The Impact of Thermal and Electrical Pretreatments and Antibrowning Solution on the Chlorogenic and Dicaffeoylquinic Acid Extraction Yield from Endive Roots
Abstract
1. Introduction
2. Results and Discussion
2.1. Extracted Yield
2.1.1. Effect of Cossette Size on Extracted Yield
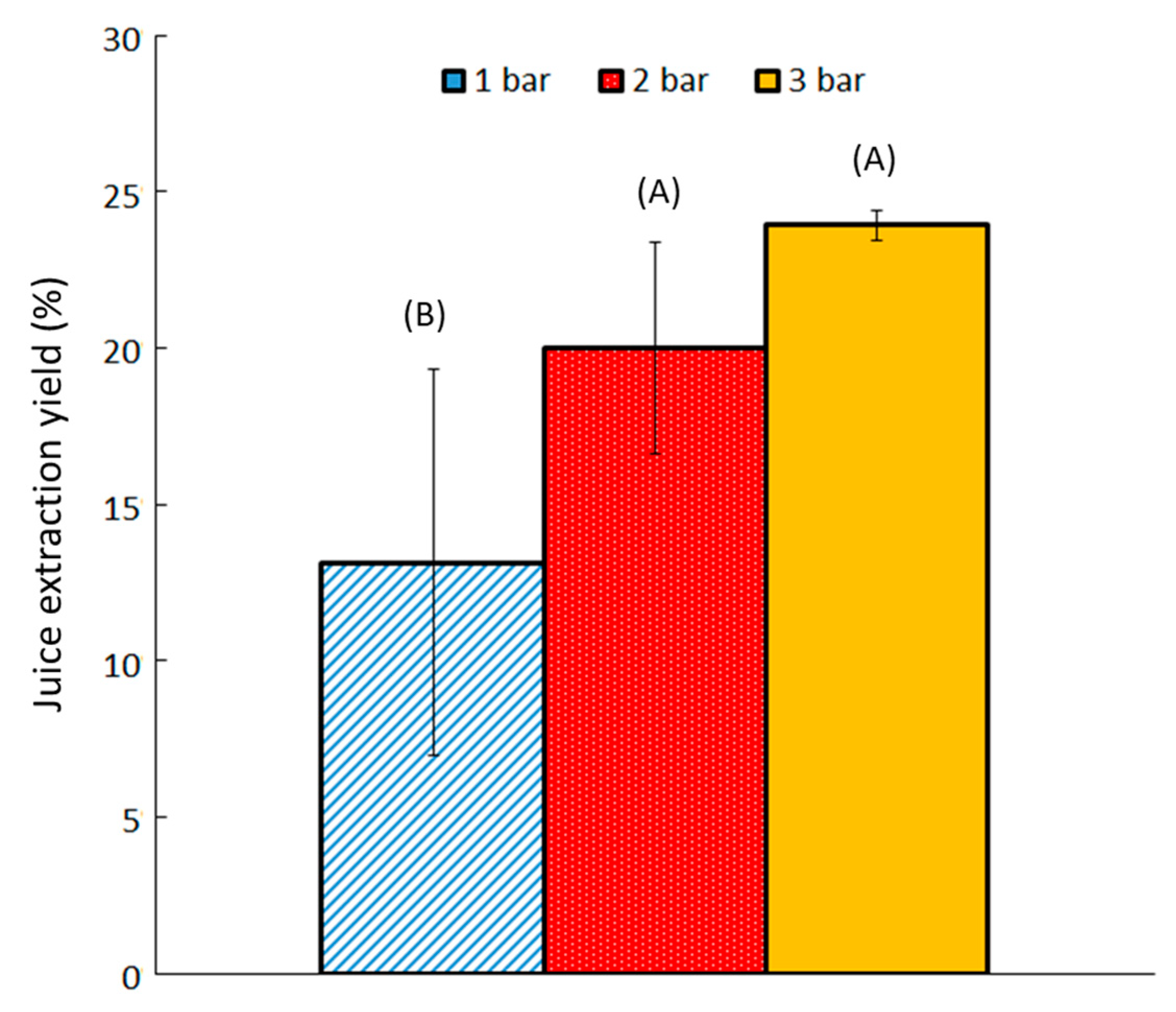
2.1.2. Effect of Working Pressure on Extracted Yield
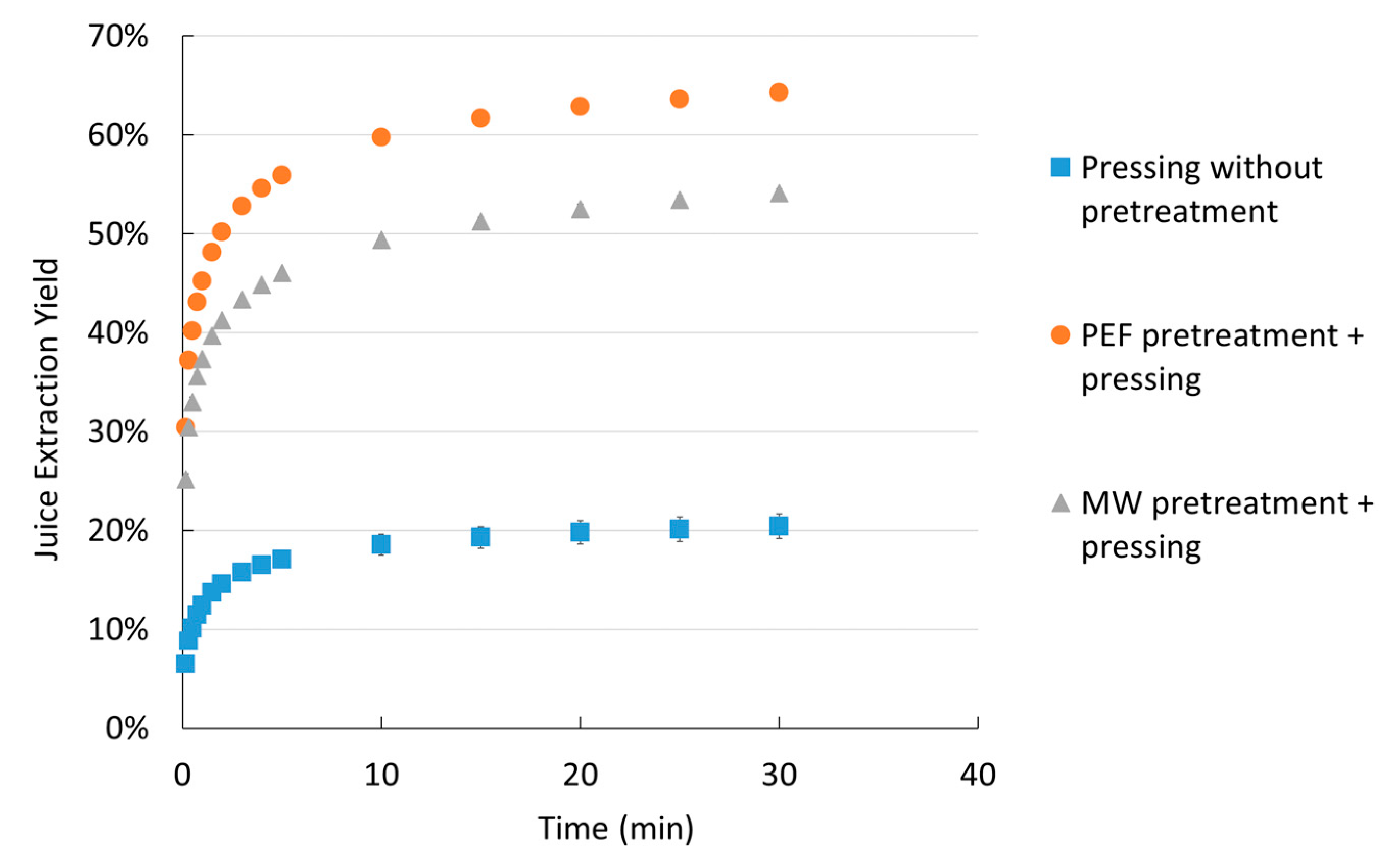
2.1.3. Effect of Pretreatment on Extracted Yield
2.2. Juice Characterization
2.2.1. Total Soluble Matter (°Brix)
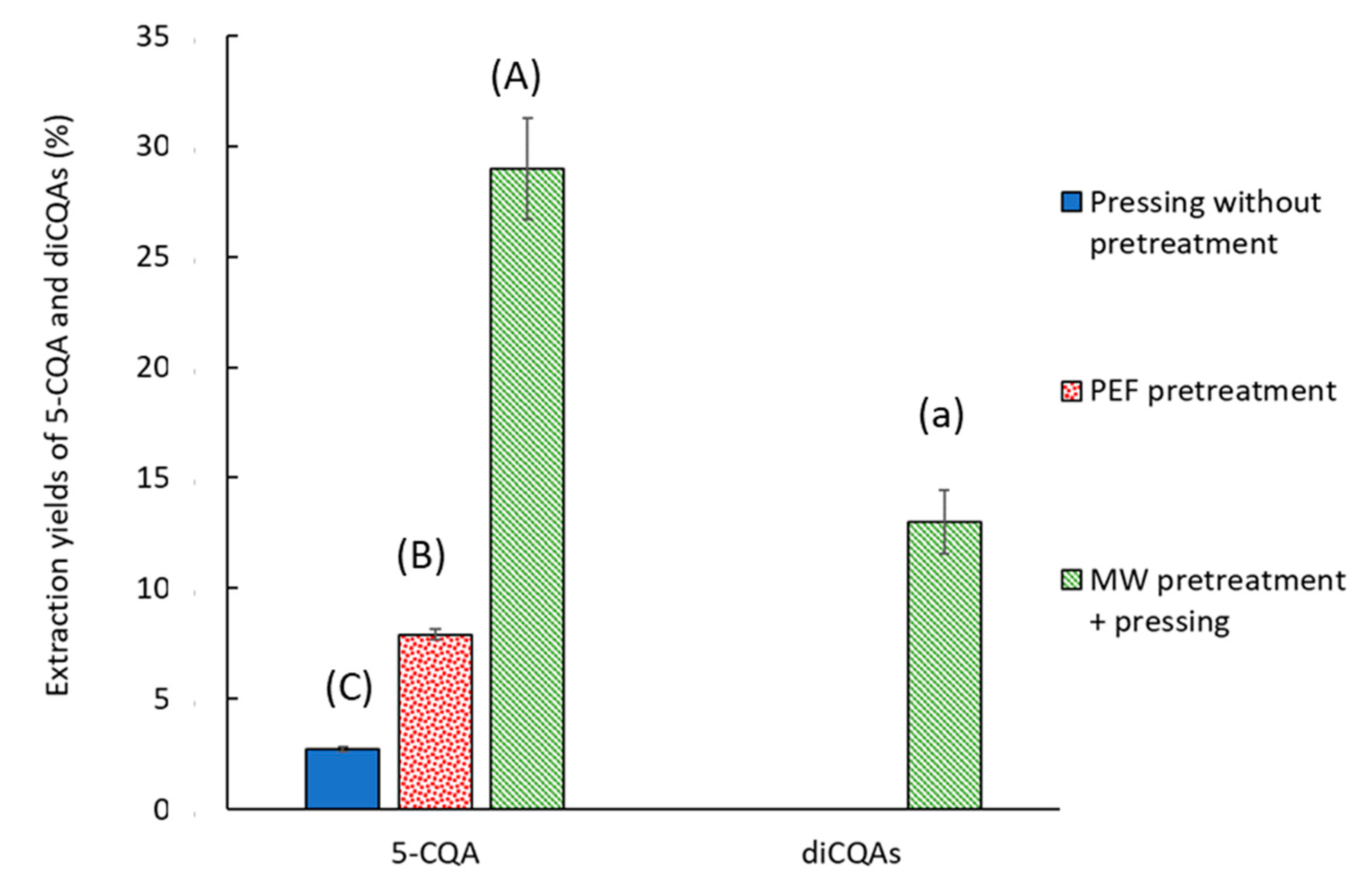
2.2.2. CQA Content
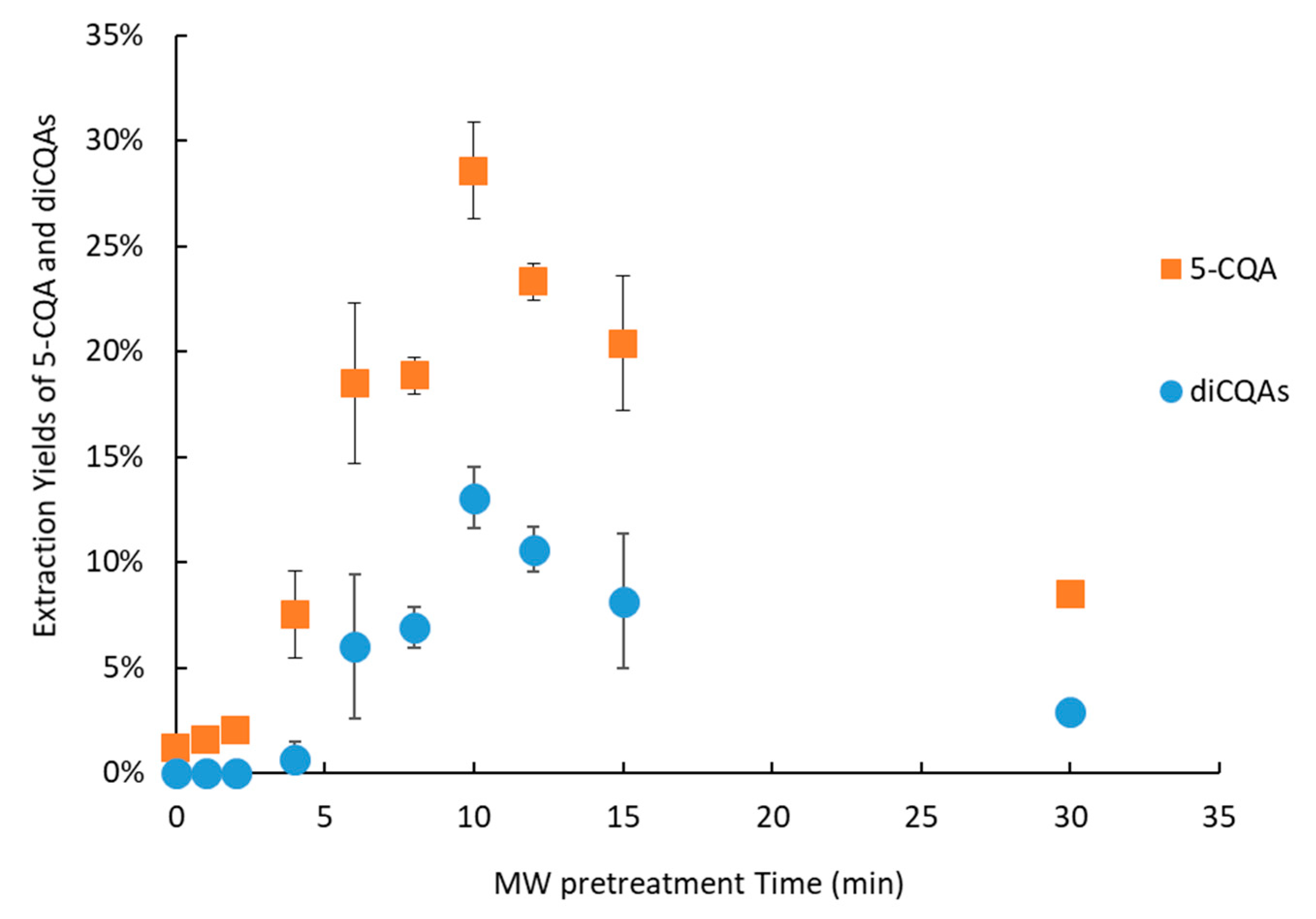
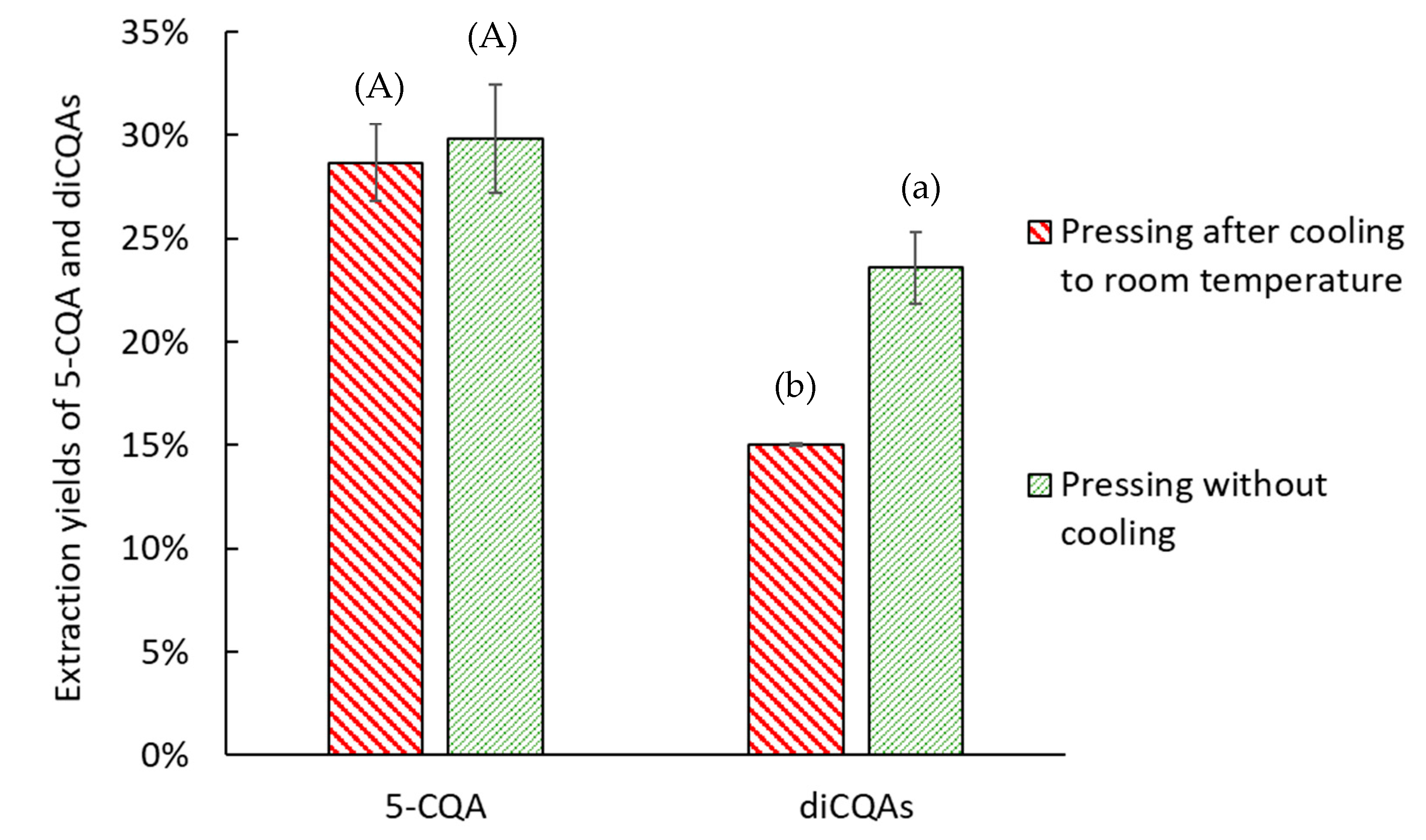
2.3. Effect of Thermal Pretreatment
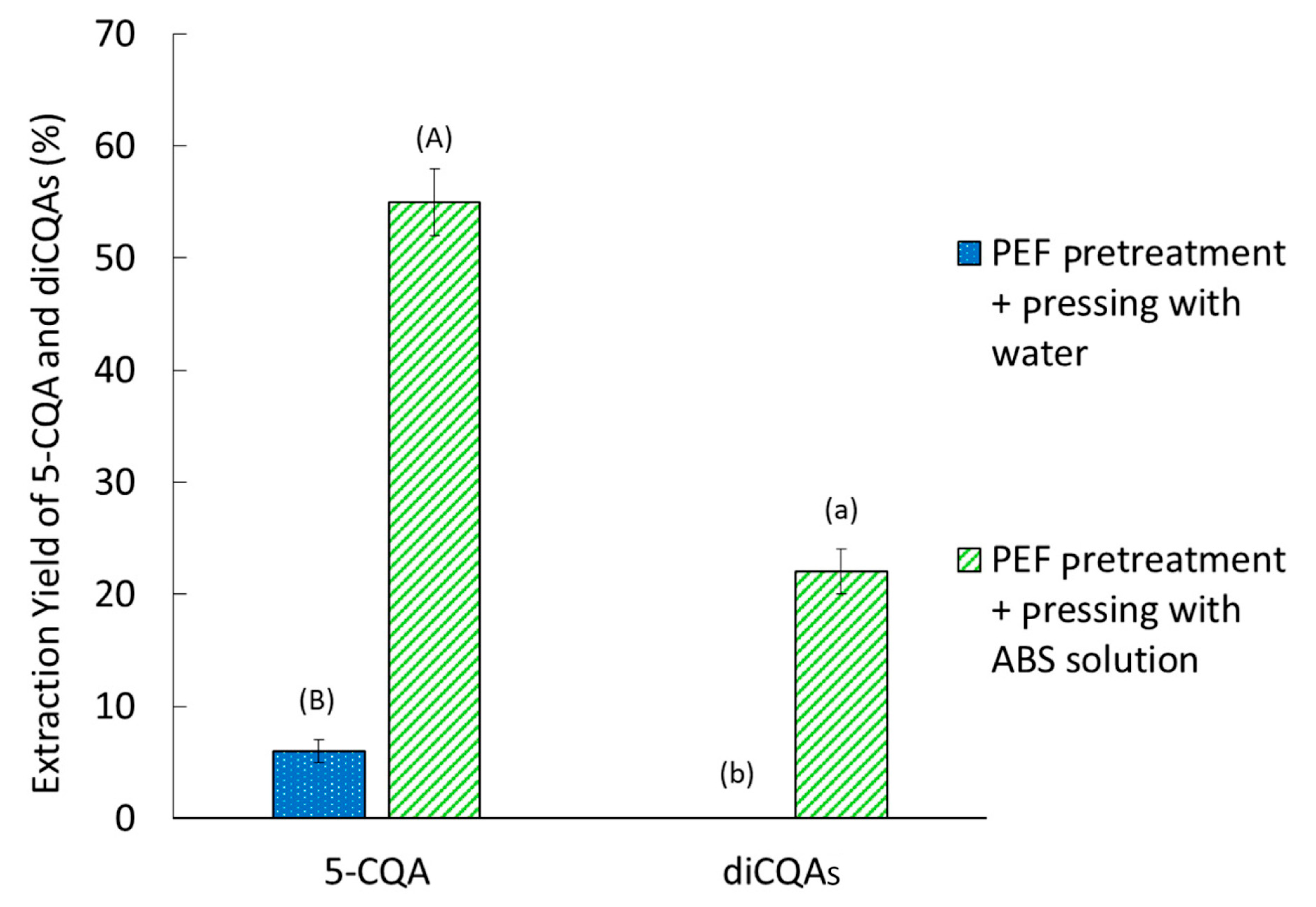
2.4. Effect of Water Addition
2.5. Effect of PEF + ABS Pretreatment on FER CQA Content
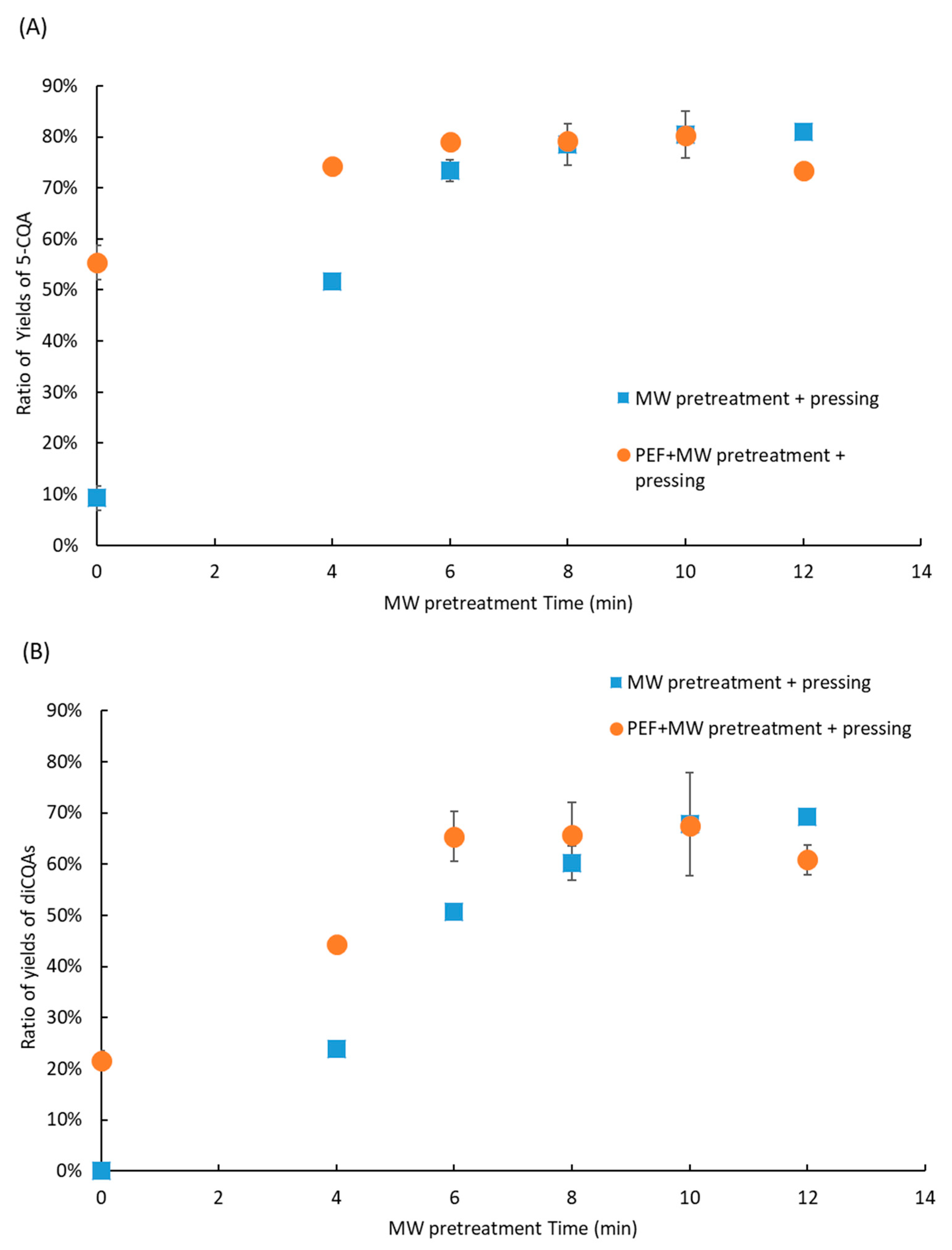
2.6. Coupling Pretreatments and Antibrowning Treatment
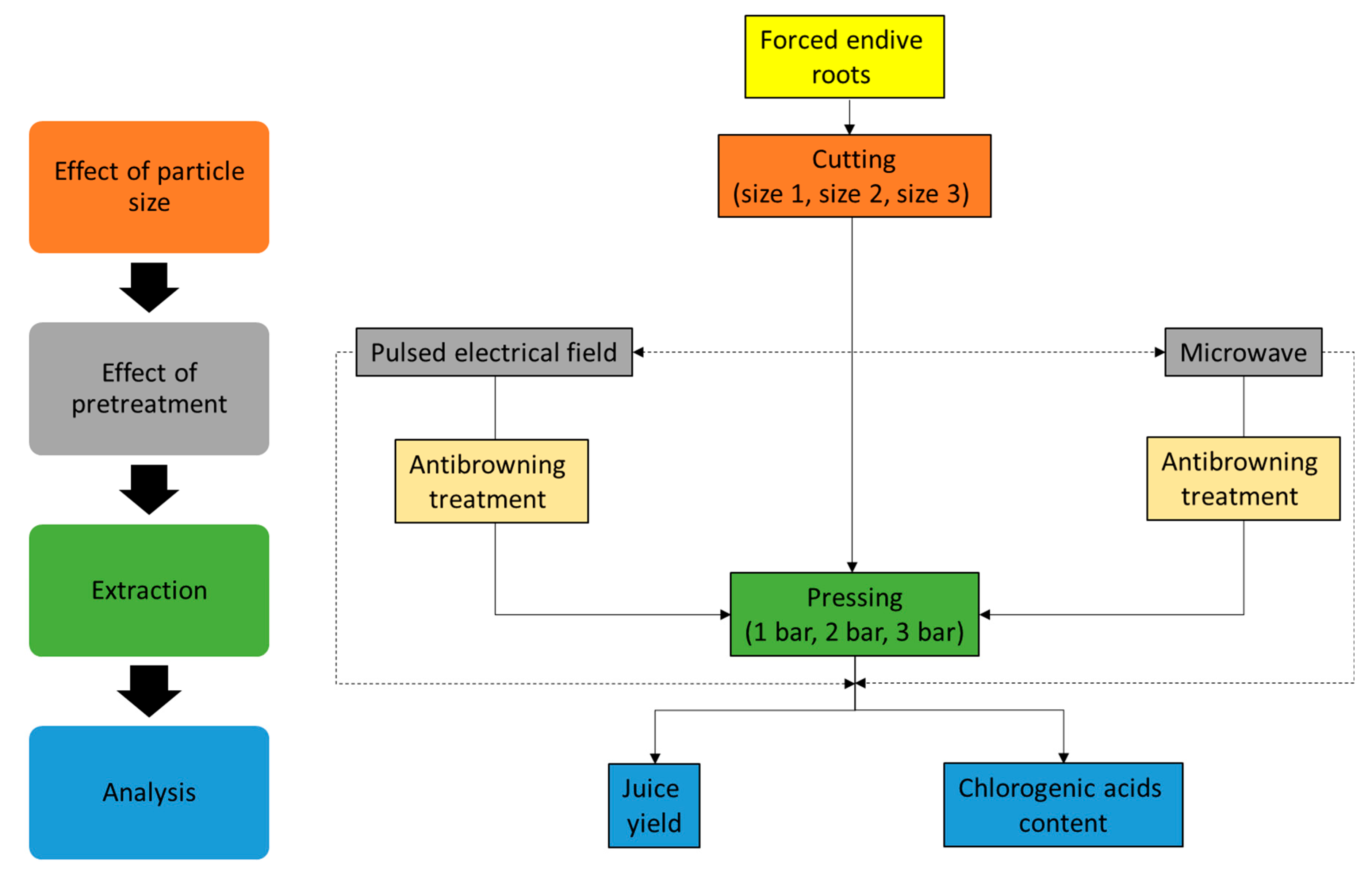
3. Materials and Methods
3.1. Raw Materials
3.2. Analytical Reagents and Chemicals
3.3. Extraction Procedure
3.3.1. Pressing Experiments
3.3.2. Diffusion Experiments
3.4. Physical and Chemical Pretreatments
3.4.1. Pulsed Electrical Field Pretreatment
3.4.2. Microwave Pretreatment
3.4.3. Antibrowning Treatment
3.5. Analytical Measurements
3.5.1. Soluble Matter
3.5.2. HPLC Analysis
3.6. CQA Extraction
3.7. Energy Consumption Measurement
3.8. Statistical Analyses
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Chadni, M.; Isidore, E.; Diemer, E.; Ouguir, O.; Brunois, F.; Catteau, R.; Cassan, L.; Ioannou, I. Optimization of Extraction Conditions to Improve Chlorogenic Acid Content and Antioxidant Activity of Extracts from Forced Witloof Chicory Roots. Foods 2022, 11, 1217. [Google Scholar] [CrossRef]
- Sinkovič, L.; Hribar, J.; Znidarcic, D.; Treutter, D.; Vidrih, R. Comparison of the Phenolics Profiles of Forced and Unforced Chicory (Cichorium intybus L.) Cultivars. Agric. Conspec. Sci. 2014, 79, 133–137. [Google Scholar]
- Stökle, K.; Hülsemann, B.; Steinbach, D.; Cao, Z.; Oechsner, H.; Kruse, A. A Biorefinery Concept Using Forced Chicory Roots for the Production of Biogas, Hydrochar, and Platform Chemicals. Biomass Convers. Biorefinery 2021, 11, 1453–1463. [Google Scholar] [CrossRef]
- Twarogowska, A.; Van Poucke, C.; Van Droogenbroeck, B. Upcycling of Belgian Endive (Cichorium intybus Var. foliosum) by-Products. Chemical Composition and Functional Properties of Dietary Fibre Root Powders. Food Chem. 2020, 332, 127444. [Google Scholar] [CrossRef]
- Dibert, K.; Cros, E.; Andrieu, J. Solvent Extraction of Oil and Chlorogenic Acid from Green Coffee. Part II. Kinetic Data. J. Food Eng. 1989, 10, 199–214. [Google Scholar] [CrossRef]
- Shen, Z.; Ji, X.; Yao, S.; Zhang, H.; Xiong, L.; Li, H.; Chen, X.; Chen, X. Solid-Liquid Extraction of Chlorogenic Acid from Eucommia Ulmoides oliver Leaves: Kinetic and Mass Transfer Studies. Ind. Crops Prod. 2023, 205, 117544. [Google Scholar] [CrossRef]
- Safdar, M.N.; Kausar, T.; Jabbar, S.; Mumtaz, A.; Ahad, K.; Saddozai, A.A. Extraction and Quantification of Polyphenols from Kinnow (Citrus reticulate L.) Peel Using Ultrasound and Maceration Techniques. J. Food Drug Anal. 2017, 25, 488–500. [Google Scholar] [CrossRef]
- Vieira, V.; Prieto, M.A.; Barros, L.; Coutinho, J.A.P.; Ferreira, O.; Ferreira, I.C.F.R. Optimization and Comparison of Maceration and Microwave Extraction Systems for the Production of Phenolic Compounds from Juglans regia L. for the Valorization of Walnut Leaves. Ind. Crops Prod. 2017, 107, 341–352. [Google Scholar] [CrossRef]
- Náthia-Neves, G.; Alonso, E. Valorization of Sunflower By-Product Using Microwave-Assisted Extraction to Obtain a Rich Protein Flour: Recovery of Chlorogenic Acid, Phenolic Content and Antioxidant Capacity. Food Bioprod. Process. 2021, 125, 57–67. [Google Scholar] [CrossRef]
- Jabbar, S.; Abid, M.; Wu, T.; Hashim, M.M.; Saeeduddin, M.; Hu, B.; Lei, S.; Zeng, X. Ultrasound-Assisted Extraction of Bioactive Compounds and Antioxidants from Carrot Pomace: A Response Surface Approach. J. Food Process. Preserv. 2015, 39, 1878–1888. [Google Scholar] [CrossRef]
- Pradal, D.; Vauchel, P.; Decossin, S.; Dhulster, P.; Dimitrov, K. Kinetics of Ultrasound-Assisted Extraction of Antioxidant Polyphenols from Food by-Products: Extraction and Energy Consumption Optimization. Ultrason. Sonochem. 2016, 32, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Bouhzam, I.; Cantero, R.; Balcells, M.; Margallo, M.; Aldaco, R.; Bala, A.; Fullana-i-Palmer, P.; Puig, R. Environmental and Yield Comparison of Quick Extraction Methods for Caffeine and Chlorogenic Acid from Spent Coffee Grounds. Foods 2023, 12, 779. [Google Scholar] [CrossRef] [PubMed]
- Caballero, A.S.; Romero-García, J.M.; Castro, E.; Cardona, C.A. Supercritical Fluid Extraction for Enhancing Polyphenolic Compounds Production from Olive Waste Extracts. J. Chem. Technol. Biotechnol. 2020, 95, 356–362. [Google Scholar] [CrossRef]
- Pataro, G.; Bobinaitė, R.; Bobinas, Č.; Šatkauskas, S.; Raudonis, R.; Visockis, M.; Ferrari, G.; Viškelis, P. Improving the Extraction of Juice and Anthocyanins from Blueberry Fruits and Their by-Products by Application of Pulsed Electric Fields. Food Bioprocess Technol. 2017, 10, 1595–1605. [Google Scholar] [CrossRef]
- Faugno, S.; Piccolella, S.; Sannino, M.; Principio, L.; Crescente, G.; Baldi, G.M.; Fiorentino, N.; Pacifico, S. Can Agronomic Practices and Cold-Pressing Extraction Parameters Affect Phenols and Polyphenols Content in Hempseed Oils? Ind. Crops Prod. 2019, 130, 511–519. [Google Scholar] [CrossRef]
- Luengo, E.; Álvarez, I.; Raso, J. Improving the Pressing Extraction of Polyphenols of Orange Peel by Pulsed Electric Fields. Innov. Food Sci. Emerg. Technol. 2013, 17, 79–84. [Google Scholar] [CrossRef]
- Laroze, L.E.; Díaz-Reinoso, B.; Moure, A.; Zúñiga, M.E.; Domínguez, H. Extraction of Antioxidants from Several Berries Pressing Wastes Using Conventional and Supercritical Solvents. Eur. Food Res. Technol. 2010, 231, 669–677. [Google Scholar] [CrossRef]
- Siger, A.; Nogala-Kalucka, M.; Lampart-Szczapa, E. The Content and Antioxidant Activity of Phenolic Compounds in Cold-Pressed Plant Oils. J. Food Lipids 2008, 15, 137–149. [Google Scholar] [CrossRef]
- Michel, T.; Destandau, E.; Elfakir, C. Evaluation of a Simple and Promising Method for Extraction of Antioxidants from Sea Buckthorn (Hippophaë rhamnoides L.) Berries: Pressurised Solvent-Free Microwave Assisted Extraction. Food Chem. 2011, 126, 1380–1386. [Google Scholar] [CrossRef]
- Hemwimon, S.; Pavasant, P.; Shotipruk, A. Microwave-Assisted Extraction of Antioxidative Anthraquinones from Roots of Morinda Citrifolia. Sep. Purif. Technol. 2007, 54, 44–50. [Google Scholar] [CrossRef]
- Oufnac, D.S.; Xu, Z.; Sun, T.; Sabliov, C.; Prinyawiwatkul, W.; Godber, J.S. Extraction of Antioxidants from Wheat Bran Using Conventional Solvent and Microwave-Assisted Methods. Cereal Chem. 2007, 84, 125–129. [Google Scholar] [CrossRef]
- Jokić, S.; Cvjetko, M.; Božić, Đ.; Fabek, S.; Toth, N.; Vorkapić-Furač, J.; Redovniković, I.R. Optimisation of Microwave-Assisted Extraction of Phenolic Compounds from Broccoli and Its Antioxidant Activity. Int. J. Food Sci. Technol. 2012, 47, 2613–2619. [Google Scholar] [CrossRef]
- Baiano, A.; Fiore, A. Enzymatic-Assisted Recovery of Antioxidants from Chicory and Fennel by-Products. Waste Biomass Valorization 2024, 16, 957–969. [Google Scholar] [CrossRef]
- Chemat, F.; Fabiano-Tixier, A.S.; Vian, M.A.; Allaf, T.; Vorobiev, E. Solvent-Free Extraction of Food and Natural Products. TrAC Trends Anal. Chem. 2015, 71, 157–168. [Google Scholar] [CrossRef]
- Jaeger, H.; Schulz, M.; Lu, P.; Knorr, D. Adjustment of Milling, Mash Electroporation and Pressing for the Development of a PEF Assisted Juice Production in Industrial Scale. Innov. Food Sci. Emerg. Technol. 2012, 14, 46–60. [Google Scholar] [CrossRef]
- Cendres, A.; Chemat, F.; Maingonnat, J.-F.; Renard, C.M.G.C. An Innovative Process for Extraction of Fruit Juice Using Microwave Heating. LWT—Food Sci. Technol. 2011, 44, 1035–1041. [Google Scholar] [CrossRef]
- Guyot, S.; Bernillon, S.; Poupard, P.; Renard, C.M.G.C. Multiplicity of Phenolic Oxidation Products in Apple Juices and Ciders, from Synthetic Medium to Commercial Products. In Recent Advances in Polyphenol Research; John Wiley & Sons, Ltd.: New York, NY, USA, 2008; pp. 278–292. ISBN 978-1-4443-0240-0. [Google Scholar]
- Wildermuth, S.R.; Young, E.E.; Were, L.M. Chlorogenic Acid Oxidation and Its Reaction with Sunflower Proteins to Form Green-Colored Complexes. Compr. Rev. Food Sci. Food Saf. 2016, 15, 829–843. [Google Scholar] [CrossRef]
- Zhang, R.; Vorobiev, E.; Zhu, Z.; Grimi, N. Effect of Pulsed Electric Fields Pretreatment on Juice Expression and Quality of Chicory. Innov. Food Sci. Emerg. Technol. 2021, 74, 102842. [Google Scholar] [CrossRef]
- Mannozzi, C.; Fauster, T.; Haas, K.; Tylewicz, U.; Romani, S.; Dalla Rosa, M.; Jaeger, H. Role of Thermal and Electric Field Effects during the Pre-Treatment of Fruit and Vegetable Mash by Pulsed Electric Fields (PEF) and Ohmic Heating (OH). Innov. Food Sci. Emerg. Technol. 2018, 48, 131–137. [Google Scholar] [CrossRef]
- Moussa-Ayoub, T.E.; Jaeger, H.; Youssef, K.; Knorr, D.; El-Samahy, S.; Kroh, L.W.; Rohn, S. Technological Characteristics and Selected Bioactive Compounds of Opuntia dillenii Cactus Fruit Juice Following the Impact of Pulsed Electric Field Pre-Treatment. Food Chem. 2016, 210, 249–261. [Google Scholar] [CrossRef]
- Lebovka, N.I.; Praporscic, I.; Vorobiev, E. Effect of Moderate Thermal and Pulsed Electric Field Treatments on Textural Properties of Carrots, Potatoes and Apples. Innov. Food Sci. Emerg. Technol. 2004, 5, 9–16. [Google Scholar] [CrossRef]
- Dongare, M.L.; Buchade, P.B.; Shaligram, A.D. Refractive Index Based Optical Brix Measurement Technique with Equilateral Angle Prism for Sugar and Allied Industries. Optik 2015, 126, 2383–2385. [Google Scholar] [CrossRef]
- Mhemdi, H.; Bals, O.; Vorobiev, E. Combined Pressing-Diffusion Technology for Sugar Beets Pretreated by Pulsed Electric Field. J. Food Eng. 2016, 168, 166–172. [Google Scholar] [CrossRef]
- Al Bittar, S.; Périno-Issartier, S.; Dangles, O.; Chemat, F. An Innovative Grape Juice Enriched in Polyphenols by Microwave-Assisted Extraction. Food Chem. 2013, 141, 3268–3272. [Google Scholar] [CrossRef]
- Liu, X.; Le Bourvellec, C.; Renard, C.M.G.C. Interactions between Cell Wall Polysaccharides and Polyphenols: Effect of Molecular Internal Structure. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3574–3617. [Google Scholar] [CrossRef]
- Padayachee, A.; Netzel, G.; Netzel, M.; Day, L.; Zabaras, D.; Mikkelsen, D.; Gidley, M.J. Binding of Polyphenols to Plant Cell Wall Analogues—Part 2: Phenolic Acids. Food Chem. 2012, 135, 2287–2292. [Google Scholar] [CrossRef]
- Diemer, E.; Chadni, M.; Grimi, N.; Ioannou, I. Optimization of the Accelerated Solvent Extraction of Caffeoylquinic Acids from Forced Chicory Roots and Antioxidant Activity of the Resulting Extracts. Foods 2022, 11, 3214. [Google Scholar] [CrossRef]
- Clifford, M.N. Chlorogenic Acids and Other Cinnamates–Nature, Occurrence, Dietary Burden, Absorption and Metabolism. J. Sci. Food Agric. 2000, 80, 1033–1043. [Google Scholar] [CrossRef]
- Diemer, E.; Morad, C.; Mouterde, L.; Grimi, N.; Ioannou, I. Postharvest Caffeoylquinic Acid Accumulation in Forced Chicory Roots: Insights into the Role of Temperature, Water Loss, and Biological Defense Mechanisms. ACS Food Sci. Technol. 2024, 4, 470–478. [Google Scholar] [CrossRef]
- Chow, Y.-N.; Louarme, L.; Bonazzi, C.; Nicolas, J.; Billaud, C. Apple Polyphenoloxidase Inactivation during Heating in the Presence of Ascorbic Acid and Chlorogenic Acid. Food Chem. 2011, 129, 761–767. [Google Scholar] [CrossRef]
- Le Bourvellec, C.; Le Quéré, J.-M.; Sanoner, P.; Drilleau, J.-F.; Guyot, S. Inhibition of Apple Polyphenol Oxidase Activity by Procyanidins and Polyphenol Oxidation Products. J. Agric. Food Chem. 2004, 52, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Altunkaya, A.; Gökmen, V. Effect of Various Anti-Browning Agents on Phenolic Compounds Profile of Fresh Lettuce (L. sativa). Food Chem. 2009, 117, 122–126. [Google Scholar] [CrossRef]
- Son, S.M.; Moon, K.D.; Lee, C.Y. Inhibitory effects of various antibrowning agents on apple slices. Food Chem. 2001, 73, 23–30. [Google Scholar] [CrossRef]


| Condition | Soluble Matter (°Brix) |
|---|---|
| Pressing without pretreatment | 6.0 ± 0.0 |
| PEF pretreatment + pressing | 7.5 ± 0.6 * |
| MW pretreatement + pressing | 9.3 ± 0.6 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diemer, E.; Chadni, M.; Ioannou, I.; Grimi, N. The Impact of Thermal and Electrical Pretreatments and Antibrowning Solution on the Chlorogenic and Dicaffeoylquinic Acid Extraction Yield from Endive Roots. Molecules 2025, 30, 2091. https://doi.org/10.3390/molecules30102091
Diemer E, Chadni M, Ioannou I, Grimi N. The Impact of Thermal and Electrical Pretreatments and Antibrowning Solution on the Chlorogenic and Dicaffeoylquinic Acid Extraction Yield from Endive Roots. Molecules. 2025; 30(10):2091. https://doi.org/10.3390/molecules30102091
Chicago/Turabian StyleDiemer, Etienne, Morad Chadni, Irina Ioannou, and Nabil Grimi. 2025. "The Impact of Thermal and Electrical Pretreatments and Antibrowning Solution on the Chlorogenic and Dicaffeoylquinic Acid Extraction Yield from Endive Roots" Molecules 30, no. 10: 2091. https://doi.org/10.3390/molecules30102091
APA StyleDiemer, E., Chadni, M., Ioannou, I., & Grimi, N. (2025). The Impact of Thermal and Electrical Pretreatments and Antibrowning Solution on the Chlorogenic and Dicaffeoylquinic Acid Extraction Yield from Endive Roots. Molecules, 30(10), 2091. https://doi.org/10.3390/molecules30102091









