Exploring the Potential of Nitrogen-Doped Graphene in ZnSe-TiO2 Composite Materials for Supercapacitor Electrode
Abstract
1. Introduction
2. Results and Discussions
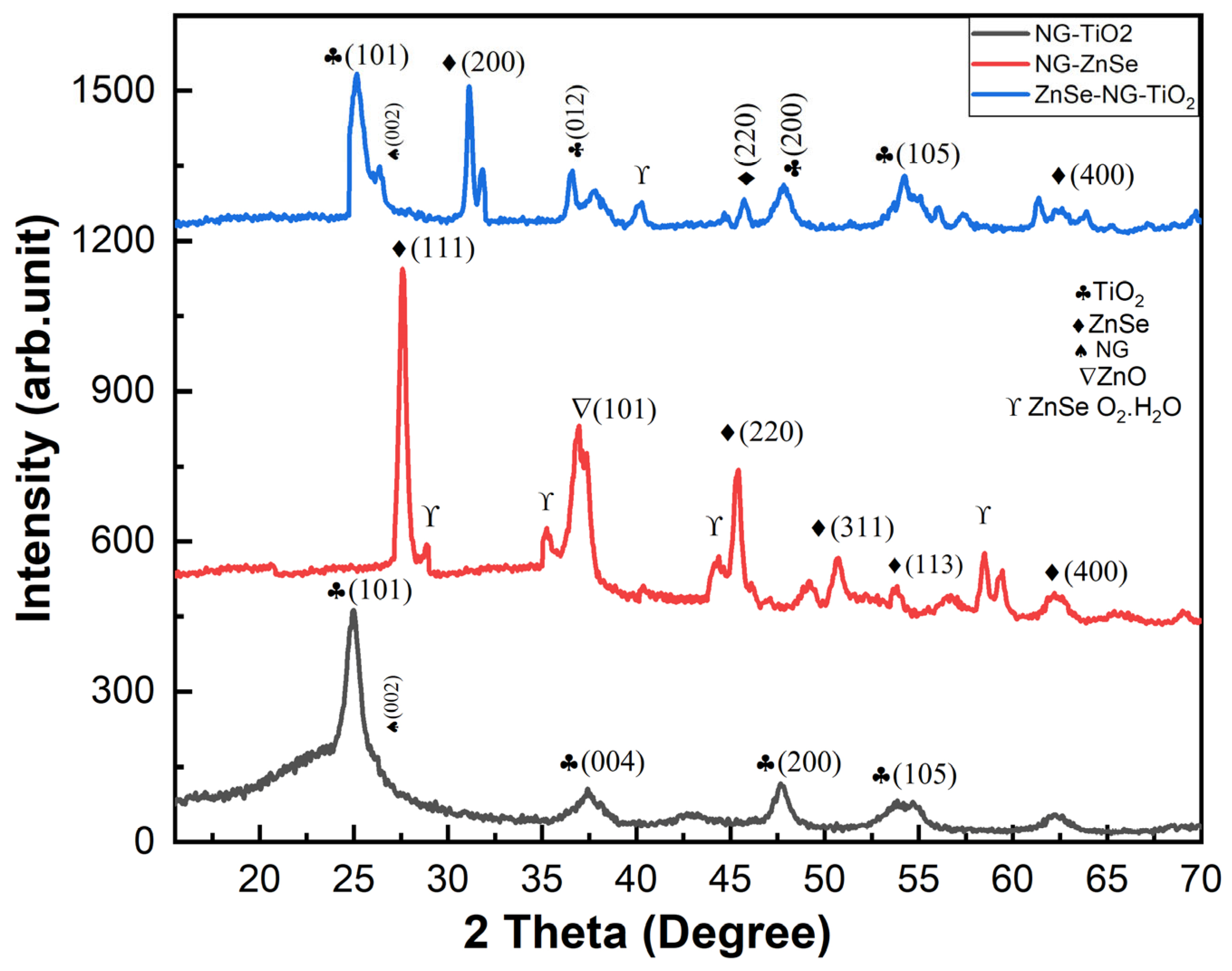
2.1. XRD Analysis
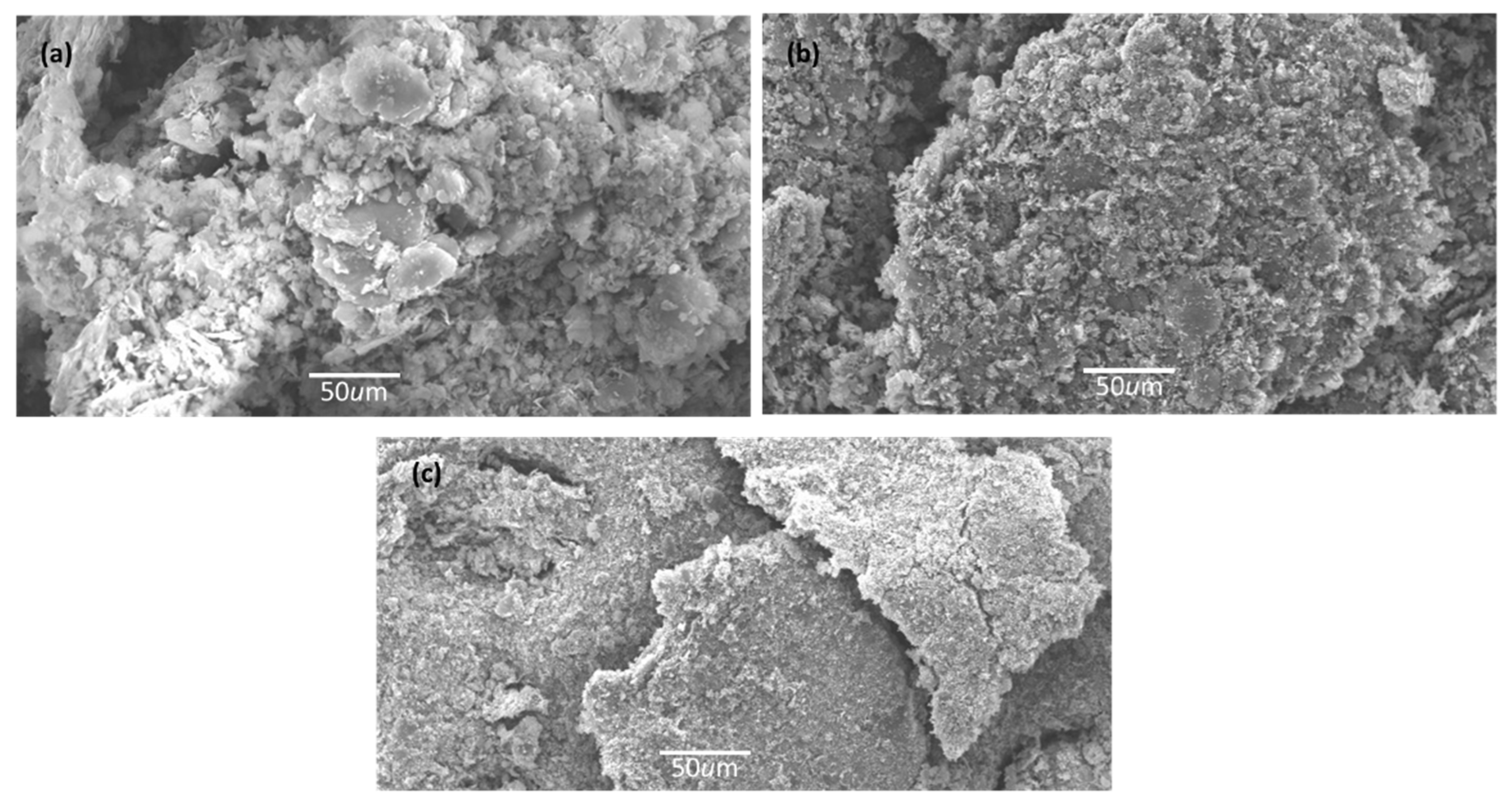
2.2. SEM
2.3. Raman Analysis
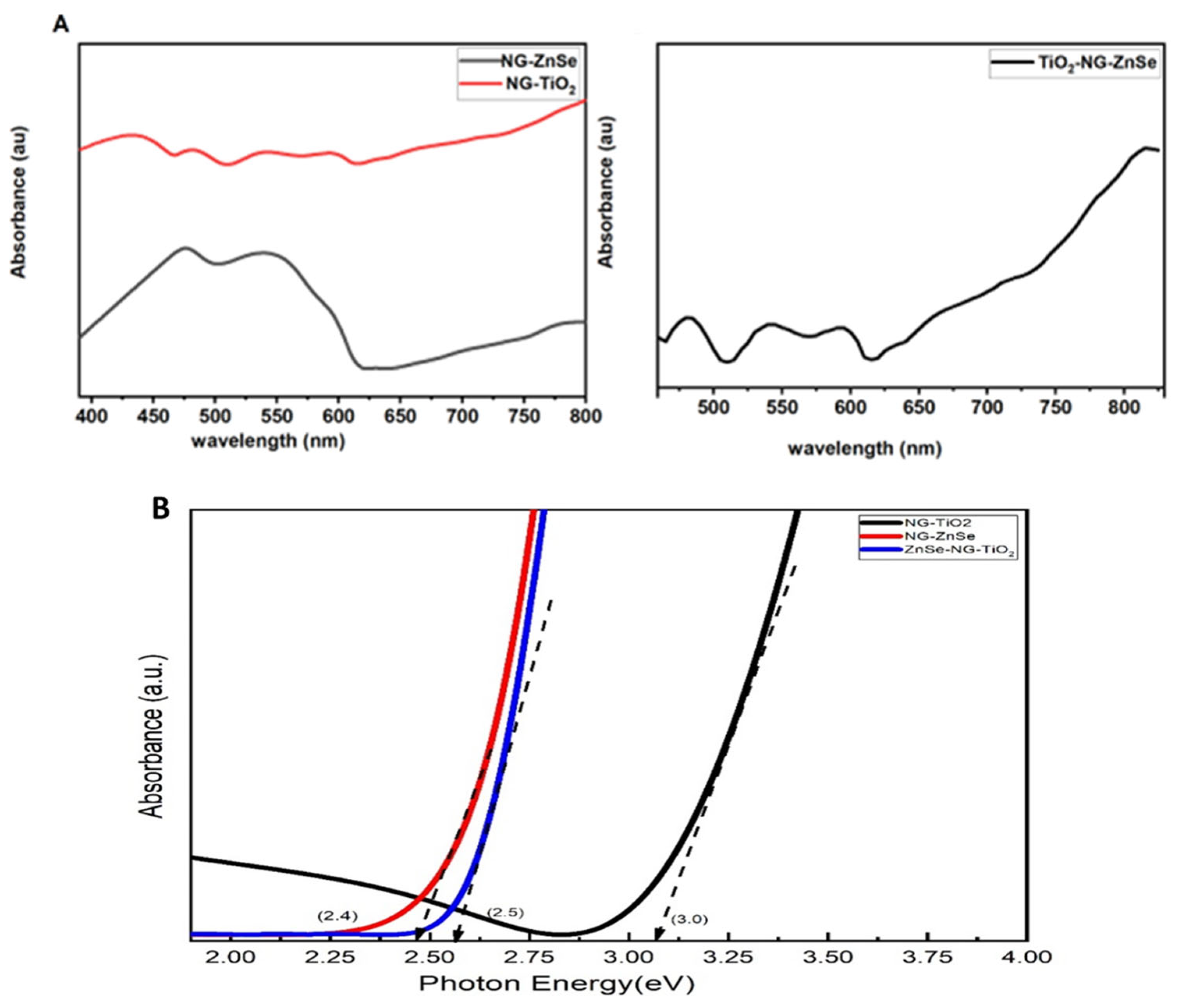
2.4. UV Analysis
3. Electrochemistry Performance
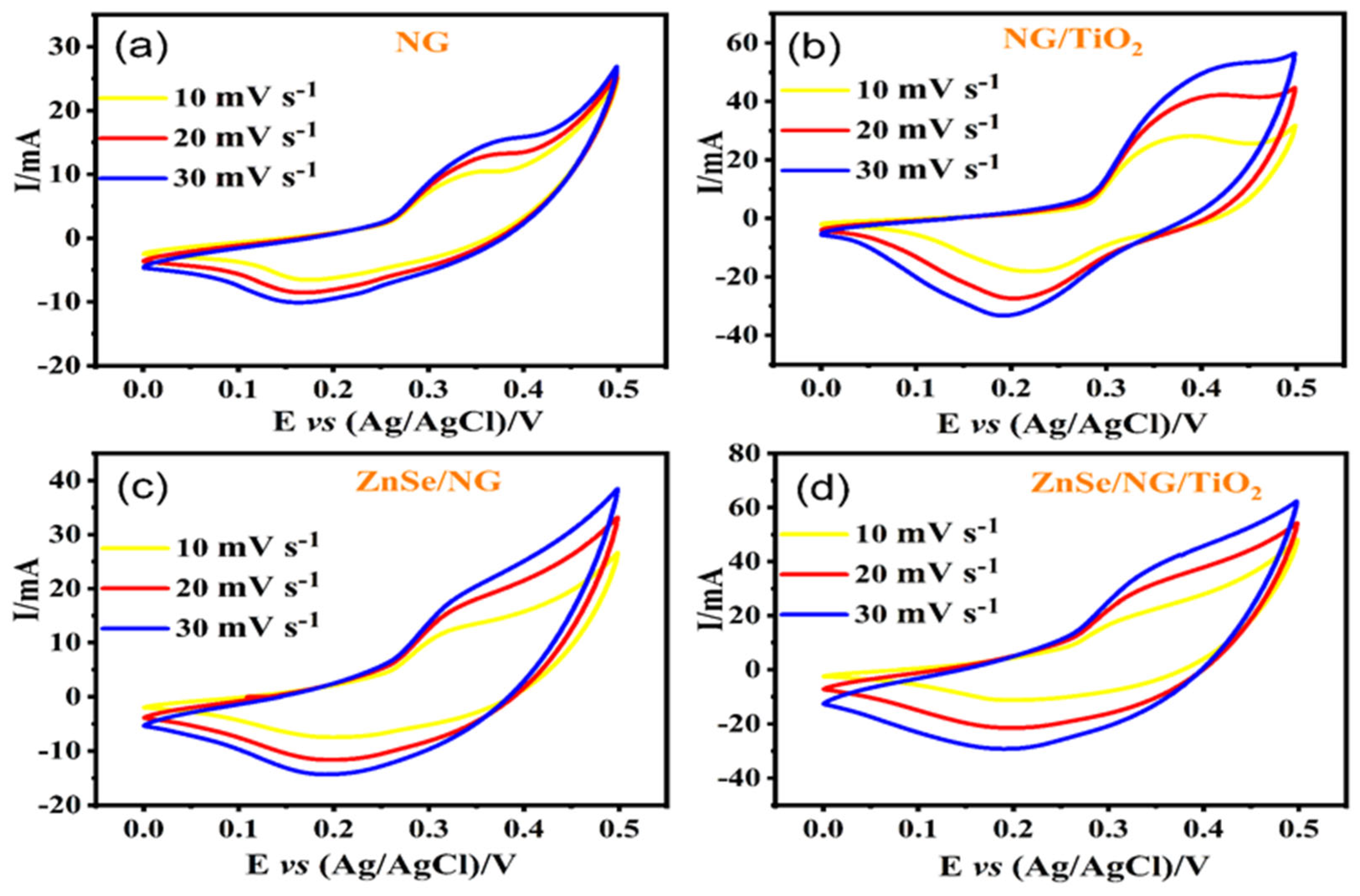
Cyclic Voltammetry
4. Experimental Work
4.1. Material Preparation
4.2. Electrode Preparation
4.3. Characterization
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tan, K.M.; Babu, T.S.; Ramachandaramurthy, V.K.; Kasinathan, P.; Solanki, S.G.; Raveendran, S.K. Empowering smart grid: A comprehensive review of energy storage technology and application with renewable energy integration. J. Energy Storage 2021, 39, 102591. [Google Scholar] [CrossRef]
- Farghali, M.; Osman, A.I.; Chen, Z.; Abdelhaleem, A.; Ihara, I.; Mohamed, I.M.; Yap, P.-S.; Rooney, D.W. Social, environmental, and economic consequences of integrating renewable energies in the electricity sector: A review. Environ. Chem. Lett. 2023, 21, 1381–1418. [Google Scholar] [CrossRef]
- Heard, B.P.; Brook, B.W.; Wigley, T.M.; Bradshaw, C.J. Burden of proof: A comprehensive review of the feasibility of 100% renewable-electricity systems. Renew. Sustain. Energy Rev. 2017, 76, 1122–1133. [Google Scholar] [CrossRef]
- Zheng, X.; Liu, C.; Yan, Y.; Wang, Q. A review of thermoelectrics research–Recent developments and potentials for sustainable and renewable energy applications. Renew. Sustain. Energy Rev. 2014, 32, 486–503. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, Z.; Liang, Q.; Gao, F.; Yi, F.; Ma, M.; Liao, Q.; Kang, Z.; Zhang, Y. Green hybrid power system based on triboelectric nanogenerator for wearable/portable electronics. Nano Energy 2019, 55, 151–163. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, L.; Zheng, Y.; Li, M.; Ding, B.; Diao, X.; Cheng, H.-M.; Tang, Y. On-demand engineerable visible spectrum by fine control of electrochemical reactions. Natl. Sci. Rev. 2024, 11, nwad323. [Google Scholar] [CrossRef] [PubMed]
- Khan, K.; Tareen, A.K.; Aslam, M.; Mahmood, A.; Zhang, Y.; Ouyang, Z.; Guo, Z.; Zhang, H. Going green with batteries and supercapacitor: Two dimensional materials and their nanocomposites based energy storage applications. Prog. Solid State Chem. 2020, 58, 100254. [Google Scholar] [CrossRef]
- Jiang, H.; Li, J.; Xie, Y.; Du, Y.; Zhao, J.; Mei, Y.; Xie, D. Rapid exfoliation and surface hydroxylation of high-quality boron nitride nanosheets enabling waterborne polyurethane with high thermal conductivity and flame retardancy. Adv. Compos. Hybrid Mater. 2024, 7, 8. [Google Scholar] [CrossRef]
- Zuo, W.; Li, R.; Zhou, C.; Li, Y.; Xia, J.; Liu, J. Battery-supercapacitor hybrid devices: Recent progress and future prospects. Adv. Sci. 2017, 4, 1600539. [Google Scholar] [CrossRef]
- Wang, M.; Jiang, C.; Zhang, S.; Song, X.; Tang, Y.; Cheng, H.-M. Reversible calcium alloying enables a practical room-temperature rechargeable calcium-ion battery with a high discharge voltage. Nat. Chem. 2018, 10, 667–672. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Wang, X.-X.; Jin, M.L.; He, P.; Zhang, S. Molecular level manipulation of charge density for solid-liquid TENG system by proton irradiation. Nano Energy 2022, 103, 107819. [Google Scholar] [CrossRef]
- Patel, K.K.; Singhal, T.; Pandey, V.; Sumangala, T.; Sreekanth, M. Evolution and recent developments of high performance electrode material for supercapacitors: A review. J. Energy Storage 2021, 44, 103366. [Google Scholar] [CrossRef]
- Zhang, S.; Pan, N. Supercapacitors performance evaluation. Adv. Energy Mater. 2015, 5, 1401401. [Google Scholar] [CrossRef]
- Cao, K.; Jiao, L.; Liu, Y.; Liu, H.; Wang, Y.; Yuan, H. Ultra-high capacity lithium-ion batteries with hierarchical CoO nanowire clusters as binder free electrodes. Adv. Funct. Mater. 2015, 25, 1082–1089. [Google Scholar] [CrossRef]
- Zhang, H.; Zhou, L.; Noonan, O.; Martin, D.J.; Whittaker, A.K.; Yu, C. Tailoring the void size of iron oxide@carbon yolk–shell structure for optimized lithium storage. Adv. Funct. Mater. 2014, 24, 4337–4342. [Google Scholar] [CrossRef]
- Zhang, G.; Hou, S.; Zhang, H.; Zeng, W.; Yan, F.; Li, C.C.; Duan, H. High-performance and ultra-stable lithium-ion batteries based on MOF-derived ZnO@ZnO quantum dots/C core-shell nanorod arrays on a carbon cloth anode. Adv. Mater. 2015, 27, 2400–2405. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Hua, C.; Li, B.; Fang, X.; Yao, C.; Zhang, Y.; Hu, Y.S.; Wang, Z.; Chen, L.; Zhao, D. Highly ordered mesoporous crystalline MoSe2 material with efficient visible-light-driven photocatalytic activity and enhanced lithium storage performance. Adv. Funct. Mater. 2013, 23, 1832–1838. [Google Scholar] [CrossRef]
- Sokolikova, M.S.; Mattevi, C. Direct synthesis of metastable phases of 2D transition metal dichalcogenides. Chem. Soc. Rev. 2020, 49, 3952–3980. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Oh, W.-C. Preparation of nanowire like WSe2-graphene nanocomposite for photocatalytic reduction of CO2 into CH3OH with the presence of sacrificial agents. Sci. Rep. 2017, 7, 1867. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, A.; Sudhaik, A.; Raizada, P.; Khan, A.A.P.; Singh, A.; Van Le, Q.; Nguyen, V.-H.; Ahamad, T.; Thakur, S.; Singh, P. Enhancement strategies for ZnSe based photocatalysts: Application to environmental remediation and energy conversion. Process Saf. Environ. Prot. 2023, 170, 415–435. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, B.; Ling, Z.; Liu, Q.; Fu, X.; Zhang, Y.; Zhang, R.; Hu, S.; Zhao, F.; Li, X. Advances in ionogels for proton-exchange membranes. Sci. Total Environ. 2024, 921, 171099. [Google Scholar] [CrossRef] [PubMed]
- Ye, B.; Cao, X.; Zhao, Q.; Wang, J. Coelectrodeposition of NiSe/ZnSe hybrid nanostructures as a battery-type electrode for an asymmetric supercapacitor. J. Phys. Chem. C 2020, 124, 21242–21249. [Google Scholar] [CrossRef]
- Hussain, M.; Alotaibi, B.; Alrowaily, A.W.; Alyousef, H.A.; Alotiby, M.F.; Abdullah, M.; Al-Sehemi, A.G.; Henaish, A.; Ahmad, Z.; Aman, S. Synthesis of high-performance supercapacitor electrode materials by hydrothermal route based on ZnSe/MnSe composite. J. Phys. Chem. Solids 2024, 188, 111919. [Google Scholar] [CrossRef]
- Zhu, Y.; Yao, J.; Yun, X.; Zhou, R.; Yang, F.; Guo, C.; Liu, X.; Gao, Z. One-step synthesis of CoSe modified ZnSe hybrid as a battery-type cathode material for supercapacitors with improved electrochemical performance. J. Mater. Sci. Mater. Electron. 2022, 33, 21075–21090. [Google Scholar] [CrossRef]
- Biel, B.; Blase, X.; Triozon, F.; Roche, S. Anomalous doping effects on charge transport in graphene nanoribbons. Phys. Rev. Lett. 2009, 102, 096803. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.-F.; Terakura, K.; Ozaki, T.; Ikeda, T.; Boero, M.; Oshima, M.; Ozaki, J.-i.; Miyata, S. First-principles calculation of the electronic properties of graphene clusters doped with nitrogen and boron: Analysis of catalytic activity for the oxygen reduction reaction. Phys. Rev. B 2009, 80, 235410. [Google Scholar] [CrossRef]
- Zhang, S.; Shao, Y.; Liao, H.; Engelhard, M.H.; Yin, G.; Lin, Y. Polyelectrolyte-induced reduction of exfoliated graphite oxide: A facile route to synthesis of soluble graphene nanosheets. ACS Nano 2011, 5, 1785–1791. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Yuwen, L.; Han, Y.; Tian, J.; Zhu, X.; Weng, L.; Wang, L. Reduced graphene oxide/PAMAM–silver nanoparticles nanocomposite modified electrode for direct electrochemistry of glucose oxidase and glucose sensing. Biosens. Bioelectron. 2012, 36, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Becton, M.; Zhang, L.; Wang, X. Effects of surface dopants on graphene folding by molecular simulations. Chem. Phys. Lett. 2013, 584, 135–141. [Google Scholar] [CrossRef]
- Wang, W.-N.; Jiang, Y.; Biswas, P. Evaporation-induced crumpling of graphene oxide nanosheets in aerosolized droplets: Confinement force relationship. J. Phys. Chem. Lett. 2012, 3, 3228–3233. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Oh, W.-C. A simple ultrasono-synthetic route of PbSe-graphene-TiO2 ternary composites to improve the photocatalytic reduction of CO2. Fuller. Nanotub. Carbon Nanostructures 2017, 25, 449–458. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, W.; Zhang, F.; Lee, C.-S.; Tang, Y. Anion-hosting cathodes for current and late-stage dual-ion batteries. Sci. China Chem. 2024, 1–25. [Google Scholar] [CrossRef]
- Zare, Y.; Rhee, K.Y.; Hui, D. Influences of nanoparticles aggregation/agglomeration on the interfacial/interphase and tensile properties of nanocomposites. Compos. Part B Eng. 2017, 122, 41–46. [Google Scholar] [CrossRef]
- Ali, A.; Cung Tien Nguyen, D.; Cho, K.-Y.; Oh, W.-C. A simple ultrasonic-synthetic route of Cu2Se-graphene-TiO2 ternary composites for carbon dioxide conversion processes. Fuller. Nanotub. Carbon Nanostruct. 2018, 26, 827–836. [Google Scholar] [CrossRef]
- Su, Y.; Shang, J.; Liu, X.; Li, J.; Pan, Q.; Tang, Y. Constructing π-π Superposition Effect of Tetralithium Naphthalenetetracarboxylate with Electron Delocalization for Robust Dual-ion Batteries. Angew. Chem. Int. Ed. 2024, e202403775. [Google Scholar] [CrossRef] [PubMed]
- Pol, S.V.; Pol, V.G.; Gedanken, A. Encapsulating ZnS and ZnSe nanocrystals in the carbon shell: A RAPET approach. J. Phys. Chem. C 2007, 111, 13309–13314. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, H.; Xie, X.; Zhang, F.; Li, L. Preparation and photocatalytic activity of hollow ZnSe microspheres via Ostwald ripening. J. Alloys Compd. 2009, 473, 65–70. [Google Scholar] [CrossRef]
- Guo, J.; He, B.; Han, Y.; Liu, H.; Han, J.; Ma, X.; Wang, J.; Gao, W.; Lü, W. Resurrected and Tunable Conductivity and Ferromagnetism in the Secondary Growth La0.7Ca0.3MnO3 on Transferred SrTiO3 Membranes. Nano Lett. 2024, 24, 1114–1121. [Google Scholar] [CrossRef] [PubMed]
- Mehraeen, S.; Taşdemir, A.; Gürsel, S.A.; Yürüm, A. Homogeneous growth of TiO2-based nanotubes on nitrogen-doped reduced graphene oxide and its enhanced performance as a Li-ion battery anode. Nanotechnology 2018, 29, 255402. [Google Scholar] [CrossRef] [PubMed]
- An, D.; Chen, H.; He, R.; Chen, J.; Liu, C.; Sun, Z.; Yu, H.; Liu, Y.; Wong, C.; Feng, W. MOF decorated boron nitride/natural rubber composites with heterostructure for thermal management application through dual passive cooling modes base on the improved thermal conductivity and water sorption-desorption process. Compos. Sci. Technol. 2024, 248, 110469. [Google Scholar] [CrossRef]
- Błoński, P.; Tucek, J.; Sofer, Z.; Mazanek, V.; Petr, M.; Pumera, M.; Otyepka, M.; Zboril, R. Doping with graphitic nitrogen triggers ferromagnetism in graphene. J. Am. Chem. Soc. 2017, 139, 3171–3180. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.-M.; Hsu, C.-Y.; Chen, D.-H. Sodium tungsten oxide nanowires-based all-solid-state flexible transparent supercapacitors with solar thermal enhanced performance. Chem. Eng. J. 2022, 431, 134086. [Google Scholar] [CrossRef]
- Zhang, X.; Tang, Y.; Zhang, F.; Lee, C.-S. A novel aluminum–graphite dual-ion battery. Adv. Energy Mater. 2016, 6, 1502588. [Google Scholar] [CrossRef]
- Xiang, J.; Liu, J.; Yang, W.; Wei, X.; Li, P.; Chen, Z.; Zheng, Y.; Huang, J. Development of novel thermal diode based on improved check valve and modified wick structure. Int. J. Therm. Sci. 2024, 200, 108977. [Google Scholar] [CrossRef]
- Hoffmann, M.R.; Martin, S.T.; Choi, W.; Bahnemann, D.W. Environmental applications of semiconductor photocatalysis. Chem. Rev. 1995, 95, 69–96. [Google Scholar] [CrossRef]
- Ali, A.; Oh, W.-C.; Ali, A.; Oh, W.-C. Preparation of Ag2Se-Graphene-TiO2 Nanocomposite and its Photocatalytic Degradation (Rh B). J. Korean Ceram. Soc. 2017, 54, 388–394. [Google Scholar] [CrossRef]
- Deshpande, M.; Chaki, S.; Patel, N.; Bhatt, S.; Soni, B. Study on nanoparticles of ZnSe synthesized by chemical method and their characterization. J. Nano-Electron. Phys. 2011, 3, 193. [Google Scholar]
- Bhattacharya, A.; Mukherjee, A.; Roy, A.; Chattopadhyay, S. Co-deposition of CuO:ZnO Nanocomposite on n-Type Si Substrate by Chemical Bath Deposition (CBD) Technique for Photovoltaic Application. J. Electron. Mater. 2024, 1–13. [Google Scholar] [CrossRef]
- Namsheer, K.; Rout, C.S. Photo-powered integrated supercapacitors: A review on recent developments, challenges and future perspectives. J. Mater. Chem. A 2021, 9, 8248–8278. [Google Scholar]
- Anjum, F.; Ahmad, R.; Afzal, N.; Murtaza, G. Characterization of InN films prepared using magnetron sputtering at variable power. Mater. Lett. 2018, 219, 23–28. [Google Scholar] [CrossRef]
- Chen, L.; Zhao, Y.; Li, M.; Li, L.; Hou, L.; Hou, H. Reinforced AZ91D magnesium alloy with thixomolding process facilitated dispersion of graphene nanoplatelets and enhanced interfacial interactions. Mater. Sci. Eng. A 2021, 804, 140793. [Google Scholar] [CrossRef]
- Jadhav, H.S.; Roy, A.; Chung, W.-J.; Seo, J.G. Growth of urchin-like ZnCo2O4 microspheres on nickel foam as a binder-free electrode for high-performance supercapacitor and methanol electro-oxidation. Electrochim. Acta 2017, 246, 941–950. [Google Scholar] [CrossRef]
- Omar, F.S.; Numan, A.; Duraisamy, N.; Ramly, M.M.; Ramesh, K.; Ramesh, S. Binary composite of polyaniline/copper cobaltite for high performance asymmetric supercapacitor application. Electrochim. Acta 2017, 227, 41–48. [Google Scholar] [CrossRef]
- Iqbal, M.Z.; Faisal, M.M.; Sulman, M.; Ali, S.R.; Alzaid, M. Facile synthesis of strontium oxide/polyaniline/graphene composite for the high-performance supercapattery devices. J. Electroanal. Chem. 2020, 879, 114812. [Google Scholar] [CrossRef]
- Iqbal, M.Z.; Faisal, M.M.; Ali, S.R.; Afzal, A.M.; Karim, M.R.A.; Kamran, M.A.; Alharbi, T. Strontium phosphide-polyaniline composites for high performance supercapattery devices. Ceram. Int. 2020, 46, 10203–10214. [Google Scholar] [CrossRef]
- Forghani, M.; Donne, S.W. Method comparison for deconvoluting capacitive and pseudo-capacitive contributions to electrochemical capacitor electrode behavior. J. Electrochem. Soc. 2018, 165, A664. [Google Scholar] [CrossRef]
- Iqbal, M.Z.; Faisal, M.M.; Ali, S.R.; Farid, S.; Afzal, A.M. Co-MOF/polyaniline-based electrode material for high performance supercapattery devices. Electrochim. Acta 2020, 346, 136039. [Google Scholar] [CrossRef]
- Iqbal, M.Z.; Faisal, M.M.; Sulman, M.; Ali, S.R.; Afzal, A.M.; Kamran, M.A.; Alharbi, T. Capacitive and diffusive contribution in strontium phosphide-polyaniline based supercapattery. J. Energy Storage 2020, 29, 101324. [Google Scholar] [CrossRef]
- Zhu, T.; Pan, J.; An, Z.; Zhe, R.; Ou, Q.; Wang, H.-E. Bifunctional NiCuOx photoelectrodes to promote pseudocapacitive charge storage by in situ photocharging. J. Mater. Chem. A 2022, 10, 20375–20385. [Google Scholar] [CrossRef]
- Kim, J.H.; Koo, S.-J.; Cheon, J.Y.; Jung, Y.; Cho, S.; Lee, D.; Choi, J.W.; Kim, T.; Song, M. Self-powered and flexible integrated solid-state fiber-shaped energy conversion and storage based on CNT yarn with efficiency of 5.5%. Nano Energy 2022, 96, 107054. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, H.; Tang, R.; Wang, X.; Wu, Y.; Yan, S.; Zhang, Y. Photo-assisted asymmetric supercapacitors based on dual photoelectrodes for enhanced photoelectric energy storage. J. Mater. Chem. A 2023, 11, 15844–15854. [Google Scholar] [CrossRef]
- Du, Y.; Liu, X.; Chen, L.; Yin, S.; Xie, Y.; Li, A.; Liang, X.; Luo, Y.; Wu, F.; Mei, Y. 3D hierarchical fireproof gel polymer electrolyte towards high-performance and comprehensive safety lithium-ion batteries. Chem. Eng. J. 2023, 476, 146605. [Google Scholar] [CrossRef]
- Iqbal, M.; Saykar, N.G.; Arya, A.; Banerjee, I.; Alegaonkar, P.S.; Mahapatra, S.K. High-performance supercapacitor based on MoS2@TiO2 composite for wide range temperature application. J. Alloys Compd. 2021, 883, 160705. [Google Scholar] [CrossRef]
- Zea, H.; Lester, K.; Datye, A.K.; Rightor, E.; Gulotty, R.; Waterman, W.; Smith, M. The influence of Pd–Ag catalyst restructuring on the activation energy for ethylene hydrogenation in ethylene–acetylene mixtures. Appl. Catal. A Gen. 2005, 282, 237–245. [Google Scholar] [CrossRef]
- Neghmouche, N.; Lanez, T. Calculation of electrochemical parameters starting from the polarization curves of ferrocene at glassy carbon electrode. Int. Lett. Chem. Phys. Astron. 2013, 4, 37–45. [Google Scholar] [CrossRef]
- Zhu, T.; He, Z.; An, Z.; Xu, R.; Li, Y.; Zhe, R.; Wang, H.-E.; Pang, H. Enhancing solar energy harvest by Cu2S/CuCl heteroarrays with enriched sulfur vacancies for photo-rechargeable pseudocapacitors. Sci. China Mater. 2023, 66, 2216–2226. [Google Scholar] [CrossRef]
- Chen, P.; Xiao, T.-Y.; Li, H.-H.; Yang, J.-J.; Wang, Z.; Yao, H.-B.; Yu, S.-H. Nitrogen-doped graphene/ZnSe nanocomposites: Hydrothermal synthesis and their enhanced electrochemical and photocatalytic activities. ACS Nano 2012, 6, 712–719. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Biswas, M.R.U.D.; Oh, W.-C. Novel and simple process for the photocatalytic reduction of CO2 with ternary Bi2O3–graphene–ZnO nanocomposite. J. Mater. Sci. Mater. Electron. 2018, 29, 10222–10233. [Google Scholar] [CrossRef]
- Han, X.; Zhao, C.; Wang, S.; Pan, Z.; Jiang, Z.; Tang, X. Multifunctional TiO2/C nanosheets derived from 3D metal–organic frameworks for mild-temperature-photothermal-sonodynamic-chemodynamic therapy under photoacoustic image guidance. J. Colloid Interface Sci. 2022, 621, 360–373. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akbar, H.; Ali, A.; Mohammad, S.; Anjum, F.; Ahmad, A.; Afzal, A.M.; Albaqami, M.D.; Mohammad, S.; Choi, J.R. Exploring the Potential of Nitrogen-Doped Graphene in ZnSe-TiO2 Composite Materials for Supercapacitor Electrode. Molecules 2024, 29, 2103. https://doi.org/10.3390/molecules29092103
Akbar H, Ali A, Mohammad S, Anjum F, Ahmad A, Afzal AM, Albaqami MD, Mohammad S, Choi JR. Exploring the Potential of Nitrogen-Doped Graphene in ZnSe-TiO2 Composite Materials for Supercapacitor Electrode. Molecules. 2024; 29(9):2103. https://doi.org/10.3390/molecules29092103
Chicago/Turabian StyleAkbar, Hassan, Asghar Ali, Shoaib Mohammad, Faiza Anjum, Ashfaq Ahmad, Amir Muhammad Afzal, Munirah D. Albaqami, Saikh Mohammad, and Jeong Ryeol Choi. 2024. "Exploring the Potential of Nitrogen-Doped Graphene in ZnSe-TiO2 Composite Materials for Supercapacitor Electrode" Molecules 29, no. 9: 2103. https://doi.org/10.3390/molecules29092103
APA StyleAkbar, H., Ali, A., Mohammad, S., Anjum, F., Ahmad, A., Afzal, A. M., Albaqami, M. D., Mohammad, S., & Choi, J. R. (2024). Exploring the Potential of Nitrogen-Doped Graphene in ZnSe-TiO2 Composite Materials for Supercapacitor Electrode. Molecules, 29(9), 2103. https://doi.org/10.3390/molecules29092103






