Enhanced Mass Activity and Durability of Bimetallic Pt-Pd Nanoparticles on Sulfated-Zirconia-Doped Graphene Nanoplates for Oxygen Reduction Reaction in Proton Exchange Membrane Fuel Cell Applications
Abstract
Highlights
- In this work, graphene nanoplates (GNPs) with a supreme medium were obtained.
- Pt particles (4.50 nm) were uniformly dispersed on the surface of S-ZrO2-GNP support.
- The Pt-Pd/S-ZrO2-GNPs exhibited higher ECSA than Pt-Pd/ZrO2-GNPs and Pt/C.
- Pt-Pd/S-ZrO2-GNPs exhibited higher ORR mass activity than other studied electrodes.
- Pt-Pd/S-ZrO2-GNPs exhibited low charge transfer resistance in EIS measurements.
Abstract
1. Introduction
2. Results and Discussion
2.1. Physical Characterization
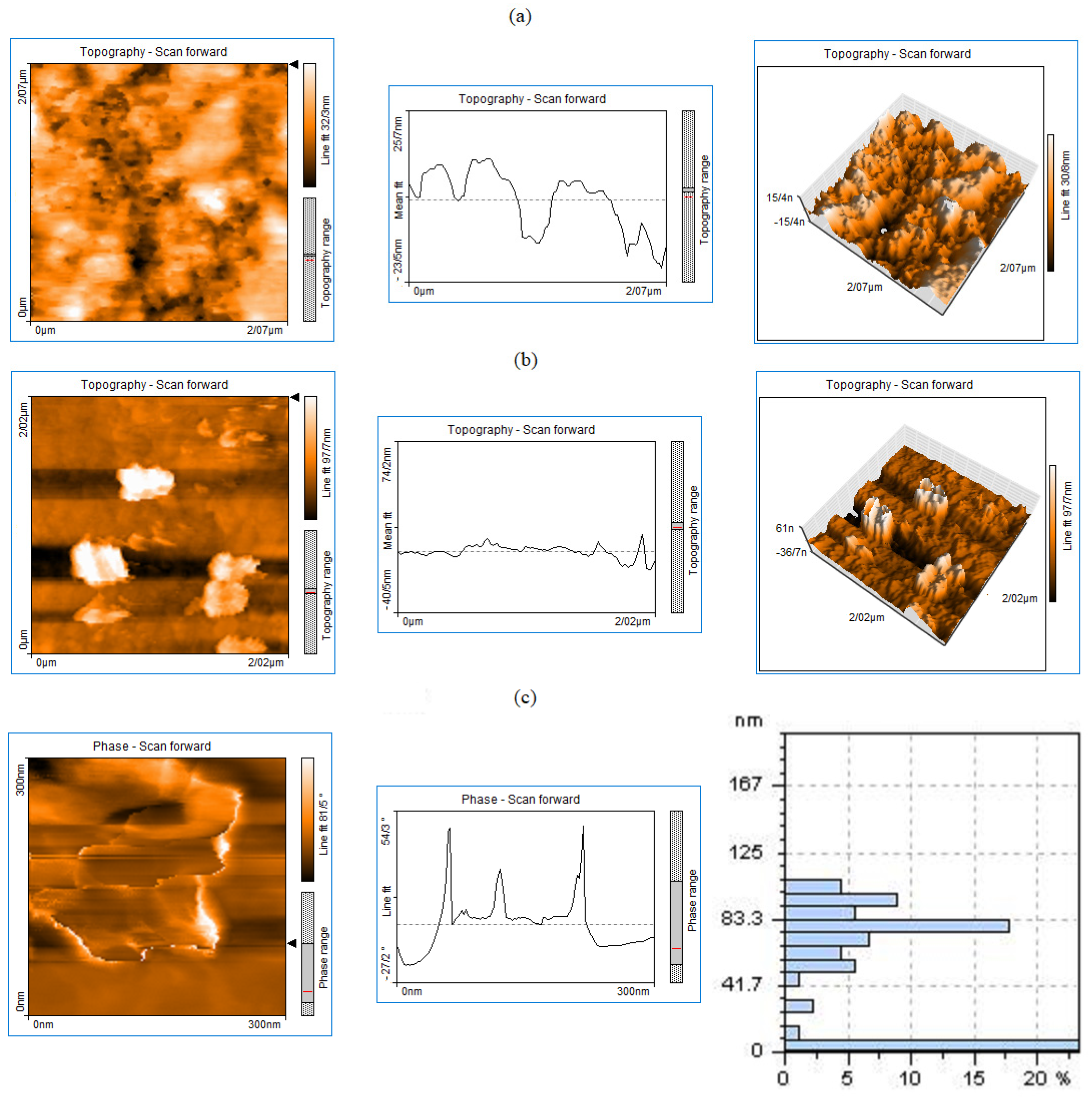
2.1.1. Topography Study of Graphene Nanoplates and S-ZrO2-GNPs
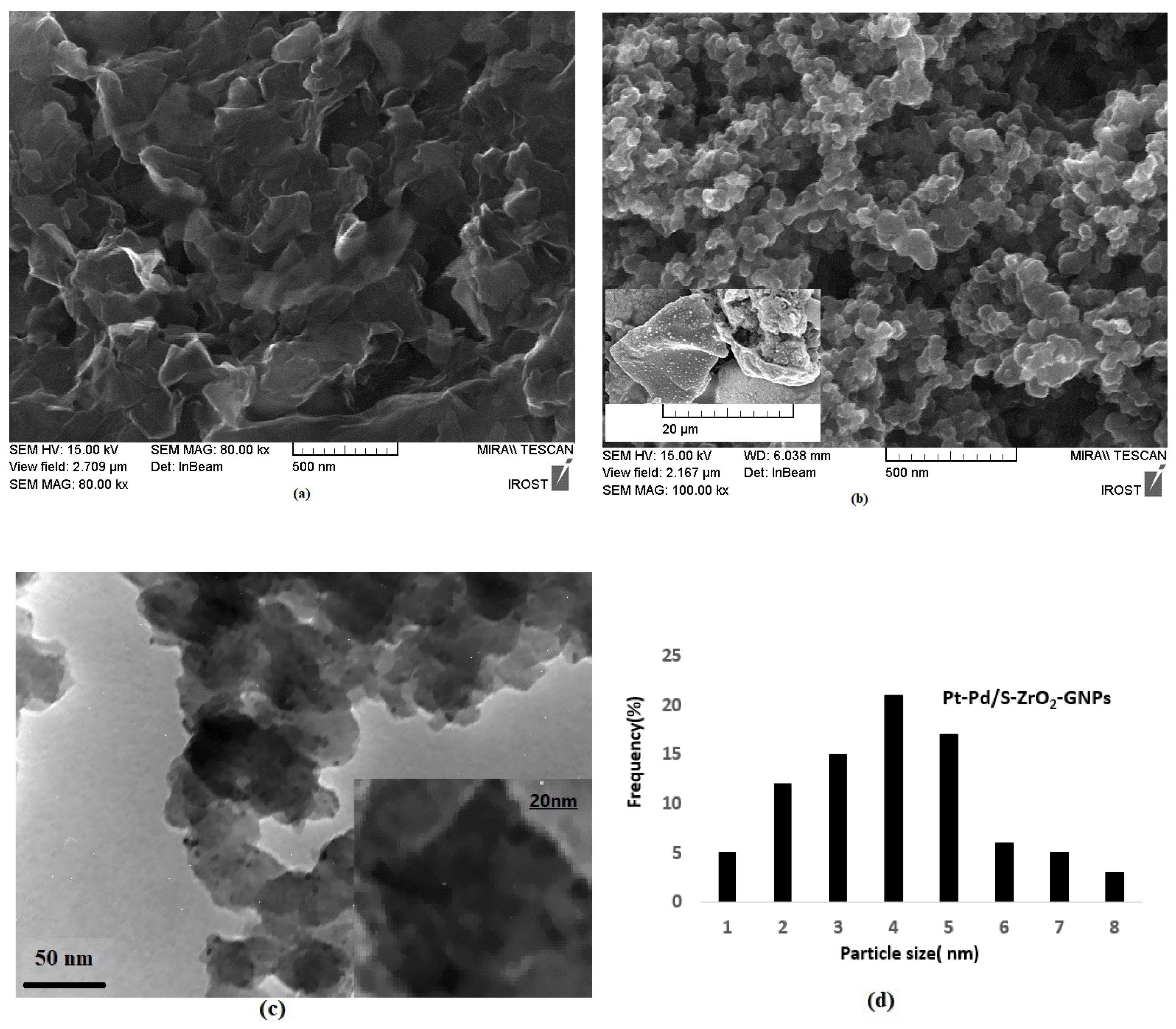
2.1.2. Morphological Study of GNPs and Pt-Pd Nanocrystals on S-ZrO2-GNPs
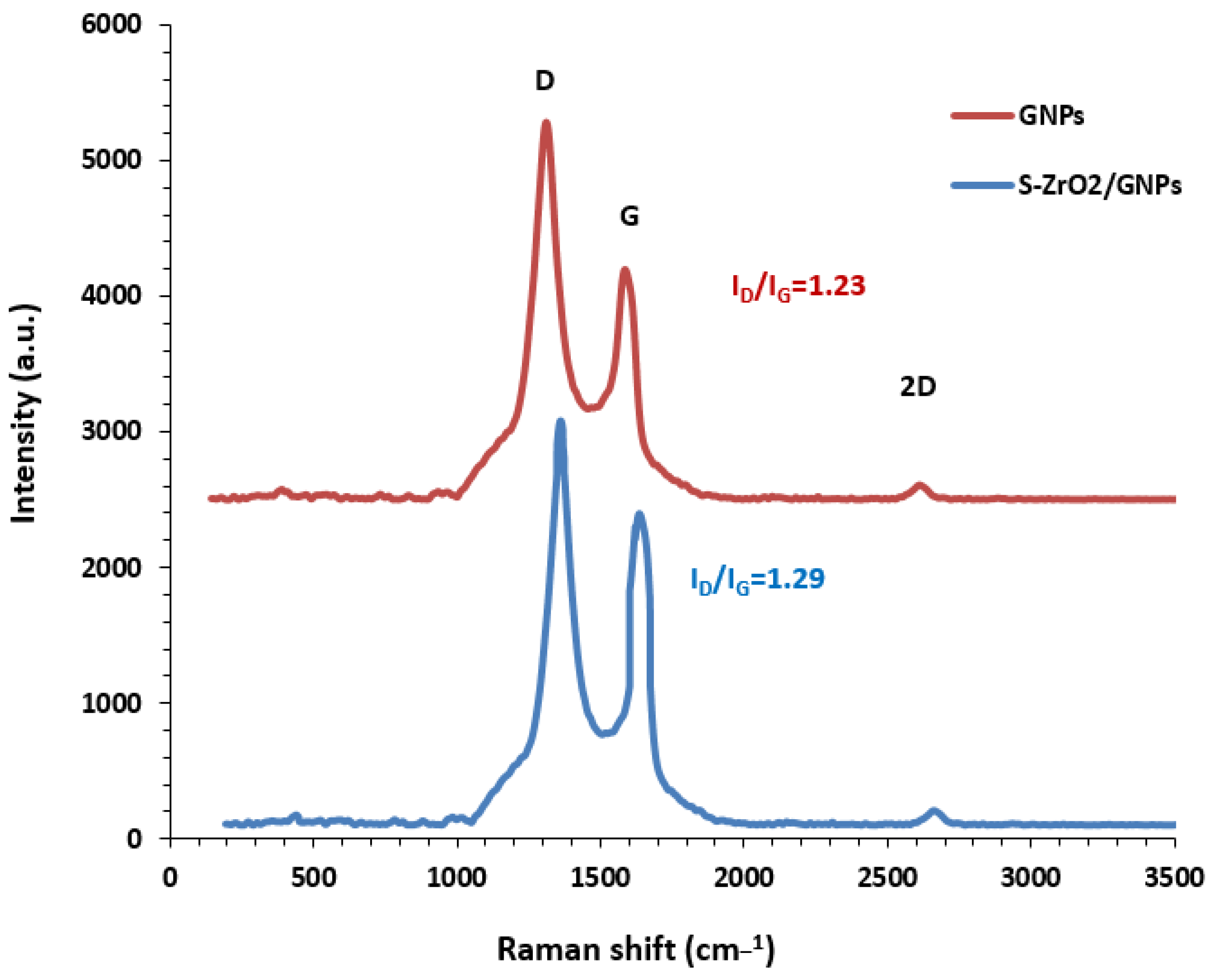
2.1.3. Structural Specifications of Synthesized Support Material
2.1.4. XRD Pattern Characterization
2.1.5. Chemical Composition of Synthesized Nanocomposite
2.2. Electrochemical Measurements
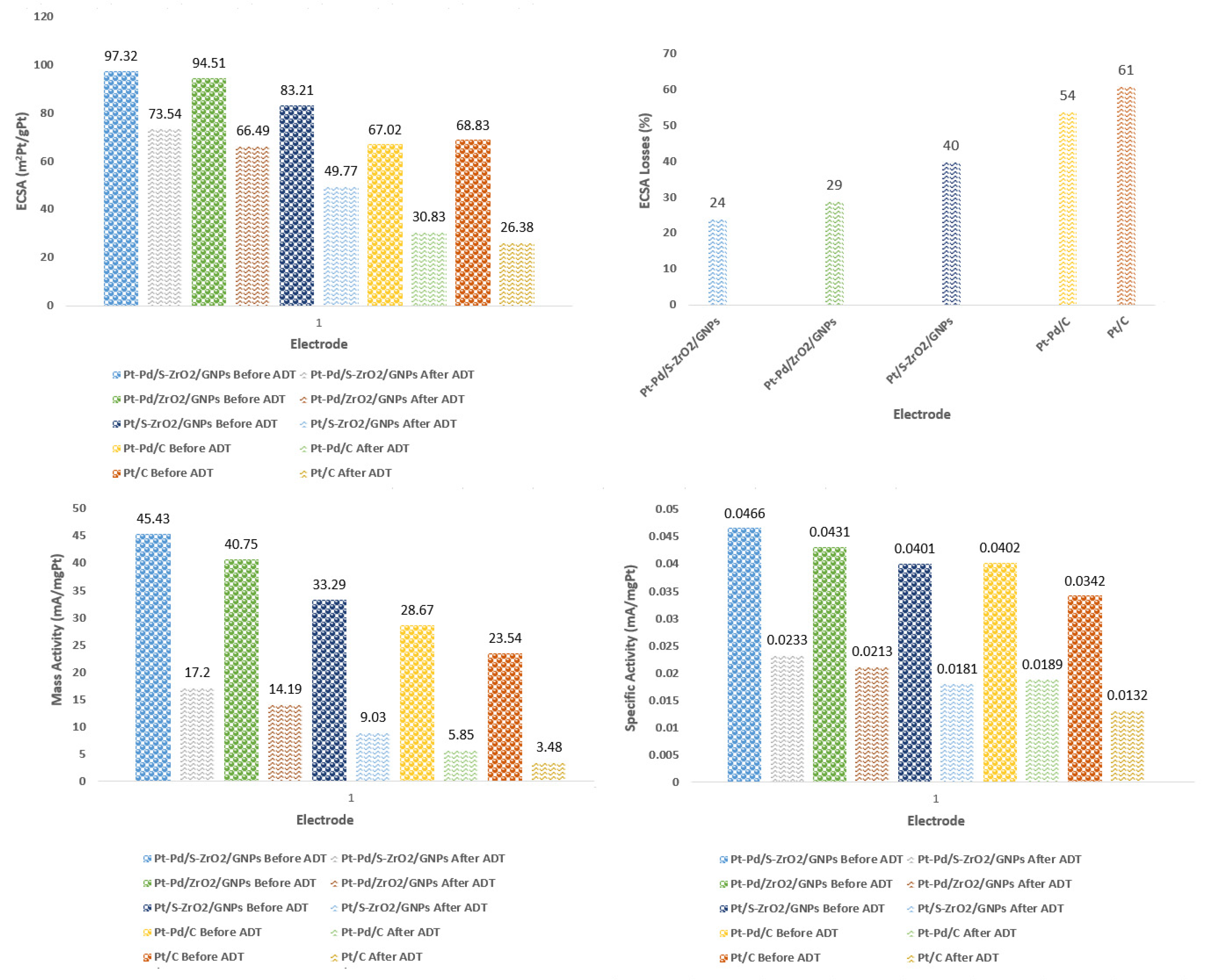
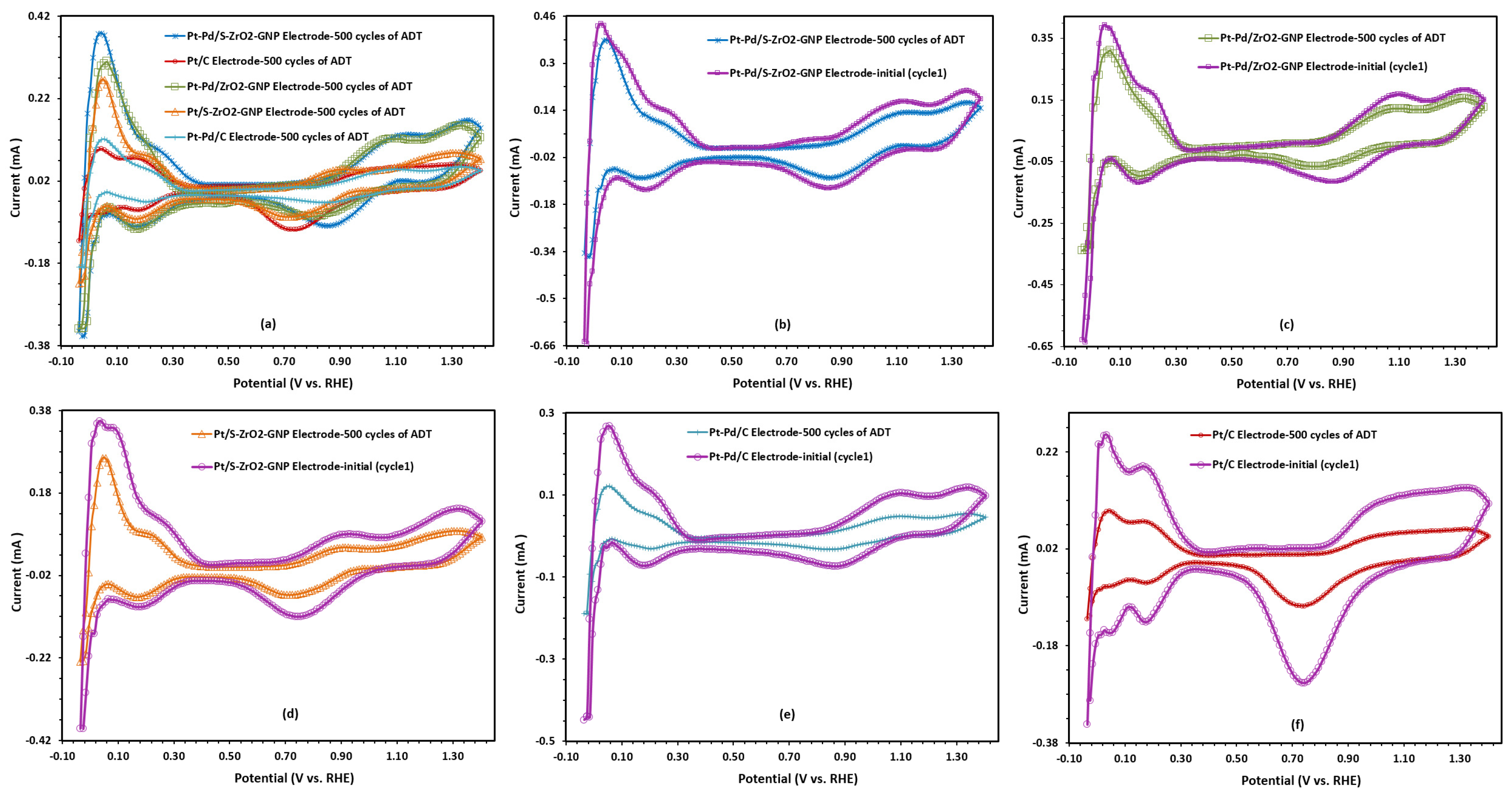
2.2.1. Electrochemical Surface Area (ECSA) of Electrodes
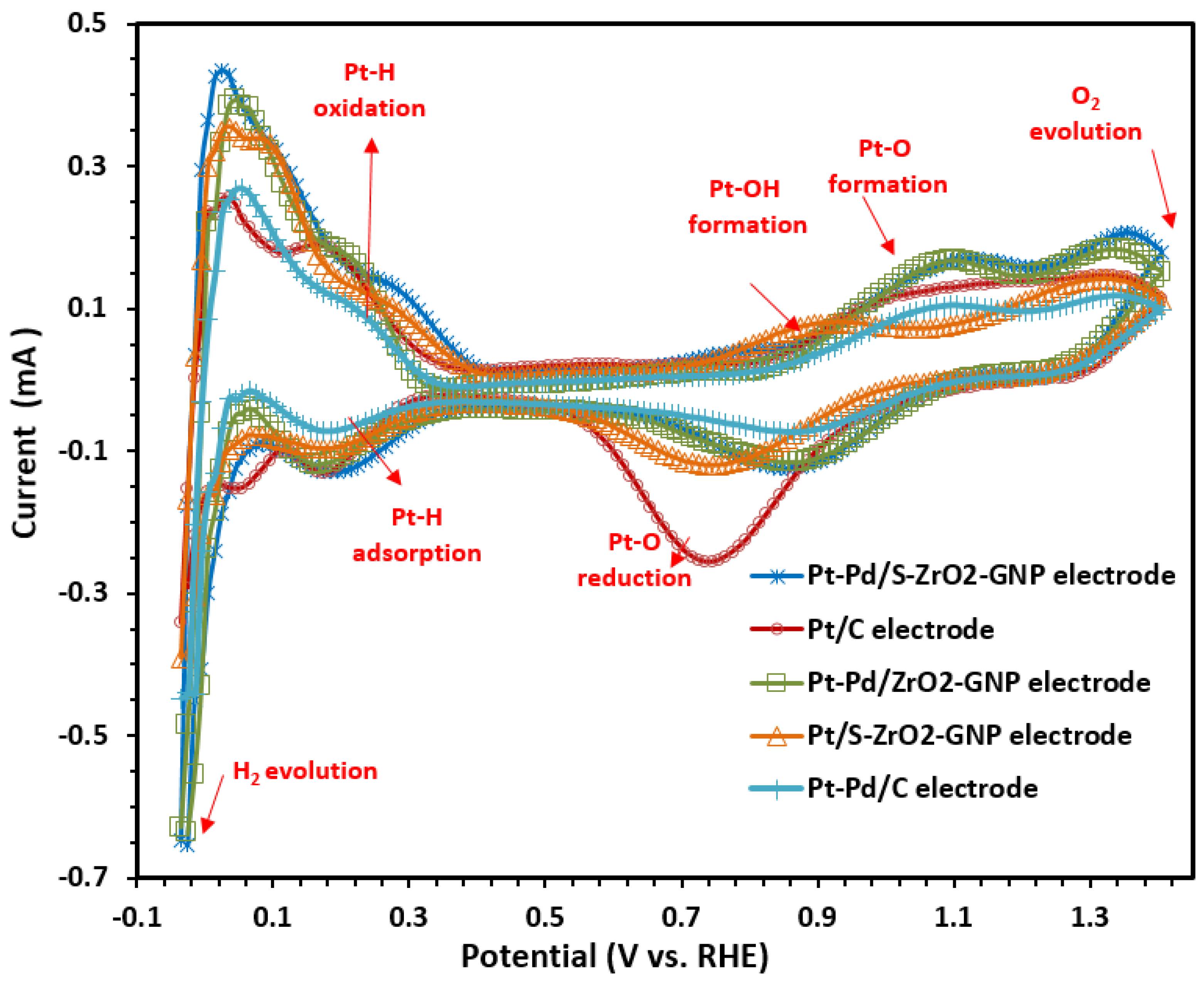
2.2.2. Mechanism of Reaction
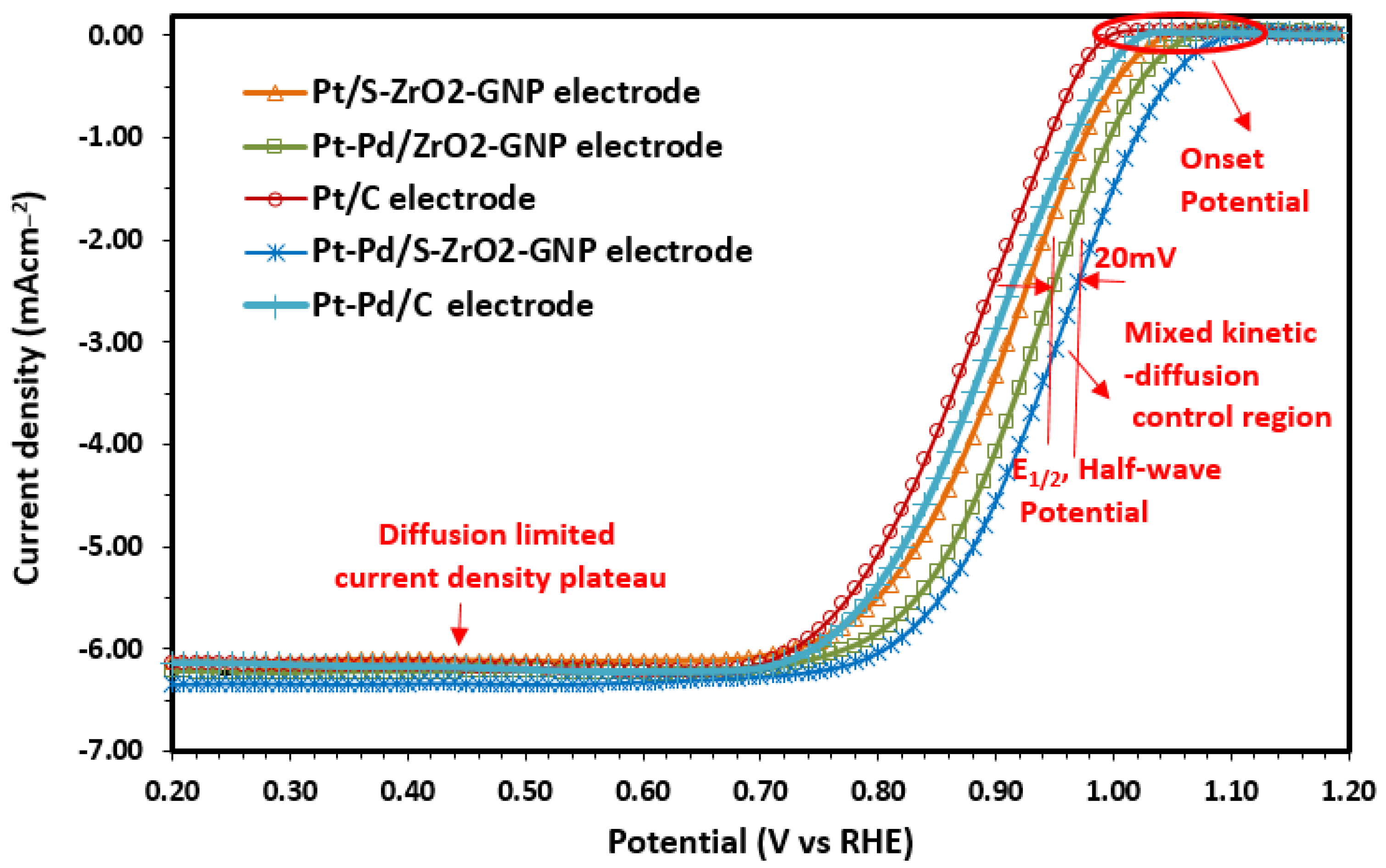
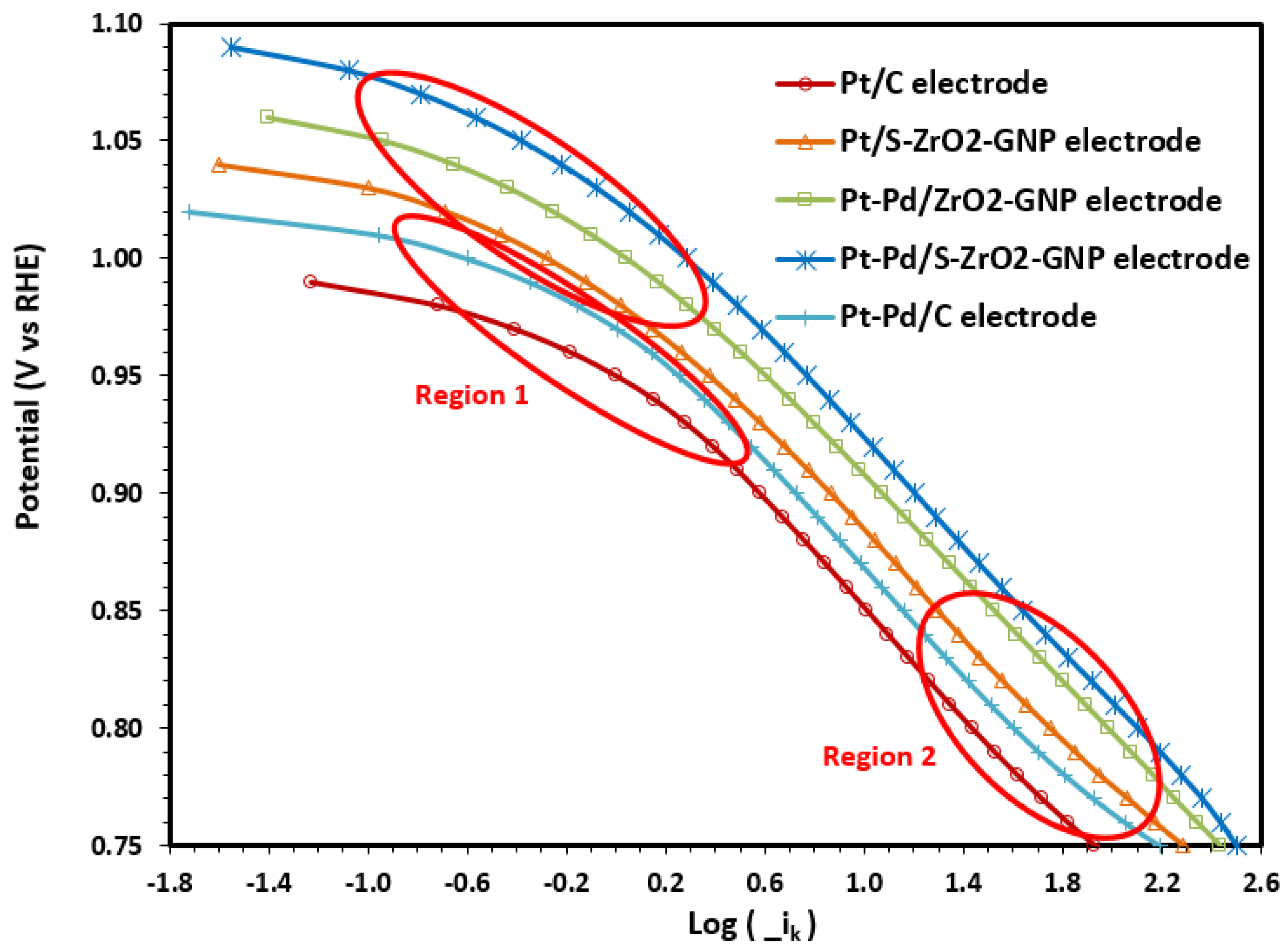
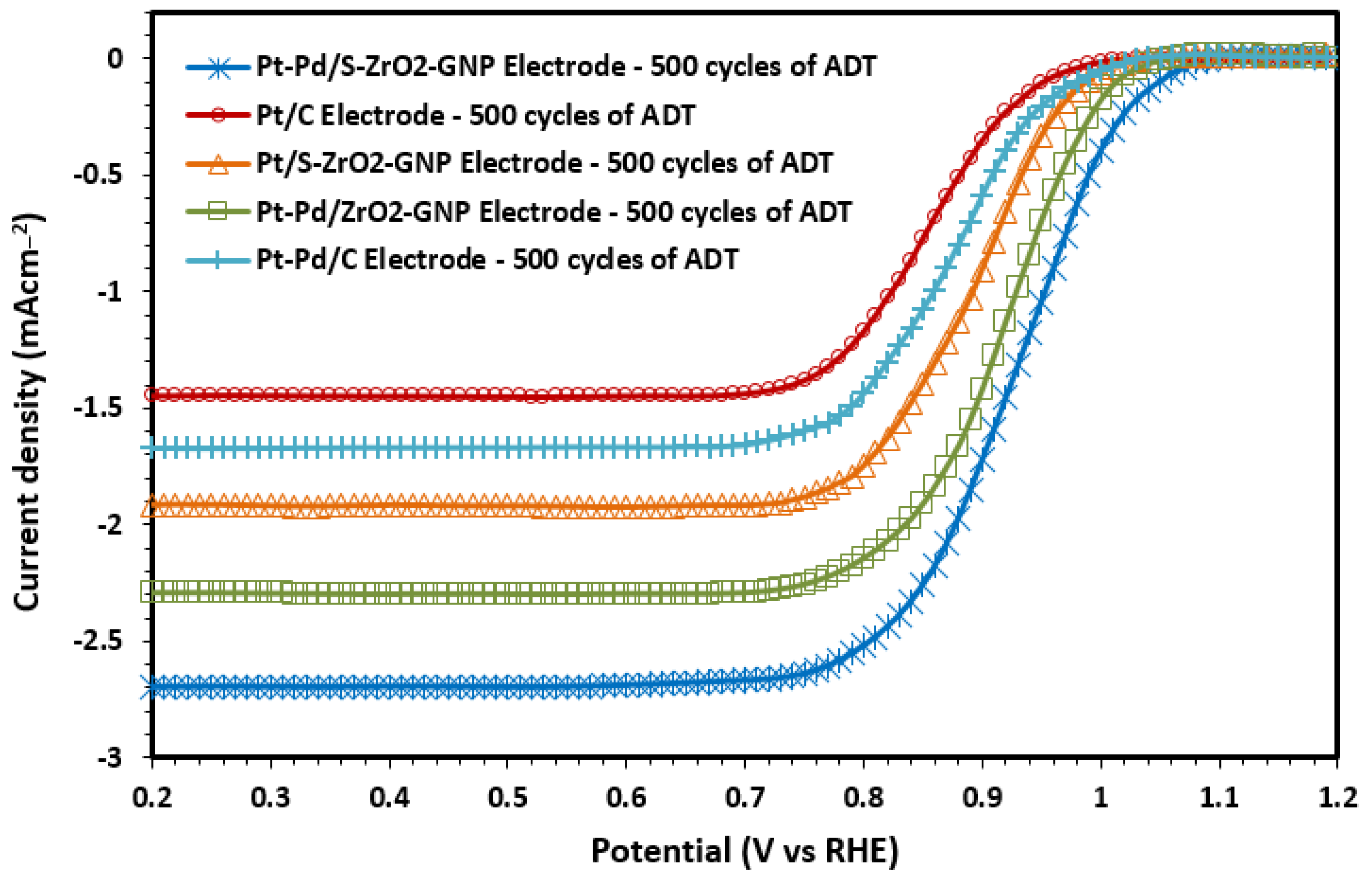
2.2.3. ORR Kinetics
Estimation of Electrocatalyst Efficiency Using the RDE Instrument
Determination of Kinetic Parameters of b and
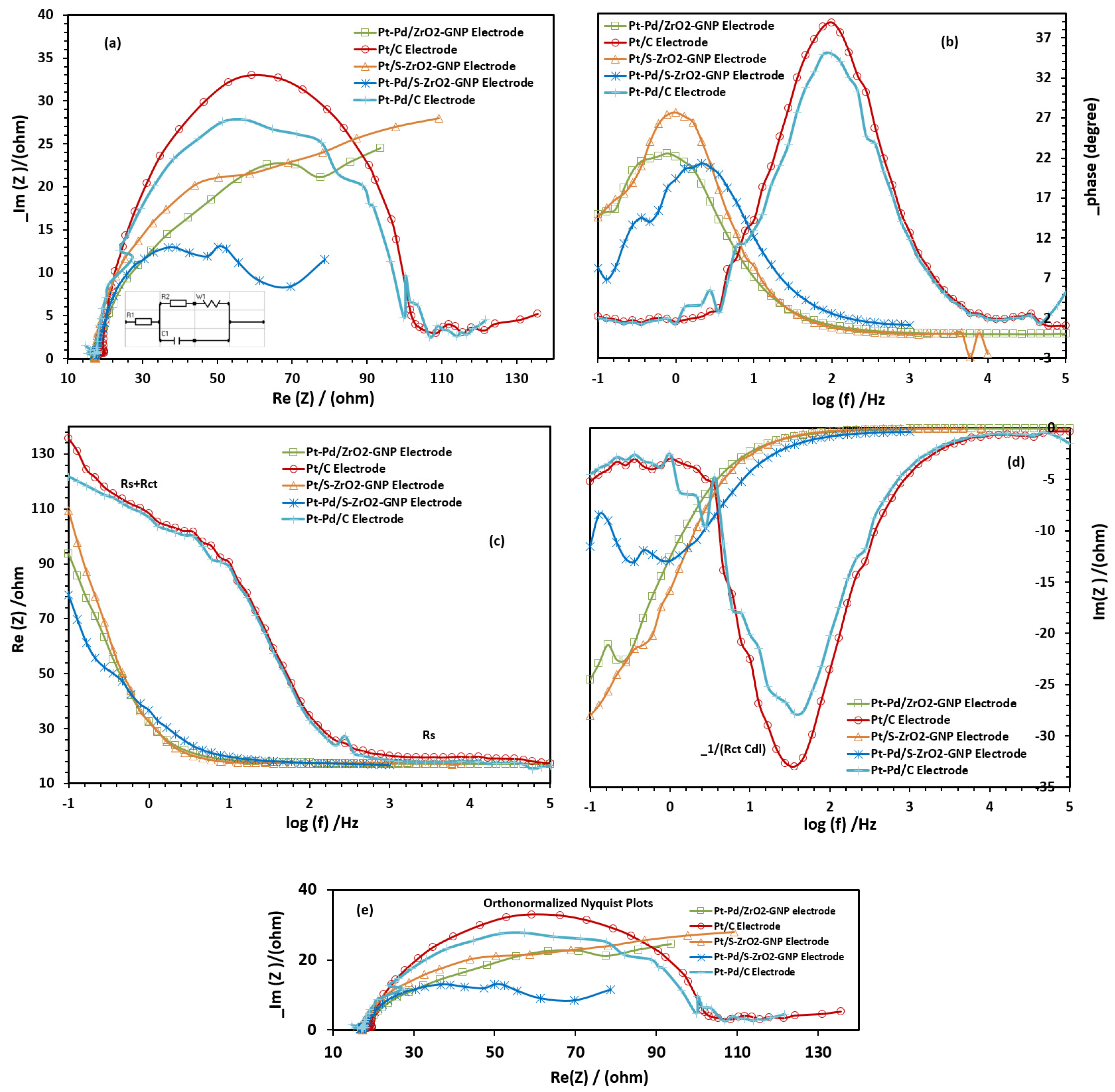
2.2.4. Electrochemical Impedance Spectroscopy (EIS) Studies of the Electrodes
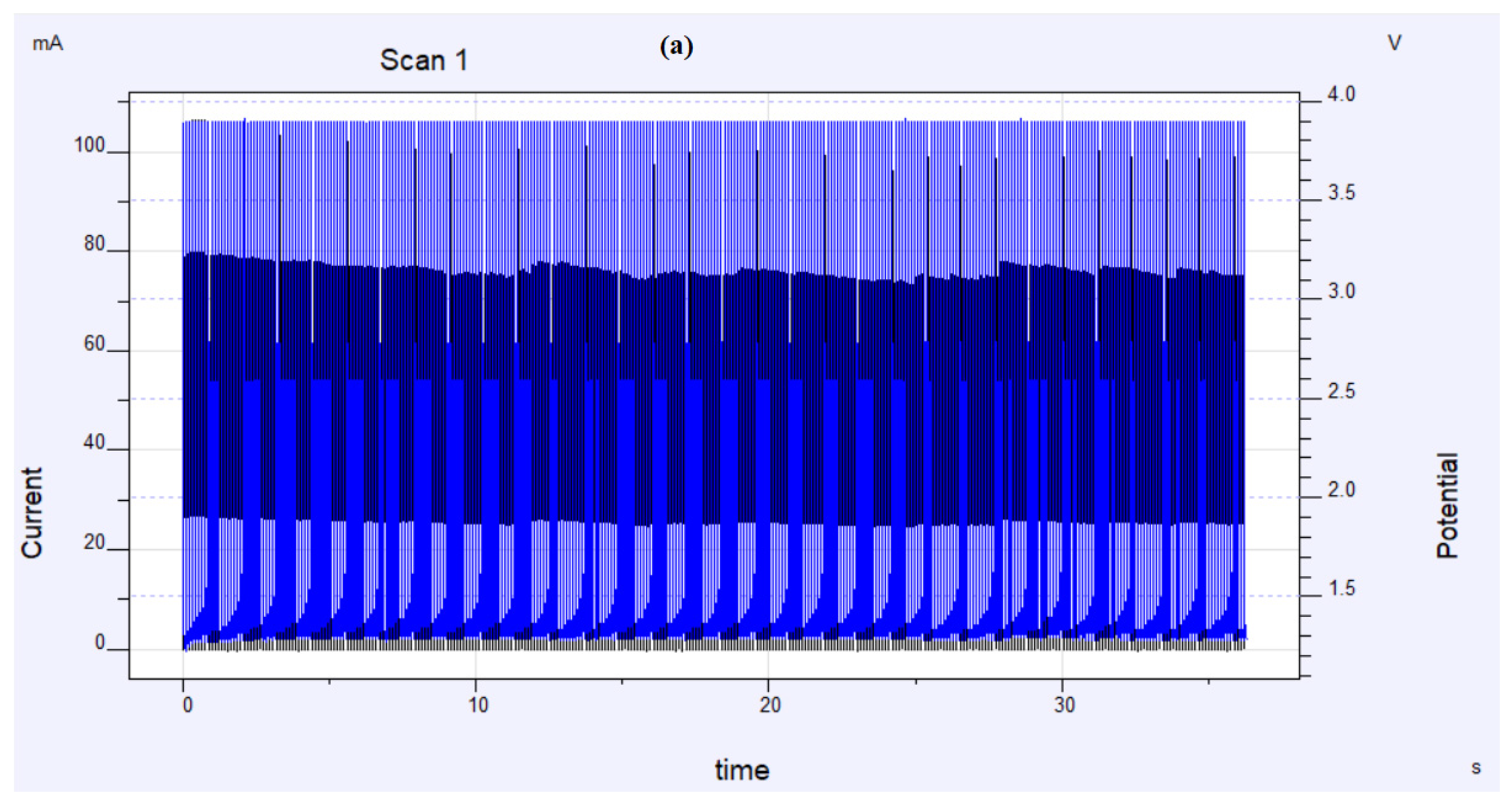
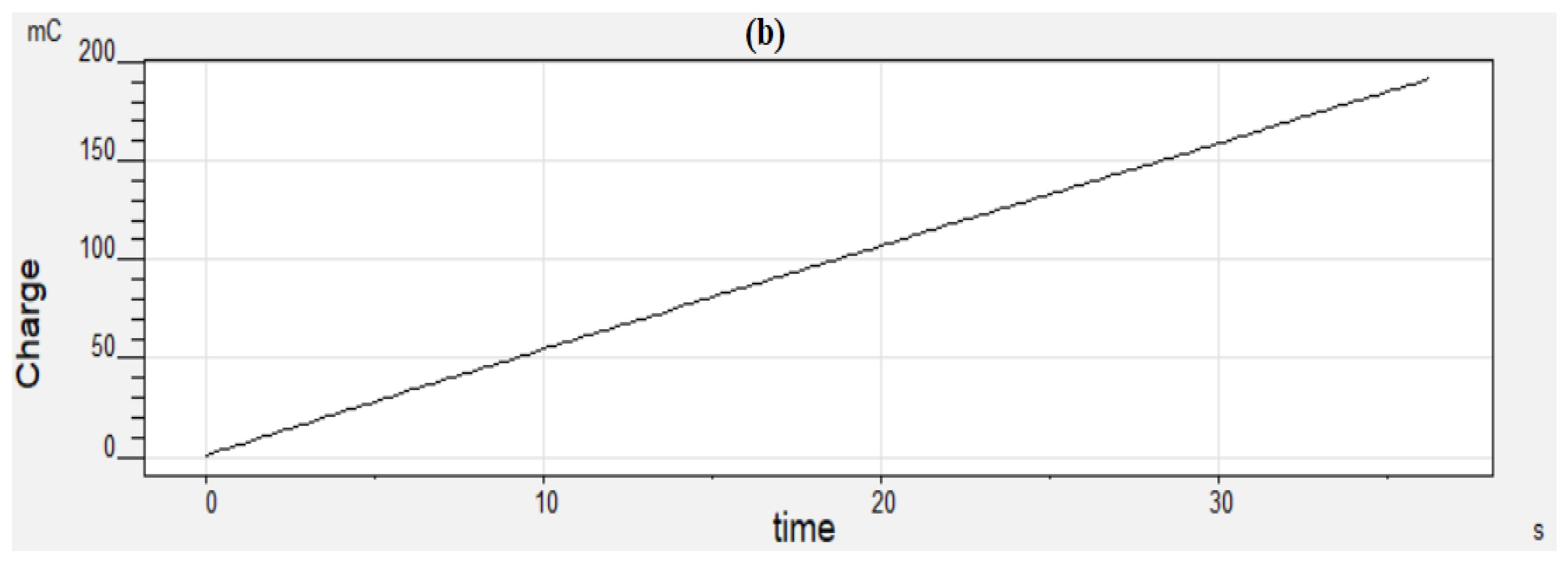
2.2.5. Long-Term Activity and Durability
3. Materials and Methods
3.1. Materials
3.2. Methods
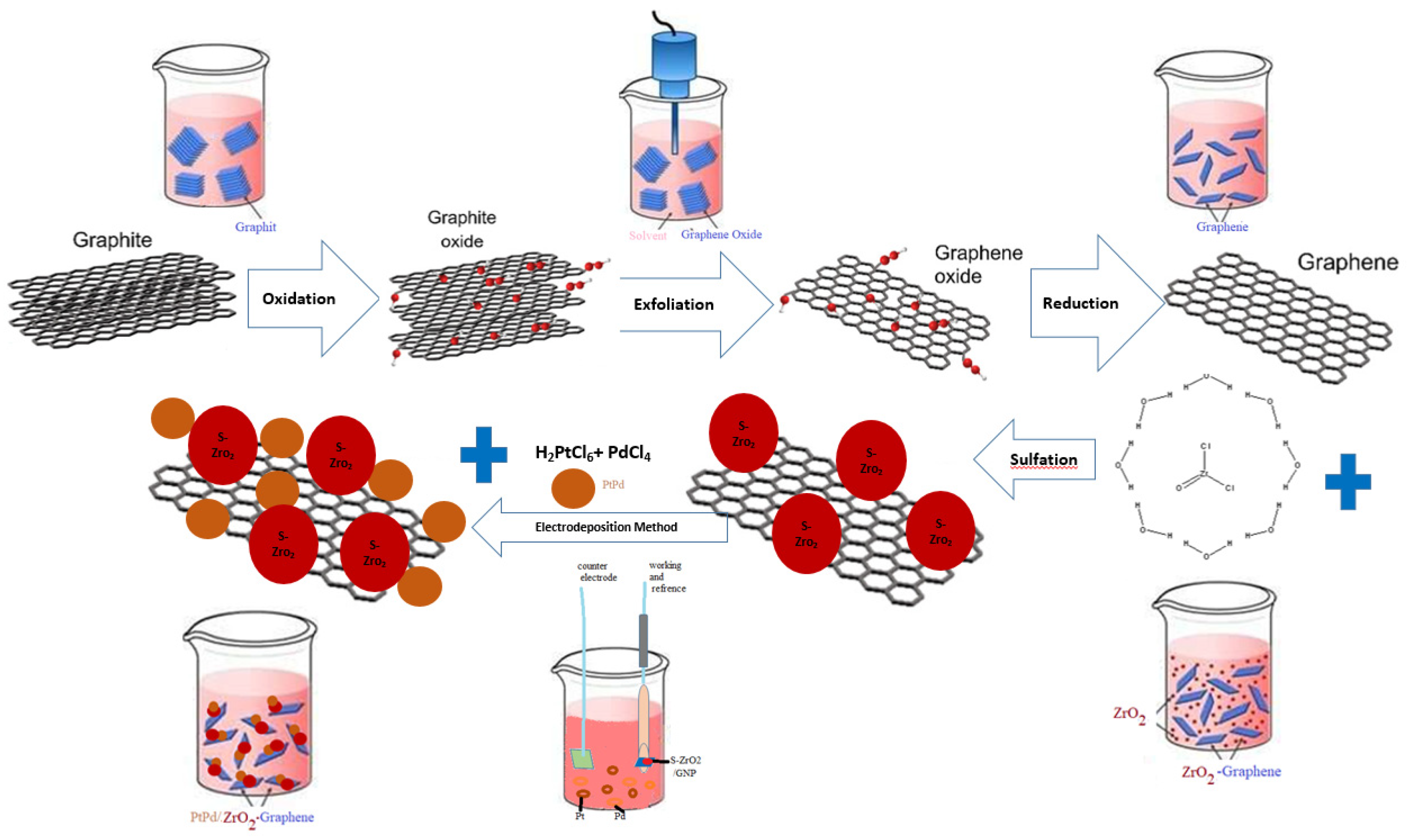
3.2.1. Synthesis of Graphene Nanoplates
3.2.2. Synthesis of S-ZrO2-GNP Support
3.2.3. Electrodeposition of Pt-Pd Nanoparticles on the S-ZrO2-GNP-Contained Glassy Carbon
3.2.4. Characterization and Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tian, N.; Lu, B.-A.; Yang, X.-D.; Huang, R.; Jiang, Y.-X.; Zhou, Z.-Y.; Sun, S.-G. Rational design and synthesis of low-temperature fuel cell electrocatalysts. Electrochem. Energy Rev. 2018, 1, 54–83. [Google Scholar] [CrossRef]
- Hu, B.; Yuan, J.; Zhang, J.; Shu, Q.; Guan, D.; Yang, G.; Zhou, W.; Shao, Z. High activity and durability of a Pt–Cu–Co ternary alloy electrocatalyst and its large-scale preparation for practical proton exchange membrane fuel cells. Compos. Part B Eng. 2021, 222, 109082. [Google Scholar] [CrossRef]
- Xu, X.; Wang, W.; Zhou, W.; Shao, Z. Recent advances in novel nanostructuring methods of perovskite electrocatalysts for energy-related applications. Small Methods 2018, 2, 1800071. [Google Scholar] [CrossRef]
- Li, Y.; Gui, F.; Wang, F.; Liu, J.; Zhu, H. Synthesis of modified, ordered mesoporous carbon-supported Pt3Cu catalyst for enhancing the oxygen reduction activity and durability. Int. J. Hydrog. Energy 2021, 46, 37802–37813. [Google Scholar] [CrossRef]
- Zhao, W.; Ye, Y.; Jiang, W.; Li, J.; Tang, H.; Hu, J.; Du, L.; Cui, Z.; Liao, S. Mesoporous carbon confined intermetallic nanoparticles as highly durable electrocatalysts for the oxygen reduction reaction. J. Mater. Chem. A 2020, 8, 15822–15828. [Google Scholar] [CrossRef]
- Han, X.-F.; Batool, N.; Wang, W.-T.; Teng, H.-T.; Zhang, L.; Yang, R.; Tian, J.-H. Templated-assisted synthesis of structurally ordered intermetallic Pt3Co with ultralow loading supported on 3D porous carbon for oxygen reduction reaction. ACS Appl. Mater. Interfaces 2021, 13, 37133–37141. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.; Zheng, T.; Chen, L.; Wang, H.; Cai, X.; Sun, Y.; Hao, Z. Anchored Pt-Co nanoparticles on honeycombed graphene as highly durable catalysts for the oxygen reduction reaction. ACS Appl. Mater. Interfaces 2021, 13, 34397–34409. [Google Scholar] [CrossRef] [PubMed]
- Britto, P.J.; Santhanam, K.S.; Rubio, A.; Alonso, J.A.; Ajayan, P.M. Improved charge transfer at carbon nanotube electrodes. Adv. Mater. 1999, 11, 154–157. [Google Scholar] [CrossRef]
- Tada, T. Handbook of Fuel Cells: Fundamentals, Technology, and Applications; Vielstich, W., Lamm, A., Gasteiger, H., Eds.; John Wiley & Sons: New York, NY, USA, 2003; Volume 3. [Google Scholar]
- Palit, G.; Elayaperumal, K. Passivity and pitting of corrosion resistant pure metals Ta, Nb, Ti, Zr, Cr and A1 in chloride solutions. Corros. Sci. 1978, 18, 169–179. [Google Scholar] [CrossRef]
- Ioroi, T.; Siroma, Z.; Fujiwara, N.; Yamazaki, S.-i.; Yasuda, K. Sub-stoichiometric titanium oxide-supported platinum electrocatalyst for polymer electrolyte fuel cells. Electrochem. Commun. 2005, 7, 183–188. [Google Scholar] [CrossRef]
- Chhina, H.; Campbell, S.; Kesler, O. An oxidation-resistant indium tin oxide catalyst support for proton exchange membrane fuel cells. J. Power Sources 2006, 161, 893–900. [Google Scholar] [CrossRef]
- Arata, K.i.; Hino, M. Preparation of superacids by metal oxides and their catalytic action. Mater. Chem. Phys. 1990, 26, 213–237. [Google Scholar] [CrossRef]
- Hara, S.; Miyayama, M. Proton conductivity of superacidic sulfated zirconia. Solid State Ion. 2004, 168, 111–116. [Google Scholar] [CrossRef]
- Uchida, M.; Fukuoka, Y.; Sugawara, Y.; Eda, N.; Ohta, A. Effects of microstructure of carbon support in the catalyst layer on the performance of polymer-electrolyte fuel cells. J. Electrochem. Soc. 1996, 143, 2245. [Google Scholar] [CrossRef]
- Wu, Z.; Sun, G.; Jin, W.; Hou, H.; Wang, S.; Xin, Q. Nafion® and nano-size TiO2–SO42− solid superacid composite membrane for direct methanol fuel cell. J. Membr. Sci. 2008, 313, 336–343. [Google Scholar] [CrossRef]
- Suzuki, Y.; Ishihara, A.; Mitsushima, S.; Kamiya, N.; Ota, K.-i. Sulfated-zirconia as a support of Pt catalyst for polymer electrolyte fuel cells. Electrochem. Solid-State Lett. 2007, 10, B105. [Google Scholar] [CrossRef]
- Choi, P.; Jalani, N.H.; Datta, R. Thermodynamics and Proton Transport in Nafion: III. Proton Transport in Nafion/Sulfated Nanocomposite Membranes. J. Electrochem. Soc. 2005, 152, A1548. [Google Scholar] [CrossRef]
- Ma, Z.; Li, S.; Wu, L.; Song, L.; Jiang, G.; Liang, Z.; Su, D.; Zhu, Y.; Adzic, R.R.; Wang, J.X. NbOx nano-nail with a Pt head embedded in carbon as a highly active and durable oxygen reduction catalyst. Nano Energy 2020, 69, 104455. [Google Scholar] [CrossRef]
- Liu, Y.; Ishihara, A.; Mitsushima, S.; Kamiya, N.; Ota, K.-i. Zirconium oxide for PEFC cathodes. Electrochem. Solid-State Lett 2005, 8, A400. [Google Scholar] [CrossRef]
- Kodama, K.; Shinohara, A.; Hasegawa, N.; Shinozaki, K.; Jinnouchi, R.; Suzuki, T.; Hatanaka, T.; Morimoto, Y. Catalyst poisoning property of sulfonimide acid ionomer on Pt (111) surface. J. Electrochem. Soc. 2014, 161, F649. [Google Scholar] [CrossRef]
- Shinozaki, K.; Morimoto, Y.; Pivovar, B.S.; Kocha, S.S. Suppression of oxygen reduction reaction activity on Pt-based electrocatalysts from ionomer incorporation. J. Power Sources 2016, 325, 745–751. [Google Scholar] [CrossRef]
- Subbaraman, R.; Strmcnik, D.; Paulikas, A.P.; Stamenkovic, V.R.; Markovic, N.M. Oxygen Reduction Reaction at Three-Phase Interfaces. ChemPhysChem 2010, 11, 2825–2833. [Google Scholar] [CrossRef] [PubMed]
- Yarlagadda, V.; Carpenter, M.K.; Moylan, T.E.; Kukreja, R.S.; Koestner, R.; Gu, W.; Thompson, L.; Kongkanand, A. Boosting fuel cell performance with accessible carbon mesopores. ACS Energy Lett. 2018, 3, 618–621. [Google Scholar] [CrossRef]
- Ho, V.T.T.; Pan, C.-J.; Rick, J.; Su, W.-N.; Hwang, B.-J. Nanostructured Ti0. 7Mo0. 3O2 support enhances electron transfer to Pt: High-performance catalyst for oxygen reduction reaction. J.Am. Chem. Soc. 2011, 133, 11716–11724. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.-Z.; Wang, Z.-B.; Chu, Y.-Y.; Gu, D.-M.; Yin, G.-P. Ultrahigh stable carbon riveted Pt/TiO2–C catalyst prepared by in situ carbonized glucose for proton exchange membrane fuel cell. Energy Environ. Sci. 2011, 4, 728–735. [Google Scholar] [CrossRef]
- Sun, W.; Sun, J.; Du, L.; Du, C.; Gao, Y.; Yin, G. Synthesis of Nitrogen-doped Niobium Dioxide and its co-catalytic effect towards the electrocatalysis of oxygen reduction on platinum. Electrochim. Acta 2016, 195, 166–174. [Google Scholar] [CrossRef]
- Orilall, M.C.; Matsumoto, F.; Zhou, Q.; Sai, H.; Abruna, H.D.; DiSalvo, F.J.; Wiesner, U. One-pot synthesis of platinum-based nanoparticles incorporated into mesoporous niobium oxide− carbon composites for fuel cell electrodes. J. Am. Chem. Soc. 2009, 131, 9389–9395. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Yang, J.; Liu, E.; Jia, Q.; Veith, G.M.; Nair, G.; DiPietro, S.; Sun, K.; Chen, J.; Pietrasz, P. Physical vapor deposition process for engineering Pt based oxygen reduction reaction catalysts on NbOx templated carbon support. J. Power Sources 2020, 451, 227709. [Google Scholar] [CrossRef]
- Geim, A.K.; Novoselov, K.S. The rise of graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Jiang, D.; Schedin, F.; Booth, T.; Khotkevich, V.; Morozov, S.; Geim, A.K. Two-dimensional atomic crystals. Proc. Natl. Acad. Sci. USA 2005, 102, 10451–10453. [Google Scholar] [CrossRef]
- Stankovich, S.; Dikin, D.A.; Dommett, G.H.; Kohlhaas, K.M.; Zimney, E.J.; Stach, E.A.; Piner, R.D.; Nguyen, S.T.; Ruoff, R.S. Graphene-based composite materials. Nature 2006, 442, 282–286. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Fu, Z.; Chou, S.Y. Graphene transistors fabricated via transfer-printing in device active-areas on large wafer. Nano Lett. 2007, 7, 3840–3844. [Google Scholar] [CrossRef]
- Eda, G.; Fanchini, G.; Chhowalla, M. Large-area ultrathin films of reduced graphene oxide as a transparent and flexible electronic material. Nat. Nanotechnol. 2008, 3, 270–274. [Google Scholar] [CrossRef] [PubMed]
- Gasteiger, H.A.; Kocha, S.S.; Sompalli, B.; Wagner, F.T. Activity benchmarks and requirements for Pt, Pt-alloy, and non-Pt oxygen reduction catalysts for PEMFCs. Appl. Catal. B 2005, 56, 9–35. [Google Scholar] [CrossRef]
- Antolini, E. Formation of carbon-supported PtM alloys for low temperature fuel cells: A review. Mater. Chem. Phys. 2003, 78, 563–573. [Google Scholar] [CrossRef]
- Yu, X.; Ye, S. Recent advances in activity and durability enhancement of Pt/C catalytic cathode in PEMFC: Part I. Physico-chemical and electronic interaction between Pt and carbon support, and activity enhancement of Pt/C catalyst. J. Power Sources 2007, 172, 133–144. [Google Scholar] [CrossRef]
- Lopes, T.; Antolini, E.; Colmati, F.; Gonzalez, E.R. Carbon supported Pt–Co (3: 1) alloy as improved cathode electrocatalyst for direct ethanol fuel cells. J. Power Sources 2007, 164, 111–114. [Google Scholar] [CrossRef]
- Neyerlin, K.; Srivastava, R.; Yu, C.; Strasser, P. Electrochemical activity and stability of dealloyed Pt–Cu and Pt–Cu–Co electrocatalysts for the oxygen reduction reaction (ORR). J. Power Sources 2009, 186, 261–267. [Google Scholar] [CrossRef]
- Koh, S.; Toney, M.F.; Strasser, P. Activity–stability relationships of ordered and disordered alloy phases of Pt3Co electrocatalysts for the oxygen reduction reaction (ORR). Electrochim. Acta 2007, 52, 2765–2774. [Google Scholar]
- Maillard, F.; Dubau, L.; Durst, J.; Chatenet, M.; André, J.; Rossinot, E. Durability of Pt3Co/C nanoparticles in a proton-exchange membrane fuel cell: Direct evidence of bulk Co segregation to the surface. Electrochem. Commun. 2010, 12, 1161–1164. [Google Scholar] [CrossRef]
- Yu, X.; Ye, S. Recent advances in activity and durability enhancement of Pt/C catalytic cathode in PEMFC: Part II: Degradation mechanism and durability enhancement of carbon supported platinum catalyst. J. Power Sources 2007, 172, 145–154. [Google Scholar] [CrossRef]
- Li, H.; Sun, G.; Li, N.; Sun, S.; Su, D.; Xin, Q. Design and preparation of highly active Pt− Pd/C catalyst for the oxygen reduction reaction. J. Phys. Chem. C 2007, 111, 5605–5617. [Google Scholar] [CrossRef]
- Wu, Z.-P.; Caracciolo, D.T.; Maswadeh, Y.; Wen, J.; Kong, Z.; Shan, S.; Vargas, J.A.; Yan, S.; Hopkins, E.; Park, K. Alloying–realloying enabled high durability for Pt–Pd-3d-transition metal nanoparticle fuel cell catalysts. Nat. Commun. 2021, 12, 859. [Google Scholar] [CrossRef] [PubMed]
- Ye, H.; Crooks, R.M. Effect of elemental composition of PtPd bimetallic nanoparticles containing an average of 180 atoms on the kinetics of the electrochemical oxygen reduction reaction. J. Am. Chem. Soc. 2007, 129, 3627–3633. [Google Scholar] [CrossRef] [PubMed]
- Hoshi, N.; Nakamura, M.; Kondo, S. Oxygen reduction reaction on the low index planes of palladium electrodes modified with a monolayer of platinum film. Electrochem. Commun. 2009, 11, 2282–2284. [Google Scholar] [CrossRef]
- Lim, B.; Jiang, M.; Camargo, P.H.; Cho, E.C.; Tao, J.; Lu, X.; Zhu, Y.; Xia, Y. Pd-Pt bimetallic nanodendrites with high activity for oxygen reduction. Science 2009, 324, 1302–1305. [Google Scholar] [CrossRef]
- Peng, Z.; Yang, H. Synthesis and oxygen reduction electrocatalytic property of Pt-on-Pd bimetallic heteronanostructures. J. Am. Chem. Soc. 2009, 131, 7542–7543. [Google Scholar] [CrossRef]
- Rego, R.; Oliveira, C.; Velázquez, A.; Cabot, P.-L. A new route to prepare carbon paper-supported Pd catalyst for oxygen reduction reaction. Electrochem. Commun. 2010, 12, 745–748. [Google Scholar] [CrossRef]
- Shao, M. Palladium-based electrocatalysts for hydrogen oxidation and oxygen reduction reactions. J. Power Sources 2011, 196, 2433–2444. [Google Scholar] [CrossRef]
- Yaldagard, M.; Seghatoleslami, N.; Jahanshahi, M. Preparation of Pt-Co nanoparticles by galvanostatic pulse electrochemical codeposition on in situ electrochemical reduced graphene nanoplates based carbon paper electrode for oxygen reduction reaction in proton exchange membrane fuel cell. Appl. Surf. Sci. 2014, 315, 222–234. [Google Scholar] [CrossRef]
- Park, S.; Ruoff, R.S. Chemical methods for the production of graphenes. Nat. Nanotechnol. 2009, 4, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Stankovich, S.; Piner, R.D.; Chen, X.; Wu, N.; Nguyen, S.T.; Ruoff, R.S. Stable aqueous dispersions of graphitic nanoplatelets via the reduction of exfoliated graphite oxide in the presence of poly (sodium 4-styrenesulfonate). J. Mater. Chem. 2006, 16, 155–158. [Google Scholar] [CrossRef]
- Stankovich, S.; Dikin, D.A.; Piner, R.D.; Kohlhaas, K.A.; Kleinhammes, A.; Jia, Y.; Wu, Y.; Nguyen, S.T.; Ruoff, R.S. Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon 2007, 45, 1558–1565. [Google Scholar] [CrossRef]
- Park, S.; An, J.; Jung, I.; Piner, R.D.; An, S.J.; Li, X.; Velamakanni, A.; Ruoff, R.S. Colloidal suspensions of highly reduced graphene oxide in a wide variety of organic solvents. Nano Lett. 2009, 9, 1593–1597. [Google Scholar] [CrossRef] [PubMed]
- Hassan, H.M.; Abdelsayed, V.; Abd El Rahman, S.K.; AbouZeid, K.M.; Terner, J.; El-Shall, M.S.; Al-Resayes, S.I.; El-Azhary, A.A. Microwave synthesis of graphene sheets supporting metal nanocrystals in aqueous and organic media. J. Mater. Chem. 2009, 19, 3832–3837. [Google Scholar] [CrossRef]
- Ferrari, A.C. Raman spectroscopy of graphene and graphite: Disorder, electron–phonon coupling, doping and nonadiabatic effects. Solid State Commun. 2007, 143, 47–57. [Google Scholar] [CrossRef]
- Berciaud, S.; Ryu, S.; Brus, L.E.; Heinz, T.F. Probing the intrinsic properties of exfoliated graphene: Raman spectroscopy of free-standing monolayers. Nano Lett. 2008, 9, 346–352. [Google Scholar] [CrossRef] [PubMed]
- Dresselhaus, M.S.; Jorio, A.; Hofmann, M.; Dresselhaus, G.; Saito, R. Perspectives on carbon nanotubes and graphene Raman spectroscopy. Nano Lett. 2010, 10, 751–758. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Zhou, Y.; Pan, H.-B.; Zhu, C.; Fu, S.; Wai, C.M.; Du, D.; Zhu, J.-J.; Lin, Y. Ultrasonic-assisted synthesis of Pd–Pt/carbon nanotubes nanocomposites for enhanced electro-oxidation of ethanol and methanol in alkaline medium. Ultrason. Sonochem. 2016, 28, 192–198. [Google Scholar] [CrossRef]
- Yıldrım, A.; Seçkin, T. In situ preparation of polyether amine functionalized MWCNT nanofiller as reinforcing agents. Adv. Mater. Sci. Eng. 2014, 2014, 1–6. [Google Scholar] [CrossRef]
- Chaudhary, B.; Panwar, V.; Roy, T.; Pal, K. Thermomechanical behaviour of zirconia–multiwalled carbon nanotube-reinforced polypropylene hybrid composites. Polym. Bull. 2019, 76, 511–521. [Google Scholar] [CrossRef]
- Yaldagard, M.; Shahbaz, M.; Kim, H.W.; Kim, S.S. Ethanol Electro-Oxidation on Catalysts with S-ZrO2-Decorated Graphene as Support in Fuel Cell Applications. Nanomaterials 2022, 12, 3327. [Google Scholar] [CrossRef]
- Mangla, O.; Roy, S. Monoclinic zirconium oxide nanostructures having tunable band gap synthesized under extremely non-equilibrium plasma conditions. Proceedings 2019, 3, 10. [Google Scholar] [CrossRef]
- Ding, S.; Zhao, J.; Yu, Q. Effect of zirconia polymorph on vapor-phase ketonization of propionic acid. Catalysts 2019, 9, 768. [Google Scholar] [CrossRef]
- Manoharan, D.; Loganathan, A.; Kurapati, V.; Nesamony, V.J. Unique sharp photoluminescence of size-controlled sonochemically synthesized zirconia nanoparticles. Ultrason. Sonochem. 2015, 23, 174–184. [Google Scholar] [CrossRef]
- Mkhize, N.; Vashistha, V.K.; Pullabhotla, V.S.R. Catalytic Oxidation of 1, 2-Dichlorobenzene over Metal-Supported on ZrO2 Catalysts. Top. Catal. 2023, 67, 409–421. [Google Scholar] [CrossRef]
- Asencios, Y.J.; Yigit, N.; Wicht, T.; Stöger-Pollach, M.; Lucrédio, A.F.; Marcos, F.C.; Assaf, E.M.; Rupprechter, G. Partial Oxidation of Bio-methane over Nickel Supported on MgO–ZrO. Top. Catal. 2023, 66, 1532–1552. [Google Scholar] [CrossRef] [PubMed]
- Abdelaziz, O.Y.; Clemmensen, I.; Meier, S.; Bjelić, S.; Hulteberg, C.P.; Riisager, A. Oxidative Depolymerization of Kraft Lignin to Aromatics Over Bimetallic V–Cu/ZrO2 Catalysts. Top. Catal. 2023, 66, 1369–1380. [Google Scholar] [CrossRef]
- Gregory, N. Elements of X-Ray Diffraction. J. Am. Chem. Soc. 1957, 79, 1773–1774. [Google Scholar] [CrossRef]
- Swanson, H.E.; Tatge, E. Standard X-ray Diffraction Powder Patterns; US Department of Commerce, National Bureau of Standards: Washington, DC, USA, 1953; Volume 1, p. 31. [Google Scholar]
- Sharma, R.; Gyergyek, S.; Andersen, S.M. Critical thinking on baseline corrections for electrochemical surface area (ECSA) determination of Pt/C through H-adsorption/H-desorption regions of a cyclic voltammogram. Appl. Catal. B 2022, 311, 121351. [Google Scholar] [CrossRef]
- Zaman, S.; Su, Y.Q.; Dong, C.L.; Qi, R.; Huang, L.; Qin, Y.; Huang, Y.C.; Li, F.M.; You, B.; Guo, W. Scalable molten salt synthesis of platinum alloys planted in metal–nitrogen–graphene for efficient oxygen reduction. Angew. Chem. Int. Ed. 2022, 61, e202115835. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.-F.; Guo, P.; Li, J.-Z.; Zhao, L.; Sui, X.-L.; Wang, Y.; Wang, Z.-B. How to appropriately assess the oxygen reduction reaction activity of platinum group metal catalysts with rotating disk electrode. IScience 2021, 24, 103024. [Google Scholar] [CrossRef] [PubMed]
- Pozio, A.; De Francesco, M.; Cemmi, A.; Cardellini, F.; Giorgi, L. Comparison of high surface Pt/C catalysts by cyclic voltammetry. J. Power Sources 2002, 105, 13–19. [Google Scholar] [CrossRef]
- Garsany, Y.; Baturina, O.A.; Swider-Lyons, K.E.; Kocha, S.S. Experimental Methods for Quantifying the Activity of Platinum Electrocatalysts for the Oxygen Reduction Reaction; ACS Publications: Washington, DC, USA, 2010. [Google Scholar]
- Jiang, L.; Sun, G.; Sun, S.; Liu, J.; Tang, S.; Li, H.; Zhou, B.; Xin, Q. Structure and chemical composition of supported Pt–Sn electrocatalysts for ethanol oxidation. Electrochim. Acta 2005, 50, 5384–5389. [Google Scholar] [CrossRef]
- Antolini, E.; Salgado, J.; Giz, M.; Gonzalez, E. Effects of geometric and electronic factors on ORR activity of carbon supported Pt–Co electrocatalysts in PEM fuel cells. Int. J. Hydrog. Energy 2005, 30, 1213–1220. [Google Scholar] [CrossRef]
- Cheng, N.; Liu, J.; Banis, M.N.; Geng, D.; Li, R.; Ye, S.; Knights, S.; Sun, X. High stability and activity of Pt electrocatalyst on atomic layer deposited metal oxide/nitrogen-doped graphene hybrid support. Int. J. Hydrogen Energy 2014, 39, 15967–15974. [Google Scholar] [CrossRef]
- Wu, H.; Wexler, D.; Wang, G. PtxNi alloy nanoparticles as cathode catalyst for PEM fuel cells with enhanced catalytic activity. J. Alloys Compd. 2009, 488, 195–198. [Google Scholar] [CrossRef]
- Justin, P.; Charan, P.H.K.; Rao, G.R. High performance Pt–Nb2O5/C electrocatalysts for methanol electrooxidation in acidic media. Appl. Catal. B. 2010, 100, 510–515. [Google Scholar] [CrossRef]
- Saha, M.S.; Zhang, Y.; Cai, M.; Sun, X. Carbon-coated tungsten oxide nanowires supported Pt nanoparticles for oxygen reduction. Int. J. Hydrogen Energy 2012, 37, 4633–4638. [Google Scholar] [CrossRef]
- Song, Y.; Duan, D.; Shi, W.; Wang, H.; Yang, S.; Sun, Z. Promotion effects of ZrO2 on mesoporous Pd prepared by a one-step dealloying method for methanol oxidation in an alkaline electrolyte. J. Electrochem. Soc. 2017, 164, F1495. [Google Scholar] [CrossRef]
- Aguilar-Vallejo, A.; Álvarez-Contreras, L.; Guerra-Balcázar, M.; Ledesma-García, J.; Gerardo Arriaga, L.; Arjona, N.; Rivas, S. Electrocatalytic evaluation of highly stable Pt/ZrO2 electrocatalysts for the methanol oxidation reaction synthesized without the assistance of any carbon support. ChemElectroChem 2019, 6, 2107–2118. [Google Scholar] [CrossRef]
- Gwebu, S.S.; Maxakato, N.W. The influence of ZrO2 promoter in Pd/fCNDs-ZrO2 catalyst towards alcohol fuel electrooxidation in alkaline media. Mater. Res. Express 2020, 7, 015607. [Google Scholar] [CrossRef]
- Wang, X.; Li, W.; Chen, Z.; Waje, M.; Yan, Y. Durability investigation of carbon nanotube as catalyst support for proton exchange membrane fuel cell. J. Power Sources 2006, 158, 154–159. [Google Scholar] [CrossRef]
- Higuchi, E.; Uchida, H.; Watanabe, M. Effect of loading level in platinum-dispersed carbon black electrocatalysts on oxygen reduction activity evaluated by rotating disk electrode. J. Electroanal. Chem. 2005, 583, 69–76. [Google Scholar] [CrossRef]
- Paulus, U.; Schmidt, T.; Gasteiger, H.; Behm, R. Oxygen reduction on a high-surface area Pt/Vulcan carbon catalyst: A thin-film rotating ring-disk electrode study. J. Electroanal. Chem. 2001, 495, 134–145. [Google Scholar] [CrossRef]
- He, Q.; Mukerjee, S. Electrocatalysis of oxygen reduction on carbon-supported PtCo catalysts prepared by water-in-oil micro-emulsion. Electrochim. Acta 2010, 55, 1709–1719. [Google Scholar] [CrossRef]
- Van Brussel, M.; Kokkinidis, G.; Vandendael, I.; Buess-Herman, C. High performance gold-supported platinum electrocatalyst for oxygen reduction. Electrochem. Commun. 2002, 4, 808–813. [Google Scholar] [CrossRef]
- Van Brussel, M.; Kokkinidis, G.; Hubin, A.; Buess-Herman, C. Oxygen reduction at platinum modified gold electrodes. Electrochim. Acta 2003, 48, 3909–3919. [Google Scholar] [CrossRef]
- Qiao, J.; Lin, R.; Li, B.; Ma, J.; Liu, J. Kinetics and electrocatalytic activity of nanostructured Ir–V/C for oxygen reduction reaction. Electrochim. Acta 2010, 55, 8490–8497. [Google Scholar] [CrossRef]
- Zaman, S.; Tian, X.; Su, Y.-Q.; Cai, W.; Yan, Y.; Qi, R.; Douka, A.I.; Chen, S.; You, B.; Liu, H. Direct integration of ultralow-platinum alloy into nanocarbon architectures for efficient oxygen reduction in fuel cells. Sci. Bull. 2021, 66, 2207–2216. [Google Scholar] [CrossRef]
- Horwood, E. Instrumental Methods in Electrochemistry; Series in Physical Chemistry; Horwood, E., Ed.; Southampton Electrochemistry Group, University of Southampton: Southampton, UK, 1985. [Google Scholar]
- Durst, J.; Siebel, A.; Simon, C.; Hasché, F.; Herranz, J.; Gasteiger, H. New insights into the electrochemical hydrogen oxidation and evolution reaction mechanism. Energy Environ. Sci. 2014, 7, 2255–2260. [Google Scholar] [CrossRef]
- Zhang, J. PEM Fuel Cell Electrocatalysts and Catalyst Layers: Fundamentals and Applications; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2008. [Google Scholar] [CrossRef]
- Markovic, N.; Gasteiger, H.; Ross, P.N. Kinetics of oxygen reduction on Pt (hkl) electrodes: Implications for the crystallite size effect with supported Pt electrocatalysts. J. Electrochem. Soc. 1997, 144, 1591. [Google Scholar] [CrossRef]
- Markovic, N.M.; Gasteiger, H.A.; Ross Jr, P.N. Oxygen reduction on platinum low-index single-crystal surfaces in sulfuric acid solution: Rotating ring-Pt (hkl) disk studies. J. Phys. Chem. 1995, 99, 3411–3415. [Google Scholar] [CrossRef]
- Perez, J.; Villullas, H.M.; Gonzalez, E.R. Structure sensitivity of oxygen reduction on platinum single crystal electrodes in acid solutions. J. Electroanal. Chem. 1997, 435, 179–187. [Google Scholar] [CrossRef]
- Marković, N.; Gasteiger, H.; Grgur, B.; Ross, P. Oxygen reduction reaction on Pt (111): Effects of bromide. J. Electroanal. Chem. 1999, 467, 157–163. [Google Scholar] [CrossRef]
- Bett, J.; Lundquist, J.; Washington, E.; Stonehart, P. Platinum crystallite size considerations for electrocatalytic oxygen reduction—I. Electrochim. Acta 1973, 18, 343–348. [Google Scholar] [CrossRef]
- Damjanovic, A.; Sepa, D. An analysis of the pH dependence of enthalpies and Gibbs energies of activation for O2 reduction at Pt electrodes in acid solutions. Electrochim. Acta 1990, 35, 1157–1162. [Google Scholar] [CrossRef]
- Sarapuu, A.; Kasikov, A.; Laaksonen, T.; Kontturi, K.; Tammeveski, K. Electrochemical reduction of oxygen on thin-film Pt electrodes in acid solutions. Electrochim. Acta 2008, 53, 5873–5880. [Google Scholar] [CrossRef]
- Zignani, S.C.; Antolini, E.; Gonzalez, E.R. Evaluation of the stability and durability of Pt and Pt–Co/C catalysts for polymer electrolyte membrane fuel cells. J. Power Sources 2008, 182, 83–90. [Google Scholar] [CrossRef]
- Salgado, J.R.C.; Antolini, E.; Gonzalez, E.R. Carbon supported Pt–Co alloys as methanol-resistant oxygen-reduction electrocatalysts for direct methanol fuel cells. Appl. Catal. B Environ. 2005, 57, 283–290. [Google Scholar] [CrossRef]
- Mustain, W.E.; Kepler, K.; Prakash, J. CoPdx oxygen reduction electrocatalysts for polymer electrolyte membrane and direct methanol fuel cells. Electrochim. Acta 2007, 52, 2102–2108. [Google Scholar] [CrossRef]
- Zaman, S.; Huang, L.; Douka, A.I.; Yang, H.; You, B.; Xia, B.Y. Oxygen reduction electrocatalysts toward practical fuel cells: Progress and perspectives. Angew. Chem. 2021, 133, 17976–17996. [Google Scholar] [CrossRef]
- Wang, G.; Shen, X.; Wang, B.; Yao, J.; Park, J. Synthesis and characterisation of hydrophilic and organophilic graphene nanosheets. Carbon 2009, 47, 1359–1364. [Google Scholar] [CrossRef]
- Woo, S.; Kim, I.; Lee, J.K.; Bong, S.; Lee, J.; Kim, H. Preparation of cost-effective Pt–Co electrodes by pulse electrodeposition for PEMFC electrocatalysts. Electrochim. Acta 2011, 56, 3036–3041. [Google Scholar] [CrossRef]
- Antolini, E.; Giorgi, L.; Pozio, A.; Passalacqua, E. Influence of Nafion loading in the catalyst layer of gas-diffusion electrodes for PEFC. J. Power Sources 1999, 77, 136–142. [Google Scholar] [CrossRef]

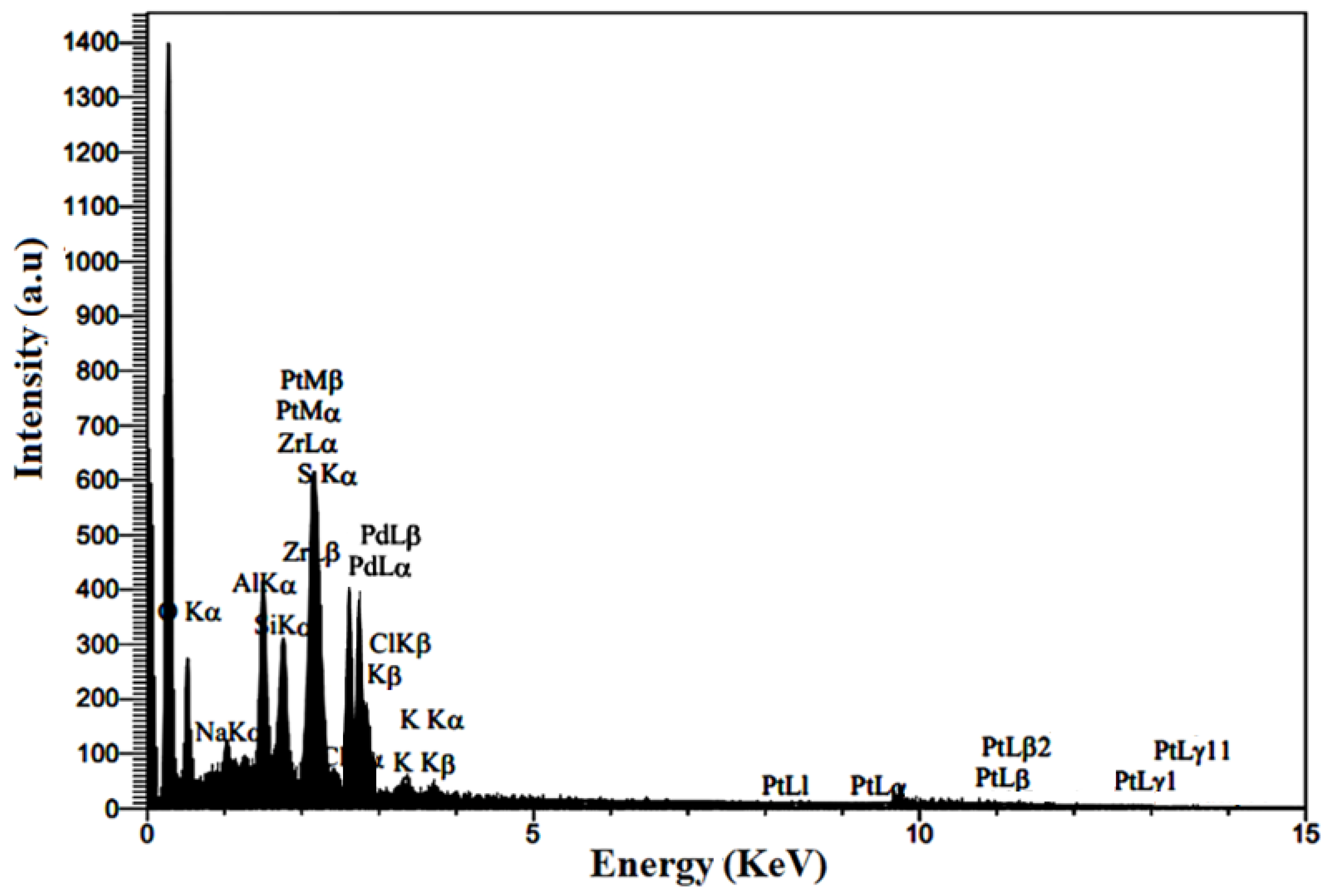

| Element | Line | wt% |
|---|---|---|
| C | Kα | 52.84 |
| O | Kα | 11.27 |
| Na | Kα | 0.95 |
| Al | Kα | 2.43 |
| Si | Kα | 1.77 |
| S | Kα | 10.30 |
| Cl | Kα | 0.28 |
| K | Kα | 0.57 |
| Zr | Lα | 1.35 |
| Pt | Lα | 10.03 |
| Pd | Lα | 8.21 |
| 100 |
| Electrode | Crystallite Size (XRD) (nm) | ) | |
|---|---|---|---|
| Pt-Pd/S-ZrO2-GNPs | 4.50 | 14.443 | 97.32 ** |
| Pt-Pd/ZrO2-GNPs | 4.54 | 14.011 | 94.51 ** |
| Pt/S-ZrO2-GNPs | 4.31 | 12.338 | 83.21 * |
| Pt-Pd/C | 4.39 | 9.94 | 67.02 ** |
| Pt/C(20 wt%) | 4.20 | 10.20 | 68.83 * |
| Electrode | in (E > 0.9 V) | in (E < 0.85 V) | i0 For (E < 0.85 V) |
|---|---|---|---|
| Pt-Pd/S-ZrO2-GNPs | −56 | −106 | 1.662 |
| Pt-Pd/ZrO2-GNPs | −57 | −107 | 1.610 |
| Pt/S-ZrO2-GNPs | −59 | −111 | 1.021 |
| Pt-Pd/C Pt/C (20 wt%) | −58 | −113 | 1.020 |
| −61 | −122 | 1.018 |
| Test | Electrode | Mass Activity at 0. 9 V (vs. RHE) (mA/mg metal) | Specific Activity (mA/mg metal) | |
|---|---|---|---|---|
| Before ADT | Pt-Pd/S-ZrO2-GNPs | 45.43 | 0.0466 | 97.32 |
| Pt-Pd/ZrO2-GNPs | 40.75 | 0.0431 | 94.51 | |
| Pt/S-ZrO2-GNPs | 33.29 | 0.0401 | 83.21 | |
| Pt-Pd/C | 28.67 | 0.0402 | 67.02 | |
| Pt/C | 23.54 | 0.0342 | 68.83 | |
| After ADT | Pt-Pd/S-ZrO2-GNPs | 17.20 | 0.0233 | 73.54 |
| Pt-Pd/ZrO2-GNPs | 14.19 | 0.0213 | 66.49 | |
| Pt/S-ZrO2-GNPs | 9.03 | 0.0181 | 49.77 | |
| Pt-Pd/C | 5.85 | 0.0189 | 30.83 | |
| Pt/C | 3.48 | 0.0132 | 26.38 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yaldagard, M.; Arkas, M. Enhanced Mass Activity and Durability of Bimetallic Pt-Pd Nanoparticles on Sulfated-Zirconia-Doped Graphene Nanoplates for Oxygen Reduction Reaction in Proton Exchange Membrane Fuel Cell Applications. Molecules 2024, 29, 2129. https://doi.org/10.3390/molecules29092129
Yaldagard M, Arkas M. Enhanced Mass Activity and Durability of Bimetallic Pt-Pd Nanoparticles on Sulfated-Zirconia-Doped Graphene Nanoplates for Oxygen Reduction Reaction in Proton Exchange Membrane Fuel Cell Applications. Molecules. 2024; 29(9):2129. https://doi.org/10.3390/molecules29092129
Chicago/Turabian StyleYaldagard, Maryam, and Michael Arkas. 2024. "Enhanced Mass Activity and Durability of Bimetallic Pt-Pd Nanoparticles on Sulfated-Zirconia-Doped Graphene Nanoplates for Oxygen Reduction Reaction in Proton Exchange Membrane Fuel Cell Applications" Molecules 29, no. 9: 2129. https://doi.org/10.3390/molecules29092129
APA StyleYaldagard, M., & Arkas, M. (2024). Enhanced Mass Activity and Durability of Bimetallic Pt-Pd Nanoparticles on Sulfated-Zirconia-Doped Graphene Nanoplates for Oxygen Reduction Reaction in Proton Exchange Membrane Fuel Cell Applications. Molecules, 29(9), 2129. https://doi.org/10.3390/molecules29092129











