Synthesis and Application of Salicylhydrazone Probes with High Selectivity for Rapid Detection of Cu2+
Abstract
1. Introduction
2. Results and Discussion
2.1. Solvent–Chromic Effect
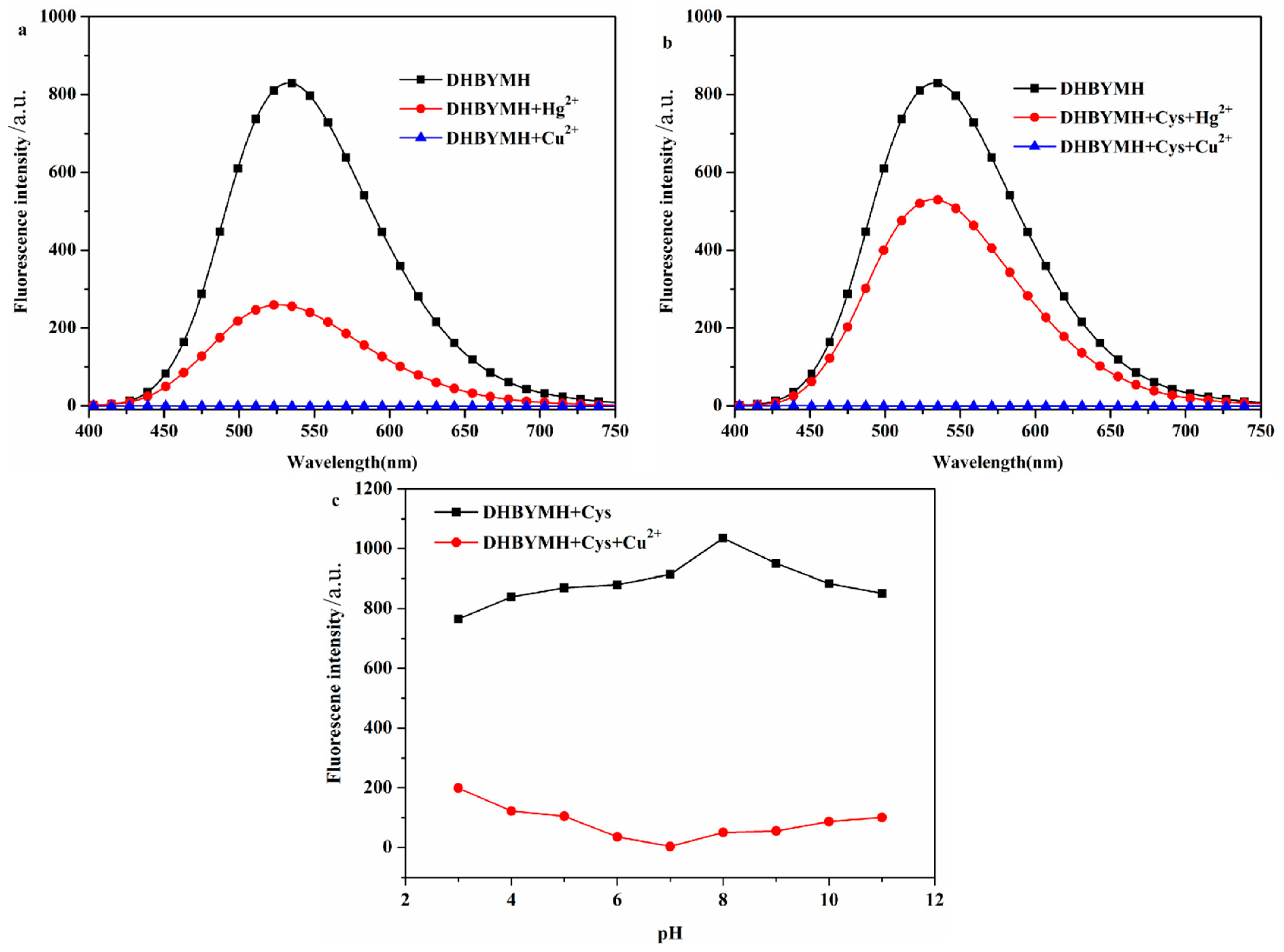
2.2. Cu2+ Response Behavior of DHBYMH
2.2.1. Screening of Test Conditions
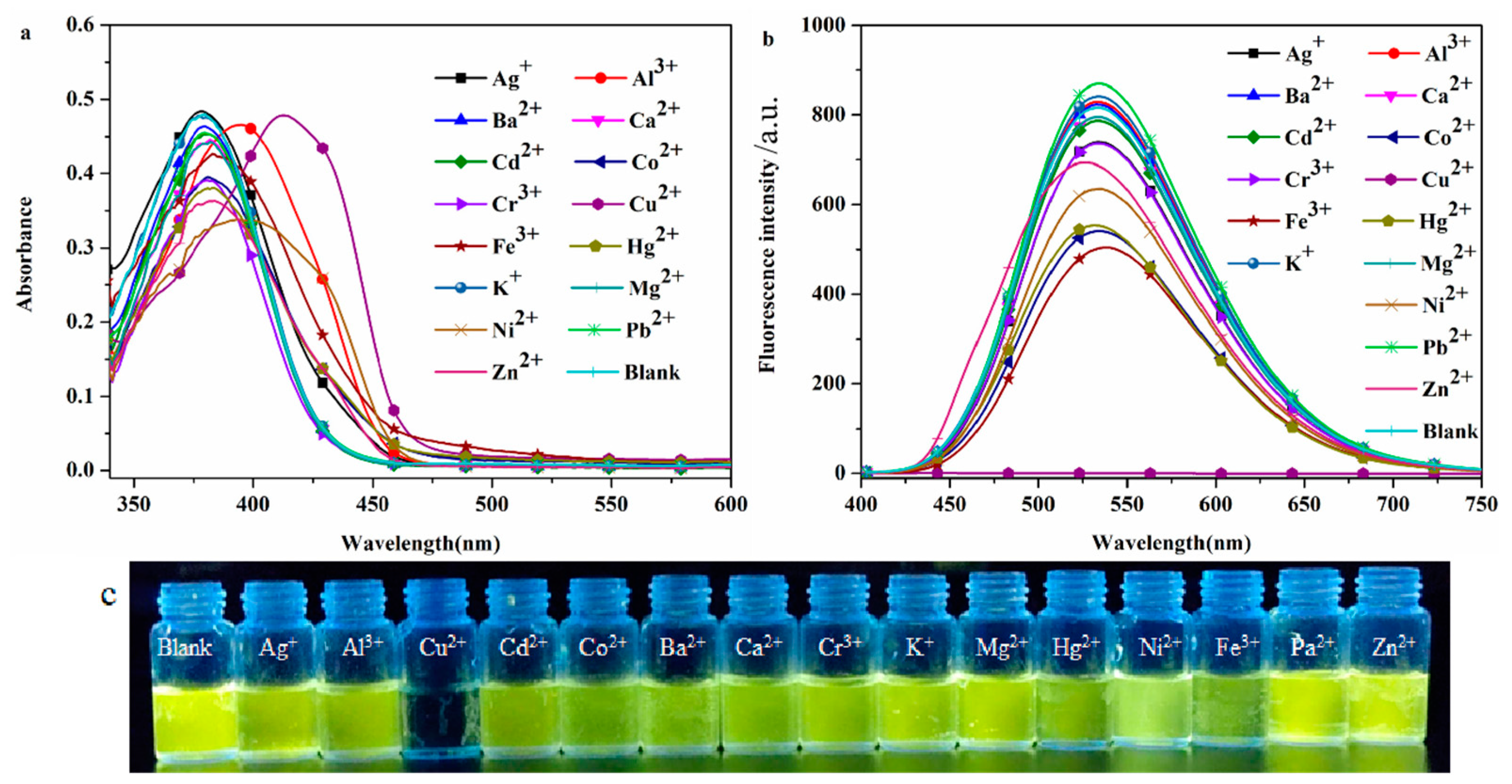
2.2.2. Selective Recognition of Cations
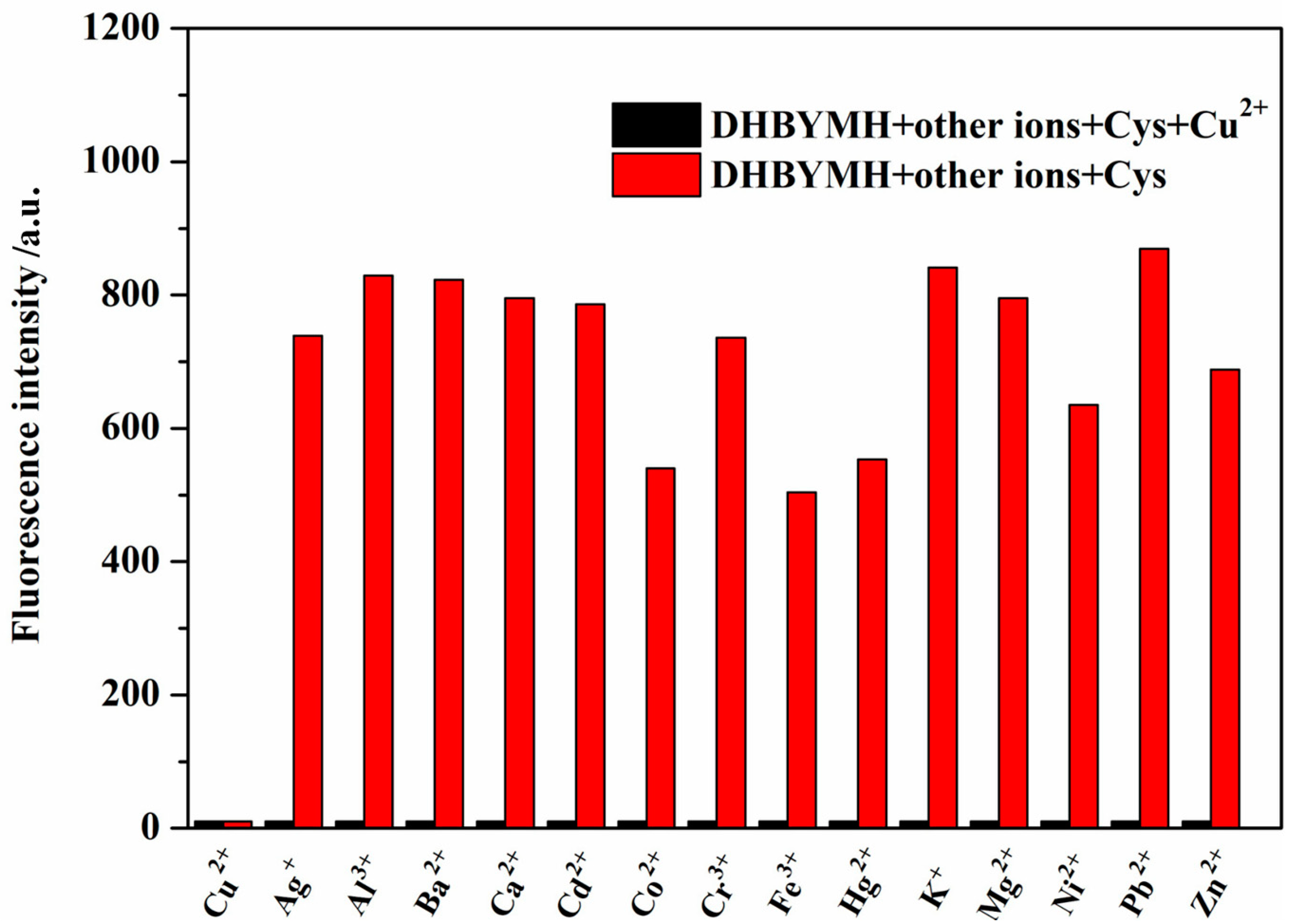
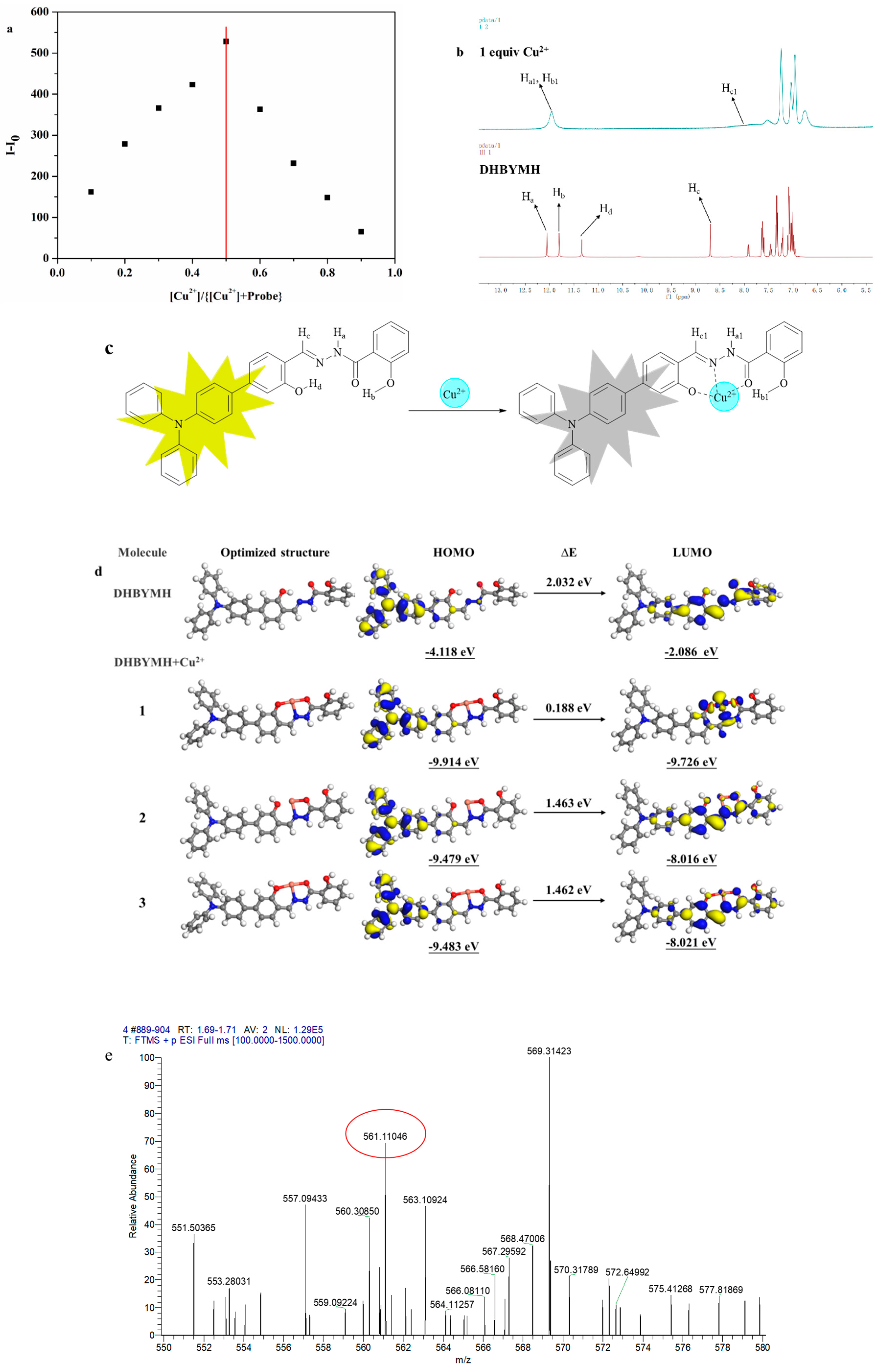
2.2.3. Competitive Recognition of Cu2+ Ions
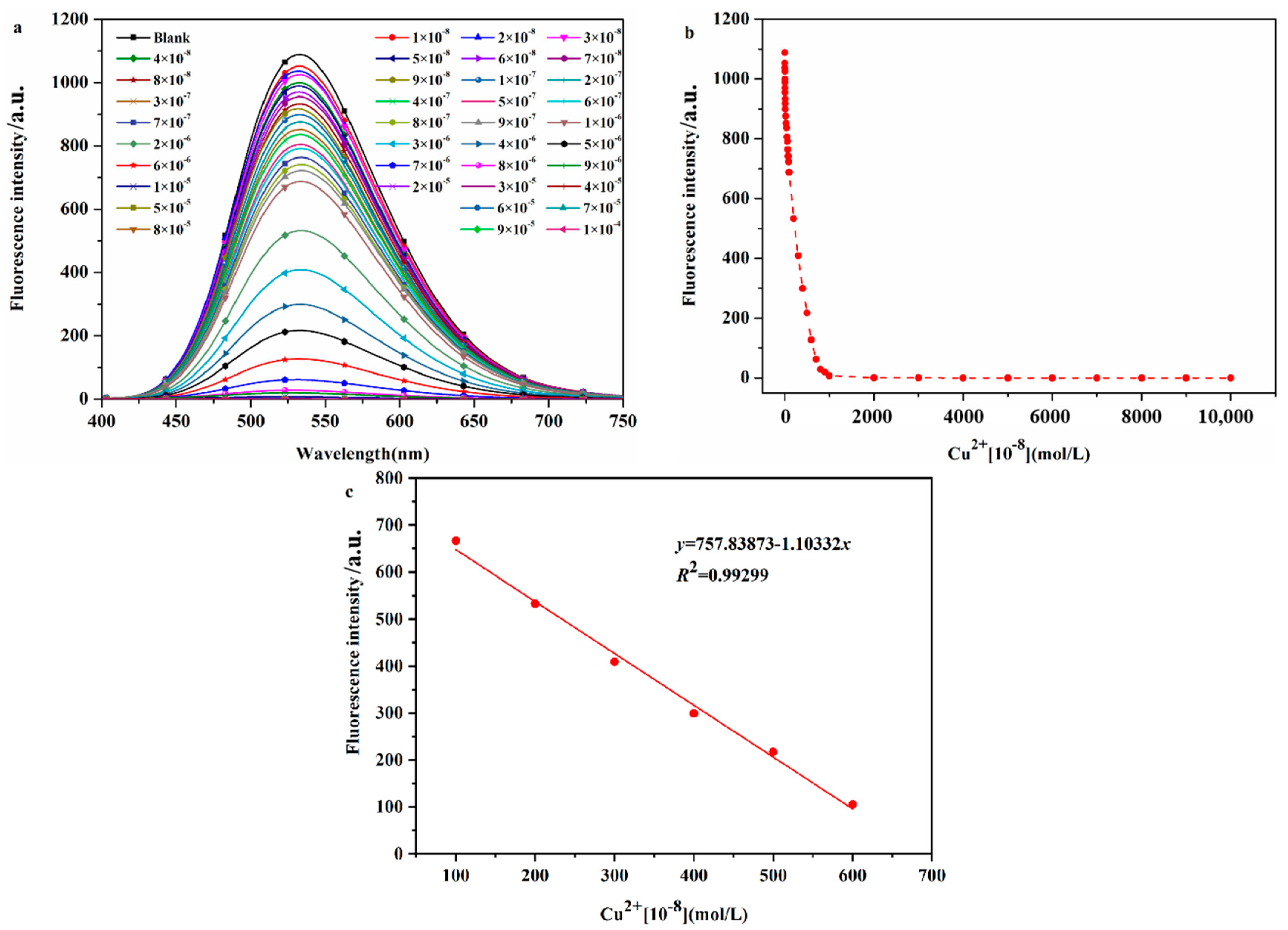
2.2.4. Determination of the Detection Limit of Probe DHBYMH for Cu2+ Ion Recognition
2.2.5. The Mechanism of Cu2+ Ion Recognition
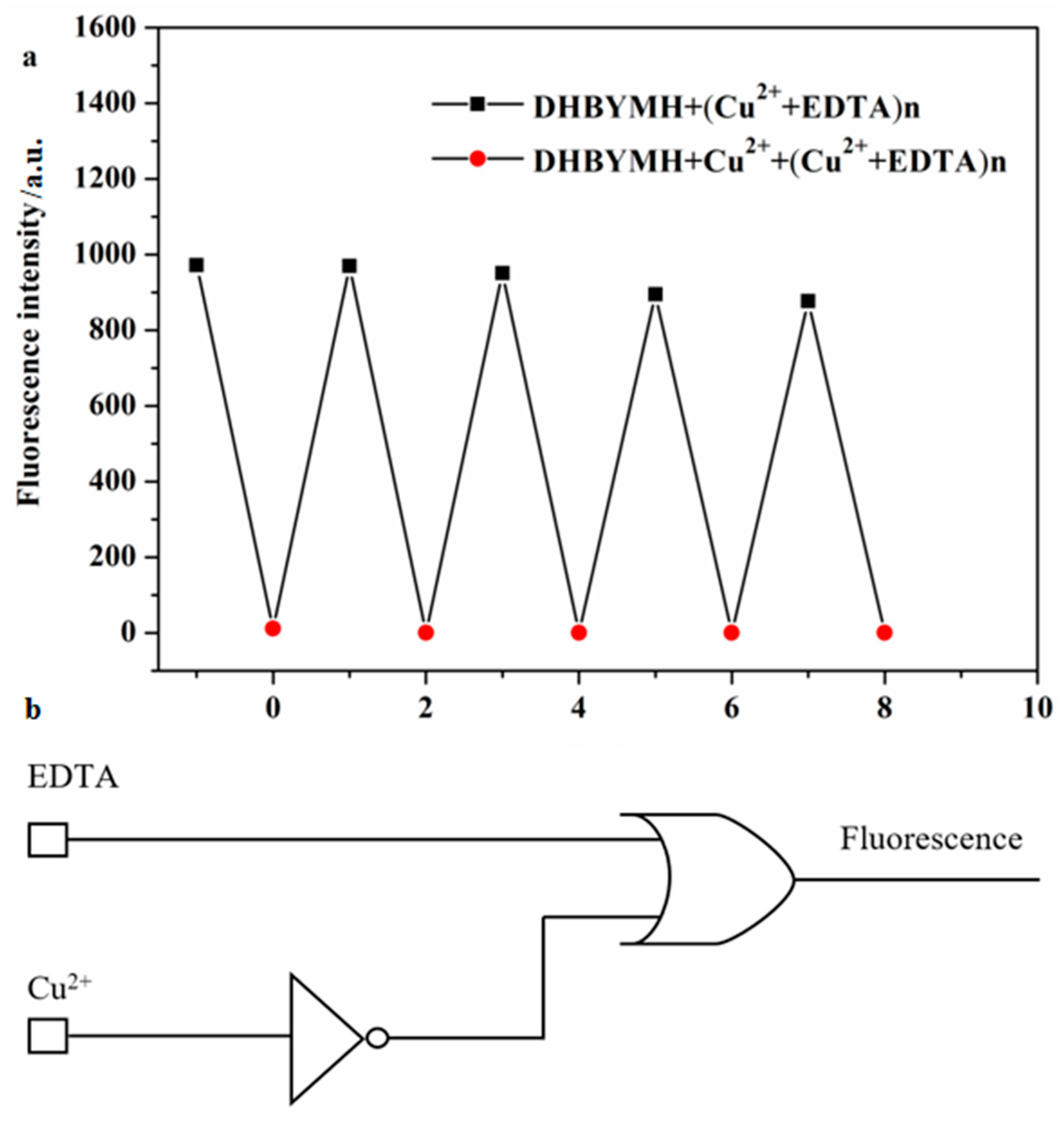
2.2.6. Construction of Molecular Logic Gates
3. Materials and Methods
3.1. Chemicals and Instruments
3.2. Design and Synthesis
3.2.1. Synthesis of 4-(Diphenylamino)-3-hydroxy-[1,1′-biphenyl]-4-formaldehyde (DHB) [42,43]
3.2.2. The Synthesis of N-((4-(Diphenylamino)-3-hydroxy-[1,1-biphenyl]-4-yl)methylene)-2-hydroxybenzohydrazide (DHBYMH)
3.3. DFT Calculations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Desai, V.; Kaler, S.G. Role of copper in human neurological disorders. Am. J. Clin. Nutr. 2008, 88, 855S–858S. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Ma, X.; Zhong, W.; Cao, Y.; Zhao, H.; Leng, X.; Yang, J.; Zhou, H.; She, M. Fluorescent sensing film decorated with ratiometric probe for visual and recyclable monitoring of Cu2+. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 249, 119217. [Google Scholar] [CrossRef] [PubMed]
- Ishida, S.; Andreux, P.; Poitry-Yamate, C.; Auwerx, J.; Hanahan, D. Bioavailable copper modulates oxidative phosphorylation and growth of tumors. Proc. Natl. Acad. Sci. USA 2013, 110, 19507–19512. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Lin, K.; Liu, X. Distribution and pollution risk assessment of heavy metals in the surface sediment of the intertidal zones of the Yellow River Estuary, China. Mar. Pollut. Bull. 2022, 174, 113286. [Google Scholar] [CrossRef]
- Liu, Z.; Fei, Y.; Shi, H.; Mo, L.; Qi, J. Prediction of high-risk areas of soil heavy metal pollution with multiple factors on a large scale in industrial agglomeration areas. Sci. Total Environ. 2022, 808, 151874. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Song, F.; Wei, G.; Wu, R.; Yan, Z.; Zhang, F.; Guang, S.; Xu, H. Molecular design for novel sensing materials with self-screening interference effect (SSIE): Reversible recognizing Cu2+ in aqueous and biologic samples. Sens. Actuators B Chem. 2019, 286, 163–172. [Google Scholar] [CrossRef]
- Hosseini, M.; Hashemimoghaddam, H. Sensitized extraction spectrophotometric determination of Hg(II) with dithizone after its flotation as ion-associate using iodide and ferroin. Talanta 2005, 67, 555–559. [Google Scholar] [CrossRef] [PubMed]
- Danet, A.F.; Bratu, M.; Radulescu, M.; Bratu, A. Portable minianalyzer based on cold vapor atomic absorption spectrometry at 184.9nm for atmospheric mercury determination. Sens. Actuators B Chem. 2009, 137, 12–16. [Google Scholar] [CrossRef]
- Gong, J.; Zhou, T.; Song, D.; Zhang, L.; Hu, X. Stripping Voltammetric Detection of Mercury(II) Based on a Bimetallic Au-Pt Inorganic-Organic Hybrid Nanocomposite Modified Glassy Carbon Electrode. Anal. Chem. 2010, 82, 567–573. [Google Scholar] [CrossRef]
- Schlöglova, K.; Wälle, M.; Heinrich, C.A. LA-ICP-MS analysis of fluid inclusions: Contamination effects challenging micro-analysis of elements close to their detection limit. J. Anal. At. Spectrom. 2017, 32, 1052–1063. [Google Scholar] [CrossRef]
- Wu, J.; Boyle, E.A. Low Blank Preconcentration Technique for the Determination of Lead, Copper, and Cadmium in Small-Volume Seawater Samples by Isotope Dilution ICPMS. Anal. Chem. 1997, 69, 2464–2470. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Wang, Q.; Zhou, D.; An, Y.; Wang, P.; Liao, F. A novel peptide-based fluorescent probe with a large stokes shift for rapid and sequential detection of Cu2+ and CN− in aqueous systems and live cells. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 264, 120257. [Google Scholar] [CrossRef]
- Wang, P.; Sun, L.; Wu, J.; Yang, X.; Lin, P.; Wang, M. A dual-functional colorimetric and fluorescent peptide-based probe for sequential detection of Cu2+ and S2- in 100% aqueous buffered solutions and living cells. J. Hazard. Mater. 2021, 407, 124388. [Google Scholar] [CrossRef] [PubMed]
- Selvaraj, M.; Rajalakshmi, K.; Ahn, D.; Yoon, S.; Nam, Y.; Lee, Y.; Xu, Y.; Song, J.; Lee, K. Tetraphenylethene-based fluorescent probe with aggregation-induced emission behavior for Hg2+ detection and its application. Anal. Chim. Acta 2021, 1148, 238178. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Pang, X.; Wang, Z.; Chai, Q.; Ye, F. A highly sensitive and selective fluorescent probe for determination of Cu (II) and application in live cell imaging. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 208, 198–205. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, Y.; Wang, E. A highly selective “turn-on” fluorescent probe for detecting Cu2+ in two different sensing mechanisms. Dye. Pigment. 2019, 163, 533–537. [Google Scholar] [CrossRef]
- Jiang, N.; Gong, X.; Zhong, T.; Zheng, Y.; Wang, G. A highly selective and sensitive “turn-on” fluorescent probe for rapid recognition and detection of Cu2+ in aqueous solution and in living cells. J. Mol. Struct. 2020, 1219, 128573. [Google Scholar] [CrossRef]
- Li, B.; Kou, J.; Mei, H.; Gu, X.; Wang, M.; Xie, X.; Xu, K. A hemicyanine-based “turn-on” fluorescent probe for the selective detection of Cu2+ ions and imaging in living cells. Anal. Methods 2020, 12, 4181–4184. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, J.; Zhao, L.; Xu, B. A dual-responsive and highly sensitive fluorescent probe for Cu2+ and pH based on a dansyl derivative. Dye. Pigment. 2020, 180, 108513. [Google Scholar] [CrossRef]
- Yin, J.; Wang, Z.; Zhao, F.; Yang, H.; Li, M.; Yang, Y. A novel dual functional pyrene-based turn-on fluorescent probe for hypochlorite and copper (II) ion detection and bioimaging applications. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 239, 118470. [Google Scholar] [CrossRef]
- Lina, G.; Gao, Y.; Han, L. Detecting Cu2+ and H2O in methanol based on aggregation-induced emission fluorescent enhancement. J. Coord. Chem. 2021, 74, 1284–1297. [Google Scholar] [CrossRef]
- Leng, X.; Wang, D.; Mi, Z.; Zhang, Y.; Yang, B.; Chen, F. Novel Fluorescence Probe toward Cu2+ Based on Fluorescein Derivatives and Its Bioimaging in Cells. Biosensors 2022, 12, 732. [Google Scholar] [CrossRef] [PubMed]
- Shi, T.; Xie, Z.; He, J.; Wu, F.; Li, H.; Guo, J.; Zhao, J. Synthesis and Application of Acylhydrazone Probe with High Selectivity and Rapid Detection of Mercury Ion. ChemistrySelect 2023, 8, e202203827. [Google Scholar] [CrossRef]
- Zhao, Y.; Qiu, H.; Liang, H.; Tao, Y.; Zhou, R.; Chu, Y.; Shi, T.; Xie, Z.; Wen, Y. Synthesis of Triazole Functionalized Triphenylamine Cu2+ Fluorescent Probe and Its Application in Detection and HeLa Cells. Chin. J. Org. Chem. 2022, 42, 1463–1473. [Google Scholar] [CrossRef]
- Xue, S.; Xie, Z.; He, J.; Zhang, T.; Xia, B.; Li, Y. Synthesis of Sulfonylhydrazone Probe with High Selectivity and Rapid Identification of Hg(Ⅱ)Ion and Its Application in Adsorption. Chin. J. Appl. Chem. 2021, 5, 760–768. [Google Scholar] [CrossRef]
- Xue, S.; Xie, Z.; Chu, Y.; Shi, W.; Liu, Y.; Zhao, Y. Highly selective and sensitive fluorescent probe possessing AIEE and ICT properties for rapid detection of Pb2+ in aqueous medium and its applications in living cells. Luminescence 2021, 37, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Shi, W.; Yue, Y.; Chu, Y.; Xie, Z.; Xue, S. Synthesis of Sulfonylhydrazone Type Probe with High Selectivity for Rapid Detection of Mercury and Its Application in Adsorption and HeLa Cell. Chin. J. Org. Chem. 2021, 41, 1138–1145. [Google Scholar] [CrossRef]
- Yue, Y.; Xie, Z.; Chu, Y.; Xue, S. Synthesis and Optical Properties of Novel Spiro[chromo(2,3-c)-pyrazole-4,1′-isobenzofuran]-3′-one Compounds. Chin. J. Org. Chem. 2020, 40, 501–510. [Google Scholar] [CrossRef]
- Xue, S.; Xie, Z.; Wen, Y.; He, J.; Liu, Y.; Shi, W. Highly Selective and Sensitive Sulfonylhydrazone Type Fluorescent Probe for Rapid Detection of Mercury(II) and Its Application in Logic Gate and Adsorption. ChemistrySelect 2021, 6, 7123–7129. [Google Scholar] [CrossRef]
- Shi, T.; Xie, Z.; Mo, X.; Shi, W.; Qiu, H.; Lan, G.; Liu, Y. Adsorption behaviors of heavy metal ions by different hydrazone-modified sodium alginate in aqueous medium: Experimental and DFT studies. Colloids Surf. A Physicochem. Eng. Asp. 2023, 659, 130754. [Google Scholar] [CrossRef]
- Shi, T.; Xie, Z.; Mo, X.; Feng, Y.; Peng, T.; Song, D. Highly Efficient Adsorption of Heavy Metals and Cationic Dyes by Smart Functionalized Sodium Alginate Hydrogels. Gels 2022, 8, 343. [Google Scholar] [CrossRef] [PubMed]
- Shi, T.; Xie, Z.; Zhu, Z.; Shi, W.; Liu, Y.; Liu, M. Highly efficient and selective adsorption of heavy metal ions by hydrazide-modified sodium alginate. Carbohydr. Polym. 2022, 276, 118797. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Liu, J.; He, J.; Zhang, J.; Zhou, H.; Gao, C. A novel red-emitting fluorescent probe for the highly selective detection of Hg2+ ion with AIE mechanism. Chem. Phys. 2020, 539, 110944. [Google Scholar] [CrossRef]
- Zhu, L.; Yang, X.; Luo, X.; Hu, B.; Huang, W. A highly selective fluorescent probe based on coumarin and pyrimidine hydrazide for Cu2+ ion detection. Inorg. Chem. Commun. 2020, 114, 107823. [Google Scholar] [CrossRef]
- Wang, L.; Li, W.; Zhi, W.; Huang, Y.; Han, J.; Wang, Y.; Ren, Y.; Ni, L. A new coumarin schiff based fluorescent-colorimetric chemosensor for dual monitoring of Zn2+ and Fe3+ in different solutions: An application to bio-imaging. Sens. Actuators B Chem. 2018, 260, 243–254. [Google Scholar] [CrossRef]
- Qin, J.; Yang, Z.; Wang, G.; Li, C. FRET-based rhodamine–coumarin conjugate as a Fe3+ selective ratiometric fluorescent sensor in aqueous media. Tetrahedron Lett. 2015, 56, 5024–5029. [Google Scholar] [CrossRef]
- Hua, C.; Zheng, H.; Zhang, K.; Xin, M.; Gao, J.; Li, Y. A novel turn off fluorescent sensor for Fe(III) and pH environment based on coumarin derivatives: The fluorescence characteristics and theoretical study. Tetrahedron 2016, 72, 8365–8372. [Google Scholar] [CrossRef]
- Erdemir, S.; Malkondu, S. A switch-on xanthene triphenylamine based fluorescent and colorimetric sensor for the detection of ultra-trace Hg2+ in food samples and living cells. Food Chem. 2022, 376, 131951. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Tong, Q.; Qin, X.; Liao, X.; Li, Q.; Yan, G. A hydrophilic naphthalimide-based fluorescence chemosensor for Cu2+ ion: Sensing properties, cell imaging and molecular logic behavior. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 230, 118029. [Google Scholar] [CrossRef]
- Mohanasundaram, D.; Vinoth Kumar, G.G.; Kumar, S.K.; Maddiboyina, B.; Raja, R.P.; Rajesh, J.; Sivaraman, G. Turn-on fluorescence sensor for selective detection of fluoride ion and its molecular logic gates behavior. J. Mol. Liq. 2020, 317, 113913. [Google Scholar] [CrossRef]
- Acharyya, S.; Gharami, S.; Sarkar, D.; Ghosh, P.; Murmu, N.; Mondal, T.K. A thioether containing reversible fluorescence “turn-on” chemosensor for selective detection of zinc(II): Applications in live cell imaging and inhibit logic gate. J. Mol. Struct. 2021, 1224, 129179. [Google Scholar] [CrossRef]
- Lin, H.; Shi, W.; Tian, Y.; Ma, F.; Xu, L.; Ma, J.; Hui, Y.; Xie, Z. A simple and highly selective ‘turn-on’ type fluorescence chemodosimeter for Hg2+ based on 1-(2-phenyl-2H-[1,2,3]triazole-4-carbonyl)thiosemicarbazide. J. Lumin. 2015, 157, 280–284. [Google Scholar] [CrossRef]
- Feng, L.; Shi, W.; Ma, J.; Chen, Y.; Kui, F.; Hui, Y.; Xie, Z. A novel thiosemicarbazone Schiff base derivative with aggregation-induced emission enhancement characteristics and its application in Hg2+ detection. Sens. Actuators B Chem. 2016, 237, 563–569. [Google Scholar] [CrossRef]
- Cao, J.; Liu, Q.-M.; Bai, S.-J.; Wang, H.-C.; Ren, X.; Xu, Y.-X. Ladder-Type Dye with Large Transition Dipole Moment for Solvatochromism and Microphase Visualization. ACS Appl. Mater. Interfaces 2019, 11, 29814–29820. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, Y.; Li, X.; Nie, S.; Liu, C.; Zhang, Y.; Guo, J.; Liu, C. A dual functional fluorescent probe based on naphthalimide for detecting Cu2+ and pH and its applications. Inorganica Chim. Acta 2023, 554, 121544. [Google Scholar] [CrossRef]
- Yang, Y.-S.; Cao, J.-Q.; Ma, C.-M.; Zhang, Y.-P.; Guo, H.-C.; Xue, J.-J. A novel pyrazoline-based fluorescence probe armed by pyrene and naphthol system for the selective detection of Cu2+ and its biological application. J. Iran. Chem. Soc. 2022, 19, 3451–3461. [Google Scholar] [CrossRef]
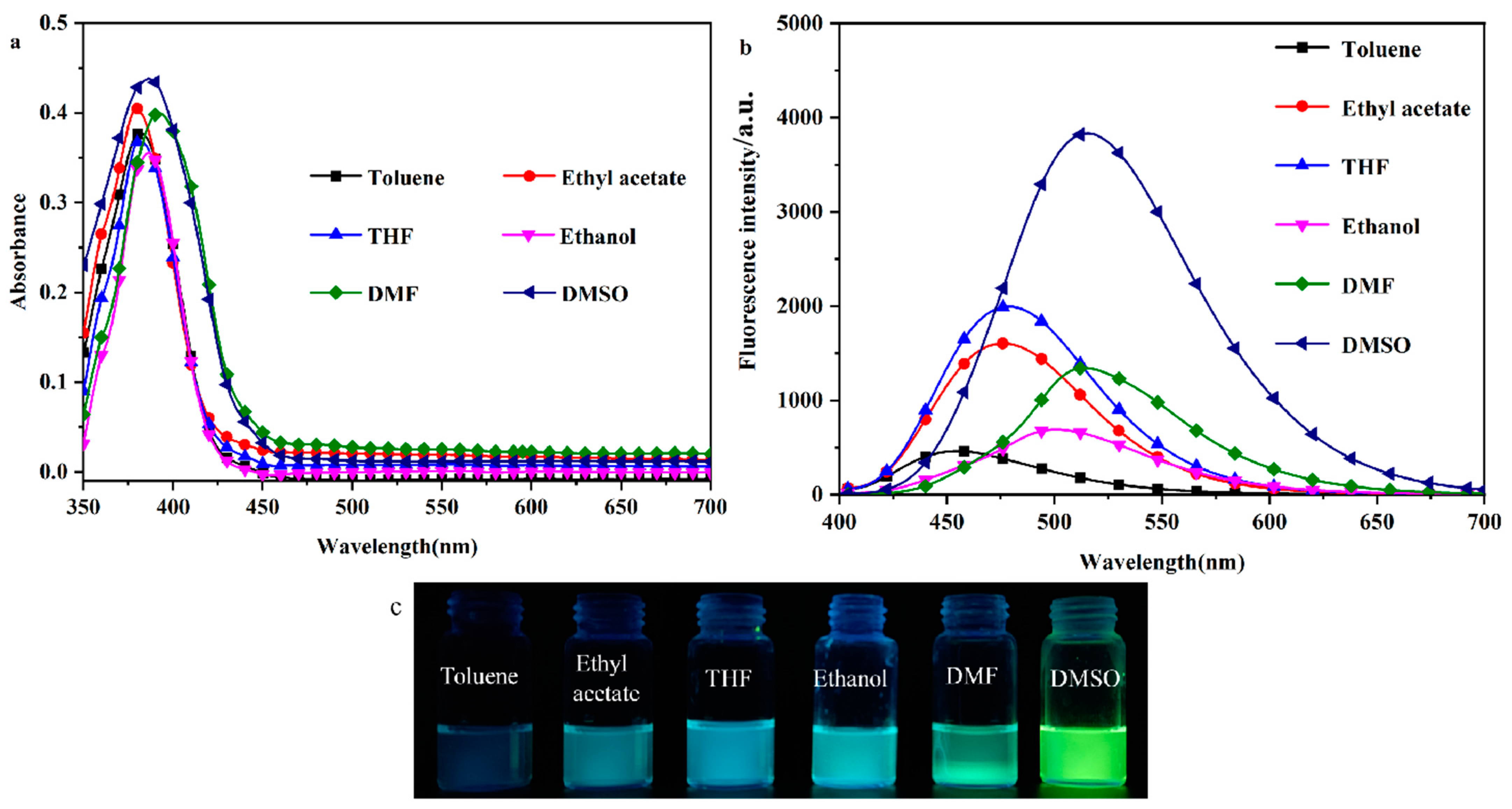

| DHBYMH | λabs (nm) | λem (nm) | Intensity | Stokes Shift (nm) | QY (%) |
|---|---|---|---|---|---|
| Toluene | 380 | 454 | 461 | 74 | 15.64 |
| Ethyl acetate | 380 | 476 | 1603 | 96 | 18.51 |
| THF | 381 | 479 | 1994 | 98 | 19.27 |
| Ethanol | 386 | 501 | 690 | 115 | 22.31 |
| DMF | 390 | 514 | 1343 | 124 | 24.19 |
| DMSO | 384 | 520 | 3833 | 136 | 25.06 |
| Entry | Input A (Cu2+) | Input B (EDTA) | Output (Fluorescence) |
|---|---|---|---|
| 1 | 0 | 0 | 1 |
| 2 | 0 | 1 | 1 |
| 3 | 1 | 0 | 0 |
| 4 | 1 | 1 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, T.; Xie, Z.; Mo, X.; Feng, Y.; Peng, T.; Wu, F.; Yu, M.; Zhao, J.; Zhang, L.; Guo, J. Synthesis and Application of Salicylhydrazone Probes with High Selectivity for Rapid Detection of Cu2+. Molecules 2024, 29, 2032. https://doi.org/10.3390/molecules29092032
Shi T, Xie Z, Mo X, Feng Y, Peng T, Wu F, Yu M, Zhao J, Zhang L, Guo J. Synthesis and Application of Salicylhydrazone Probes with High Selectivity for Rapid Detection of Cu2+. Molecules. 2024; 29(9):2032. https://doi.org/10.3390/molecules29092032
Chicago/Turabian StyleShi, Tianzhu, Zhengfeng Xie, Xinliang Mo, Yulong Feng, Tao Peng, Fuyong Wu, Mei Yu, Jingjing Zhao, Li Zhang, and Ju Guo. 2024. "Synthesis and Application of Salicylhydrazone Probes with High Selectivity for Rapid Detection of Cu2+" Molecules 29, no. 9: 2032. https://doi.org/10.3390/molecules29092032
APA StyleShi, T., Xie, Z., Mo, X., Feng, Y., Peng, T., Wu, F., Yu, M., Zhao, J., Zhang, L., & Guo, J. (2024). Synthesis and Application of Salicylhydrazone Probes with High Selectivity for Rapid Detection of Cu2+. Molecules, 29(9), 2032. https://doi.org/10.3390/molecules29092032





