Voltammetric Sensing of Chloride Based on a Redox-Active Complex: A Terpyridine-Co(II)-Dipyrromethene Functionalized Anion Receptor Deposited on a Gold Electrode
Abstract
1. Introduction
2. Results and Discussion
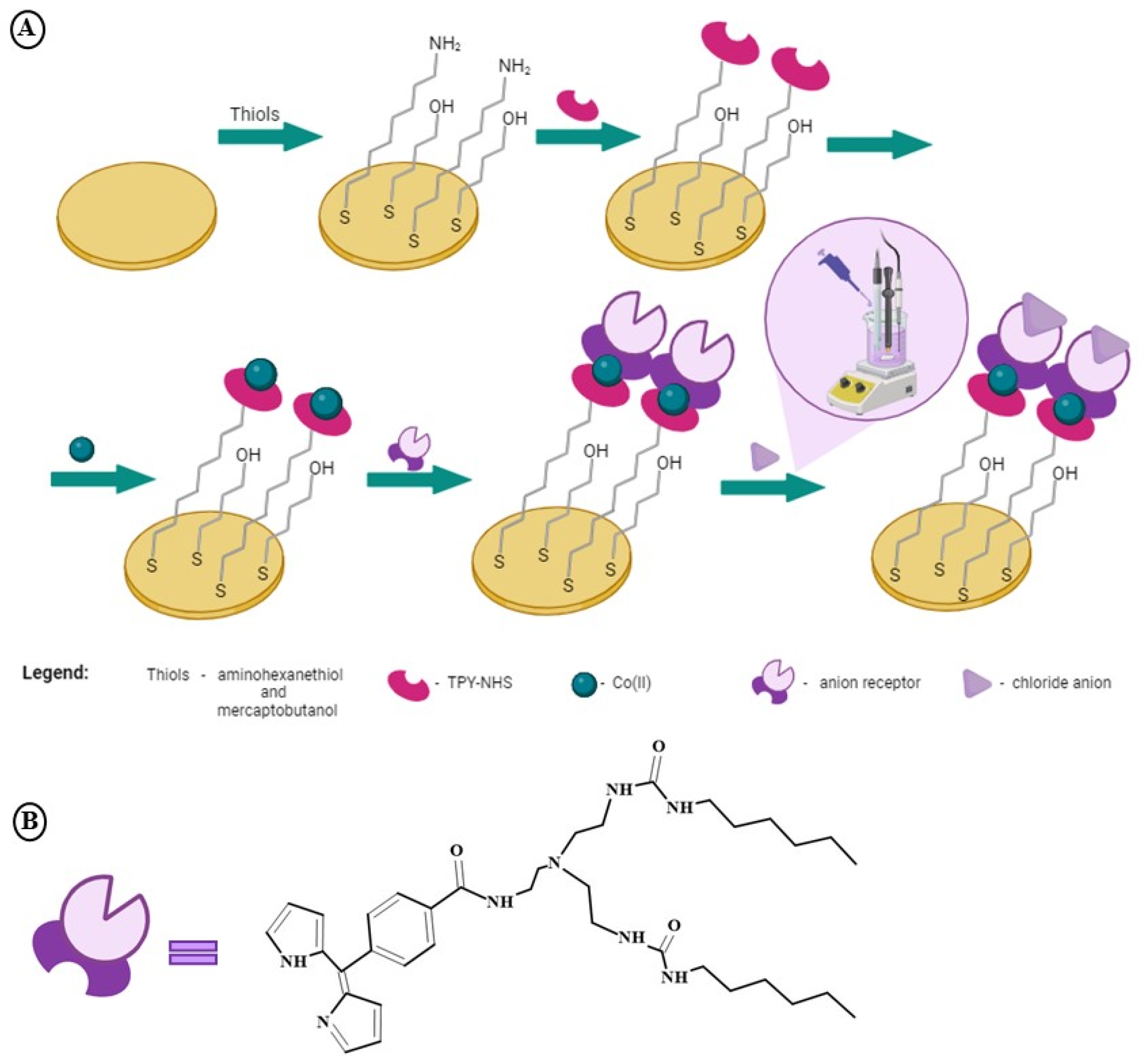
2.1. The Procedure of Au/MBL, AHT/TPY/Co(II)/DPM-AR Layer Preparation
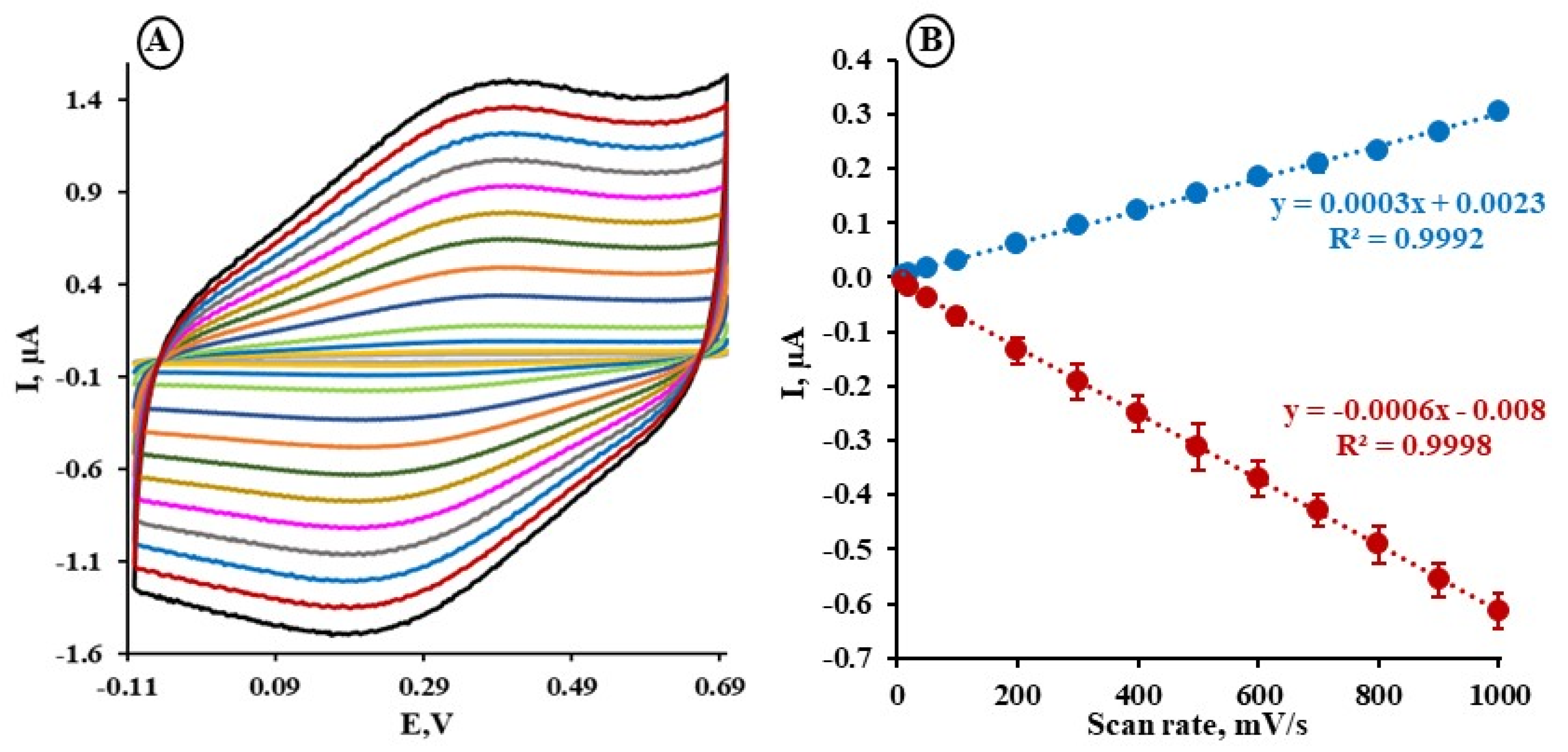
2.2. The Electrochemical Characterization of the Au/MBL, AHT/TPY/Co(II)/DPM-AR Layer
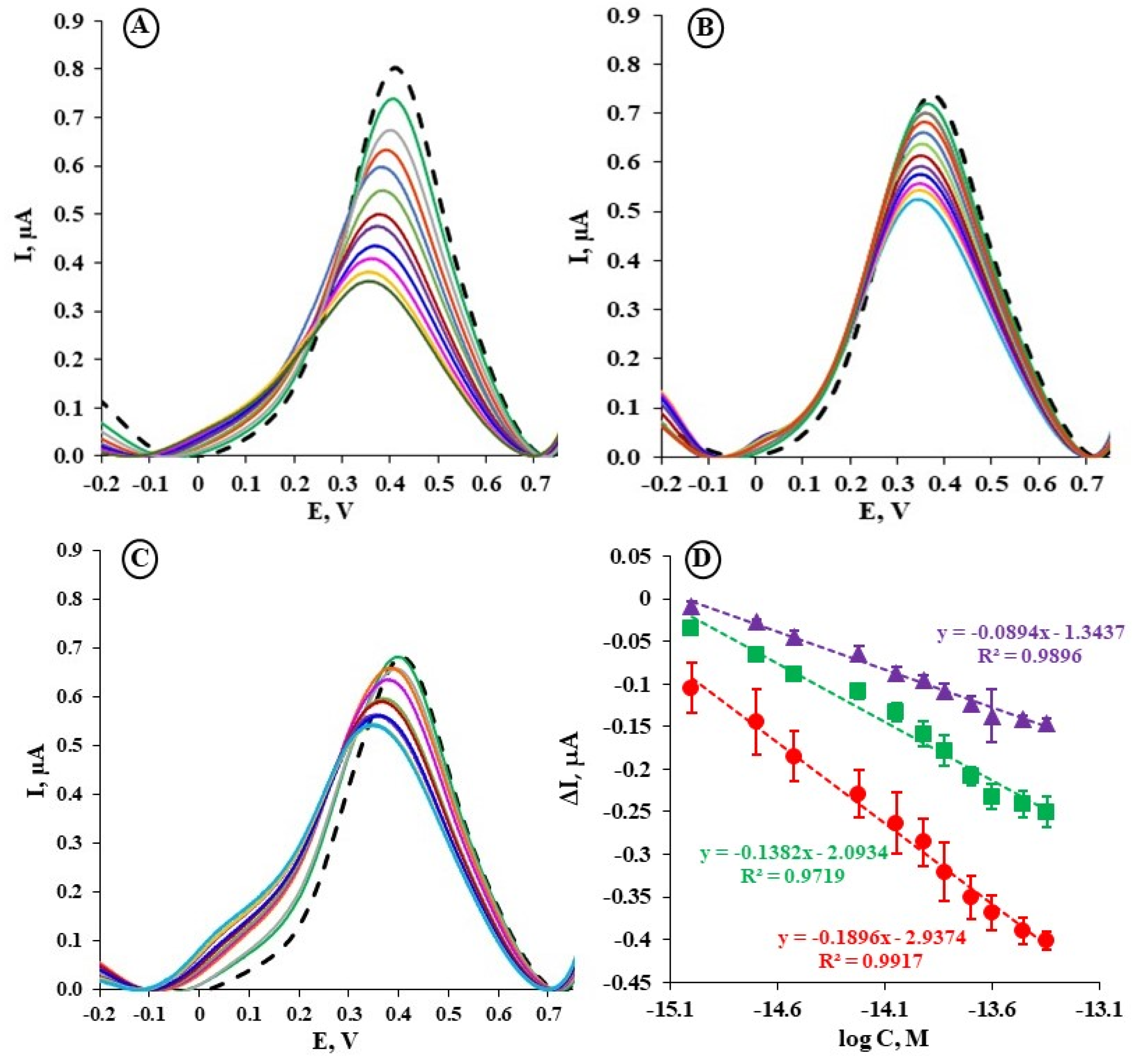
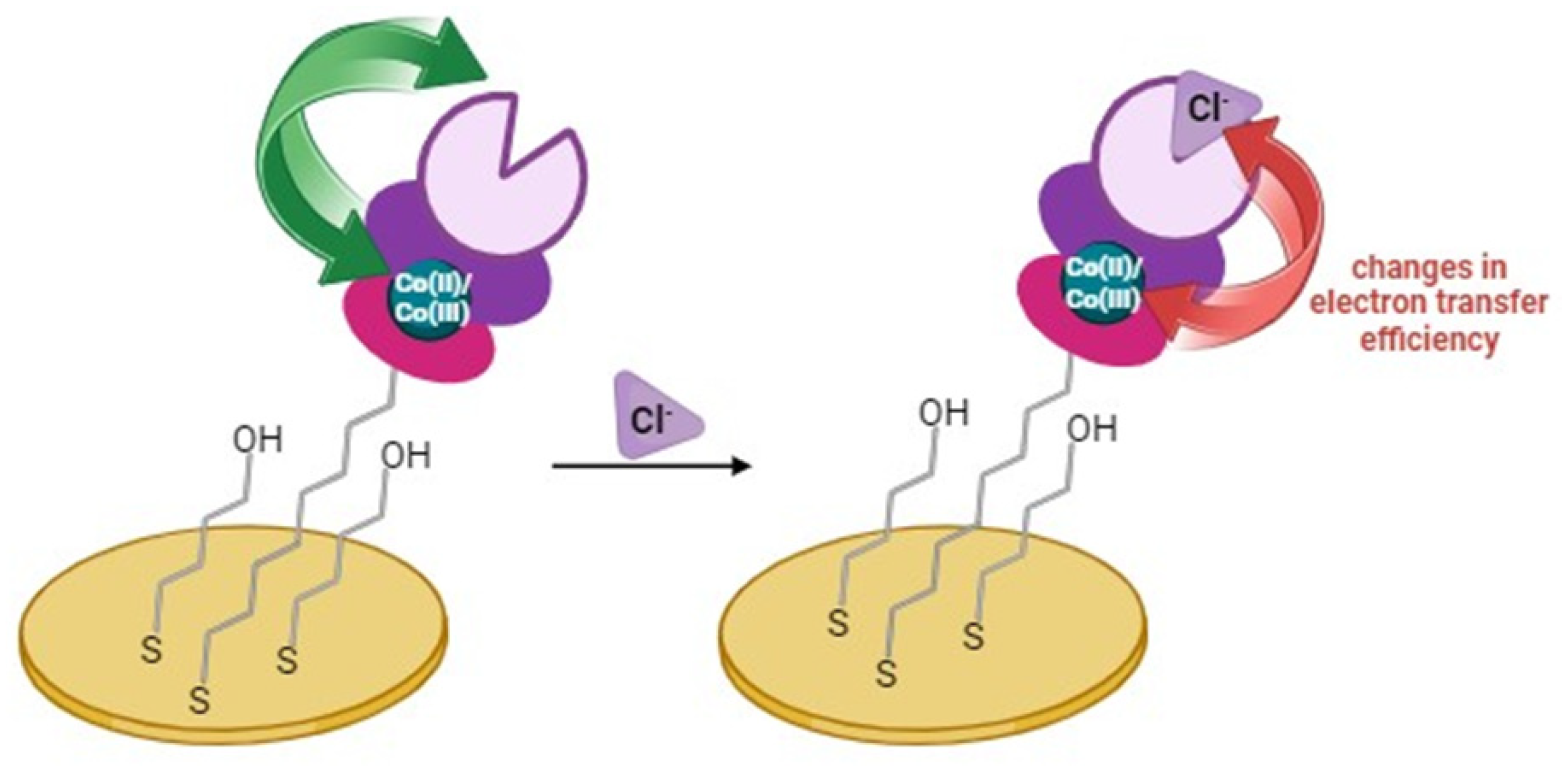
2.3. The Electrochemical Detection of Chloride Using the Au/MBL, AHT/TPY/Co(II)/DPM-AR layer
2.4. The Repeatability, Reproducibility and Stability of the Sensing Layer
3. Materials and Methods
3.1. Materials
3.2. Electrochemical Measurements
3.3. Fabrication of Sensing Layer for Detection of Chloride
- The gold electrodes were immersed in a mixture of 0.01 mM AHT and 1 mM MBL—3 h at room temperature (RT), DCM:MeOH (1:1, v/v); after modification, electrodes were carefully rinsed with a mixture of DCM:MeOH;
- The reaction between the amine groups of AHT and NHS of 0.1 mM TPY-NHS during 1 h, RT, DCM:MeOH (1:1); after this step, electrodes were carefully washed with a mixture of DCM:MeOH;
- The complexation of Co(II) ions was achieved by immersing electrodes in 1 mM of Co(OAc)2—1 h, RT, DCM:MeOH (1:1); after modification, electrodes were carefully rinsed with a mixture of DCM:MeOH;
- The coordination sphere of the Co(II) metal ions was closed by 0.1 mM DPM-AR—1 h, RT, DCM-MeOH (1:1); after modification, electrodes were carefully washed with: a mixture of DCM:MeOH, MeOH, Milli-Q water, and 0.1 M NaNO3 + 0.01 M H3BO3 pH 4.0 buffer and stored for approximately 36 h in the same buffer at 4 °C.
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Beer, P.D.; Gale, P.A. Anion Recognition and Sensing: The State of the Art and Future Perspectives. Angew. Chem. Int. Ed. 2001, 40, 486–516. [Google Scholar] [CrossRef]
- Kubik, S. Anion recognition in water. Chem. Soc. Rev. 2010, 39, 3648–3663. [Google Scholar] [CrossRef]
- Langton, M.J.; Serpell, C.J.; Beer, P.D. Anion Recognition in Water: Recent Advances from a Supramolecular and Macromolecular Perspective. Angew. Chem. Int. Ed. 2016, 55, 1974–1987. [Google Scholar] [CrossRef]
- Sessler, J.L.; Gale, P.; Cho, W.-S. Receptors for Ion-Pairs. In Anion Receptor Chemistry; Sessler, J.L., Gale, P.A., Cho, W.-S., Rowan, S.J., Aida, T., Rowan, A.E., Stoddart, J.F., Eds.; The Royal Society of Chemistry: London, UK, 2006. [Google Scholar]
- Pal, A.; Karmakar, M.; Bhatta, S.R.; Thakur, A. A detailed insight into anion sensing based on intramolecular charge transfer (ICT) mechanism: A comprehensive review of the years 2016 to 2021. Coord. Chem. Rev. 2021, 448, 214167. [Google Scholar] [CrossRef]
- McNaughton, D.A.; Fares, M.; Picci, G.; Gale, P.A.; Caltagirone, C. Advances in fluorescent and colorimetric sensors for anionic species. Coord. Chem. Rev. 2021, 427, 213573. [Google Scholar] [CrossRef]
- Hein, R.; Beer, P.D.; Davis, J.J. Electrochemical Anion Sensing: Supramolecular Approaches. Chem. Rev. 2020, 120, 1888–1935. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Zheng, Y.; Wang, E.; Yang, T.; Wang, H.; Hou, X. Ti3C2Tx (MXene)/Pt nanoparticle electrode for the accurate detection of DA coexisting with AA and UA. Dalton Trans. 2022, 51, 4549–4559. [Google Scholar] [CrossRef]
- Jia, D.; Yang, T.; Wang, K.; Zhou, L.; Wang, E.; Chou, K.-C.; Wang, H.; Hou, X. Facile in-situ synthesis of Ti3C2Tx/TiO2 nanowires toward simultaneous determination of ascorbic acid, dopamine and uric acid. J. Alloys Compd. 2024, 985, 173392. [Google Scholar] [CrossRef]
- Li, G.; Qi, X.; Wu, J.; Wan, X.; Wang, T.; Liu, Y.; Chen, Y.; Xia, Y. Highly stable electrochemical sensing platform for the selective determination of pefloxacin in food samples based on a molecularly imprinted-polymer-coated gold nanoparticle/black phosphorus nanocomposite. Food Chem. 2024, 436, 137753. [Google Scholar] [CrossRef]
- Li, G.; Wu, J.; Qi, X.; Wan, X.; Liu, Y.; Chen, Y.; Xu, L. Molecularly imprinted polypyrrole film-coated poly(3,4-ethylenedioxythiophene):polystyrene sulfonate-functionalized black phosphorene for the selective and robust detection of norfloxacin. Mater. Today Chem. 2022, 26, 101043. [Google Scholar] [CrossRef]
- Wan, X.; Du, H.; Tuo, D.; Qi, X.; Wang, T.; Wu, J.; Li, G. UiO-66/Carboxylated Multiwalled Carbon Nanotube Composites for Highly Efficient and Stable Voltammetric Sensors for Gatifloxacin. ACS Appl. Nano Mater. 2023, 6, 19403–19413. [Google Scholar] [CrossRef]
- Zhao, J.; Yang, D.; Yang, X.-J.; Wu, B. Anion coordination chemistry: From recognition to supramolecular assembly. Coord. Chem. Rev. 2019, 378, 415–444. [Google Scholar] [CrossRef]
- Khurana, R.; Alami, F.; Nijhuis, C.A.; Keinan, E.; Huskens, J.; Reany, O. Selective Perchlorate Sensing Using Electrochemical Impedance Spectroscopy with Self-Assembled Monolayers of semiaza-Bambusurils. Chem. A Eur. J. 2024, 30, e202302968. [Google Scholar] [CrossRef] [PubMed]
- Hein, R.; Li, X.; Beer, P.D.; Davis, J.J. Enhanced voltammetric anion sensing at halogen and hydrogen bonding ferrocenyl SAMs. Chem. Sci. 2021, 12, 2433–2440. [Google Scholar] [CrossRef] [PubMed]
- Beer, P.D.; Gale, P.A.; Chen, G.Z. Mechanisms of electrochemical recognition of cations, anions and neutral guest species by redox-active receptor molecules. Coord. Chem. Rev. 1999, 185–186, 3–36. [Google Scholar] [CrossRef]
- Ke, X. Micro-fabricated electrochemical chloride ion sensors: From the present to the future. Talanta 2020, 211, 120734. [Google Scholar] [CrossRef] [PubMed]
- Patella, B.; Aiello, G.; Drago, G.; Torino, C.; Vilasi, A.; O‘Riordan, A.; Inguanta, R. Electrochemical detection of chloride ions using Ag-based electrodes obtained from compact disc. Anal. Chim. Acta 2022, 1190, 339215. [Google Scholar] [CrossRef] [PubMed]
- Kwak, B.; Park, S.; Lee, H.S.; Kim, J.; Yoo, B. Improved Chloride Ion Sensing Performance of Flexible Ag-NPs/AgCl Electrode Sensor Using Cu-BTC as an Effective Adsorption Layer. Front. Chem. 2019, 7, 637. [Google Scholar] [CrossRef] [PubMed]
- Bin, Q.; Wang, M.; Wang, L. Ag nanoparticles decorated into metal-organic framework (Ag NPs/ZIF-8) for electrochemical sensing of chloride ion. Nanotechnology 2020, 31, 125601. [Google Scholar] [CrossRef]
- Bujes-Garrido, J.; Arcos-Martínez, M.J. Disposable sensor for electrochemical determination of chloride ions. Talanta 2016, 155, 153–157. [Google Scholar] [CrossRef]
- Bujes-Garrido, J.; Arcos-Martínez, M.J. Development of a wearable electrochemical sensor for voltammetric determination of chloride ions. Sens. Actuators B Chem. 2017, 240, 224–228. [Google Scholar] [CrossRef]
- Kaur, B.; Erdmann, C.A.; Daniëls, M.; Dehaen, W.; Rafiński, Z.; Radecka, H.; Radecki, J. Highly Sensitive Electrochemical Sensor for the Detection of Anions in Water Based on a Redox-Active Monolayer Incorporating an Anion Receptor. Anal. Chem. 2017, 89, 12756–12763. [Google Scholar] [CrossRef] [PubMed]
- Malecka, K.; Menon, S.; Palla, G.; Kumar, K.G.; Daniels, M.; Dehaen, W.; Radecka, H.; Radecki, J. Redox-Active Monolayers Self-Assembled on Gold Electrodes—Effect of Their Structures on Electrochemical Parameters and DNA Sensing Ability. Molecules 2020, 25, 607. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Hu, Y.; Liu, Y.; Zhang, R. Review of Chloride Ion Detection Technology in Water. Appl. Sci. 2021, 11, 11137. [Google Scholar] [CrossRef]
- Zaki, M.H.M.; Mohd, Y.; Chin, L.Y. Surface Properties of Nanostructured Gold Coatings Electrodeposited at Different Potentials. Int. J. Electrochem. Sci. 2020, 15, 11401–11415. [Google Scholar] [CrossRef]
- Xie, X.; Holze, R. Electrode Kinetic Data: Geometric vs. Real Surface Area. Batteries 2022, 8, 146. [Google Scholar] [CrossRef]
- Bard, A.J.; Faulkner, L.R.; White, H.S. Electrochemical Methods: Fundamentals and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2022. [Google Scholar]
- Szymańska, I.; Stobiecka, M.; Orlewska, C.; Rohand, T.; Janssen, D.; Dehaen, W.; Radecka, H. Electroactive Dipyrromethene−Cu(II) Self-Assembled Monolayers: Complexation Reaction on the Surface of Gold Electrodes. Langmuir 2008, 24, 11239–11245. [Google Scholar] [CrossRef] [PubMed]
- Laviron, E. General expression of the linear potential sweep voltammogram in the case of diffusionless electrochemical systems. J. Electroanal. Chem. Interfacial Electrochem. 1979, 101, 19–28. [Google Scholar] [CrossRef]
- Eckermann, A.L.; Feld, D.J.; Shaw, J.A.; Meade, T.J. Electrochemistry of redox-active self-assembled monolayers. Coord. Chem. Rev. 2010, 254, 1769–1802. [Google Scholar] [CrossRef]
- Maccà, C.; Wang, J. Experimental procedures for the determination of amperometric selectivity coefficients. Anal. Chim. Acta 1995, 303, 265–274. [Google Scholar] [CrossRef]
- Amouzadeh Tabrizi, M.; Acedo, P. An Electrochemical Immunosensor for the Determination of Procalcitonin Using the Gold-Graphene Interdigitated Electrode. Biosensors 2022, 12, 771. [Google Scholar] [CrossRef]
- Scholten, K.; Merten, C. Anion-binding of a chiral tris(2-aminoethyl)amine-based tripodal thiourea: A spectroscopic and computational study. Phys. Chem. Chem. Phys. 2022, 24, 4042–4050. [Google Scholar] [CrossRef]
- Swartz, M.E.; Krull, I.S. (Eds.) Analytical Method Development and Validation, 1st ed.; CRC Press: Boca Raton, FL, USA, 1997. [Google Scholar]
- Maaroof, Y.T.; Mahmoud, K.M. Silver nanoparticle-modified graphite pencil electrode for sensitive electrochemical detection of chloride ions in pharmaceutical formulations. Bull. Chem. Soc. Ethiop. 2023, 37, 491–503. [Google Scholar] [CrossRef]
- Pięk, M.; Paczosa-Bator, B.; Smajdor, J.; Piech, R. Molecular organic materials intermediate layers modified with carbon black in potentiometric sensors for chloride determination. Electrochim. Acta 2018, 283, 1753–1762. [Google Scholar] [CrossRef]
- Cinti, S.; Fiore, L.; Massoud, R.; Cortese, C.; Moscone, D.; Palleschi, G.; Arduini, F. Low-cost and reagent-free paper-based device to detect chloride ions in serum and sweat. Talanta 2018, 179, 186–192. [Google Scholar] [CrossRef] [PubMed]
- de Araujo, W.R.; Paixão, T.R.L.C. Fabrication of disposable electrochemical devices using silver ink and office paper. Analyst 2014, 139, 2742–2747. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Cheng, C.; Yuan, W.; Liu, Z.; Zhu, L.; Li, X.; Lu, Y.; Chen, Z.; Liu, J.; Cui, Z.; et al. Smartphone-based battery-free and flexible electrochemical patch for calcium and chloride ions detections in biofluids. Sens. Actuators B Chem. 2019, 297, 126743. [Google Scholar] [CrossRef]
- Sikarwar, B.; Singh, V.V.; Sharma, P.K.; Kumar, A.; Thavaselvam, D.; Boopathi, M.; Singh, B.; Jaiswal, Y.K. DNA-probe-target interaction based detection of Brucella melitensis by using surface plasmon resonance. Biosens. Bioelectron. 2017, 87, 964–969. [Google Scholar] [CrossRef]
- Gobi, K.V.; Ohsaka, T. Anion recognition and electrochemical characteristics of the self-assembled monolayer of nickel(II) azamacrocyclic complex. J. Electroanal. Chem. 2000, 485, 61–70. [Google Scholar] [CrossRef]
- Beer, P.D.; Gale, P.A.; Chen, Z. Electrochemical Recognition of Charged and Neutral Guest Species by Redox-active Receptor Molecules††Manuscript received 9th October 1995. In Advances in Physical Organic Chemistry; Bethell, D., Ed.; Academic Press: Cambridge, MA, USA, 1999; Volume 31, pp. 1–90. [Google Scholar]
- Malecka-Baturo, K.; Żółtowska, P.; Jackowska, A.; Kurzątkowska-Adaszyńska, K.; Grabowska, I. Electrochemical Aptasensing Platform for the Detection of Retinol Binding Protein-4. Biosensors 2024, 14, 101. [Google Scholar] [CrossRef]

| Sensor | Method | LOD, M | Interferents | Ref. |
|---|---|---|---|---|
| GPE/AgNPs | CV | 4.1 × 10−5 | CO32−, NO3−, NO2−, PO43−, SO42− | [36] |
| CD/Ag | LSV | 2 × 10−5 | NO2−, PO42−, Na+, K+, SO42− | [18] |
| PTFE/MWCNT/(Ag-NPs/AgCl)/Cu-BTC | ChA | nd | NO3−, SO42−, S2−, OH− | [19] |
| GCE/ZIF-8/AgNPs | DPV | 6.1 × 10−7 | NO3−, SO42−, CO32−, PO42− | [20] |
| ISE/TCNQ-TTF/CB | Pm | 2.51 × 10−6 | NO3−, NO2−, SO42−, CO32−, HCO3−, CH3COO− | [37] |
| FP/Ag Ink | CV | 1 × 10−3 | HPO42−, H2PO42−, CO32− HCO3−, Br−, I−, CN− | [38] |
| OP/Ag Ink | CV | 7 × 10−6 | nd | [39] |
| ISE/PDMS/AgNWs | Vm | 5 × 10−4 | HCO3−, CO32−, OH−, NO3− | [40] |
| CSPE/FcMeOH CSPE/K4[Fe(CN)6] CSPE/K3[Fe(CN)6] | DPV | 0.01 2 2 | AsO42−, PO43−, I−, CN−, Br−, CO32−, CrO42−, Cr2O72−, IO3−, BrO3−, SO42−, Hg22+, Pb2+ | [21] |
| CSPE/Gore-Tex/FcMeOH | DPV | 2 × 10−4 | nd | [22] |
| Au/DPM/Cu(II)/DPM-AR Au/DPM/Co(II)/DPM-AR | SWV | 1.0 × 10−12 1.1 × 10−12 | SO42−, Br− | [23] |
| Au/AHT,MBL/TPY/Co(II)/DPM-AR | SWV | 5.0 × 10−16 | SO42−, Br− | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malecka-Baturo, K.; Daniels, M.; Dehaen, W.; Radecka, H.; Radecki, J.; Grabowska, I. Voltammetric Sensing of Chloride Based on a Redox-Active Complex: A Terpyridine-Co(II)-Dipyrromethene Functionalized Anion Receptor Deposited on a Gold Electrode. Molecules 2024, 29, 2102. https://doi.org/10.3390/molecules29092102
Malecka-Baturo K, Daniels M, Dehaen W, Radecka H, Radecki J, Grabowska I. Voltammetric Sensing of Chloride Based on a Redox-Active Complex: A Terpyridine-Co(II)-Dipyrromethene Functionalized Anion Receptor Deposited on a Gold Electrode. Molecules. 2024; 29(9):2102. https://doi.org/10.3390/molecules29092102
Chicago/Turabian StyleMalecka-Baturo, Kamila, Mathias Daniels, Wim Dehaen, Hanna Radecka, Jerzy Radecki, and Iwona Grabowska. 2024. "Voltammetric Sensing of Chloride Based on a Redox-Active Complex: A Terpyridine-Co(II)-Dipyrromethene Functionalized Anion Receptor Deposited on a Gold Electrode" Molecules 29, no. 9: 2102. https://doi.org/10.3390/molecules29092102
APA StyleMalecka-Baturo, K., Daniels, M., Dehaen, W., Radecka, H., Radecki, J., & Grabowska, I. (2024). Voltammetric Sensing of Chloride Based on a Redox-Active Complex: A Terpyridine-Co(II)-Dipyrromethene Functionalized Anion Receptor Deposited on a Gold Electrode. Molecules, 29(9), 2102. https://doi.org/10.3390/molecules29092102








