Abstract
Leishmaniasis and Human African trypanosomiasis pose significant public health threats in resource-limited regions, accentuated by the drawbacks of the current antiprotozoal treatments and the lack of approved vaccines. Considering the demand for novel therapeutic drugs, a series of BODIPY derivatives with several functionalizations at the meso, 2 and/or 6 positions of the core were synthesized and characterized. The in vitro activity against Trypanosoma brucei and Leishmania major parasites was carried out alongside a human healthy cell line (MRC-5) to establish selectivity indices (SIs). Notably, the meso-substituted BODIPY, with 1-dimethylaminonaphthalene (1b) and anthracene moiety (1c), were the most active against L. major, displaying IC50 = 4.84 and 5.41 μM, with a 16 and 18-fold selectivity over MRC-5 cells, respectively. In contrast, the mono-formylated analogues 2b and 2c exhibited the highest toxicity (IC50 = 2.84 and 6.17 μM, respectively) and selectivity (SI = 24 and 11, respectively) against T. brucei. Further insights on the activity of these compounds were gathered from molecular docking studies. The results suggest that these BODIPYs act as competitive inhibitors targeting the NADPH/NADP+ linkage site of the pteridine reductase (PR) enzyme. Additionally, these findings unveil a range of quasi-degenerate binding complexes formed between the PRs and the investigated BODIPY derivatives. These results suggest a potential correlation between the anti-parasitic activity and the presence of multiple configurations that block the same site of the enzyme.
1. Introduction
Leishmania major and Trypanosoma brucei are the pathogenic parasites responsible for two neglected tropical diseases (NTDs): leishmaniasis and Human African trypanosomiasis (HAT, also known as sleeping sickness). Due to the absence of an approved vaccination for these pathogenic diseases and the high toxicity and drug resistance associated with current antileishmanial and antitrypanosomal drugs, ongoing efforts are being dedicated to develop improved molecules to address the limitations of the existing treatments [1]. Notably, in 2021, the US Food and Drug Administration (FDA) approved fexinidazole as the first all-oral treatment for both stages of the Trypanosoma brucei gambiense form of sleeping sickness. In 2023, the approval by the European Medicines Agency followed. In any case, new potential treatments are advisable for HAT and also for leishmaniasis.
Recently, our group reported the investigation of bis(indolyl)methane (BIM) derivatives substituted with different (hetero)aromatic moieties as antiparasitic agents. We found that the triphenylamine-functionalized compound exhibited an IC50 of 3.21 and 3.30 μM against Trypanosoma brucei (T. brucei) and Leishmania major (L. major), respectively, and an eight-fold selectivity over a healthy cell line (MRC-5) [2].
Identifying and understanding molecular targets associated with the survival of parasites plays an important role in advancing drug discovery towards antiparasitic agents. Numerous studies have elucidated the potential of target-based drug discovery for parasitic diseases, including HAT and leishmaniasis. These findings have been comprehensively reviewed previously [3,4]. For example, dihydrofolate reductase (DHFR) and pteridine reductase 1 (PTR1) are NADPH-dependent enzymes involved in the reduction of pterins and folates of the trypanosomatid parasites. A greater understanding of the folates pathway in trypanosomatid biology highlighted their crucial role in cellular mechanisms, such as DNA and protein synthesis [5]. Therefore, by disrupting these cellular processes, drugs targeting the folate pathway (also known as antifolates) have the potential to effectively combat trypanosomatid infections. Inhibitors of DHFR, such as trimethoprim and chloroguanide, have been used for the treatment of bacterial infections and malaria, respectively [6,7,8]. However, blockers of the DHRF have shown less efficacy against Trypanosoma and Leishmania parasites, mainly due to the presence of the PTR1 enzyme [9]. Studies demonstrated that PTR1 is upregulated under DHFR inhibition, thus contributing to the parasites’ resistance to antifolates [10]. Hence, additional PTR1 inhibition has been proposed as an alternative to suppress the trypanosomatidae folate pathway in order to avoid this mechanism of resistance [11,12]. In the past years, several compounds targeting PTR1 have been developed, for example, quinoxaline-based, 2,4-diaminopteridine-based and thiadiazoles-based derivatives [11,13,14,15]. However, additional advancements are required to enhance their selectivity and effectiveness in combating these pathogenic parasites.
The derivatives based on the boron-dipyrromethene (BODIPY) scaffold represent a versatile class of fluorophores widely employed in different scientific fields. The first BODIPY was synthesized by Alfred Treibs and Franz-Heinreich Kreuzer in 1968 [16] and since then, there has been an exponential emergence in the design and synthesis of new compounds. The relevance of these derivatives arises from their facile synthesis and structural versatility. There are numerous functionalization strategies to develop a desirable framework pattern with specific features, including pre-functionalization of the pyrrole/aldehyde and post-functionalization of each position of the core [17,18]. The synthetic flexibility of this scaffold has given rise to a plethora of derivatives with fine-tuned properties, making them versatile tools for diverse applications, such as organic light-emitting diodes (OLEDs), chemosensors, fluorescence probes for bioimaging and photosensitizers in Photodynamic Therapy (PDT) [19,20,21,22,23].
As a matter of fact, BODIPY derivatives are demonstrating a growing success in the field of Photodynamic Therapy (PDT) and Photodynamic Inactivation (PDI) [24,25]. These two therapeutic approaches have become an alternative, especially for cancer treatment and against resistant pathogenic agents, such as bacteria, viruses and fungi. Several approaches have been attempted to fine-tune the BODIPY framework and improve its efficiency in generating reactive oxygen species [26,27,28].
BODIPY-based molecules are also increasingly prominent in the bioimaging field, ascribed to their excellent photophysical properties and great photostability in biological conditions [20]. Interestingly, BODIPY-based fluorescence probes have been developed to investigate the cellular internalization and biodistribution of antiparasitic drugs [29,30,31]. A BODIPY-fluorophore based probe for an anti-Chagas agent was developed and investigated for its potential as an in vivo theranostic probe [30]. Moreover, fluorescent analogues of the leishmanicidal drug miltefosine were successfully developed by integrating a BODIPY moiety on the alkyl chain. These derivatives demonstrated in vitro antiparasitic activity, comparable to that of the original alkylphosphocholine compound [31]. Nevertheless, to the best of our knowledge, the investigation of BODIPY derivatives as the active moiety of antiparasitic agents has not been reported previously.
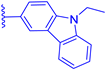
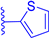
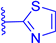
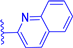
In this work, we report on the therapeutic activity of BODIPY derivatives against protozoan parasites. We prepared a series of BODIPY derivatives featuring different functionalizations at the meso, 2 and/or 6 positions of the scaffold structure (Cf. Figure 1). The in vitro effectiveness of these compounds was assessed against T. brucei and L. major, along with the human lung fibroblast cell line (MRC-5), to establish their selectivity indices (SIs). Additional understanding regarding the antitrypanosomal and antileishmanial activity of the BODIPY derivatives was obtained by employing molecular modeling methods to analyze the interactions of the compounds with the pteridine reductase (PR) enzyme.
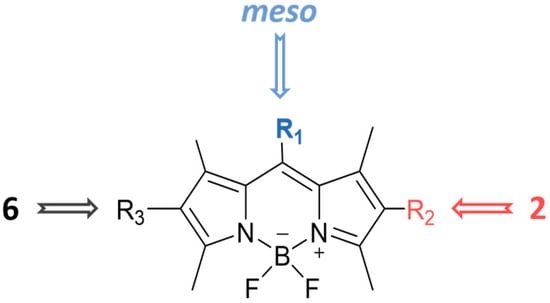
Figure 1.
Functionalization of the BODIPY core at meso position, 2 position and 6 position with different substituents groups.
2. Results and Discussion
2.1. Synthesis and Characterization of BODIPY Derivatives
A series of BODIPY derivatives were prepared and characterized in order to investigate the effects of the different chemical modifications at positions 2, 6 and meso of the core on their biological activity against T. brucei and L. major parasites. The route for the synthesis of the BODIPY derivatives is represented in Scheme 1, and the functionalization groups at each position of the BODIPY core (R1, R2 and R3), the reaction yields and the photophysical characterization in acetonitrile solution are compiled in Table 1. In this work, the nomenclature employed is as follows: the precursor compounds (R2 = R3 = H, Cf. Figure 1) of the core are denominated with number 1; the successor compounds functionalized at R2 and/or R3 are denominated with numbers from 2 to 4; and the suffix letters a to i denote the meso substituent (R1). The synthesis and characterization of the BODIPY derivatives 1a, 2a, 3a, 1b, 2b, 3b, 1c, 2c, 4c, 1d, 2d, 1e and 2e have been previously reported by our group [27,28,32,33,34,35,36,37,38,39,40]. The synthesis of the BODIPY derivatives 1f–h has been reported previously elsewhere [41,42,43].

Scheme 1.
Synthetic route for the BODIPY derivatives functionalized at meso position and 2 and/or 6 positions.

Table 1.
Synthesis and photophysical data for the BODIPY derivatives. The photophysical studies were conducted in acetonitrile solutions with concentrations of the compounds ranging from 10−5 to 10−6 M (absorption, λabs, and fluorescence emission maxima, λfluo, and fluorescence quantum yields, ΦF).
Firstly, to obtain the BODIPY scaffold substituted at meso position with several aromatic and heteroaromatic groups (1a–i), we followed the established Lindsey’s method, as reported by our group previously [27,32,33,34,35,36,44], with yields ranging from 3% to 74%, probably explained by the lower reactivity of the precursor aldehydes, and the difficulty in the purification, by silica gel column chromatography, especially for the thiophene, thiazole and thieno[3,2-b]thiophene derivatives 1f–i. The BODIPYs formylated in position 2 and/or 6 (2a–f) were obtained from the precursors 1a–f via the Vilsmeier-Haack reaction [27,28,32,36,37,38,39] in moderate to excellent yields (47–91%). In the case of the formylation of precursor 1d, the reaction resulted in two products: the monoformylated compound 2d (yield 25%), with an aldehyde group on the BODIPY core and the diformylated compound 3d (yield 26%), with a second aldehyde group on the triphenylamine moiety. The diformylated BODIPY 3c substituted with two aldehydes groups on the BODIPY core was obtained by a second formylation of its precursor 2c, with a lower yield (10%). The derivatives functionalized with a benzimidazole at position 2 of the core (3a and 3b) were obtained, in fair to good yields (77% and 31%, respectively), by the condensation between the aldehyde group and o-phenylenediamine, followed by an intramolecular cyclisation reaction [32,36]. Finally, derivative 4c was obtained by the direct halogenation of the precursor 1c with N-iodosuccinimide (NIS), with a yield of 57% [28]. The NMR spectroscopic characterization of compounds 1f, 1g and 1h were in agreement with the previously published data [41,42,43]. The new compounds 3c, 3d, 2f and 1i were characterized through NMR spectroscopy and high-resolution mass spectrometry. The synthetic methods and characterizations are described in the supporting information.
A comprehensive study focused on the influence of the electron donor/withdrawing substituents at the meso and 2 positions of the BODIPY core and solvent polarity on the photophysical properties of the BODIPY derivatives 3a, 1b, 2b, 1c and 2c has been recently reported by our research group [27,36]. Overall, the derivatives are characterized by an absorption band, with maxima at 492–512 nm, and emission maxima at 507–524 nm, the typical features for the BODIPY chromophore [19]. Nevertheless, the anthracene-BODIPY substituted with iodine atoms at positions 2 and 6 (compound 4c) displayed the highest absorption maxima of the series at 540 nm and emission maxima at 559 nm. Comparison of derivatives 1d and 3d shows that the introduction of the electron-withdrawing formyl group, at position 2 of the BODIPY core and at the triphenylamine moiety, leads to a red-shift of the maximum emission band (λfluo = 519 nm vs. λfluo = 549 nm, respectively), whereas the absorption maximum did not suffer significant changes. Regarding the relative fluorescence quantum yield (ΦF), in general, the BODIPY derivatives demonstrated low fluorescence intensity, with the exception of compounds 1a, 2a and 2f, which exhibited values of 0.68, 0.84 and 0.30, respectively. Comparison between compounds 1a and 2a with their counterpart 3a shows that the modification with the benzimidazole induces a fluorescence quenching (ΦF = 0.68 and ΦF = 0.84 vs. ΦF = 0.10, respectively). In the case of derivative 2f, bearing a thiophene moiety at the meso position of the BODIPY core and an electron acceptor formyl group at position 2, the compound demonstrated a higher fluorescence emission’s efficiency when compared to its analogue 1f, non-substituted with the formyl group (ΦF = 0.30 vs. ΦF = 0.045, respectively).
2.2. Antiparasitic and Cytotoxicity Activity
The in vitro antitrypanosomal and antileishmanial activity of the BODIPY derivatives were evaluated on the bloodstream form (BSF) of T. brucei and the promastigote form of L. major parasites. The toxicity of the compounds was determined after 72 h of incubation using the alamarBlue assay (for T. brucei) and the MTT assay (for L. major). The cytotoxicity of the compounds on a healthy human cell line (human lung fibroblast, MRC-5) was also determined after 72 h of incubation using the alamarBlue assay. The data were expressed as the half maximal inhibitory concentration (IC50). The selectivity index (SI) of each derivative was calculated by dividing the IC50 of MRC-5 cells into the IC50 of T. brucei or L. major. The results are shown in Table 2.

Table 2.
Antiparasitic activity against T. brucei BSF and L. major promastigotes and cytotoxicity in MRC-5 represented as IC50 values (μM) with the error reported as σ. The selectivity index was calculated related to a healthy cell line (MRC-5). The best selectivity values (SI > 10) are highlighted in bold.
We observed that the BODIPY derivatives 1b, meso-substituted with 1-dimethylaminonaphthalene, and 1c, meso-substituted with anthracene moiety, were the most active of the series against L. major, exhibiting IC50 values of 4.84 and 5.41 μM, together with a 16 and 18-fold selectivity over the healthy cell line (MRC-5), respectively. In contrast, the cytotoxicity of the derivative 3a, functionalized at the meso-position with a phenyl group and with a benzimidazole moiety at position 2, was higher for the healthy cell line than towards the L. major (IC50 = 63 μM vs. IC50 > 100 μM, respectively), which resulted in a SI lower than one. Interestingly, the compound exhibited an inhibitory effect on T. brucei with an IC50 value of 5.5 μM and an SI value of 11.38. The formylated derivatives 2b and 2c also exhibited good inhibitory activity against T. brucei, particularly compound 2b (substituted with 1-dimethylaminonaphthalene at meso position). This compound displayed the best IC50 2.84 μM and selectivity index of the series and a 24-fold selectivity over MRC-5, which suggests an interesting therapeutic potential of 2b against T. brucei parasites. Moreover, inhibitory concentration values of 3.6 μM against L. major and 2.7 μM against T. brucei have been reported for methotrexate, a reference antifolate drug [12,45]. These values fall within the range of the toxicity exhibited by the most active BODIPY derivatives 1b, 2c and 3c towards L. major and compound 2b towards T. brucei.
The functionalization of the BODIPY core with a formyl group seems to influence, in certain cases, their antiparasitic activity. The unsubstituted compounds 1b and 1c were more active against L. major, whilst the mono-formyl analogues 2b and 2c were more cytotoxic against T. brucei parasites. Additionally, in T. brucei, the BODIPYs functionalized at R3 with formyl were overall more toxic than the unsubstituted analogues, hence with higher selectivity indices (C.f. Figure S1). For instance, compound 2b has an IC50 of 2.84 μM (SI = 24.34), whilst its precursor 1b has an IC50 = 18.24 μM (SI = 4.21); compound 2c shows an IC50 = 6.17 μM (SI = 10.84) and its unsubstituted analogue 1c an IC50 = 77.11 μM (SI = 1.07); compound 2f displays an IC50 of 12.37 μM (SI = 6.54), whilst its counterpart 1f shows an IC50 = 36.93 μM (SI = 1.57). However, this tendency seems not to be so relevant for antileishmanial activity (C.f. Figure S2).
2.3. Molecular Docking Studies
In order to rationalize the antileishmanial and antitrypanosomal activity data presented in Table 2 for the 20 BODIPY derivatives, their interaction with pteridine reductase native to L. major (PRLm) and T. brucei (PRTb) was studied using a molecular docking protocol.
The selection of Pteridine reductase as the probable target for these BODIPY derivatives was predicated on its structural resemblance to known antileishmanial and antitrypanosomal drugs. A quantitative assessment of the structural similarities was conducted through the analysis of Morgan fingerprint bit differences (Cf. Table S1).
Figure 2 summarizes the main results from the molecular docking studies. In general, all BODIPY derivatives present good binding energies for the most stable binding mode, and is generally lower for PRTb than for PRLm, with the noticeable exception of 1d. As it will be detailed later, the attachment of the BODYPY derivatives is mostly established via hydrophobic interactions between the sp2 carbon atoms of the BODIPY derivative and the carbon atoms on the side chains of a handful of amino acid residues (Cf. Figures S3–S11). The only significant exceptions noted involved the possible hydrogen bonding between PRLm and compounds 1b, 2b and 3b, involving the H atoms of the amine group and the backbone N of Ala15, the heterocyclic N of Hys241 and the O atom of Ser111, respectively. Compound 3b also forms a hydrogen bond with the amino moiety at the side chain of Arg14 of PRTb.
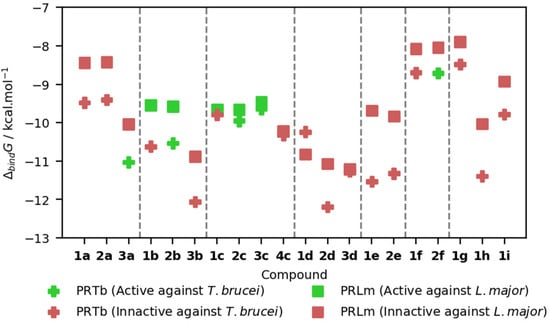
Figure 2.
Binding Gibbs energies (, in kcal/mol) for the best binding mode of each enzyme-BODIPY derivative. Compounds 1b, 1c, 2b, 2c, and 3c present antileishmanial activity, whereas compounds 2b, 2c, 2f, 3a, and 3c present antitrypanosomal activity.
Although the results depicted in Figure 2 do not allow for a clear distinction between active and inactive compounds, the docking studies found additional low-energy binding modes, some of which translate into a significantly different geometry of the enzyme-ligand complex.
In most cases, the set of 30 binding modes with binding energies lower than 5 kcal·mol−1, relative to the most stable mode, could be classified into 2 or 3 families of nearly degenerate modes. The importance of these observations was first accessed by estimating the probability of finding each enzyme-BODIPY derivative complex at a given binding mode i:
where = 298.15 K, is the gas constant (in kcal/mol) and is the degeneracy of each binding mode. Because the modes are nearly, but not completely, degenerate, all calculations were carried out using = 1. Assuming an exhaustive search of the conformational space and that the collected modes are representative of the lowest energy states of the system, these probabilities reflect the population of each binding mode [46]. The complete results from these calculations in the form of the population of each binding mode for each enzyme-BODIPY derivative are given in Tables S2 and S3 in the Supporting Information.
In general, the results show that there is a high probability that the most stable binding mode is not representative of the thermally available complexes. Indeed, the higher values were found for the lowest energy binding mode of PRLm-3d (48%) and PRTb-3b (44%). These, however, are exceptions, with the second higher being observed for the complexes of 2d with PRLm and PRTb, which corresponded to populations of only 26% and 39%, respectively. It should be noted that the ligand in each of these highly stable complexes is not active against L. major nor T. brucei. On the other hand, the results show that most complexes with antileishmanial and antitrypanosomal compounds present a population of the lowest energy binding mode smaller than 20%, with the exception of PRTb-2b, for which the lowest energy binding mode represents 36% of the population.
Thus far, the results from the molecular docking studies strongly suggest that most BODIPY derivatives have some freedom to move within the binding cavity of the tested enzymes, making the lowest energy binding mode less representative than what ought to be expected. As a consequence, the affinity of each compound for the enzyme cannot be rationalized solely based on the geometry of this binding mode, prompting us to explore more sophisticated ways of understanding the formation of these complexes; why are only a handful of BODIPY derivatives biologically active, and why some are specific to a given parasite? For this purpose, the geometry of each binding mode was analyzed in order to find the 10 closest contacts between the enzyme and the ligand; for each contact, we recorded the amino acid involved, the atoms of the ligand and amino acid involved, as well as the distance between the atoms at close contact. For each ligand, the contact to each amino acid in the enzyme was weighted by the of the binding mode. Thus, for each amino acid and ligand , the overall affinity between a given amino acid, and a ligand is the number of closest contacts between and , weighted by :
where is the set of amino acids containing atoms in close contact with the ligand in the binding mode (limited to the closest contacts) and —cardinality of —denotes the count of amino acid A in the set of closest contacts.
The results from using this unitless affinity metric were quite interesting, as they highlighted the affinity of the BODIPY derivatives to certain amino acids in and around the binding pocket. Considering all the 20 BODIPY derivatives, only 57 of the 301 amino acids in PRLm’s chain A have some affinity for these ligands, representing about 19% of the enzyme’s primary structure. In the case of PRTb, affinity toward the BODIPY derivatives was found for 61 of the 267 amino acids in chain A, which covers about 23% of the primary structure. Nevertheless, the values of vary by more than three orders of magnitude, from less than 0.1 to about 325 (measured for (Val206, 2b) in PRTb).
Although some patterns in were apparent by a simple visual depiction of the data, a Principal Component Analysis (PCA) of the affinity data provided a much-needed simplification of the data. In the case of PRLm, the first Principal Component (PC), representing 43% of the variance in the data, clearly classifies the active from the inactive compounds, as shown in Figure 3a, with the exception of 4c. This erroneous classification of 4c may be due to its low solubility in water, which may have prevented it from reaching PRTb during the biological assays.

Figure 3.
Principal Component Analysis (PCA) scores of the affinity data for PRLm (a) and PRTb (b) from the molecular docking studies of the 20 BODIPY derivatives binding to the active site at chain A of each enzyme. The variance explained by each PC is given in parenthesis, as a percentage of the total variance in each data set. Compounds with antileishmanial (a) or antitrypanosomal (b) activity are depicted in green and are further labeled in (b), and inactive ones are depicted in red.
On the other hand, PCA was unable to resolve the compounds for which antitrypanosomal activity was observed from those lacking it, as shown in Figure 3b, which depicts the distribution of the data along PC1 and PC2, covering 62% of the variance in the data.
This intriguing result prompted more details of the loadings associated with PC1 for the data concerning PRLm. Table 3 shows the seven most prominent loadings along the positive (pointing toward antileishmanial activity) and negative (pointing toward lack of antileishmanial activity) directions of PC1. These results highlight that affinity toward Ser40 and Ala15 of PRLm are desirable traits when searching for antileishmanial activity. On the other hand, significant affinity toward Phe113, as well as affinity toward a number of hydrophobic amino acids in the 224 to 230 positions, appear to hinder the antileishmanial activity of the BODIPY derivatives.

Table 3.
Most prominent PCA loadings associated with PC1 of the affinity data for PRLm and the set of 20 BODIPY derivatives.
With the results from PRLm in mind, we sought to find in the primary structure of PRTb, the homologous amino acids to those listed in Table 3 using the aligned primary structures of both compounds, as shown in Figure 4. As shown in Figure 4, almost all amino acids listed in Table 3 are present in both PRLm and PRTb. Despite that, a few of these amino acids are replaced by similar ones: Ser112 is replaced in PRTb by alanine (Ala96) and Ser146 is replaced by threonine (Thr126), only one amino acid mentioned in Table 3 (Val230) is replaced by a substantially different one (Pro210). Moreover, the two enzymes align quite well, with the amino acids highlighted by PCA in PRLm overlaying their homologues in PRTb, as shown in Figure 5.

Figure 4.
Alignment of the primary structure of PRLm (PDB code 2QHX) and PRTb (PDB code 4CM7). In the structure of PRLm, affinity toward the highlighted amino acids determines antileishmanial activity (green), or lack thereof (red), according to the data shown in Table 3. The initial sequence of 21 amino acids is due to the technique used to express these proteins during the sequencing procedure. The initial 21 amino acids, marked in grey, derive from the cloning strategy used in the sequencing protocol.
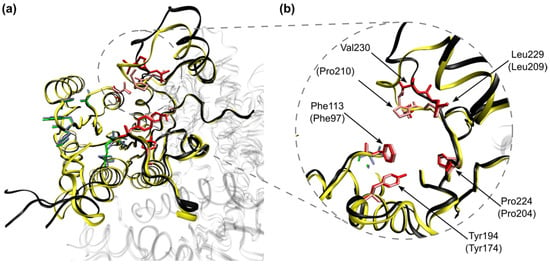
Figure 5.
Aligned structures of PRLm (black) and PRTb (yellow) in ribbons representation. Amino acid residues targeted for antileishmanial activity (positive loading value in Table 3) are shown in the structure of PRLm in green, and their homologues in PRTb are highlighted in light blue. Amino acid residues associated with a lack of antileishmanial activity (negative loading value in Table 3) are highlighted in red (PRLm), whereas their homologues in PRTb are depicted in pink. (a) The overall structure of chain A (chains B, C and D of both enzymes are depicted using high transparency). (b) Detailed view of the pocket surrounding Val230 in PRLm, with the structural homologues in PRTb labeled inside parenthesis.
Looking at the aligned structures shown in Figure 5, it is clear that the antileishmanial activity is linked to the compound’s ability to bind to the more peripheral side of the binding cavity, whereas affinity towards the internal face of this cavity will likely lead to a lack of antileishmanial activity. Furthermore, Figure 5a shows that the amino acids more likely to bind to an antileishmanial BODIPY derivative occupy the same position and orientation in both PRLm and PRTb. In both cases, these amino acids form the pocket where the adenine moiety of NADPH binds to these enzymes, allowing one to postulate that these BODIPY derivatives compete with NADPH for the binding cavity.
Although most amino acids considered as undesirable binding spots (Figure 5a, depicted in red) also line up well between PRLm and PRTb (depicted in pink), it is noticeable that replacing Val230 with Pro210 leads to a local discrepancy in the three-dimensional structure of these enzymes, which is highlighted in Figure 5b. This is evident by the lack of overlap between PRLm and PRTb in the turn domain following Pro210, as well as some misalignment of the α-helix that follows. Moreover, Pro210 is oriented towards Phe113, forming a narrow space that may trap planar molecules or groups, such as some of the meso substituents of the BODIPY derivatives described in this work. In a similar manner, the narrowing of the space close to Phe113 may drive some bulkier compounds to seek the binding spots in the peripheral face of the binding cavity, hindering the enzyme’s ability to bind to NADPH.
This rationale leads to the hypothesis that the inhibition of PRTb is driven by the affinity to the amino acids that are homologues of the ones driving the inhibition of PRLm. In order to test this hypothesis, the affinities of each BODIPY derivative toward the amino acids listed in Table 3, or their homologues in PRTb, are depicted in Figure 6.

Figure 6.
Dimensional affinities (Cf. Equation (2)) towards key amino acid residues in PRLm impacting the antileishmanial activity of BODIPY derivatives: compounds of the (a) a and b series; (b) c and d series; (c) e and f series, as well as 1g–1i. Likewise, for the structural homologues in PRTb (d) a and b series; (e) c and d series; (f) e and f series, as well as 1g–1i. Compounds showing antileishmanial activity are depicted in various shades of green, whereas those lacking activity are shown in various shades of red (a–c). A similar color coding is applied for antitrypanosomal activity (d–f).
The results depicted in Figure 6 provide some validation of our hypothesis. Indeed, all antileishmanial compounds show considerable affinity toward Ala15, whereas none of the compounds inactive against L. major show affinity toward this amino acid residue, as depicted in Figure 6a–c. The same observation can be noted for Tyr37 and Ser146. On the other hand, BODIPY derivatives lacking antileishmanial activity have an increased affinity toward Phe113, Arg117 and (to a lesser extent) toward Pro224 and Val230. It should be noted that some of the BODIPY derivatives with the smallest IC50 against L. major also feature some affinity toward Ser40. It is also noteworthy that the preferential orientation of the active BODIPY derivatives toward these amino acid residues involves pointing the group in the meso position towards said amino acid (regardless of the amino acid being associated with antileishmanial activity, or lack thereof).
Thus far, the results from the ensemble of configurations collected in the docking studies show that the affinity of the meso group toward specific amino acid residues is a good indicator of the possible antileishmanial activity, with the already justified exception of compounds 4c. In order to fully validate our hypothesis, some rules concerning antitrypanosomal activity should also be inferred from the data concerning the docking studies carried out using PRTb. Indeed, some commonalities are found between Figure 6a–c and their PRTb counterparts (Figure 6d, Figure 6e, and Figure 6f, respectively), such as the overall high affinity of non-antitrypanosomal BODIPY derivatives toward Phe97 (homologue of Phe113 in PRLm) or the relatively high affinity of antitrypanosomal compounds 2c and 3c towards Ala12 (homologue of Ala15 in PRLM, Figure 6e). On the other hand, a plain application of the rules devised for PRLm may be misleading. For example, antitrypanosomal compounds 3a and 2b show high affinity toward Phe97 and Arg14 and low or no noticeable affinity towards amino acids that would be associated with the inactivation of PRTb such as Ala12 (homologue of Ala15 in PRLm). Interestingly, these two compounds show some affinity toward Pro210, suggesting the possibility that these compounds can stay “pinched” between Phe97 and Pro210, effectively preventing the nicotinamide moiety of NADPH to bind.
The BODIPY derivatives from the c and d series show similar affinity profiles in both PRLm (C.f. Figure 6b) and PRTb (C.f. Figure 6e), suggesting that they may share the same inactivation mechanism for both enzymes. However, all antileishmanial compounds from the c series show a decreased affinity toward Tyr34. Moreover, compound 1c also shows increased affinity toward Arg14, which suggests that this compound is more labile inside PRTb’s cavity, which may lead to its displacement by NADPH (or water, or another cofactor).
Finally, compound 2f does not present antileishmanial activity, showing an affinity profile toward PRLm similar to other non-antileishmanial BODIPY derivatives (C.f. Figure 6c). In the presence of PRTb, this compound shows some significant affinity toward Pro210, suggesting again a stabilization of its binding configuration at the location where the nicotinamide moiety of NADPH binds to PRTb.
Overall, the results from the docking studies show that the BODIPY derivatives considered in this work have two inactivation mechanisms towards pteridine reductase: they may bind to the Ser, Ala and Tyr residues in the most peripheral area of pteridine reductase’s active cavity, preventing the adenine moiety of NADPH from binding, or they may attach closer to Phe113, Pro204 and Val230 (or their homologues in PRTb) and interfere with the binding of NADPH’s nicotinamide moiety. This latter mechanism is much less effective in PRLm and usually leads to an incomplete inactivation of PRLm, allowing the parasite to survive. On the other hand, the structural differences between PRLm and PRTb allow the nicotinamide approach to be an effective way to inhibit PRTb, via the formation of stable enzyme-BODIPY derivatives in which the latter are kept stable between Phe97 and Pro204. Most importantly, the results from this study showed a multitude of quasi-degenerate binding complexes between either enzyme and the BODIPY derivatives under study, suggesting that antileishmanial and antitrypanosomal activities may be linked to the existence of a number of quasi-degenerated configurations blocking the same moiety of the target enzyme. Indeed, the results from the docking studies strongly suggest that this effect, closely related to the entropy of the binding complexes, surpasses the mere evaluation of the binding energy between the enzyme and its ligands when it comes to predicting anti-parasitic activity.
3. Conclusions
In conclusion, we prepared a series of BODIPY derivatives functionalized at the meso, 2 and/or 6 positions of the core and evaluated their antileishmanial (L. major parasites) and antitrypanosomal (T. brucei parasites) activity. The studies revealed promising anti-parasitic activities, with compounds 1b and 1c, meso-substituted with 1-dimethylaminonaphthalene and anthracene moieties, standing out as the most potent of the series against L. major. These derivatives exhibited IC50 values of 4.84 and 5.41 μM, along with selectivity indices of 16 and 18-fold over healthy cells, respectively. Concerning the antitrypanosomal activity, the formylated derivatives 2b and 2c displayed significant efficacy against T. brucei, with compound 2b demonstrating the highest inhibitory effect and selectivity (IC50 = 2.84 μM and SI = 24.34) within the series.
Additionally, the docking studies conducted on the BODIPY derivatives unveil two distinct inactivation mechanisms for pteridine reductase: (a) these derivatives exhibit an ability to bind to specific residues, including Ser, Ala and Tyr, preventing the adenine moiety of NADPH from binding; (b) blocking NADPH’s nicotinamide moiety from binding by interference with Phe113, Pro204 and Val230 in PRLm (or their homologues in PRTb). On the one hand, the second mechanism seems to be less effective against PRLm, leading to an incomplete inactivation of the pteridine reductase in L. major. On the other hand, structural disparities between PRLm and PRTb underscore the effectiveness of the nicotinamide approach in inhibiting the enzyme in T. brucei. Moreover, this study highlights the significance of quasi-degenerate binding complexes, indicating a potential correlation between antileishmanial and antitrypanosomal activities and various configurations hindering the target enzyme’s moiety. This emphasizes the importance of considering these multiple binding configurations beyond traditional binding energy assessments in predicting anti-parasitic activity.
4. Experimental Section
4.1. General
NMR spectra were obtained on a Bruker Avance III 400 at an operating frequency of 400 MHz for 1H and 100.6 MHz for 13C, at 25 °C using the solvent peak as an internal reference. All chemical shifts are given in ppm using δH Me4Si = 0 ppm as a reference. Peak assignments were supported by spin decoupling-double resonance and bidimensional heteronuclear techniques. High-resolution mass spectrometry analysis was performed at the “C.A.C.T.I.—Unidad de Espectrometria de Masas” at the University of Vigo, Spain. All reagents were purchased from Sigma–Aldrich, Acros and Fluka and used as received. Thin-layer chromatography (TLC) was carried out on 0.25 mm thick precoated silica plates (Merck Fertigplatten Kieselgel 60F254), and spots were visualized under ultraviolet (UV) light. Mps were determined on a Gallenkamp apparatus. UV-visible absorption spectra (200–700 nm) were obtained using a Shimadzu UV/2501PC spectrophotometer. Fluorescence spectra were collected using a FluoroMax-4 spectrofluorometer. Fluorescence quantum yields were measured using Rhodamine 6G solution in ethanol as standard (ΦF = 0.95) [47]. The synthesis and characterization of the BODIPY derivatives 1–3a, 1–3b, 1c, 2c, 4c, 1d, 2d, 1e and 2e has been previously reported by our group [27,28,32,33,34,35,36,37,38,39,40]. The synthesis of the BODIPY derivatives 1f–h has been reported previously elsewhere [41,42,43]. The synthesis and characterization of BODIPY derivatives 1f–i, 2f, 3c and 3d are given in the Supporting Information.
4.2. Biological Activity
4.2.1. Parasite and Cell Culturing
T. brucei (Lister 427, antigenic type MiTat 1.2, clone 221a, bloodstream forms, “single marker” S427 (S16)) [48] were cultured at 37 °C, 5% CO2 in HMI-9 medium supplemented with 10% heat-inactivated fetal bovine serum (hiFBS, Invitrogen, Carlsbad, CA, USA), as previously described [49].
L. major (MHOM/IL/80/Friedlin) promastigotes were cultured at 28 °C, 5% CO2 in modified RPMI-1640 medium (Invitrogen, Carlsbad, CA, USA) supplemented with 10% hiFBS [49]. Parasites were maintained in culture in their experimental growth phase (below 2 million parasites per mL for T. brucei and 10 million parasites per mL for L. major).
MRC-5 cell line (human lung fibroblast) was grown in a monolayer at 37 °C, 5% CO2 in DMEM medium (1 g/L glucose) supplemented with 10% hiFBS, 100 U/mL penicillin, 100 mg/mL streptomycin and 2 mM L-glutamine [50].
4.2.2. Antiparasitic Activity
The antitrypanosomal activity of the BODIPY derivatives was determined by the alamarBlue® assay (ThermoFisher Scientific, Waltham, MA, USA) [50,51]. The stock solutions of the compounds were prepared in DMSO and the final DMSO percentage in each well was adjusted to be less than 1%. A total of 2 × 104 parasites per mL were incubated at 37 °C, 5% CO2 in 96-well plates (50 μL/well) alone or in the presence of an increasing concentration of compounds for 72 h. A total of 20 μL of resazurin solution (110 ng/mL) was then added to each well, and the parasites were incubated for 4 h at 37 °C. Finally, cells were lysed with 50 μL per well of SDS 3%. The plate was incubated at 37 °C for an extra hour and then, fluorescence intensity was measured with an Infinite F200 plate reader (Tecan Austria, GmbH, Grödig, Austria), exciting at 550 nm and recording the emission at 590 nm. The results are expressed as the concentration of a compound that reduces cell growth by 50% versus untreated control cells (IC50). Data are presented as the average of at least three independent measurements, all conducted in triplicate conditions.
The antileishmanial activity of the BODIPY derivatives was assessed using an MTT-based assay (Sigma-Aldrich, St. Louis, MO, USA) [52]. The stock solutions of the compounds were prepared in DMSO and the final DMSO percentage in each well was adjusted to be less than 1%. A total of 4 × 106 parasites per mL were incubated at 28 °C in 96-well plates (50 μL/well) alone or in the presence of an increasing concentration of compounds DMSO for 72 h. A total of 10 μL of MTT (5 mg/mL) were added to each well and parasites were incubated for 4 h at 28 °C. Finally, cells were lysed with 50 μL/well of 20% SDS. The plate was incubated at 37 °C for an extra hour and then, absorbance was measured at the Infinite F200 plate reader (TECAN Austria, GmbH, Grödig, Austria) at a wavelength of 540 nm. The IC50 was calculated as described above. Data are presented as the average of at least three independent measurements, all conducted in triplicate conditions.
4.2.3. Cytoxicity
Cytotoxicity was measured in MRC-5 through alamarBlue® assay (ThermoFisher Scientific) [50,51]. The stock solutions of the compounds were prepared in DMSO and the final DMSO percentage in each well was adjusted to be less than 1%. A total of 5 × 103 cells per mL were seeded in 96-well plates (100 μL/well) with 24 h of incubation time before compound addition at 37 °C, 5% CO2. Then, the compounds were added at increasing concentrations (from 0 to 100 μM) and the plates were incubated for 72 h. A total of 20 μL of resazurin solution (110 ng/mL) was added to each well and cells were incubated for 4 h. Then, cells were lysed with 50 μL/well of 3% SDS. The plate was incubated at 37 °C for an extra hour and then, the fluorescence intensity was measured at the Infinite F200 plate reader (TECAN Austria, GmbH), exciting at 550 nm and recording the emission at 590 nm. The results are expressed as the concentration of a compound that reduces cell growth by 50% versus untreated control cells (IC50). Data are presented as the average of at least three independent measurements, all conducted in triplicate conditions.
4.2.4. Molecular Docking
The structures of the BODIPY derivatives were optimized using Density Functional Theory (DFT) calculations using the B3LYP functional [53] in conjunction with Alrichs’ Def2-TZVP basis set [54] for all atoms, except iodine, which was described using the relativistic pseudopotential by Peterson et al. [55]. The equilibrium geometries of these compounds were converted to the pdbqt format to be used in the molecular docking studies, which were updated with the CHELPG charges [56,57] derived from the molecular electrostatic potential obtained from the DFT calculations. All DFT calculations were carried out using the Orca software package, version 5.0.3 [58].
The structures of the native form of pteridine reductase from Leishmania major (PRLm) and Trypanosoma brucei (PRTb) were obtained from the Protein Data Bank (PDB) [59], with codes 2QHX and 4CM7, respectively. These structures were filtered to remove water, ions, NADPH and any substrate or inhibitor declared in the PDB records. Alignment of the primary structure of the two enzymes was performed using the BLAST software, version 2.15.0 [60,61] linked from inside PDB.
The docking studies were prepared using the MGLTools software suite, version 1.5.7 [62] and the binding mode search and ranking were carried out using AutoDock Vina, version 1.2.5 [63,64]. Both enzymes exist as a tetramer, and the conformational search was restricted to the binding region of chain A, using a grid spacing of 1 Å, an exhaustiveness setting of 24, and collecting the 30 lowest energy binding modes within 5 kcal.mol−1 of the lowest energy mode. The specific details concerning the grid box used in each enzyme are given in the Supporting Information. The subsequent treatment of the results from the docking studies was carried out using in-house developed Python scripts, whose source code is transcribed in the Supporting Information.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules29092072/s1. Synthesis and characterization of meso-substituted BODIPY derivatives 1f–i; Synthesis and characterization of formylated BODIPY derivatives 2f, 3c and 3d; Figure S1. Analysis of the chemical structure—antitrypanosomal activity relationship according to the selectivity index (SI) values of the BODIPY derivatives; Figure S2. Analysis of the chemical structure—antileishmanial activity relationship according to the selectivity index values of the BODIPY derivatives; Table S1. Inter-compound distances based on difference counts of Morgan fingerprints of radius 2, normalized so that the maximum distance between the BODYPI derivatives reported in this work was 1.0; Figure S3. Images of the most stable complexes of 1a (a), 1b (b), 1c (c), 1d (d), 1e (e), and 1f (f) with PRLm, as found in the molecular docking studies; Figure S4. Images of the most stable complexes of 1g (a), 1i (b), 1h (c), 2a (d), 2b (e), and 2c (f) with PRLm, as found in the molecular docking studies; Figure S5. Images of the most stable complexes of 2d (a), 2e (b), 2f (c), 3a (d), 3b (e), and 3c (f) with PRLm, as found in the molecular docking studies; Figure S6. Images of the most stable complexes of 3d (a) and 4c (b) with PRLm, as found in the molecular docking studies; Figure S7. Images of the most stable complexes of 1a (a), 1b (b), 1c (c), 1d (d), 1e (e), and 1f (f) with PRTb, as found in the molecular docking studies. Figure S8. Images of the most stable complexes of 1g (a), 1i (b), 1h (c), 2a (d), 2b (e), and 2c (f) with PRTb, as found in the molecular docking studies; Figure S9. Images of the most stable complexes of 2d (a), 2e (b), 2f (c), 3a (d), 3b (e), and 3c (f) with PRTb, as found in the molecular docking studies; Figure S10. Images of the most stable complexes of 3d (a), 4c (b) with PRTb, as found in the molecular docking studies; Figure S11. Adimensional affinities towards the amino acid residues in PRLm impacting the antileishmanial activity of BODIPY derivatives; Table S2. Population of each binding mode for each complex PRLm-BODIPY derivative complex; Table S3. Population of each binding mode for each complex PRTb-BODIPY derivative; Configuration of Autodock Vina used in the docking studies; Python Scripts.
Author Contributions
Conceptualization, R.C.R.G., P.P., J.C.M. and M.M.M.R.; methodology, R.C.R.G., P.P., J.C.M. and M.M.M.R.; validation, P.P. and M.M.M.R.; formal analysis, F.T., R.C.R.G., P.P., J.C.M. and M.M.M.R.; investigation, R.C.R.G., F.T. and P.P.; resources, S.P.G.C., J.C.M. and M.M.M.R.; writing—original draft preparation, R.C.R.G. and F.T.; writing—review and editing, R.C.R.G., P.P., J.C.M. and M.M.M.R.; supervision, S.P.G.C., J.C.M. and M.M.M.R.; project administration, S.P.G.C. and M.M.M.R.; funding acquisition, S.P.G.C. and M.M.M.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Fundação para a Ciência e Tecnologia (FCT) and FEDER (European Fund for Regional Development)-COMPETE-QRENEU through the Chemistry Research Centre of the University of Minho (ref. CQ/UM (UID/QUI/00686/2020), a contract CEECINST/00156/2018/CP1642/CT0011, and a PhD grant of R.C.R. Gonçalves (SFRH/BD/05278/2020, https://doi.org/10.54499/2020.05278.BD). The NMR spectrometer Bruker Avance III 400 is part of the National NMR Network and was purchased within the framework of the National Program for Scientific Re-equipment, contract REDE/1517/RMN/2005 with funds from POCI 2010 (FEDER) and FCT.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article and Supplementary Materials.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- De Rycker, M.; Wyllie, S.; Horn, D.; Read, K.D.; Gilbert, I.H. Anti-Trypanosomatid Drug Discovery: Progress and Challenges. Nat. Rev. Microbiol. 2023, 21, 35–50. [Google Scholar] [CrossRef]
- Gonçalves, R.C.R.; Peñalver, P.; Costa, S.P.G.; Morales, J.C.; Raposo, M.M.M. Polyaromatic Bis(Indolyl)Methane Derivatives with Antiproliferative and Antiparasitic Activity. Molecules 2023, 28, 7728. [Google Scholar] [CrossRef] [PubMed]
- Kourbeli, V.; Chontzopoulou, E.; Moschovou, K.; Pavlos, D.; Mavromoustakos, T.; Papanastasiou, I.P. An Overview on Target-Based Drug Design against Kinetoplastid Protozoan Infections: Human African Trypanosomiasis, Chagas Disease and Leishmaniases. Molecules 2021, 26, 4629. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, I.H. Drug Discovery for Neglected Diseases: Molecular Target-Based and Phenotypic Approaches: Miniperspectives Series on Phenotypic Screening for Antiinfective Targets. J. Med. Chem. 2013, 56, 7719–7726. [Google Scholar] [CrossRef]
- Pozzi, C.; Tassone, G.; Mangani, S. X-Ray Crystallography Contributions to Drug Discovery Against Parasite. In Annual Reports in Medicinal Chemistry; Elsevier: Amsterdam, The Netherlands, 2018; Volume 51, pp. 175–230. ISBN 978-0-12-815143-3. [Google Scholar]
- Hawser, S.; Lociuro, S.; Islam, K. Dihydrofolate Reductase Inhibitors as Antibacterial Agents. Biochem. Pharmacol. 2006, 71, 941–948. [Google Scholar] [CrossRef] [PubMed]
- Wróbel, A.; Arciszewska, K.; Maliszewski, D.; Drozdowska, D. Trimethoprim and Other Nonclassical Antifolates an Excellent Template for Searching Modifications of Dihydrofolate Reductase Enzyme Inhibitors. J. Antibiot. 2020, 73, 5–27. [Google Scholar] [CrossRef]
- Nzila, A. The Past, Present and Future of Antifolates in the Treatment of Plasmodium Falciparum Infection. J. Antimicrob. Chemother. 2006, 57, 1043–1054. [Google Scholar] [CrossRef]
- Gilbert, I.H. Inhibitors of Dihydrofolate Reductase in Leishmania and Trypanosomes. Biochim. Biophys. Acta BBA-Mol. Basis Dis. 2002, 1587, 249–257. [Google Scholar] [CrossRef]
- Gourley, D.G.; Schüttelkopf, A.W.; Leonard, G.A.; Luba, J.; Hardy, L.W.; Beverley, S.M.; Hunter, W.N. Pteridine Reductase Mechanism Correlates Pterin Metabolism with Drug Resistance in Trypanosomatid Parasites. Nat. Struct. Biol. 2001, 8, 521–525. [Google Scholar] [CrossRef]
- Panecka-Hofman, J.; Poehner, I. Structure and Dynamics of Pteridine Reductase 1: The Key Phenomena Relevant to Enzyme Function and Drug Design. Eur. Biophys. J. 2023, 52, 521–532. [Google Scholar] [CrossRef]
- Cavazzuti, A.; Paglietti, G.; Hunter, W.N.; Gamarro, F.; Piras, S.; Loriga, M.; Allecca, S.; Corona, P.; McLuskey, K.; Tulloch, L.; et al. Discovery of Potent Pteridine Reductase Inhibitors to Guide Antiparasite Drug Development. Proc. Natl. Acad. Sci. USA 2008, 105, 1448–1453. [Google Scholar] [CrossRef] [PubMed]
- Kimuda, M.P.; Laming, D.; Hoppe, H.C.; Tastan Bishop, Ö. Identification of Novel Potential Inhibitors of Pteridine Reductase 1 in Trypanosoma Brucei via Computational Structure-Based Approaches and in Vitro Inhibition Assays. Molecules 2019, 24, 142. [Google Scholar] [CrossRef] [PubMed]
- Panecka-Hofman, J.; Poehner, I.; Wade, R.C. Anti-Trypanosomatid Structure-Based Drug Design—Lessons Learned from Targeting the Folate Pathway. Expert Opin. Drug Discov. 2022, 17, 1029–1045. [Google Scholar] [CrossRef] [PubMed]
- Pöhner, I.; Quotadamo, A.; Panecka-Hofman, J.; Luciani, R.; Santucci, M.; Linciano, P.; Landi, G.; Di Pisa, F.; Dello Iacono, L.; Pozzi, C.; et al. Multitarget, Selective Compound Design Yields Potent Inhibitors of a Kinetoplastid Pteridine Reductase 1. J. Med. Chem. 2022, 65, 9011–9033. [Google Scholar] [CrossRef] [PubMed]
- Treibs, A.; Kreuzer, F. Difluorboryl-Komplexe von Di- Und Tripyrrylmethenen. Justus Liebigs Ann. Chem. 1968, 718, 208–223. [Google Scholar] [CrossRef]
- Ulrich, G.; Ziessel, R.; Harriman, A. The Chemistry of Fluorescent Bodipy Dyes: Versatility Unsurpassed. Angew. Chem. Int. Ed. 2008, 47, 1184–1201. [Google Scholar] [CrossRef] [PubMed]
- Boens, N.; Verbelen, B.; Dehaen, W. Postfunctionalization of the BODIPY Core: Synthesis and Spectroscopy. Eur. J. Org. Chem. 2015, 2015, 6577–6595. [Google Scholar] [CrossRef]
- Loudet, A.; Burgess, K. BODIPY Dyes and Their Derivatives: Syntheses and Spectroscopic Properties. Chem. Rev. 2007, 107, 4891–4932. [Google Scholar] [CrossRef] [PubMed]
- Kowada, T.; Maeda, H.; Kikuchi, K. BODIPY-Based Probes for the Fluorescence Imaging of Biomolecules in Living Cells. Chem. Soc. Rev. 2015, 44, 4953–4972. [Google Scholar] [CrossRef]
- Bañuelos, J. BODIPY Dye, the Most Versatile Fluorophore Ever? Chem. Rec. 2016, 16, 335–348. [Google Scholar] [CrossRef]
- Poddar, M.; Misra, R. Recent Advances of BODIPY Based Derivatives for Optoelectronic Applications. Coord. Chem. Rev. 2020, 421, 213462. [Google Scholar] [CrossRef]
- Kaur, P.; Singh, K. Recent Advances in the Application of BODIPY in Bioimaging and Chemosensing. J. Mater. Chem. C 2019, 7, 11361–11405. [Google Scholar] [CrossRef]
- Prieto-Montero, R.; Prieto-Castañeda, A.; Sola-Llano, R.; Agarrabeitia, A.R.; García-Fresnadillo, D.; López-Arbeloa, I.; Villanueva, A.; Ortiz, M.J.; De La Moya, S.; Martínez-Martínez, V. Exploring BODIPY Derivatives as Singlet Oxygen Photosensitizers for PDT. Photochem. Photobiol. 2020, 96, 458–477. [Google Scholar] [CrossRef]
- Carpenter, B.; Situ, X.; Scholle, F.; Bartelmess, J.; Weare, W.; Ghiladi, R. Antiviral, Antifungal and Antibacterial Activities of a BODIPY-Based Photosensitizer. Molecules 2015, 20, 10604–10621. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Gong, Q.; Wang, L.; Hao, E.; Jiao, L. The Main Strategies for Tuning BODIPY Fluorophores into Photosensitizers. J. Porphyr. Phthalocyanines 2020, 24, 603–635. [Google Scholar] [CrossRef]
- Gonçalves, R.C.R.; Pina, J.; Costa, S.P.G.; Raposo, M.M.M. Synthesis and Characterization of Aryl-Substituted BODIPY Dyes Displaying Distinct Solvatochromic Singlet Oxygen Photosensitization Efficiencies. Dye. Pigment. 2021, 196, 109784. [Google Scholar] [CrossRef]
- Gonçalves, R.C.R.; Pinto, S.C.S.; Pina, J.; Gomes-da-Silva, L.C.; Costa, S.P.G.; Raposo, M.M.M. Investigating the Photophysical Properties and Biological Efficacy of BODIPY Derivatives as Photosensitizers in Photodynamic Therapy. Chem. Proc. 2023, 14, 71. [Google Scholar] [CrossRef]
- De La Torre, B.G.; Hornillos, V.; Luque-Ortega, J.R.; Abengózar, M.A.; Amat-Guerri, F.; Ulises Acuña, A.; Rivas, L.; Andreu, D. A BODIPY-Embedding Miltefosine Analog Linked to Cell-Penetrating Tat(48-60) Peptide Favors Intracellular Delivery and Visualization of the Antiparasitic Drug. Amino Acids 2014, 46, 1047–1058. [Google Scholar] [CrossRef]
- Rodríguez, G.; Nargoli, J.; López, A.; Moyna, G.; Álvarez, G.; Fernández, M.; Osorio-Martínez, C.A.; González, M.; Cerecetto, H. Synthesis and in Vivo Proof of Concept of a BODIPY-Based Fluorescent Probe as a Tracer for Biodistribution Studies of a New Anti-Chagas Agent. RSC Adv. 2017, 7, 7983–7989. [Google Scholar] [CrossRef]
- Hornillos, V.; Carrillo, E.; Rivas, L.; Amat-Guerri, F.; Acuña, A.U. Synthesis of BODIPY-Labeled Alkylphosphocholines with Leishmanicidal Activity, as Fluorescent Analogues of Miltefosine. Bioorg. Med. Chem. Lett. 2008, 18, 6336–6339. [Google Scholar] [CrossRef]
- Gonçalves, R.C.R.; Nogueira, M.B.; Costa, S.P.G.; Raposo, M.M.M. BODIPY Derivatives: Synthesis and Evaluation of Their Optical Properties. Proceedings 2019, 9, 10. [Google Scholar] [CrossRef]
- Pinto, S.C.S.; Gonçalves, R.C.R.; Costa, S.P.G.; Raposo, M.M.M. Synthesis and Characterization of a Meso-Anthracene-BODIPY Derivative for Colorimetric Recognition of Cu2+ and Fe3+. Chem. Proc. 2021, 3, 79. [Google Scholar] [CrossRef]
- Pinto, S.C.S.; Gonçalves, R.C.R.; Costa, S.P.G.; Raposo, M.M.M. Synthesis, Characterization and Evaluation of a Carbazolyl-BODIPY as a Fluorimetric Chemosensor for F−. Chem. Proc. 2022, 8, 20. [Google Scholar] [CrossRef]
- Gonçalves, R.C.R.; Boland, M.L.; Costa, S.P.G.; Raposo, M.M.M. A BODIPY Derivative for Selective Fluorescent Chemosensing of Iron (III). Eng. Proc. 2022, 27, 7. [Google Scholar] [CrossRef]
- Gonçalves, R.C.R.; Belmonte-Reche, E.; Pina, J.; Costa Da Silva, M.; Pinto, S.C.S.; Gallo, J.; Costa, S.P.G.; Raposo, M.M.M. Bioimaging of Lysosomes with a BODIPY pH-Dependent Fluorescent Probe. Molecules 2022, 27, 8065. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, R.C.R.; Pinto, S.C.S.; Costa, S.P.G.; Raposo, M.M.M. Synthesis, Characterization and Evaluation of a Novel BODIPY Derivative as a Colorimetric Chemosensor for Fe3+ Recognition. Proceedings 2019, 41, 40. [Google Scholar] [CrossRef]
- Gonçalves, R.C.R.; Pinto, S.C.S.; Costa, S.P.G.; Raposo, M.M.M. A Meso-Triphenylamine-BODIPY Derivative for the Optical Chemosensing of Metal Ions. Chem. Proc. 2021, 3, 65. [Google Scholar] [CrossRef]
- Gonçalves, R.C.R.; Boland, M.L.; Costa, S.P.G.; Raposo, M.M.M. Anion Dual Mode Fluoro-Chromogenic Chemosensor Based on a BODIPY Core. Eng. Proc. 2022, 27, 6. [Google Scholar] [CrossRef]
- Pinto, S.C.S.; Gonçalves, R.C.R.; Costa, S.P.G.; Raposo, M.M.M. Colorimetric Chemosensor for Cu2+ and Fe3+ Based on a Meso-Triphenylamine-BODIPY Derivative. Sensors 2023, 23, 6995. [Google Scholar] [CrossRef]
- Cunha Dias De Rezende, L.; Menezes Vaidergorn, M.; Biazzotto Moraes, J.C.; Da Silva Emery, F. Synthesis, Photophysical Properties and Solvatochromism of Meso-Substituted Tetramethyl BODIPY Dyes. J. Fluoresc. 2014, 24, 257–266. [Google Scholar] [CrossRef]
- Shi, W.-J.; Yan, X.-H.; Yang, J.; Wei, Y.-F.; Huo, Y.-T.; Su, C.-L.; Yan, J.; Han, D.; Niu, L. Development of Meso -Five-Membered Heterocycle BODIPY-Based AIE Fluorescent Probes for Dual-Organelle Viscosity Imaging. Anal. Chem. 2023, 95, 9646–9653. [Google Scholar] [CrossRef] [PubMed]
- Kukoyi, A.; He, H.; Wheeler, K. Quinoline-Functionalized BODIPY Dyes: Structural and Photophysical Properties. J. Photochem. Photobiol. Chem. 2022, 425, 113686. [Google Scholar] [CrossRef]
- Gonçalves, R.C.R.; Nogueira, M.B.; Costa, S.P.G.; Raposo, M.M.M. Functionalized BODIPY Derivatives as Potential Fluorescent Labels. Proceedings 2019, 9, 36. [Google Scholar] [CrossRef]
- Tulloch, L.B.; Martini, V.P.; Iulek, J.; Huggan, J.K.; Lee, J.H.; Gibson, C.L.; Smith, T.K.; Suckling, C.J.; Hunter, W.N. Structure-Based Design of Pteridine Reductase Inhibitors Targeting African Sleeping Sickness and the Leishmaniases. J. Med. Chem. 2010, 53, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Voroshylova, I.V.; Teixeira, F.; Costa, R.; Pereira, C.M.; Cordeiro, M.N.D.S. Interactions in the Ionic Liquid [EMIM][FAP]: A Coupled Experimental and Computational Analysis. Phys. Chem. Chem. Phys. 2016, 18, 2617–2628. [Google Scholar] [CrossRef] [PubMed]
- Crosby, G.A.; Demas, J.N. Measurement of Photoluminescence Quantum Yields. Review. J. Phys. Chem. 1971, 75, 991–1024. [Google Scholar] [CrossRef]
- Wirtz, E.; Leal, S.; Ochatt, C.; Cross, G.M. A Tightly Regulated Inducible Expression System for Conditional Gene Knock-Outs and Dominant-Negative Genetics in Trypanosoma brucei. Mol. Biochem. Parasitol. 1999, 99, 89–101. [Google Scholar] [CrossRef]
- Cabello-Donayre, M.; Malagarie-Cazenave, S.; Campos-Salinas, J.; Gálvez, F.J.; Rodríguez-Martínez, A.; Pineda-Molina, E.; Orrego, L.M.; Martínez-García, M.; Sánchez-Cañete, M.P.; Estévez, A.M.; et al. Trypanosomatid Parasites Rescue Heme from Endocytosed Hemoglobin through Lysosomal HRG Transporters: Trypanosomatid HRG Proteins Rescue Heme from Hb. Mol. Microbiol. 2016, 101, 895–908. [Google Scholar] [CrossRef]
- Belmonte-Reche, E.; Martínez-García, M.; Guédin, A.; Zuffo, M.; Arévalo-Ruiz, M.; Doria, F.; Campos-Salinas, J.; Maynadier, M.; López-Rubio, J.J.; Freccero, M.; et al. G-Quadruplex Identification in the Genome of Protozoan Parasites Points to Naphthalene Diimide Ligands as New Antiparasitic Agents. J. Med. Chem. 2018, 61, 1231–1240. [Google Scholar] [CrossRef]
- Larson, E.M.; Doughman, D.J.; Gregerson, D.S.; Obritsch, W.F. A New, Simple, Nonradioactive, Nontoxic in Vitro Assay to Monitor Corneal Endothelial Cell Viability. Investig. Ophtalmol. Vis. Sci. 1997, 38, 1929–1933. [Google Scholar]
- Pérez-Victoria, J.M.; Bavchvarov, B.I.; Torrecillas, I.R.; Martínez-García, M.; López-Martín, C.; Campillo, M.; Castanys, S.; Gamarro, F. Sitamaquine Overcomes ABC-Mediated Resistance to Miltefosine and Antimony in Leishmania. Antimicrob. Agents Chemother. 2011, 55, 3838–3844. [Google Scholar] [CrossRef] [PubMed]
- Becke, A.D. A New Mixing of Hartree–Fock and Local Density-Functional Theories. J. Chem. Phys. 1993, 98, 1372–1377. [Google Scholar] [CrossRef]
- Weigend, F.; Ahlrichs, R. Balanced Basis Sets of Split Valence, Triple Zeta Valence and Quadruple Zeta Valence Quality for H to Rn: Design and Assessment of Accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297. [Google Scholar] [CrossRef] [PubMed]
- Peterson, K.A.; Figgen, D.; Goll, E.; Stoll, H.; Dolg, M. Systematically Convergent Basis Sets with Relativistic Pseudopotentials. II. Small-Core Pseudopotentials and Correlation Consistent Basis Sets for the Post- d Group 16–18 Elements. J. Chem. Phys. 2003, 119, 11113–11123. [Google Scholar] [CrossRef]
- Breneman, C.M.; Wiberg, K.B. Determining Atom-centered Monopoles from Molecular Electrostatic Potentials. The Need for High Sampling Density in Formamide Conformational Analysis. J. Comput. Chem. 1990, 11, 361–373. [Google Scholar] [CrossRef]
- Teixeira, F.; Mosquera, R.; Melo, A.; Freire, C.; Cordeiro, M.N.D.S. Charge Distribution in Mn(Salen) Complexes. Int. J. Quantum Chem. 2014, 114, 525–533. [Google Scholar] [CrossRef]
- Neese, F.; Wennmohs, F.; Becker, U.; Riplinger, C. The ORCA Quantum Chemistry Program Package. J. Chem. Phys. 2020, 152, 224108. [Google Scholar] [CrossRef] [PubMed]
- wwPDB consortium. Protein Data Bank: The Single Global Archive for 3D Macromolecular Structure Data. Nucleic Acids Res. 2019, 47, D520–D528. [CrossRef] [PubMed]
- Altschul, S. Gapped BLAST and PSI-BLAST: A New Generation of Protein Database Search Programs. Nucleic Acids Res. 1997, 25, 3389–3402. [Google Scholar] [CrossRef]
- Altschul, S.F.; Wootton, J.C.; Gertz, E.M.; Agarwala, R.; Morgulis, A.; Schäffer, A.A.; Yu, Y. Protein Database Searches Using Compositionally Adjusted Substitution Matrices. FEBS J. 2005, 272, 5101–5109. [Google Scholar] [CrossRef]
- Harris, R.; Olson, A.J.; Goodsell, D.S. Automated Prediction of Ligand-binding Sites in Proteins. Proteins Struct. Funct. Bioinform. 2008, 70, 1506–1517. [Google Scholar] [CrossRef] [PubMed]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the Speed and Accuracy of Docking with a New Scoring Function, Efficient Optimization, and Multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Eberhardt, J.; Santos-Martins, D.; Tillack, A.F.; Forli, S. AutoDock Vina 1.2.0: New Docking Methods, Expanded Force Field, and Python Bindings. J. Chem. Inf. Model. 2021, 61, 3891–3898. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).