Ethylene Elimination Using Activated Carbons Obtained from Baru (Dipteryx alata vog.) Waste and Impregnated with Copper Oxide
Abstract
1. Introduction
2. Results and Discussion
2.1. Characterization of the Precursor Material and the Prepared Activated Carbons
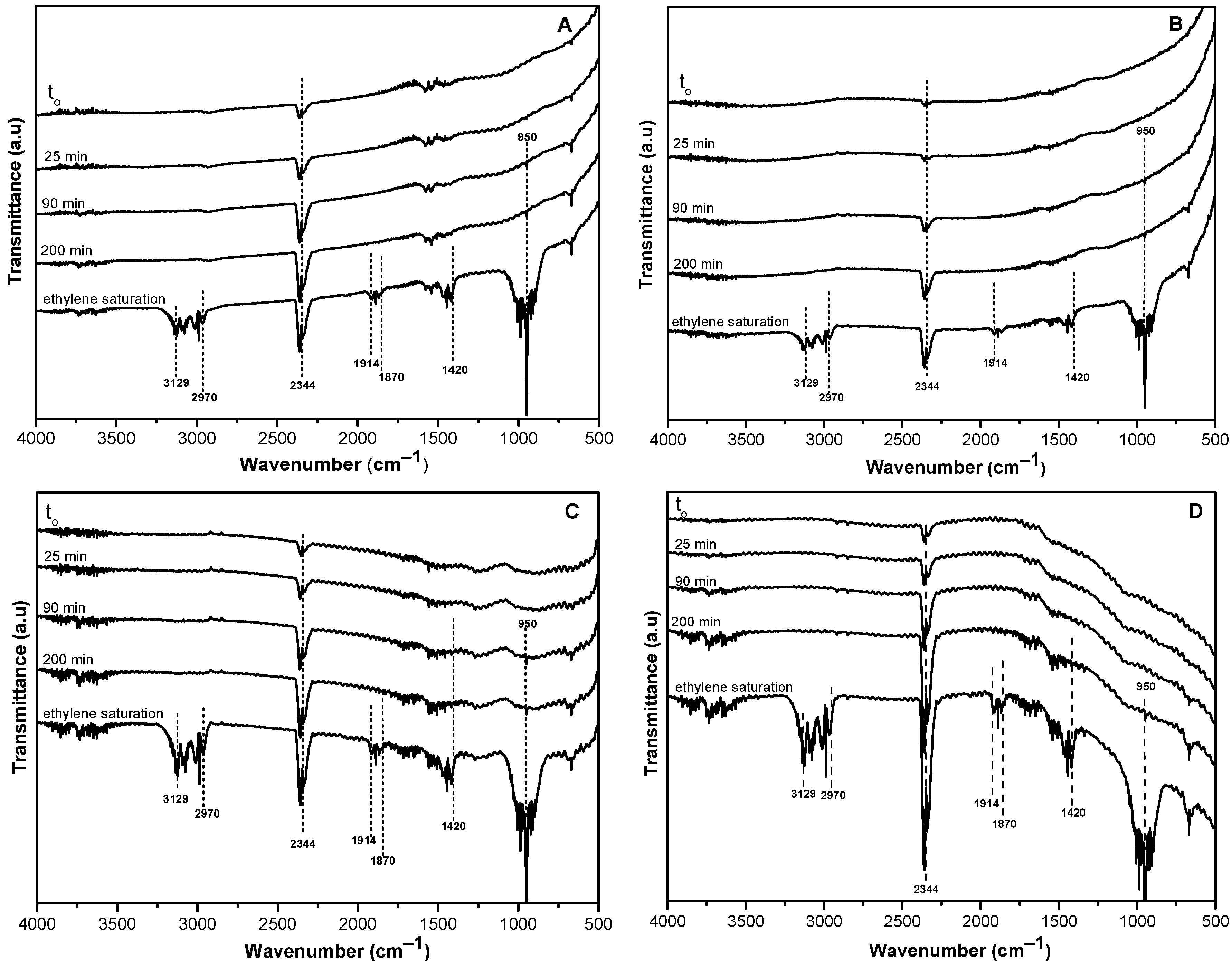
2.2. Chemical Surface Interaction Assessments by Operando IR Spectroscopy
2.3. Mechanistic Approach of Ethylene Adsorption onto Baru-Based Carbon Samples
3. Materials and Methods
3.1. Raw Materials and Reagents
3.2. Preparation of Porous Materials
3.2.1. Preparation and Characterization of the Precursor Material
3.2.2. Generation of Activated Carbon Materials
3.3. Preparation of Baru Activated Carbon Impregnated with Copper Oxide (ACB/CuO)
3.4. Characterization of AC Samples
3.5. Chemical Surface Interaction Assessments by Operando Transmission IR Spectroscopy
3.6. Determination of Ethylene Adsorption Isotherms
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saltveit, M.E. Effect of Ethylene on Quality of Fresh Fruits and Vegetables. Postharvest Biol. Technol. 1999, 15, 279–292. [Google Scholar] [CrossRef]
- Ku, V.V.V.; Shohet, D.; Wills, R.B.H.; Kim, G.H. Importance of Low Ethylene Levels to Delay Senescence of Non-Climacteric Fruit and Vegetables. Aust. J. Exp. Agric. 1999, 39, 221–224. [Google Scholar] [CrossRef]
- Wei, H.; Seidi, F.; Zhang, T.; Jin, Y.; Xiao, H. Ethylene Scavengers for the Preservation of Fruits and Vegetables: A Review. Food Chem. 2021, 337, 127750. [Google Scholar] [CrossRef] [PubMed]
- Keller, N.; Ducamp, M.-N.; Robert, D.; Keller, V. Ethylene Removal and Fresh Product Storage: A Challenge at the Frontiers of Chemistry. Toward an Approach by Photocatalytic Oxidation. Chem. Rev. 2013, 113, 5029–5070. [Google Scholar] [CrossRef] [PubMed]
- Abreu, N.J.; Valdés, H.; Zaror, C.A.; Azzolina-Jury, F.; Meléndrez, M.F. Ethylene Adsorption onto Natural and Transition Metal Modified Chilean Zeolite: An Operando DRIFTS Approach. Microporous Mesoporous Mater. 2019, 274, 138–148. [Google Scholar] [CrossRef]
- Hu, B.; Sun, D.-W.; Pu, H.; Wei, Q. Recent Advances in Detecting and Regulating Ethylene Concentrations for Shelf-Life Extension and Maturity Control of Fruit: A Review. Trends Food Sci. Technol. 2019, 91, 66–82. [Google Scholar] [CrossRef]
- An, Y.; Fu, Q.; Zhang, D.; Wang, Y.; Tang, Z. Performance Evaluation of Activated Carbon with Different Pore Sizes and Functional Groups for VOC Adsorption by Molecular Simulation. Chemosphere 2019, 227, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Shenoy, S.; Pathak, N.; Molins, A.; Toncheva, A.; Schouw, T.; Hemberg, A.; Laoutid, F.; Mahajan, P.V. Impact of Relative Humidity on Ethylene Removal Kinetics of Different Scavenging Materials for Fresh Produce Industry. Postharvest Biol. Technol. 2022, 188, 111881. [Google Scholar] [CrossRef]
- Regadera-Macías, A.M.; Morales-Torres, S.; Pastrana-Martínez, L.M.; Maldonado-Hódar, F.J. Ethylene Removal by Adsorption and Photocatalytic Oxidation Using Biocarbon–TiO2 Nanocomposites. Catal. Today 2023, 413–415, 113932. [Google Scholar] [CrossRef]
- Zhu, L.; Shen, D.; Luo, K.H. A Critical Review on VOCs Adsorption by Different Porous Materials: Species, Mechanisms and Modification Methods. J. Hazard. Mater. 2020, 389, 122102. [Google Scholar] [CrossRef]
- Zhang, X.; Gao, B.; Creamer, A.E.; Cao, C.; Li, Y. Adsorption of VOCs onto Engineered Carbon Materials: A Review. J. Hazard. Mater. 2017, 338, 102–123. [Google Scholar] [CrossRef]
- Zhou, J.; Luo, A.; Zhao, Y. Preparation and Characterisation of Activated Carbon from Waste Tea by Physical Activation Using Steam. J. Air Waste Manag. Assoc. 2018, 68, 1269–1277. [Google Scholar] [CrossRef] [PubMed]
- Lei, B.; Liu, B.; Zhang, H.; Yan, L.; Xie, H.; Zhou, G. CuO-Modified Activated Carbon for the Improvement of Toluene Removal in Air. J. Environ. Sci. 2020, 88, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Chen, Z.; Gong, H.; Wang, X. Copper Oxide Modified Activated Carbon for Enhanced Adsorption Performance of Siloxane: An Experimental and DFT Study. Appl. Surf. Sci. 2022, 601, 154200. [Google Scholar] [CrossRef]
- He, X.; Gui, Y.; Xie, J.; Liu, X.; Wang, Q.; Tang, C. A DFT Study of Dissolved Gas (C2H2, H2, CH4) Detection in Oil on CuO-Modified BNNT. Appl. Surf. Sci. 2020, 500, 144030. [Google Scholar] [CrossRef]
- de Miranda Monteiro, G.; Carvalho, E.E.N.; Boas, E.V.B.V. Baru (Dipteryx Alata Vog.): Fruit or Almond? A Review on Applicability in Food Science and Technology. Food Chem. Adv. 2022, 1, 100103. [Google Scholar] [CrossRef]
- Rambo, M.K.D.; Nemet, Y.K.S.; Júnior, C.C.S.; Pedroza, M.M.; Rambo, M.C.D. Comparative Study of the Products from the Pyrolysis of Raw and Hydrolyzed Baru Wastes. Biomass Convers. Biorefinery 2021, 11, 1943–1953. [Google Scholar] [CrossRef]
- Boundzanga, H.M.; Cagnon, B.; Roulet, M.; de Persis, S.; Vautrin-Ul, C.; Bonnamy, S. Contributions of Hemicellulose, Cellulose, and Lignin to the Mass and the Porous Characteristics of Activated Carbons Produced from Biomass Residues by Phosphoric Acid Activation. Biomass Convers. Biorefinery 2022, 12, 3081–3096. [Google Scholar] [CrossRef]
- Babas, H.; Khachani, M.; Warad, I.; Ajebli, S.; Guessous, A.; Guenbour, A.; Safi, Z.; Berisha, A.; Bellaouchou, A.; Abdelkader, Z.; et al. Sofosbuvir Adsorption onto Activated Carbon Derived from Argan Shell Residue: Optimization, Kinetic, Thermodynamic and Theoretical Approaches. J. Mol. Liq. 2022, 356, 119019. [Google Scholar] [CrossRef]
- Neme, I.; Gonfa, G.; Masi, C. Activated Carbon from Biomass Precursors Using Phosphoric Acid: A Review. Heliyon 2022, 8, e11940. [Google Scholar] [CrossRef]
- Nahil, M.A.; Williams, P.T. Pore Characteristics of Activated Carbons from the Phosphoric Acid Chemical Activation of Cotton Stalks. Biomass Bioenergy 2012, 37, 142–149. [Google Scholar] [CrossRef]
- Amran, F.; Zaini, M.A.A. Valorization of Casuarina Empty Fruit-Based Activated Carbons for Dyes Removal—Activators, Isotherm, Kinetics and Thermodynamics. Surf. Interfaces 2021, 25, 101277. [Google Scholar] [CrossRef]
- Danish, M.; Hashim, R.; Ibrahim, M.N.M.; Sulaiman, O. Optimization Study for Preparation of Activated Carbon from Acacia Mangium Wood Using Phosphoric Acid. Wood Sci. Technol. 2014, 48, 1069–1083. [Google Scholar] [CrossRef]
- Luo, Y.; Li, D.; Chen, Y.; Sun, X.; Cao, Q.; Liu, X. The Performance of Phosphoric Acid in the Preparation of Activated Carbon-Containing Phosphorus Species from Rice Husk Residue. J. Mater. Sci. 2019, 54, 5008–5021. [Google Scholar] [CrossRef]
- Ayalkie Gizaw, B.; Gabbiye Habtu, N. Catalytic Wet Air Oxidation of Azo Dye (Reactive Red 2) over Copper Oxide Loaded Activated Carbon Catalyst. J. Water Process Eng. 2022, 48, 102797. [Google Scholar] [CrossRef]
- Djilani, C.; Zaghdoudi, R.; Modarressi, A.; Rogalski, M.; Djazi, F.; Lallam, A. Elimination of Organic Micropollutants by Adsorption on Activated Carbon Prepared from Agricultural Waste. Chem. Eng. J. 2012, 189–190, 203–212. [Google Scholar] [CrossRef]
- Ofgea, N.M.; Tura, A.M.; Fanta, G.M. Activated Carbon from H3PO4 -Activated Moringa Stenopetale Seed Husk for Removal of Methylene Blue: Optimization Using the Response Surface Method (RSM). Environ. Sustain. Indic. 2022, 16, 100214. [Google Scholar] [CrossRef]
- Liu, X.; Geng, B.; Du, Q.; Ma, J.; Liu, X. Temperature-Controlled Self-Assembled Synthesis of CuO, Cu2O and Cu Nanoparticles through a Single-Precursor Route. Mater. Sci. Eng. A 2007, 448, 7–14. [Google Scholar] [CrossRef]
- Mishra, S.R.; Ahmaruzzaman, M. CuO and CuO-Based Nanocomposites: Synthesis and Applications in Environment and Energy. Sustain. Mater. Technol. 2022, 33, e00463. [Google Scholar] [CrossRef]
- Chatir, E.M.; El Hadrami, A.; Ojala, S.; Brahmi, R. Production of Activated Carbon with Tunable Porosity and Surface Chemistry via Chemical Activation of Hydrochar with Phosphoric Acid under Oxidizing Atmosphere. Surf. Interfaces 2022, 30, 101849. [Google Scholar] [CrossRef]
- Lee, C.L.; H’ng, P.S.; Paridah, M.T.; Chin, K.L.; Rashid, U.; Maminski, M.; Go, W.Z.; Nazrin, R.A.R.; Rosli, S.N.A.; Khoo, P.S. Production of Bioadsorbent from Phosphoric Acid Pretreated Palm Kernel Shell and Coconut Shell by Two-Stage Continuous Physical Activation via N2 and Air. R. Soc. Open Sci. 2018, 5, 180775. [Google Scholar] [CrossRef] [PubMed]
- De Rose, E.; Bartucci, S.; Poselle Bonaventura, C.; Conte, G.; Agostino, R.G.; Policicchio, A. Effects of Activation Temperature and Time on Porosity Features of Activated Carbons Derived from Lemon Peel and Preliminary Hydrogen Adsorption Tests. Colloids Surf. Physicochem. Eng. Asp. 2023, 672, 131727. [Google Scholar] [CrossRef]
- Khajonrit, J.; Sichumsaeng, T.; Kalawa, O.; Chaisit, S.; Chinnakorn, A.; Chanlek, N.; Maensiri, S. Mangosteen Peel-Derived Activated Carbon for Supercapacitors. Prog. Nat. Sci. Mater. Int. 2022, 32, 570–578. [Google Scholar] [CrossRef]
- Wang, J.; Li, X.; Fang, Y.; Huang, Q.; Wang, Y. Efficient Adsorption of Tetracycline from Aqueous Solution Using Copper and Zinc Oxides Modified Porous Boron Nitride Adsorbent. Colloids Surf. Physicochem. Eng. Asp. 2023, 666, 131372. [Google Scholar] [CrossRef]
- Santos, M.P.F.; Porfírio, M.C.P.; Junior, E.C.S.; Bonomo, R.C.F.; Veloso, C.M. Pepsin Immobilization: Influence of Carbon Support Functionalization. Int. J. Biol. Macromol. 2022, 203, 67–79. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, M.; Garmarudi, A.B.; Khanmohammadi, M.; Rouchi, M.B. Infrared Spectrometric Evaluation of Carbon Nanotube Sulfonation. Fuller. Nanotub. Carbon Nanostructures 2016, 24, 219–224. [Google Scholar] [CrossRef]
- Ghaedi, A.M.; Karamipour, S.; Vafaei, A.; Baneshi, M.M.; Kiarostami, V. Optimization and Modeling of Simultaneous Ultrasound-Assisted Adsorption of Ternary Dyes Using Copper Oxide Nanoparticles Immobilized on Activated Carbon Using Response Surface Methodology and Artificial Neural Network. Ultrason. Sonochem. 2019, 51, 264–280. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.G.; Veloso, C.M.; da Silva, N.M.; de Sousa, L.F.; Bonomo, R.C.F.; de Souza, A.O.; da Guarda Souza, M.O.; Fontan, R.D.C.I. Preparation of Activated Carbons from Cocoa Shells and Siriguela Seeds Using H3PO4 and ZnCL2 as Activating Agents for BSA and α-Lactalbumin Adsorption. Fuel Process. Technol. 2014, 126, 476–486. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, Q.; Hao, M.; Zhang, Y.; Ren, X.; Cao, F.; Zhang, L.; Sun, Q.; Wennersten, R. Effect of Functional Groups on VOCs Adsorption by Activated Carbon: DFT Study. Surf. Sci. 2023, 736, 122352. [Google Scholar] [CrossRef]
- Sue-aok, N.; Srithanratana, T.; Rangsriwatananon, K.; Hengrasmee, S. Study of Ethylene Adsorption on Zeolite NaY Modified with Group I Metal Ions. Appl. Surf. Sci. 2010, 256, 3997–4002. [Google Scholar] [CrossRef]
- Zhou, K.; Ma, W.; Zeng, Z.; Ma, X.; Xu, X.; Guo, Y.; Li, H.; Li, L. Experimental and DFT Study on the Adsorption of VOCs on Activated Carbon/Metal Oxides Composites. Chem. Eng. J. 2019, 372, 1122–1133. [Google Scholar] [CrossRef]
- Wang, S.-H.; Hwang, Y.-K.; Choi, S.W.; Yuan, X.; Lee, K.B.; Chang, F.-C. Developing Self-Activated Lignosulfonate-Based Porous Carbon Material for Ethylene Adsorption. J. Taiwan Inst. Chem. Eng. 2020, 115, 315–320. [Google Scholar] [CrossRef]
- OAC. Official Methods of Analysis of the Association of Official Analytical Chemists: Official Methods of Analysis of AOAC International, 21st ed.; AOAC: Washington, DC, USA, 2019. [Google Scholar]
- Rabemanolontsoa, H.; Ayada, S.; Saka, S. Quantitative Method Applicable for Various Biomass Species to Determine Their Chemical Composition. Biomass Bioenergy 2011, 35, 4630–4635. [Google Scholar] [CrossRef]
- Barrett, E.P.; Joyner, L.G.; Halenda, P.P. The Determination of Pore Volume and Area Distributions in Porous Substances. I. Computations from Nitrogen Isotherms. J. Am. Chem. Soc. 1951, 73, 373–380. [Google Scholar] [CrossRef]
- Kuśmierek, K.; Szala, M.; Świątkowski, A. Adsorption of 2,4-Dichlorophenol and 2,4-Dichlorophenoxyacetic Acid from Aqueous Solutions on Carbonaceous Materials Obtained by Combustion Synthesis. J. Taiwan Inst. Chem. Eng. 2016, 63, 371–378. [Google Scholar] [CrossRef]








| Samples | SBET [m2 g−1] | VT [cm3 g−1] | Vmeso [cm3 g−1] | Vmicro [cm3 g−1] | L0 [nm] | pHPZC |
|---|---|---|---|---|---|---|
| CB | 291 | 0.16 | 0.05 | 0.11 | 4.35 | 6.98 |
| ACB | 886 | 0.46 | 0.09 | 0.37 | 1.72 | 3.81 |
| ACB/CuO | 628 | 0.34 | 0.04 | 0.30 | 1.37 | 3.65 |
| ACB/O2 | 747 | 0.39 | 0.21 | 0.18 | 2.71 | 3.53 |
| Precursor | Adsorbents | SBET [m2 g−1] | References |
|---|---|---|---|
| Baru activated carbon | ACB/CuO | 628 | This work |
| Natural zeolite | NH4Z2-Cu | 351 | [5] |
| Commercial activated carbon | 10-CuO/AC-800 | 667 | [14] |
| Commercial activated carbon | CuO/AC08 | 947 | [13] |
| Porous boron nitride | BN-Cu | 626 | [34] |
| Samples | qmax [μg g−1] | KL [dm3 μg−1] | R2 |
|---|---|---|---|
| CB | 769 | 0.00314 | 0.98 |
| ACB | 1111 | 0.00143 | 0.98 |
| ACB/CuO | 1667 | 0.00191 | 0.98 |
| ACB/O2 | 588 | 0.00358 | 0.99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliveira, A.C.d.J.; Rodrigues, C.A.P.; de Almeida, M.C.; Mársico, E.T.; Scalize, P.S.; de Oliveira, T.F.; Solar, V.A.; Valdés, H. Ethylene Elimination Using Activated Carbons Obtained from Baru (Dipteryx alata vog.) Waste and Impregnated with Copper Oxide. Molecules 2024, 29, 2717. https://doi.org/10.3390/molecules29122717
Oliveira ACdJ, Rodrigues CAP, de Almeida MC, Mársico ET, Scalize PS, de Oliveira TF, Solar VA, Valdés H. Ethylene Elimination Using Activated Carbons Obtained from Baru (Dipteryx alata vog.) Waste and Impregnated with Copper Oxide. Molecules. 2024; 29(12):2717. https://doi.org/10.3390/molecules29122717
Chicago/Turabian StyleOliveira, Ana Carolina de Jesus, Camilla Alves Pereira Rodrigues, Maria Carolina de Almeida, Eliane Teixeira Mársico, Paulo Sérgio Scalize, Tatianne Ferreira de Oliveira, Victor Andrés Solar, and Héctor Valdés. 2024. "Ethylene Elimination Using Activated Carbons Obtained from Baru (Dipteryx alata vog.) Waste and Impregnated with Copper Oxide" Molecules 29, no. 12: 2717. https://doi.org/10.3390/molecules29122717
APA StyleOliveira, A. C. d. J., Rodrigues, C. A. P., de Almeida, M. C., Mársico, E. T., Scalize, P. S., de Oliveira, T. F., Solar, V. A., & Valdés, H. (2024). Ethylene Elimination Using Activated Carbons Obtained from Baru (Dipteryx alata vog.) Waste and Impregnated with Copper Oxide. Molecules, 29(12), 2717. https://doi.org/10.3390/molecules29122717











