Synthesis, Structure and Biological Activity of 2-Methyl-5-nitro-6-phenylnicotinohydrazide-Based Hydrazones
Abstract
1. Introduction
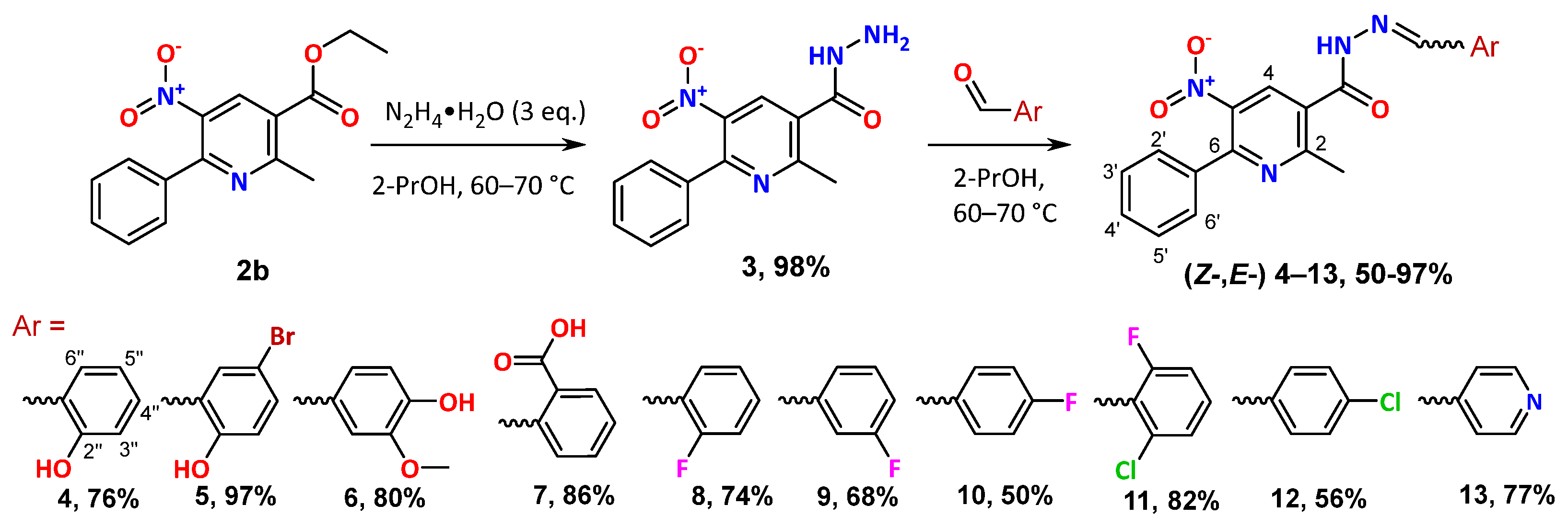
2. Results and Discussion
2.1. Study of NMR Spectra
2.2. Isomer Modeling
2.3. Antibacterial and Antifungal Properties
3. Materials and Methods
3.1. Instrumentation and Synthetic Procedures
3.2. DFT Calculations
3.3. Biological Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alam, M.; Verma, G.; Shaquiquzzaman, M.; Marella, A.; Akhtar, M.; Ali, M. A Review Exploring Biological Activities of Hydrazones. J. Pharm. Bioallied. Sci. 2014, 6, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.S.; Siddiqui, S.P.; Tarannum, N. A systematic review on the synthesis and biological activity of hydrazide derivatives. Hygeia. J. Drugs Med. 2017, 9, 61–79. [Google Scholar]
- Mali, S.N.; Thorat, B.R.; Gupta, D.R.; Pandey, A. Mini-Review of the Importance of Hydrazides and Their Derivatives—Synthesis and Biological Activity. Eng. Proc. 2021, 11, 21. [Google Scholar] [CrossRef]
- Myasoedova, Y.V.; Garifullina, L.R.; Nurieva, E.R.; Ishmuratov, G.Y. Hydrazides of Organic Acids in the Transformations of the Peroxide Products of Non-1-ene Ozonolysis. Russ. J. Org. Chem. 2019, 55, 1712–1715. [Google Scholar] [CrossRef]
- Popiołek, Ł.; Biernasiuk, A. Synthesis and Investigation of Antimicrobial Activities of Nitrofurazone Analogues Containing Hydrazide-Hydrazone Moiety. Saudi Pharm. J. 2017, 25, 1097–1102. [Google Scholar] [CrossRef] [PubMed]
- Nurkenov, O.A.; Fazylov, S.D.; Satpaeva, Z.B.; Seilkhanov, T.M.; Turdybekov, D.M.; Mendibayeva, A.Z.; Akhmetova, S.B.; Shulgau, Z.T.; Alkhimova, L.E.; Kulakov, I.V. Synthesis, Structure and Biological Activity of Hydrazones Derived from 2- and 4-Hydroxybenzoic Acid Hydrazides. Chem. Data Collect. 2023, 48, 101089. [Google Scholar] [CrossRef]
- Rodrigues, F.A.R. Biological Evaluation of Isoniazid Derivatives as an Anticancer Class. Sci. Pharm. 2014, 82, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Naveen Kumar, H.S.; Parumasivam, T.; Jumaat, F.; Ibrahim, P.; Asmawi, M.Z.; Sadikun, A. Synthesis and evaluation of isonicotinoyl hydrazone derivatives as antimycobacterial and anticancer agents. Med. Chem. Res. 2014, 23, 269–279. [Google Scholar] [CrossRef]
- Hu, Y.-Q.; Zhang, S.; Zhao, F.; Gao, C.; Feng, L.-S.; Lv, Z.-S.; Xu, Z.; Wu, X. Isoniazid Derivatives and Their Anti-Tubercular Activity. Eur. J. Med. Chem. 2017, 133, 255–267. [Google Scholar] [CrossRef]
- Narang, R.; Narasimhan, B.; Sharma, S.; Sriram, D.; Yogeeswari, P.; De Clercq, E.; Pannecouque, C.; Balzarini, J. Synthesis, Antimycobacterial, Antiviral, Antimicrobial Activities, and QSAR Studies of Nicotinic Acid Benzylidene Hydrazide Derivatives. Med. Chem. Res. 2012, 21, 1557–1576. [Google Scholar] [CrossRef]
- Altaf, A.A.; Shahzad, A.; Gul, Z.; Rasool, N.; Badshah, A.; Lal, B.; Khan, E. A Review on the Medicinal Importance of Pyridine Derivatives. J. Drug Des. Med. Chem. 2015, 1, 1. [Google Scholar]
- Nurkenov, O.A.; Fazylov, S.D.; Seilkhanov, T.M.; Abulyaissova, L.K.; Turdybekov, K.M.; Zhivotova, T.S.; Kabieva, S.K.; Mendibayeva, A.Z. Interaction of Isonicotinic Acid Hydrazide with Carboxylic Acid Anhydrides. Eurasian J. Chem. 2023, 110, 29–35. [Google Scholar] [CrossRef]
- Kulakov, I.V.; Oleshchuk, A.L.; Koveza, V.A.; Palamarchuk, I.V. Multicomponent synthesis of 4-unsubstituted 5-nitropyridine derivatives. Synth. Commun. 2020, 50, 2432–2439. [Google Scholar] [CrossRef]
- Stalinskaya, A.L.; Chikunov, S.Y.; Pustolaikina, I.A.; Gatilov, Y.V.; Kulakov, I.V. Synthesis of new structural analogues of natural Integrastatins with a basic epoxybenzo[7,8]oxocine skeleton: Combined experimental and computational study. Synthesis 2024, 56, 329–345. [Google Scholar]
- Neese, F. The ORCA program system. WIRES Comput. Molec. Sci. 2012, 2, 73–78. [Google Scholar] [CrossRef]
- Neese, F. Software update: The ORCA program system, version 5.0. WIRES Comput. Molec. Sci. 2022, 12, e1606. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef]
- Vosko, S.H.; Wilk, L.; Nusair, M. Accurate spin-dependent electron liquid correlation energies for local spin density calculations: A critical analysis. Can. J. Phys. 1980, 58, 1200–1211. [Google Scholar] [CrossRef]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef] [PubMed]
- Weigend, F.; Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef] [PubMed]
- York, D.M.; Karplus, M. A Smooth Solvation Potential Based on the Conductor-Like Screening Model. J. Phys. Chem. A 1999, 103, 11060–11079. [Google Scholar] [CrossRef]
- Marenich, A.V.; Cramer, C.J.; Truhlar, D.G. Universal Solvation Model Based on Solute Electron Density and on a Continuum Model of the Solvent Defined by the Bulk Dielectric Constant and Atomic Surface Tensions. J. Phys. Chem. B 2009, 113, 6378–6396. [Google Scholar] [CrossRef] [PubMed]
- Grishin, S.Y.; Domnin, P.A.; Kravchenko, S.V.; Azev, V.N.; Mustaeva, L.G.; Gorbunova, E.Y.; Kobyakova, M.I.; Surin, A.K.; Makarova, M.A.; Kurpe, S.R.; et al. Is It Possible to Create Antimicrobial Peptides Based on the Amyloidogenic Sequence of Ribosomal S1 Protein of P. aeruginosa? Int. J. Mol. Sci. 2021, 22, 9776. [Google Scholar] [CrossRef]

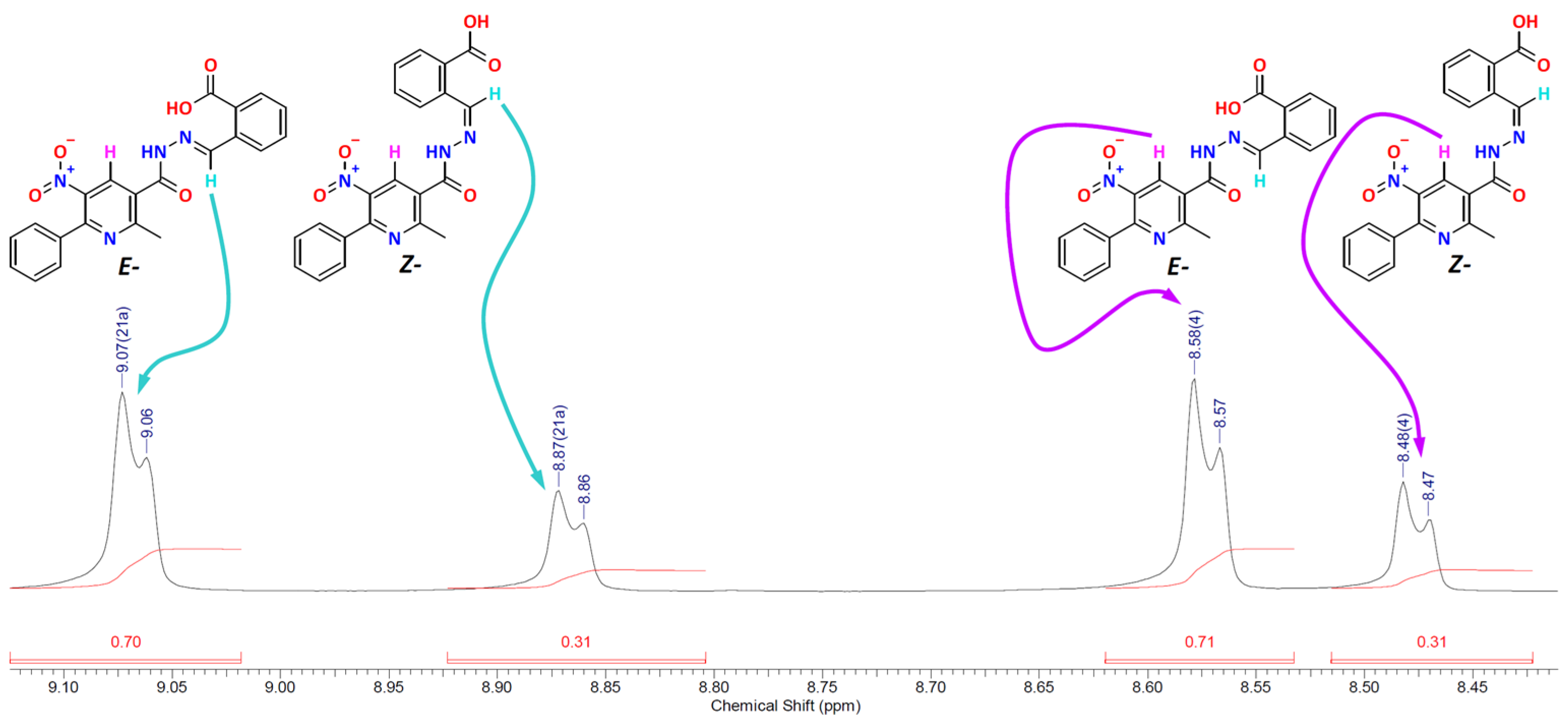
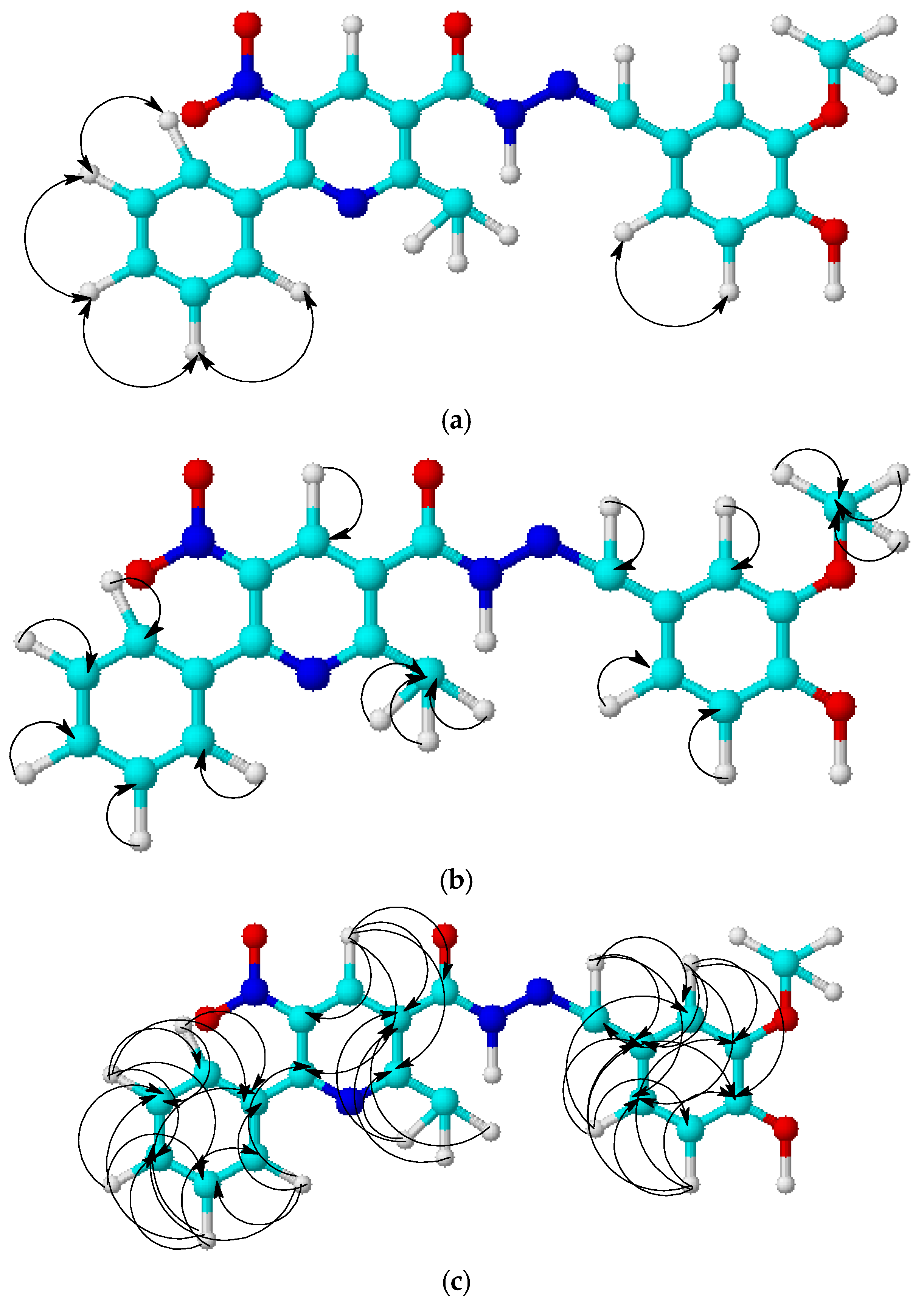

| Compound | δ (H-4 (E-)) | δ (H-4 (Z-)) | δ (=CH (E-)) | δ (=CH (Z-)) |
|---|---|---|---|---|
| 3 | 8.35 | – | – | – |
| 4 | 8.56 | 8.45 | 8.65 | 8.52 |
| 5 | 8.58 | 8.48 | 8.48 | 8.32 |
| 6 | 8.53 | 8.48 | 8.17 | 7.99 |
| 7 | 8.58 | 8.48 | 9.07 | 8.87 |
| 8 | 8.53 | 8.53 | 8.65 | 8.34 |
| 9 | 8.58 | 8.49 | 8.31 | 8.13 |
| 10 | 8.57 | 8.47 | 8.29 | 8.13 |
| 11 | 8.57 | 8.37 | 8.67 | 8.47 |
| 12 | 8.62 | 8.51 | 8.31 | 8.14 |
| 13 | 8.64 | 8.53 | 8.31 | 8.13 |
| Compound | Z-I 1 | Z-II | E-I | E-II |
|---|---|---|---|---|
| 4 | 0.0 | −1.5 | −9.7 | −10.0 |
| 5 | 0.0 | −1.5 | −9.9 | −10.1 |
| 6 | 0.0 | −0.5 | −6.3 | −6.6 |
| 7 | 0.0 | −1.9 | −8.0 | −8.3 |
| 8 | 0.0 | −0.5 | −3.5 | −3.8 |
| 9 | 0.0 | −0.4 | −4.5 | −4.9 |
| 10 | 0.0 | −0.4 | −4.3 | −4.7 |
| 11 | 0.0 | −0.7 | −1.8 | −2.1 |
| 12 | 0.0 | −0.5 | −4.5 | −4.8 |
| 13 | 0.0 | −0.4 | −4.8 | −5.2 |
| Compound | δ (H-4 (Z-I)) | δ (H-4 (Z-II)) | δ (H-4 (E-I)) | δ (H-4 (E-II)) | δ (=CH (Z-I)) | δ (=CH (Z-II)) | δ (=CH (E-I)) | δ (=CH (E-II)) |
|---|---|---|---|---|---|---|---|---|
| 4 | 8.74 | 8.73 | 8.67 | 8.67 | 8.84 | 8.86 | 10.84 | 10.81 |
| 5 | 8.74 | 8.7 | 8.78 | 8.55 | 8.76 | 8.73 | 10.81 | 10.78 |
| 6 | 8.96 | 8.66 | 8.83 | 8.52 | 8.27 | 8.34 | 10.89 | 10.84 |
| 7 | 8.87 | 8.88 | 8.82 | 8.63 | 8.93 | 9.02 | 10.93 | 10.98 |
| 8 | 8.85 | 8.65 | 8.78 | 8.57 | 8.48 | 8.93 | 10.89 | 10.84 |
| 9 | 8.83 | 8.71 | 8.83 | 8.6 | 8.49 | 8.56 | 10.87 | 10.73 |
| 10 | 8.55 | 8.61 | 8.78 | 8.56 | 8.49 | 8.51 | 10.82 | 10.85 |
| 11 | 8.47 | 8.63 | 8.73 | 8.66 | 8.72 | 8.69 | 11.39 | 11.34 |
| 12 | 8.84 | 8.7 | 8.77 | 8.57 | 8.47 | 8.63 | 10.82 | 10.83 |
| 13 | 8.68 | 8.69 | 8.72 | 8.69 | 8.6 | 8.65 | 10.77 | 10.78 |
| Bacteria | Parameter 1 | Compound | Control (1% DMSO) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | |||
| Staphylococcus aureus 209P | GI | − | − | + | − | + | − | − | − | − | − | − | − |
| Staphylococcus aureus ATCC 29923 | GI | − | − | − | − | + | − | − | − | − | − | − | − |
| Staphylococcus epidermidis | GI | − | − | − | − | − | − | − | − | − | − | − | − |
| Enterococcus. faecium 79 OSAU | GI | − | − | − | − | − | − | − | − | − | − | − | − |
| Bacillus cereus IP 5832 | GI | + | − | + | + | + | − | − | − | − | − | − | − |
| Escherichia coli K12 | GI | − | − | − | − | − | − | − | − | − | − | − | − |
| Pectobacterium carotovorum VKM-B-1247 | GI | + | − | + | − | + | − | − | − | − | − | − | − |
| Chromobacterium substugae ATCC 31532 | GI | − | − | − | − | + | − | − | − | − | − | − | − |
| − | − | − | + | − | − | − | − | − | − | − | − | ||
| Bacteria | Parameter 1 | Compound | Control (1% DMSO) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | |||
| Candida albicans ATCC 10231 | GI | − | − | − | − | − | − | − | − | − | − | − | − |
| Aspergillus niger INA 007760 | GI | − | − | − | − | − | − | − | − | − | − | − | − |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nurkenov, O.A.; Mendibayeva, A.Z.; Fazylov, S.D.; Seilkhanov, T.M.; Kabieva, S.K.; Syzdykov, A.K.; Kulakov, I.I.; Iashnikov, A.V.; Vasilchenko, A.S.; Alkhimova, L.E.; et al. Synthesis, Structure and Biological Activity of 2-Methyl-5-nitro-6-phenylnicotinohydrazide-Based Hydrazones. Molecules 2025, 30, 169. https://doi.org/10.3390/molecules30010169
Nurkenov OA, Mendibayeva AZ, Fazylov SD, Seilkhanov TM, Kabieva SK, Syzdykov AK, Kulakov II, Iashnikov AV, Vasilchenko AS, Alkhimova LE, et al. Synthesis, Structure and Biological Activity of 2-Methyl-5-nitro-6-phenylnicotinohydrazide-Based Hydrazones. Molecules. 2025; 30(1):169. https://doi.org/10.3390/molecules30010169
Chicago/Turabian StyleNurkenov, Oralgazy A., Anel Z. Mendibayeva, Serik D. Fazylov, Tulegen M. Seilkhanov, Saule K. Kabieva, Ardak K. Syzdykov, Ilya I. Kulakov, Aleksandr V. Iashnikov, Alexey S. Vasilchenko, Larisa E. Alkhimova, and et al. 2025. "Synthesis, Structure and Biological Activity of 2-Methyl-5-nitro-6-phenylnicotinohydrazide-Based Hydrazones" Molecules 30, no. 1: 169. https://doi.org/10.3390/molecules30010169
APA StyleNurkenov, O. A., Mendibayeva, A. Z., Fazylov, S. D., Seilkhanov, T. M., Kabieva, S. K., Syzdykov, A. K., Kulakov, I. I., Iashnikov, A. V., Vasilchenko, A. S., Alkhimova, L. E., & Kulakov, I. V. (2025). Synthesis, Structure and Biological Activity of 2-Methyl-5-nitro-6-phenylnicotinohydrazide-Based Hydrazones. Molecules, 30(1), 169. https://doi.org/10.3390/molecules30010169










