Poly(Acrylic Acid)/TiO2 Nanocomposite Hydrogels for Paper Artwork Cleaning and Protection
Abstract
1. Introduction
- Intrinsic characteristics of the paper, including the raw materials used during production and manufacturing methods.
- Nature of materials applied to the paper such as inks, pigments, binders, etc.
- Possible presence of pathogens and air pollutants in the storage environment, as well as light exposure.
2. Results
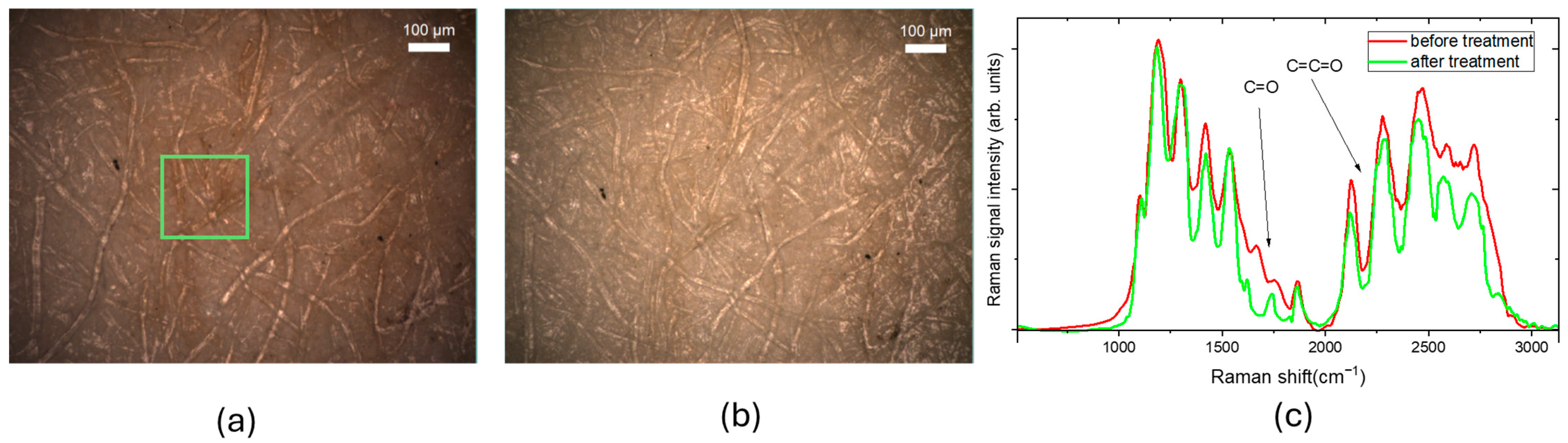
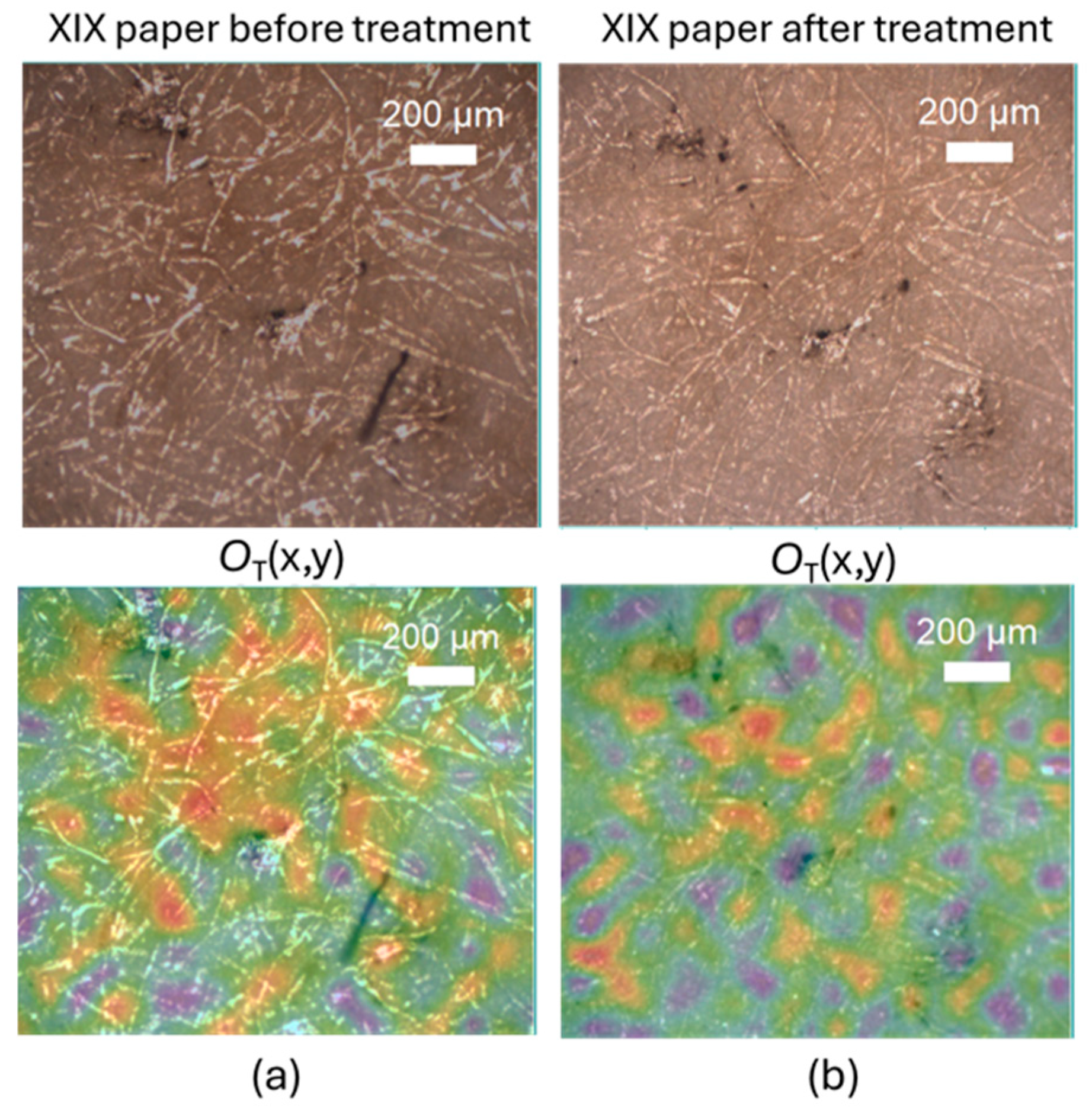
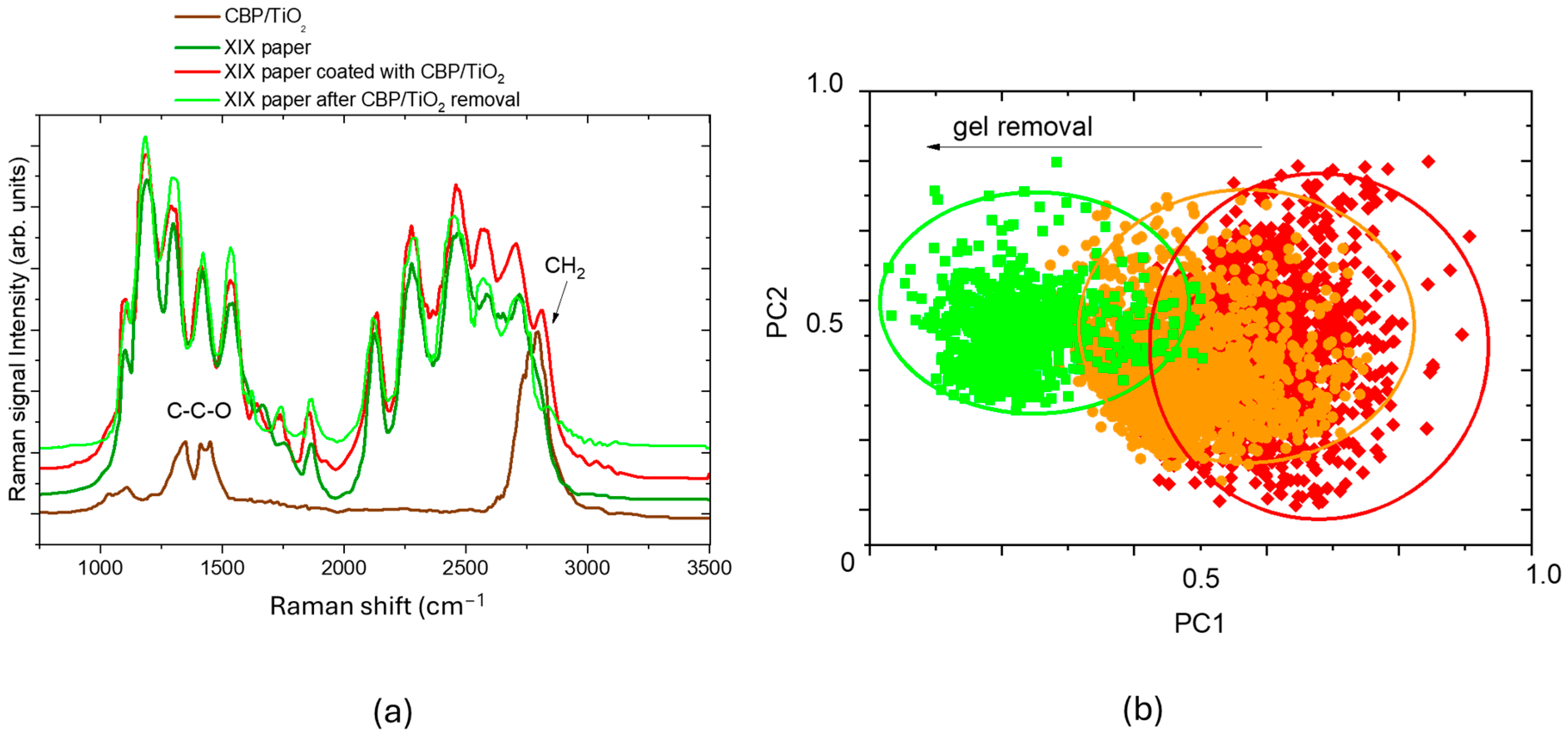
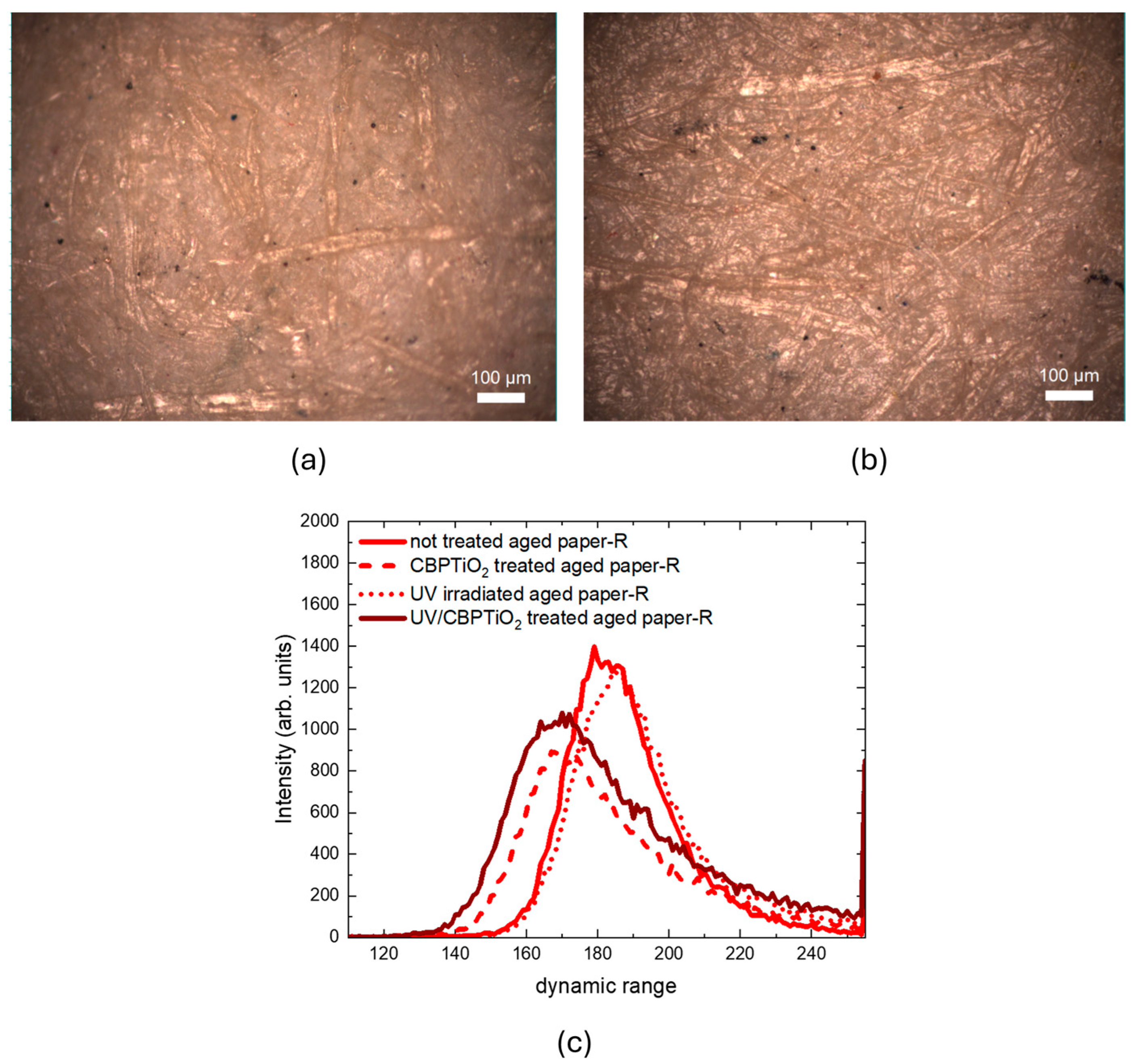
2.1. Characterization of Cleaning Process with Raman Micro-Spectroscopy and Confocal Laser Microscopy
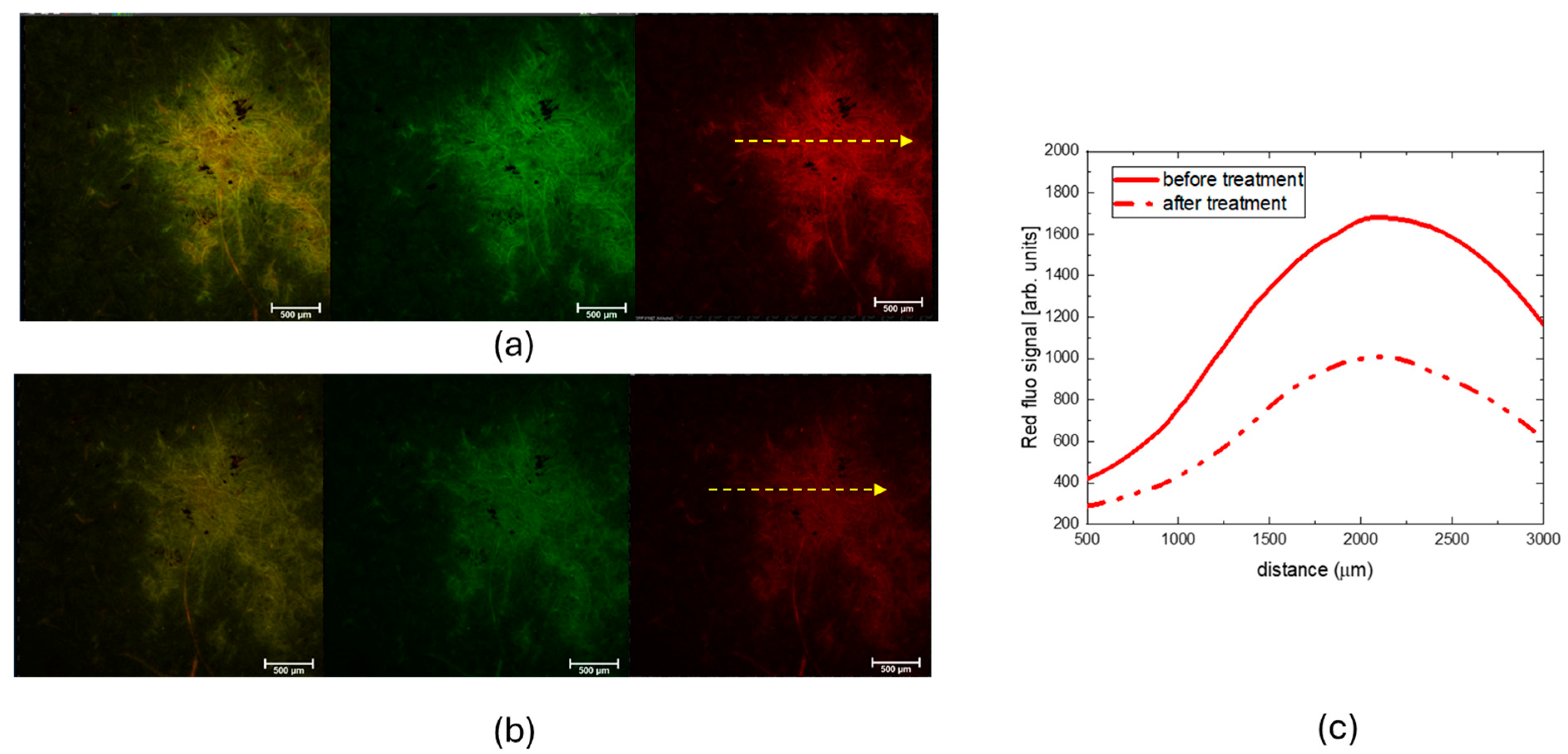
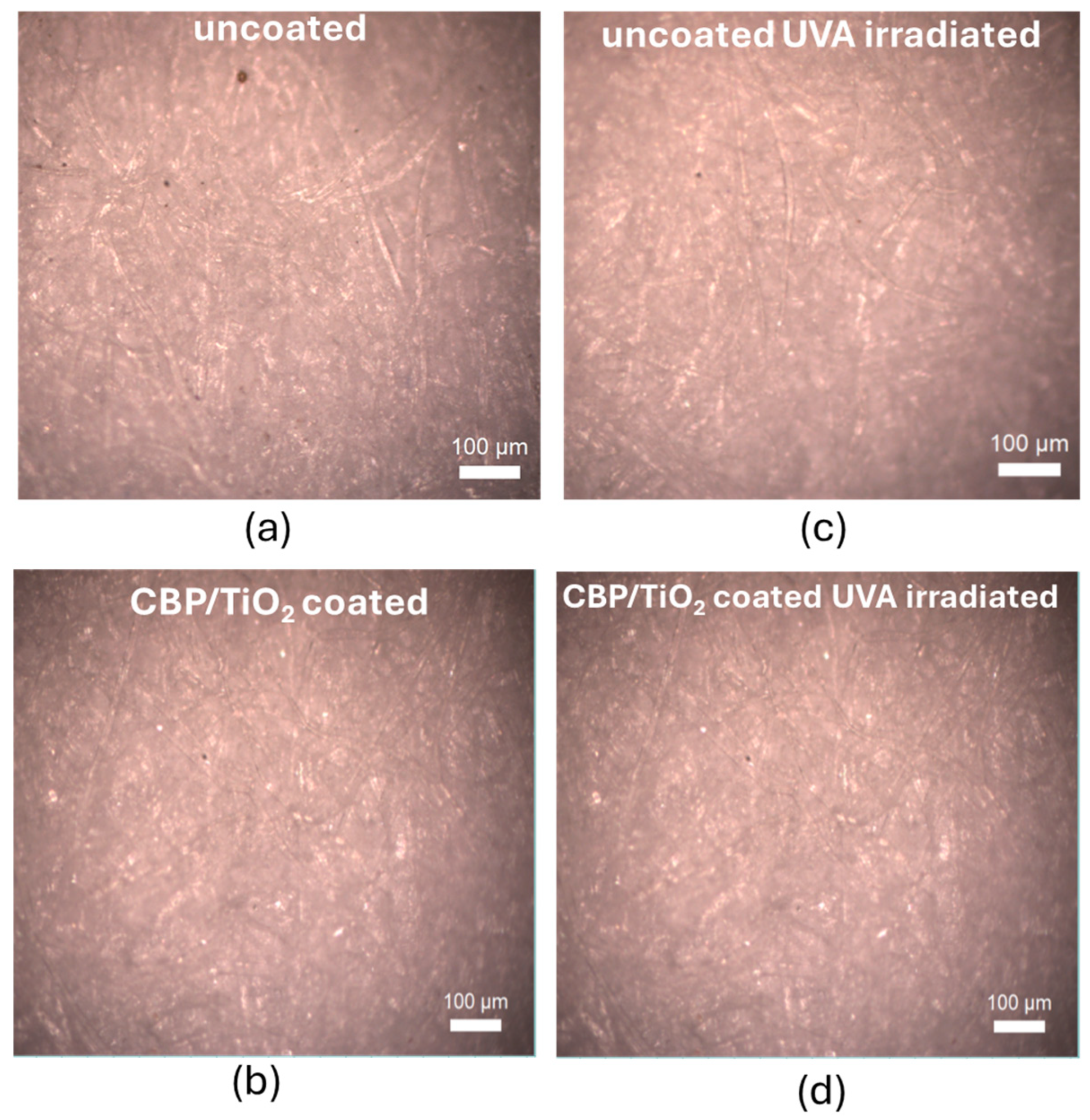
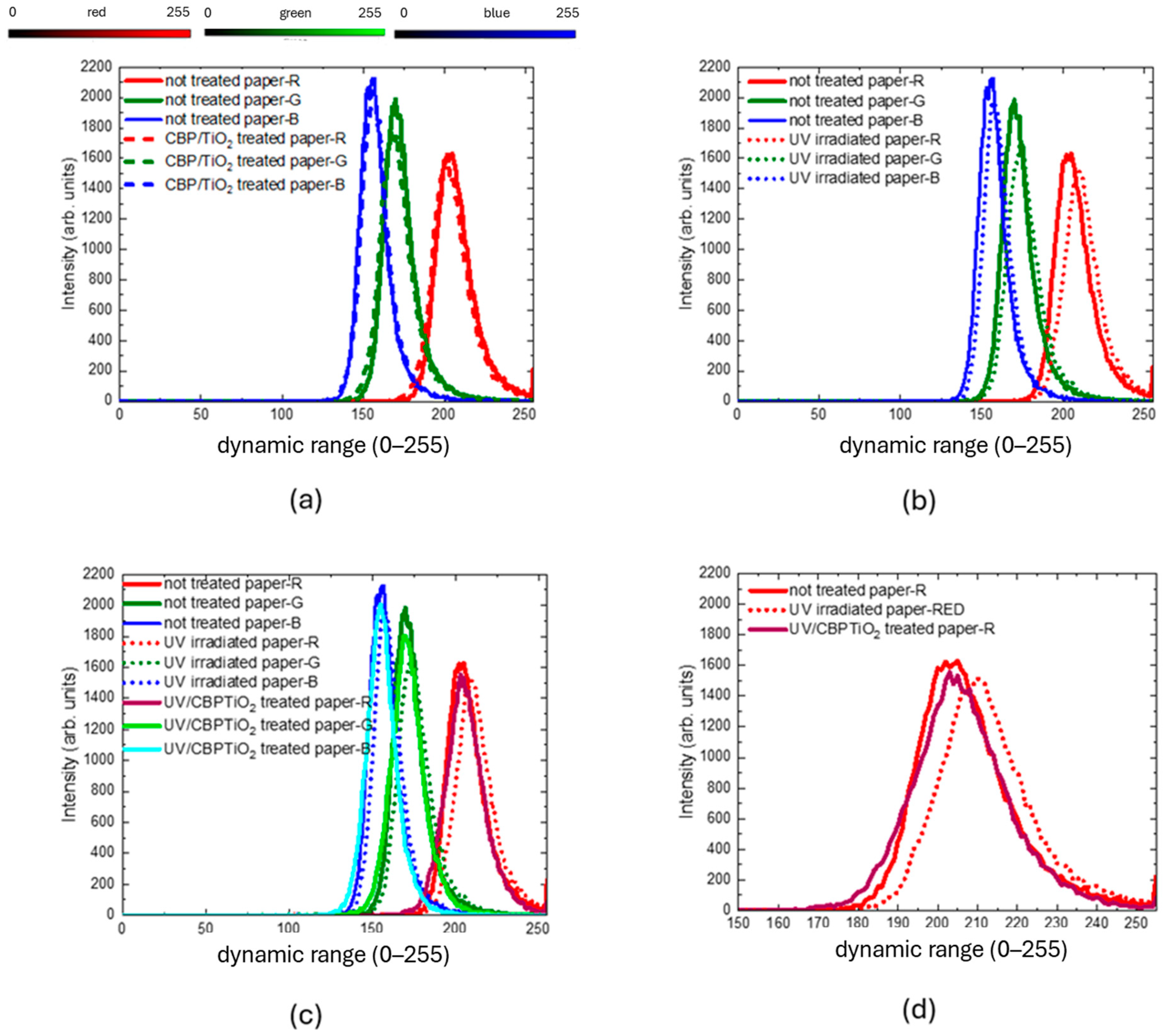
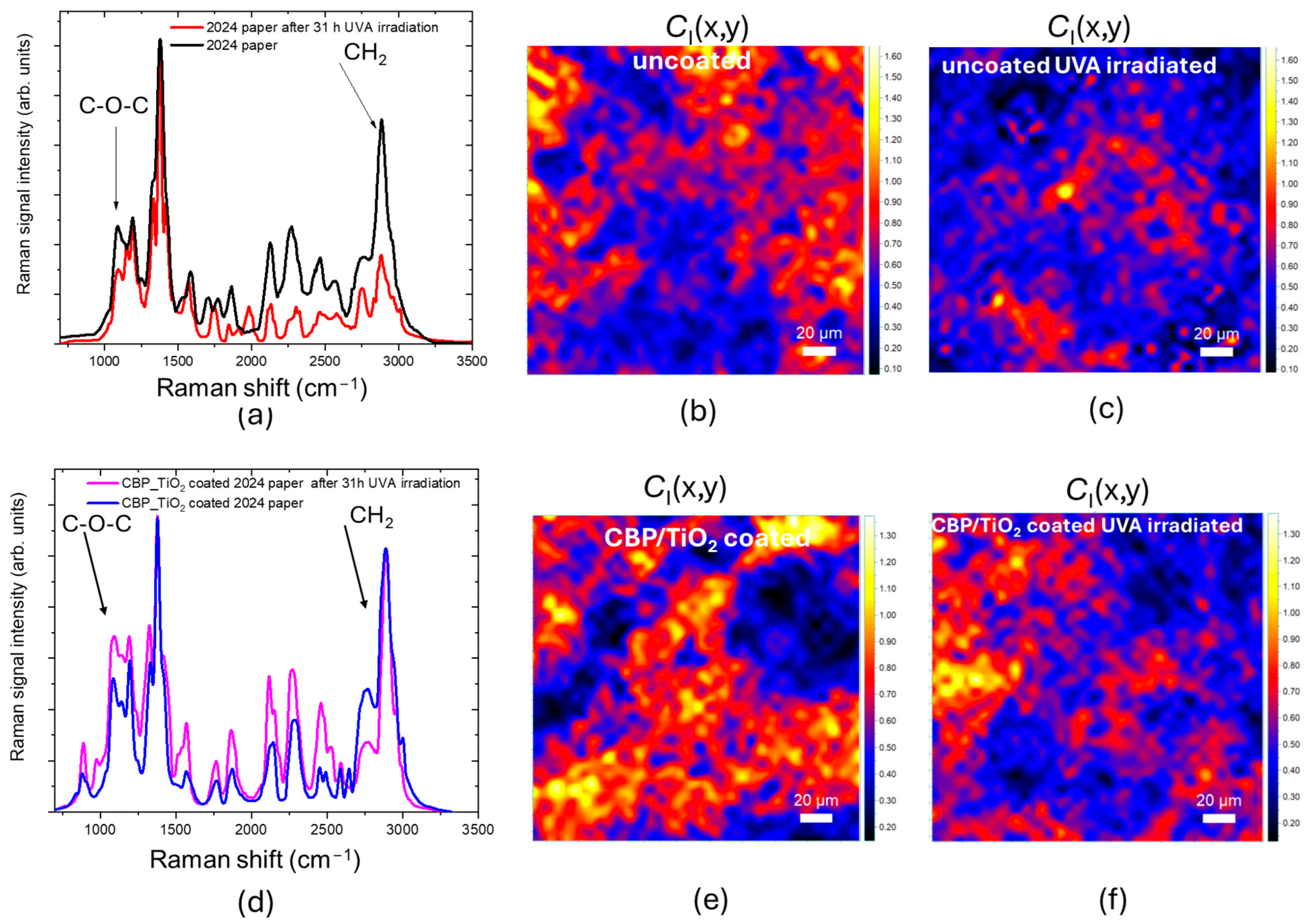
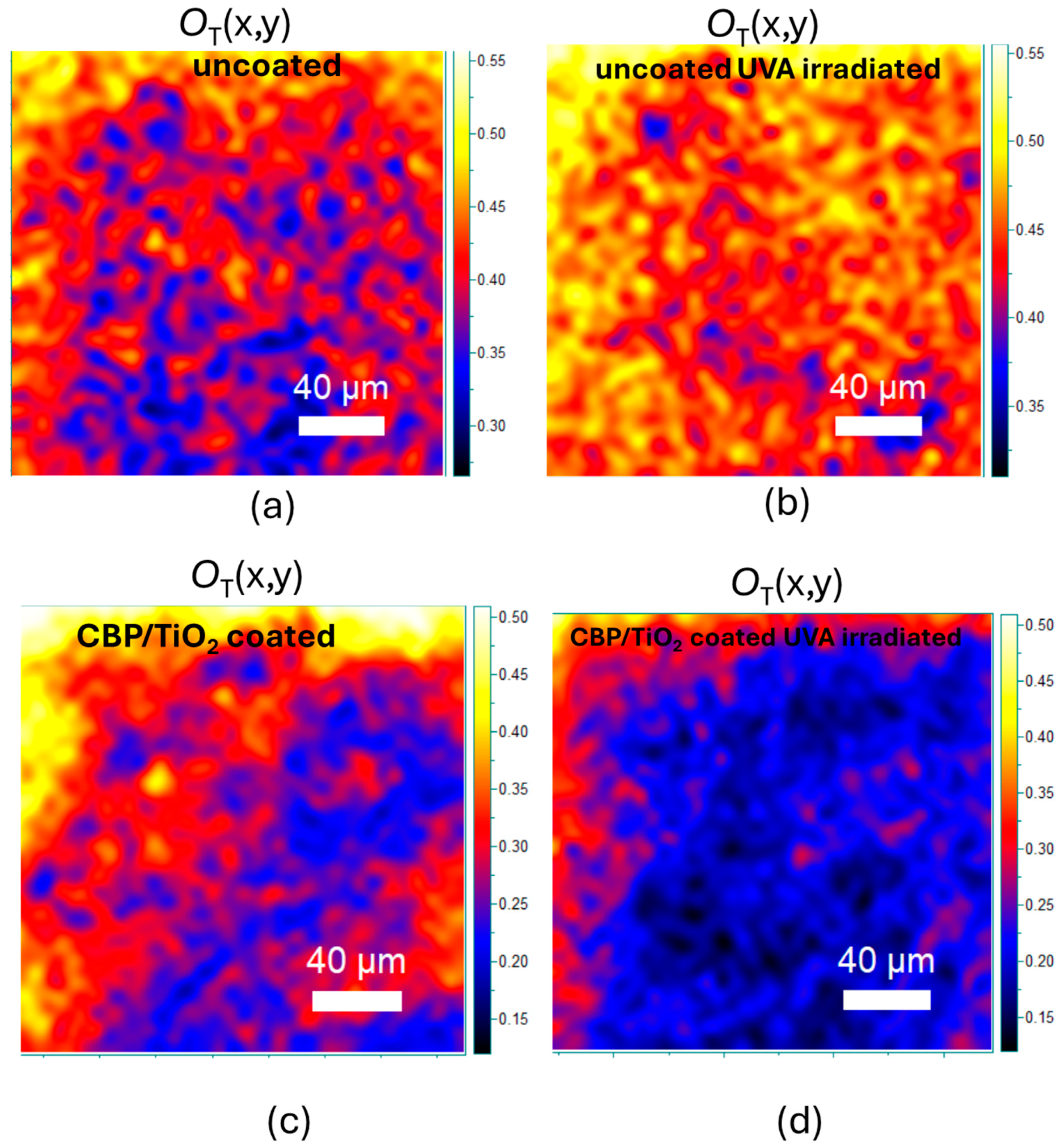
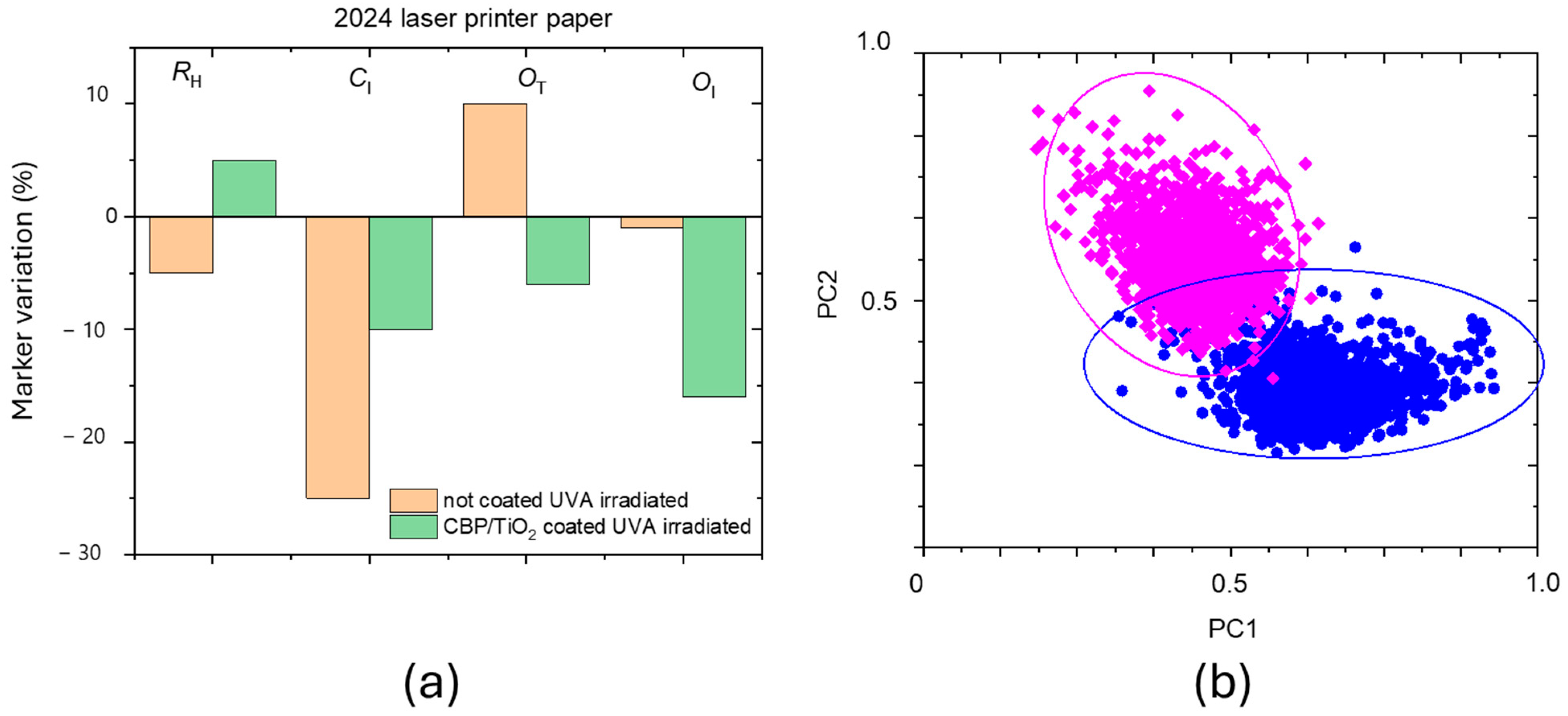
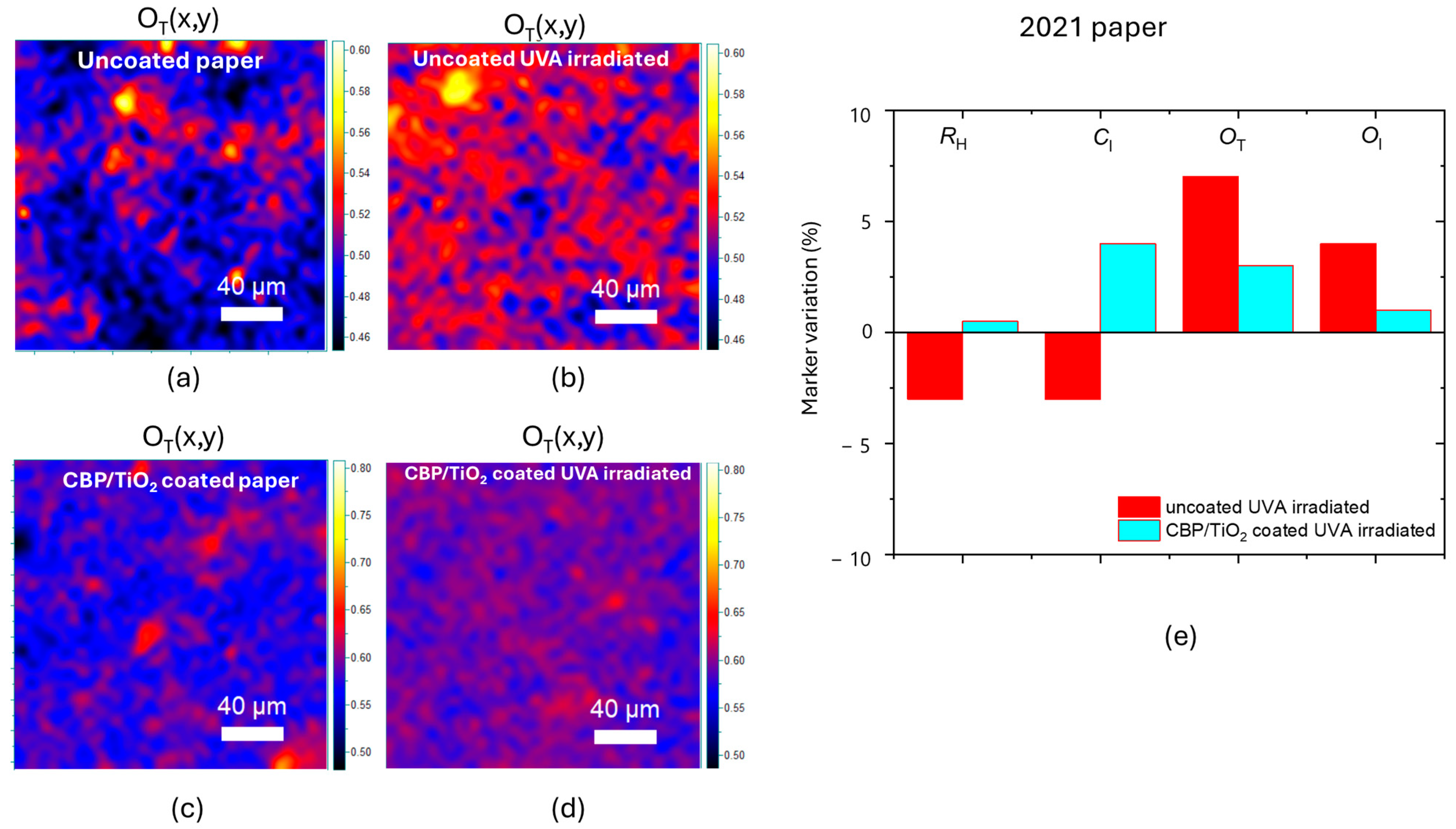
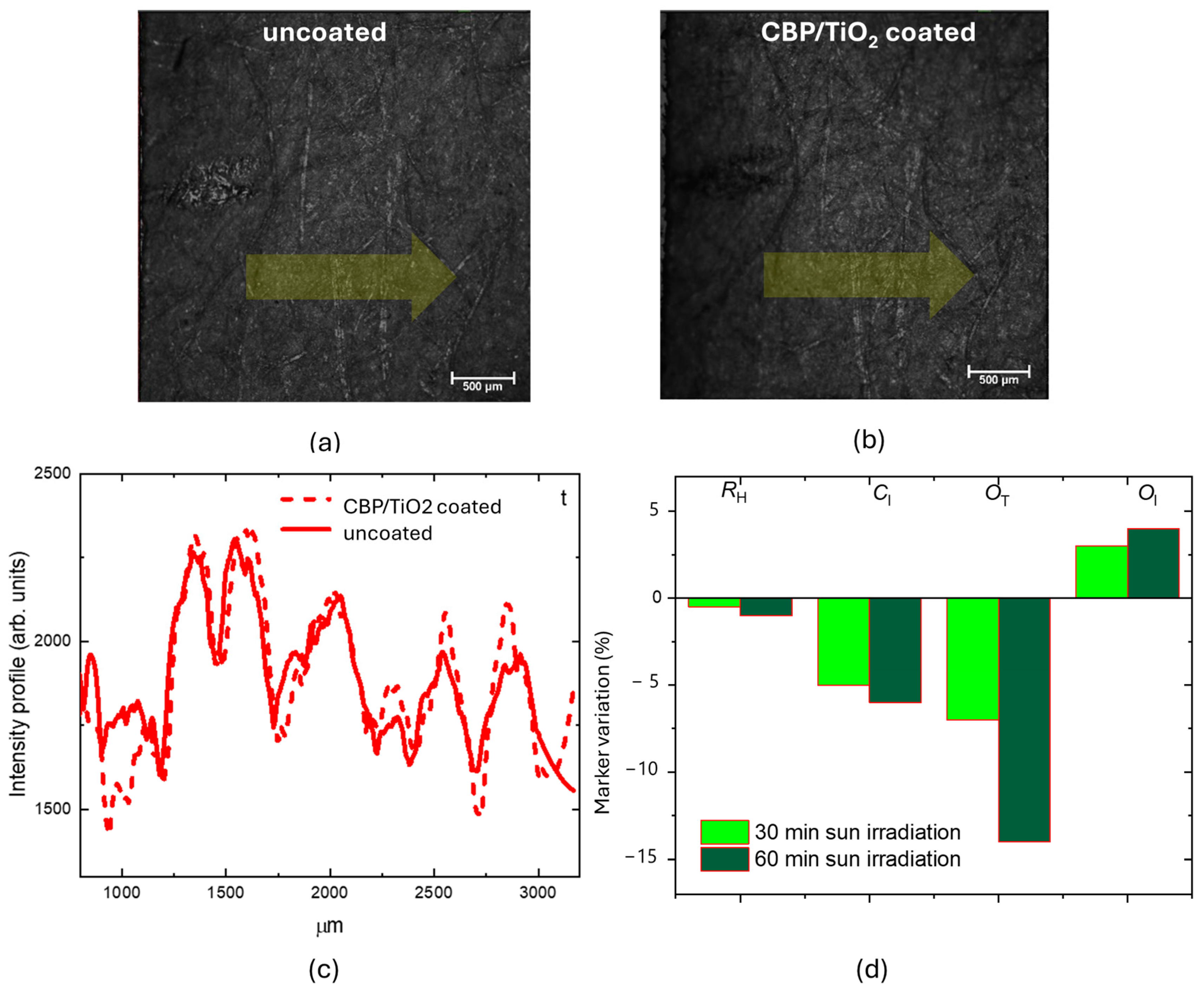
2.2. Characterization of Protective Effect of CBP/TiO2 Coating
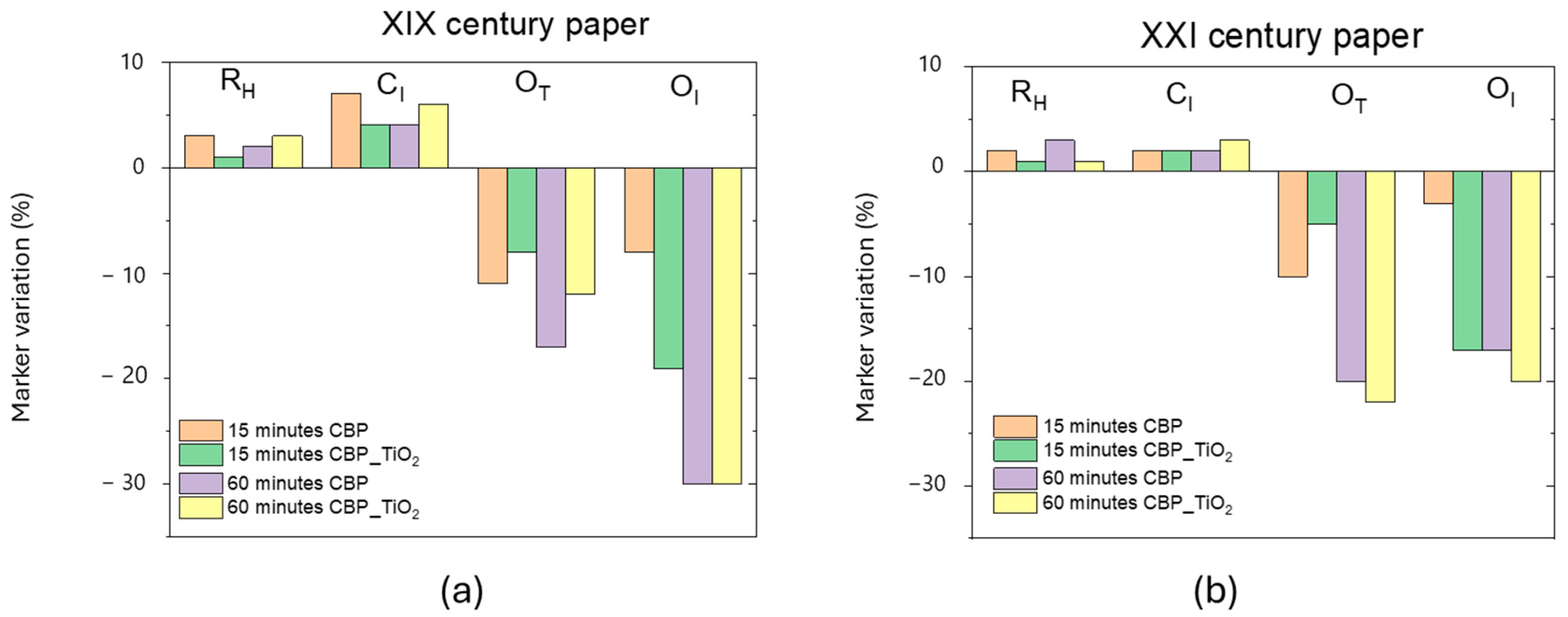
3. Discussion
4. Materials and Methods
4.1. Nanocomposite Hydrogel Preparation
4.2. Paper Samples
4.3. Gel Application Procedure
4.4. UV Source
4.5. Raman Confocal Spectrometer
4.6. Confocal Laser Scanning Optical Microscope
4.7. Analysis with ImageJ Software of Optical Microscope Images
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Manso, M.; Carvalho, M.L. Application of spectroscopic techniques for the study of paper documents: A survey. Spectrochim. Acta Part B At. Spectrosc. 2009, 64, 482–490. [Google Scholar] [CrossRef]
- Bitossi, G.; Giorgi, R.; Mauro, M.; Salvadori, B. Spectroscopic techniques on cultural heritage conservation: A survey. Appl. Spectrosc. Rev. 2005, 40, 187–228. [Google Scholar] [CrossRef]
- Chiriu, D.; Ricci, P.C.; Cappellini, G.; Carbonaro, C.M. Ancient and modern paper: Study on ageing and degradation process by means of portable NIR μ-Raman spectroscopy. Microchem. J. 2018, 138, 26–34. [Google Scholar] [CrossRef]
- Librando, V.; Minniti, Z.; Lorusso, S. Ancient and modern paper characterization by FTIR and Micro-Raman Spectroscopy. Conserv. Sci. Cult. Herit. 2011, 11, 249–268. [Google Scholar]
- Balakhnina, I.A.; Brandt, N.N.; Chikishev, A.Y.; Rebrikova, N.L. Raman microspectroscopy of old paper samples with foxing. Appl. Spec. 2018, 68, 495–500. [Google Scholar] [CrossRef]
- Lojewska, J.; Miskowiec, P.; Lojesewski, T.; Proniewicz, L.M. Cellulose oxidative and hydrolytic degradation: In situ FTIR approach. Polym. Degrad. Stabil. 2005, 88, 512–520. [Google Scholar] [CrossRef]
- Botti, S.; Bonfigli, F.; Nigro, V.; Rufoloni, A.; Vannozzi, A. Evaluating the Conservation State of Naturally Aged Paper with Raman and Luminescence Spectral Mapping: Toward a Non-Destructive Diagnostic Protocol. Molecules 2022, 27, 1712. [Google Scholar] [CrossRef] [PubMed]
- Refugio Martínez, J.; Nieto-Villena, A.; De la Cruz-Mendoza, J.A.; Ortega-Zarzosa, G.; Lobo Guerrero, A. Monitoring the natural aging degradation of paper by fluorescence. J. Cult. Herit. 2017, 26, 22–27. [Google Scholar] [CrossRef]
- Chiriu, D.; Ricci, P.C.; Cappellini, G.; Salis, M.; Loddo, G.; Carbonaro, C.M. Ageing of ancient paper: A kinetic model of cellulose degradation from Raman spectra. J. Raman Spectrosc. 2018, 49, 1802–1811. [Google Scholar] [CrossRef]
- Teygeler, R. Preserving paper: Recent advances. In Managing Preservation for Libraries and Archives; Current Practice and Future Developments; Feather, J., Ed.; Ashgate: Aldershot, UK, 2004. [Google Scholar]
- Mazzuca, C.; Micheli, L.; Carbone, M.; Basoli, F.; Cervelli, E.; Iannuccelli, S.; Sotgiu, S.; Palleschi, A. Gellan hydrogel as a powerful tool in paper cleaning process: A detailed Study. J. Colloid Interface Sci. 2014, 416, 205–211. [Google Scholar] [CrossRef]
- De Filipo, G.; Palermo, A.M.; Tolmino, R.; Formoso, P.; Nicoletta, F.P. Gellan gum hybrid hydrogels for the cleaning of paper artworks contaminated with Aspergillus versicolor. Cellulose 2016, 23, 3265–3279. [Google Scholar] [CrossRef]
- Botti, S.; Bonfigli, F.; D’Amato, R.; Rodesi, J.; Santonicola, M.G. Colorimetric Sensors Based on Poly(acrylic Acid)/TiO2 Nanocomposite Hydrogels for Monitoring UV Radiation Exposure. Gels 2023, 9, 797. [Google Scholar] [CrossRef]
- Talarico, F.; Caldi, C.; Valanzuela, M.; Zaccheo, C.; Zampa, A.; Nugari, M.P. Applicazione dei gel come supportanti nel restauro. Boll. ICR 2001, 3, 101–118. [Google Scholar]
- De Filpo, G.; Mormile, S.; Nicoletta, F.P.; Chidichimo, G. Fast, self-supplied, all-solid photoelectrochromic film. J. Power Sources 2010, 195, 4365–4369. [Google Scholar] [CrossRef]
- De Filpo, G.; Palermo, A.M.; Rachiele, F.; Nicoletta, F.P. Preventing fungal growth in wood by titanium dioxide nanoparticles. Int. Biodeterior. Biodegrad. 2013, 85, 217–222. [Google Scholar] [CrossRef]
- De Filpo, G.; Palermo, A.M.; Munno, R.; Molinaro, L.; Formoso, P.; Nicoletta, F.P. Gellan gum/titanium dioxide nanoparticle hybrid hydrogels for the cleaning and disinfection of parchment. Int. Biodeterior. Biodegrad. 2015, 103, 51–58. [Google Scholar] [CrossRef]
- Rantala, J.T.; Kärkkäinen, A.H.O. Optical properties of spin-on deposited low temperature titanium oxide thin films. Opt. Express 2003, 11, 1406–1410. [Google Scholar] [CrossRef]
- Afsharpour, M.; Rad, F.T.; Malekian, H. New cellulosic titanium dioxide nanocomposite as a protective coating for preserving paper-art-works. J. Cult. Herit. 2011, 12, 380–383. [Google Scholar] [CrossRef]
- D’Amato, R.; Falconieri, M.; Gagliardi, S.; Popovici, E.; Serra, E.; Terranova, G.; Borsella, E. Synthesis of ceramic nanoparticles by laser pyrolysis: From research to applications. J. Anal. Appl. Pyrolysis 2013, 104, 461–469. [Google Scholar] [CrossRef]
- Wiley, J.H.; Atalla, R.H. Band assignments in the Raman spectra of celluloses. Carbohydr. Res. 1987, 160, 113–129. [Google Scholar] [CrossRef]
- Botti, S.; Di Lazzaro, P.; Flora, F.; Mezi, L.; Murra, D. Raman spectral mapping reveal molecular changes in cellulose aging induced by ultraviolet and extreme ultraviolet radiation. Cellulose 2024, 31, 749–758. [Google Scholar] [CrossRef]
- Kacík, F.; Kacíková, D.; Jablonsky, M.; Katuscak, S. Cellulose degradation in newsprint paper ageing. Polym. Deg. Stab. 2009, 94, 1509–1514. [Google Scholar] [CrossRef]
- Malesi, J.; Kolar, J.; Stirlic, M.; Kocar, D.; Fromageot, D.; Lemaire, J. Photoinduced degradation of cellulose. Polym. Deg. Stab. 2005, 89, 64–69. [Google Scholar] [CrossRef]
- Lubrizol. Optimizing Performance of Carbopol®ETD 2020 and Ultrez 10 Polymers with Partial Neutralization of Polymer Dispersions. Technical Data Sheet TDS-243, Ed. Available online: https://www.lubrizol.com/-/media/Lubrizol/Health/TDS/TDS243OptimizingPerformanceCarbopolETD2020Ultrez10PartialNeutralizationPolymerDispersions.pdf (accessed on 15 October 2007).
- Lubrizol. Viscosity of Carbopol®Polymers in Aqueous Systems. Technical Data Sheet TDS-730, Ed. Available online: https://www.lubrizol.com/-/media/Lubrizol/Health/TDS/TDS-730_Viscosity_Carbopol_in_Aqueous-Systems.pdf (accessed on 13 August 2010).
- Gutowski, I.A.; Lee, D.; de Bruyn, J.R.; Frisken, B.J. Scaling and mesostructure of Carbopol dispersions. Rheol. Acta 2012, 51, 441–450. [Google Scholar] [CrossRef]
- Huang, C.; Chu, P.K. Recommended practices and benchmarking of foam electrodes in water splitting. Trends Chem. 2022, 4, 1065–1077. [Google Scholar] [CrossRef]
- Diaspro, A. Confocal and Two-Photon Microscopy. In Foundations, Applications and Advances; Diaspro, A., Ed.; Wiley-Liss: New York, NY, USA, 2002. [Google Scholar]

| Material | Structure | Function | Concentration or Volume |
|---|---|---|---|
| Carbopol® Ultrez 10 | 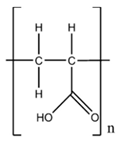 | Polymer | 3.33 wt% |
| Deionized water | 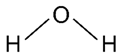 | Solvent | 15 mL |
| Sodium hydroxide | 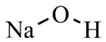 | Neutralizing agent | 260 μL |
| Titanium dioxide |  | Photoactive element | 800 ppm |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Botti, S.; Bonfigli, F.; D’Amato, R.; Rodesi, J.; Santonicola, M.G. Poly(Acrylic Acid)/TiO2 Nanocomposite Hydrogels for Paper Artwork Cleaning and Protection. Molecules 2025, 30, 75. https://doi.org/10.3390/molecules30010075
Botti S, Bonfigli F, D’Amato R, Rodesi J, Santonicola MG. Poly(Acrylic Acid)/TiO2 Nanocomposite Hydrogels for Paper Artwork Cleaning and Protection. Molecules. 2025; 30(1):75. https://doi.org/10.3390/molecules30010075
Chicago/Turabian StyleBotti, Sabina, Francesca Bonfigli, Rosaria D’Amato, Jasmine Rodesi, and Maria Gabriella Santonicola. 2025. "Poly(Acrylic Acid)/TiO2 Nanocomposite Hydrogels for Paper Artwork Cleaning and Protection" Molecules 30, no. 1: 75. https://doi.org/10.3390/molecules30010075
APA StyleBotti, S., Bonfigli, F., D’Amato, R., Rodesi, J., & Santonicola, M. G. (2025). Poly(Acrylic Acid)/TiO2 Nanocomposite Hydrogels for Paper Artwork Cleaning and Protection. Molecules, 30(1), 75. https://doi.org/10.3390/molecules30010075






