1. Introduction
Postbiotics were defined as “inanimate microorganisms and/or their components that confer a health benefit on the host” by the International Scientific Association of Probiotics and Prebiotics (ISAPP) [
1]. Postbiotics are distinguished by their elemental composition, including organic acids, phenolic acids, bioactive peptides, and polysaccharides [
2]. It has multiple bioactive effects, such as antioxidant, anti-inflammatory, and immunomodulatory properties [
3]. Exopolysaccharides (EPS) are high molecular weight, long-chain polymers secreted into the extracellular environment by microorganisms during growth and metabolism [
4,
5]. As an important member of the postbiotics, EPS also has a variety of biological functions.
Although exopolysaccharides have been isolated and characterized from various types of microorganisms in the last decades, due to low yield, only a few EPSs have been used in practical production, like xanthan from
Xanthomonas campestris and gellan from
Sphingomonas spp., which are used as thickeners and stabilizers in the food industry [
6,
7]. To improve the EPS yield, the strategies primarily revolve around genetic manipulation and optimization of fermentation conditions. The genetic approaches typically involve altering the genes of enzymes critical to EPS production [
8]. However, genetic approaches remain challenging due to the complexity of EPS synthesis. More advanced techniques like Plackett–Burman design (PBD) and response surface methodology (RSM) have become powerful tools for the optimization of EPS production by considering multiple factors simultaneously [
9]. RSM optimization has been demonstrated to increase EPS yield by 31.32% in
Bacillus velezensis [
10]. After optimization using PBD and RSM, the EPS production from
Lactobacillus plantarum increased from 53.34 mg/L to 97.85 mg/L, representing an 84.70% enhancement [
11]. Optimizing the fermentation conditions of EPS-producing bacteria with the help of these experimental and analytical tools is important for improving EPS yield.
Oxidative stress and inflammation play a vital role in the development of many chronic diseases [
12]. Oxidative stress results from the imbalance between the body’s production of reactive oxygen species (ROS) and the scavenging of ROS by the antioxidant systems [
13]. Furthermore, oxidative stress and inflammation are inextricably linked and often occur simultaneously [
14]. Therefore, there is an urgent need to find a substance with both antioxidant and anti-inflammatory properties.
Bacillus species exist in various ecosystems and produce exopolysaccharides with excellent biological properties such as antioxidant, anti-inflammatory, anti-bacterial, and antiviral activities [
15,
16,
17].
Bacillus subtilis is one of the known Gram-positive model organisms and is recognized as a safe strain by the Food and Drug Administration [
18]. Current research on EPS of
Bacillus subtilis is mostly focused on the production optimization, biological functions, or physicochemical properties [
19,
20,
21]. However, systematic studies on the physicochemical properties and biological functions of EPS from
Bacillus subtilis are limited.
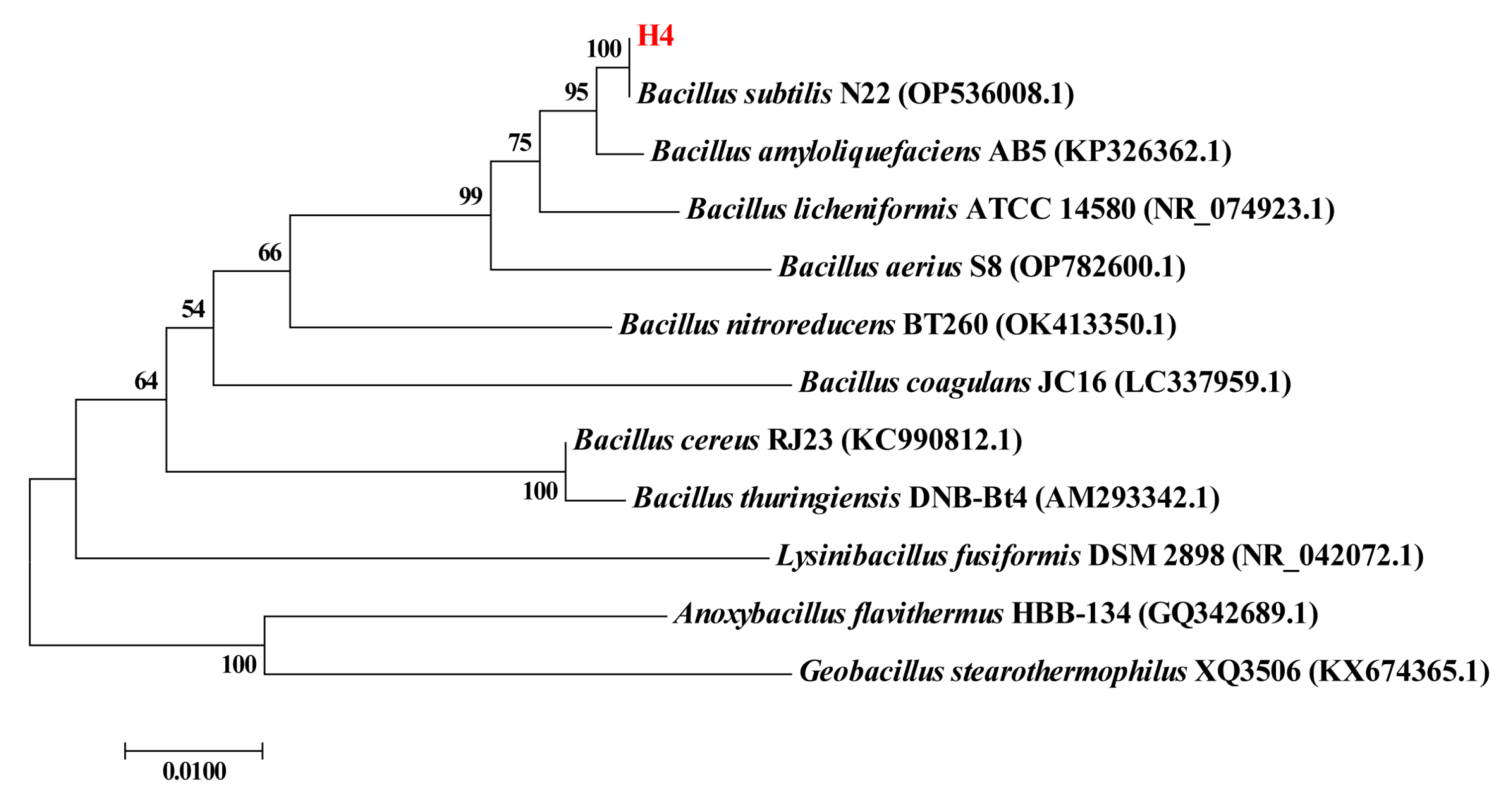
In this study, the strain Bacillus subtilis H4 was screened from the intestine of a healthy broiler. This study aimed to exploit an efficient production optimization strategy and investigate the physicochemical properties and biological activities of novel postbiotic-crude EPS from Bacillus subtilis H4. The ability of Bacillus subtilis H4 to produce EPS was significantly enhanced by systematic optimization of fermentation conditions using Plackett–Burman and Box–Behnken design (BBD). The EPS exhibited outstanding thermostability, antioxidant, and anti-inflammatory activities. These findings provide powerful theoretical guidance and technical support for the application of EPS postbiotics in the food field, as well as their industrialization and exploitation.
3. Discussion
Several EPSs exhibited multiple biological abilities, while the low EPS yield extremely restrained the application of EPS in practical production. EPS from
Lacticaseibacillus rhamnosus ACS5 had strong antioxidant and antidiabetic activities, but the crude EPS yield was 16.91 mg/L, which was far from the demand in production [
22]. It’s urgent to explore an optimization strategy for the fermentation conditions. Traditional optimization methods, such as the one-factor-at-a-time method, focus on individual variables without considering their interactions. The two-step approach (PBD screening of key factors and BBD assessment of their individual and interactive effects) has become an excellent method for fermentation optimization [
9]. In this study, EPS production was optimized by using a combination of single-factor analysis, PBD, PSA, and BBD. The EPS yield of
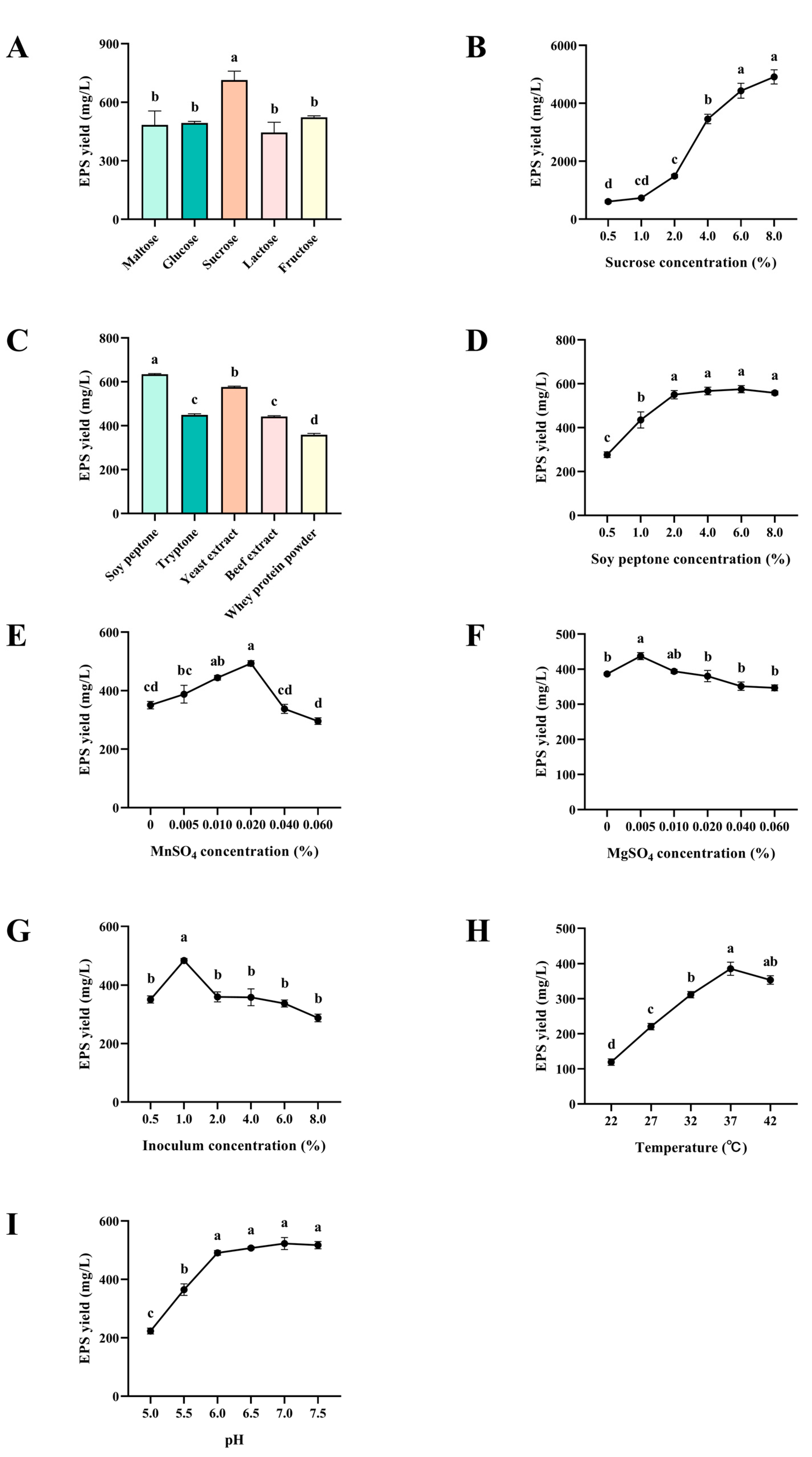
B. subtilis was remarkably elevated after single-factor optimization. During the fermentation, the carbon source played a crucial role in the production of EPS as a substrate for EPS. Among the carbon sources tested, sucrose proved to be the most productive in increasing the EPS yield, which is in agreement with previous findings [
10,
23,
24]. In addition to these conventional nutrients, metal ions such as Mg
2+ and Mn
2+ were found to play a pivotal role in EPS synthesis, likely by promoting microbial growth and metabolism [
25]. MnSO
4 was more effective than MgSO
4, which may contribute to its usage as a co-factor or catalyst in microbial fermentation processes. To maximize the EPS yield, PBD combined with the BBD method was conducted to further improve the parameters. RSM optimization has proven to be 1.675 times more effective than one-factor optimization [
26]. In the present study, the combined optimization strategies resulted in an EPS yield of 15.01 g/L, which was a significant increase from the initial 5.32 g/L and exceeded the yields of other
Bacillus strains, such as
Bacillus spp. NRC5 (5.2 g/L) [
27],
Bacillus subtilis (0.6 g/L) [
28],
Bacillus circulans (1.09 g/L) [
26], and
Bacillus velezensis (1.97 g/L) [
10]. Although the EPS yield was significantly improved after an efficient optimization strategy, the optimization in the study was at the laboratory level, and it should be further optimized at the fermenter level in order to facilitate its industrial production. Besides, high costs are a crucial constraint in industrialized production. Finding economical raw materials like molasses, agricultural waste, and industrial by-products to replace expensive sugars and peptones is necessary to reduce costs. The utilization of
Jerusalem artichoke and date seeds powder as carbon sources for EPS production by
Bacillus sp. significantly reduced fermentation costs [
28,
29]. The fermentation optimization strategies solve the problem of low EPS yield and provide a theoretical basis for its industrialization.
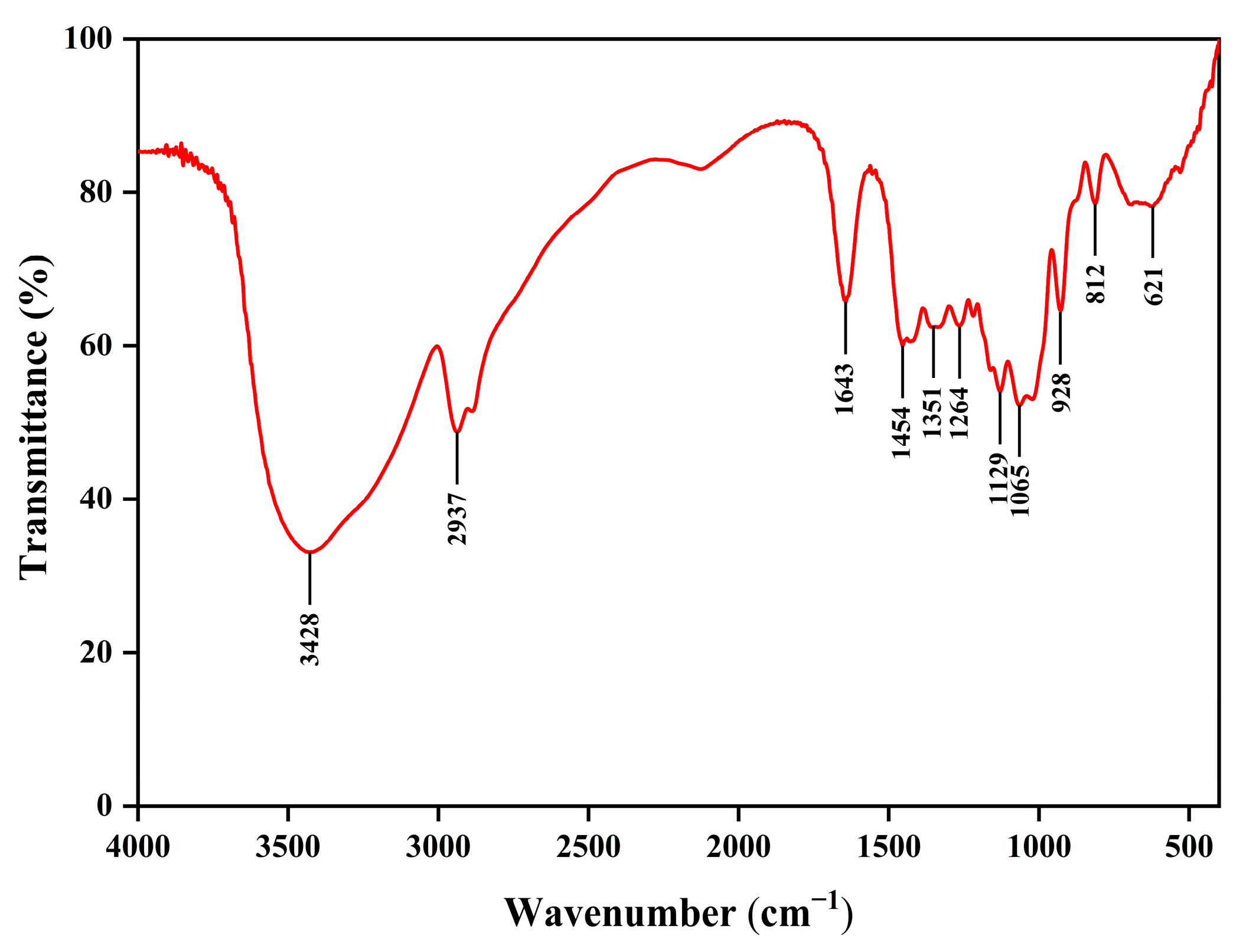
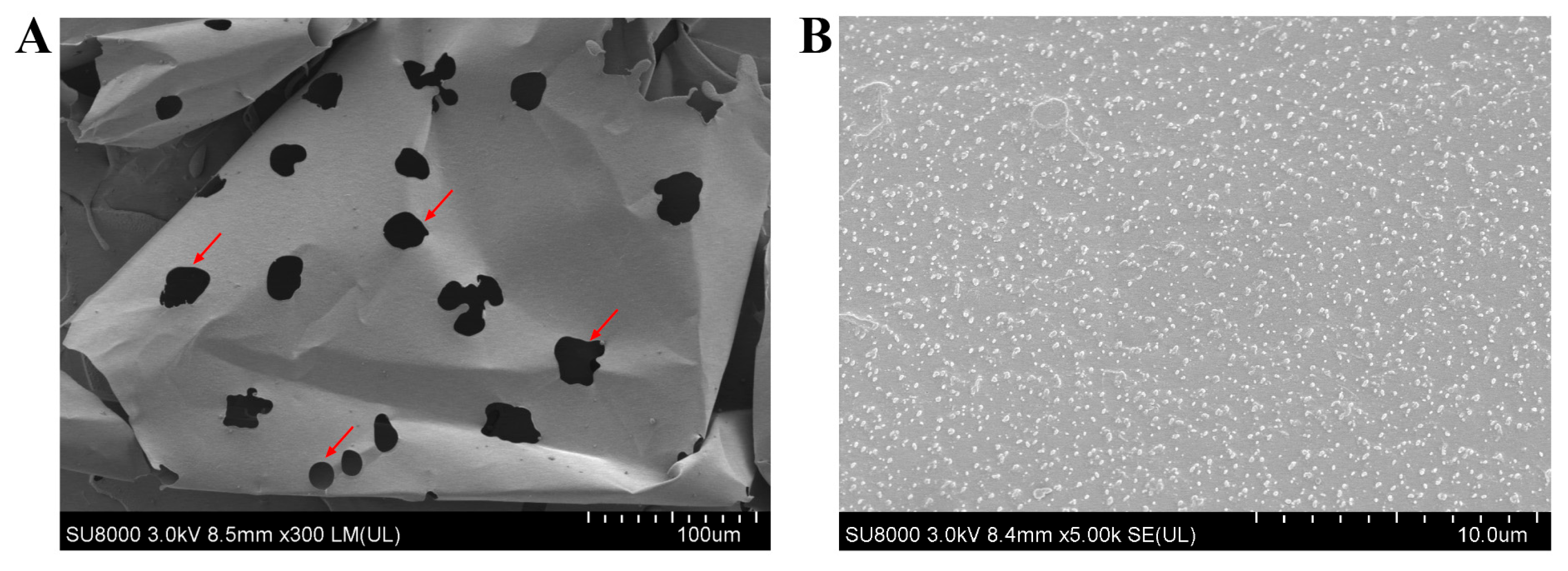
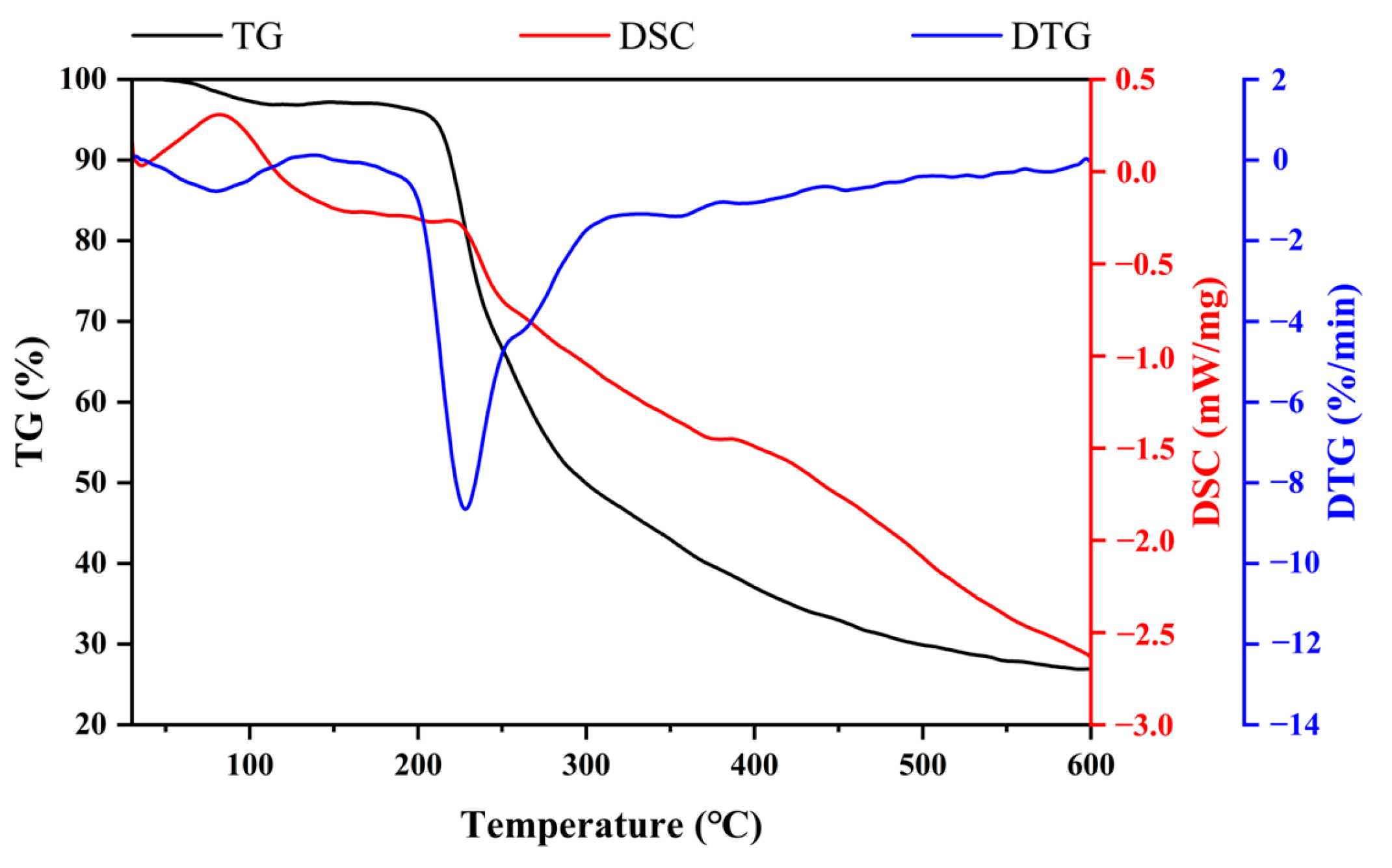
In this study, we preliminarily evaluated the basic properties of EPS, a novel postbiotic from
Bacillus subtilis H4. EPS consisted of 85.37% total carbohydrate and 0.25% protein. The content of the total carbohydrate of EPS was higher than that of EPS from
Bacillus sp. BS11 (49.5%) [
30] and
Streptomyces violaceus (61.4%) [
31], but lower than EPS from
Naematelia aurantialba (91.03%) [
32]. Due to the complexity of the chemical composition of EPS, it is difficult to characterize each chemical component. Further investigations are necessary to elucidate the unknown chemical composition of EPS. EPS molecular weights vary among species. The molecular weight of the primary EPS in the study was 44,304.913 kDa, which is comparable to that of the EPS from
Bacillus licheniformis (44,565 kDa) [
33] and
Bacillus paralicheniformis (55,170 kDa) [
34], and much larger than that of the EPS from
Bacillus spp. (89 kDa) [
35] and
Bacillus amyloliquefaciens (8 kDa) [
17]. EPS consisted of galactose, glucose, glucosamine, arabinose, and mannose, and was free of uronic acid, which was in accordance with the results of the chemical composition of EPS. EPS monosaccharides were composed of all pyranose rings except mannose. These findings were confirmed by FTIR spectroscopy, which found the featured peaks of the pyranose ring at 1065 cm
−1 and 1021 cm
−1. Previous studies have suggested that glucose and mannose form the core structure of water-soluble polysaccharide chains [
36,
37,
38,
39]. SEM results of EPS revealed a flaky, porous surface morphology, which is commonly observed in polysaccharides, and likely contributes to their excellent water solubility [
39,
40]. The thermal stability of EPS is crucial for its industrial applications. EPS from
B. subtilis exhibited great thermostability with a degradation temperature of 228 °C. The temperature was higher than that of EPS from
Bacillus enclensis at 130.5 °C [
41] and
Bacillus megaterium at 168 °C [
42], but lower than EPS from
Bacillus tequilensis FR9 at 239.72 °C [
43] and
Bacillus velezensis at 261.63 °C [
44]. Even though these bacteria belong to the same genus, the thermal stability of EPS is highly variable. DSC determined the change in thermodynamic properties to evaluate the physical or chemical change process [
23]. The DSC curve of EPS exhibited an endothermic peak of EPS with a melting point of 218 °C. Both TGA and DSC confirmed that EPS has excellent thermostability and can be safely used in different industries with high thermal processing requirements.
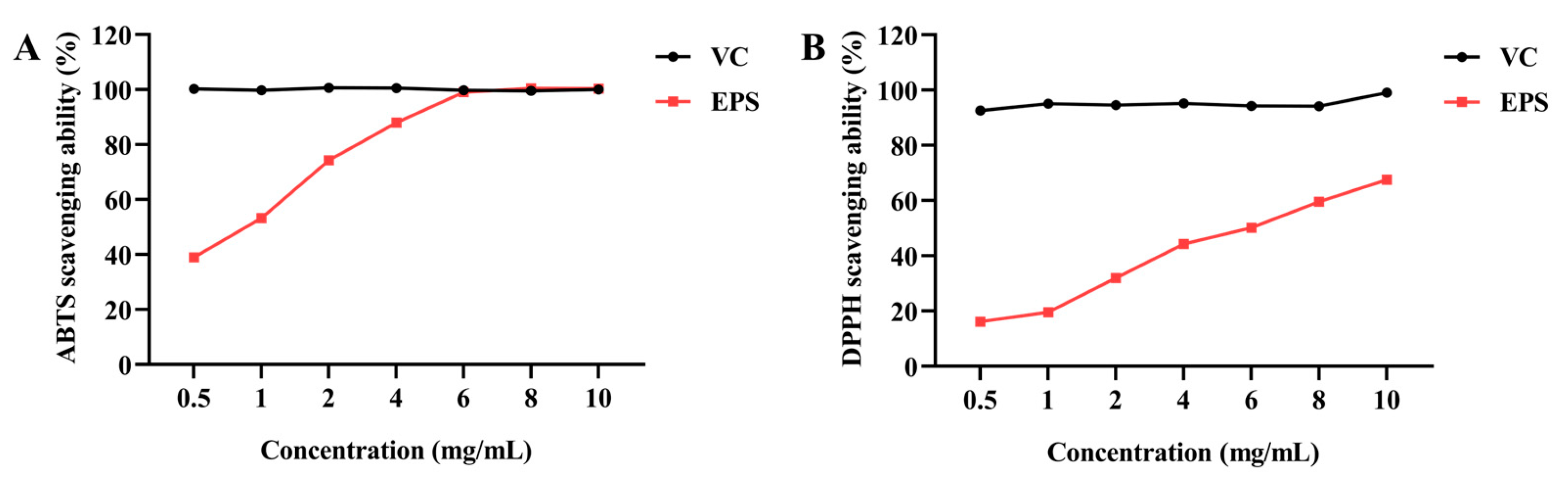
Subsequently, the biological capacities of EPS were evaluated. These facilitated our in-depth understanding of the relationship between the structure and function of EPS. The antioxidant capacities of EPS were assessed through ABTS and DPPH radical scavenging tests, with IC50 values of 1 mg/mL and 6 mg/mL, respectively. These results were consistent with previous findings demonstrating the antioxidant potential of EPS from
B. tequilensis FR9 and
Bacillus spp. NRC5 [
27,
43]. The ABTS and DPPH radical scavenging capacities of EPS were higher than L-EPS-ACS5 from
Lacticaseibacillus rhamnosus ACS5, but lower than the EPS from
Lactobacillus plantarum R301 [
11,
22]. This study assessed the capacity of EPS to scavenge free radicals; further studies are needed to elucidate the functional mechanism of its antioxidant activity both in vivo and in vitro.
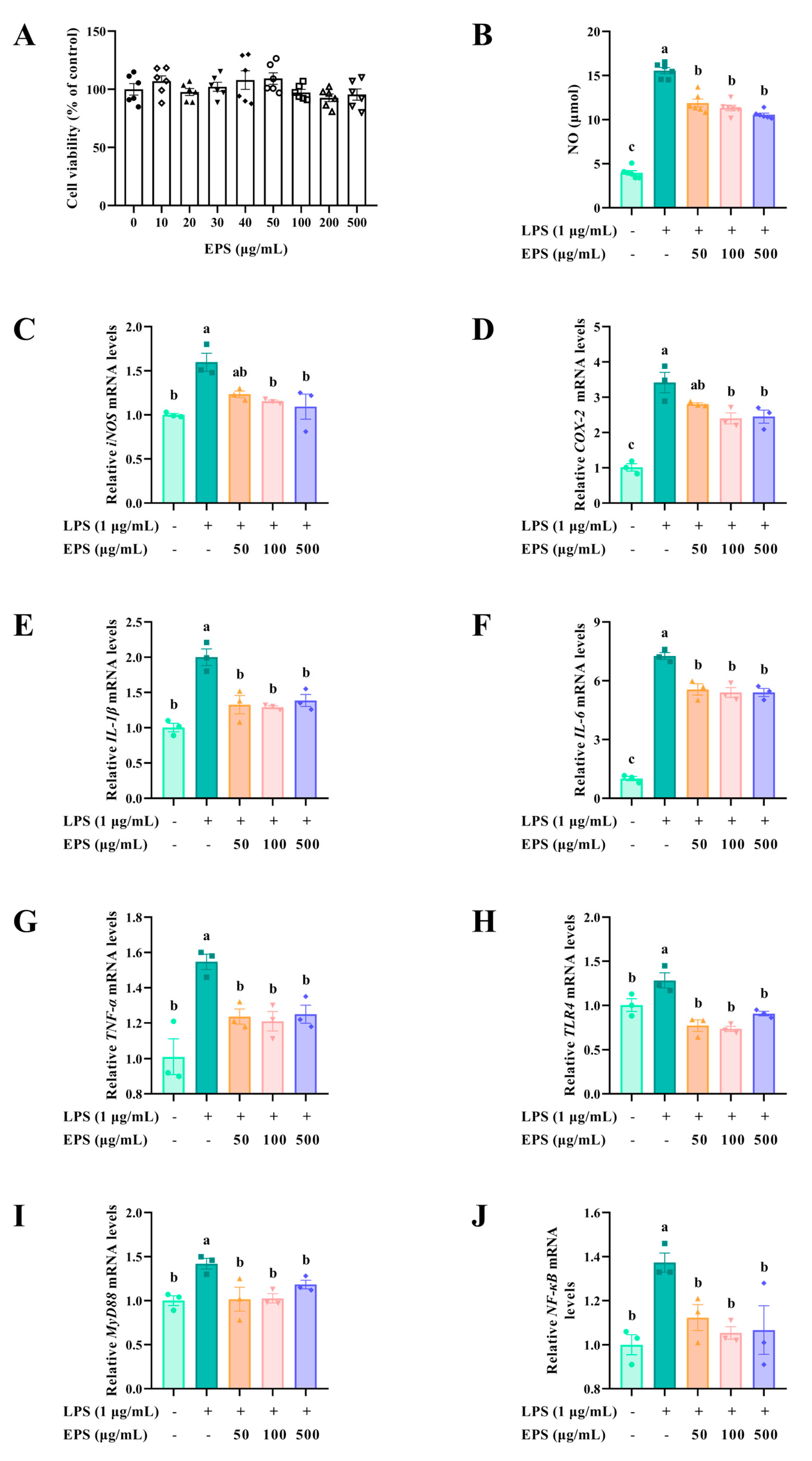
In addition to its antioxidant effects, EPS exhibited significant anti-inflammatory properties. LPS, an endotoxin in the cell wall of Gram-negative bacteria, triggers inflammatory responses and promotes the expression of inflammatory cytokines [
40]. NO, an important biomarker of the inflammatory response, is primarily catalyzed by inducible nitric oxide synthase, and plays a vital role in many inflammatory diseases [
45]. In addition, prostaglandin E2 is also a vital inflammatory mediator induced by COX-2, which is usually absent or expressed at low levels in normal conditions but is overexpressed in inflammatory states [
46]. In the present study, we found that EPS from
B. subtilis H4 has excellent anti-inflammatory activity by inhibiting LPS-induced NO release and reducing the expression of proinflammatory markers such as iNOS and COX-2.
LPS is recognized by toll-like receptor 4 and signals into cells in a MyD88-dependent manner [
47,
48]. Increased expression of MyD88 triggers the following inflammatory cascade in NF-κB and stimulates the secretion of downstream pro-inflammatory cytokines, such as IL-1β, IL-6, and TNF-α [
49,
50]. The results manifested that EPS significantly inhibited the expression of IL-1β, IL-6, and TNF-α. Similarly, EPSs from
Bacillus spp.,
Bacillus thuringiensis, and other microbial sources like
Lactobacillus plantarum,
Limosilactobacillus fermentum, and
Leuconostoc mesenteroides inhibited the LPS-induced release of pro-inflammatory mediators, such as NO, IL-6, and TNF-α [
11,
51,
52,
53,
54]. The inhibitory effect of EPS in this study on the mRNA levels of pro-inflammatory cytokines was stronger than that of EPS from
Lactobacillus plantarum and
Limosilactobacillus fermentum [
11,
54]. The inflammatory mediators were reduced, which revealed that the activity of signaling pathways related to inflammation was suppressed. Our results demonstrated that EPS restored LPS-induced elevated levels of TLR4, MyD88, and NF-κB to normal levels. EPS exhibited anti-inflammatory effects via suppression of the TLR4/MyD88/NF-κB pathway, consistent with studies on EPS from
Cordyceps sinensis [
55] and
Crypthecodinium cohnii [
56]. EPS is widely used in the food industry due to its functional properties, including its ability to act as a thickener, stabilizer, emulsifier, and gelling agent. In the present study, the thermostability enhanced its suitability for food applications. Taken together, these findings suggest that EPS is a promising new antioxidant and anti-inflammatory agent, laying a solid foundation for its further application in the food, pharmaceutical, and feed industries.
4. Materials and Methods
4.1. Materials
The strain B. subtilis H4 was isolated from the intestine of a healthy broiler, and subsequently stored in the China General Microbiological Culture Collection Center (CGMCC No. 20463). Then the strain H4 was identified by 16S rRNA sequencing. Soy peptone, beef extract, and whey protein powder were acquired from Aobox Biotechnology Co., Ltd. (Beijing, China). Maltose, glucose, sucrose, lactose, fructose, and other salts were purchased from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). Tryptone and yeast extract, monosaccharide standards, 1-phenyl-3-methyl-5-pyrazolone (PMP), and acetonitrile (HPLC grade) were obtained from Sigma-Aldrich (St. Louis, MO, USA). LPS, 2,2′-azino-bis-3-ethylbenzothiazoline-6-sulfonic acid (ABTS), 1,1-diphenyl-2-picrylhydrazyl (DPPH), dialysis bag, penicillin, streptomycin, and cell counting kit-8 were provided by Solarbio Science &Technology Co., Ltd. (Beijing, China). Dulbecco’s Modified Eagle Medium (DMEM) was acquired from Thermo Fisher Scientific Inc. (Waltham, MA, USA). Fetal bovine serum (FBS) was purchased from Wuhan Procell Life Technology Co., Ltd. (Wuhan, China). All reagents were analytical grade unless otherwise specified.
The basic fermentation medium contained (per liter distilled water) 5.0 g of yeast extract, 10.0 g of tryptone, 10.0 g of glucose, 1.5 g of NaH2PO4, and 1.5 g of Na2HPO4.
4.2. Preparation of Postbiotics from B. Subtilis H4
The preserved strain B. subtilis H4 was streaked on a fresh beef peptone yeast (BPY) plate to obtain a single colony. The isolates were subsequently inoculated in the BPY medium at 37 °C overnight to activate the strain. Finally, the seed culture was incubated in the basic fermentation medium at 37 °C for 36 h. The fermented medium was heated at 100 °C for 15 min to inactivate the EPS-degrading enzymes. After centrifuging at 12,000 rpm for 20 min, the supernatant containing postbiotics was obtained.
4.3. Separation and Measurement of EPS
EPS was extracted and purified from the supernatant using the procedure provided by Sathishkumar et al. [
57] with some adjustments. To precipitate the protein, 80% (
w/
v) trichloroacetic acid was mixed with 2 mL of supernatant and incubated at 4 °C for 8 h. Following the centrifugation at 12,000 rpm for 15 min, the supernatant was mixed with prechilled absolute ethanol (1:3,
v/
v) and incubated at 4 °C for 12 h to precipitate EPS. After precipitation, EPS was collected by centrifuging at 12,000 rpm for 15 min and then dissolved in 2 mL of distilled water. Finally, EPS was dialyzed in distilled water using a dialysis bag (8000–14,000 Da) at 4 °C for 24 h to remove monosaccharides. The rest of the EPS was lyophilized to explore the antioxidant and anti-inflammation activities.
The EPS content was measured using the phenol-sulfate acid method [
58]. Briefly, glucose was used as a standard, and 1 mL of glucose with different concentrations ranging from 0 to 1000 μg/mL was added to 1 mL of 6% (
w/
v) phenol solution, respectively. Then, 5 mL concentrated sulfuric acid was blended into the above solution and stirred. Following a half-hour incubation at room temperature, the mixture’s absorbance was assayed at 490 nm. Similarly, the 1 mL dialyzed EPS was treated according to the above procedures, and the yield was calculated using the glucose standard curve (absorbance value vs. concentration).
4.4. Optimization Strategies for EPS Production
4.4.1. Single-Factor Experiment
A preliminary investigation of the various factors affecting the EPS yield was conducted using the single-factor optimization approach. The following EPS fermentation parameters were evaluated: carbon source (maltose, glucose, sucrose, lactose, and fructose), sucrose concentration (0.5, 1.0, 2.0, 4.0, 6.0, and 8.0%), nitrogen source (tryptone, yeast extract, soy peptone, beef extract, and whey protein powder), soy peptone concentration (0.5, 1.0, 2.0, 4.0, 6.0, and 8.0%), MnSO
4 concentration (0, 0.005, 0.010, 0.020, 0.040, and 0.060%), MgSO
4 concentration (0, 0.005, 0.010, 0.020, 0.040, and 0.060%), inoculum concentration (0.5, 1.0, 2.0, 4.0, 6.0, and 8.0%), fermentation temperature (22, 27, 32, 37, and 42 °C) and pH (5.0, 5.5, 6.0, 6.5, 7.0, and 7.5). The EPS yield was measured as described in
Section 4.3.
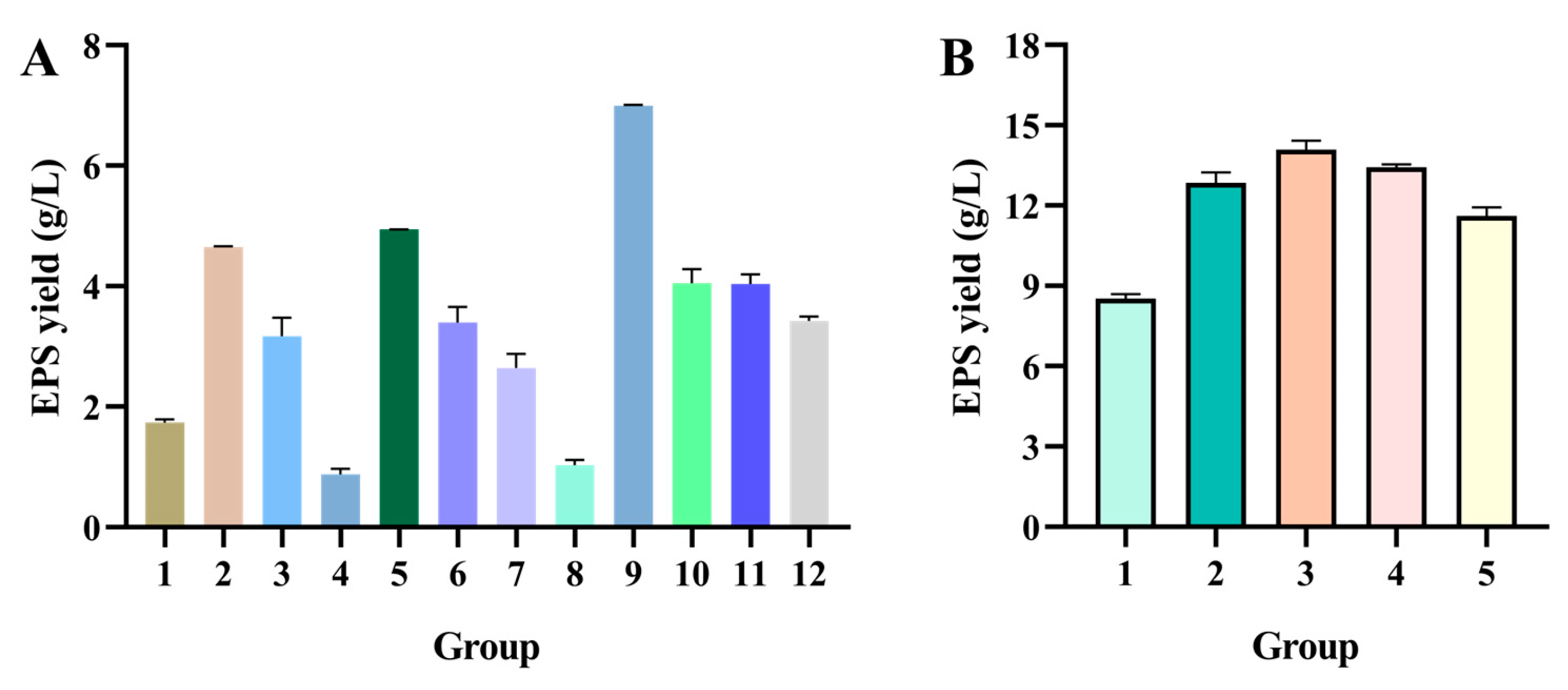
4.4.2. Selection of Critical Factors Affected the EPS Yield Using PBD
Considering the results of the one-factor tests, the PBD was used to screen the primary factors impacting the crude EPS production from the concentration of (A) sucrose, (B) soy peptone, (C) MnSO
4, and (D) MgSO
4, (E) inoculum concentration, (F) temperature, and (G) initial pH. Each factor set a high level (+1) and a low level (−1), as displayed in
Table 5. PBD was conducted by a design expert, and the experimental design is displayed in
Table 6.
Table 5.
Factors and corresponding levels of Plackett–Burman design.
Table 5.
Factors and corresponding levels of Plackett–Burman design.
| Factors | Level |
|---|
| −1 | +1 |
|---|
| A-Sucrose (%) | 4 | 8 |
| B-Soy peptone (%) | 1 | 2 |
| C-MnSO4 (%) | 0.01 | 0.04 |
| D-MgSO4 (%) | 0.005 | 0.020 |
| E-Inoculum concentration (%) | 0.5 | 2 |
| F-Temperature (°C) | 27 | 37 |
| G-pH | 5.5 | 6.5 |
Table 6.
Experimental design of Plackett–Burman experiment.
Table 6.
Experimental design of Plackett–Burman experiment.
| Run | A-Sucrose | B-Soy Peptone | C-MnSO4 | D-MgSO4 | E-Inoculum Concentration | F-Temperature | G-pH |
|---|
| 1 | −1 | −1 | −1 | −1 | 1 | −1 | −1 |
| 2 | 1 | −1 | 1 | 1 | −1 | 1 | −1 |
| 3 | −1 | −1 | 1 | −1 | −1 | 1 | −1 |
| 4 | 1 | −1 | −1 | −1 | 1 | 1 | 1 |
| 5 | −1 | 1 | 1 | 1 | 1 | −1 | −1 |
| 6 | −1 | 1 | 1 | −1 | 1 | 1 | 1 |
| 7 | −1 | 1 | −1 | 1 | −1 | 1 | 1 |
| 8 | −1 | −1 | −1 | 1 | −1 | −1 | 1 |
| 9 | 1 | 1 | 1 | −1 | −1 | −1 | 1 |
| 10 | 1 | 1 | −1 | 1 | 1 | 1 | −1 |
| 11 | 1 | 1 | −1 | −1 | −1 | −1 | -1 |
| 12 | 1 | -1 | 1 | 1 | 1 | -1 | 1 |
4.4.3. Path of Steepest Ascent Method
The dominant factors and the direction of the movement were determined by PBD. Subsequently, the PSA method was used to confirm the center point of the response surface. The increments of three significant factors included soy peptone, sucrose, and MnSO
4 were 1.5%, 1.5%, and 0.15%, respectively. The details of the experimental design are presented in
Table 7.
Table 7.
Experimental design of the path of steepest ascent experiment.
Table 7.
Experimental design of the path of steepest ascent experiment.
| Run | X1-Soy Peptone (%) | X2-Sucrose (%) | X3-MnSO4 (%) |
|---|
| 1 | 1.5 | 5.5 | 0.025 |
| 2 | 3.0 | 7.0 | 0.040 |
| 3 | 4.5 | 8.5 | 0.055 |
| 4 | 6.0 | 10.0 | 0.070 |
| 5 | 7.5 | 11.5 | 0.085 |
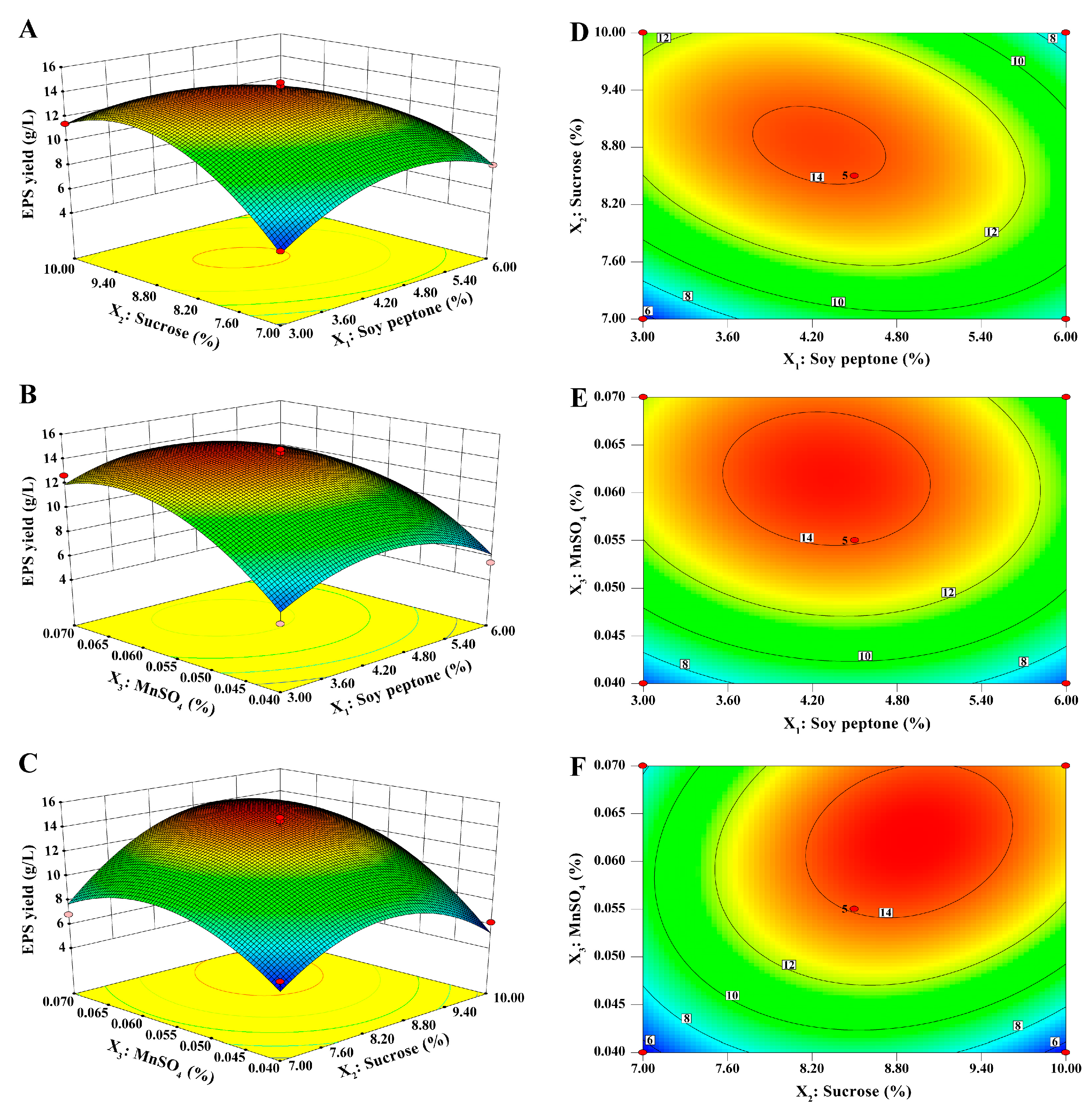
4.4.4. Response Surface Methodology
The relationship between the response values and different variables was determined using RSM. According to the results of the steepest ascent path, a BBD was conducted to further optimize the fermentation parameters of factors (soy peptone, sucrose, and MnSO
4). As shown in
Table 8, a three-variable and three-level (−1, 0, and +1) response surface design with 17 runs was generated by Design-Expert software 8.0.6. The levels were set by the PSA experiment.
4.5. Chemical Composition of EPS
One milligram of EPS was dissolved in 1 mL of H
2O. The concentration of total carbohydrate was determined using the phenol-sulfate acid method [
58]. The protein concentration was assessed by the Bradford method with bovine serum albumin as a standard [
59]. The uronic acid content was determined by the sulfuric acid-carbazole method with galacturonic acid as standard [
60]. The reducing sugar content was measured by the dinitrosalicylic acid method with glucose as standard [
61]. The sulfate concentration was measured by the barium chloride-gelatin method with potassium sulfate as standard [
62]. The total polyphenol content was determined using the Folin–Ciocalteu assay using gallic acid as a standard [
63].
4.6. Molecular Weight
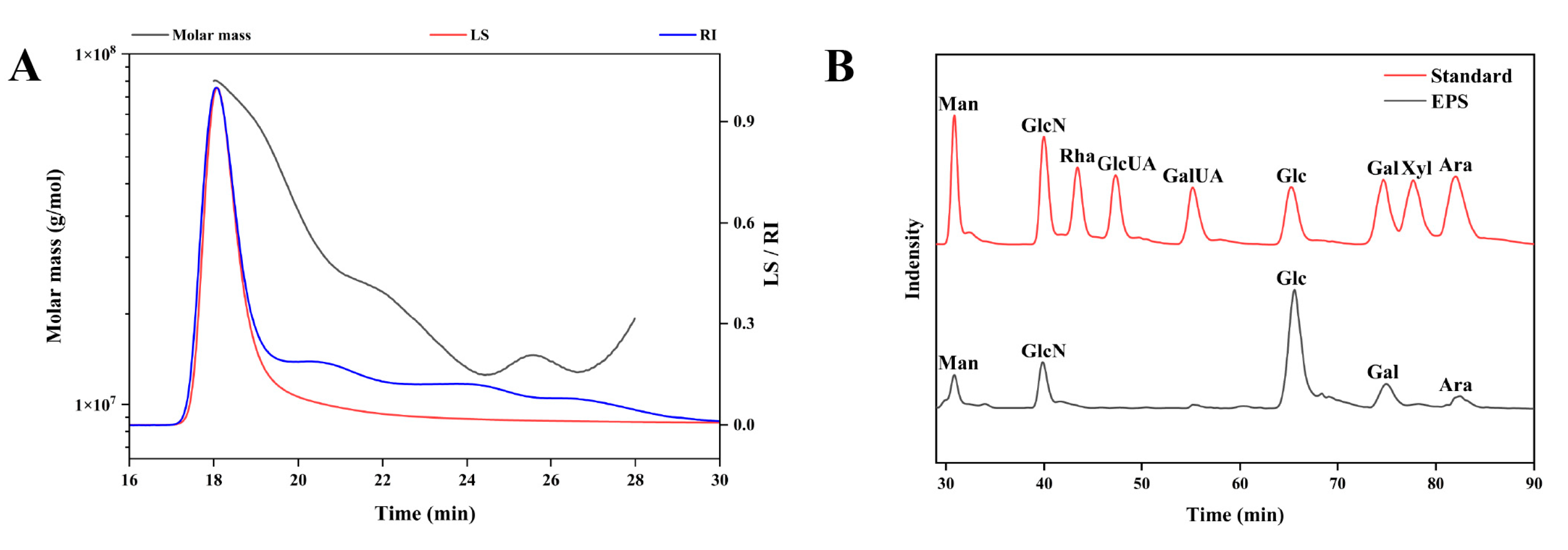
A HPGPC system (UltiMate 3000, Thermo Fisher Scientific, Waltham, MA, USA) combined with a multi-angle laser light scattering detector (DAWN HELEOS II, Wyatt technology, Santa Barbara, CA, USA) and a differential refractive index detector (Optilab T-rEX, Wyatt technology, Santa Barbara, CA, USA) was employed to determine the molecular weight. Both gel permeation columns, OHpak SB-805 HQ (300 × 8 mm, 13 μm) and SB-803 HQ (300 × 8 mm, 6 μm) (Showa Denko Co., Ltd., Tokyo, Japan), were equipped. A total of 1 mg of EPS was dissolved in 1 mL of 0.1 mol/L NaNO3 and filtered through a 0.22 μm filter before analysis. Finally, a 20 μL sample was isocratically eluted with a 0.1 mol/L NaNO3 (containing 0.02% NaN3) mobile phase at 0.8 mL/min.
4.7. Monosaccharide Compositions
A reverse-phase HPLC system (LC-20ADXR, Shimadzu, Tokyo, Japan) fitted with a Shim-pack GIST C18 Column (250 × 4.6 mm, 5 μm) (Shimadzu, Tokyo, Japan) and an ultraviolet monitor (SPD-20A, Shimadzu, Tokyo, Japan) set at 254 nm was used to analyze the monosaccharide compositions [
64]. In brief, 10 mg of EPS was mixed with 5 mL of 2 mol/L trifluoroacetic acid (TFA), filled with nitrogen, and reacted at 105 °C for 6 h. The hydrolysate was dried with nitrogen, added with methanol, and evaporated with a rotary evaporator 5 times to remove the TFA. The EPS residue was dissolved in distilled water. EPS samples, nine monosaccharide standards (glucose, Glc; rhamnose, Rha; galactose, Gla; mannose, Man; arabinose, Ara; xylose, Xyl; glucuronic acid, GlcUA; galacturonic acid, GlaUA; and glucosamine, GlcN), and 0.05–0.5 mg/mL mixed monosaccharide standards were respectively added to 0.5 mol/L PMP methanol solution and 0.3 mol/L NaOH solution, and incubated at 65 °C for 1 h. For the neutralization of the reaction, 0.3 mol/L hydrochloric acid was blended into the reaction solution after it was cooled to room temperature. PMP was extracted three times by adding trichloromethane to the mixture and then centrifuging it at 12,000 rpm for 15 min to obtain the supernatant. Before being subjected to HPLC, the supernatant was filtered using a 0.22 μm filter. The mobile phase consisted of 0.1 mol/L phosphate buffer (pH = 6.7) and acetonitrile (83:17,
v/
v). The injection volume was set as 20 μL, and the flow rate was 0.8 mL/min.
4.8. FTIR Spectrum
FTIR was conducted to ascertain the functional groups of EPS. After mixing the lyophilized EPS and KBr in a ratio of 1:100, the mixture was compressed into a thin slice. Then, an FTIR spectrometer (Nicolet IS10, Thermo Fisher Scientific, Waltham, MA, USA) was used to scan the slice between 4000 and 400 cm−1.
4.9. Surface Morphology
Five milligrams of lyophilized EPS were glued to the sample stage with conductive adhesive and then coated with gold in a sputter coater (JFC-1100, JEOL, Tokyo, Japan). The surface morphology of the EPS was viewed by cold field emission scanning electron microscope (Hitachi SU8000, Tokyo, Japan) at various magnifications of 300× and 5000×. The measured accelerating voltage was set to 3 kV.
4.10. Thermogravimetric and Differential Scanning Calorimetric Analysis
The thermogravimetric properties of the EPS were analyzed using a TG-DSC 3+ thermogravimetric analyzer (Mettler Toledo, Zurich, Switzerland). The ground EPS (10 mg) was placed into an aluminum crucible and heated from 30 °C to 600 °C at a rate of 10 °C/min in a nitrogen atmosphere.
4.11. Antioxidant Capacity of EPS
4.11.1. ABTS Radical Scavenging Assay
The ABTS radical scavenging capacity of EPS was evaluated in line with the method described by Wang et al. [
11] with some modifications. The ABTS (7 mmol/L) and potassium persulfate (2.45 mmol/L) were blended thoroughly in an equal volume and reacted overnight at room temperature in the dark to generate ABTS radicals. Subsequently, 600 μL ABTS solution was mixed with 300 μL of EPS at various concentrations (0.5–10 mg/mL), respectively, and the solution was reacted for 10 min. The reaction solution was detected at 734 nm. As a positive control, VC at an equal concentration was employed. The ABTS scavenging rate of EPS was calculated according to the following equation:
The absorbance of the EPS group (containing EPS and ABTS) was set as Asample, the absorbance of the negative control group (containing distilled water and ABTS) was set as Acontrol, and the absorbance of the blank group (containing EPS and distilled water) was set as Ablank.
4.11.2. DPPH Radical Scavenging Assay
An adjusted protocol was performed to assess the scavenging capacity of DPPH radical [
65]. An aliquot of EPS (0.5–10 mg/mL) or VC (0.5–10 mg/mL) was mixed with an equal volume of 0.2 mmol/L DPPH solution (prepared in methanol) and kept at room temperature for half an hour. The mixture was examined at 517 nm. The following equation was used to calculate the DPPH scavenging activity of EPS.
The absorbance of the EPS group (EPS and DPPH) was denoted as Asample, negative control (distilled water and DPPH) as Acontrol, and the absorbance of the blank group (EPS and methanol) as Ablank.
4.12. Anti-Inflammatory Activity of EPS
4.12.1. Cell Culture
The RAW264.7 cells were cultured in DMEM supplemented with 10% FBS, 100 U/mL penicillin, and 100 μg/mL streptomycin. The cells were grown at 37 °C in a humidified atmosphere containing 5% CO2 in an incubator (Forma 3, Thermo Fisher Scientific, Waltham, MA, USA).
4.12.2. Cell Viability of EPS
The effect of EPS on cell viability was evaluated by the cell counting kit-8 (CCK-8). RAW264.7 cells (1.0 × 10
4 cells per well) were inoculated in 96-well plates and cultured overnight. Then, lyophilized EPS was diluted with DMEM at a final concentration of 0, 10, 20, 30, 40, 50, 100, 200, and 500 μg/mL and administered to RAW264.7 cells for 24 h. After incubation, DMEM and CCK-8 solution were mixed in a ratio of 10:1, applied to the well, and incubated for 2 h at 37 °C in the dark. The supernatant’s absorbance was examined at 450 nm. The cell viability was calculated using the equation:
where A
sample represented the absorbance of EPS-treated cells and CCK-8, A
blank represented the absorbance of DMEM and CCK-8, and A
control represented the absorbance of EPS-untreated cells and CCK-8.
4.12.3. Establishment of LPS-Induced Inflammatory Model
LPS from Escherichia coli O111: B4 was used to construct a macrophage inflammatory model. Briefly, 1.0 × 104 RAW264.7 cells were incubated in 96-well plates for 12 h. In addition, cells were subjected to 50, 100, and 500 μg/mL EPS and cultured for 12 h. Subsequently, 1 μg/mL LPS stimulated the cells for 24 h.
To determine whether the model was successfully constructed, the Griess method was used to measure the level of the inflammatory mediator NO [
66]. An equal volume of Griess A and Griess B was successively mixed with the supernatant, and then the absorbance of the solution was measured at 540 nm. The NO content was calculated using NaNO
2 as a standard.
4.12.4. RNA Extraction and Quantitative Real-Time Polymerase Chain Reaction
RAW264.7 cells were seeded at a density of 2.0 × 10
6 cells per well into 6-well plates, followed by induction of the inflammatory model as previously described. Total RNA was isolated from the RAW264.7 using TRIzol reagent following the protocol of the manufacturer. Agarose gel electrophoresis and NanoDrop were conducted to detect the RNA integrity and purity, respectively. The total RNA was used for reverse transcription of cDNA by the M5 Super plus qPCR RT kit (Mei5 Biotechnology Co., Ltd., Beijing, China). qPCR was conducted using M5 HiPer SYBR Premix EsTaq (Mei5 Biotechnology Co., Ltd., Beijing, China).
Table S1 presents the details of the primers. Two-step amplification reactions were used in this study: 1 cycle of 95 °C for 30 s, 40 cycles of 95 °C for 10 s and 60 °C for 30 s, and then 1 cycle of 95 °C for 5 s, 60 °C for 60 s, and 95 °C for 1 s for the melting curve. The data were analyzed according to the 2
−ΔΔCt method with glyceraldehyde-3-phosphate dehydrogenase as an internal reference [
67].
4.13. Statistical Analysis
The data are presented using the mean values ± standard error of the mean (SEM). Each experiment was repeated three times, except for the cell viability and NO detection assays, which were six replicates. Multiple comparisons using Tukey’s test were conducted after the differences between the groups were examined by one-way analysis of variance (ANOVA) in SPSS software 26.0. Statistical significances were represented as p < 0.05.