A New BODIPY-Based Receptor for the Fluorescent Sensing of Catecholamines
Abstract
1. Introduction
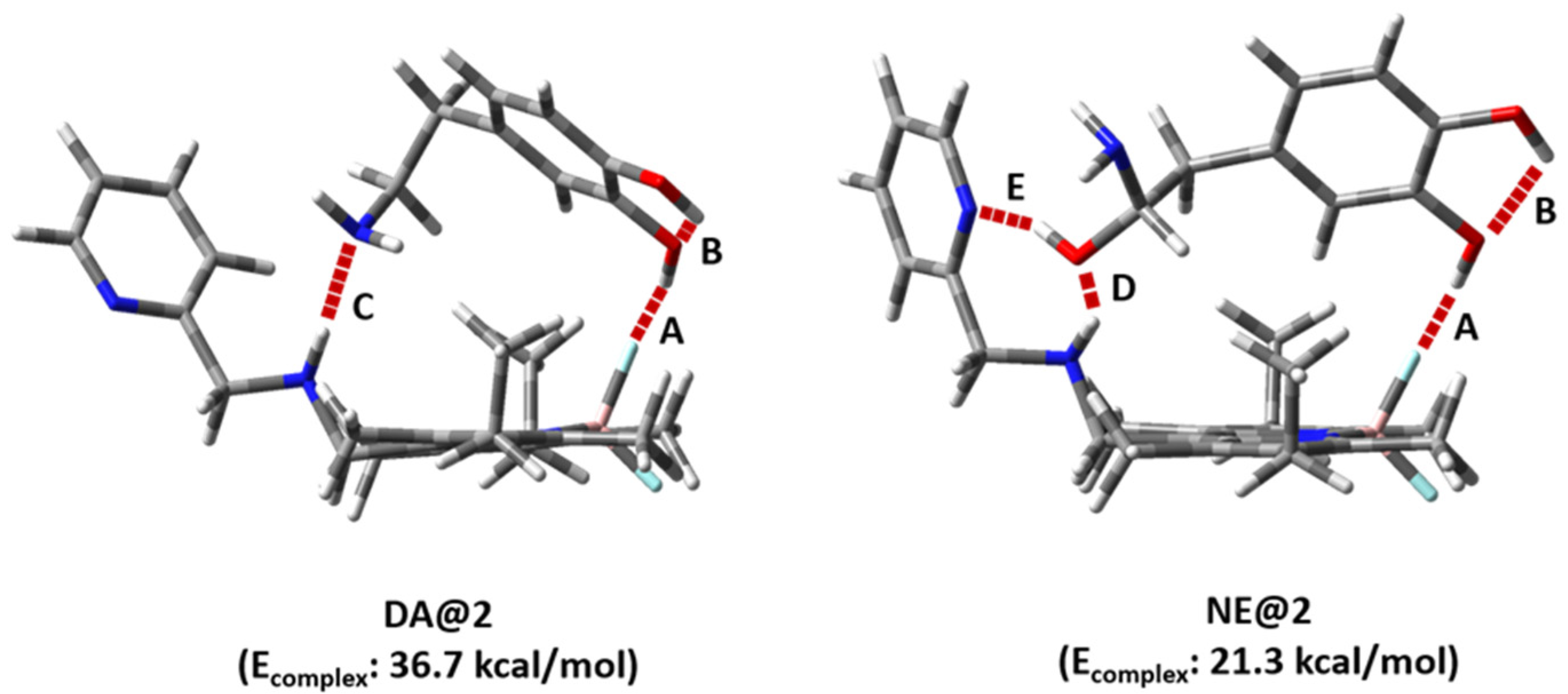
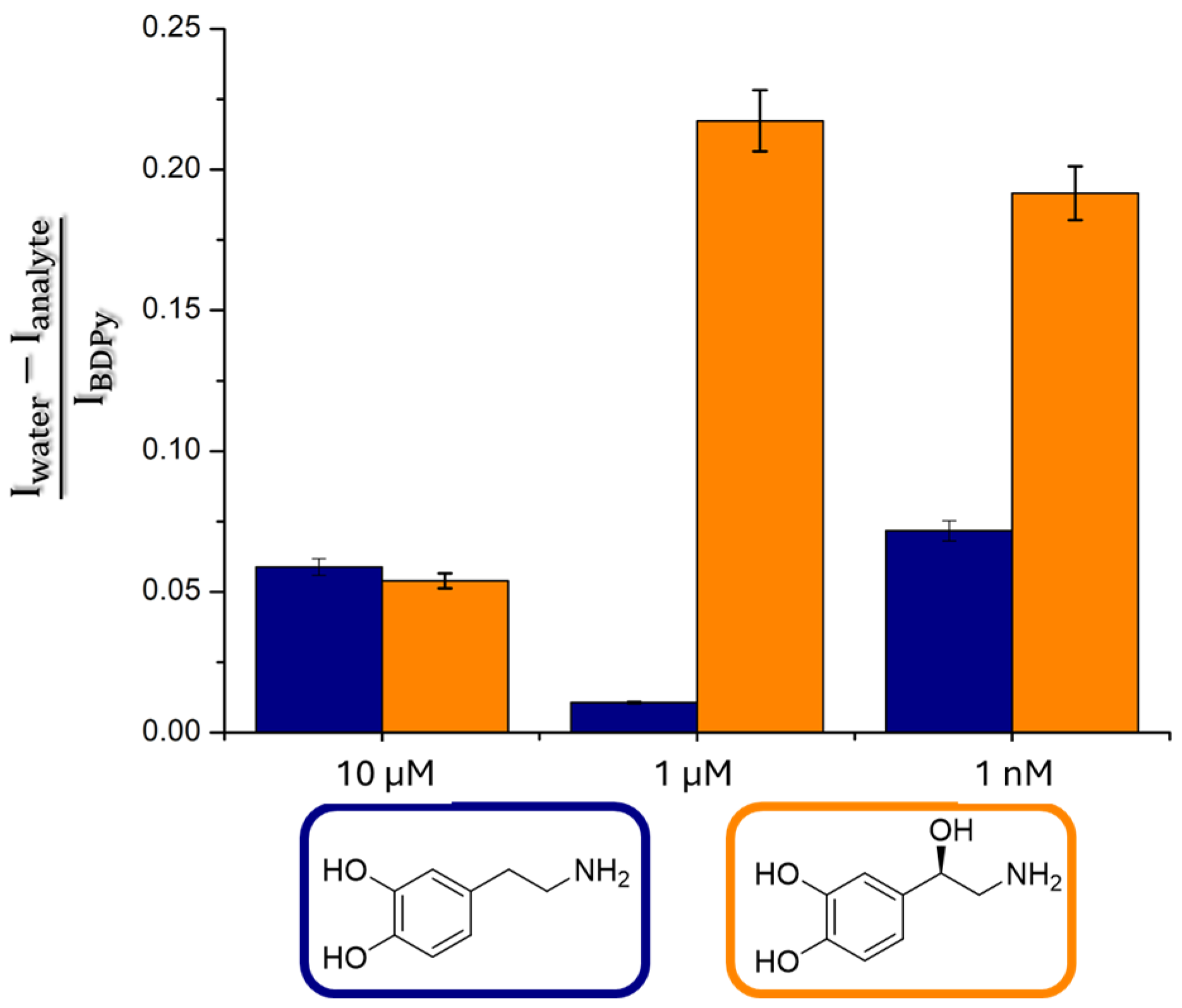
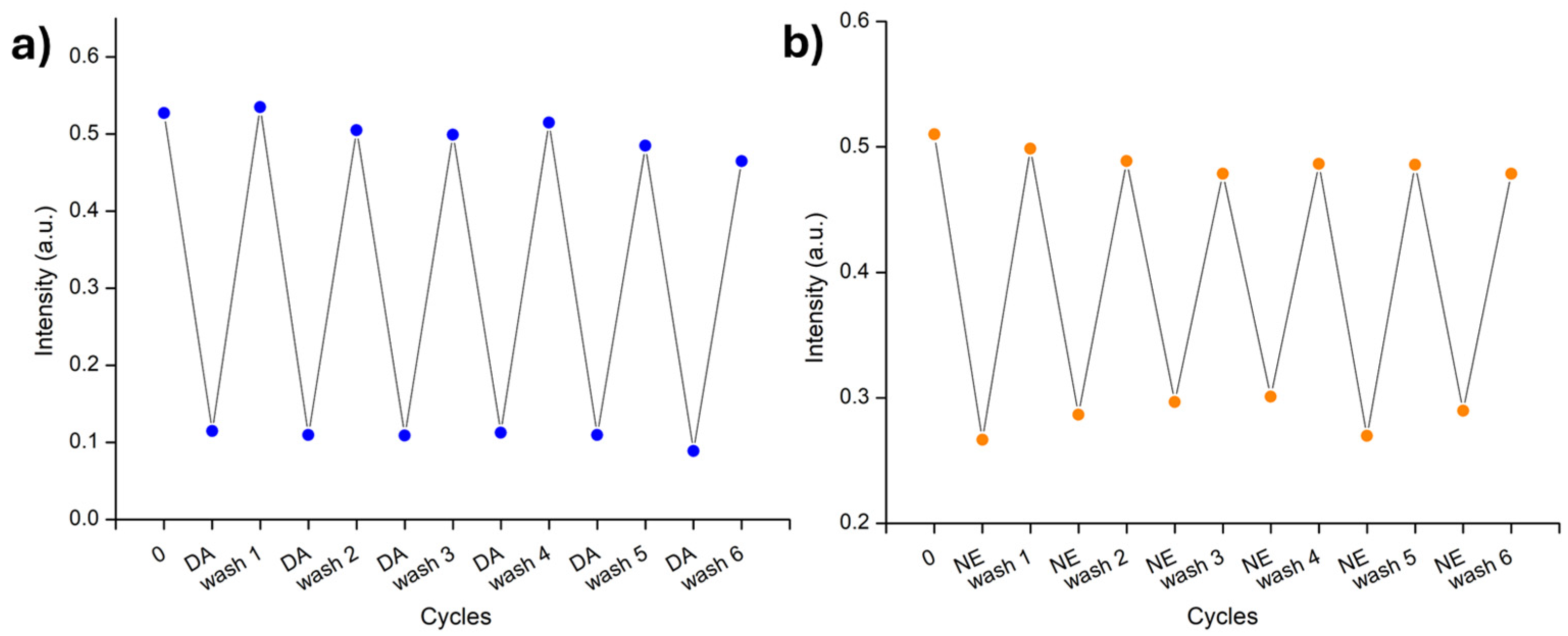
2. Results and Discussion
Sensing by the Strip Test
3. Materials and Methods
3.1. General Experimental Methods
3.2. Synthesis of (2)
3.3. The Procedure for the Fluorescence Titrations
3.4. Stoichiometry
3.5. Procedure for the Strip Test
3.6. Recovery
3.7. Computational Details
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mazloum-Ardakani, M.; Beitollahi, H.; Amini, M.K.; Mirkhalaf, F.; Mirjalili, B.-F. A highly sensitive nanostructure-based electrochemical sensor for electrocatalytic determination of norepinephrine in the presence of acetaminophen and tryptophan. Biosens. Bioelectron. 2011, 26, 2102. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Kang, K.; Wang, S.; Kang, W.; Cheng, C.; Niu, L.M.; Guo, Z. A novel label-free fluorescence aptasensor for dopamine detection based on an Exonuclease III- and SYBR Green I- aided amplification strategy. Sens. Actuators B 2020, 305, 127348. [Google Scholar] [CrossRef]
- Meyer-Lindenberg, A.; Miletich, R.S.; Kohn, P.D.; Esposito, G.; Carson, R.E.; Quarantelli, M.; Weinberger, D.R.; Berman, K.F. Reduced prefrontal activity predicts exaggerated striatal dopaminergic function in schizophrenia. Nat. Neurosci. 2002, 5, 267–271. [Google Scholar] [CrossRef] [PubMed]
- Vidoni, C.; Secomandi, E.; Castiglioni, A.; Melone, M.A.B.; Isidoro, C. Resveratrol protects neuronal-like cells expressing mutant Huntingtin from dopamine toxicity by rescuing ATG4-mediated autophagosome formation. Neurochem. Int. 2018, 117, 174–187. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Wang, W.; Yu, P.; Xiong, E.; Zhang, X.; Chen, J. A simple label-free electrochemical aptasensor for dopamine detection. RSC Adv. 2014, 4, 52250–52255. [Google Scholar] [CrossRef]
- Li, B.-R.; Hsieh, Y.-J.; Chen, Y.-X.; Chung, Y.-T.; Pan, C.-Y.; Chen, Y.-T. An ultrasensitive nanowire-transistor biosensor for detecting dopamine release from living PC12 cells under hypoxic stimulation. J. Am. Chem. Soc. 2013, 135, 16034–16037. [Google Scholar] [CrossRef]
- Santonocito, R.; Tuccitto, N.; Pappalardo, A.; Trusso Sfrazzetto, G. Smartphone-based dopamine detection by fluorescent supramolecular sensor. Molecules 2022, 27, 7503. [Google Scholar] [CrossRef] [PubMed]
- Hettie, K.S.; Liu, X.; Gillis, K.D.; Glass, T.E. Selective catecholamine recognition with NeuroSensor 521: A fluorescent sensor for the visualization of norepinephrine in fixed and live cells. ACS Chem. Neurosci. 2013, 4, 918–923. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, T.; Jung, H.S.; Jang, J.H.; Kim, T.W.; Kang, C.; Kim, J.S. Chemical sensing of neurotransmitters. Chem. Soc. Rev. 2014, 43, 4684–4713. [Google Scholar] [CrossRef]
- Feng, J.; Zhang, C.; Lischinsky, J.E.; Jing, M.; Zhou, J.; Wang, H.; Zhang, Y.; Dong, A.; Wu, Z.; Wu, H.; et al. A genetically encoded fluorescent sensor for rapid and specific in vivo detection of norepinephrine. Neuron 2019, 102, 745–761. [Google Scholar] [CrossRef]
- Okumura, T.; Nakajima, Y.; Matsuoka, M.; Takamatsu, T. Study of salivary catecholamines using fully automated column-switching high-performance liquid chromatography. J. Chromatogr. B. 1997, 694, 305–316. [Google Scholar] [CrossRef] [PubMed]
- De Lima, L.F.; Ferreira, A.L.; do Nascimento, G.H.M.; Cardoso, L.P.; de Jesus, M.B.; de Araujo, W.R. Tongue depressor (bio) sensors: A fast decentralized self-testing of salivary biomarkers for personalized medicine. Chem. Eng. J. 2024, 494, 152885. [Google Scholar] [CrossRef]
- Wang, J.; Li, M.; Shi, Z.; Li, N.; Gu, Z. Electrocatalytic oxidation of norepinephrine at a glassy carbon electrode modified with single wall carbon nanotubes. Electroanalysis 2002, 14, 225–230. [Google Scholar] [CrossRef]
- Zhao, H.; Zhang, Y.; Yuan, Z. Electrochemical behavior of norepinephrine at poly (2,4,6-trimethylpyridine) modified glassy carbon electrode. Electroanalysis 2002, 14, 445–448. [Google Scholar] [CrossRef]
- Mphuthi, N.G.; Adekunle, A.S.; Ebenso, E.E. Electrocatalytic oxidation of Epinephrine and Norepinephrine at metal oxide doped phthalocyanine/MWCNT composite sensor. Sci. Rep. 2016, 6, 26938. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Lin, X.; Luo, H.; Huang, L. Electrocatalytic oxidation and determination of norepinephrine at poly (cresol red) modified glassy carbon electrode. Electroanalysis 2005, 17, 941–945. [Google Scholar] [CrossRef]
- Ribeiro, J.A.; Fernandes, P.M.V.; Pereira, C.M.; Silva, F. Electrochemical sensors and biosensors for determination of catecholamine neurotransmitters: A review. Talanta 2016, 160, 653–679. [Google Scholar] [CrossRef]
- Beitollahi, H.; Karimi-Maleh, H.; Khabazzadeh, H. Nanomolar and selective determination of epinephrine in the presence of norepinephrine using carbon paste electrode modified with carbon nanotubes and novel 2-(4-oxo-3-phenyl-3, 4-dihydro-quinazolinyl)-N0-phenyl-hydrazinecarbothioamide. Anal. Chem. 2008, 80, 9848–9851. [Google Scholar] [CrossRef]
- Eddin, F.B.K.; Fen, Y.W. Recent Advances in Electrochemical and Optical Sensing of Dopamine. Sensors 2020, 20, 1039. [Google Scholar] [CrossRef]
- Butera, E.; Zammataro, A.; Pappalardo, A.; Sfrazzetto, G.T. Supramolecular Sensing of Chemical Warfare Agents. ChemPlusChem 2021, 86, 681–695. [Google Scholar] [CrossRef]
- Gorbunova, M.V.; Apyari, V.V.; Zolotov, I.I.; Dmitrienko, S.G.; Garshev, A.V.; Volkov, P.A.; Bochenkov, V.E. A new nanocomposite optical sensor based on polyurethane foam and gold nanorods for solid-phase spectroscopic determination of catecholamines. Gold Bull. 2019, 52, 115–124. [Google Scholar] [CrossRef]
- Jafarinejad, S.; Ghazi-Khansari, M.; Ghasemi, F.; Sasanpour, P.; Hormozi-Nezhad, M.R. Colorimetric Fingerprints of Gold Nanorods for Discriminating Catecholamine Neurotransmitters in Urine Samples. Anal. Chim. Acta 2016, 917, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Qu, K.; Wang, J.; Ren, J.; Qu, X. Carbon dots prepared by hydrothermal treatment of dopamine as an effective fluorescent sensing platform for the label-free detection of iron (III) ions and dopamine. Chem. Eur. J. 2013, 19, 7243–7249. [Google Scholar] [CrossRef] [PubMed]
- Hassani-Marand, M.; Fahimi-Kashani, N.; Hormozi-Nezhad, M.R. Machine-learning assisted multiplex detection of catecholamine neurotransmitters with a colorimetric sensor array. Anal. Methods 2023, 15, 1123–1134. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, X.A.; Gillis, K.D.; Glass, T.E. A High-Affinity Fluorescent Sensor for Catecholamine: Application to Monitoring Norepinephrine Exocytosis. Angew. Chem. Int. Ed. 2019, 58, 7611. [Google Scholar]
- Amat-Guerri, F.; Liras, M.; Carrascoso, M.L.; Sastre, R. Methacrylate-tethered analogs of the laser dye PM567—Synthesis, copolymerization with methyl methacrylate and photostability of the copolymers. Photochem. Photobiol. 2003, 77, 577–584. [Google Scholar] [CrossRef]
- Velapoldi, R.A.; Tonnesen, H.H. Corrected Emission Spectra and Quantum Yields for a Series of Fluorescent Compounds in the Visible Spectral Region. J. Fluoresc. 2004, 14, 465–472. [Google Scholar] [CrossRef]
- Caruso, M.; Petroselli, M.; Cametti, M. Design and Synthesis of Multipurpose Derivatives for N-Hydroxyimide and NHPI-based Catalysis Applications. ChemistrySelect 2021, 6, 12975–12980. [Google Scholar] [CrossRef]
- Petroselli, M.; Saccone, M.; Cametti, M. Aryl Boronic Acids in Columnar Stacked Co-crystalline Materials: Key-Factors Governing the Assembly with Quinones. ChemPhysChem 2023, 24, e202200883. [Google Scholar] [CrossRef]
- Petroselli, M.; Chen, Y.-Q.; Zhao, M.-Z.; Rebek, J., Jr.; Yu, Y. CH⋯XC bonds in alkyl halides drive reverse selectivities in confined spaces. Chin. Chem. Lett. 2023, 34, 107834. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, H.; Han, G.; Li, Z.; Mugo, S.M.; Wang, H.; Zhang, Q. Portable Saliva Sensor Based on Dual Recognition Elements for Detection of Caries Pathogenic Bacteria. Anal. Chem. 2024, 96, 9780–9789. [Google Scholar] [CrossRef] [PubMed]
- Santonocito, R.; Spina, M.; Puglisi, R.; Pappalardo, A.; Tuccitto, N.; Trusso Sfrazzetto, G. Detection of a Nerve Agent Simulant by a Fluorescent Sensor Array. Chemosensors 2023, 11, 503. [Google Scholar] [CrossRef]
- Legnani, L.; Puglisi, R.; Pappalardo, A.; Chiacchio, M.A.; Trusso Sfrazzetto, G. Supramolecular recognition of phosphocholine by an enzyme-like cavitand receptor. Chem. Commun. 2020, 56, 539–542. [Google Scholar] [CrossRef] [PubMed]
- Puglisi, R.; Mineo, P.G.; Pappalardo, A.; Gulino, A.; Trusso Sfrazetto, G. Supramolecular Detection of a Nerve Agent Simulant by Fluorescent Zn–Salen Oligomer Receptors. Molecules 2019, 24, 2160. [Google Scholar] [CrossRef]
- Trusso Sfrazzetto, G.; Millesi, S.; Pappalardo, A.; Tomaselli, G.A.; Ballistreri, F.P.; Toscano, R.M.; Fragalà, I.; Gulino, A. Nerve Gas Simulant Sensing by an Uranyl-Salen Monolayer Covalently Anchored on Quartz Substrates. Chem. Eur. J. 2017, 23, 1576–1583. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalamani, G.; Barone, V.; Mennucci, B.; Peterson, G.A.; et al. Gaussian 09, Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2009.

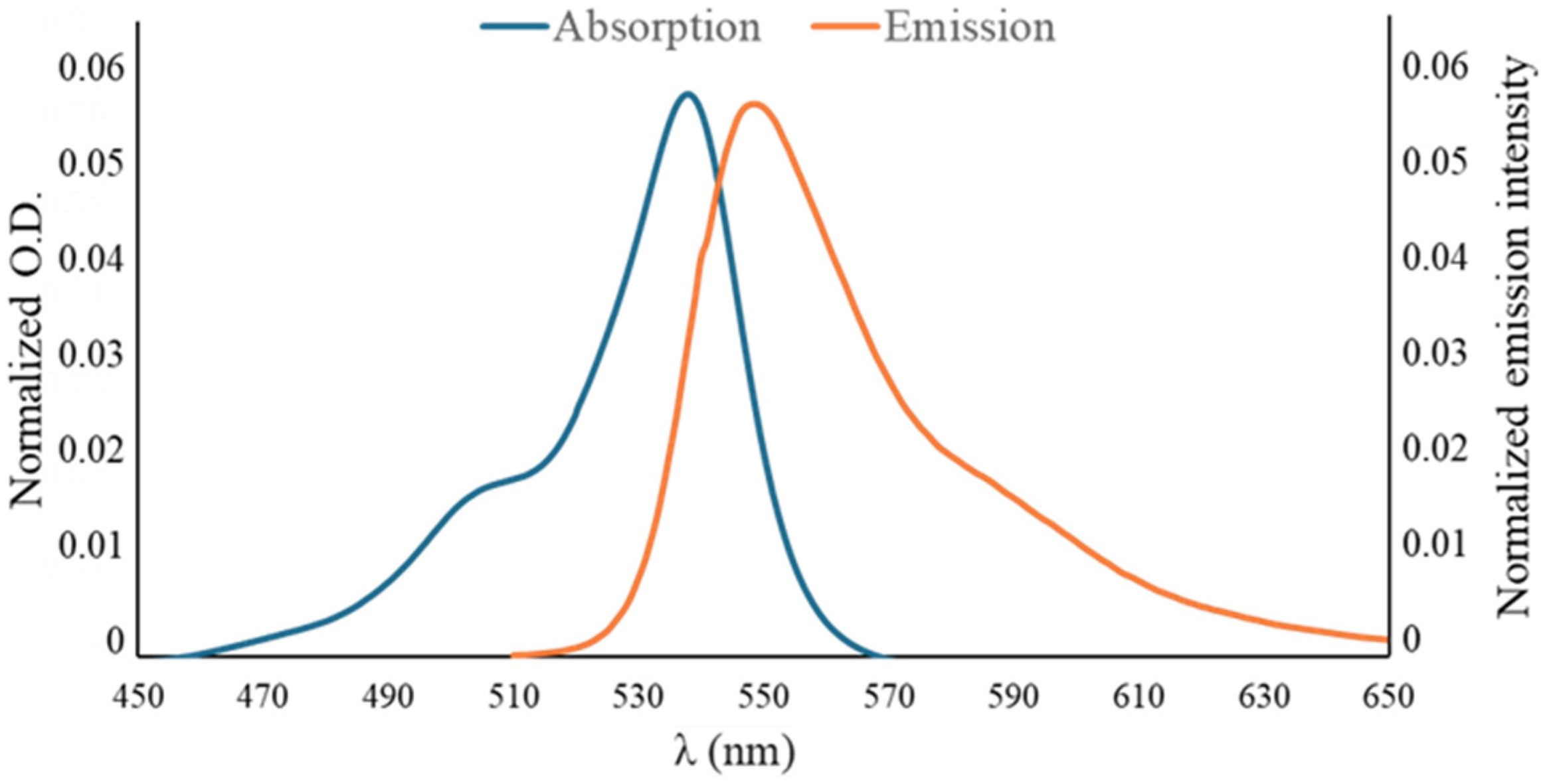



| BODIPY | Abs (nm) a | Em (nm) a,b | Qy c | Stokes Shifts (nm) |
|---|---|---|---|---|
| (1) | 519, 550 | 540 | 0.62 | 40 |
| (2) | 500, 538 | 550 | 0.37 | 50 |
| Entry | Hydrogen Bond | HB Length (Å) | HB Angle (°) | ||
|---|---|---|---|---|---|
| DA@2 | NE@2 | DA@2 | NE@2 | ||
| 1 | A | 1.748 | 1.778 | 137.03 | 145.57 |
| 2 | B | 2.099 | 2.108 | 115.85 | 115.72 |
| 3 | C | 2.069 | - | 100.97 | - |
| 4 | D | - | 2.108 | - | 150.92 |
| 5 | E | - | 1.934 | - | 157.60 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puglisi, R.; Cavallaro, A.; Pappalardo, A.; Petroselli, M.; Santonocito, R.; Trusso Sfrazzetto, G. A New BODIPY-Based Receptor for the Fluorescent Sensing of Catecholamines. Molecules 2024, 29, 3714. https://doi.org/10.3390/molecules29153714
Puglisi R, Cavallaro A, Pappalardo A, Petroselli M, Santonocito R, Trusso Sfrazzetto G. A New BODIPY-Based Receptor for the Fluorescent Sensing of Catecholamines. Molecules. 2024; 29(15):3714. https://doi.org/10.3390/molecules29153714
Chicago/Turabian StylePuglisi, Roberta, Alessia Cavallaro, Andrea Pappalardo, Manuel Petroselli, Rossella Santonocito, and Giuseppe Trusso Sfrazzetto. 2024. "A New BODIPY-Based Receptor for the Fluorescent Sensing of Catecholamines" Molecules 29, no. 15: 3714. https://doi.org/10.3390/molecules29153714
APA StylePuglisi, R., Cavallaro, A., Pappalardo, A., Petroselli, M., Santonocito, R., & Trusso Sfrazzetto, G. (2024). A New BODIPY-Based Receptor for the Fluorescent Sensing of Catecholamines. Molecules, 29(15), 3714. https://doi.org/10.3390/molecules29153714










