Valorisation of Agri-Food Waste for Bioactive Compounds: Recent Trends and Future Sustainable Challenges
Abstract
1. Introduction
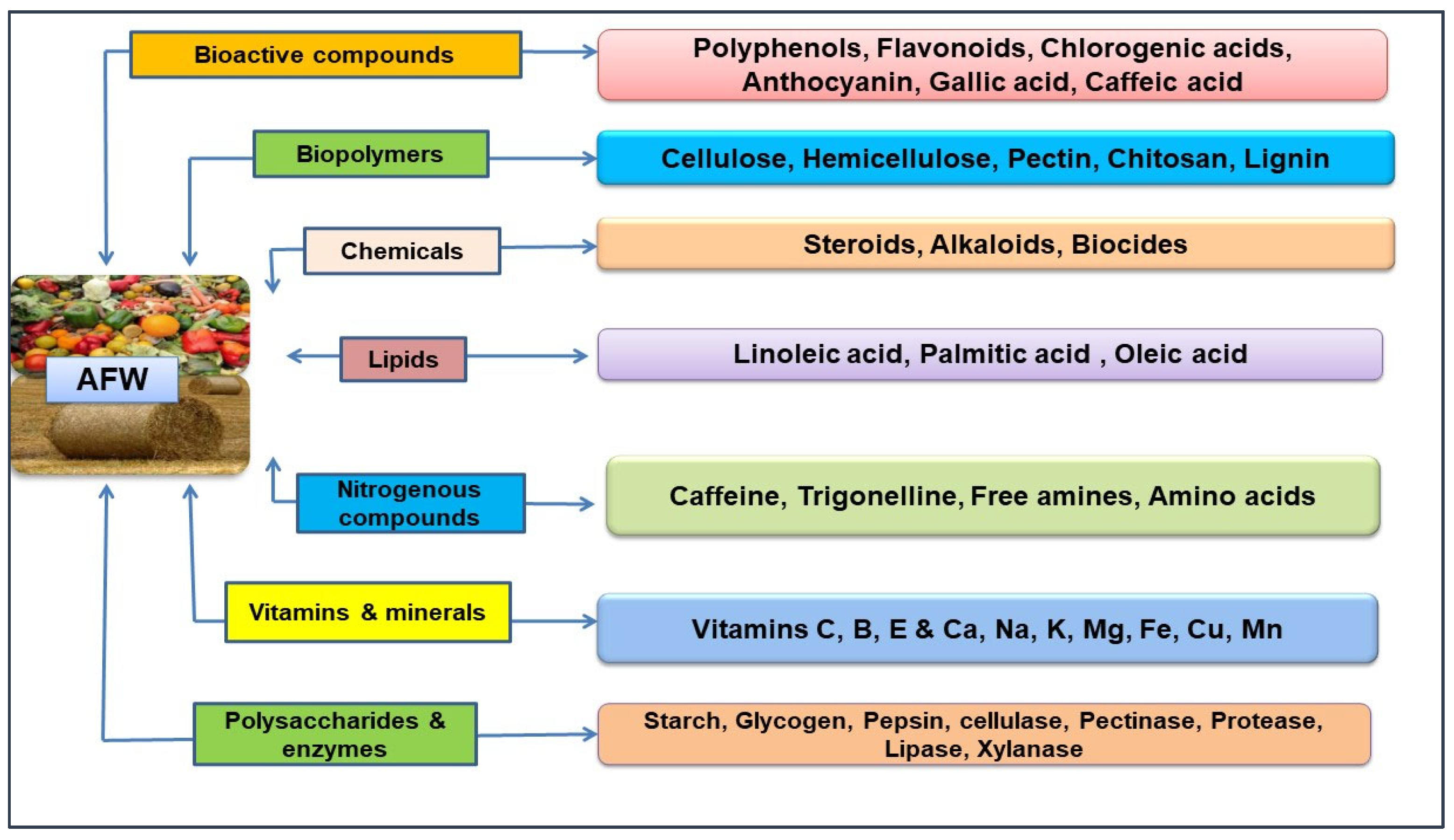
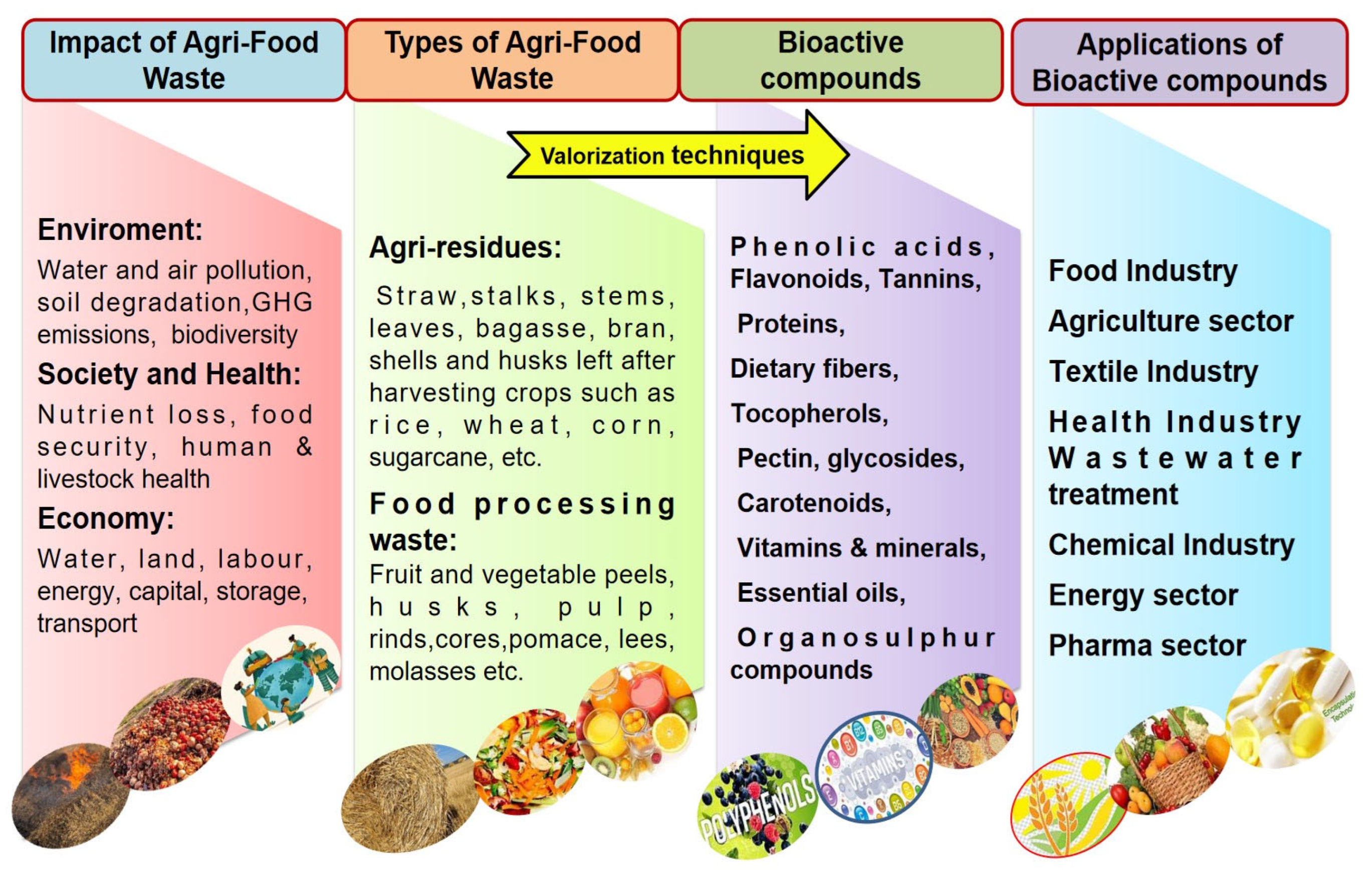
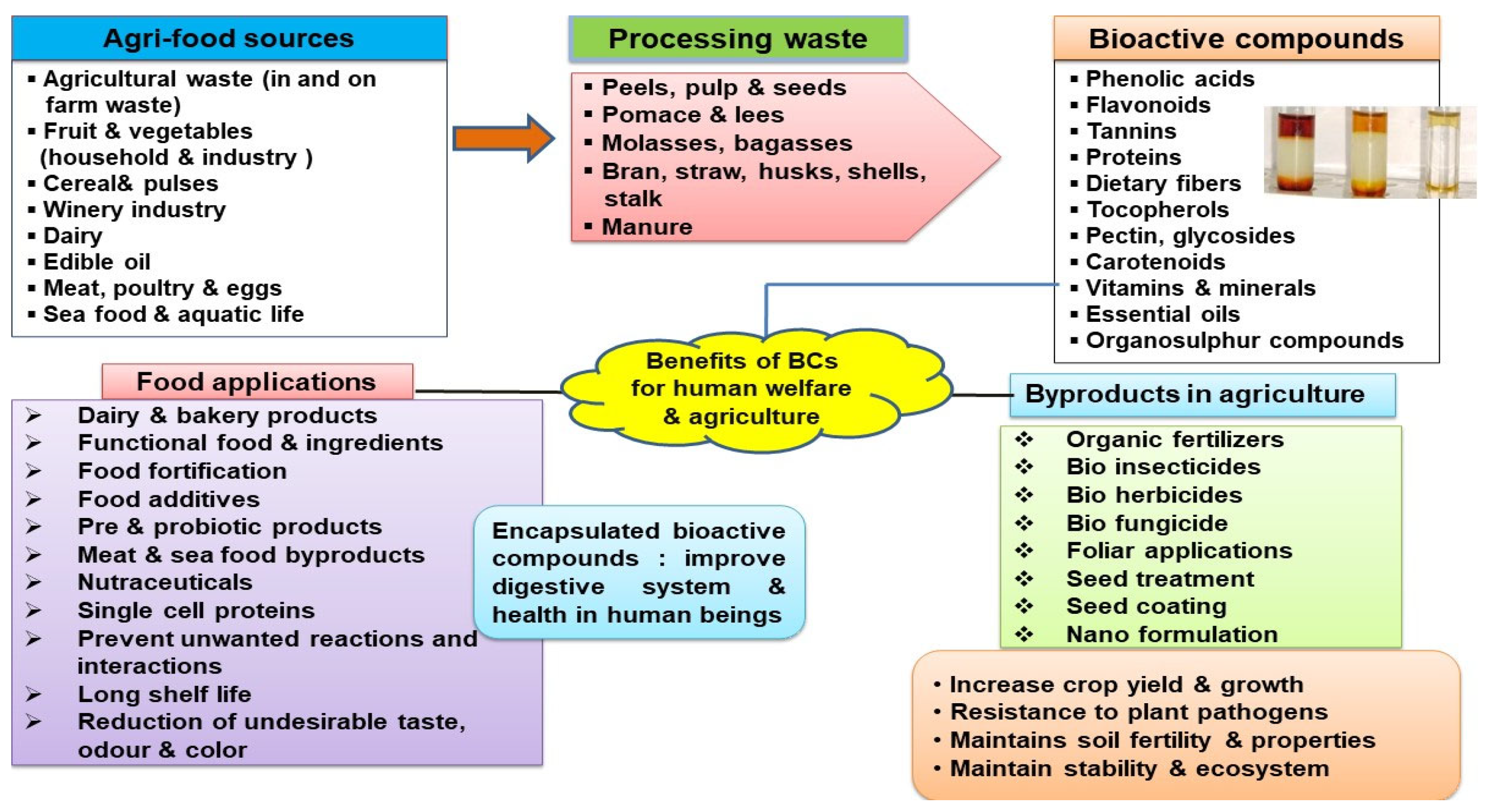
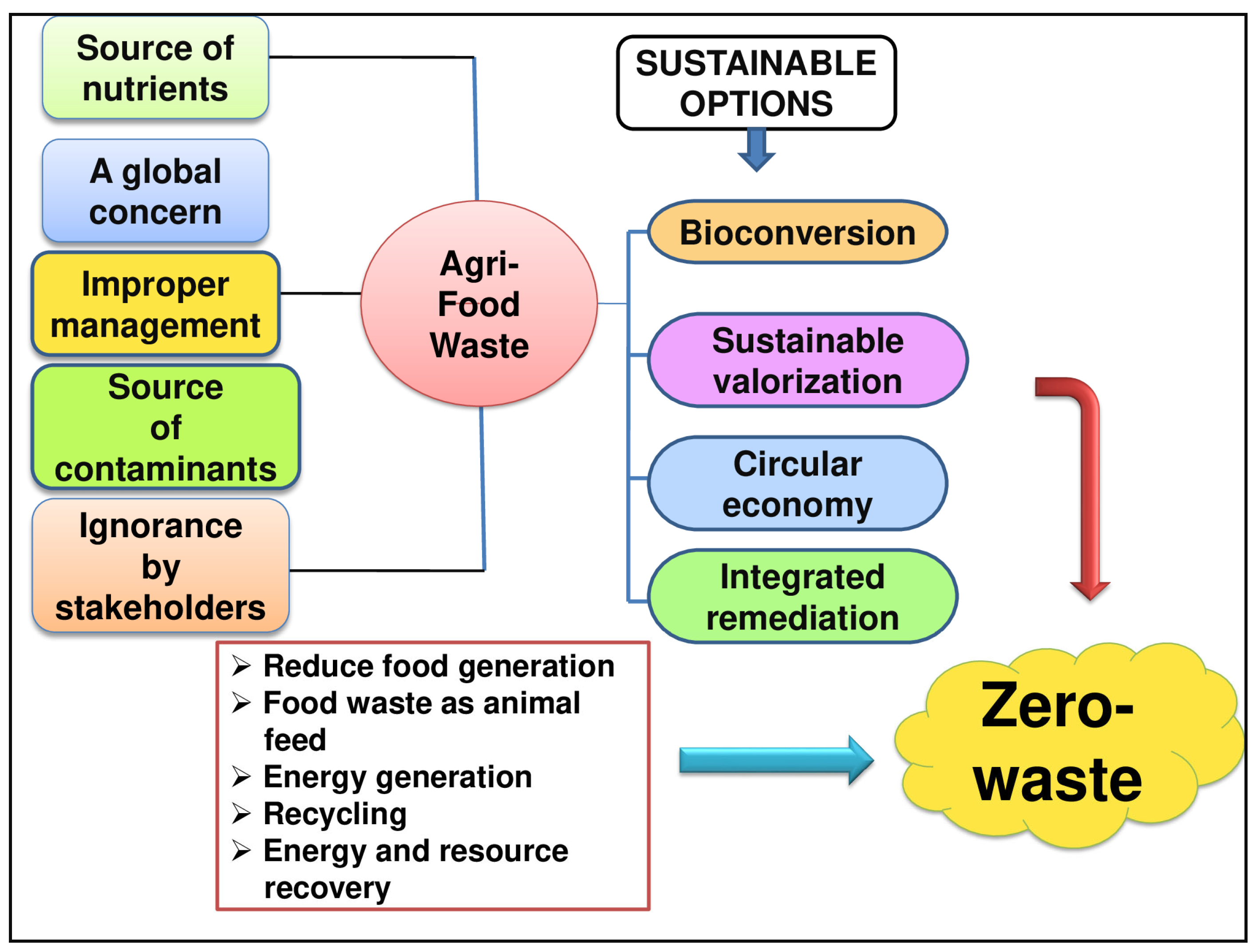
2. Agri-Food Waste as a Source of Natural Bioactive Compounds (BCs)
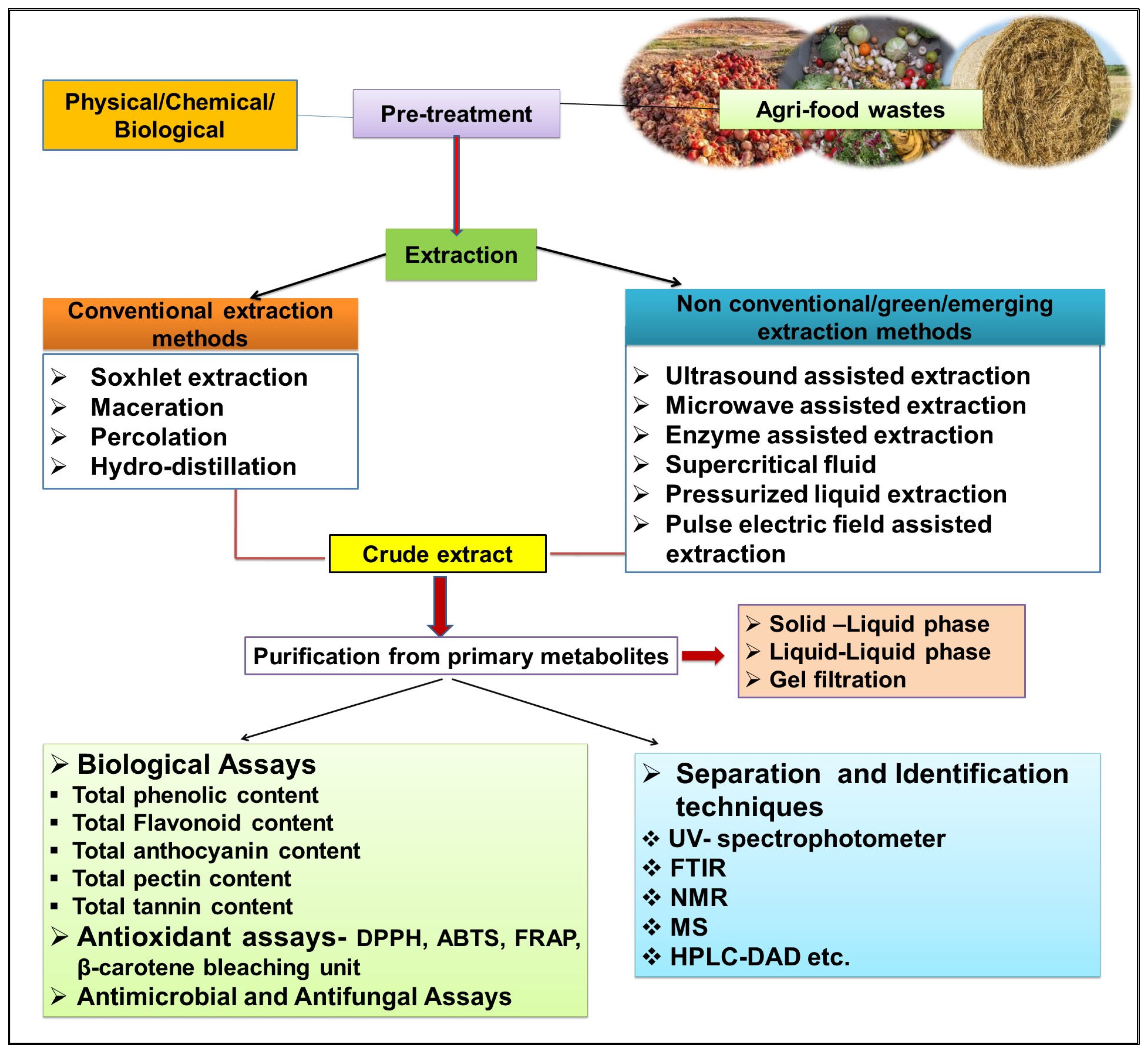
3. Valorisation Methods for Agri-Food Wastes
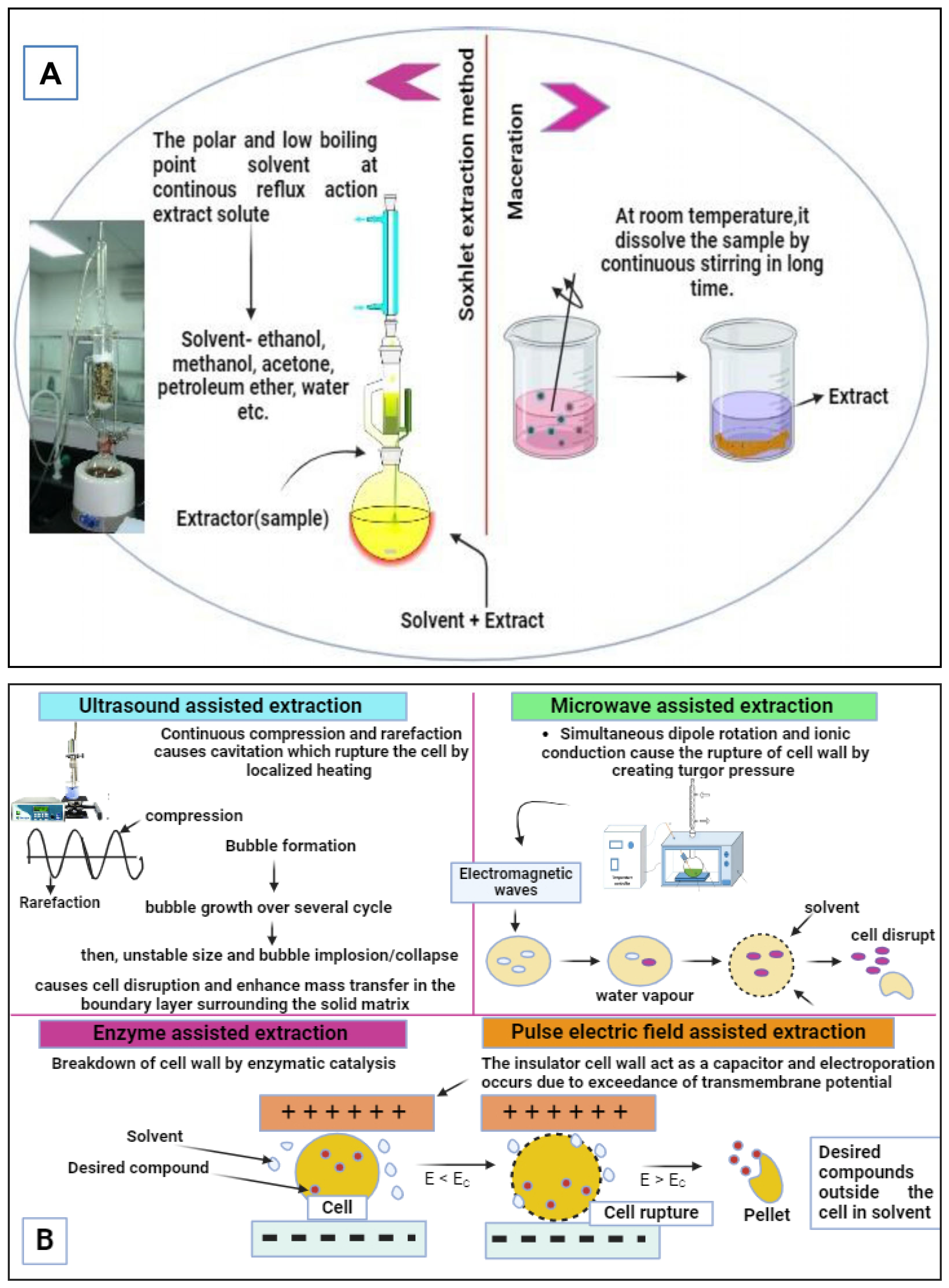
Conventional and Non-Conventional Extraction Methods for the Recovery of Bioactive Compounds from Agri-Food Wastes/By-Products
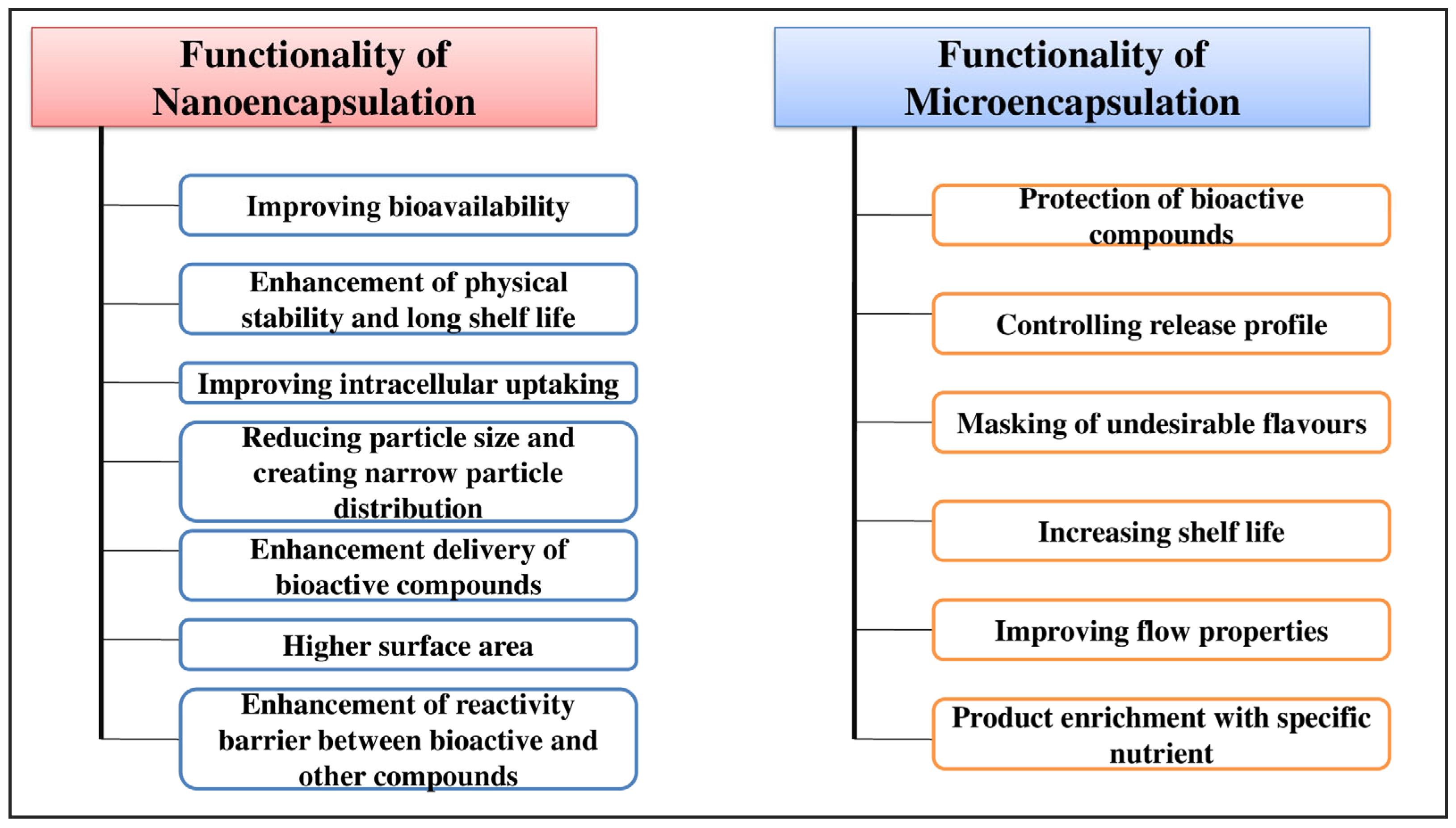
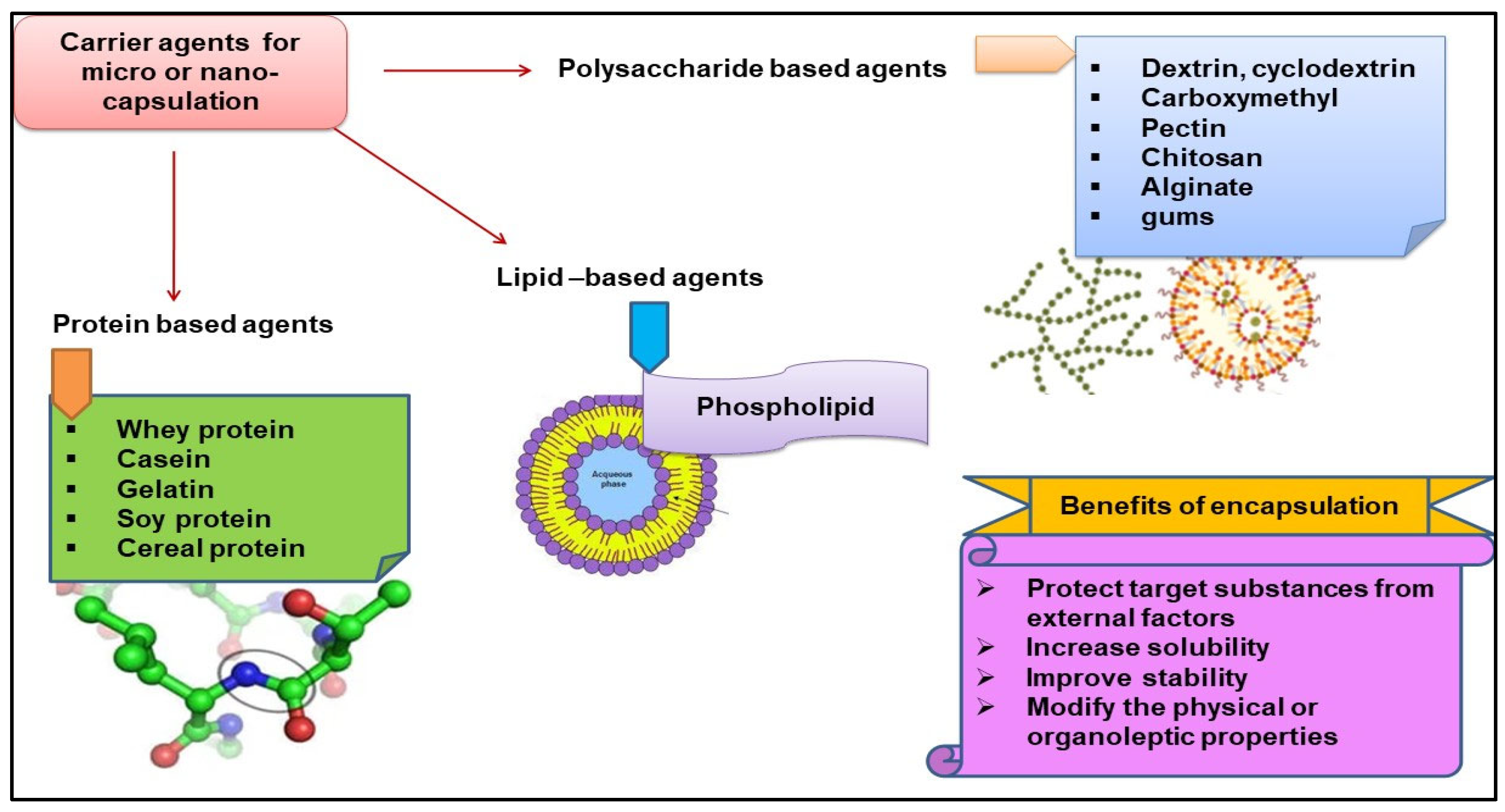
4. Encapsulation of Bioactive Compounds in Food and Agriculture Sectors
| Encapsulation Techniques | Methods/Techniques | Nanocarriers | Applications | Reference |
|---|---|---|---|---|
| Physical | Spray-drying Spray-chilling Spray-coating Supercritical micro-encapsulation Micronization Ionic gelation Freeze-drying Fluidized bed coating Centrifugal extrusion | Nano-capsules powder | Phenolic acid, carotenoids Pigments Pigments Carotenoids Nutraceutical Carotenoids, Pigments Carotenoids Food ingredients | [49] |
| Chemical | Interfacial polymerization Molecular inclusion Insitu polymerization | --- | Nutraceutical | [50,51] |
| Physical-chemical | Coacervation Complex coacervation Emulsion solvent evaporation Solidification emulsion Liposomes | Hydrogel β-ciclodextrine Liposomes | Volatile flavour oils Lycopene Food ingredients Nutraceuticals | [50,51] |
- Phase Separation: Utilizing controlled phase separation to encapsulate natural antioxidants within distinct phases of a system.
- Spray-Drying: Converting liquid formulations into powdered forms by atomization and drying, thus encapsulating antioxidants.
- Freeze-Drying: Preservation of antioxidants by freezing the formulation and then sublimating the frozen solvent under vacuum.
- Nano-emulsions: Formulating emulsions on the nano scale, offering efficient encapsulation of antioxidants.
- Liposomal Entrapment: Enveloping antioxidants within lipid bilayers, forming liposomes that enhance stability and controlled release.
- Coacervation: Phase separation of polymers, leading to the encapsulation of antioxidants incoacervate droplets.
- Inclusion Complexation: Formation of inclusion complexes, often with cyclodextrins, to encapsulate antioxidants.
- Ionic Gelation: Creation of gel-like structures through ionic interactions to encapsulate antioxidants.
- Solvent Evaporation: Dissolving antioxidants in a solvent, which is subsequently evaporated to leave behind encapsulated particles.
- Supercritical Fluid Precipitation: Employing supercritical fluids to precipitate antioxidants and form encapsulated particles.
5. Potential Applications of Encapsulated Bioactive Compounds in Agriculture and Food Industry
| AFW/By-Products | Carrier Agents | Encapsulation Methods | Encapsulated Bioactive Compounds | Model Food | Application | Reference |
|---|---|---|---|---|---|---|
| Carrot waste | Sodium alginate | Electrostatic extrusion | Carotenoids | Yoghurt | [61] | |
| Pomegranate peels | Maltodextrin | Spray-drying | Punicalagin (phenolic compound) | Cookies, ice cream | [62] | |
| Tomato peel | Whey protein | Freeze-drying | Lycopene | Salad dressing | Good functional and mechanical characteristics; reduced weight loss, increased firmness and good looks | [63] |
| Byrsonima crassifolia leaf extract | Chitosan | Coacervation | Ascorbic acid | Biodegradable film | Antimicrobial activity | [64] |
| Cocoa hulls | Maltodextrin | Spray-drying | Polyphenol | Biscuit | [65] | |
| Grape skin | Maltodextrin | Freeze-drying | Carvacrol | Biodegradable film (To reduce post-harvest Loss) | Decreased respiratory rate and increased mechanical resistance | [66] |
| Apple pomace | Chitosan | Coacervation | Polyphenol, flavonoids, hydroxycinnamic acid, dihydroxy alkaloids | Gluten-free crackers, ice cream | Reduction of moisture loss, slowing down of respiration | [67] |
| Banana peel | Maltodextrin | spray-drying | Ascorbic acid | - | [68] | |
| Grape pomace | Chitosan | Freeze-drying | Polyphenols Polyphenols | Yoghurt cheese Bread | [69] | |
| Tamarind seed | Maltodextrin | Spray-drying | β-cartoene | Cookies and mango juice | [70] | |
| Beetroot pomace | Maltodextrin | Emulsion | Betalain, β-cyanins | Candy, biscuits | [71] | |
| Curcum longa L. root | Chitosan | Spray-drying Emulsion and ultrasonication | Curcumin | Colourant (fortified rice) Food colourant | Antioxidant activity | [72] |
| Tomato peel/pomace | Inulin | Spray-drying | Lycopene | - | Antioxidant activity | [73] |
| Black Beet root pomace | Maltodextrin | Emulsion | Cyanidin, delphinidin, malvidin, pelargonidin, peonidin, petunidin | -- | Antioxidant activity | [74] |
| Blueberry pomace | Whey protein | Emulsion | Phenolics (p-hydroxybenzoic acid, epicatechin gallic acid), anthocyanins | - | Antioxidant activity | [75] |
6. Recent Trends and Challenges in Encapsulation Process
7. Conclusions and Future Prospective
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- EC—European Commission. Closing the loop—An EU action plan for the circular economy. In Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions; European Environment Agency: Brussels, Belgium, 2015. [Google Scholar]
- Lynch, J.P.; Strock, C.F.; Schneider, H.M.; Sidhu, J.S.; Ajmera, I.; Galindo-Castañeda, T. Root anatomy and soil resource capture. Plant Soil 2021, 466, 21–63. [Google Scholar] [CrossRef]
- Forster-Carneiro, T.; Berni, M.D.; Dorileo, I.L.; Rostagno, M.A. Biorefinery study of availability of agriculture residues and wastes for integrated biorefineries in Brazil. Resour. Conserv. Recycl. 2013, 77, 78–88. [Google Scholar] [CrossRef]
- Zuin, V.G.; Ramin, L.Z. Green and Sustainable Separation of Natural Products from Agro-Industrial Waste: Challenges, Potentialities, and Perspectives on Emerging Approaches. In Chemistry and Chemical Technologies in Waste Valorization; Springer: Cham, Switzerland, 2018; Volume 376, pp. 229–282. [Google Scholar] [CrossRef]
- Ayala-Zavala, J.F.; González-Aguilar, G.; Siddiqui, M.W. Plant Food Byproducts: Industrial Relevance for Food Additives and Nutraceuticals; Apple Academic Press: California, CA, USA, 2018; p. 363. [Google Scholar]
- Chilakamarry, C.R.; Sakinah, A.M.; Zularisam, A.W.; Sirohi, R.; Khilji, I.A.; Ahmad, N.; Pandey, A. Advances in solid-state fermentation for bioconversion of agricultural wastes to value-added products: Opportunities and challenges. Bioresour. Technol. 2022, 343, 126065. [Google Scholar] [CrossRef] [PubMed]
- Campos, D.A.; Gómez-García, R.; Vilas-Boas, A.A.; Madureira, A.R.; Pintado, M. Management of Fruit Industrial By-Products—A Case Study on Circular Economy Approach. Molecules 2020, 25, 320. [Google Scholar] [CrossRef] [PubMed]
- Sindhu, R.; Gnansounou, E.; Rebello, S.; Binod, P.; Varjani, S.; Thakur, I.S.; Pandey, A. Conversion of food and kitchen waste to value-added products. J. Environ. Manag. 2019, 241, 619–630. [Google Scholar] [CrossRef] [PubMed]
- Paritosh, K.; Kushwaha, S.K.; Yadav, M.; Pareek, N.; Chawade, A.; Vivekanand, V. Food Waste to Energy: An Overview of Sustainable Approaches for Food Waste Management and Nutrient Recycling. BioMed Res. Int. 2017, 2017, 2370927. [Google Scholar] [CrossRef]
- Prasad, M.; Ranjan, R.; Ali, A.; Goyal, D.; Yadav, A.; Singh, T.B.; Shrivastav, P.; Dantu, P.K. Efficient transformation of agricultural waste in India. In Contaminants in Agriculture: Sources, Impacts and Management; Springer: Berlin/Heidelberg, Germany, 2020; pp. 271–287. [Google Scholar]
- Haq, I.U.; Qaisar, K.; Ali, N.; Fatima, A.; Hamid, M.; Yong, X.; Muhammad, W.M.; Umer, R.; Wan, A.; Wan, A.K.G.; et al. Advances in valorization of lignocellulosic biomass towards energy generation. Catalysts 2021, 11, 309. [Google Scholar] [CrossRef]
- Ben-Othman, S.; Jõudu, I.; Bhat, R. Bioactives from Agri-Food Wastes: Present Insights and Future Challenges. Molecules 2020, 25, 510. [Google Scholar] [CrossRef]
- Berenguer, C.V.; Andrade, C.; Pereira, J.A.M.; Perestrelo, R.; Câmara, J.S. Current Challenges in the Sustainable Valorisation of Agri-Food Wastes: A Review. Processes 2023, 11, 20. [Google Scholar] [CrossRef]
- Plaza, M.; Santoyo, S.; Jaime, L.; Reina, G.G.B.; Herrero, M.; Senorans, F.J.; Ibáñez, E. Screening for bioactive compounds from algae. J. Pharm. Biomed. Anal. 2010, 51, 450–455. [Google Scholar] [CrossRef]
- Altemimi, A.; Lakhssassi, N.; Baharlouei, A.; Watson, D.G.; Lightfoot, D.A. Phytochemicals: Extraction, isolation, and identification of bioactivecompounds from plant extracts. Plants 2017, 6, 42. [Google Scholar] [CrossRef] [PubMed]
- Marić, M.; Grassino, A.N.; Zhu, Z.; Barba, F.J.; Brnčić, M.; Brnčić, S.R. An overview of the traditional and innovative approaches for pectin extraction from plant food wastes and by-products: Ultrasound-, microwaves-, and enzyme-assisted extraction. Trends Food Sci. Technol. 2018, 76, 28–37. [Google Scholar] [CrossRef]
- Azmir, J.; Zaidul, I.S.M.; Rahman, M.; Sharif, K.; Mohamed, A.; Sahena, F.; Jahurul, M.; Ghafoor, K.; Norulaini, N.; Omar, A. Techniques for extraction of bioactive compounds from plant materials: A review. J. Food Eng. 2013, 117, 426–436. [Google Scholar] [CrossRef]
- Sasidharan, S.; Chen, Y.; Saravanan, D.; Sundram, K.; Latha, L.Y.; Medicines, A. Extraction, isolation and characterization of bioactive compounds from plants’ extracts. Afr. J. Tradit. Complem. 2011, 8, 1–10. [Google Scholar] [CrossRef]
- Gong, P.; Wang, S.; Liu, M.; Chen, F.; Yang, W.; Chang, X.; Liu, N.; Zhao, Y.; Wang, J.; Chen, X. Extraction methods, chemical characterizations and biological activities of mushroom polysaccharides: A mini-review. Carbohydr. Res. 2020, 494, 108037. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A.; Ibsen, K.; Iwao, Y.; Zakrewsky, M.; Mitragotri, S. Transdermal protein delivery using choline and geranate (CAGE) deep eutectic solvent. Adv. Health Care Mater. 2017, 6, 1601411. [Google Scholar] [CrossRef]
- Mohamad, N.; Ramli, N.; Abd-Aziz, S.; Ibrahim, M.F. Comparison of hydro-distillation, hydro-distillation with enzyme-assisted and supercritical fluid for the extraction of essential oil from pineapple peels. 3 Biotech 2019, 9, 234. [Google Scholar] [CrossRef] [PubMed]
- Attard, T.M.; Natalia, B.; Daniel, E.; Mehrdad, A.; Paul, G.; Urban, B.; Vitaliy, L.B.; James, H.C.; Andrew, J.H. Supercritical extraction of waxes and lipids from biomass: A valuable first step towards an integrated biorefinery. J. Clean. Prod. 2018, 177, 684–698. [Google Scholar] [CrossRef]
- Setyaningsih, W.; Saputro, I.E.; Carrera, C.A.; Palma, M. Optimization of an ultrasound-assisted extraction method for the simultaneous determination of phenolics in rice grains. Food Chem. 2019, 288, 221–227. [Google Scholar] [CrossRef]
- Sabaragamuwa, R.; Perera, C.O. Total Triterpenes, Polyphenols, Flavonoids, and Antioxidant Activity of Bioactive Phytochemicals of Centella asiatica by Different Extraction Techniques. Foods 2023, 12, 3972. [Google Scholar] [CrossRef]
- Ekinci, M.S. Supercritical fluid extraction of quercetin from sumac (Rhus coriaria L.): Effects of supercritical extraction parameters. Sep. Sci. Technol. 2022, 57, 256–262. [Google Scholar] [CrossRef]
- Ferreira-Santos, P.; Genisheva, Z.; Botelho, C.; Santos, J.; Ramos, C.; Teixeira, J.A.; Rocha, C.M.R. Unravelling the biological potential of Pinus pinaster bark extracts. Antioxidants 2020, 9, 334. [Google Scholar] [CrossRef]
- García, P.; Fredes, C.; Cea, I.; Lozano-Sánchez, J.; Leyva-Jiménez, F.J.; Robert, P.; Jimenez, P. Recovery of bioactive compounds from pomegranate (Punica granatum L.) peel using pressurized liquid extraction. Foods 2021, 10, 203. [Google Scholar] [CrossRef]
- Uribe, E.; Delgadillo, A.; Giovagnoli-Vicuña, C.; Quispe-Fuentes, I.; Zura-Bravo, L. Extraction techniques for bioactive compounds and antioxidant capacity determination of chilean papaya (Vasconcellea pubescens) Fruit. J. Chem. 2015, 2015, 347532. [Google Scholar] [CrossRef]
- Hung, V.P.; Nhi, Y.N.H.; Ting, L.Y.; Phi, L.N.T. Chemical composition and biological activities of extracts from pomelo peel by-products under enzyme and ultrasound-assisted extractions. J. Chem. 2020, 2020, 1043251. [Google Scholar]
- Wang, K.; Xie, X.; Zhang, Y.; Huang, Y.; Zhou, S.; Zhang, W.; Lin, Y.; Fan, H. Combination of microwave-assisted extraction and ultrasonic-assisted dispersive liquid-liquid microextraction for separation and enrichment of pyrethroids residues in Litchi fruit prior to HPLC determination. Food Chem. 2018, 240, 1233–1242. [Google Scholar] [CrossRef]
- Fan, Y.; Li, Z.; Liu, L.; Xi, J. Combination of liquid-phase pulsed discharge and ultrasonic for saponins extraction from lychee seeds. Ultrason. Sonochem. 2020, 69, 105264. [Google Scholar] [CrossRef] [PubMed]
- Rodsamran, P.; Sothornvit, R. Extraction of phenolic compounds from lime peel waste using ultrasonic-assisted and microwave-assisted extractions. Food Biosci. 2019, 28, 66–73. [Google Scholar] [CrossRef]
- El Kantar, S.; Boussetta, N.; Rajha, H.N.; Maroun, R.G.; Louka, N.; Vorobiev, E. High voltage electrical discharges combined with enzymatic hydrolysis for extraction of polyphenols and fermentable sugars from orange peels. Food Res. Int. 2018, 107, 755–762. [Google Scholar] [CrossRef]
- Đurđević, S.; Milovanović, S.; Šavikin, K.; Ristić, M.; Menković, N.; Pljevljakušić, D.; Petrović, S.; Bogdanović, A. Improvement of supercritical CO2 and n-hexane extraction of wild growing pomegranate seed oil by microwave pretreatment. Ind. Crops Prod. 2017, 104, 21–27. [Google Scholar] [CrossRef]
- Medina-Meza, I.G.; Barbosa-Cánovas, G.V. Assisted extraction of bioactive compounds from plum and grape peels by ultrasonics and pulsed electric felds. J. Food Eng. 2015, 166, 268–275. [Google Scholar] [CrossRef]
- Rifna, E.J.; Misra, N.N.; Dwivedi, M. Recent advances in extraction technologies for recovery of bioactive compounds derived from fruit and vegetable waste peels: A review. Crit. Rev. Food Sci. Nutr. 2023, 63, 719–752. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, H.; Parastouei, K.; Mokhtarian, M.; Rostami, H.; Niakousari, M.; Mohsenpour, Z. Application of innovative processing methods for the extraction of bioactive compounds from saffron (Crocus sativus) petals. J. Appl. Res. Med. Aromat. Plants 2020, 19, 100264. [Google Scholar] [CrossRef]
- Montiel-S’anchez, M.; García-Cayuela, T.; G’omez-Maqueo, A.; García, H.S.; Cano, M.P. In vitro gastrointestinal stability, bioaccessibility and potential biological activities of betalains and phenolic compounds in cactus berry fruits (Myrtillocactus geometrizans). Food Chem. 2021, 342, 128087. [Google Scholar] [CrossRef] [PubMed]
- Tunç, M.T.; Koca, I. Ohmic heating assisted hydrodistillation of clove essential oil. Ind. Crops Prod. 2019, 141, 111763. [Google Scholar] [CrossRef]
- Jasso de Rodríguez, D.; Gaytán-Sánchez, N.A.; Rodríguez, R.; Hernandez, F.D.; Díaz, L.; Villarreal, J.A.; Flores, M.L.; Carrillo, D.A.; Peña-Ramos, F.M. Antifungal activity of Juglans spp. and Carya sp. ethanol extracts against Fusarium oxysporum on tomato under greenhouse conditions. Ind. Crops Prod. 2019, 138, 111442. [Google Scholar] [CrossRef]
- Osorio, L.L.; Flórez-López, E.; Grande-Tovar, C.D. The Potential of Selected Agri-Food Loss and Waste to Contribute to a Circular Economy: Applications in the Food, Cosmetic and Pharmaceutical Industries. Molecules 2021, 26, 515. [Google Scholar] [CrossRef]
- Gong, C.; Singh, A.; Singh, P.; Singh, A. Anaerobic Digestion of Agri-Food Wastes for Generating Biofuels. Indian J. Microbiol. 2021, 61, 427–440. [Google Scholar] [CrossRef]
- Rodriguez, J.; Martin, M.J.; Ruiz, M.A.; Clares, B. Current encapsulation strategies for bioactive oils: From alimentary to pharmaceutical perspectives. Food Res. Int. 2016, 83, 41–59. [Google Scholar] [CrossRef]
- Sobel, R.; Versic, R.; Gaonkar, A.G. Introduction to microencapsulation and controlled delivery in foods. In Microencapsulation in the Food Industry; Gaonkar, A.G., Vasisht, N., Khare, A.R., Sobe, R.L., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 3–12. [Google Scholar] [CrossRef]
- Ali, E.A.; Nada, A.A.; Al-Moghazy, M. Self-Stick Membrane Based on Grafted Gum Arabic as Active Food Packaging for Cheese Using Cinnamon Extract. Int. J. Biol. Macromol. 2021, 189, 114–123. [Google Scholar] [CrossRef]
- Sohail, M.I.; Waris, A.A.; Ayub, M.A.; Usman, M.; Rehman, M.Z.; Sabir, M.; Faiz, T. Environmental application of nanomaterials: A promise to sustainable future. In Comprehensive Analytical Chemistry; Elsevier: Amsterdam, The Netherlands, 2019; Volume 87, pp. 1–54. [Google Scholar]
- de Souza Simoes, L.; Madalena, D.A.; Pinheiro, A.C.; Teixeira, J.A.; Vicente, A.A.; Ramos, Ó.L. Micro-and nano bio-based delivery systems for food applications: In vitro behavior. Adv. Colloid. Interface Sci. 2017, 243, 23–45. [Google Scholar] [CrossRef] [PubMed]
- Fasihi, H.; Noshirvani, N.; Hashemi, M.; Fazilati, M.; Salavati, H.; Coma, V. Antioxidant and Antimicrobial Properties of Carbohydrate-Based Films Enriched with Cinnamon Essential Oil by Pickering Emulsion Method. Food Packag. Shelf Life 2019, 19, 147–154. [Google Scholar] [CrossRef]
- Janiszewska-Turak, E. Carotenoids Microencapsulation by Spray Drying Method and Supercritical Micronization. Food Res. Int. 2017, 99, 891–901. [Google Scholar] [CrossRef] [PubMed]
- De Freitas Santos, P.D.; Rubio, F.T.V.; Da Silva, M.P.; Pinho, L.S.; Favaro-Trindade, C.S. Microencapsulation of Carotenoid-Rich Materials: A Review. Food Res. Int. 2021, 147, 110571. [Google Scholar] [CrossRef]
- O’Toole, M.G.; Richard, M.H.; Patricia, A.S.; Brigitte, H.F.; Patrick, J.H.; Robert, S.K.; William, D.E.; Andrea, S.G. Curcumin encapsulation in submicrometer spray-dried chitosan/tween 20 particles. Biomacromolecules 2012, 13, 2309–2314. [Google Scholar] [CrossRef] [PubMed]
- Yadav, P.; Yadav, A.; Labhasetwar, P.K. Sustainable adsorptive removal of antibiotics from aqueous streams using Fe3O4-functionalized MIL101 (Fe) chitosan composite beads. Environ. Sci. Pollut. Res. 2022, 29, 37204–37217. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, A.C.; Coimbra, M.A.; Vicente, A.A. In vitro behaviour of curcumin nanoemulsions stabilized by biopolymer emulsifiers- effect of interfacial composition. Food Hydrocoll. 2016, 52, 460–467. [Google Scholar] [CrossRef]
- Piao, X.; Zhang, L.; Zhang, S.; Yi, F. Nematicidal action of microencapsulated essential oil of flesh fingered citron. J. Chem. 2015, 82, 25–35. [Google Scholar] [CrossRef]
- De Oliveira, J.L.; Fraceto, L.F.; Bravo, A.; Polanczyk, R.A. Encapsulation Strategies for Bacillus thuringiensis: From Now to the Future. J. Agric. Food Chem. 2021, 69, 4564–4577. [Google Scholar] [CrossRef]
- Vassilev, N.; Vassileva, M.; Martos, V.; Garcia del Moral, L.F.; Kowalska, J.; Tylkowski, B.; Malusá, E. Formulation of Microbial Inoculants by Encapsulation in Natural Polysaccharides: Focus on Beneficial Properties of Carrier Additives and Derivatives. Front. Plant Sci. 2020, 11, 270. [Google Scholar] [CrossRef]
- Bae, M.; Lewis, A.; Liu, S.; Arcot, Y.; Lin, Y.-T.; Bernal, J.S.; Cisneros-Zevallos, L.; Akbulut, M. Novel Biopesticides Based on Nanoencapsulation of Azadirachtin withWhey Protein to Control Fall Armyworm. J. Agric. Food Chem. 2022, 70, 7900–7910. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, L.; Tavakoilpour, H.; Roozbeh-Nasiraie, L.; Kalbasi-Ashtari, A. Ultrasonication and encapsulation of Butcher broom (Ruscus Hyrcanus L.) extract and its bioactive effects on qualitative properties, oxidative stability and shelf life of cake. Sustain. Chem. Pharm. 2020, 17, 100295. [Google Scholar] [CrossRef]
- Stoll, L.; Martins da Silva, A.; Iahnke, A.O.E.S.; Costa, T.M.H.; Flôres, S.H.; Rios, A.D.O. Active biodegradable film with encapsulated anthocyanins: Effect on the quality attributes of extra-virgin olive oil during storage. J. Food Process. Preserv. 2017, 41, e13218. [Google Scholar] [CrossRef]
- Seregelj, V.; Pezo, L.; Sovljanski, O.; Levic, S.; Nedovic, V.; Markov, S.; Tomic, A.; Canadanovic-Brunet, J.; Vulic, J.; Saponjac, V.T.; et al. New concept of fortified yogurt formulation with encapsulated carrot waste extract. Lebensm. Wiss. Technol. 2021, 138, 110732. [Google Scholar] [CrossRef]
- Kaderides, K.; Mourtzinos, I.; Goula, A.M. Stability of pomegranate peel polyphenols encapsulated in orange juice industry by-product and their incorporation in cookies. Food Chem. 2020, 310, 125849. [Google Scholar] [CrossRef] [PubMed]
- Gheonea, I.; Aprodu, I.; Cîrciumaru, A.; Râpeanu, G.; Bahrim, G.E.; Stanciuc, N. Microencapsulation of lycopene from tomatoes peels by complex coacervation and freeze-drying: Evidences on phytochemical profile, stability and food applications. J. Food Eng. 2021, 288, 110166. [Google Scholar] [CrossRef]
- Gutierrez, J.; Corona, M.L.; Ventura, R.I.; Barrera, L.L.; Bautista, S.; Correa, Z.N. Chitosan and Byrsonima crassifolia-based nanostructured coatings: Characterization and effect on tomato preservation during refrigerated storage. Food Biosci. 2021, 42, 101212. [Google Scholar] [CrossRef]
- Papillo, V.A.; Locatelli, M.; Travaglia, F.; Bordiga, M.; Garino, C.; Coïsson, J.D.; Arlorio, M. Cocoa hulls polyphenols stabilized by microencapsulation as functional ingredient for bakery applications. Food Res. Int. 2019, 115, 511–518. [Google Scholar] [CrossRef]
- Alves, V.L.C.D.; Rico, B.P.M.; Cruz, R.M.S.; Vicente, A.A.; Khmelinskii, I.; Vieira, M.C. Preparation and characterization of a chitosan film with grape seed extract-carvacrol microcapsules and its effect on the shelf-life of refrigerated Salmon (Salmo salar). Lebensm. Wiss. Technol. 2018, 89, 525–534. [Google Scholar] [CrossRef]
- Tsali, A.; Goula, A.M. Valorization of grape pomace: Encapsulation and storage stability of its phenolic extract. Powder Technol. 2018, 340, 194–207. [Google Scholar] [CrossRef]
- Saffarzadeh-Matin, S.; Shahbazi, M. Modeling and Optimization of process conditions in nanoencapsulation of the polyphenolic extract of industrial apple pomace. Innov. Food Technol. 2016, 3, 1–13. [Google Scholar]
- Ortiz, L.; Dorta, E.; Lobo, M.G.; González-Mendoza, L.A.; Díaz, C.; González, M. Use of banana (Musa acuminata Colla AAA) peel extract as an antioxidant source in orange juices. Plant Foods Hum. Nutr. 2017, 72, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Marchiani, R.; Bertolino, M.; Ghirardello, D.; McSweeney, P.L.; Zeppa, G. Physico-chemical and nutritional qualities of grape pomace powder-fortified semi-hard cheeses. J. Food Sci. Technol. 2016, 53, 1585–1596. [Google Scholar] [CrossRef] [PubMed]
- Natukunda, S.; Muyonga, J.H.; Mukisa, I.M. Effect of tamarind (Tamarindus indica L.) seed on antioxidant activity, phytocompounds, physicochemical characteristics, and sensory acceptability of enriched cookies and mango juice. Food Sci. Nutr. 2016, 4, 494–507. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo, A.; Brandolini, A.; Čanadanović-Brunet, J.; Ćetković, G.; Šaponjac, V.T. Microencapsulates and extracts from red beetroot pomace modify antioxidant capacity, heat damage and colour of pseudocereals enriched einkorn water biscuits. Food Chem. 2018, 268, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, V.M.C.; Silva, R.F.G.; Gonçalves, O.H.; Pereira, C.; Barros, L.; Ferreira, I.C.F.R.; Bona, E.; Leimann, F.V. Chemometric approaches to evaluate the substitution of synthetic food dyes by natural compounds: The case of nanoencapsulated curcumin, spirulina and hibiscus extracts. LWT Food Sci. Technol. 2022, 154, 112786. [Google Scholar] [CrossRef]
- Correa Filho, L.C.; Lourenco, S.C.; Duarte, D.F.; Moldão-Martins, M.; Alves, V.D. Microencapsulation of Tomato (Solanum lycopersicum L.) Pomace Ethanolic Extract by Spray Drying: Optimization of Process Conditions. Appl. Sci. 2019, 9, 612. [Google Scholar] [CrossRef]
- Kaur, G.; Sharma, N.; Singh, A.; Kapoor, S.; Khatkar, S.K. Ultrasound-assisted microemulsions of anthocyanins extracted from black carrot pomace and its utilisation as functional component in kulfi: Implication on in vitro release, bio-functional components and rheological characteristics. Int. J. Food Sci. Technol. 2022, 58, 2744–2753. [Google Scholar] [CrossRef]
- Do Nascimento Junior, D.R.; Tabernero, A.; Cabral Albuquerque, E.C.d.M.; Vieira de Melo, S.A.B. Biopesticide Encapsulation Using Supercritical CO2: A Comprehensive Review and Potential Applications. Molecules 2021, 26, 4003. [Google Scholar] [CrossRef]
- Zabot, G.L.; Silva, E.K.; Emerick, L.B.; Felisberto, M.H.F.; Clerici, M.T.P.S.; Meireles, M.A.A. Physicochemical, Morphological, Thermal and Pasting Properties of a Novel Native Starch Obtained from Annatto Seeds. Food Hydrocoll. 2019, 89, 321–329. [Google Scholar] [CrossRef]
- Yan, Z.; Shi, J.; Gao, L.; Wang, Q.; Zuo, J. The combined treatment of broccoli florets with kojic acid and calcium chloride maintains post-harvest quality and inhibits off-odor production. Sci. Hortic. 2020, 262, 109019. [Google Scholar] [CrossRef]
- Marcillo-Parra, V.; Tupuna-Yerovi, D.S.; González, Z.; Ruales, J. Encapsulation of Bioactive Compounds from Fruit and Vegetable By-Products for Food Application—A Review. Trends Food Sci. Technol. 2021, 116, 11–23. [Google Scholar] [CrossRef]
- Pavoni, L.; Benelli, G.; Maggi, F.; Bonacucina, G. Green Nanoemulsion Interventions for Biopesticide Formulations; Elsevier: Amsterdam, The Netherlands, 2019; ISBN 9780128158296. [Google Scholar]
- Maes, C.; Bouquillon, S.; Fauconnier, M.L. Encapsulation of Essential Oils for the Development of Biosourced Pesticides with Controlled Release: A Review. Molecules 2019, 24, 2539. [Google Scholar] [CrossRef]
- Luiz De Oliveira, J.; Ramos Campos, E.V.; Fraceto, L.F. Recent Developments and Challenges for Nanoscale Formulation of Botanical Pesticides for Use in Sustainable Agriculture. J. Agric. Food Chem. 2018, 66, 8898–8913. [Google Scholar] [CrossRef] [PubMed]
- Jina, Y.; Chihib, N.E.; Gharsallaoui, A.; Ismail, A.; Karam, L. Advances in essential oils encapsulation: Development, characterization and release mechanisms. Polymer Bull. 2024, 81, 3837–3882. [Google Scholar]
- Maruyama, C.R.; Bilesky-José, N.; de Lima, R.; Fraceto, L.F. Encapsulation of Trichoderma Harzianum Preserves Enzymatic Activity and Enhances the Potential for Biological Control. Front. Bioeng. Biotechnol. 2020, 8, 225. [Google Scholar] [CrossRef] [PubMed]
- Pour, M.M.; Saberi-Riseh, R.; Mohammadinejad, R.; Hosseini, A. Investigating the Formulation of Alginate- Gelatin Encapsulated Pseudomonas fluorescens (VUPF5 and T17-4 Strains) for Controlling Fusarium solani on Potato. Int. J. Biol. Macromol. 2019, 133, 603–613. [Google Scholar] [CrossRef]
- Panichikkal, J.; Prathap, G.; Nair, R.A.; Krishnankutty, R.E. Evaluation of Plant Probiotic Performance of Pseudomonas Sp. Encapsulated in Alginate Supplemented with Salicylic Acid and Zinc Oxide Nanoparticles. Int. J. Biol. Macromol. 2021, 166, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Amar Feldbaum, R.; Yaakov, N.; Ananth Mani, K.; Yossef, E.; Metbeev, S.; Zelinger, E.; Belausov, E.; Koltai, H.; Ment, D.; Mechrez, G. Single Cell Encapsulation in a Pickering Emulsion Stabilized by TiO2 Nanoparticles Provides Protection against UV Radiation for a Biopesticide. Colloids Surf. B Biointerfaces 2021, 206, 111958. [Google Scholar] [CrossRef]
- Riseh, R.S.; Ebrahimi-Zarandi, M.; Tamanadar, E.; Pour, M.M.; Thakur, V.K. Salinity Stress: Toward Sustainable Plant Strategies and Using Plant Growth-Promoting Rhizobacteria Encapsulation for Reducing It. Sustainability 2021, 13, 12758. [Google Scholar] [CrossRef]
- Kadmiri, I.M.; El Mernissi, N.; Azaroual, S.E.; Mekhzoum, M.E.M.; Qaiss, A.E.K.; Bouhfid, R. Bioformulation of Microbial Fertilizer Based on Clay and Alginate Encapsulation. Curr. Microbiol. 2021, 78, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Panzella, L.; Moccia, F.; Nasti, R.; Marzorati, S.; Verotta, L.; Napolitano, A. Bioactive Phenolic Compounds from Agri-Food Wastes: An Update on Green and Sustainable Extraction Methodologies. Front. Nutr. 2020, 7, 60. [Google Scholar] [CrossRef] [PubMed]
- Directive of the European Parliament and Council. Directive No 2006/12/EC of the European Parliament and Council of 5 April 2006 on Waste. Official Journal of European Union, L114/9. Available online: https://eurlex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2006:114:0009:0021:en:PDF (accessed on 10 October 2023).
- Popplewell, L.M. Evaluating Encapsulation Economics; Allured Publishing Corp.: Carol Stream, IL, USA, 2001; Volume 26. [Google Scholar]
- Ozkan, G.; Franco, P.; De Marco, I.; Xiao, J.; Capanoglu, E. A review of microencapsulation methods for food antioxidants: Principles, advantages, drawbacks and applications. Food Chem. 2019, 272, 494–506. [Google Scholar] [CrossRef] [PubMed]
- Sarker, A.; Ahmmed, R.; Ahsan, S.M.; Rand, J.; Ghosh, M.K.; Nandi, R. A comprehensive review of food waste valorization for the sustainable management of global food waste. Sustain. Food Technol. 2024, 2, 48–69. [Google Scholar] [CrossRef]
- Capanoglu, E.; Nemli, E.; Tomas-Barberan, F. Novel Approaches in the Valorization of Agricultural Wastes and Their Applications. J. Agric. Food. Chem. 2022, 70, 6787–6804. [Google Scholar] [CrossRef] [PubMed]
- Malik, K.; Sharma, A.; Dandu, H.K.; Rani, V.; Arya, N.; Malik, A.; Rani, S.; Sangwan, P.; Bhatia, T. Deciphering the biochemical and functional characterization of rice straw cultivars for industrial applications. Heliyon 2023, 9, 16339. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Malik, K.; Sindhu, M.; Kumari, N.; Mehta, S.; Malik, K.; Ranga, P.; Sharma, K.; Arya, N. Biostimulation of anaerobic digestion using iron oxide nanoparticles (IONPS) for increasing biogas production from cattle manure. Nanomaterials 2022, 12, 497. [Google Scholar] [CrossRef] [PubMed]
- Shweta Capareda, S.C.; Kamboj, B.R.; Malik, K.; Singh, K.; Bhisnoi, D.K.; Arya, S. Biomass resources and biofuel technologies: A focus on Indian development. Energies 2024, 17, 382. [Google Scholar] [CrossRef]
- Hari, A.; Jarkko, L.; Malik, K. How can we build inclusive circular supply chains? Examining the case of agricultural residue usage in India. Business Strategy. Development 2023, 2023, 641–654. [Google Scholar]


| Agri-Food Waste (AFW) | Techniques | Extraction Conditions | Recovery | Reference |
|---|---|---|---|---|
| Rice grains Tomato skin Blueberry pomace Papaya peel Red beetroot juice | UAE | ET—45 °C Extraction time—25 min Solvent—methanol (80%) Ratio (solvent: solid)—5:1 | Phenolic acids, anthocyanin, flavonol, carotenoids flavonoids | [23] |
| C. asiatica powder Potato peel Picrasma quassioides | MWAE UMAE (combination) | ET—30–35 °C Time—10 min Solvent—methanol Ratio (solvent:solid)—25:1 Isopropanol (solvent) (150 °C) | High yield of madicassoside, carotenoids, anthocyanin, phenols Highest amount of phenlic compounds | [24] |
| Sumac (Rhus coriaria L.) Tomato skin | SFE | ET: 40–42 °C Extraction Time: 45 min Solvent: Carbon dioxide + Ethanol | Extraction of high quercetin, carotenoids, phenolics | [25] |
| Pinus pinaster bark Blueberry pomace | PEFAE | Extraction Time: 30 min Extraction Solvent: 50% Ethanol Ratio (Solid-Solvent)—1:10, EC—12 mS/cm | Higher yields (up 30%) in comparison with conventional extraction, phenolic acids, anthocyanin | [26] |
| Pomegranate peel Onion skin | PLE | Extraction Temperature: 200 °C Extraction Time: 20 min Solvent: 77% Ethanol Pressure: 103 bar | Mainly ellagitannins extracted at high concentration, flavonoids | [27] |
| Chilean papaya (Vasconcellea pubescens) | HHPE | ET—room temp Time—10 min Pressure—500 MPa Solvent—Methanol (80%) | Four different phenolic compounds—caffeic, trans- ferulic and p-coumaric acids and rutin | [28] |
| Pomelo peel | EAE | ET—50 °C Time—60 min Enzyme—Pectinex Ultra SP-L (0, 1, 2, 3, or 4%, v/w)—0.9% | Total phenolic content higher as compared to others | [29] |
| Litchi peel | MAE + UAE | MAE—700C in 4 min of extraction with 40:1 solvent and material ratio | Pyrethroid | [30] |
| UAE + PEFAE | UAE—3 min of extraction and chlorobenzene as a solvent, 30% aqueous ethanol, 62.66 mL/g ration of liquid: solid ratio, 123 mL/min flow velocity, 276 W ultrasonic probe, 47 °C ultrasonic temperature | Saponin | [31] | |
| Lime peel | MAE + UAE | Microwave power 140 W with 55% ethanol and 45s ultrasound energy of 38% amplitude for 4 min | Phenolic compounds, antioxidants | [32] |
| Orange peel | EAE + PEFAE | High voltage of energy input of 222 kg/kg with enzymatic hydrolysis viscozyme of 12FBGU/g | Polyphenols and reducing sugars | [33] |
| Pomegranate seed | SFE + MAE + CE | Microwave radiation of 250 W with 6 min and then SFE and Soxhlet extraction | Punicic acid | [34] |
| Potato waste Yellow onion skin Red beetroot peel | CE | Maceration, hot water extraction, methanolic extraction | Phenolic, flavonoids, betalains | [34] |
| Grape peel | UAE + PEFAE | Ultrasonic energy with 50 °C with pulse of flow 290 L/h, diameter of chamber 25 mm, gap 26 mm and 25 kV voltage | Anthocyanin and flavonoid | [35] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yadav, S.; Malik, K.; Moore, J.M.; Kamboj, B.R.; Malik, S.; Malik, V.K.; Arya, S.; Singh, K.; Mahanta, S.; Bishnoi, D.K. Valorisation of Agri-Food Waste for Bioactive Compounds: Recent Trends and Future Sustainable Challenges. Molecules 2024, 29, 2055. https://doi.org/10.3390/molecules29092055
Yadav S, Malik K, Moore JM, Kamboj BR, Malik S, Malik VK, Arya S, Singh K, Mahanta S, Bishnoi DK. Valorisation of Agri-Food Waste for Bioactive Compounds: Recent Trends and Future Sustainable Challenges. Molecules. 2024; 29(9):2055. https://doi.org/10.3390/molecules29092055
Chicago/Turabian StyleYadav, Sujeeta, Kamla Malik, Janie McClurkin Moore, Baldev Raj Kamboj, Shweta Malik, Vinod Kumar Malik, Sandeep Arya, Karmal Singh, Shikhadri Mahanta, and Dalip Kumar Bishnoi. 2024. "Valorisation of Agri-Food Waste for Bioactive Compounds: Recent Trends and Future Sustainable Challenges" Molecules 29, no. 9: 2055. https://doi.org/10.3390/molecules29092055
APA StyleYadav, S., Malik, K., Moore, J. M., Kamboj, B. R., Malik, S., Malik, V. K., Arya, S., Singh, K., Mahanta, S., & Bishnoi, D. K. (2024). Valorisation of Agri-Food Waste for Bioactive Compounds: Recent Trends and Future Sustainable Challenges. Molecules, 29(9), 2055. https://doi.org/10.3390/molecules29092055








