Thiosemicarbazone-Based Compounds: A Promising Scaffold for Developing Antibacterial, Antioxidant, and Anticancer Therapeutics
Abstract
1. Introduction
2. Results and Discussion
2.1. Synthesis and Physicochemical Characterization
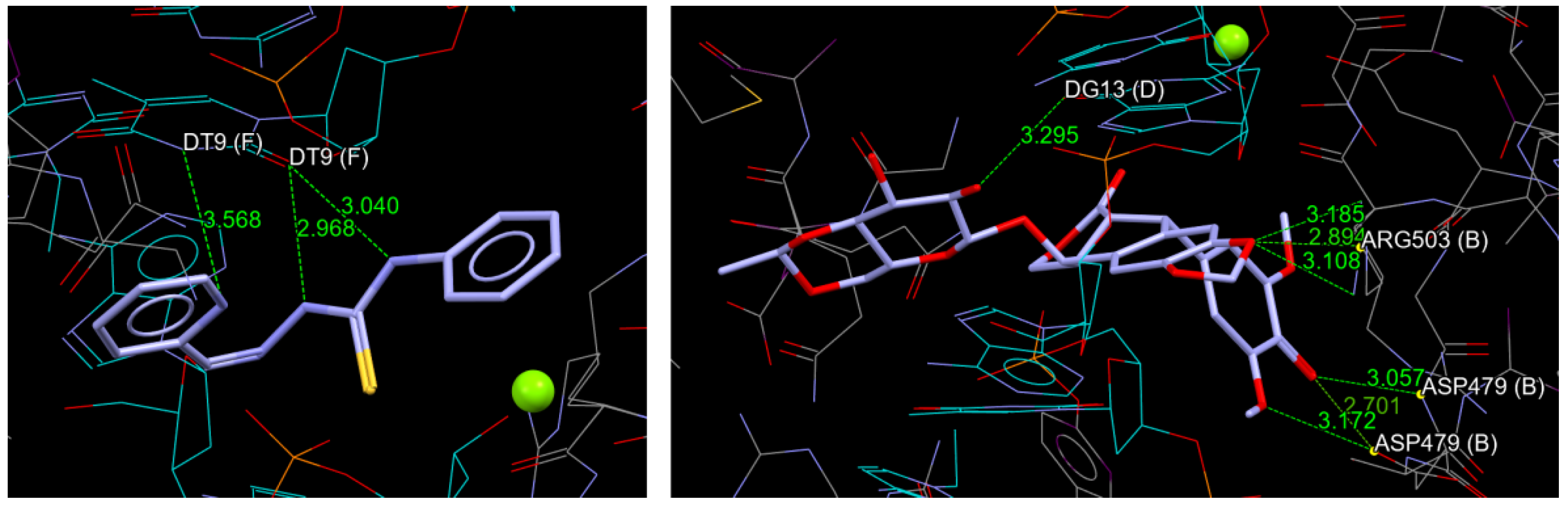
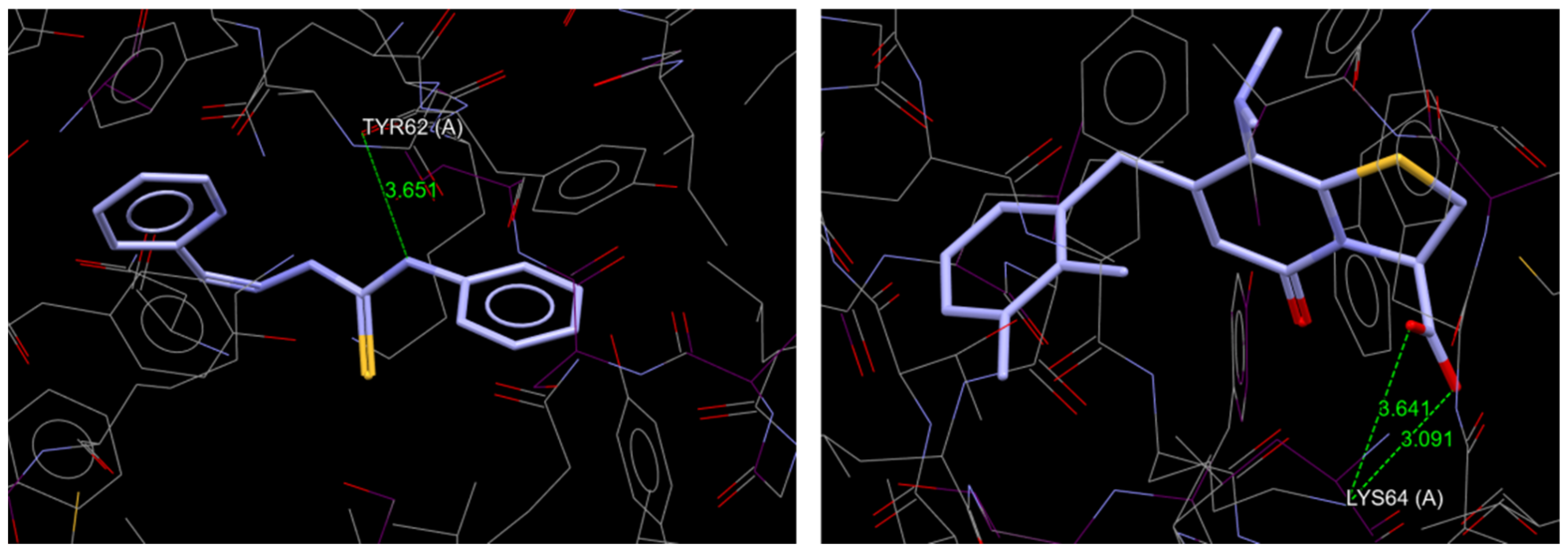
2.2. Molecular Docking
2.3. In Silico Biological Activity Predictions
2.4. Antibacterial Activity
2.5. Antioxidant Activity
2.5.1. ABTS
2.5.2. DPPH
2.5.3. ORAC-FL
2.5.4. Cell Culture and MTT Cytotoxicity Assay
3. Materials and Methods
3.1. Chemicals
3.2. Mass Spectrometry
3.3. Infrared Spectra
3.4. Minimum Inhibitory Concentration
3.5. Molecular Docking
3.6. ADME
3.7. ABTS
3.8. DPPH
3.9. ORAC-FL
3.10. Cell Culture and MTT Cytotoxicity Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dilović, I.; Rubčić, M.; Vrdoljak, V.; Pavelić, S.K.; Kralj, M.; Piantanida, I.; Cindrić, M. Novel thiosemicarbazone derivatives as potential antitumor agents: Synthesis, physicochemical and structural properties, DNA interactions and antiproliferative activity. Bioorganic Med. Chem. 2008, 16, 5189–5198. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.; Abser, M.N.; Kumer, A.; Bhuiyan, M.M.H.; Akter, P.; Hossain, M.E.; Chakma, U. Synthesis, characterization, antibacterial activity of thiosemicarbazones derivatives and their computational approaches: Quantum calculation, molecular docking, molecular dynamic, ADMET, QSAR. Heliyon 2023, 9, e16222. [Google Scholar] [CrossRef]
- Pitucha, M.; Woś, M.; Miazga-Karska, M.; Klimek, K.; Mirosław, B.; Pachuta-Stec, A.; Gładysz, A.; Ginalska, G. Synthesis, antibacterial and antiproliferative potential of some new 1-pyridinecarbonyl-4-substituted thiosemicarbazide derivatives. Med. Chem. Res. 2016, 25, 1666–1677. [Google Scholar] [CrossRef] [PubMed]
- Vandresen, F.; Falzirolli, H.; Batista, S.A.A.; Da Silva-Giardini, A.P.B.; De Oliveira, D.N.; Catharino, R.R.; Ruiz, A.L.T.G.; De Carvalho, J.E.; Foglio, M.A.; Da Silva, C.C. Novel R-(+)-limonene-based thiosemicarbazones and their antitumor activity against human tumor cell lines. Eur. J. Med. Chem. 2014, 79, 110–116. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Wang, X.; Zhao, X.; Liang, Y.; He, H.; Fu, L. Synthesis and antitumor activity of novel quinazoline derivatives containing thiosemicarbazide moiety. Eur. J. Med. Chem. 2012, 54, 925–930. [Google Scholar] [CrossRef] [PubMed]
- Rogalewicz, B.; Pitucha, M.; Świątkowski, M.; Humeniuk, E.; Adamczuk, G.; Drózd, M.; Karczmarzyk, Z.; Kuśmierek, E.; Strzelec, K.; Raducka, A.; et al. Structure-activity relationship and cytotoxicity of the new thiosemicarbazide derivatives and their Cu(II) complexes against prostate and melanoma cancer cells. Arch. Biochem. Biophys. 2024, 755, 109955. [Google Scholar] [CrossRef]
- Cebotari, D.; Buils, J.; Garbuz, O.; Balan, G.; Marrot, J.; Guérineau, V.; Touboul, D.; Haouas, M.; Segado-Centelles, M.; Bo, C.; et al. A new series of bioactive Mo(V)2O2S2-based thiosemicarbazone complexes: Solution and DFT studies, and antifungal and antioxidant activities. J. Inorg. Biochem. 2023, 245, 112258. [Google Scholar] [CrossRef]
- Nguyen, D.T.; Le, T.H.; Bui, T.T.T. Antioxidant activities of thiosemicarbazones from substituted benzaldehydes and N-(tetra-O-acetyl-b-D-galactopyranosyl)thiosemicarbazide. Eur. J. Med. Chem. 2013, 60, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Umamatheswari, S.; Balaji, B.; Ramanathan, M.; Kabilan, S. Synthesis, stereochemistry, antimicrobial evaluation and QSAR studies of 2,6-diaryltetrahydropyran-4-one thiosemicarbazones. Eur. J. Med. Chem. 2011, 46, 1415–1424. [Google Scholar] [CrossRef]
- Siwek, A.; Stefanska, J.; Dzitko, K.; Ruszczak, A. Antifungal effect of 4-arylthiosemicarbazides against Candida species. Search for molecular basis of antifungal activity of thiosemicarbazide derivatives. J. Mol. Model. 2012, 18, 4159–4170. [Google Scholar] [CrossRef]
- Pacca, C.C.; Marques, R.E.; Espindola, J.W.P.; Filho, G.B.O.O.; Leite, A.C.L.; Teixeira, M.M.; Nogueira, M.L. Thiosemicarbazones and Phthalyl-Thiazoles compounds exert antiviral activity against yellow fever virus and Saint Louis encephalitis virus. Biomed. Pharmacother. 2017, 87, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Basyouni, W.M.; Abbas, S.Y.; El-Bayouki, K.A.M.; Daawod, R.M.; Elawady, M.K. Synthesis and antiviral evaluation of 5 (arylazo)salicylaldehyde thiosemicarbazone derivatives as potent anti-bovine viral diarrhea virus agents. Synth. Commun. 2021, 51, 2168–2174. [Google Scholar] [CrossRef]
- Saeed, K.; Rafiq, M.; Khalid, M.; Hussain, A.; Siddique, F.; Hanif, M.; Hussain, S.; Mahmood, K.; Ameer, N.; Ahmed, M.M.; et al. Synthesis, characterization, computational assay and anti-inflammatory activity of thiosemicarbazone derivatives: Highly potent and efficacious for COX inhibitors. Int. Immunopharmacol. 2024, 126, 111259. [Google Scholar] [CrossRef] [PubMed]
- Sachdeva, H.; Dwivedi, D.; Arya, K.; Khaturia, S.; Saroj, R. Synthesis, anti-inflammatory activity, and QSAR study of some Schiff bases derived from 5-mercapto-3-(40-pyridyl)-4H-1,2,4 triazol-4-yl-thiosemicarbazide. Med. Chem. Res. 2013, 22, 4953–4963. [Google Scholar] [CrossRef]
- Li, M.X.; Chen, C.L.; Ling, C.S.; Zhou, J.; Ji, B.S.; Wu, Y.J.; Niu, J.Y. Cytotoxicity and structure–activity relationships of four α-N-heterocyclic thiosemicarbazone derivatives crystal structure of 2-acetylpyrazine thiosemicarbazone. Bioorganic Med. Chem. Lett. 2009, 19, 2704–2706. [Google Scholar] [CrossRef]
- Stefani, C.; Jansson, P.J.; Gutierrez, E.; Bernhardt, P.V.; Richardson, D.R.; Kalinowski, D.S. Alkyl Substituted 2′-Benzoylpyridine Thiosemicarbazone Chelators with Potent and Selective Anti-Neoplastic Activity: Novel Ligands that Limit Methemoglobin Formation. J. Med. Chem. 2012, 56, 357–370. [Google Scholar] [CrossRef] [PubMed]
- Dobek, A.S.; Klayman, D.L.; Scovill, J.P.; Dickson, E.T. Antibacterial Properties of 2-Acetylpyridine-l-Oxide Thiosemicarbazones. Chemotherapy 1986, 30, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Enumula, S.; Mudam, S.; Ahmed, K. 3-Quinonlinecarbaldehyde thiosemicarbazones: Synthesis from N-arylacetamide, Characterization, Antibacterial and Antioxidant activities. Int. J. Eng. Appl. Sci. 2017, 4, 33–36. [Google Scholar]
- Korkmaz, G.; Karaman, I.; Gezegen, H. A review of recent research on the antimicrobial activities of thiosemicarbazone-based compounds. J. New Results Sci. 2024, 13, 61–83. [Google Scholar] [CrossRef]
- Kowalczyk, A.; Paneth, A.; Trojanowski, D.; Paneth, P.; Zakrzewska-Czerwińska, J.; Stączek, P. Thiosemicarbazide Derivatives Decrease the ATPase Activity of Staphylococcus aureus Topoisomerase IV, Inhibit Mycobacterial Growth, and Affect Replication in Mycobacterium smegmatis. Int. J. Mol. Sci. 2021, 22, 3881. [Google Scholar] [CrossRef] [PubMed]
- Siwek, A.; Stączek, P.; Stefańska, J. Synthesis and structure activity relationship studies of 4-arylthiosemicarbazides as topoisomerase IV inhibitors with Gram-positive antibacterial activity. Search for molecular basis of antibacterial activity of thiosemicarbazides. Eur. J. Med. Chem. 2011, 46, 5717–5726. [Google Scholar] [CrossRef] [PubMed]
- Laxmi, S.V.; Rajitha, G.; Rajitha, B.; Rao, A.J. Photochemical synthesis and anticancer activity of barbituric acid, thiobarbituric acid, thiosemicarbazide, and isoniazid linked to 2-phenyl indole derivatives. J. Chem. Biol. 2016, 9, 57–63. [Google Scholar] [CrossRef]
- Wos, M.; Miazga-Karska, M.; Kaczor, A.A.; Klimek, K.; Karczmarzyk, Z.; Kowalczuk, D.; Wysocki, W.; Ginalska, G.; Urbanczyk-Lipkowska, Z.; Morawiak, M.; et al. Novel thiosemicarbazide derivatives with 4-nitrophenyl group as multi-target drugs: a-glucosidase inhibitors with antibacterial and antiproliferative activity. Biomed. Pharmacother. 2017, 93, 1269–1276. [Google Scholar] [CrossRef]
- Chen, R.; Huo, L.; Jaiswal, Y.; Huang, J.; Zhong, Z.; Zhong, J.; Williams, L.; Xia, X.; Liang, Y.; Yan, Z. Design, Synthesis, Antimicrobial, and Anticancer Activities of Acridine Thiosemicarbazides Derivatives. Molecules 2019, 24, 2065. [Google Scholar] [CrossRef]
- Ayoup, M.S.; Wahby, Y.; Abdel-Hamid, H.; Abu-Serie, M.M.; Ramadan, S.; Barakat, A.; Teleb, M.; Ismail, M.M.F. Reinvestigation of Passerini and Ugi scaffolds as multistep apoptotic inducers via dual modulation of caspase 3/7 and P53-MDM2 signaling for halting breast cancer. RSC Adv. 2023, 13, 27722–27737. [Google Scholar] [CrossRef]
- Sever, B.; Çiftçi, G.A.; Özdemir, A.; Altintop, M.D. Design, synthesis and biological evaluation of new bis(thiosemicarbazone) derivatives as potential targeted anticancer agents for non-small cell lung cancer. J. Res. Pharm. 2020, 24, 670–680. [Google Scholar] [CrossRef]
- Sever, B.; Çiftçi, G.A.; Özdemir, A.; Altintop, M.D. Design, synthesis and in vitro evaluation of new thiosemicarbazone derivatives as potential anticancer agents. Marmara Pharm. J. 2019, 23, 16–24. [Google Scholar] [CrossRef]
- Sever, B.; Altıntop, M.D.; Çiftçi, G.A.; Özdemir, A. A New Series of Triazolothiadiazines as Potential Anticancer Agents for Targeted Therapy of Non-Small Cell Lung and Colorectal Cancers: Design, Synthesis, In silico and In vitro Studies Providing Mechanistic Insight into Their Anticancer Potencies. Med. Chem. 2021, 17, 1104–1128. [Google Scholar] [CrossRef]
- Šarkanj, B.; Molnar, M.; Čačić, M.; Gille, L. 4-Methyl-7-hydroxycoumarin antifungal and antioxidant activity enhancement by substitution with thiosemicarbazide and thiazolidinone moieties. Food Chem. 2013, 139, 488–495. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, M.U.; Da Silva, A.P.B.; Ueda-Nakamura, T.; Filho, B.P.D.; Da Silva, C.C.; Nakamura, C.V. Effects of a Thiosemicarbazide Camphene Derivative on Trichophyton mentagrophytes. Molecules 2009, 14, 1796–1807. [Google Scholar] [CrossRef]
- Doss, P.A.; Haribabu, J.; Balakrishnan, N.; Swaminathan, S.; Pino, J.A.; Bhuvanesh, N.; Karvembu, R. Cytotoxicity of copper(I) complexes containing indole-based thiosemicarbazones and triphenylphosphine. ChemistrySelect 2023, 8, 1–8. [Google Scholar]
- Kovač, T.; Kovač, M.; Strelec, I.; Nevistić, A.; Molnar, M. Antifungal and antiaflatoxigenic activities of coumarinyl thiosemicarbazides against Aspergillus flavus NRRL 3251. Arh. Hig. Rada Toksikol. 2017, 68, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.P.D.; Sampiron, E.G.; Meneguello, J.E.; Leque, A.L.; Ferracioli, K.R.C.; Cardoso, R.F.; Vandresen, F.; Scodro, R.B.L. Cytotoxicity and activity of thiosemicarbazones and semicarbazones in Mycobacterium tuberculosis: A systematic review. Cuad. Educ. Desarro. 2024, 16, 1–38. [Google Scholar] [CrossRef]
- Reshi, Z.A.; Ahmad, W.; Lukatkin, A.S.; Javed, S.B. From Nature to Lab: A Review of Secondary Metabolite Biosynthetic Pathways, Environmental Influences, and In Vitro Approaches. Metabolites 2023, 13, 895. [Google Scholar] [CrossRef] [PubMed]
- Rahim, F.; Ullah, H.; Taha, M.; Hussain, R.; Sarfaz, M.; Iqbal, R.; Iqbal, N.; Khan, S.; Shah, S.A.A.; Albalawi, M.A.; et al. Synthesis of New Triazole-Based Thiosemicarbazone Derivatives as Anti-Alzheimer’s Disease Candidates: Evidence-Based In Vitro Study. Molecules 2023, 28, 21. [Google Scholar] [CrossRef] [PubMed]
- Ullah, H.; Khan, S.; Rahim, F.; Taha, M.; Iqbal, R.; Sarfraz, M.; Shah, S.A.A.; Sajid, M.; Awad, M.F.; Omran, A.; et al. Benzimidazole Bearing Thiosemicarbazone Derivatives Act as Potent-Amylase and-Glucosidase Inhibitors; Synthesis, Bioactivity Screening and Molecular Docking Study. Molecules 2022, 27, 6921. [Google Scholar] [CrossRef] [PubMed]
- Khalaji, A.D.; Grivani, G.; Akerdi, S.J.; Gotoh, K.; Ishida, H.; Mighani, H. Synthesis, spectroscopic characterization, crystal structures, and theoretical studies of (E)-2-(2,4-dimethoxybenzylidene) thiosemicarbazone and (E)-2-(2,5-dimethoxybenzylidene) thiosemicarbazone. Struct. Chem. 2010, 21, 995–1003. [Google Scholar] [CrossRef]
- Sagnou, M.; Mavroidi, B.; Kaminari, A.; Boukos, N.; Pelecanou, M. Novel Isatin Thiosemicarbazone Derivatives as Potent Inhibitors of β-Amyloid Peptide Aggregation and Toxicity. ACS Chem. Neurosci. 2020, 11, 15. [Google Scholar] [CrossRef]
- Sousa, G.; de Almeida, M.C.F.; Lócio, L.L.; Santos, V.L.D.; Bezerra, D.P.; Silva, V.R.; de Almeida, S.M.V.; Simon, A.; Honório, T.D.S.; Cabral, L.M.; et al. Synthesis and Evaluation of Antiproliferative Activity, Topoisomerase IIα Inhibition, DNA Binding and Non-Clinical Toxicity of New Acridine–Thiosemicarbazone Derivatives. Pharmaceuticals 2022, 15, 1098. [Google Scholar] [CrossRef] [PubMed]
- Altıntop, M.D.; Temel, H.E.; Sever, B.; CiftCi, G.A.; Kaplancıklı, Z.A. Synthesis and Evaluation of New Benzodioxole-Based Thiosemicarbazone Derivatives as Potential Antitumor Agents. Molecules 2016, 21, 1598. [Google Scholar] [CrossRef] [PubMed]
- Bermejo, M.R.; Gonza, A.M.; Martı, M.; Pedrido, R.; Romero, M.J.; Ferna, M.I.; Maneiro, M.Z. Electrochemical Synthesis: A Convenient Method for the Preparation of Neutral Metal Complexes with a Thiosemicarbazone Ligand. Anorg. Allg. Chem. 2007, 633, 807. [Google Scholar] [CrossRef]
- Ibrahim, M.B.A.; Farh, M.K.; El-Gyar, S.A.; EL-Gahami, M.A.; Fouad, D.M.; Silva, F.; Santos, I.C.; Paulo, A. Synthesis, structural studies and antimicrobial activities of manganese, nickel and copper complexes of two new tridentate 2-formylpyridine thiosemicarbazone ligands. Inorg. Chem. Comm. 2018, 96, 194–201. [Google Scholar] [CrossRef]
- Li, J.-Q.; Sun, L.-Y.; Jiang, Z.; Chen, C.; Gao, H.; Chigan, J.-Z.; Ding, H.-H.; Yang, K.-W. Diaryl-substituted thiosemicarbazone: A potent scaffold for the development of New Delhi metallo-β-lactamase-1 inhibitors. Bioorganic Chem. 2021, 107, 104576. [Google Scholar] [CrossRef] [PubMed]
- Pitucha, M.; Korga-Plewko, A.; Czylkowska, A.; Rogalewicz, B.; Drozd, M.; Iwan, M.; Kubik, J.; Humeniuk, E.; Adamczuk, G.; Karczmarzyk, Z.; et al. Influence of Complexation of Thiosemicarbazone Derivatives with Cu (II) Ions on Their Antitumor Activity against Melanoma Cells. Int. J. Mol. Sci. 2021, 22, 3104. [Google Scholar] [CrossRef]
- Nitiss, J.L. Targeting DNA topoisomerase II in cancer chemotherapy. Nat. Rev. Cancer 2009, 9, 338–350. [Google Scholar] [CrossRef]
- Li, J.; Qiao, M.; Zhang, X.; Li, J.; Meng, Q.; Qiao, J.; Li, Y.; Wang, X.; Zhang, G.; Zhang, K.; et al. Effects of Lmo2672 Deficiency on Environmental Adaptability, Biofilm Formation, and Motility of Listeria monocytogenes. Jundishapur J. Microbiol. 2020, 13, 1–8. [Google Scholar] [CrossRef]
- Veber, D.F.; Johnson, S.R.; Cheng, H.Y.; Smith, B.R.; Ward, K.W.; Kopple, K.D. Molecular Properties That Influence the Oral Bioavailability of Drug Candidates. J. Med. Chem. 2002, 45, 2615–2623. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2012, 64, 4–17. [Google Scholar] [CrossRef]
- Elkina, N.A.; Grishchenko, M.V.; Shchegolkov, E.V.; Makhaeva, G.F.; Kovaleva, N.V.; Rudakova, E.V.; Boltneva, N.P.; Lushchekina, S.V.; Astakhova, T.Y.; Radchenko, E.V.; et al. New Multifunctional Agents for Potential Alzheimer’s Disease Treatment Based on Tacrine Conjugates with 2-Arylhydrazinylidene-1,3-Diketones. Biomolecules 2022, 12, 1551. [Google Scholar] [CrossRef]
- Maciejewska, K.; Czarnecka, K.; Kręcisz, P.; Niedziałek, D.; Wieczorek, G.; Skibiński, R.; Szymański, P. Novel Cyclopentaquinoline and Acridine Analogs as Multifunctional, Potent Drug Candidates in Alzheimer’s Disease. Int. J. Mol. Sci. 2022, 23, 5876. [Google Scholar] [CrossRef] [PubMed]
- Floegel, A.; Kim, D.O.; Chung, S.J.; Koo, S.I.; Chun, O.K. Comparison of ABTS/DPPH assays to measure antioxidant capacity in popular antioxidant-rich US foods. J. Food Compos. Anal. 2011, 24, 1043–1048. [Google Scholar] [CrossRef]
- Giske, C.G.; Turnidge, J.; Cantón, R.; Kahlmeter, G.; Gatermann, S.; Lina, G.; Lindemann, C.; MacGowan, A.; Meletiadis, J.; Das, S.; et al. Update from the European Committee on Antimicrobial Susceptibility Testing (EUCAST). J. Clin. Microbiol. 2022, 60, 1–10. [Google Scholar] [CrossRef]
- Jones, G.; Willett, P.; Glen, R.C.; Leach, A.R.; Taylor, R.J. Development and validation of a genetic algorithm for flexible docking. Mol. Biol. 1997, 267, 727–748. [Google Scholar] [CrossRef] [PubMed]
- Verdonk, M.L.; Cole, J.C.; Hartshorn, M.J.; Murray, C.W.; Taylor, R.D. Improved protein-ligand docking using GOLD. Proteins 2003, 52, 609–623. [Google Scholar] [CrossRef] [PubMed]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge Structural Database. Acta Crystallogr. Sect. B Struct. Sci. Cryst. Eng. Mater. 2016, 72, 171–179. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 1–13. [Google Scholar] [CrossRef]
- Daina, A.; Zoete, V. A BOILED-Egg To Predict Gastrointestinal Absorption and Brain Penetration of Small Molecules. ChemMedChem 2016, 11, 1117–1121. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, P.; Eckert, A.O.; Schrey, A.K.; Preissner, R. ProTox-II: A webserver for the prediction of toxicity of chemicals. Nucleic Acids Res. 2018, 46, W257–W263. [Google Scholar] [CrossRef]
- Lee, C.; Yoon, J. UV direct photolysis of 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonate) (ABTS) in aqueous solution: Kinetics and mechanism. J. Photochem. Photobiol. A Chem. 2008, 197, 232–238. [Google Scholar] [CrossRef]
- Kumar, V.; Kaur, K.; Gupta, G.K.; Sharma, A.K. Pyrazole containing natural products: Synthetic preview and biological significance. Eur. J. Med. Chem. 2013, 69, 735–753. [Google Scholar] [CrossRef] [PubMed]
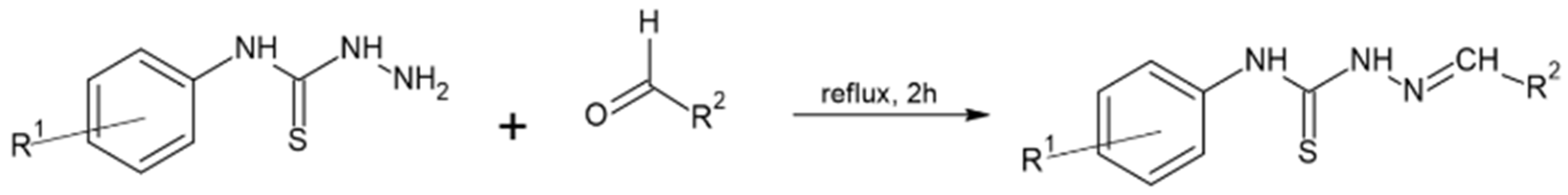
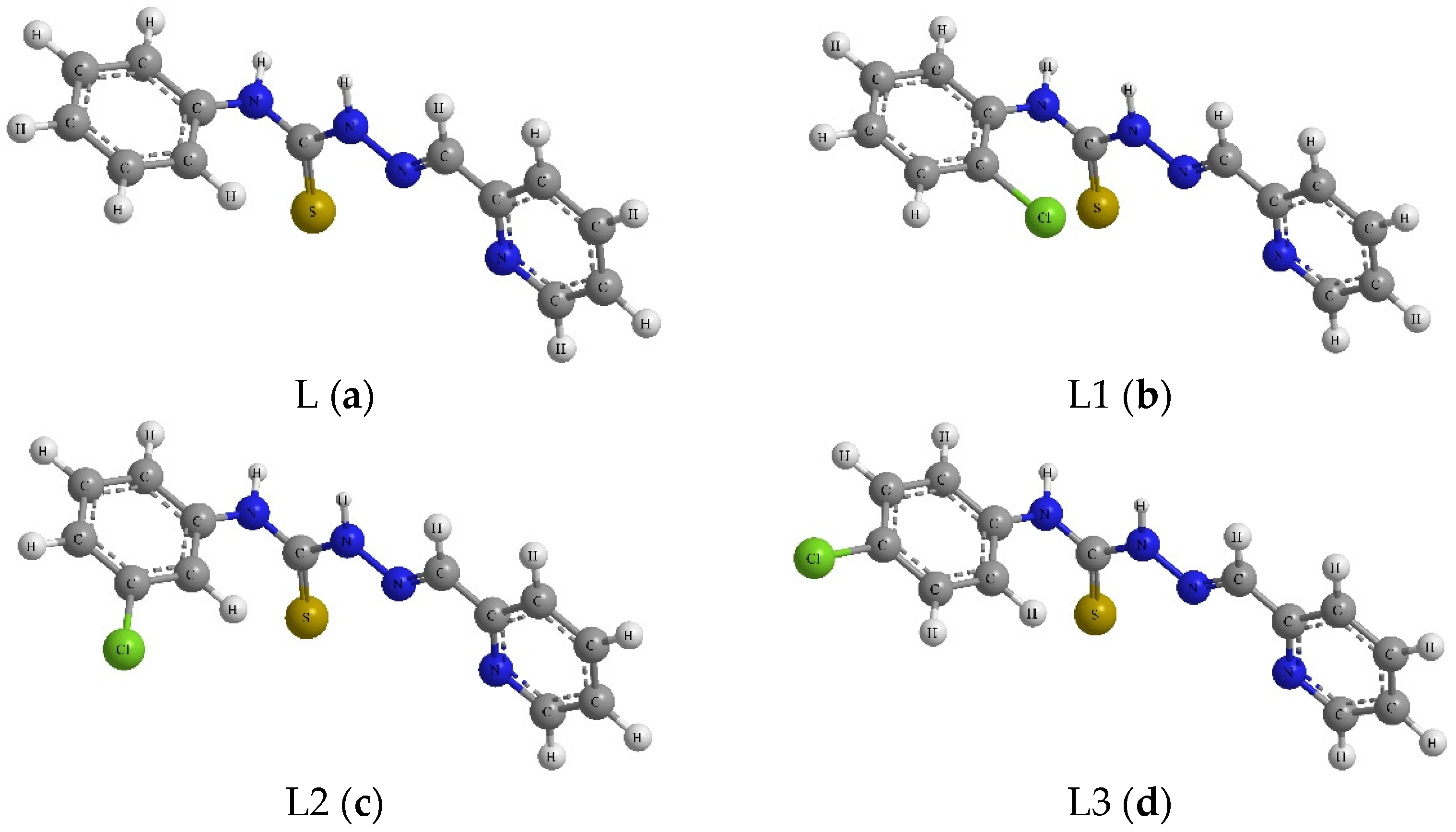
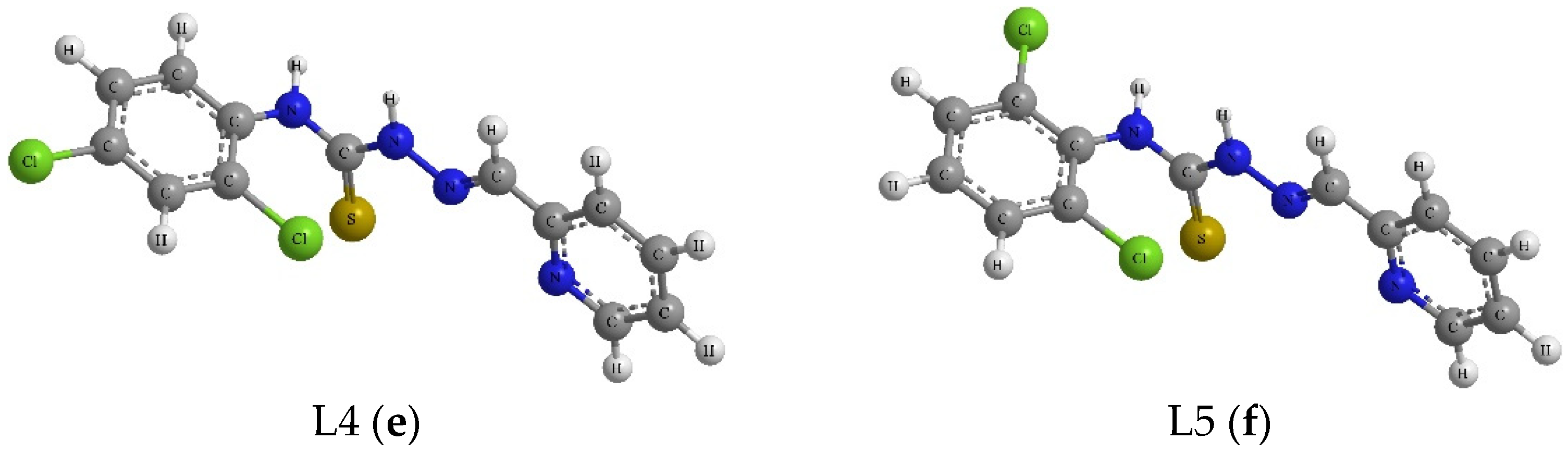


| Compound | R1 | R2 |
|---|---|---|
| L | H | 2-pyridine |
| L1 | 2-Cl | 2-pyridine |
| L2 | 3-Cl | 2-pyridine |
| L3 | 4-Cl | 2-pyridine |
| L4 | 2,4-diCl | 2-pyridine |
| L5 | 2,6-diCl | 2-pyridine |
| Compound | [M+H]+theor. | [M+H]+meas. | Delta [ppm] |
|---|---|---|---|
| L | 257.0855 | 257.0847 | −3.4 |
| L1 | 291.0466 | 291.0458 | −2.7 |
| L2 | 291.0466 | 291.0458 | −2.7 |
| L3 | 291.0466 | 291.0456 | −3.3 |
| L4 | 325.0076 | 325.0065 | −3.1 |
| L5 | 325.0076 | 325.0069 | −2.1 |
| Compound | TopoII | PrfA |
|---|---|---|
| L | 76.72 | 64.35 |
| L1 | 72.70 | 58.56 |
| L2 | 72.81 | 58.86 |
| L3 | 70.15 | 55.80 |
| L4 | 73.10 | 54.40 |
| L5 | 71.47 | 56.60 |
| Original inhibitor | 110.45 * | 73.18 ** |
| Chemicals | L | L1 | L2 | L3 | L4 | L5 | Ref. (Van/Cip) |
|---|---|---|---|---|---|---|---|
| Microorganism | MIC [mg/L] | ||||||
| Bacillus cereus ŁOCK 0807 | 1000 | 10 | >1000 | 100 | 50 | >1000 | 1 |
| Bacillus subtilis ATCC 6633 | 200 | 100 | 50 | 200 | 1000 | >1000 | 1 |
| Enterococcus faecalis ATCC 29212 | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 | 2 |
| Staphylococcus aureus ATCC 25923 | 1000 | 1000 | 50 | 1000 | >1000 | 1000 | 1 |
| Staphylococcus aureus ATCC 6538 | 1000 | 100 | 500 | 1000 | 100 | 1000 | 1 |
| Staphylococcus epidermidis ATCC 12228 | 200 | 100 | >1000 | >1000 | >1000 | >1000 | 1 |
| Listeria monocytogenes ATCC 19115 | 200 | 100 | 500 | >1000 | 1000 | 1000 | 2 |
| Escherichia coli ATCC 10530 | 1000 | >1000 | 1000 | >1000 | >1000 | 1000 | 1 |
| Salmonella typhimurium ATCC 14028 | >1000 | >1000 | >1000 | >1000 | >1000 | 1000 | 1 |
| Compound | IC50 (mg/mL) | SD (+/−) |
|---|---|---|
| L | 0.010 | 0.001 |
| L1 | 0.017 | 0.004 |
| L2 | 0.012 | 0.003 |
| L3 | 0.015 | 0.002 |
| L4 | 0.107 | 0.053 |
| L5 | 0.017 | 0.052 |
| Trolox (STD) * | 0.015 | 0.002 |
| Compound | IC50 (mg/mL) | SD (+/−) |
|---|---|---|
| L | 0.403 | 0.058 |
| L1 | 0.088 | 0.008 |
| L2 | 0.025 | 0.001 |
| L3 | - | - |
| L4 | 0.160 | 0.003 |
| L5 | 0.141 | 0.026 |
| Trolox (STD) * | 0.013 | 0.002 |
| Compound | IC50 (mg/mL) | SD (+/−) |
|---|---|---|
| L | 0.028 | 0.001 |
| L1 | 0.010 | 0.001 |
| L2 | 0.021 | 0.001 |
| L3 | 0.020 | 0.002 |
| L4 | 0.025 | 0.002 |
| L5 | 0.033 | 0.002 |
| Trolox (STD) * | 1.000 | 0.000 |
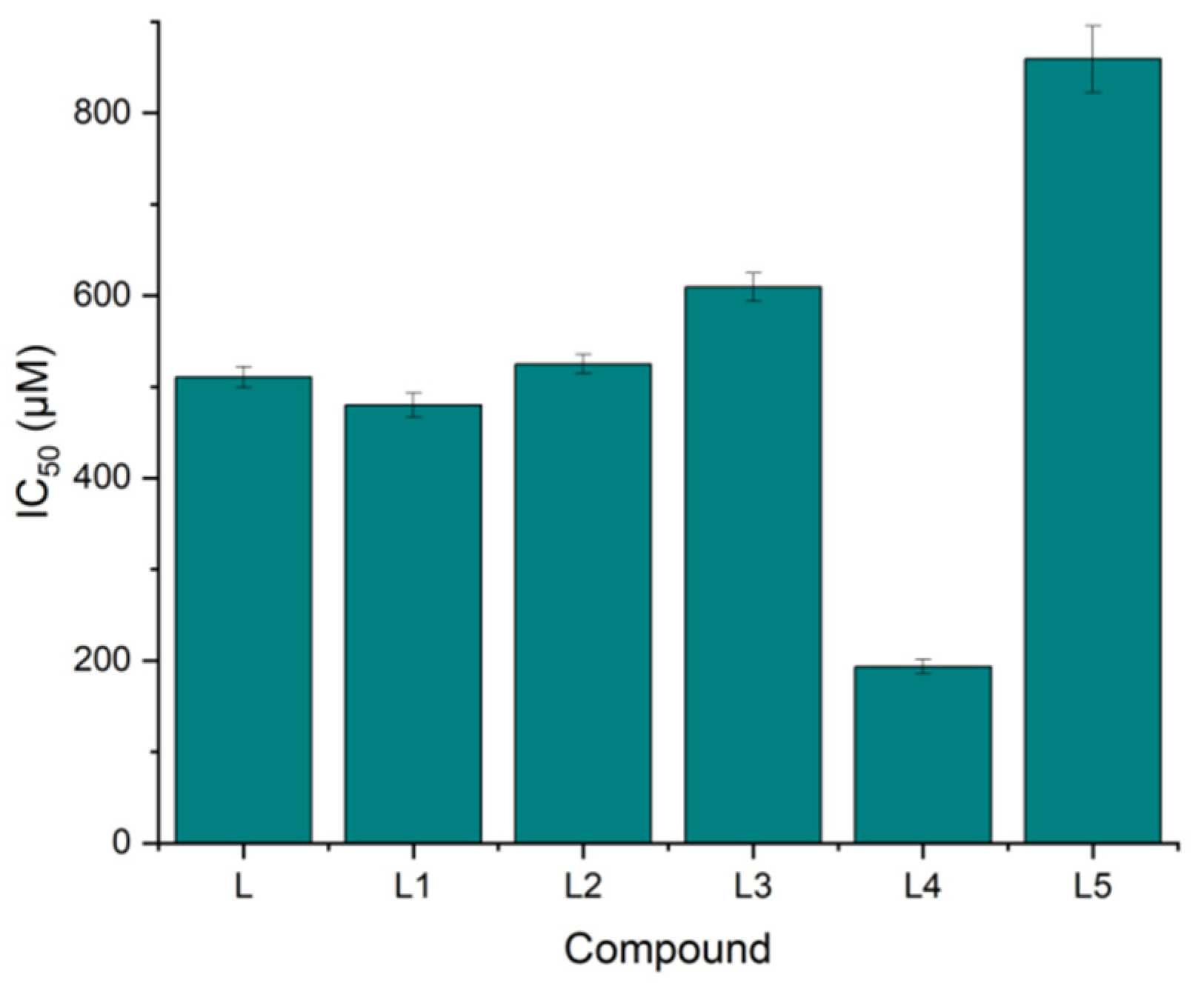
| Compound | IC50 (µM) | SD (+/−) |
|---|---|---|
| L | 510.61 | 11.53 |
| L1 | 479.96 | 13.12 |
| L2 | 524.89 | 10.25 |
| L3 | 609.18 | 15.21 |
| L4 | 193.44 | 8.20 |
| L5 | 858.93 | 36.21 |
| Cisplatin | 841.25 | 12.02 |
| Doxorubicin | 194.65 | 21.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Czylkowska, A.; Pitucha, M.; Raducka, A.; Fornal, E.; Kordialik-Bogacka, E.; Ścieszka, S.; Smoluch, M.; Burdan, F.; Jędrzejec, M.; Szymański, P. Thiosemicarbazone-Based Compounds: A Promising Scaffold for Developing Antibacterial, Antioxidant, and Anticancer Therapeutics. Molecules 2025, 30, 129. https://doi.org/10.3390/molecules30010129
Czylkowska A, Pitucha M, Raducka A, Fornal E, Kordialik-Bogacka E, Ścieszka S, Smoluch M, Burdan F, Jędrzejec M, Szymański P. Thiosemicarbazone-Based Compounds: A Promising Scaffold for Developing Antibacterial, Antioxidant, and Anticancer Therapeutics. Molecules. 2025; 30(1):129. https://doi.org/10.3390/molecules30010129
Chicago/Turabian StyleCzylkowska, Agnieszka, Monika Pitucha, Anita Raducka, Ewelina Fornal, Edyta Kordialik-Bogacka, Sylwia Ścieszka, Marek Smoluch, Franciszek Burdan, Mateusz Jędrzejec, and Paweł Szymański. 2025. "Thiosemicarbazone-Based Compounds: A Promising Scaffold for Developing Antibacterial, Antioxidant, and Anticancer Therapeutics" Molecules 30, no. 1: 129. https://doi.org/10.3390/molecules30010129
APA StyleCzylkowska, A., Pitucha, M., Raducka, A., Fornal, E., Kordialik-Bogacka, E., Ścieszka, S., Smoluch, M., Burdan, F., Jędrzejec, M., & Szymański, P. (2025). Thiosemicarbazone-Based Compounds: A Promising Scaffold for Developing Antibacterial, Antioxidant, and Anticancer Therapeutics. Molecules, 30(1), 129. https://doi.org/10.3390/molecules30010129









