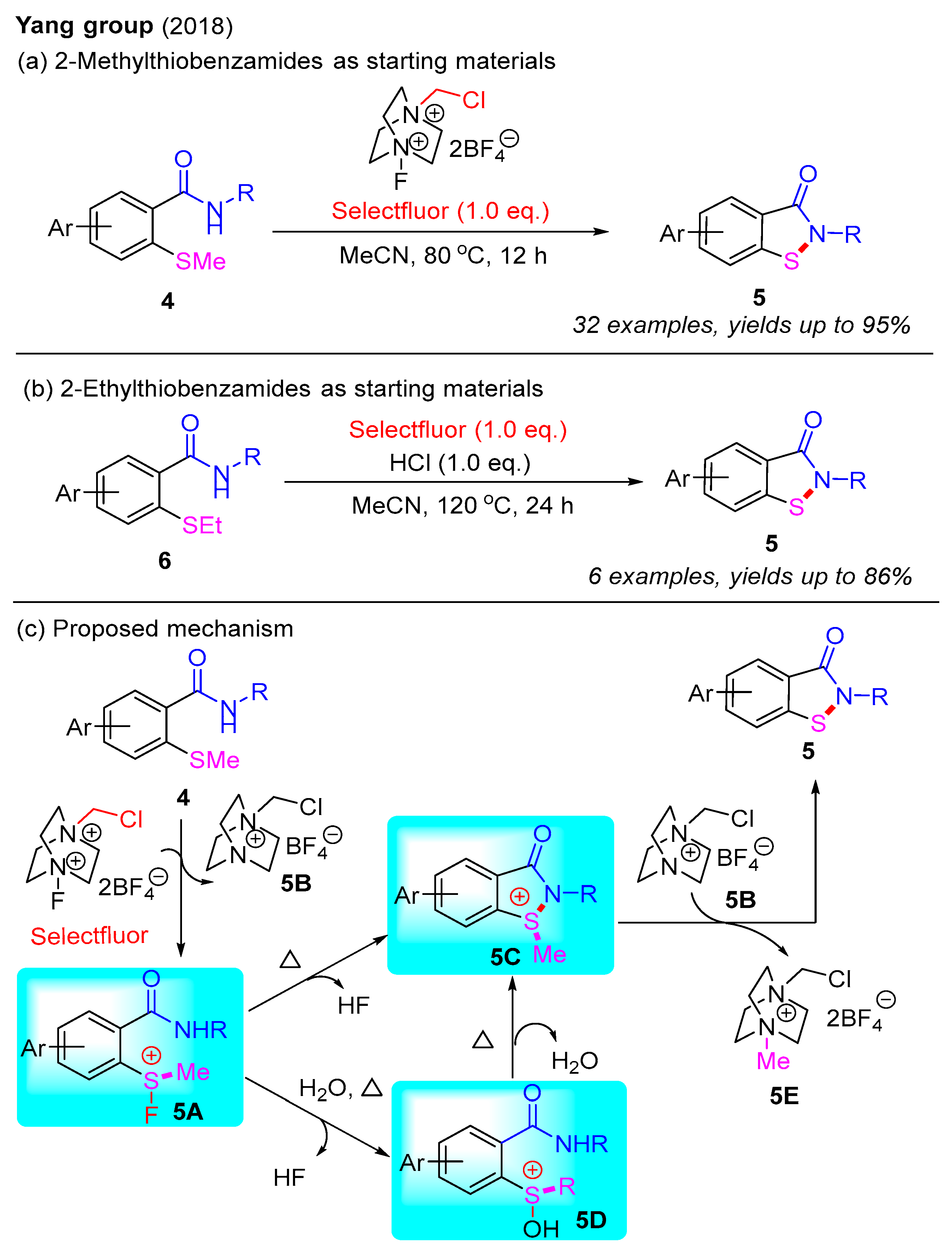
In 2018, the Yang group pioneered the use of Selectfluor for the synthesis of benzo[d]isothiazol-3(2H)-ones via a cascade N–S bond formation and the C(sp3)–S bond cleavage of 2-methylthiobenzamides (Scheme 5a) [38]. They subsequently found that the addition of HCl facilitated the conversion of 2-ethylthiobenzamides into benzo[d]isothiazol-3(2H)-ones (Scheme 5b) [39]. In these reactions, Selectfluor serves as a source of the fluorine cation (F+), which activates the methylthio group to form a transient fluorosulfonium salt. A plausible mechanism for this is presented in Scheme 5c. Initially, 2-methylthiobenzamide 4 reacts with Selectfluor to generate a transient fluorosulfonium salt 5A and an intermediate salt 5B. Subsequently, the fluorosulfonium salt 5A can either directly cyclize to form the cyclic sulfonium salt 5C, or it can be converted into salt 5C via the intermediate formation of salt 5D. Finally, benzo[d]isothiazol-3(2H)-one 5 is synthesized through nucleophilic substitution of salt 5C by salt 5B.
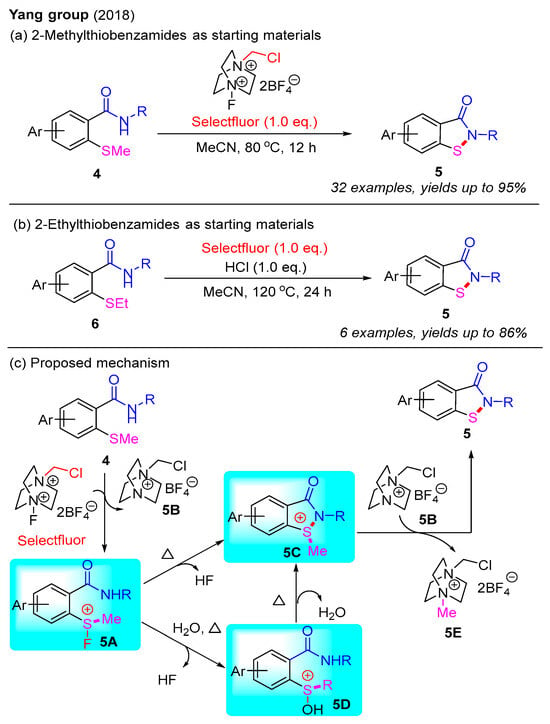
Scheme 5.
Selectfluor-mediated intramolecular N–S bond formation for the synthesis of benzo[d]isothiazol-3(2H)-ones [38].
2.2. Synthesis of Benzo[d]isothiazol-3(2H)-Ones via Intermolecular Pathways
In addition to intramolecular pathways, 2-halobenzamides can undergo intermolecular reactions with sulfur powder (S8), potassium thiocyanate (KSCN), and carbon disulfide (CS2) to construct benzo[d]isothiazol-3(2H)-ones in the presence of transition-metal catalysts.
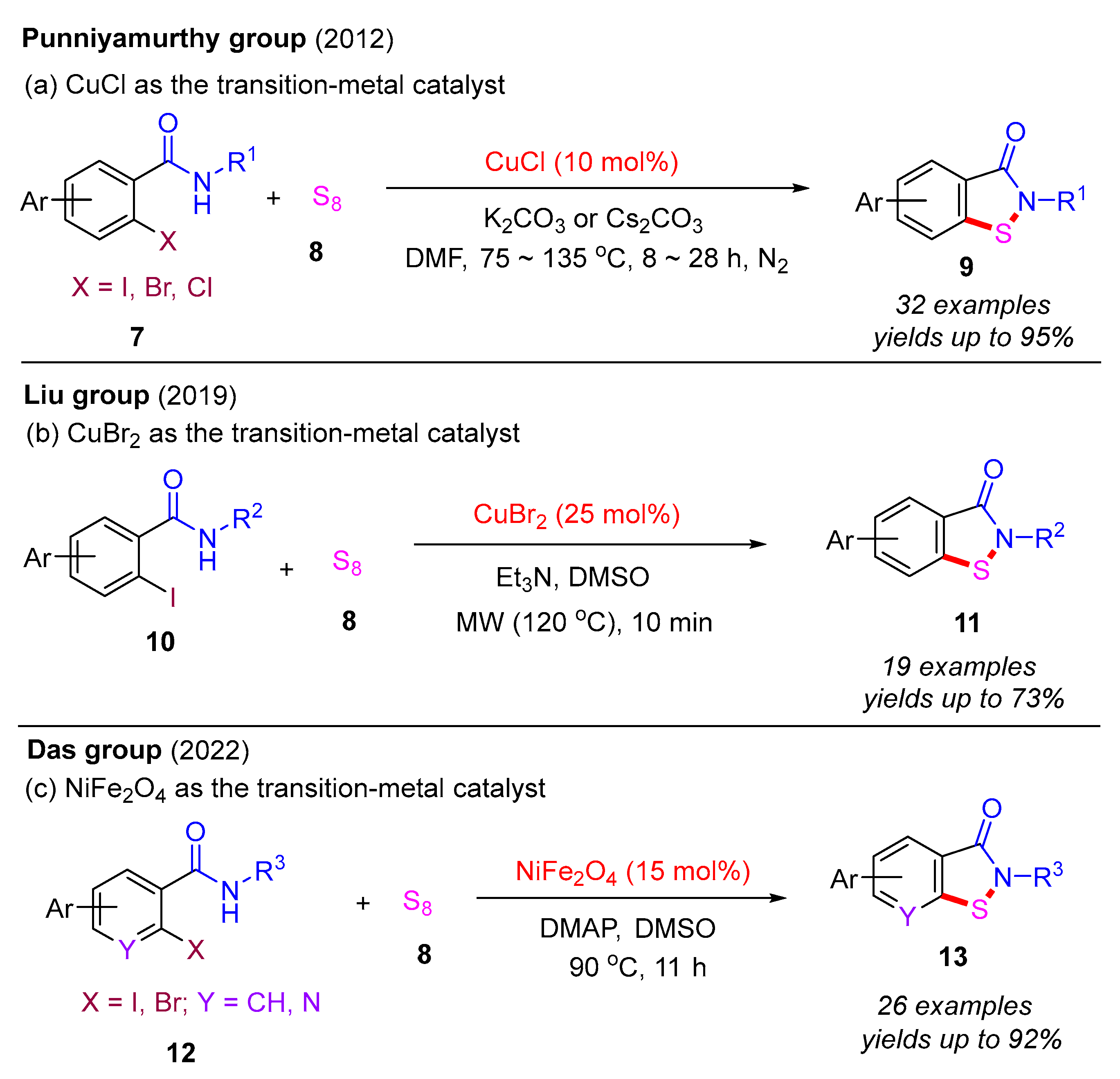
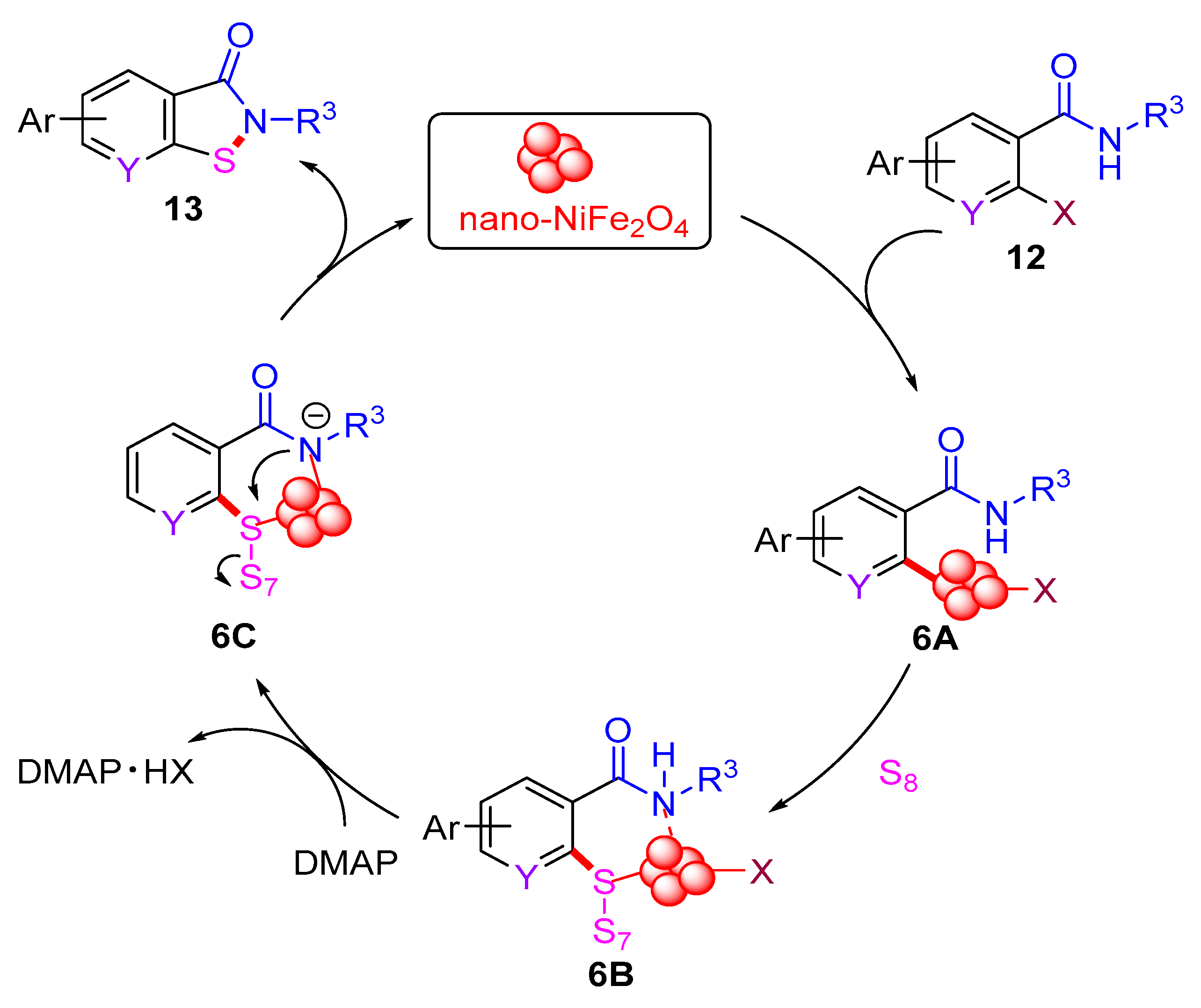
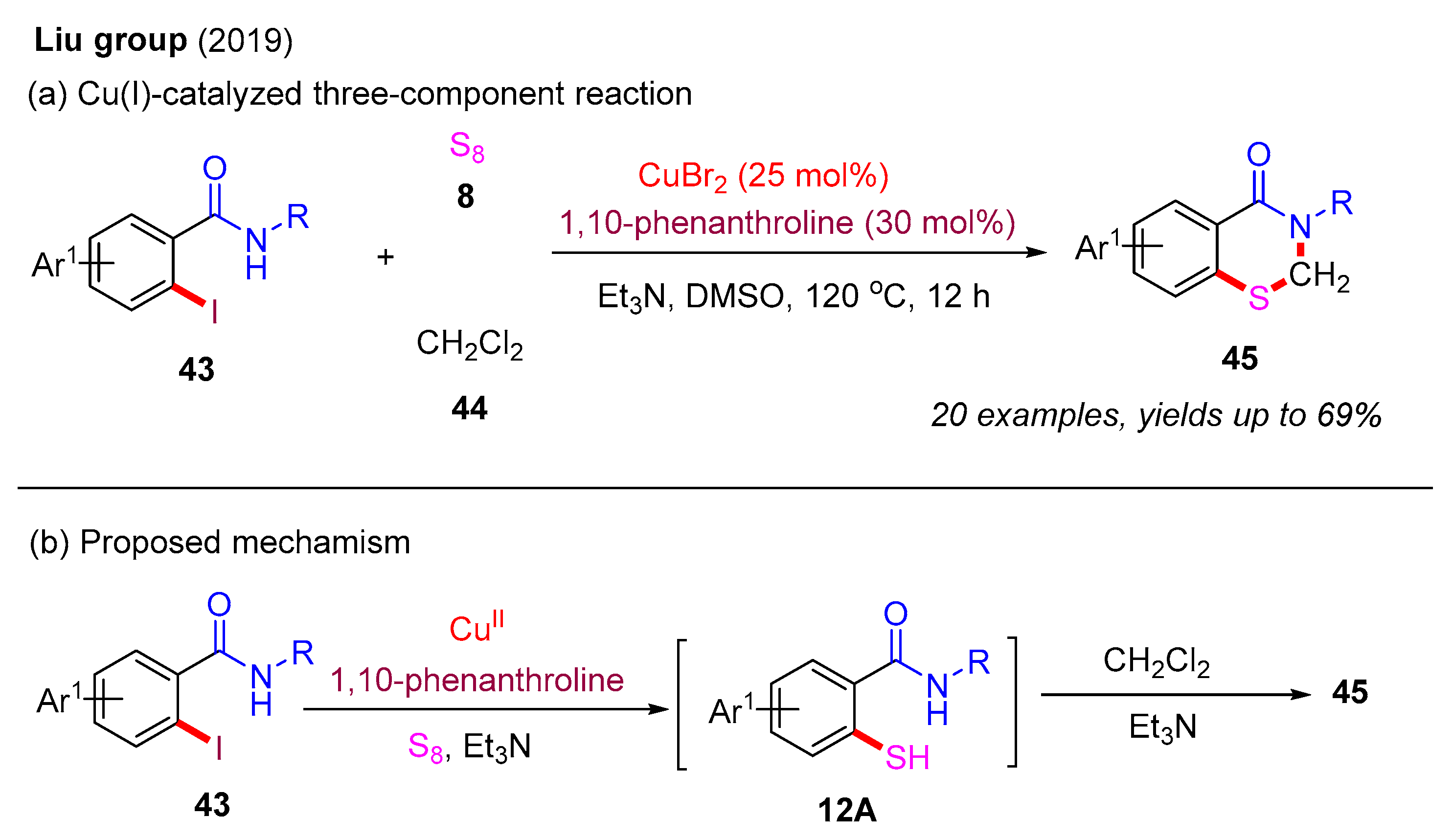
In 2012, the Punniyamurthy group developed a CuCl-catalyzed cascade reaction involving C–S bond formation followed by N–S bond cyclization (Scheme 6a) [16]. This protocol enabled the synthesis of benzo[d]isothiazol-3(2H)-ones in moderate to good yields via the coupling of various 2-halobenzamides with S8. The reactivity order of 2-halobenzamides follows the sequence 2-iodobenzamide > 2-bromobenzamide > 2-chlorobenzamide. Subsequently, in 2019, the Liu group employed the CuBr2-catalyzed approach to synthesize benzo[d]isothiazol-3(2H)-ones from 2-iodobenzamides and S8 under microwave irradiation (MW) (Scheme 6b) [40]. In 2022, the Das group used a recyclable nano-nickel ferrite catalyst (nano-NiFe2O4) to perform this cascade reaction, employing 2-halobenzamides with S8 as starting materials (Scheme 6c) [41]. A plausible mechanism for this process is proposed (Scheme 7). Initially, 2-halobenzamide 12 reacts with nano-NiFe2O4 to form intermediate 6A, which then reacts with S8 to yield intermediate 6B. In the presence of DMAP, intermediate 6B undergoes transformation into 6C via the elimination of DMAP·HX. Finally, the cyclization of the N–S bond in intermediate 6C yields the desired product 13 and regenerates the catalyst nano-NiFe2O4 [42].
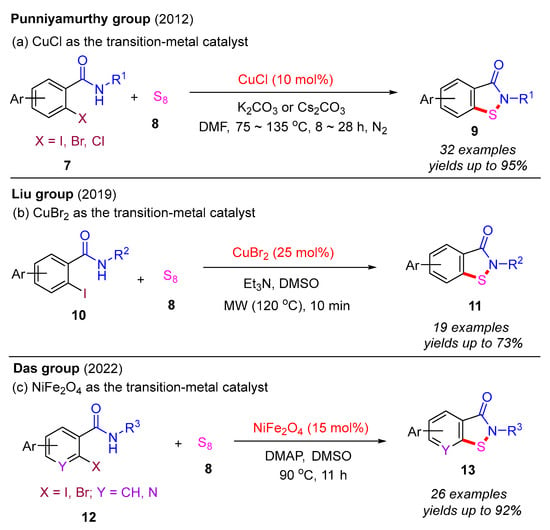
Scheme 6.
Transition-metal-catalyzed cascade reaction involving C–S bond formation followed by N–S bond cyclization for the synthesis of benzo[d]isothiazol-3(2H)-ones from 2-halobenzamides and S8 [16,40,41].
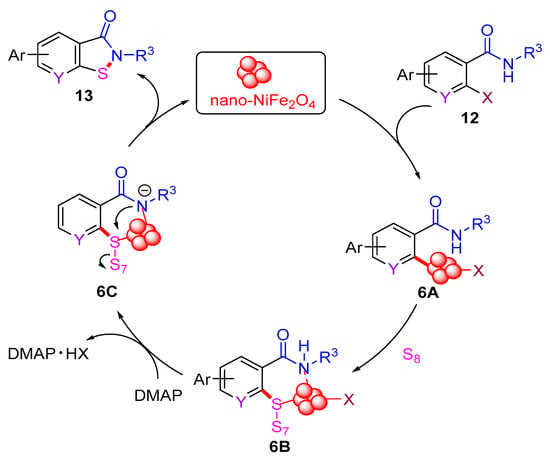
Scheme 7.
The plausible mechanism of nano-NiFe2O4-catalyzed cascade reaction involving C–S bond formation followed by N–S bond cyclization.
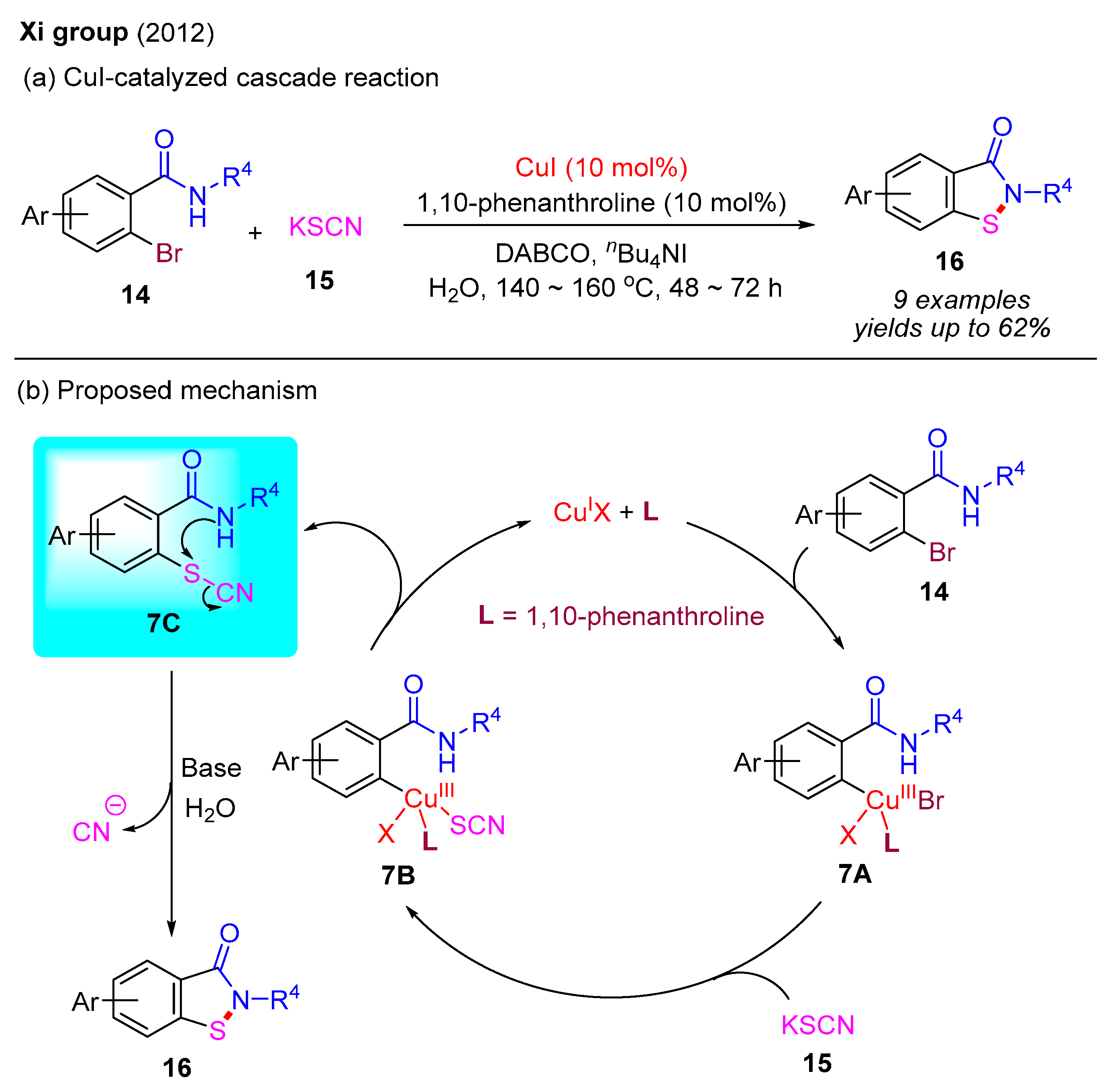
In 2012, the Xi group employed KSCN as the sulfur source to react with 2-bromobenzamides, constructing benzo[d]isothiazol-3(2H)-ones via a similar Cu(I)-catalyzed cascade reaction with C–S bond and N–S bond formation in an aqueous medium (Scheme 8a) [17]. In this process, 1,10-phenanthroline served as the ligand, DABCO functioned as the base, and Bu4NI was utilized as an additive. Notably, this reaction required higher temperatures and extended reaction times, leading to only moderate yields of the desired products. A plausible reaction mechanism is provided as shown in Scheme 8b. The initial oxidative addition between 2-bromobenzamide 14 with the Cu(I) catalyst generates intermediate 7A, which subsequently undergoes ligand exchange with KSCN to form intermediate 7B. The ensuing reductive elimination of 7B produces thiocyanate intermediate 7C and regenerates the Cu(I) catalyst. In the presence of a base and H2O, intermediate 7C is converted into the final product 16 via an intramolecular nucleophilic substitution reaction. Concurrently, the in situ generated CN anion can be hydrolyzed by the base during this process.
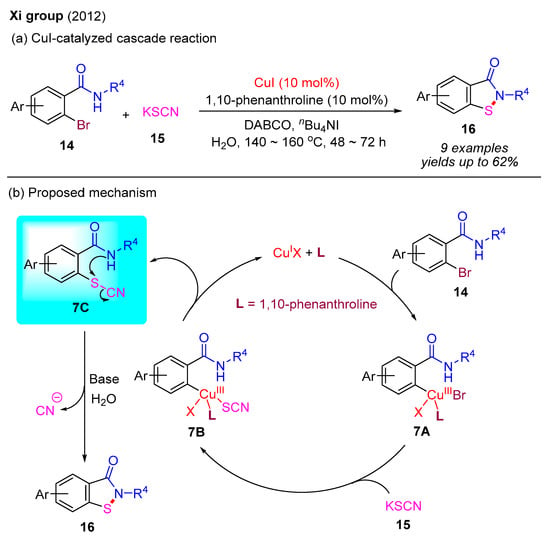
Scheme 8.
CuI-catalyzed cascade reaction for the synthesis of benzo[d]isothiazol-3(2H)-ones using 2-bromobenzamides and KSCN [17].
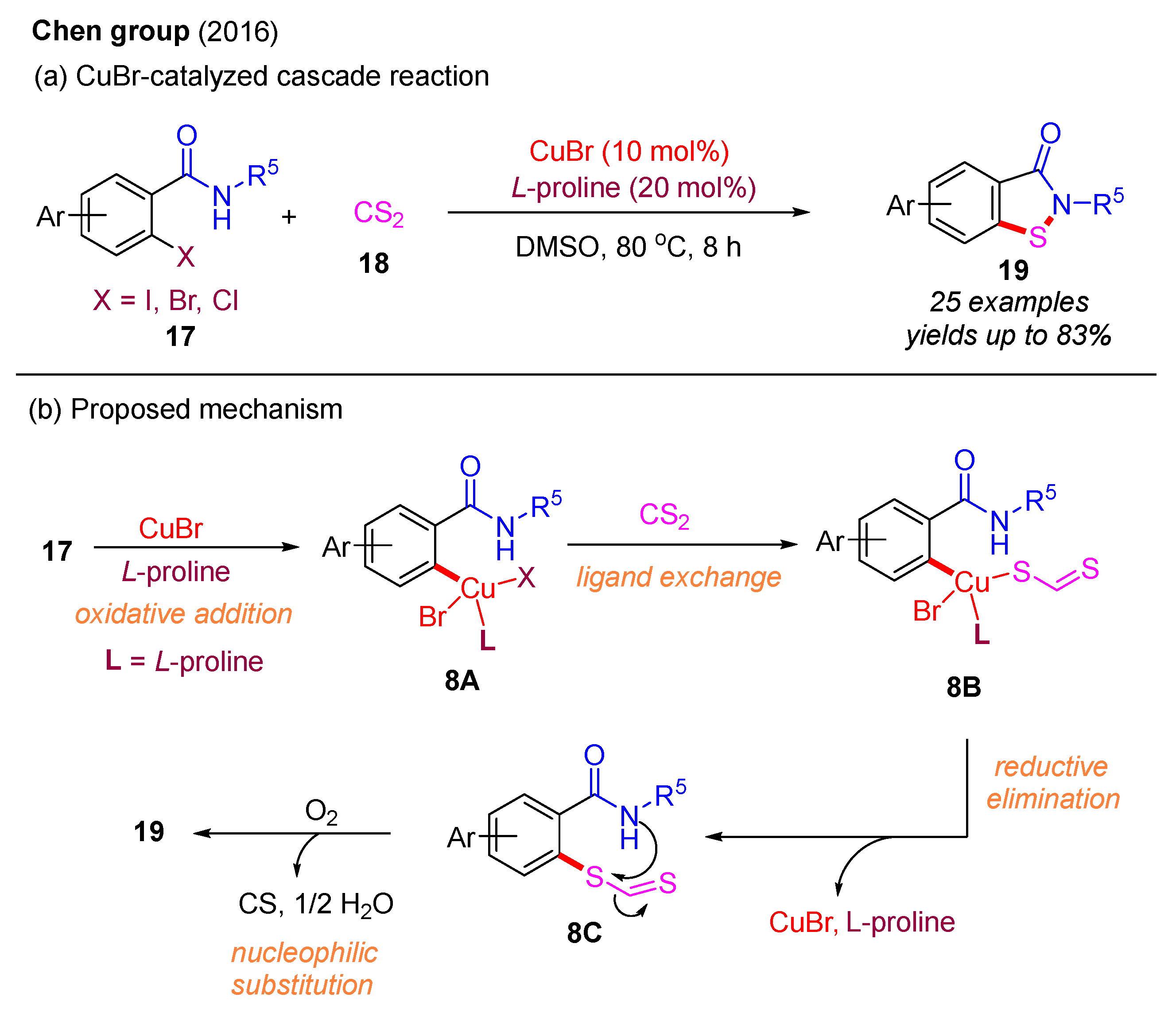
Subsequently, in 2016, the Chen group reported that CS2 could serve as a sulfur source to react with 2-halobenzamides for the preparation of benzo[d]isothiazol-3(2H)-ones under a Cu(I)-catalyzed system (Scheme 9a). Over 25 examples were synthesized with moderate to good yields. Notably, L-proline was identified as the optimal ligand, exhibiting superior catalytic performance compared to 1,10-phenanthroline. The reaction mechanism involves an initial oxidative addition, yielding intermediate 8A, which is followed by ligand exchange to form intermediate 8B. Subsequent reductive elimination of 8B affords the intermediate 8C, which then undergoes nucleophilic substitution to provide the desired product 19 (Scheme 9b) [43].
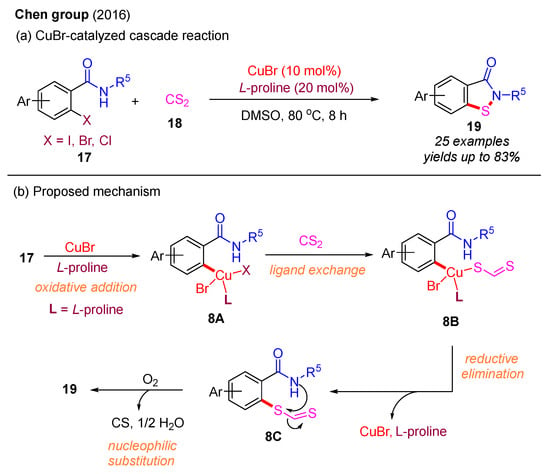
Scheme 9.
CuBr-catalyzed cascade reaction with C−S bond and N−S bond formation using 2-halobenzamides and CS2 [43].
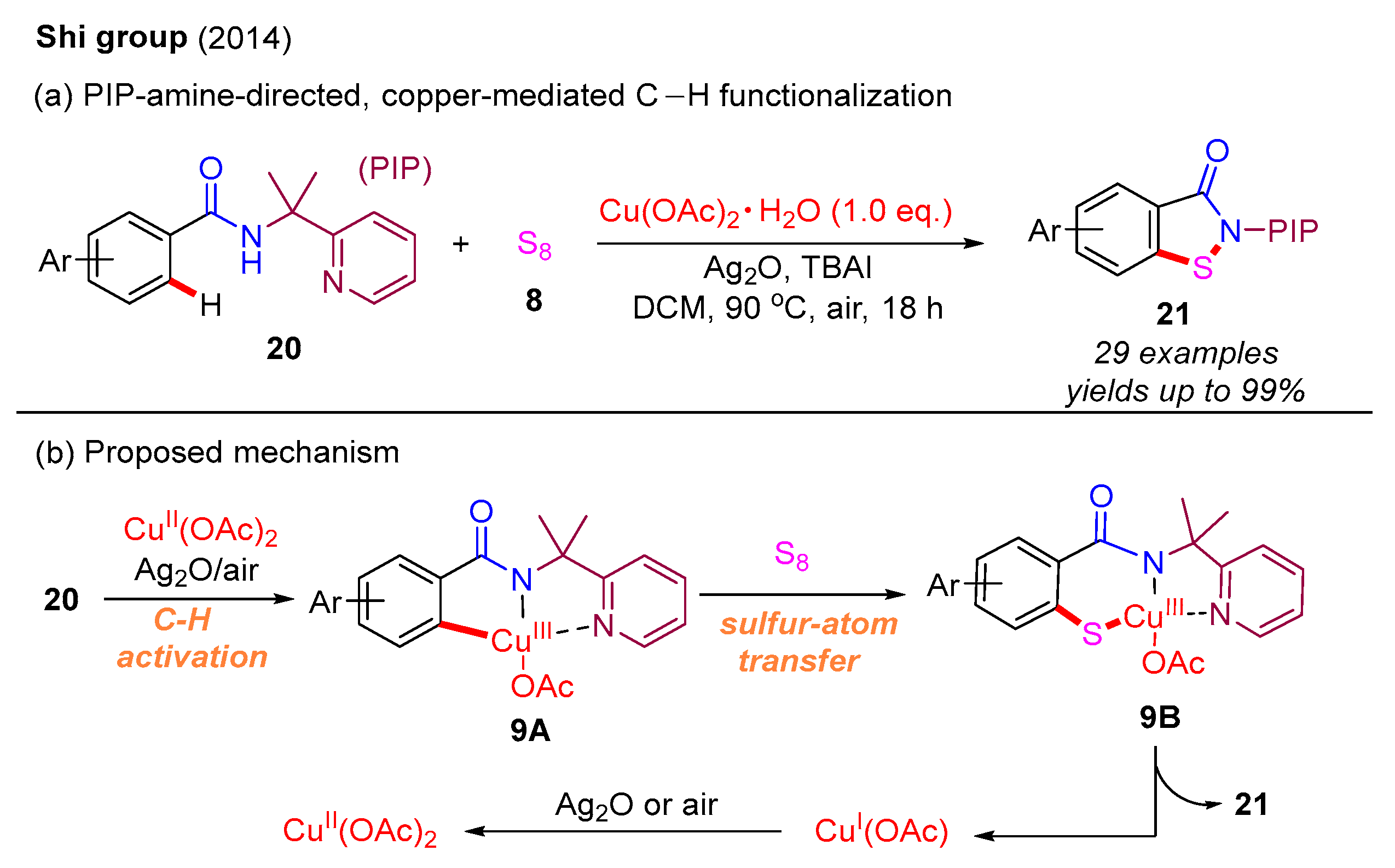
Recently, C–H functionalization has emerged as an exceptionally efficient method for constructing C–C and C–heteroatom bonds [44,45,46,47,48,49]. In 2014, the Shi group first developed a PIP-amine [(pyridin-2-yl)isopropylamine]-directed, copper-mediated C–H functionalization strategy to construct benzo[d]isothiazol-3(2H)-ones. Within this approach, various benzamides react with S8 to produce the desired products, with good yields, via C–S bond and N–S bond formation (Scheme 10a). Based on mechanistic studies and previous work, the authors propose that the benzamide substrate 20 undergoes initial C–H functionalization to form intermediate 9A in the presence of Cu(II) species, Ag2O, and air. This intermediate is subsequently transformed into intermediate 9B through a sulfur-atom transfer. Following this, the N–S reductive elimination of 9B yields the final product 21 along with Cu(I) species. Importantly, the Cu(I) species is re-oxidized by Ag2O or air to regenerate Cu(II) species (Scheme 10b) [50].
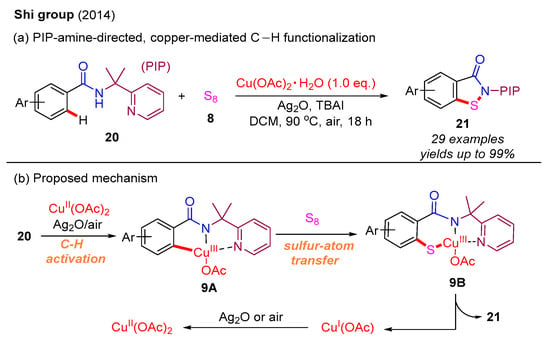
Scheme 10.
PIP-amine-directed, copper-mediated C–H functionalization strategy for the synthesis of benzo[d]isothiazol-3(2H)-ones [50].
3. Synthesis of 2,3-Dihydro-4H-Benzo[e][1,3]Thiazin-4-Ones via Intramolecular and Intermolecular Pathways
3.1. Synthesis of 2,3-Dihydro-4H-Benzo[e][1,3]Thiazin-4-Ones via Intramolecular Pathways
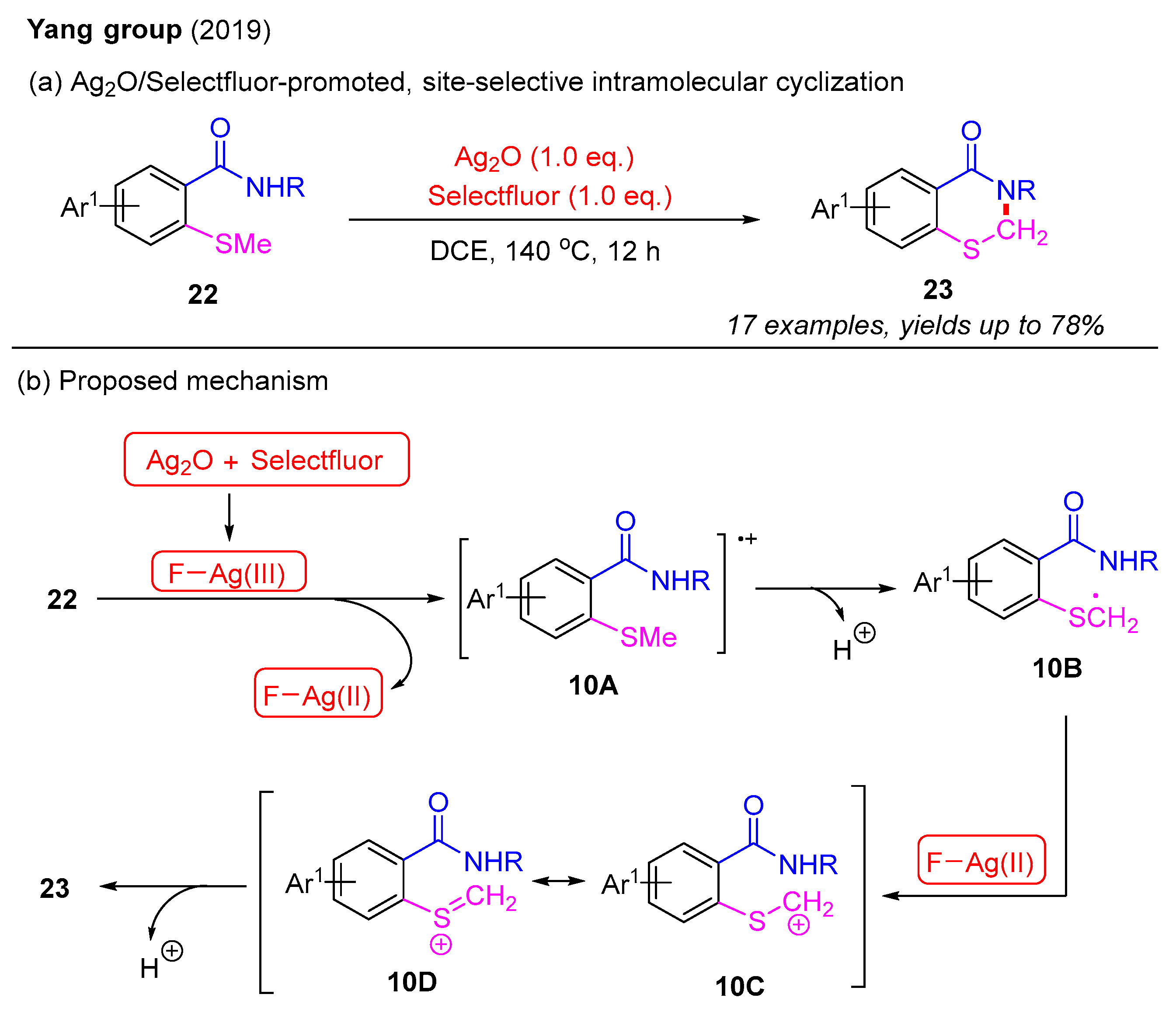
It is imperative to highlight that the literature on the intramolecular synthesis of 2,3-dihydro-4H-benzo[e][1,3]thiazin-4-ones remains limited. In 2019, the Yang group introduced an Ag2O/Selectfluor-promoted, site-selective intramolecular cyclization of 2-methylthiobenzamides to synthesize 2,3-dihydro-4H-benzo[e][1,3]thiazin-4-ones (Scheme 11a). In this process, the α-C(sp3)–H bond functionalization of the methyl group yielded various 2,3-dihydro-4H-benzo[e][1,3]thiazin-4-ones in good yields. However, attempts to substitute the methyl group on the sulfur atom with an ethyl group failed to produce the desired product. A plausible mechanism for this is proposed in Scheme 11b. The initial oxidation between Ag2O and Selectfluor forms the F–Ag(III) species, which further reacts with 2-methylthiobenzamide 22 to produce intermediate 10A and Ag(II)–F species. The subsequent deprotonation of 10A leads to the formation of intermediate 10B. The second oxidation of 10B by the Ag(II)–F species provides intermediate 10C and its resonant intermediate 10D. Finally, intramolecular cyclization of either 10C or 10D results in the formation of the desired product 23 [51].
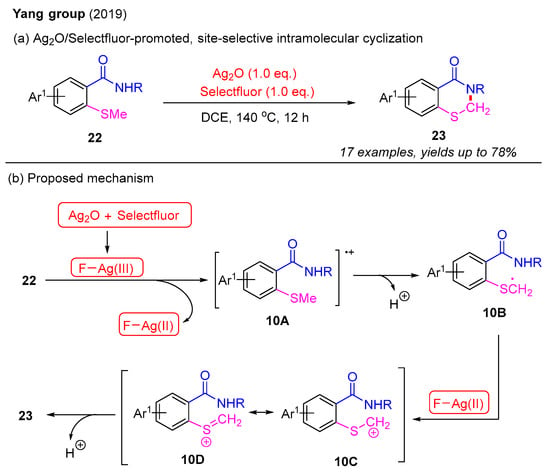
Scheme 11.
Ag2O/Selectfluor-promoted, site-selective intramolecular cyclization of 2-methylthiobenzamides for the synthesis of 2,3-dihydro-4H-benzo[e][1,3]thiazin-4-ones [51].
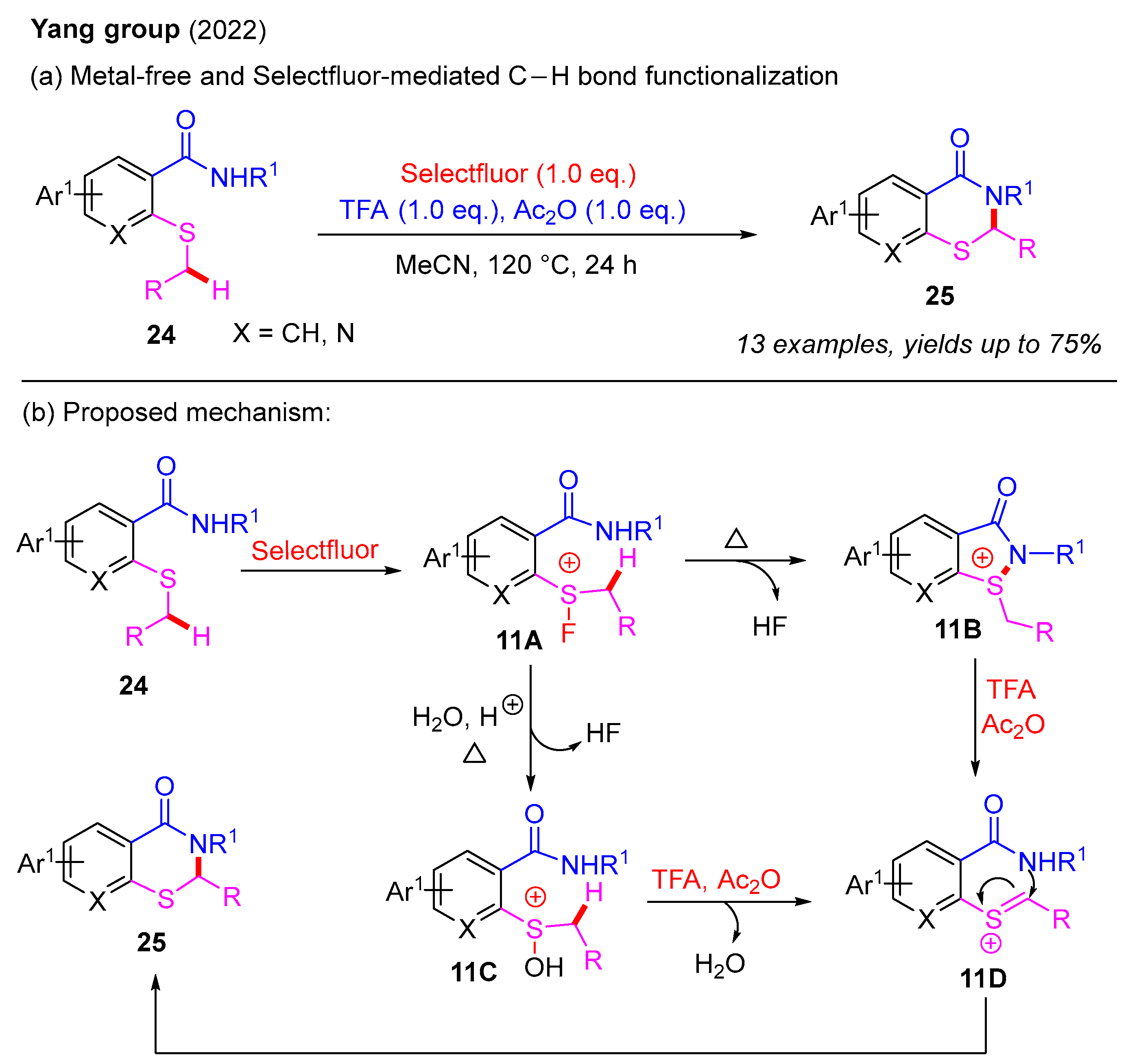
In 2022, the same group further advanced this field by developing a metal-free and Selectfluor-mediated α-C(sp3)–H bond functionalization of 2-alkylthiobenzamides to construct 2-substituted 2,3-dihydro-4H-benzo[e][1,3]thiazin-4-ones (Scheme 12a). In this process, trifluoroacetic acid (TFA) and acetic anhydride (Ac2O) played critical roles as additives. Moreover, Selectfluor acted as a F+ initiator to activate the thioether group to facilitate this reaction. A proposed reaction pathway is also provided in Scheme 12b. Firstly, 2-alkylthiobenzamide 24 reacts with Selectfluor to form fluorosulfonium salt 11A. Next, fluorosulfonium salt 11A can be converted into either cyclic sulfonium salt 11B or hydroxy sulfonium intermediate 11C. In the presence of TFA and Ac2O, sulfonium salt 11D is generated from intermediates 11B or 11C. Finally, the intramolecular cyclization of 11D affords the desired product 25 [39].
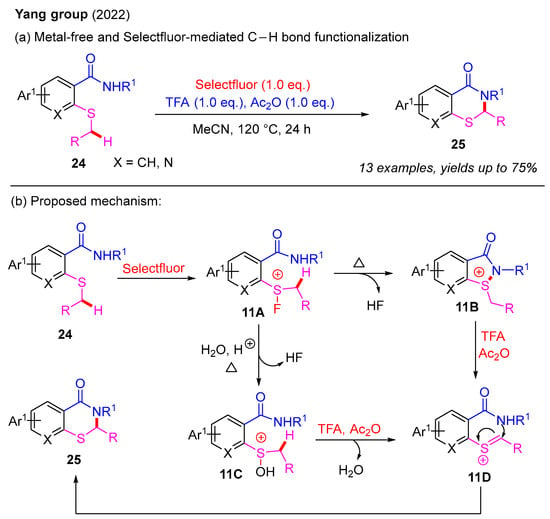
Scheme 12.
Metal-free and Selectfluor-mediated α-C(sp3)–H bond functionalization of 2-alkylthiobenzamides for the synthesis of 2-substituted 2,3-dihydro-4H-benzo[e][1,3]thiazin-4-ones [39].
3.2. Synthesis of 2,3-Dihydrobenzothiazin-4-Ones via Intermolecular Pathways
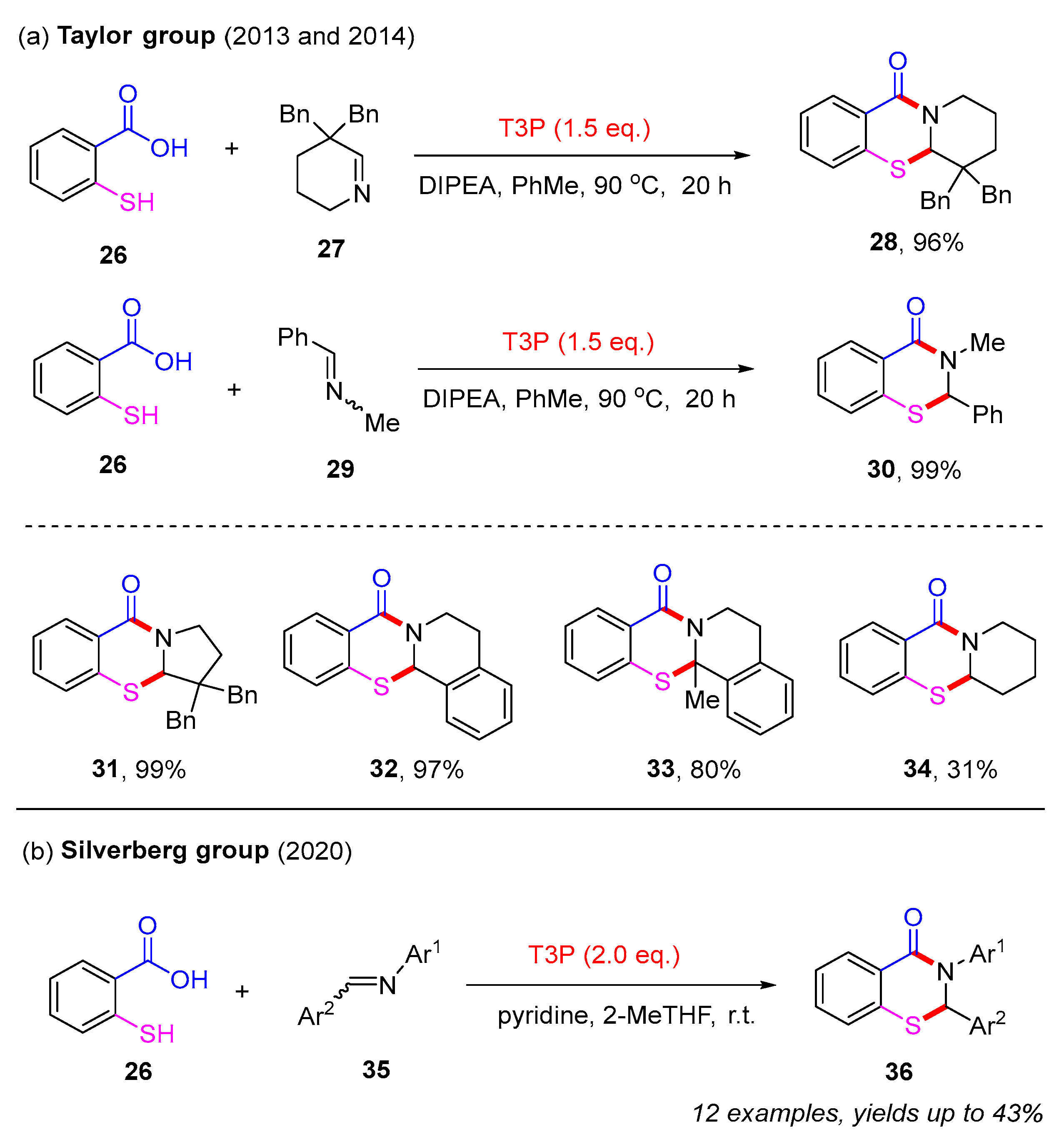
In contrast to the limited intramolecular pathways, the synthesis of 2,3-dihydro-4H-benzo[e][1,3]thiazin-4-ones primarily depends on intermolecular strategies. In 2013, the Taylor group reported a simple and direct imine acylation approach for the synthesis of 2,3-dihydro-4H-benzo[e][1,3]thiazin-4-ones. By utilizing 2-mercaptobenzoic acid 26 and cyclic imine 27 as starting materials, the desired product 28 was isolated in a 96% yield in the presence of T3P (propylphosphonic acid anhydride) and DIPEA (diisopropylethylamine). Additionally, acylic imine 29 was also found to produce product 30 with excellent yield (Scheme 13a). Mechanistic studies suggested that this process likely proceeds via the initial formation of an N-acyliminium ion, which is subsequently captured by an intramolecular S–H bond [52].
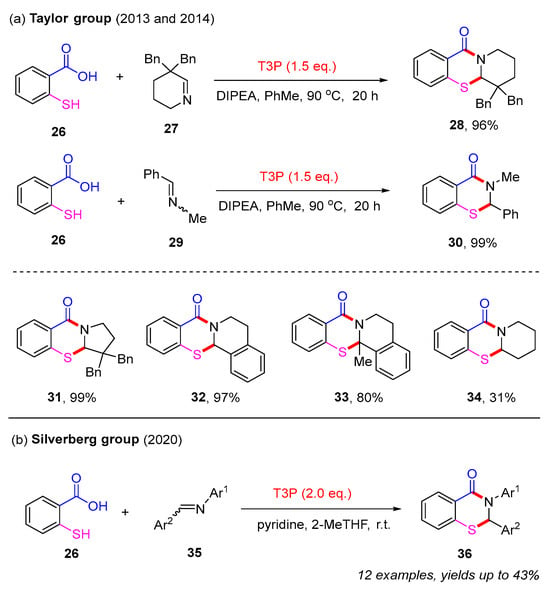
Scheme 13.
The synthesis of 2,3-dihydro-4H-benzo[e][1,3]thiazin-4-ones from 2-mercaptobenzoic acids and imines [52,53,54].
In subsequent studies, the Taylor group extended this approach to various cyclic imines in combination with 2-mercaptobenzoic acid. Different 2,3-dihydro-4H-benzo[e][1,3]thiazin-4-ones 31–33 were isolated in good to excellent yields. However, product 34 was only obtained in 31% yield due to this kind of imine existing primarily in its trimeric form, dodecahydro-4a,8a,12a-triazatriphenylene (Scheme 13a) [53]. In 2020, the Silverberg group used diaryl imines to react with 2-mercaptobenzoic acids in the presence of T3P. However, this strategy only afforded a limited range of 2,3-dihydro-4H-benzo[e][1,3]thiazin-4-ones 36 with low yields (Scheme 13b) [54].
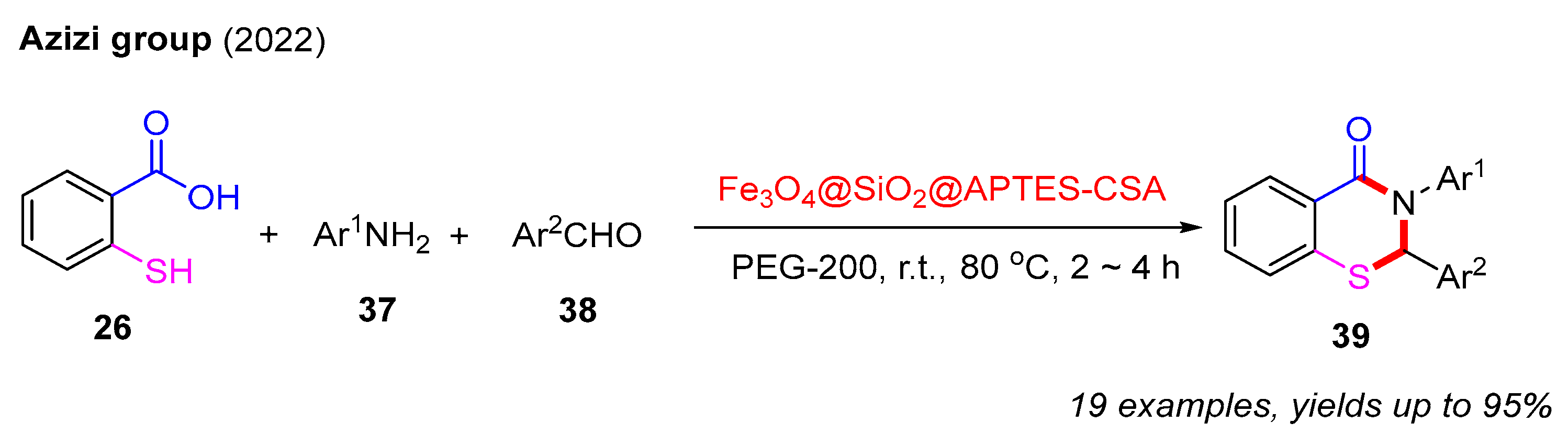
To our delight, in 2022, the Azizi group successfully developed a recyclable heterogeneous catalyst, Fe3O4@SiO2@APTES-CSA. This catalyst was employed to achieve the efficient synthesis of 2,3-dihydro-4H-benzo[e][1,3]thiazin-4-ones via a three-component reaction involving 2-mercaptobenzoic acids, aldehydes, and amines in the green solvent PEG-200. Notably, this catalyst can be reused at least five times without any observable decrease in its catalytic performance (Scheme 14) [55].
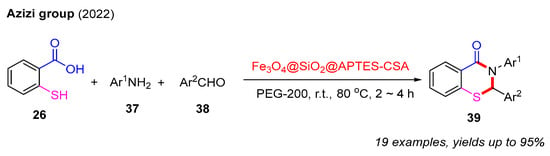
Scheme 14.
The synthesis of 2,3-dihydro-4H-benzo[e][1,3]thiazin-4-ones via a three-component reaction involving 2-mercaptobenzoic acids, aldehydes, and amines [55].
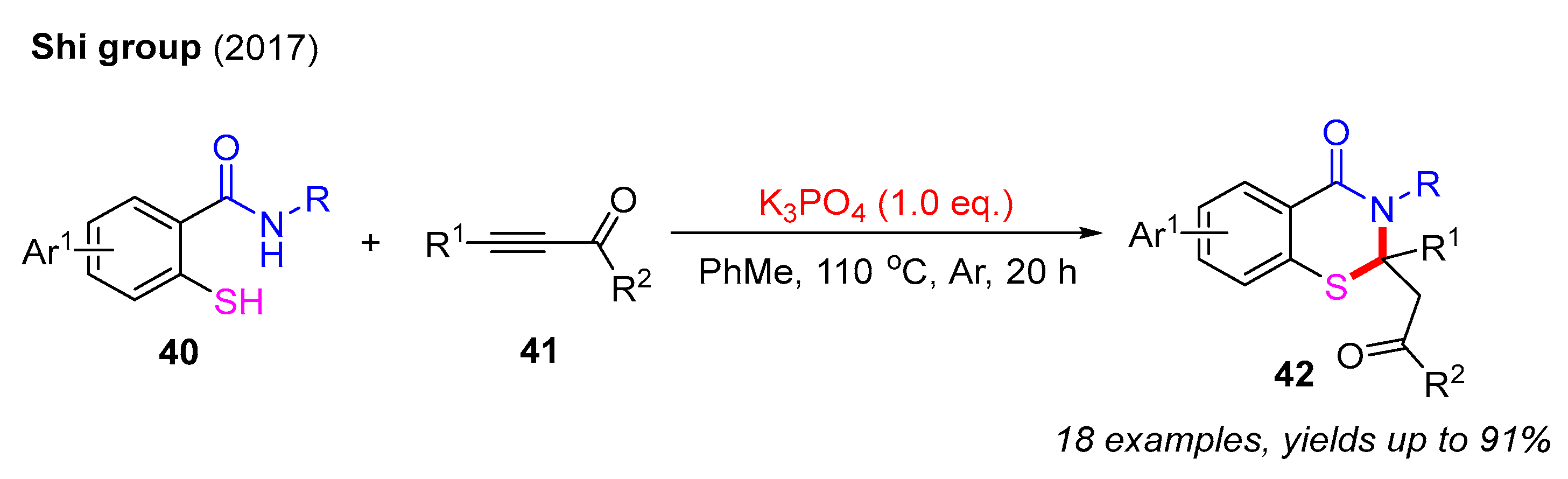
In 2017, the Shi group successfully synthesized a series of 2,3-dihydro-4H-benzo[e][1,3]thiazin-4-ones using 2-mercaptobenzamides and propiolate derivatives for preparation in the presence of K3PO4. This reaction yielded a diverse range of products 42 with satisfactory yields. Mechanistic studies revealed that the initial 1,4-addition between 2-mercaptobenzamides and propiolate derivatives provides alkene intermediates that undergo intramolecular cyclization under basic conditions to form the final products (Scheme 15) [56].
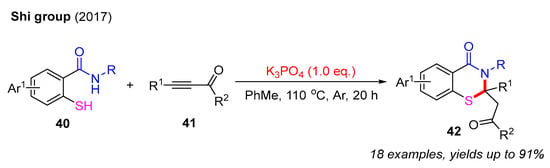
Scheme 15.
The synthesis of 2,3-dihydro-4H-benzo[e][1,3]thiazin-4-ones using 2-mercaptobenzamides with propiolate derivatives [56].
In addition to 2-mercaptobenzamides and 2-mercaptobenzoic acids, 2-iodobenzamides can also serve as effective precursors for the construction of 2,3-dihydro-4H-benzo[e][1,3]thiazin-4-ones. In 2019, the Liu group reported a Cu(I)-catalyzed three-component reaction involving 2-iodobenzamides, S8, and dichloromethane (DCM) (Scheme 16a). This protocol enabled the synthesis of various 2,3-dihydrobenzothiazin-4-ones in moderate to good yields through a cascade thiolation and annulation process. Notably, 1,10-phenanthroline was identified as the optimal ligand, significantly enhancing the reaction yield. A more reasonable proposed mechanism is presented in Scheme 16b. In the presence of a Cu(II) catalyst, 1,10-phenanthroline, Et3N, and S8, 2-iodobenzamide 43 is first converted into 2-mercaptobenzamide 12A via a typical Ullmann-type thiolation reaction. Next, 2-mercaptobenzamide 12A can directly react with DCM in the presence of Et3N to produce the desired product 45 [40].
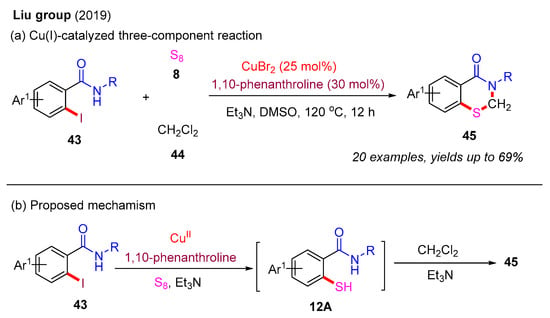
Scheme 16.
Cu(I)-catalyzed three-component reaction of 2-iodobenzamides, S8, and DCM for the construction of 2,3-dihydro-4H-benzo[e][1,3]thiazin-4-ones [40].
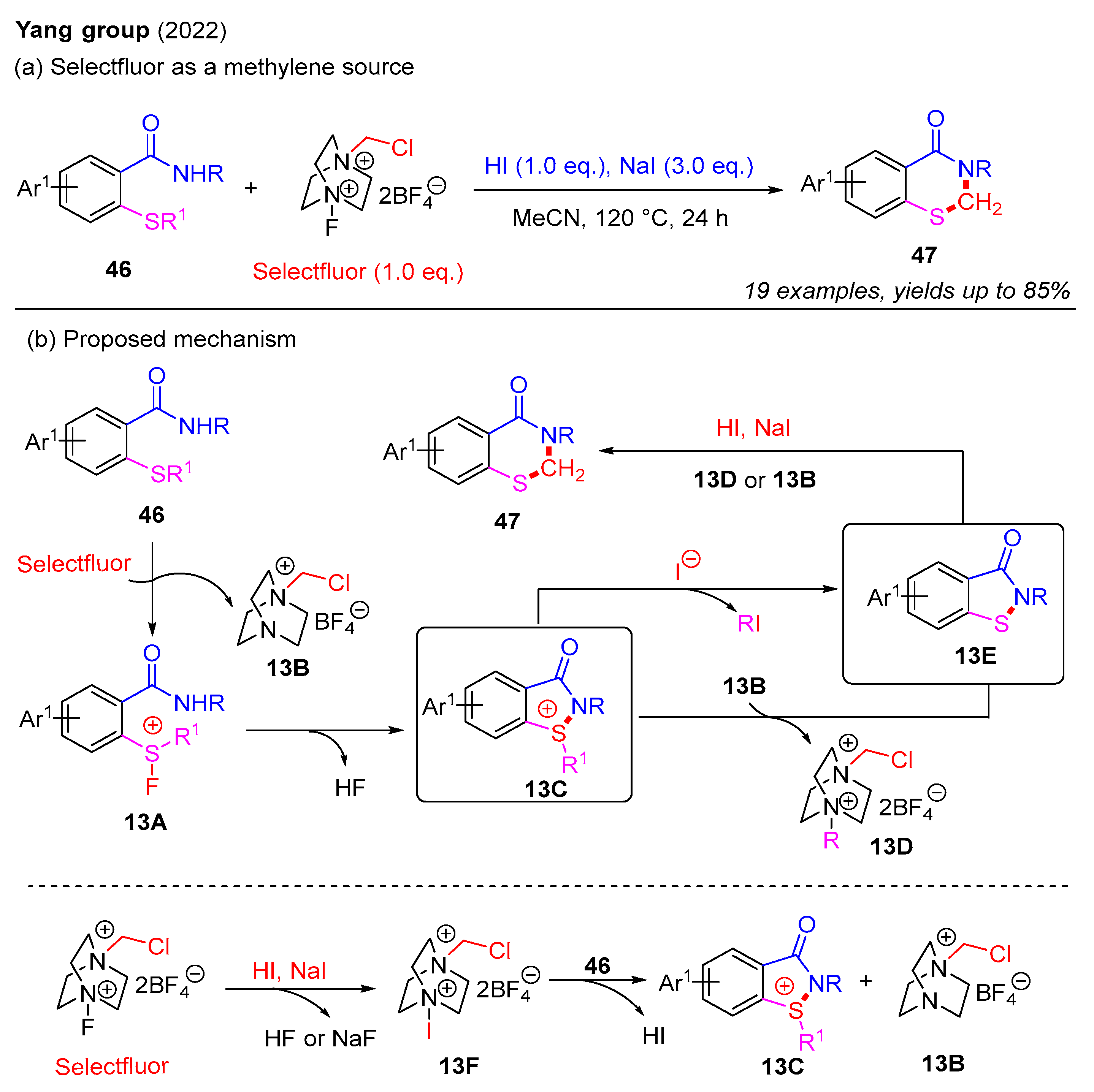
In 2022, the Yang group utilized Selectfluor as a methylene source to synthesize 2,3-dihydrobenzothiazin-4-ones from 2-alkylthiobenzamides in the NaI-HI system (Scheme 17a) [39]. This process featured the selective cleavage of C(sp3)–S bonds in 2-alkylthiobenzamides, resulting in the formation of various 2,3-dihydro-4H-benzo[e][1,3]thiazin-4-ones with satisfactory yields. Furthermore, 2-alkylthiobenzamides bearing diverse sulfur substituents, including n-propyl, n-butyl, benzyl, isopropyl, and methyl groups, were well-tolerated in this reaction, affording the desired products in good yields. Mechanistic studies indicated that Selectfluor functions as a bifunctional reagent, serving both F+ initiator and methylene sources in the NaI-HI system. A plausible mechanism for this is presented in Scheme 17b. Initially, the reaction between 2-alkylthiobenzamide 46 and Selectfluor forms a transient fluorosulfonium salt 13A and a chloromethyl salt 13B. Subsequently, intramolecular cyclization of 13A produces sulfonium salt 13C. Next, nucleophilic displacement of 13C by 13B or an iodide ion affords intermediate 13E in the presence of NaI and HI. Finally, ring expansion between intermediate 13E and 13D or 13B provides the desired product 47. Moreover, it is also proposed that Selectfluor in the NaI-HI system may generate intermediate 13F, which reacts with 46 to form intermediate 13C and chloromethyl salt 13B.
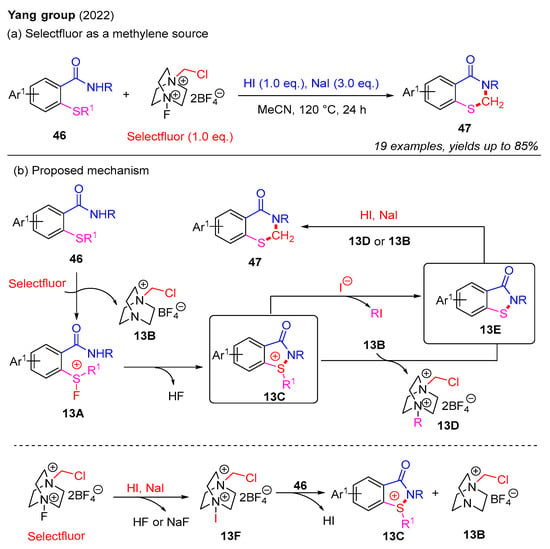
Scheme 17.
The use of Selectfluor as a methylene source for the construction of 2,3-dihydro-4H-benzo[e][1,3]thiazin-4-ones from 2-alkylthiobenzamides in the NaI-HI system [39].
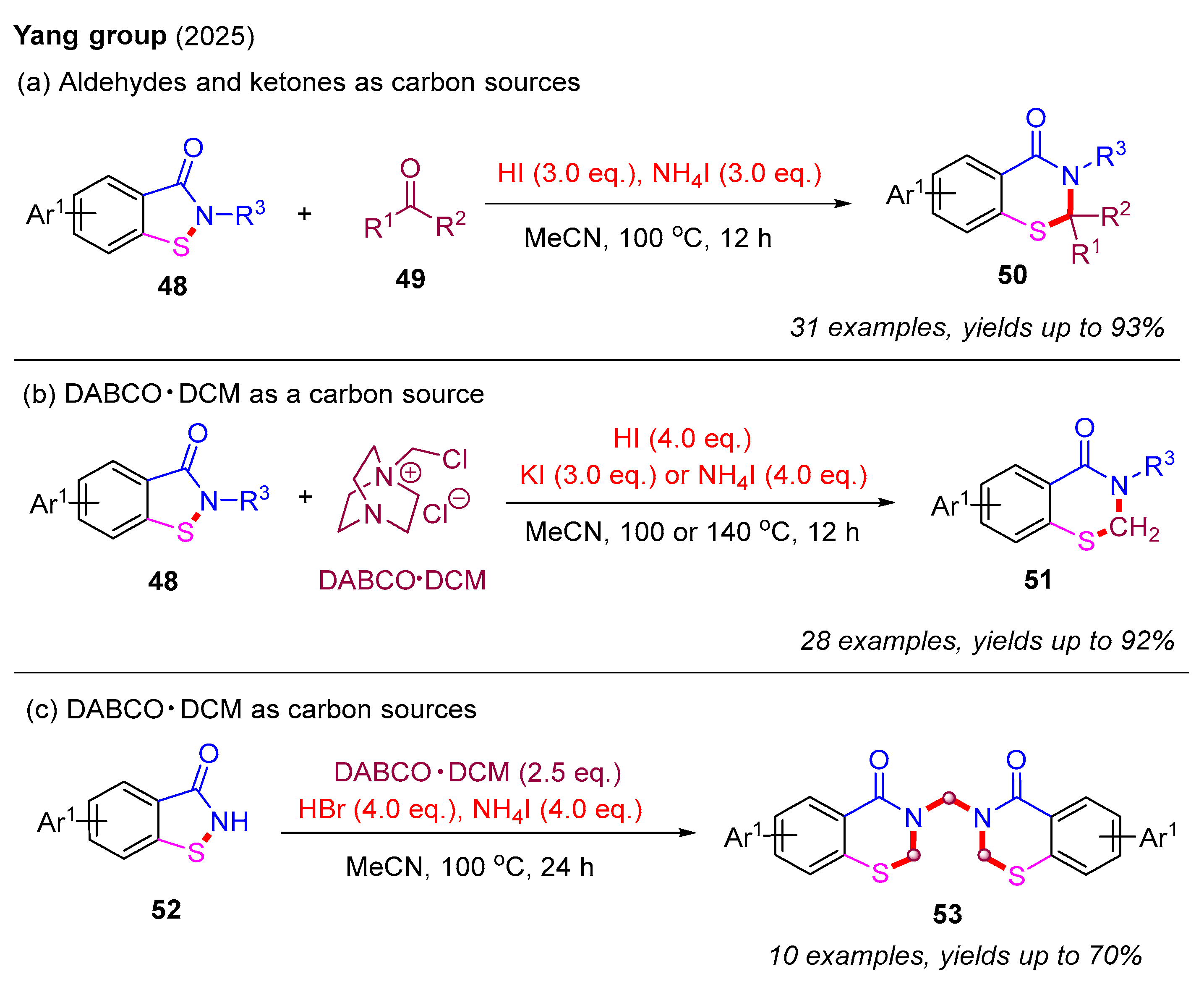
Recently, the same group also developed a novel metal-free ring-expansion strategy to construct 2,3-dihydro-4H-benzo[e][1,3]thiazin-4-ones from benzoisothiazol-3-ones through a single-carbon insertion approach. In this strategy, both 2-substituted and 2,2-disubstituted 2,3-dihydro-4H-benzo[e][1,3]thiazin-4-ones 50 were isolated in good to excellent yields. Specifically, in the presence of HI and NH4I, aldehydes and ketones served as single-carbon sources for the selective formation of 2-substituted and 2,2-disubstituted 2,3-dihydrobenzothiazin-4-ones, respectively (Scheme 18a). Notably, DABCO·DCM [1-(chloromethyl)-1,4-diazabicyclo [2.2.2]octan-1-ium chloride] was utilized for the first time as a single-carbon source for constructing 2,3-dihydro-4H-benzo[e][1,3]thiazin-4-ones 51 in the presence of HI and KI or NH4I (Scheme 18b). Furthermore, bis-2,3-dihydro-4H-benzo[e][1,3]thiazin-4-ones 53 were synthesized in moderate to good yields using DABCO·DCM in the presence of HBr and NH4I (Scheme 18c) [57].
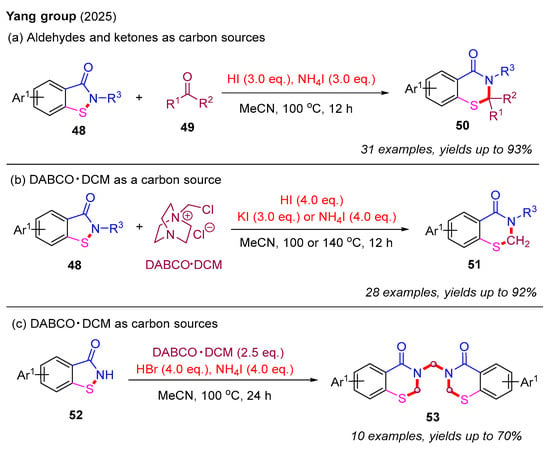
Scheme 18.
The metal-free ring-expansion strategy for the construction of 2,3-dihydro-4H-benzo[e][1,3]thiazin-4-ones through a single-carbon insertion approach [57].
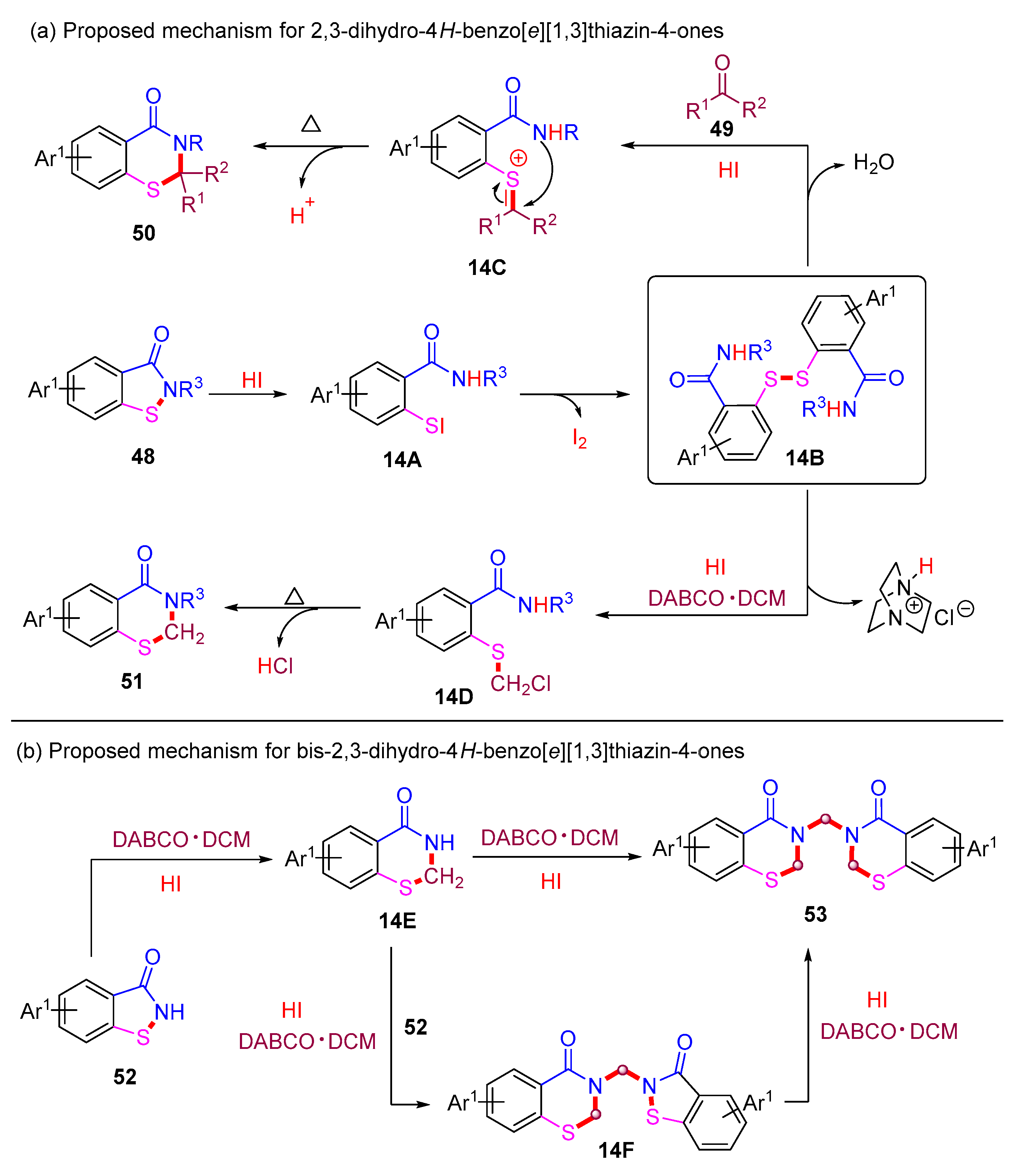
The proposed reaction mechanisms for 2,3-dihydro-4H-benzo[e][1,3]thiazin-4-ones are depicted in Scheme 19a. In the presence of HI, the initial ring-opening of benzoisothiazol-3-one 48 proceeds to form intermediate 14A. Subsequently, intermediate 14A undergoes rapid transformation into the crucial disulfide intermediate 14B. Next, intermediate 14B reacts with an aldehyde or ketone to form intermediate 14C, which subsequently undergoes intramolecular cyclization to yield the desired product 50. On the other hand, the reaction between 14B and DABCO·DCM affords intermediate 14D, which is further transformed into the desired product 51 through HCl elimination.
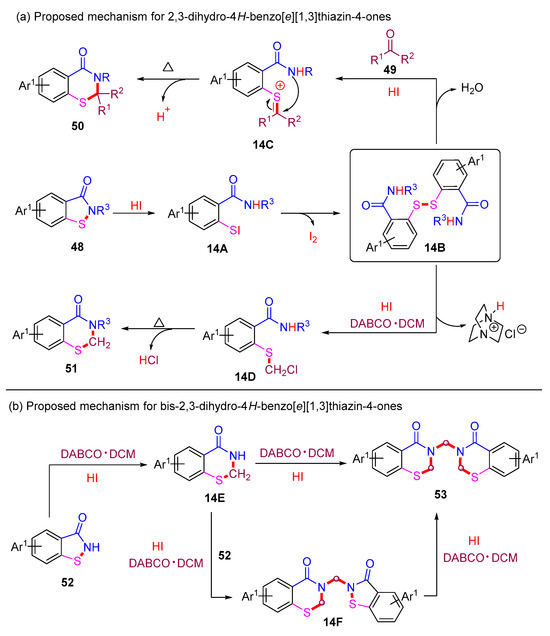
Scheme 19.
Proposed mechanisms for 2,3-dihydro-4H-benzo[e][1,3]thiazin-4-ones via metal-free ring-expansion strategies.
The proposed mechanism for the construction of bis-2,3-dihydro-4H-benzo[e][1,3]thiazin-4-ones is provided in Scheme 19b. The HI-mediated ring-expansion of benzoisothiazol-3-one 52 with DABCO·DCM generates intermediate 14E, which further reacts with DABCO·DCM to yield the desired product 53 through a self-dimerization process. Concurrently, intermediate 14E can react with 52 and DABCO·DCM to form intermediate 14F, which then undergoes a second ring-expansion with DABCO·DCM to generate the desired product 53.
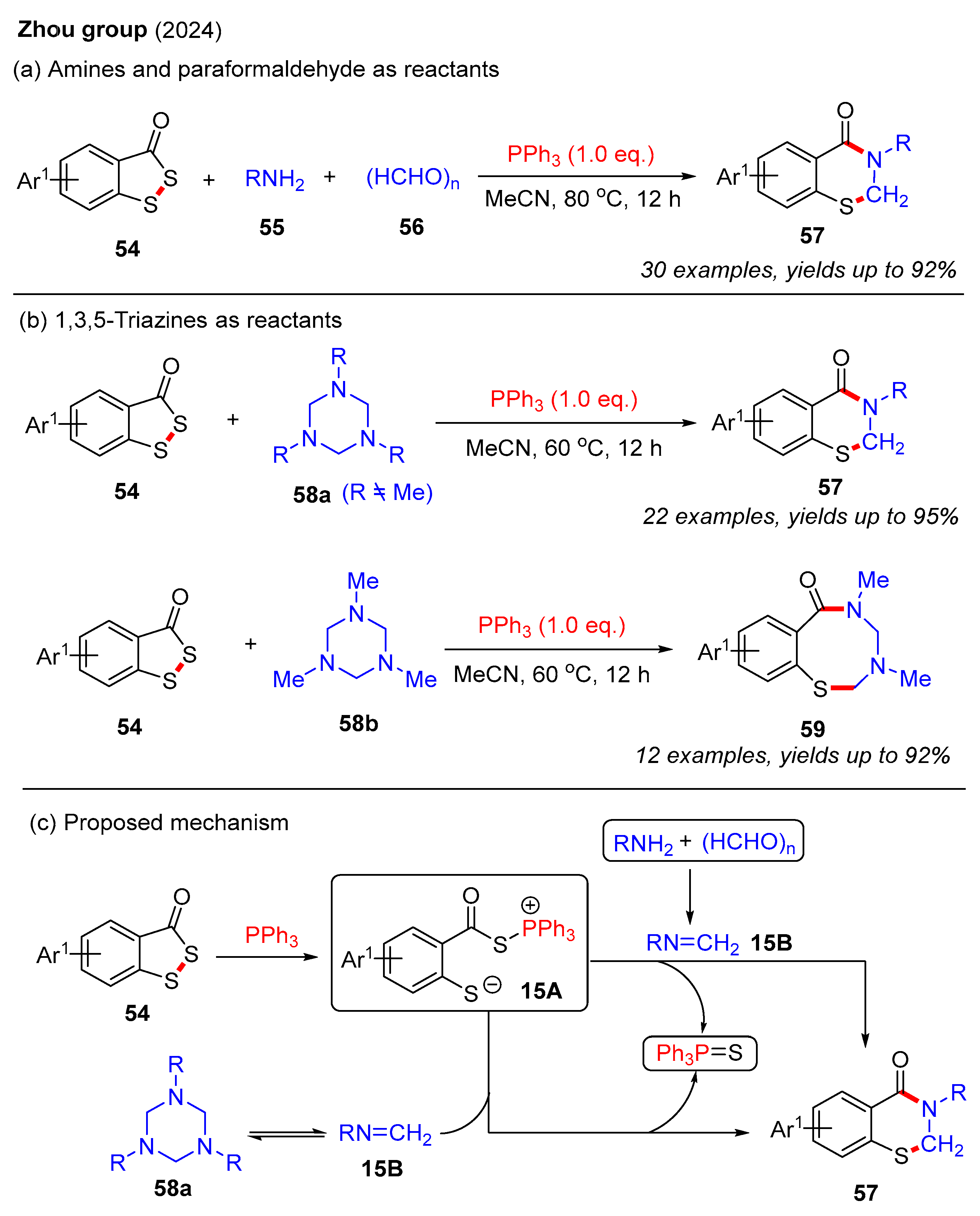
Recently, the Zhou group developed a PPh3-mediated three-component cyclization reaction of benzodithiol-3-ones, amines, and paraformaldehyde to construct 2,3-dihydro-4H-benzo[e][1,3]thiazin-4-ones (Scheme 20a) [58]. This method enabled the synthesis of various 2,3-dihydro-4H-benzo[e][1,3]thiazin-4-ones 57 in good yields using different amines. Furthermore, the same group also used the same strategy to achieve the synthesis of 2,3-dihydrobenzothiazin-4-ones employing benzodithiol-3-ones and 1,3,5-triazines as starting materials (Scheme 20b) [59]. To our surprise, when using 1,3,5-trimethyl-1,3,5-triazinane, only eight-membered benzothiadiazocin-6-ones 59 were isolated. In contrast, other substituted 1,3,5-triazinanes yielded the desired 2,3-dihydro-4H-benzo[e][1,3]thiazin-4-ones, likely due to steric effects. The plausible reaction mechanisms are outlined in Scheme 20c. Initially, the PPh3-mediated ring-opening of benzodithiol-3-ones 54 generates intermediate 15A. Next, the imine intermediate 15B is formed via the condensation of amine and paraformaldehyde or through the self-dissociation of 1,3,5-triazine. Finally, intermediate 15A reacts with imine intermediate 15B to form the desired product 57, accompanied by the elimination of Ph3P=S.
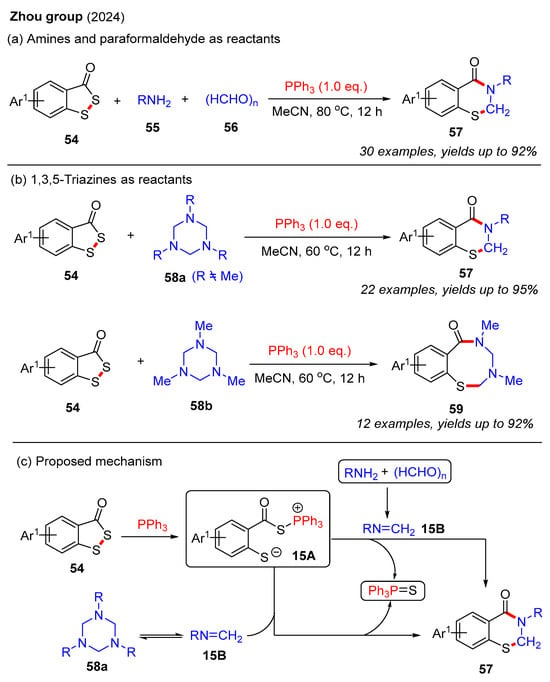
Scheme 20.
PPh3-mediated cyclization reaction of benzodithiol-3-ones for the construction of 2,3-dihydro-4H-benzo[e][1,3]thiazin-4-ones [58,59].
4. Synthesis of 4H-Benzo[e][1,3]Thiazin-4-Ones via Intermolecular Pathways
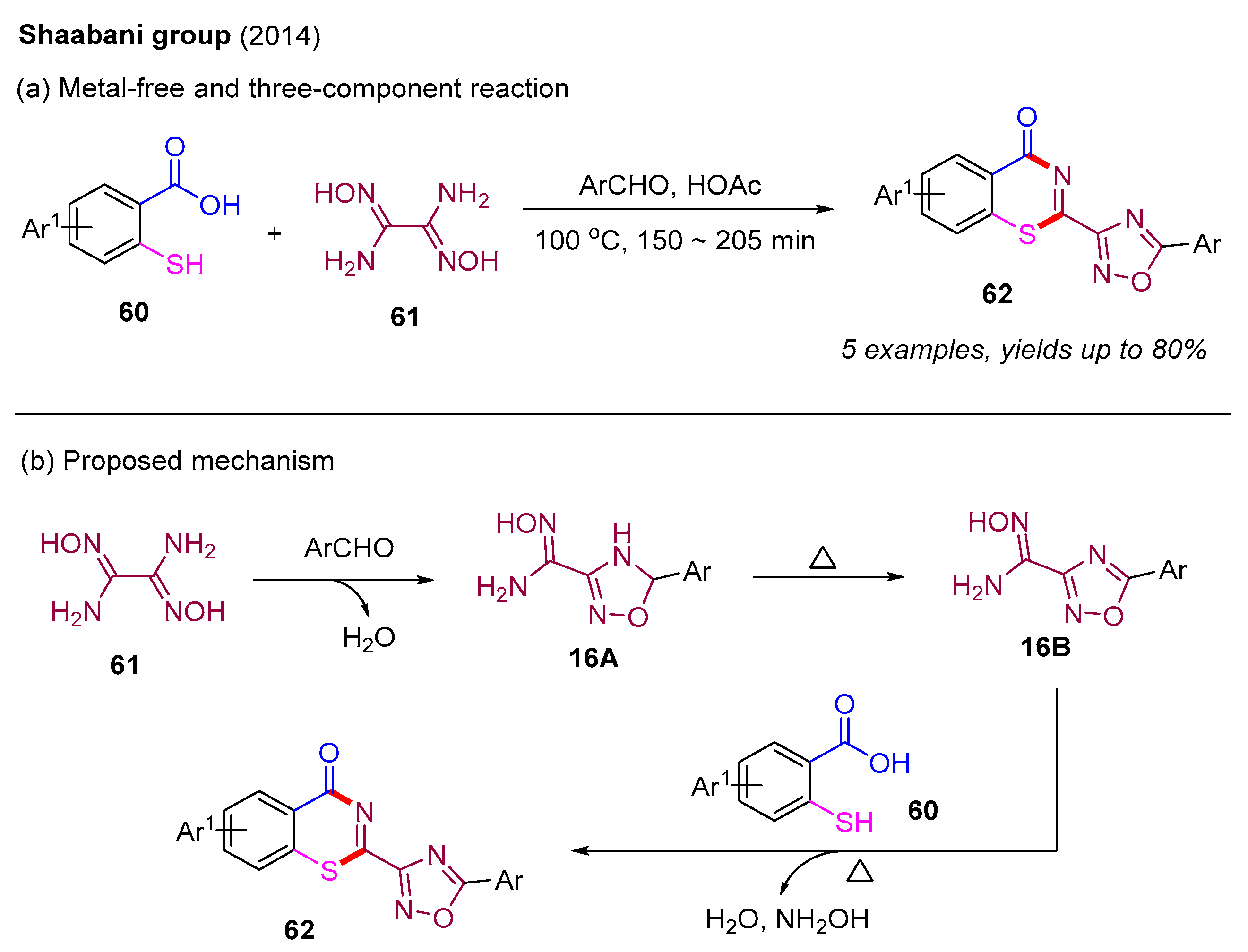
Currently, there are limited intermolecular methods available for constructing 4H-benzo[e][1,3]thiazin-4-ones. Early synthesis methods involve the condensation of 2-mercaptobenzoic acids with various nitrile compounds [60]. In 2014, the Shaabani group prepared a series of 4H-benzo[e][1,3]thiazin-4-one derivatives using 2-mercaptobenzoic acid, diaminoglyoxime, and aryl aldehydes in HOAc solvent (Scheme 21a) [61]. In this process, different substituted aryl aldehydes (MeO and Cl) at the para-, meta-, or ortho-position were suitable, providing the desired products in good yields. Additionally, a plausible mechanism is also depicted in Scheme 21b. The initial condensation reaction between diaminoglyoxime 61 and aryl aldehyde in the solvent of HOAc provides the intermediate 16A, which undergoes a subsequent oxidation to form the intermediate 16B. Next, 2-mercaptobenzoic acid 60 reacts with the intermediate 16B to produce the final product 62.
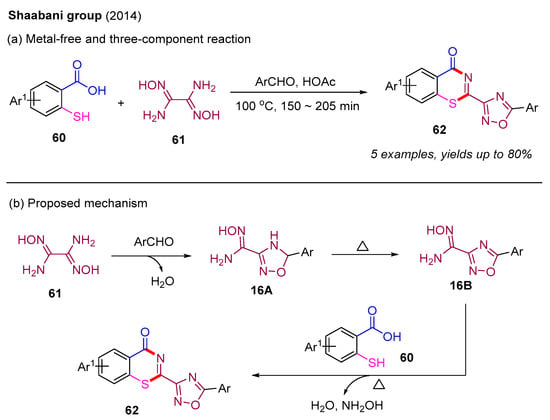
Scheme 21.
The synthesis of 4H-benzo[e][1,3]thiazin-4-one derivatives using 2-mercaptobenzoic acid, diaminoglyoxime, and aryl aldehydes [61].
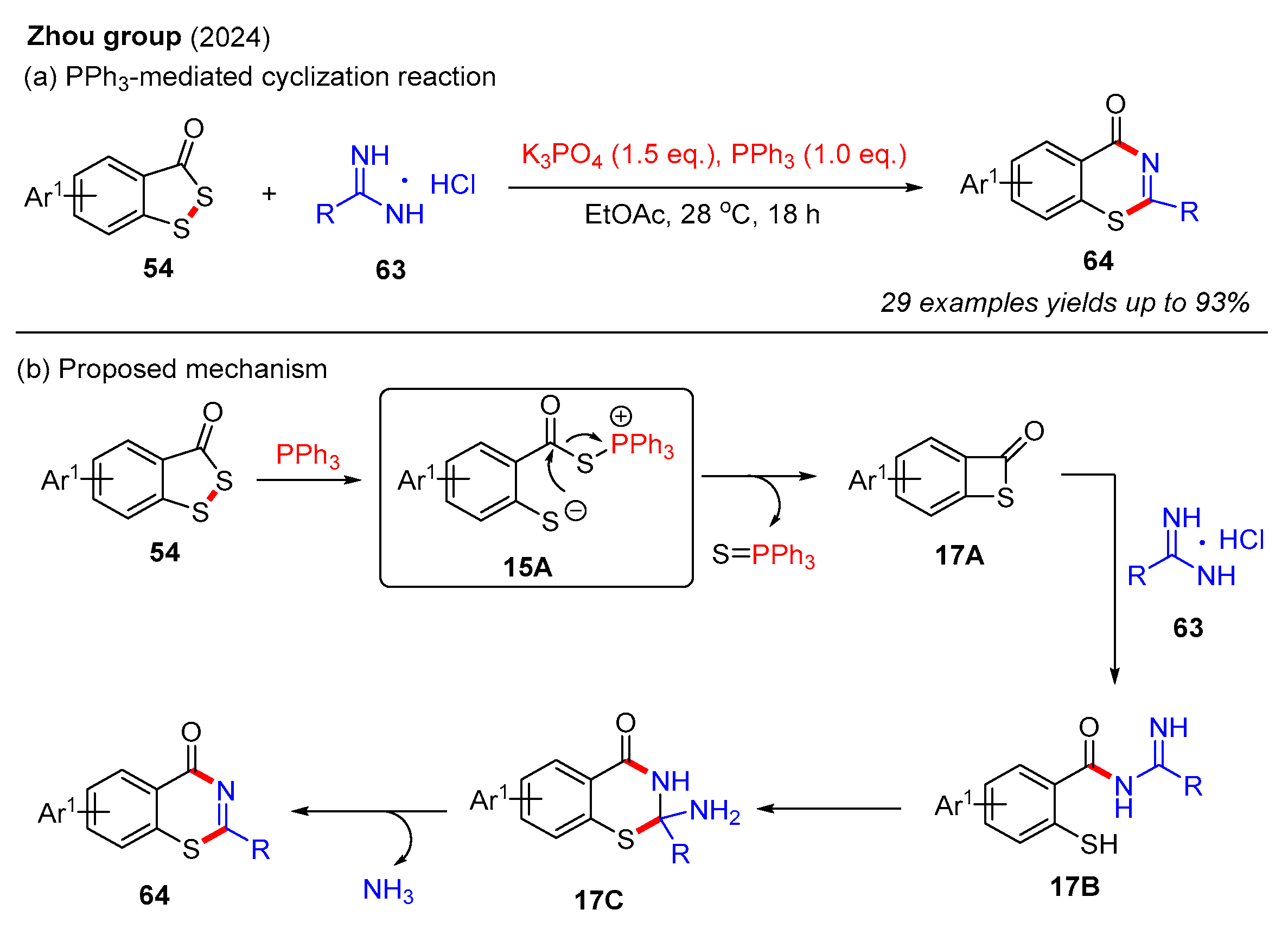
Recently, the Zhou group reported a novel PPh3-mediated cyclization reaction between benzodithiol-3-ones and amidines, resulting in the efficient synthesis of 4H-benzo[e][1,3]thiazin-4-one (Scheme 22a) [62]. This method facilitated the preparation of a diverse range of 4H-benzo[e][1,3]thiazin-4-one in moderate to good yields through the use of various benzodithiol-3-ones and amidines. In this reaction, K3PO4 is a crucial base for activating amidines. It should be noted that the reduction of 4H-benzo[e][1,3]thiazin-4-one with NaBH4 and MeOH afforded 2,3-dihydro-4H-benzo[e][1,3]thiazin-4-ones in good yields. A plausible reaction mechanism for this process is described in Scheme 22b. Initially, benzodithiol-3-one 54 reacts with PPh3 to form intermediate 15A, which is further converted into intermediate 17A via intramolecular nucleophilic attack. Subsequently, the unstable intermediate 17A is easily attacked by amidine 63 to generate intermediate 17B, which then undergoes intramolecular cyclization to afford intermediate 17C. Finally, the elimination of NH3 produces the desired product 64.
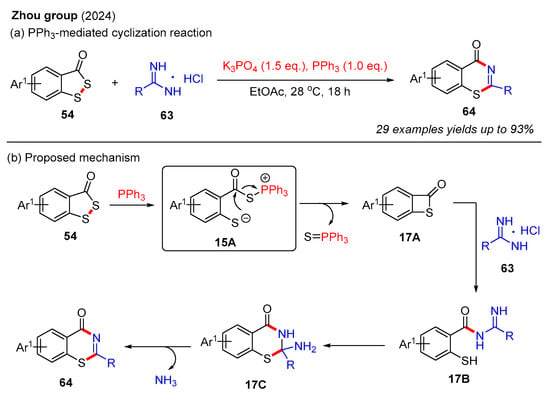
Scheme 22.
PPh3-mediated cyclization reaction of benzodithiol-3-ones and amidines for the construction of 4H-benzo[e][1,3]thiazin-4-ones [62].
5. Conclusions
In this review, we present a comprehensive overview of recent advancements in the synthesis of benzo[d]isothiazol-3(2H)-one and benzo[e][1,3]thiazin-4-one derivatives. The initial section outlines the intramolecular and intermolecular synthetic methodologies for benzo[d]isothiazol-3(2H)-ones. The intramolecular pathways primarily involve the formation of N–S bonds in 2-mercaptobenzamides or 2-alkylthiobenzamides through different methods, including thermal and electrochemical approaches. In contrast, intermolecular pathways are mainly achieved using 2-halobenzamides with S8, KSCN, and CS2 via transition-metal catalysis under thermal conditions. The second section primarily discusses the synthesis of 2,3-dihydro-4H-benzo[e][1,3]thiazin-4-ones via intermolecular strategies, which involve various sulfur-containing substrates (2-mercaptobenzoic acids, 2-mercaptobenzamides, 2-alkylthiobenzamides, benzoisothiazol-3-ones, and benzodithiol-3-ones) with carbon or amine sources. However, the intramolecular methods are only achieved by using 2-alkylthiobenzamides. The final section describes the synthesis of 4H-benzo[e][1,3]thiazin-4-ones, which can only be achieved via intermolecular pathways. Recent methods involve a three-component reaction among 2-mercaptobenzoic acid, diaminoglyoxime, and aryl aldehydes, as well as a PPh3-mediated cyclization reaction between benzodithiol-3-ones and amidines.
While some significant reports have been published, numerous aspects in this field still require further improvement. (1) The development of electrochemical or photochemical methods for the construction of heterocyclic compounds is currently a hot topic [63]. To date, only one electrochemical example for the synthesis of benzo[d]isothiazol-3(2H)-ones has been reported. Therefore, electrochemical and photochemical approaches may represent important future directions for the synthesis of these scaffolds. (2) It is worth noting that further studies on the synthesis of chiral 2,3-dihydro-4H-benzo[e][1,3]thiazin-4-ones are likely to become a key research focus. The aim of this review is to offer readers valuable insights and to inspire them to investigate innovative strategies for the synthesis of benzo[d]isothiazol-3(2H)-one and benzo[e][1,3]thiazin-4-one derivatives.
Author Contributions
Writing—original draft preparation, Y.H. and D.Y.; writing—review and editing, K.Y. and B.L. All authors have read and agreed to the published version of the manuscript.
Funding
K.Y. is grateful for the financial support from Changzhou University.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Kaur, B.; Singh, G.; Sharma, V.; Singh, I. Sulphur containing heterocyclic compounds as anticancer agents. Anticancer Agents Med. Chem. 2023, 23, 869–881. [Google Scholar] [PubMed]
- Obaid, R.J.; Mughal, E.U.; Naeem, N.; Al-Rooqi, M.M.; Sadiq, A.; Jassas, R.S.; Moussa, Z.; Ahmed, S.A. Pharmacological significance of nitrogen-containing five and six-membered heterocyclic scaffolds as potent cholinesterase inhibitors for drug discovery. Process Biochem. 2022, 120, 250–259. [Google Scholar] [CrossRef]
- Hemming, K. Heterocyclic chemistry. Annu. Rep. Prog. Chem. Sect. B Org. Chem. 2011, 107, 118–137. [Google Scholar] [CrossRef]
- Gopinath, P.; Yadav, R.K.; Shukla, P.K.; Srivastava, K.; Puri, S.K.; Muraleedharan, K.M. Broad spectrum anti-infective properties of benzisothiazolones and the parallels in their anti-bacterial and anti-fungal effects. Bioorg. Med. Chem. Lett. 2017, 27, 1291–1295. [Google Scholar] [CrossRef]
- Lai, H.; Dou, D.; Aravapalli, S.; Teramoto, T.; Lushington, G.H.; Mwania, T.M.; Alliston, K.R.; Eichhorn, D.M.; Padmanabhan, R.; Groutas, W.C. Design, synthesis and characterization of novel 1,2-benzisothiazol-3(2H)-one and 1,3,4-oxadiazole hybrid derivatives: Potent inhibitors of dengue and west nile virus NS2B/NS3 proteases. Bioorg. Med. Chem. 2013, 21, 102–113. [Google Scholar] [CrossRef] [PubMed]
- Obermeyer, L.; Dicke, K.; Skudlik, C.; Brans, R. Occupational allergic contact dermatitis from 2-butyl-1,2-benzisothiazol-3-one in cutting fluids: A case series. Contact Dermat. 2024, 90, 520–522. [Google Scholar] [CrossRef]
- Dahl, R.; Bravo, Y.; Sharma, V.; Ichikawa, M.; Dhanya, R.-P.; Hedrick, M.; Brown, B.; Rascon, J.; Vicchiarelli, M.; Mangravita-Novo, A.; et al. Potent, selective, and orally available benzoisothiazolone phosphomannose isomerase inhibitors as probes for congenital disorder of glycosylation la. J. Med. Chem. 2011, 54, 3661–3668. [Google Scholar] [CrossRef]
- Smith, S.M.E.; Min, J.; Ganesh, T.; Diebold, B.; Kawahara, T.; Zhu, Y.; McCoy, J.; Sun, A.; Snyder, J.P.; Fu, H.; et al. Ebselen and congeners inhibit NADPH oxidase 2-dependent superoxide generation by interrupting the binding of regulatory subunits. Chem. Biol. 2012, 19, 752–763. [Google Scholar] [CrossRef]
- Li, Q.; Yuan, D.; Liu, C.; Herington, F.; Yang, K.; Ge, H. Selective oxidation of benzo[d]isothiazol-3(2H)-ones enabled by Selectfluor. Molecules 2024, 29, 3899. [Google Scholar] [CrossRef]
- Solomon, V.R.; Haq, W.; Srivastava, K.; Puri, S.K.; Katti, S.B. Synthesis and antimalarial activity of side chain modified 4-aminoquinoline serivatives. J. Med. Chem. 2007, 50, 394–398. [Google Scholar] [CrossRef]
- Zarghi, A.; Zebardast, T.; Daraie, B.; Hedayati, M. Design and synthesis of new 1,3-benzthiazinan-4-one derivatives as selective cyclooxygenase (COX-2) inhibitors. Bioorg. Med. Chem. 2009, 17, 5369–5373. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Fang, K.; Dong, G.; Chen, S.; Liu, N.; Miao, Z.; Yao, J.; Li, J.; Zhang, W.; Sheng, C. Scaffold diversity inspired by the natural product evodiamine: Discovery of highly potent and multitargeting antitumor agents. J. Med. Chem. 2015, 58, 6678–6696. [Google Scholar] [CrossRef]
- Kimura, H.; Sato, Y.; Tajima, Y.; Suzuki, H.; Yukitake, H.; Imaeda, T.; Kajino, M.; Oki, H.; Takizawa, M.; Tanida, S. BTZO-1, a cardioprotective agent, reveals that macrophage migration inhibitory factor regulates ARE-mediated gene expression. Chem. Biol. 2010, 17, 1282–1294. [Google Scholar] [CrossRef]
- Tiwari, R.; Miller, P.A.; Chiarelli, L.R.; Mori, G.; Sarkan, M.; Centarova, I.; Cho, S.; Mikusova, K.; Franzblau, S.G.; Oliver, A.G.; et al. Design, syntheses, and anti-TB activity of 1,3-benzothiazinone azide and click chemistry products inspired by BTZ043. ACS Med. Chem. Lett. 2016, 7, 266–270. [Google Scholar] [CrossRef] [PubMed]
- Ohno, K.; Tsutsumi, R.; Matsumoto, N.; Yamashita, H.; Amada, Y.; Shishikura, J.-I.; Yatsugi, H.I.S.I.; Okada, M.; Sakamoto, S.; Yamaguchi, T. Functional characterization of YM928, a novel moncompetitive alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor antagonist. J. Pharmacol. Exp. Ther. 2003, 306, 66–72. [Google Scholar] [CrossRef]
- Paul, R.; Punniyamurthy, T. Copper-catalysed one-pot synthesis of N-substituted benzo[d]isothiazol-3(2H)-ones via C−S/N−S bond formation. RSC Adv. 2012, 2, 7057–7060. [Google Scholar] [CrossRef]
- Wang, F.; Chen, C.; Deng, G.; Xi, C. Concise approach to benzisothiazol-3(2H)-one via copper-catalyzed tandem reaction of o-bromobenzamide and potassium thiocyanate in water. J. Org. Chem. 2012, 77, 4148–4151. [Google Scholar] [CrossRef]
- Clerici, F.; Gelmi, M.L.; Pellegrino, S.; Pocar, D. Chemistry of biologically active isothiazoles. Top. Heterocycl. Chem. 2007, 9, 179–264. [Google Scholar]
- Li, S.; Hong, H.; Zhu, N.; Han, L.; Lu, J. Review about the synthesis of 1,3-benzothiazinone derivatives. Chin. J. Org. Chem. 2016, 36, 2024–2038. [Google Scholar] [CrossRef][Green Version]
- Nosova, E.V.; Lipunova, G.N.; Charushin, V.N.; Chupakhin, O.N. Synthesis and biological activity of 2-amino- and 2-aryl (heteryl)substituted 1,3-benzothiazin-4-ones. Mini-Rev. Med. Chem. 2019, 19, 999–1014. [Google Scholar] [CrossRef]
- Sainsbury, M. Oxazines, thiazines and their benzo derivatives. In Comprehensive Heterocyclic Chemistry, 1st ed.; Katritzky, A.R., Rees, C.W., Eds.; Pergamon Press: Oxford, UK, 1984; Volume 3, pp. 995–1038. [Google Scholar]
- Potts, K.T. Synthesis of five-membered rings with two or more heteroatoms. In Comprehensive Heterocyclic Chemistry, 1st ed.; Katritzky, A.R., Rees, C.W., Eds.; Pergamon Press: Oxford, UK, 1984; Volume 5, pp. 111–166. [Google Scholar]
- Pain, D.L.; Peart, B.J.; Wooldridge, K.R.H. Isothiazoles and their benzo derivatives. In Comprehensive Heterocyclic Chemistry, 1st ed.; Katritzky, A.R., Rees, C.W., Eds.; Pergamon Press: Oxford, UK, 1984; Volume 6, pp. 131–175. [Google Scholar]
- Ivanova, Y.; Smoljo, M.; De Jonghe, S.; Dehaen, W. Synthesis of benzo[d]isothiazoles: An update. Archivoc 2024, 202312146. [Google Scholar] [CrossRef]
- Wang, Z.; Kuninobu, Y.; Kanai, M. Copper-catalyzed intramolecular N−S bond formation by oxidative dehydrogenative cyclization. J. Org. Chem. 2013, 78, 7337–7342. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Song, L.; Tang, S.; Li, L.; Li, H.; Yuan, B.; Yang, G. Co-catalyzed intramolecular S−N bond formation in water for 1,2-benzisothiazol-3(2H)-ones and 1,2,4-thiadiazoles synthesis. Eur. J. Org. Chem. 2019, 2019, 1281–1285. [Google Scholar] [CrossRef]
- Yu, T.-Q.; Hou, Y.-S.; Jiang, Y.; Xu, W.-X.; Shi, T.; Wu, X.; Zhang, J.-C.; He, D.; Wang, Z. Potassium bromide catalyzed N−S bond formation via oxidative dehydrogenation. Tetrahedron Lett. 2017, 58, 2084–2087. [Google Scholar] [CrossRef]
- Gopinath, P.; Nilaya, S.; Debi, T.R.; Ramkumar, V.; Muraleedharan, K.M. As many as six tandem reactions in one step! Unprecedented formation of highly functionalized benzothiophenes. Chem. Commun. 2009, 7131–7133. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Xu, H.-C. Electrochemical generation of nitrogen-centered radicals for organic synthesis. Green Synth. Catal. 2021, 2, 165–178. [Google Scholar] [CrossRef]
- Wu, Y.; Chen, J.Y.; Liao, H.R.; Shu, X.R.; He, W.-M. Electrochemical transient iodination and coupling for selenylated 4-anilinocoumarin synthesis. Green Synth. Catal. 2021, 2, 233–236. [Google Scholar] [CrossRef]
- Zhong, Q.; Xiong, Z.; Sheng, S.; Chen, J. Electrochemical synthesis for benzisothiazol-3(2H)-ones by dehydrogenative N−S bond formation. Tetrahedron Lett. 2021, 80, 153323. [Google Scholar] [CrossRef]
- Laudadio, G.; Barmpoutsis, E.; Schotten, C.; Struik, L.; Govaerts, S.; Browne, D.L.; Noel, T. Sulfonamide synthesis through electrochemical oxidative coupling of amines and thiols. J. Am. Chem. Soc. 2019, 141, 5664–5668. [Google Scholar] [CrossRef]
- Nyffeler, P.T.; Duron, S.G.; Burkart, M.D.; Vincent, S.P.; Wong, C.-H. Selectfluor: Mechanistic insight and applications. Angew. Chem. Int. Ed. 2005, 44, 192–212. [Google Scholar] [CrossRef]
- Stavber, S. Recent advances in the application of SelectfluorTMF-TEDA-BF4 as a versatile mediator or catalyst in organic synthesis. Molecules 2011, 16, 6432–6464. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Song, M.; Ali, A.; Mudassir, S.; Ge, H. Recent advances in the application of selectfluor as a “fluorine-free” functional reagent in organic synthesis. Chem. Asian J. 2020, 15, 729–741. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Li, Y.; Ma, Z.; Tang, L.; Yin, Y.; Zhang, H.; Li, Z.; Sun, X. Metal-free C−S bond cleavage to access N-substituted acrylamide and β-aminopropanamide. Eur. J. Org. Chem. 2019, 2019, 5812–5814. [Google Scholar] [CrossRef]
- Yang, K.; Li, Y.; Song, M.; Dai, S.; Li, Z.-Y.; Sun, X. Metal-free direct C(sp3)−H functionalization of 2-alkylthiobenzoic acid to access 1,3-benzooxathiin-4-one. Chin. Chem. Lett. 2021, 32, 146–149. [Google Scholar] [CrossRef]
- Yang, K.; Zhang, H.; Niu, B.; Tang, T.; Ge, H. Benzisothiazol-3-ones through a metal-free intramolecular N–S bond formation. Eur. J. Org. Chem. 2018, 2018, 5520–5523. [Google Scholar] [CrossRef]
- Dai, S.; Yang, K.; Luo, Y.; Xu, Z.; Li, Z.; Li, Z.-Y.; Li, B.; Sun, X. Metal-free and Selectfluor-mediated diverse transformations of 2-alkylthiobenzamides to access 2,3-dihydrobenzothiazin-4-ones, benzoisothiazol-3-ones and 2-alkylthiobenzonitriles. Org. Chem. Front. 2022, 9, 4016–4022. [Google Scholar] [CrossRef]
- Xiong, J.; Zhong, G.; Liu, Y. Domino reactions initiated by copper-catalyzed aryl-I bond thiolation for the switchable synthesis of 2,3-dihydrobenzothiazinones and benzoisothiazolones. Adv. Synth. Catal. 2019, 361, 550–555. [Google Scholar] [CrossRef]
- Dhara, S.; Saha, M.; Das, A.R. Ligand-free access to benzisothiazolones and benzisoselenazolones through NiFe2O4 catalyzed concomitant annulation of 2-halobenzanilides with chalcogens and their late-stage transformations. New J. Chem. 2022, 46, 19501–19513. [Google Scholar] [CrossRef]
- Paul, S.; Pradhan, K.; Ghosh, S.; De, S.K.; Das, A.R. Magnetically retrievable nano crystalline nickel ferrite-catalyzed aerobic, ligand-free C–N, C–O and C–C cross-coupling reactions for the synthesis of a diversified library of heterocyclic molecules. Adv. Synth. Catal. 2014, 356, 1301–1306. [Google Scholar] [CrossRef]
- Li, T.; Yang, L.; Ni, K.; Shi, Z.; Li, F.; Chen, D. An efficient approach to construct benzisothiazol3(2H)-ones via copper-catalyzed consecutive reaction of 2-halobenzamides and carbon disulfide. Org. Biomol. Chem. 2016, 14, 6297–6303. [Google Scholar] [CrossRef]
- Yang, K.; Song, M.; Liu, H.; Ge, H. Palladium-catalyzed direct asymmetric C–H bond functionalization enabled by the directing group strategy. Chem. Sci. 2020, 11, 12616–12632. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Wang, H.; Li, Z.; Tang, L.; Sun, X.; Yang, K. Transition-metal-catalyzed remote C–H functionalization of thioethers. RSC Adv. 2022, 12, 10835–10845. [Google Scholar] [CrossRef] [PubMed]
- Niu, B.; Yang, K.; Lawrence, B.; Ge, H. Transient ligand-enabled transition metal-catalyzed C–H functionalization. ChemSusChem 2019, 12, 2955–2969. [Google Scholar] [CrossRef]
- Yang, K.; Song, M.; Ma, Z.; Li, Y.; Li, Z.; Sun, X. The decarboxylative C−H heteroarylation of azoles catalysed by nickel catalysts to access unsymmetrical biheteroaryls. Org. Chem. Front. 2019, 6, 3996–3999. [Google Scholar] [CrossRef]
- Xu, Z.; Li, Z.; Liu, C.; Yang, K.; Ge, H. Palladium-catalyzed β-C(sp3)–H bond arylation of tertiary aldehydes facilitated by 2-pyridone ligands. Molecules 2024, 29, 259. [Google Scholar] [CrossRef]
- Yuan, D.; Xu, Z.; Zhou, Y.; Herington, F.; Liu, C.; Yang, K.; Ge, H. Palladium-catalyzed cascade reactions for synthesis of heterocycles initiated by C(sp3)–H functionalization. Catalysts 2025, 15, 72. [Google Scholar] [CrossRef]
- Chen, F.-J.; Liao, G.; Li, X.; Wu, J.; Shi, B.-F. Cu(II)-mediated C−S/N−S bond formation via C−H activation: Access to benzoisothiazolones using elemental sulfur. Org. Lett. 2014, 16, 5644–5647. [Google Scholar] [CrossRef]
- Yang, K.; Niu, B.; Ma, Z.; Wang, H.; Lawrence, B.; Ge, H. Silver-promoted site-selective intramolecular cyclization of 2-methylthiobenzamide through α-C(sp3)−H functionalization. J. Org. Chem. 2019, 84, 14045–14052. [Google Scholar] [CrossRef] [PubMed]
- Unsworth, W.P.; Kitsiou, C.; Taylor, R.J.K. Direct imine acylation: Rapid access to diverse heterocyclic scaffolds. Org. Lett. 2013, 15, 258–261. [Google Scholar] [CrossRef]
- Kitsiou, C.; Unsworth, W.P.; Coulthard, G.; Taylor, R.J.K. Substrate scope in the direct imine acylation of ortho-substituted benzoic acid derivatives: The total synthesis (±)-cavidine. Tetrahedron 2014, 70, 7172–7180. [Google Scholar] [CrossRef]
- Silverberg, L.J.; Pacheco, C.; Sahu, D.; Scholl, P.; Sobhi, H.F.; Bachert, J.T.; Bandholz, K.; Bendinsky, R.V.; Bradley, H.G.; Colburn, B.K.; et al. T3P-promoted synthesis of a series of novel 3-aryl-2-phenyl-2,3-dihydro-4H-1,3-benzothiazin-4-ones. Heterocyclic Chem. 2020, 57, 1797–1805. [Google Scholar] [CrossRef]
- Azizi, N.; Farzaneh, F.; Habibnejad, N. Recyclable magnetic camphor sulfonic acid: A reliable and highly efficient ionic organocatalyst for benzothiazin-4-one synthesis in green media. Catal. Lett. 2022, 152, 3146–3157. [Google Scholar] [CrossRef]
- Wang, H.-H.; Shi, T.; Gao, W.-W.; Zhang, H.-H.; Wang, Y.-Q.; Li, J.-F.; Hou, Y.-S.; Chen, J.-H.; Peng, X.; Wang, Z. Double 1,4-addition of (thio)salicylamides/thiosalicylic acids with propiolate derivatives: A direct, general synthesis of diverse heterocyclic scaffolds. Org. Biomol. Chem. 2017, 15, 8013–8017. [Google Scholar] [CrossRef]
- Yang, K.; Li, Q.; Luo, Y.; Yuan, D.; Qi, C.; Li, Z.; Li, B.; Sun, X. Transition-metal-free skeletal editing of benzoisothiazol-3-ones to 2,3-dihydrobenzothiazin-4-ones via single-carbon insertion. Org. Chem. Front. 2025, 12, 478–484. [Google Scholar] [CrossRef]
- Zhang, G.; Wan, H.; Dong, N.; Zhu, A.; Zhou, Y.; Song, Q. Metal-free three-component tandem cyclization for modular synthesis of 2,3-dihydrobenzothiazin4-ones. Org. Chem. Front. 2024, 11, 2021–2026. [Google Scholar] [CrossRef]
- Zhang, B.; He, S.; Dong, N.; Zhu, A.; Duan, H.; Wang, D.; Zhou, Y. Substituent-controlled divergent cyclization reactions of benzo[c][1,2]dithiol-3-ones and hexahydro-1,3,5-triazines. Org. Chem. Front. 2024, 11, 3302–3307. [Google Scholar] [CrossRef]
- Ibrahim, N.S.; Abed, N.M.; Kandeel, Z.E. Nitriles in heterocyclic synthesis: A new approach for the synthesis of thiazinones. Heterocycles 1984, 22, 1677–1682. [Google Scholar] [CrossRef]
- Khanmiri, R.H.; Moghimi, A.; Shaabani, A.; Valizadeh, H.; Ng, S.W. Diaminoglyoxime as a versatile reagent in the synthesis of bis(1,2,4-oxadiazoles), 1,2,4-oxadiazolyl-quinazolines and 1,2,4-oxadiazolyl-benzothiazinones. Mol. Divers. 2014, 18, 769–776. [Google Scholar] [CrossRef]
- Liu, X.; Lv, W.; Dong, J.; Liu, Z.; Hu, F.; Zhou, C.; Zhou, Y. Synthesis of benzo[e][1,3]thiazin-4-ones via PPh3-promoted cyclization of benzo[c][1,2]dithiol-3-ones and amidines. Adv. Synth. Catal. 2024, 366, 1978–1982. [Google Scholar] [CrossRef]
- Tian, X.; Liu, Y.; Yakubov, S.; Schutte, J.; Chiba, S.; Barham, J.P. Photo- and electro-chemical strategies for the activations of strong chemical bonds. Chem. Soc. Rev. 2024, 53, 263–316. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).