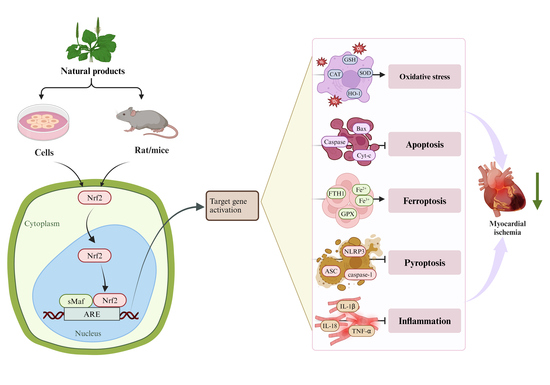
Potential Targets of Natural Products for Improving Cardiac Ischemic Injury: The Role of Nrf2 Signaling Transduction
Abstract
1. Introduction
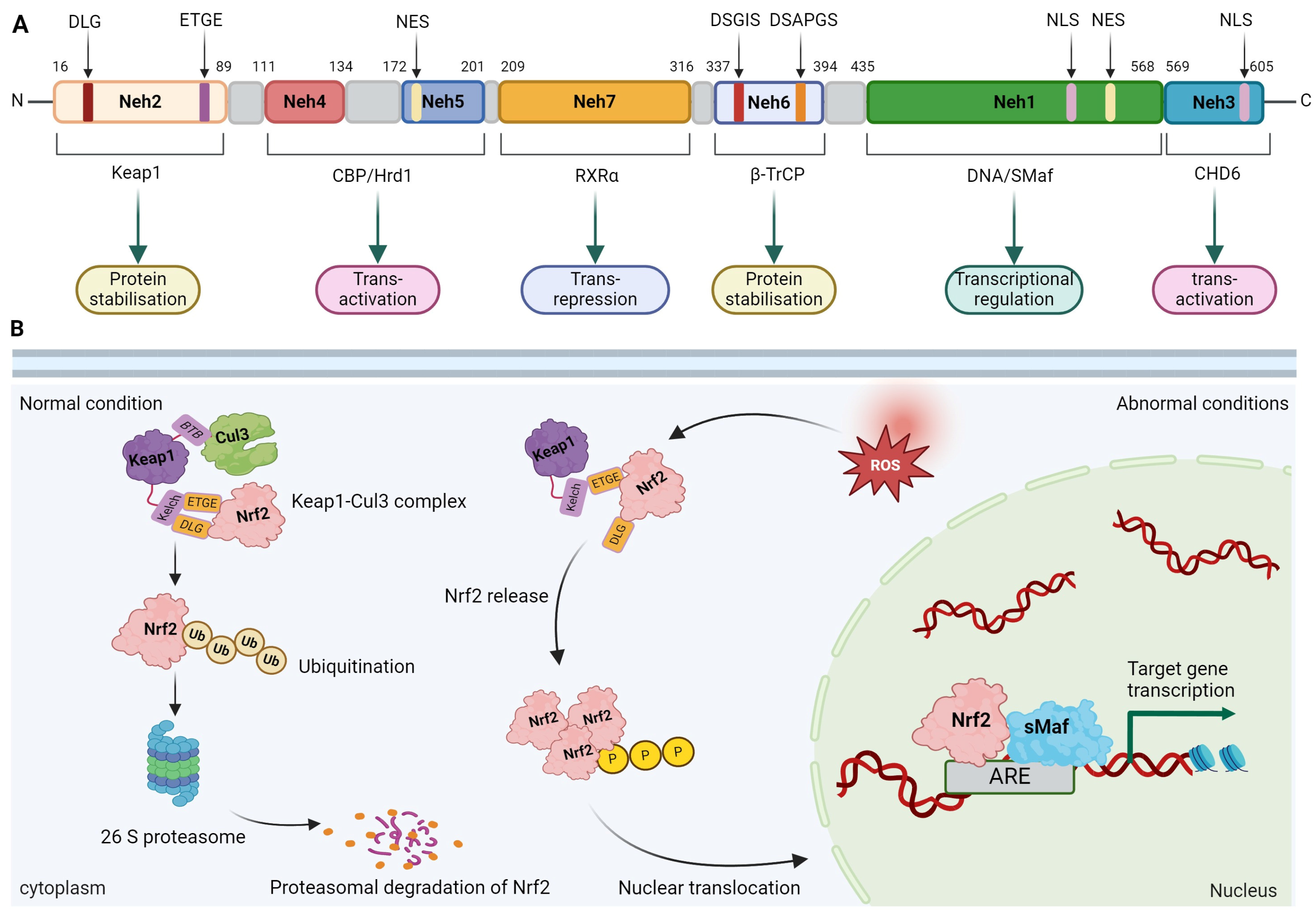
2. Nrf2 Overview
2.1. Molecular Structure of Nrf2
2.2. Regulation of Nrf2 Activity
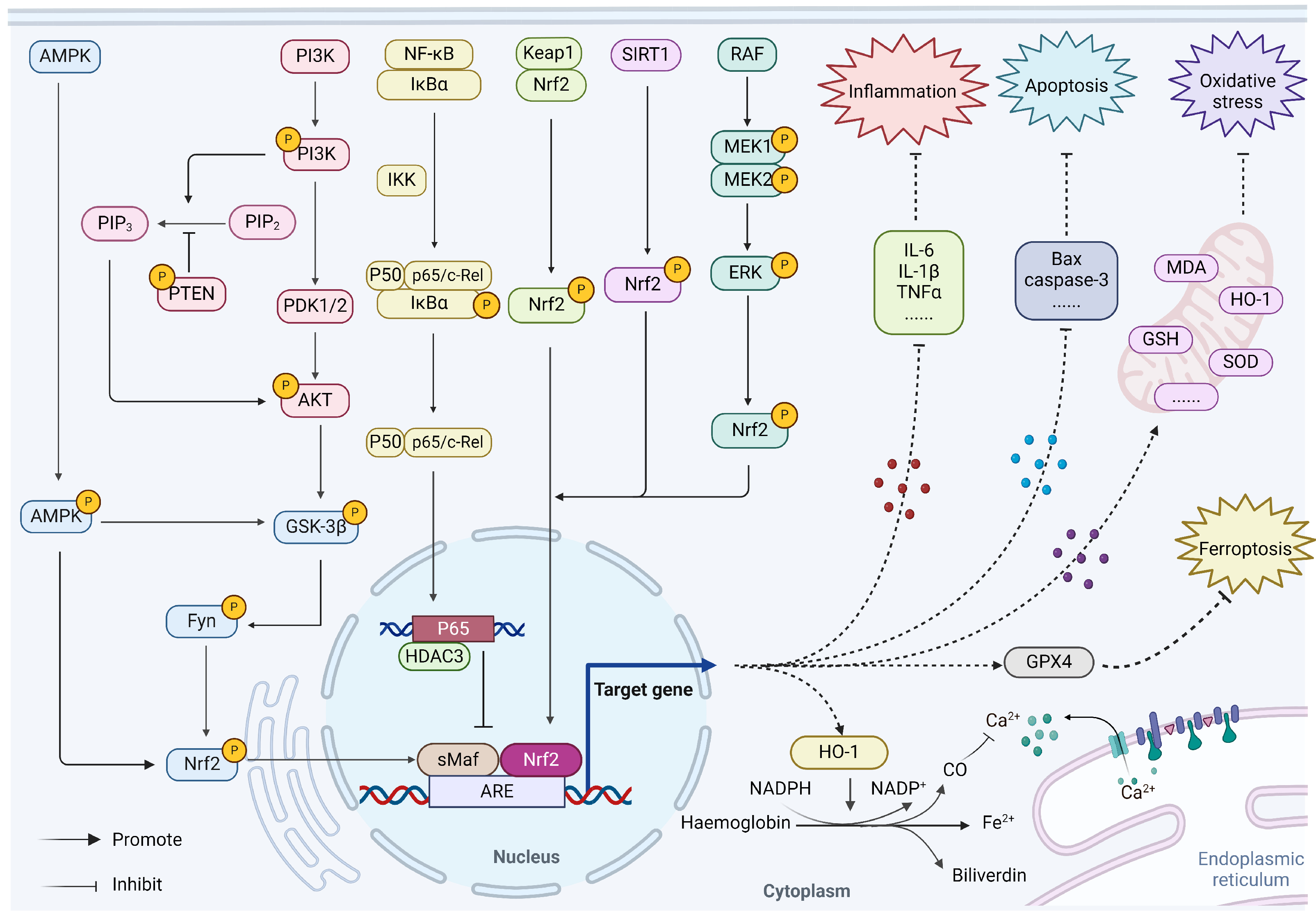
3. Regulation of Nrf2 on Myocardial Ischemia
3.1. Nrf2/HO-1
3.2. AMPK/GSK-3β/Nrf2
3.3. PI3K/Akt/Nrf2
3.4. NF-κB/p65/Nrf2
3.5. Sirt1/Nrf2
3.6. MAPK/ERK/Nrf2
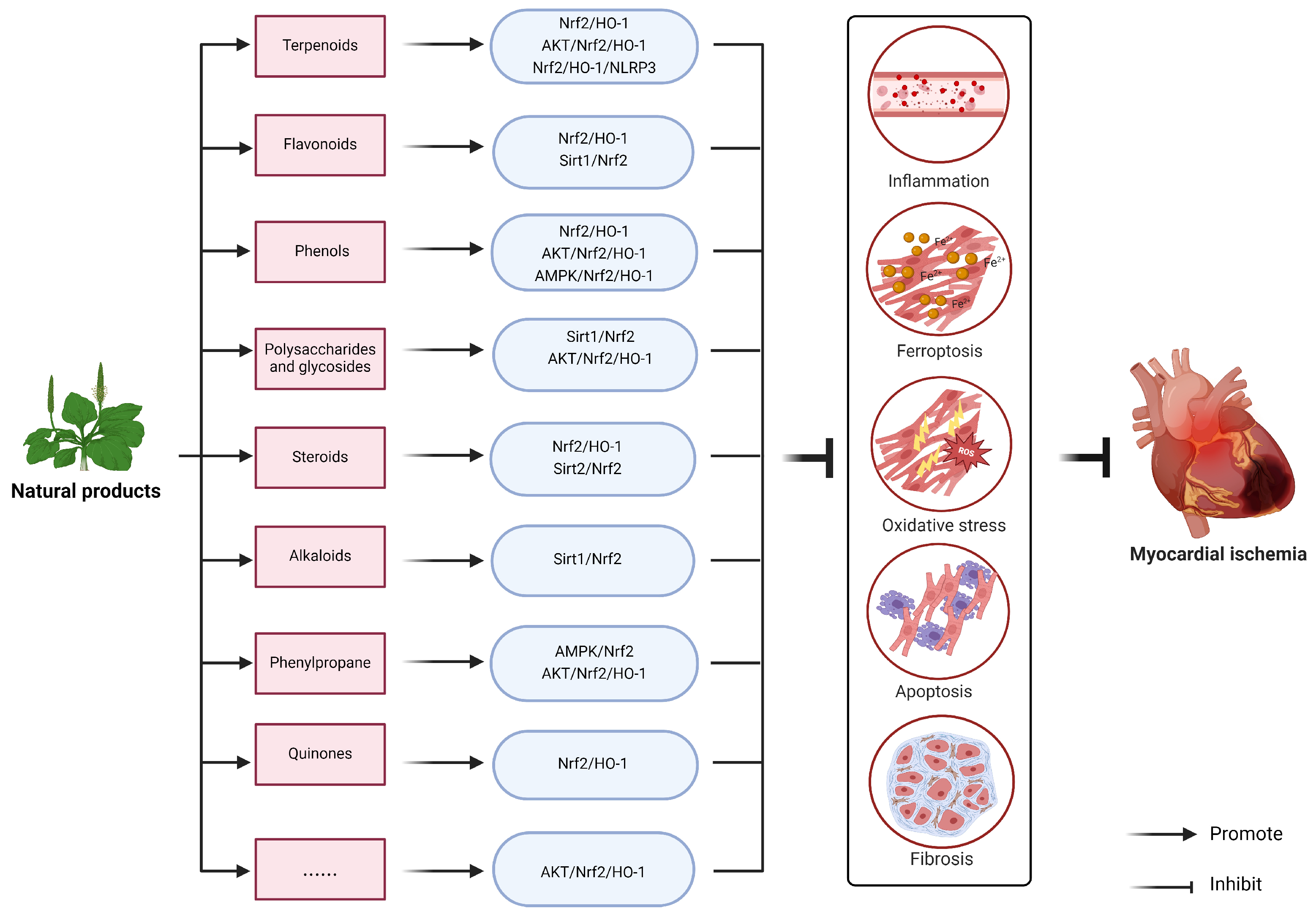
4. Natural Products Target Nrf2 to Improve Myocardial Ischemia Injury
4.1. Terpenoids
4.2. Flavonoids
4.3. Phenols
4.4. Polysaccharides and Glycosides
4.5. Steroids
4.6. Alkaloids
4.7. Phenylpropane
4.8. Quinones
4.9. Others
5. Conclusions and Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, Z.; Lin, L.; Wu, H.; Yan, L.; Wang, H.; Yang, H.; Li, H. Global, Regional, and National Death, and Disability-Adjusted Life-Years (DALYs) for Cardiovascular Disease in 2017 and Trends and Risk Analysis From 1990 to 2017 Using the Global Burden of Disease Study and Implications for Prevention. Front. Public Health 2021, 9, 559751. [Google Scholar] [CrossRef] [PubMed]
- Roth, G.A.; Johnson, C.; Abajobir, A.; Abd-Allah, F.; Abera, S.F.; Abyu, G.; Ahmed, M.; Aksut, B.; Alam, T.; Alam, K.; et al. Global, Regional, and National Burden of Cardiovascular Diseases for 10 Causes, 1990 to 2015. J. Am. Coll. Cardiol. 2017, 70, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Divoky, L.; Maran, A.; Ramu, B. Gender Differences in Ischemic Cardiomyopathy. Curr. Atheroscler. Rep. 2018, 20, 50. [Google Scholar] [CrossRef] [PubMed]
- Hands, M.E.; Rutherford, J.D.; Muller, J.E.; Davies, G.; Stone, P.H.; Parker, C.; Braunwald, E. The in-hospital development of cardiogenic shock after myocardial infarction: Incidence, predictors of occurrence, outcome and prognostic factors. The MILIS Study Group. J. Am. Coll. Cardiol. 1989, 14, 40–46; discussion 47–48. [Google Scholar] [CrossRef] [PubMed]
- Welsh, R.C.; Cantor, W.J.; Traboulsi, M.; Schampaert, E.; May, M.L. Primary Percutaneous Coronary Intervention and Application of the Pharmacoinvasive Approach Within ST-Elevation Myocardial Infarction Care Networks. Can. J. Cardiol. 2022, 38, S5–S16. [Google Scholar] [CrossRef] [PubMed]
- Reed, G.W.; Rossi, J.E.; Cannon, C.P. Acute myocardial infarction. Lancet 2017, 389, 197–210. [Google Scholar] [CrossRef]
- Piper, H.M.; Garcia-Dorado, D.; Ovize, M. A fresh look at reperfusion injury. Cardiovasc. Res. 1998, 38, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Severino, P.; D’Amato, A.; Pucci, M.; Infusino, F.; Adamo, F.; Birtolo, L.I.; Netti, L.; Montefusco, G.; Chimenti, C.; Lavalle, C.; et al. Ischemic Heart Disease Pathophysiology Paradigms Overview: From Plaque Activation to Microvascular Dysfunction. Int. J. Mol. Sci. 2020, 21, 8118. [Google Scholar] [CrossRef]
- Chevillard, G.; Blank, V. NFE2L3 (NRF3): The Cinderella of the Cap’n’Collar transcription factors. Cell. Mol. Life Sci. 2011, 68, 3337–3348. [Google Scholar] [CrossRef]
- Loboda, A.; Damulewicz, M.; Pyza, E.; Jozkowicz, A.; Dulak, J. Role of Nrf2/HO-1 system in development, oxidative stress response and diseases: An evolutionarily conserved mechanism. Cell. Mol. Life Sci. 2016, 73, 3221–3247. [Google Scholar] [CrossRef]
- Moi, P.; Chan, K.; Asunis, I.; Cao, A.; Kan, Y.W. Isolation of NF-E2-related factor 2 (Nrf2), a NF-E2-like basic leucine zipper transcriptional activator that binds to the tandem NF-E2/AP1 repeat of the beta-globin locus control region. Proc. Natl. Acad. Sci. USA 1994, 91, 9926–9930. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, K.; Kataoka, K.; Itoh, K.; Hayashi, N.; Nishizawa, M.; Yamamoto, M. Regulation of transcription by dimerization of erythroid factor NF-E2 p45 with small Maf proteins. Nature 1994, 367, 568–572. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.M.; Maltagliati, A.J. Nrf2 at the heart of oxidative stress and cardiac protection. Physiol. Genom. 2018, 50, 77–97. [Google Scholar] [CrossRef]
- Saeedi, B.J.; Liu, K.H.; Owens, J.A.; Hunter-Chang, S.; Camacho, M.C.; Eboka, R.U.; Chandrasekharan, B.; Baker, N.F.; Darby, T.M.; Robinson, B.S.; et al. Gut-Resident Lactobacilli Activate Hepatic Nrf2 and Protect Against Oxidative Liver Injury. Cell Metab. 2020, 31, 956–968.e955. [Google Scholar] [CrossRef] [PubMed]
- Xin, L.; Zhou, F.; Zhang, C.; Zhong, W.; Xu, S.; Jing, X.; Wang, D.; Wang, S.; Chen, T.; Song, J. Four-Octyl itaconate ameliorates periodontal destruction via Nrf2-dependent antioxidant system. Int. J. Oral Sci. 2022, 14, 27. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Jin, Y.; Liu, J.; Liu, Q.; Shen, Y.; Zuo, S.; Yu, Y. EP1 activation inhibits doxorubicin-cardiomyocyte ferroptosis via Nrf2. Redox Biol. 2023, 65, 102825. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Liu, J.; Wang, Z.; Qian, X.; Zhao, Y.; Wang, Q.; Dai, N.; Xie, Y.; Zeng, W.; Yang, W.; et al. Dexmedetomidine abates myocardial ischemia reperfusion injury through inhibition of pyroptosis via regulation of miR-665/MEF2D/Nrf2 axis. Biomed. Pharmacother. 2023, 165, 115255. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Li, Z.; Liang, Y.; Yang, C.; Ou, W.; Mo, H.; Tang, M.; Chen, D.; Zhong, C.; Que, D.; et al. Fucoxanthin alleviated myocardial ischemia and reperfusion injury through inhibition of ferroptosis via the NRF2 signaling pathway. Food Funct. 2023, 14, 10052–10068. [Google Scholar] [CrossRef] [PubMed]
- Pieters, F.A.; Woonink, F.; Zuidema, J. Influence of once-monthly rifampicin and daily clofazimine on the pharmacokinetics of dapsone in leprosy patients in Nigeria. Eur. J. Clin. Pharmacol. 1988, 34, 73–76. [Google Scholar] [CrossRef]
- Suzuki, T.; Motohashi, H.; Yamamoto, M. Toward clinical application of the Keap1-Nrf2 pathway. Trends Pharmacol. Sci. 2013, 34, 340–346. [Google Scholar] [CrossRef]
- Syed, A.M.; Ram, C.; Murty, U.S.; Sahu, B.D. A review on herbal Nrf2 activators with preclinical evidence in cardiovascular diseases. Phytother. Res. 2021, 35, 5068–5102. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Tan, B.K.; Huang, S.H.; Whiteman, M.; Zhu, Y.Z. Effects of natural products on ischemic heart diseases and cardiovascular system. Acta Pharmacol. Sin. 2002, 23, 1142–1151. [Google Scholar] [PubMed]
- Gouda, N.A.; Alshammari, S.O.; Abourehab, M.A.S.; Alshammari, Q.A.; Elkamhawy, A. Therapeutic potential of natural products in inflammation: Underlying molecular mechanisms, clinical outcomes, technological advances, and future perspectives. Inflammopharmacology 2023, 31, 2857–2883. [Google Scholar] [CrossRef] [PubMed]
- Tognola, C.; Alessandro, M.; Milani, M.; Cartella, I.; Tavecchia, G.; Grasso, E.; Sun, J.; Giannattasio, C. Nutraceuticals in Chronic Coronary Syndromes: Preclinical Data and Translational Experiences. High. Blood Press. Cardiovasc. Prev. 2021, 28, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Wei, J.; Yi, Y.; Gong, Q.; Gao, J. Activation of Nrf2 signaling: A key molecular mechanism of protection against cardiovascular diseases by natural products. Front. Pharmacol. 2022, 13, 1057918. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Zhao, T.; Zha, B.; Zhang, G.; Qian, W.; Wang, X.; Zhao, Q.; Chen, S.; Hu, Z.; Dong, L. Piceatannol protects against myocardial ischemia/reperfusion injury by inhibiting ferroptosis via Nrf-2 signaling-mediated iron metabolism. Biochem. Biophys. Res. Commun. 2024, 700, 149598. [Google Scholar] [CrossRef]
- Sykiotis, G.P.; Bohmann, D. Stress-activated cap’n’collar transcription factors in aging and human disease. Sci. Signal. 2010, 3, re3. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q. Role of nrf2 in oxidative stress and toxicity. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 401–426. [Google Scholar] [CrossRef]
- Saha, S.; Buttari, B.; Panieri, E.; Profumo, E.; Saso, L. An Overview of Nrf2 Signaling Pathway and Its Role in Inflammation. Molecules 2020, 25, 5474. [Google Scholar] [CrossRef]
- Sun, Z.; Chin, Y.E.; Zhang, D.D. Acetylation of Nrf2 by p300/CBP augments promoter-specific DNA binding of Nrf2 during the antioxidant response. Mol. Cell Biol. 2009, 29, 2658–2672. [Google Scholar] [CrossRef]
- Jaramillo, M.C.; Zhang, D.D. The emerging role of the Nrf2-Keap1 signaling pathway in cancer. Genes Dev. 2013, 27, 2179–2191. [Google Scholar] [CrossRef]
- Itoh, K.; Chiba, T.; Takahashi, S.; Ishii, T.; Igarashi, K.; Katoh, Y.; Oyake, T.; Hayashi, N.; Satoh, K.; Hatayama, I.; et al. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem. Biophys. Res. Commun. 1997, 236, 313–322. [Google Scholar] [CrossRef]
- Venugopal, R.; Jaiswal, A.K. Nrf1 and Nrf2 positively and c-Fos and Fra1 negatively regulate the human antioxidant response element-mediated expression of NAD(P)H:quinone oxidoreductase1 gene. Proc. Natl. Acad. Sci. USA 1996, 93, 14960–14965. [Google Scholar] [CrossRef]
- Sivinski, J.; Zhang, D.D.; Chapman, E. Targeting NRF2 to treat cancer. Semin. Cancer Biol. 2021, 76, 61–73. [Google Scholar] [CrossRef]
- McMahon, M.; Thomas, N.; Itoh, K.; Yamamoto, M.; Hayes, J.D. Dimerization of substrate adaptors can facilitate cullin-mediated ubiquitylation of proteins by a “tethering” mechanism: A two-site interaction model for the Nrf2-Keap1 complex. J. Biol. Chem. 2006, 281, 24756–24768. [Google Scholar] [CrossRef]
- Mohan, S.; Gupta, D. Crosstalk of toll-like receptors signaling and Nrf2 pathway for regulation of inflammation. Biomed. Pharmacother. 2018, 108, 1866–1878. [Google Scholar] [CrossRef]
- Nioi, P.; Nguyen, T.; Sherratt, P.J.; Pickett, C.B. The carboxy-terminal Neh3 domain of Nrf2 is required for transcriptional activation. Mol. Cell. Biol. 2005, 25, 10895–10906. [Google Scholar] [CrossRef]
- Zhang, W.; Feng, C.; Jiang, H. Novel target for treating Alzheimer’s Diseases: Crosstalk between the Nrf2 pathway and autophagy. Ageing Res. Rev. 2021, 65, 101207. [Google Scholar] [CrossRef]
- Niture, S.K.; Khatri, R.; Jaiswal, A.K. Regulation of Nrf2—An update. Free Radic. Biol. Med. 2014, 66, 36–44. [Google Scholar] [CrossRef]
- Chang, M.; Wilson, C.J.; Karunatilleke, N.C.; Moselhy, M.H.; Karttunen, M.; Choy, W.Y. Exploring the Conformational Landscape of the Neh4 and Neh5 Domains of Nrf2 Using Two Different Force Fields and Circular Dichroism. J. Chem. Theory Comput. 2021, 17, 3145–3156. [Google Scholar] [CrossRef] [PubMed]
- Katoh, Y.; Itoh, K.; Yoshida, E.; Miyagishi, M.; Fukamizu, A.; Yamamoto, M. Two domains of Nrf2 cooperatively bind CBP, a CREB binding protein, and synergistically activate transcription. Genes Cells 2001, 6, 857–868. [Google Scholar] [CrossRef]
- Chowdhry, S.; Zhang, Y.; McMahon, M.; Sutherland, C.; Cuadrado, A.; Hayes, J.D. Nrf2 is controlled by two distinct beta-TrCP recognition motifs in its Neh6 domain, one of which can be modulated by GSK-3 activity. Oncogene 2013, 32, 3765–3781. [Google Scholar] [CrossRef]
- Rada, P.; Rojo, A.I.; Evrard-Todeschi, N.; Innamorato, N.G.; Cotte, A.; Jaworski, T.; Tobon-Velasco, J.C.; Devijver, H.; Garcia-Mayoral, M.F.; Van Leuven, F.; et al. Structural and functional characterization of Nrf2 degradation by the glycogen synthase kinase 3/beta-TrCP axis. Mol. Cell. Biol. 2012, 32, 3486–3499. [Google Scholar] [CrossRef]
- Wang, H.; Liu, K.; Geng, M.; Gao, P.; Wu, X.; Hai, Y.; Li, Y.; Li, Y.; Luo, L.; Hayes, J.D.; et al. RXRalpha inhibits the NRF2-ARE signaling pathway through a direct interaction with the Neh7 domain of NRF2. Cancer Res. 2013, 73, 3097–3108. [Google Scholar] [CrossRef]
- Saha, S.; Buttari, B.; Profumo, E.; Tucci, P.; Saso, L. A Perspective on Nrf2 Signaling Pathway for Neuroinflammation: A Potential Therapeutic Target in Alzheimer’s and Parkinson’s Diseases. Front. Cell. Neurosci. 2021, 15, 787258. [Google Scholar] [CrossRef]
- Itoh, K.; Wakabayashi, N.; Katoh, Y.; Ishii, T.; Igarashi, K.; Engel, J.D.; Yamamoto, M. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev. 1999, 13, 76–86. [Google Scholar] [CrossRef]
- Kansanen, E.; Kivela, A.M.; Levonen, A.L. Regulation of Nrf2-dependent gene expression by 15-deoxy-Delta12,14-prostaglandin J2. Free Radic. Biol. Med. 2009, 47, 1310–1317. [Google Scholar] [CrossRef]
- Ogura, T.; Tong, K.I.; Mio, K.; Maruyama, Y.; Kurokawa, H.; Sato, C.; Yamamoto, M. Keap1 is a forked-stem dimer structure with two large spheres enclosing the intervening, double glycine repeat, and C-terminal domains. Proc. Natl. Acad. Sci. USA 2010, 107, 2842–2847. [Google Scholar] [CrossRef]
- Cardozo, L.F.; Pedruzzi, L.M.; Stenvinkel, P.; Stockler-Pinto, M.B.; Daleprane, J.B.; Leite, M., Jr.; Mafra, D. Nutritional strategies to modulate inflammation and oxidative stress pathways via activation of the master antioxidant switch Nrf2. Biochimie 2013, 95, 1525–1533. [Google Scholar] [CrossRef]
- Zipper, L.M.; Mulcahy, R.T. The Keap1 BTB/POZ dimerization function is required to sequester Nrf2 in cytoplasm. J. Biol. Chem. 2002, 277, 36544–36552. [Google Scholar] [CrossRef]
- Huang, Y.; Li, W.; Su, Z.Y.; Kong, A.N. The complexity of the Nrf2 pathway: Beyond the antioxidant response. J. Nutr. Biochem. 2015, 26, 1401–1413. [Google Scholar] [CrossRef]
- Vomhof-Dekrey, E.E.; Picklo, M.J., Sr. The Nrf2-antioxidant response element pathway: A target for regulating energy metabolism. J. Nutr. Biochem. 2012, 23, 1201–1206. [Google Scholar] [CrossRef]
- Hayes, J.D.; McMahon, M. NRF2 and KEAP1 mutations: Permanent activation of an adaptive response in cancer. Trends Biochem. Sci. 2009, 34, 176–188. [Google Scholar] [CrossRef]
- Kobayashi, A.; Kang, M.I.; Okawa, H.; Ohtsuji, M.; Zenke, Y.; Chiba, T.; Igarashi, K.; Yamamoto, M. Oxidative stress sensor Keap1 functions as an adaptor for Cul3-based E3 ligase to regulate proteasomal degradation of Nrf2. Mol. Cell. Biol. 2004, 24, 7130–7139. [Google Scholar] [CrossRef]
- Wakabayashi, N.; Itoh, K.; Wakabayashi, J.; Motohashi, H.; Noda, S.; Takahashi, S.; Imakado, S.; Kotsuji, T.; Otsuka, F.; Roop, D.R.; et al. Keap1-null mutation leads to postnatal lethality due to constitutive Nrf2 activation. Nat. Genet. 2003, 35, 238–245. [Google Scholar] [CrossRef]
- Tong, K.I.; Katoh, Y.; Kusunoki, H.; Itoh, K.; Tanaka, T.; Yamamoto, M. Keap1 recruits Neh2 through binding to ETGE and DLG motifs: Characterization of the two-site molecular recognition model. Mol. Cell. Biol. 2006, 26, 2887–2900. [Google Scholar] [CrossRef]
- Dinkova-Kostova, A.T.; Kostov, R.V.; Kazantsev, A.G. The role of Nrf2 signaling in counteracting neurodegenerative diseases. FEBS J. 2018, 285, 3576–3590. [Google Scholar] [CrossRef]
- Hayes, J.D.; McMahon, M.; Chowdhry, S.; Dinkova-Kostova, A.T. Cancer chemoprevention mechanisms mediated through the Keap1-Nrf2 pathway. Antioxid. Redox Signal. 2010, 13, 1713–1748. [Google Scholar] [CrossRef]
- Qiao, H.; Sai, X.; Gai, L.; Huang, G.; Chen, X.; Tu, X.; Ding, Z. Association between heme oxygenase 1 gene promoter polymorphisms and susceptibility to coronary artery disease: A HuGE review and meta-analysis. Am. J. Epidemiol. 2014, 179, 1039–1048. [Google Scholar] [CrossRef]
- Ji, H.; Xiao, F.; Li, S.; Wei, R.; Yu, F.; Xu, J. GRP78 effectively protect hypoxia/reperfusion-induced myocardial apoptosis via promotion of the Nrf2/HO-1 signaling pathway. J. Cell. Physiol. 2021, 236, 1228–1236. [Google Scholar] [CrossRef]
- Yeh, C.H.; Chen, T.P.; Wang, Y.C.; Lin, Y.M.; Lin, P.J. HO-1 activation can attenuate cardiomyocytic apoptosis via inhibition of NF-kappaB and AP-1 translocation following cardiac global ischemia and reperfusion. J. Surg. Res. 2009, 155, 147–156. [Google Scholar] [CrossRef]
- Kusmic, C.; Barsanti, C.; Matteucci, M.; Vesentini, N.; Pelosi, G.; Abraham, N.G.; L’Abbate, A. Up-regulation of heme oxygenase-1 after infarct initiation reduces mortality, infarct size and left ventricular remodeling: Experimental evidence and proof of concept. J. Transl. Med. 2014, 12, 89. [Google Scholar] [CrossRef]
- Duckles, H.; Boycott, H.E.; Al-Owais, M.M.; Elies, J.; Johnson, E.; Dallas, M.L.; Porter, K.E.; Giuntini, F.; Boyle, J.P.; Scragg, J.L.; et al. Heme oxygenase-1 regulates cell proliferation via carbon monoxide-mediated inhibition of T-type Ca2+ channels. Pflugers Arch. 2015, 467, 415–427. [Google Scholar] [CrossRef]
- Cai, J.; Chen, X.; Liu, X.; Li, Z.; Shi, A.; Tang, X.; Xia, P.; Zhang, J.; Yu, P. AMPK: The key to ischemia-reperfusion injury. J. Cell. Physiol. 2022, 237, 4079–4096. [Google Scholar] [CrossRef]
- Xing, Y.; Musi, N.; Fujii, N.; Zou, L.; Luptak, I.; Hirshman, M.F.; Goodyear, L.J.; Tian, R. Glucose metabolism and energy homeostasis in mouse hearts overexpressing dominant negative alpha2 subunit of AMP-activated protein kinase. J. Biol. Chem. 2003, 278, 28372–28377. [Google Scholar] [CrossRef]
- Wang, Z.; Yao, M.; Jiang, L.; Wang, L.; Yang, Y.; Wang, Q.; Qian, X.; Zhao, Y.; Qian, J. Dexmedetomidine attenuates myocardial ischemia/reperfusion-induced ferroptosis via AMPK/GSK-3beta/Nrf2 axis. Biomed. Pharmacother. 2022, 154, 113572. [Google Scholar] [CrossRef]
- Jiang, W.; Song, J.; Zhang, S.; Ye, Y.; Wang, J.; Zhang, Y. CTRP13 Protects H9c2 Cells Against Hypoxia/Reoxygenation (H/R)-Induced Injury Via Regulating the AMPK/Nrf2/ARE Signaling Pathway. Cell Transplant. 2021, 30, 9636897211033275. [Google Scholar] [CrossRef]
- Qin, Y.; Shi, Y.; Yu, Q.; Yang, S.; Wang, Y.; Dai, X.; Li, G.; Cheng, Z. Vitamin B12 alleviates myocardial ischemia/reperfusion injury via the SIRT3/AMPK signaling pathway. Biomed. Pharmacother. 2023, 163, 114761. [Google Scholar] [CrossRef]
- Liu, C.W.; Yang, F.; Cheng, S.Z.; Liu, Y.; Wan, L.H.; Cong, H.L. Rosuvastatin postconditioning protects isolated hearts against ischemia-reperfusion injury: The role of radical oxygen species, PI3K-Akt-GSK-3beta pathway, and mitochondrial permeability transition pore. Cardiovasc. Ther. 2017, 35, 3–9. [Google Scholar] [CrossRef]
- Feng, Q.; Li, X.; Qin, X.; Yu, C.; Jin, Y.; Qian, X. PTEN inhibitor improves vascular remodeling and cardiac function after myocardial infarction through PI3k/Akt/VEGF signaling pathway. Mol. Med. 2020, 26, 111. [Google Scholar] [CrossRef]
- Duan, H.; Li, M.; Liu, J.; Sun, J.; Wu, C.; Chen, Y.; Guo, X.; Liu, X. An Integrated Approach Based on Network Analysis Combined With Experimental Verification Reveals PI3K/Akt/Nrf2 Signaling Is an Important Way for the Anti-Myocardial Ischemia Activity of Yi-Qi-Tong-Luo Capsule. Front. Pharmacol. 2022, 13, 794528. [Google Scholar] [CrossRef] [PubMed]
- Zheng, T.; Yang, J.; Zhang, J.; Yang, C.; Fan, Z.; Li, Q.; Zhai, Y.; Liu, H.; Yang, J. Downregulated MicroRNA-327 Attenuates Oxidative Stress-Mediated Myocardial Ischemia Reperfusion Injury through Regulating the FGF10/Akt/Nrf2 Signaling Pathway. Front. Pharmacol. 2021, 12, 669146. [Google Scholar] [CrossRef] [PubMed]
- Deng, C.; Sun, Z.; Tong, G.; Yi, W.; Ma, L.; Zhao, B.; Cheng, L.; Zhang, J.; Cao, F.; Yi, D. alpha-Lipoic acid reduces infarct size and preserves cardiac function in rat myocardial ischemia/reperfusion injury through activation of PI3K/Akt/Nrf2 pathway. PLoS ONE 2013, 8, e58371. [Google Scholar] [CrossRef] [PubMed]
- Ajzashokouhi, A.H.; Rezaee, R.; Omidkhoda, N.; Karimi, G. Natural compounds regulate the PI3K/Akt/GSK3beta pathway in myocardial ischemia-reperfusion injury. Cell Cycle 2023, 22, 741–757. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Hong, S.; He, H.; Zeng, Y.; Chen, Y.; Mo, X.; Li, J.; Li, L.; Steinmetz, R.; Liu, Q. NFkappaB promotes oxidative stress-induced necrosis and ischemia/reperfusion injury by inhibiting Nrf2-ARE pathway. Free Radic. Biol. Med. 2020, 159, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Gong, Y.; Chen, T.; Li, B.; Zhang, W.; Yin, L.; Zhao, H.; Tang, Y.; Wang, X.; Huang, C. Maresin1 ameliorates ventricular remodelling and arrhythmia in mice models of myocardial infarction via NRF2/HO-1 and TLR4/NF-kB signalling. Int. Immunopharmacol. 2022, 113, 109369. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Li, Z.; Li, T.; Liu, C.; Lu, C. Involvement of the crosstalk between Nrf2 and NF-kappaB pathways regulated by SIRT1 in myocardial ischemia/reperfusion injury. Int. J. Cardiol. 2022, 355, 44. [Google Scholar] [CrossRef] [PubMed]
- Ning, H.; Chen, H.; Deng, J.; Xiao, C.; Xu, M.; Shan, L.; Yang, C.; Zhang, Z. Exosomes secreted by FNDC5-BMMSCs protect myocardial infarction by anti-inflammation and macrophage polarization via NF-kappaB signaling pathway and Nrf2/HO-1 axis. Stem Cell Res. Ther. 2021, 12, 519. [Google Scholar] [CrossRef] [PubMed]
- Casper, E. The crosstalk between Nrf2 and NF-kappaB pathways in coronary artery disease: Can it be regulated by SIRT6? Life Sci. 2023, 330, 122007. [Google Scholar] [CrossRef]
- Jin, Y.; Liu, Z.; Li, Z.; Li, H.; Zhu, C.; Li, R.; Zhou, T.; Fang, B. Histone demethylase JMJD3 downregulation protects against aberrant force-induced osteoarthritis through epigenetic control of NR4A1. Int. J. Oral Sci. 2022, 14, 34. [Google Scholar] [CrossRef]
- Guo, R.; Liu, W.; Liu, B.; Zhang, B.; Li, W.; Xu, Y. SIRT1 suppresses cardiomyocyte apoptosis in diabetic cardiomyopathy: An insight into endoplasmic reticulum stress response mechanism. Int. J. Cardiol. 2015, 191, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.J.; Cui, J.; Lin, Q.; Chen, X.Y.; Zhang, J.; Gao, E.H.; Wei, B.; Zhao, W. Protection of the enhanced Nrf2 deacetylation and its downstream transcriptional activity by SIRT1 in myocardial ischemia/reperfusion injury. Int. J. Cardiol. 2021, 342, 82–93. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Shi, Y.; Yu, Q.; Peng, Y.; Zhao, F.; Cui, J.; Chen, Y.; Liu, L.; Zhang, Y.; Zhang, J.; et al. Coumarin-derived imino sulfonate 5h ameliorates cardiac injury induced by myocardial infarction via activating the Sirt1/Nrf2 signaling pathway. Eur. J. Pharmacol. 2023, 945, 175615. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Li, S.; Tang, X.; Li, Z.; Zhang, J.; Xue, X.; Han, J.; Liu, Y.; Zhang, Y.; Zhang, Y.; et al. Diallyl trisulfide ameliorates myocardial ischemia-reperfusion injury by reducing oxidative stress and endoplasmic reticulum stress-mediated apoptosis in type 1 diabetic rats: Role of SIRT1 activation. Apoptosis 2017, 22, 942–954. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Wang, Y.; Guo, W.; Tan, H. FOXO6 transcription inhibition of CTRP3 promotes OGD/R-triggered cardiac microvascular endothelial barrier disruption via SIRT1/Nrf2 signaling. Folia Morphol. 2023, 83, 125–138. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.J.; Qi, G.Q.; Ma, Y. Effect of propofol on myocardial ischemia-reperfusion injury through MAPK/ERK pathway. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 11051–11061. [Google Scholar] [CrossRef]
- Sun, G.Y.; Chen, Z.; Jasmer, K.J.; Chuang, D.Y.; Gu, Z.; Hannink, M.; Simonyi, A. Quercetin Attenuates Inflammatory Responses in BV-2 Microglial Cells: Role of MAPKs on the Nrf2 Pathway and Induction of Heme Oxygenase-1. PLoS ONE 2015, 10, e0141509. [Google Scholar] [CrossRef] [PubMed]
- Peake, B.F.; Nicholson, C.K.; Lambert, J.P.; Hood, R.L.; Amin, H.; Amin, S.; Calvert, J.W. Hydrogen sulfide preconditions the db/db diabetic mouse heart against ischemia-reperfusion injury by activating Nrf2 signaling in an Erk-dependent manner. Am. J. Physiol. Heart Circ. Physiol. 2013, 304, H1215–H1224. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Gao, H.; Cao, X.; Li, Z.; Kuang, Y.; Ji, Y.; Li, Y. Role of GADD45A in myocardial ischemia/reperfusion through mediation of the JNK/p38 MAPK and STAT3/VEGF pathways. Int. J. Mol. Med. 2022, 50, 144. [Google Scholar] [CrossRef]
- Du, X.J.; Wei, J.; Tian, D.; Yan, C.; Hu, P.; Wu, X.; Yang, W.; Hu, X. NEAT1 promotes myocardial ischemia-reperfusion injury via activating the MAPK signaling pathway. J. Cell. Physiol. 2019, 234, 18773–18780. [Google Scholar] [CrossRef]
- Xie, S.; Deng, W.; Chen, J.; Wu, Q.Q.; Li, H.; Wang, J.; Wei, L.; Liu, C.; Duan, M.; Cai, Z.; et al. Andrographolide Protects Against Adverse Cardiac Remodeling After Myocardial Infarction through Enhancing Nrf2 Signaling Pathway. Int. J. Biol. Sci. 2020, 16, 12–26. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Xie, Q.; He, Q.; Zeng, L.; Long, J.; Gong, Y.; Li, X.; Li, X.; Liu, W.; Xu, Z.; et al. Pretreatment with Panaxatriol Saponin Attenuates Mitochondrial Apoptosis and Oxidative Stress to Facilitate Treatment of Myocardial Ischemia-Reperfusion Injury via the Regulation of Keap1/Nrf2 Activity. Oxid. Med. Cell Longev. 2022, 2022, 9626703. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Li, J.; Li, Z. Ginsenoside Rd mitigates myocardial ischemia-reperfusion injury via Nrf2/HO-1 signaling pathway. Int. J. Clin. Exp. Med. 2015, 8, 14497–14504. [Google Scholar]
- Fan, Z.X.; Yang, C.J.; Li, Y.H.; Yang, J.; Huang, C.X. Ginsenoside Rh2 attenuates myocardial ischaemia-reperfusion injury by regulating the Nrf2/HO-1/NLRP3 signalling pathway. Exp. Ther. Med. 2023, 25, 35. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, W. Effect of Ginsenoside Rb2 on a Myocardial Cell Model of Coronary Heart Disease through Nrf2/HO-1 Signaling Pathway. Biol. Pharm. Bull. 2022, 45, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Shi, L.; Zhao, S.; Sun, Y.; Gao, Y.; Sun, Y.; Qi, G. Triptolide Attenuates Myocardial Ischemia/Reperfusion Injuries in Rats by Inducing the Activation of Nrf2/HO-1 Defense Pathway. Cardiovasc. Toxicol. 2016, 16, 325–335. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Chen, T.; Liu, F. Betulinic acid alleviates myocardial hypoxia/reoxygenation injury via inducing Nrf2/HO-1 and inhibiting p38 and JNK pathways. Eur. J. Pharmacol. 2018, 838, 53–59. [Google Scholar] [CrossRef]
- Li, Q.; Li, Z.; Liu, C.; Xu, M.; Li, T.; Wang, Y.; Feng, J.; Yin, X.; Du, X.; Lu, C. Maslinic Acid Ameliorates Myocardial Ischemia Reperfusion Injury-Induced Oxidative Stress via Activating Nrf2 and Inhibiting NF-[Formula: See text]B Pathways. Am. J. Chin. Med. 2023, 51, 929–951. [Google Scholar] [CrossRef]
- Peng, Z.; Zhang, R.; Pan, L.; Pei, H.; Niu, Z.; Wang, H.; Lv, J.; Dang, X. Glaucocalyxin A Protects H9c2 Cells Against Hypoxia/Reoxygenation-Induced Injury through the Activation of Akt/Nrf2/HO-1 Pathway. Cell Transplant. 2020, 29, 963689720967672. [Google Scholar] [CrossRef]
- Li, W.; Luo, Y.; Huang, Z.; Shen, S.; Dai, C.; Shen, S.; Qi, X.; Liang, G.; Luo, W. Costunolide Protects Myocardium From Ischemia Reperfusion Injury by Inhibiting Oxidative Stress Through Nrf2/Keap1 Pathway Activation. J. Cardiovasc. Pharmacol. 2023, 82, 117–127. [Google Scholar] [CrossRef]
- Ouyang, B.; Li, Z.; Ji, X.; Huang, J.; Zhang, H.; Jiang, C. The protective role of lutein on isoproterenol-induced cardiac failure rat model through improving cardiac morphology, antioxidant status via positively regulating Nrf2/HO-1 signalling pathway. Pharm. Biol. 2019, 57, 529–535. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Li, J.; Liu, M.; Zhang, M.; Xue, Y.; Zhang, Y.; Han, X.; Jing, X.; Chu, L. Hesperetin modulates the Sirt1/Nrf2 signaling pathway in counteracting myocardial ischemia through suppression of oxidative stress, inflammation, and apoptosis. Biomed. Pharmacother. 2021, 139, 111552. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Chen, B.; Ren, Q. Baicalin relieves hypoxia-aroused H9c2 cell apoptosis by activating Nrf2/HO-1-mediated HIF1alpha/BNIP3 pathway. Artif. Cells Nanomed. Biotechnol. 2019, 47, 3657–3663. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Huang, H.; Liu, C.; Jiang, B.; Li, M.; Liu, L.; Zhang, S. Pinocembrin inhibited cardiomyocyte pyroptosis against doxorubicin-induced cardiac dysfunction via regulating Nrf2/Sirt3 signaling pathway. Int. Immunopharmacol. 2021, 95, 107533. [Google Scholar] [CrossRef]
- Chen, X.; Wan, W.; Guo, Y.; Ye, T.; Fo, Y.; Sun, Y.; Qu, C.; Yang, B.; Zhang, C. Pinocembrin ameliorates post-infarct heart failure through activation of Nrf2/HO-1 signaling pathway. Mol. Med. 2021, 27, 100. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.J.; Lv, Y.F.; Cui, W.Z.; Li, Y.; Liu, Y.; Xue, Y.T.; Dong, F. Icariin inhibits hypoxia/reoxygenation-induced ferroptosis of cardiomyocytes via regulation of the Nrf2/HO-1 signaling pathway. FEBS Open Bio 2021, 11, 2966–2976. [Google Scholar] [CrossRef]
- Zhang, B.; Xu, D. Wogonoside preserves against ischemia/reperfusion-induced myocardial injury by suppression of apoptosis, inflammation, and fibrosis via modulating Nrf2/HO-1 pathway. Immunopharmacol. Immunotoxicol. 2022, 44, 877–885. [Google Scholar] [CrossRef] [PubMed]
- Abukhalil, M.H.; Hussein, O.E.; Aladaileh, S.H.; Althunibat, O.Y.; Al-Amarat, W.; Saghir, S.A.; Alfwuaires, M.A.; Algefare, A.I.; Alanazi, K.M.; Al-Swailmi, F.K.; et al. Visnagin prevents isoproterenol-induced myocardial injury by attenuating oxidative stress and inflammation and upregulating Nrf2 signaling in rats. J. Biochem. Mol. Toxicol. 2021, 35, e22906. [Google Scholar] [CrossRef]
- Yao, D.; Shi, B.; Wang, S.; Bao, L.; Tan, M.; Shen, H.; Zhang, Z.; Pan, X.; Yang, Y.; Wu, Y.; et al. Isoliquiritigenin Ameliorates Ischemia-Induced Myocardial Injury via Modulating the Nrf2/HO-1 Pathway in Mice. Drug Des. Dev. Ther. 2022, 16, 1273–1287. [Google Scholar] [CrossRef]
- Shen, Y.; Shen, X.; Wang, S.; Zhang, Y.; Wang, Y.; Ding, Y.; Shen, J.; Zhao, J.; Qin, H.; Xu, Y.; et al. Protective effects of Salvianolic acid B on rat ferroptosis in myocardial infarction through upregulating the Nrf2 signaling pathway. Int. Immunopharmacol. 2022, 112, 109257. [Google Scholar] [CrossRef]
- Zhang, M.; Wei, L.; Xie, S.; Xing, Y.; Shi, W.; Zeng, X.; Chen, S.; Wang, S.; Deng, W.; Tang, Q. Activation of Nrf2 by Lithospermic Acid Ameliorates Myocardial Ischemia and Reperfusion Injury by Promoting Phosphorylation of AMP-Activated Protein Kinase α (AMPKα). Front. Pharmacol. 2021, 12, 794982. [Google Scholar] [CrossRef]
- Zhang, Q.; Dang, Y.Y.; Luo, X.; Fu, J.J.; Zou, Z.C.; Jia, X.J.; Zheng, G.D.; Li, C.W. Kazinol B protects H9c2 cardiomyocytes from hypoxia/reoxygenation-induced cardiac injury by modulating the AKT/AMPK/Nrf2 signalling pathway. Pharm. Biol. 2023, 61, 362–371. [Google Scholar] [CrossRef]
- Li, H.; Song, F.; Duan, L.R.; Sheng, J.J.; Xie, Y.H.; Yang, Q.; Chen, Y.; Dong, Q.Q.; Zhang, B.L.; Wang, S.W. Paeonol and danshensu combination attenuates apoptosis in myocardial infarcted rats by inhibiting oxidative stress: Roles of Nrf2/HO-1 and PI3K/Akt pathway. Sci. Rep. 2016, 6, 23693. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Ma, H.; Zhang, Y.; Jiang, H.; Xia, B.; Sberi, H.A.; Elhefny, M.A.; Lokman, M.S.; Kassab, R.B. Protocatechuic acid reverses myocardial infarction mediated by beta-adrenergic agonist via regulation of Nrf2/HO-1 pathway, inflammatory, apoptotic, and fibrotic events. J. Biochem. Mol. Toxicol. 2023, 37, e23270. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.M.; Guan, P.; Luo, L.F.; Qin, L.Y.; Wang, N.; Zhao, Y.S.; Ji, E.S. Resveratrol protects against CIH-induced myocardial injury by targeting Nrf2 and blocking NLRP3 inflammasome activation. Life Sci. 2020, 245, 117362. [Google Scholar] [CrossRef]
- Cheng, L.; Jin, Z.; Zhao, R.; Ren, K.; Deng, C.; Yu, S. Resveratrol attenuates inflammation and oxidative stress induced by myocardial ischemia-reperfusion injury: Role of Nrf2/ARE pathway. Int. J. Clin. Exp. Med. 2015, 8, 10420–10428. [Google Scholar]
- Chen, G.; Liu, G.; Cao, D.; Jin, M.; Guo, D.; Yuan, X. Polydatin protects against acute myocardial infarction-induced cardiac damage by activation of Nrf2/HO-1 signaling. J. Nat. Med. 2019, 73, 85–92. [Google Scholar] [CrossRef]
- Ge, H.; Lin, W.; Lou, Z.; Chen, R.; Shi, H.; Zhao, Q.; Lin, Z. Catalpol alleviates myocardial ischemia reperfusion injury by activating the Nrf2/HO-1 signaling pathway. Microvasc. Res. 2022, 140, 104302. [Google Scholar] [CrossRef] [PubMed]
- Sui, Y.B.; Zhang, K.K.; Ren, Y.K.; Liu, L.; Liu, Y. The role of Nrf2 in astragaloside IV-mediated antioxidative protection on heart failure. Pharm. Biol. 2020, 58, 1192–1198. [Google Scholar] [CrossRef]
- Yang, P.; Zhou, Y.; Xia, Q.; Yao, L.; Chang, X. Astragaloside IV Regulates the PI3K/Akt/HO-1 Signaling Pathway and Inhibits H9c2 Cardiomyocyte Injury Induced by Hypoxia-Reoxygenation. Biol. Pharm. Bull. 2019, 42, 721–727. [Google Scholar] [CrossRef]
- Wang, X.; Yuan, B.; Cheng, B.; Liu, Y.; Zhang, B.; Wang, X.; Lin, X.; Yang, B.; Gong, G. Crocin Alleviates Myocardial Ischemia/Reperfusion-Induced Endoplasmic Reticulum Stress via Regulation of miR-34a/Sirt1/Nrf2 Pathway. Shock 2019, 51, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Fu, F.; Lai, Q.; Hu, J.; Zhang, L.; Zhu, X.; Kou, J.; Yu, B.; Li, F. Ruscogenin Alleviates Myocardial Ischemia-Induced Ferroptosis through the Activation of BCAT1/BCAT2. Antioxidants 2022, 11, 583. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Tao, X.; Qi, Y.; Xu, L.; Yin, L.; Peng, J. Protective effect of dioscin against doxorubicin-induced cardiotoxicity via adjusting microRNA-140-5p-mediated myocardial oxidative stress. Redox Biol. 2018, 16, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Jiang, B.; Xu, J.; Zhang, X.; Jiang, N. Neferine protected cardiomyocytes against hypoxia/oxygenation injury through SIRT1/Nrf2/HO-1 signaling. J. Biochem. Mol. Toxicol. 2023, 37, e23398. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Wu, Y.; Zhang, X.; Gu, W.; Ning, Z. Stachydrine ameliorates hypoxia reoxygenation injury of cardiomyocyte via enhancing SIRT1-Nrf2 pathway. J. Cardiothorac. Surg. 2023, 18, 265. [Google Scholar] [CrossRef] [PubMed]
- Zeng, G.; An, H.; Fang, D.; Wang, W.; Han, Y.; Lian, C. Plantamajoside protects H9c2 cells against hypoxia/reoxygenation-induced injury through regulating the akt/Nrf2/HO-1 and NF-kappaB signaling pathways. J. Recept. Signal Transduct. Res. 2022, 42, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Li, G.P.; Peng, J.J.; Ren, L.H.; Lei, L.C.; Ye, H.M.; Wang, Z.Y.; Zhao, S. Schizandrin B attenuates hypoxia/reoxygenation injury in H9c2 cells by activating the AMPK/Nrf2 signaling pathway. Exp. Ther. Med. 2021, 21, 220. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.X.; Wang, J.; Shao, J.B.; Tang, W.N.; Zhong, J.Q. Plumbagin Mediates Cardioprotection Against Myocardial Ischemia/Reperfusion Injury through Nrf-2 Signaling. Med. Sci. Monit. 2016, 22, 1250–1257. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Wang, Z.; Sun, M.; Huang, W.; Wang, Y.; Wang, Y. Aloin antagonizes stimulated ischemia/reperfusion-induced damage and inflammatory response in cardiomyocytes by activating the Nrf2/HO-1 defense pathway. Cell Tissue Res. 2021, 384, 735–744. [Google Scholar] [CrossRef]
- Deng, Y.J.; Li, Z.; Wang, B.; Li, J.; Ma, J.; Xue, X.; Tian, X.; Liu, Q.C.; Zhang, Y.; Yuan, B. Immune-related gene IL17RA as a diagnostic marker in osteoporosis. Front. Genet. 2023, 14, 1219894. [Google Scholar] [CrossRef]
- Younis, N.S.; Mohamed, M.E. Anethole’s effects against myocardial infarction: The role of TLR4/NFkappaB and Nrf2/HO1 pathways. Chem. Biol. Interact. 2022, 360, 109947. [Google Scholar] [CrossRef]
- Zhang, H.J.; Chen, R.C.; Sun, G.B.; Yang, L.P.; Zhu, Y.D.; Xu, X.D.; Sun, X.B. Protective effects of total flavonoids from Clinopodium chinense (Benth.) O. Ktze on myocardial injury in vivo and in vitro via regulation of Akt/Nrf2/HO-1 pathway. Phytomedicine 2018, 40, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.J.; Ren, S.M.; Dong, J.Z.; Qiu, C.G.; Chen, Y.W.; Tao, H.L. Ginkgo biloba extract-761 protects myocardium by regulating Akt/Nrf2 signal pathway. Drug Des. Dev. Ther. 2019, 13, 647–655. [Google Scholar] [CrossRef]
- Chao, C.Y.; Lii, C.K.; Tsai, I.T.; Li, C.C.; Liu, K.L.; Tsai, C.W.; Chen, H.W. Andrographolide inhibits ICAM-1 expression and NF-kappaB activation in TNF-alpha-treated EA.hy926 cells. J. Agric. Food Chem. 2011, 59, 5263–5271. [Google Scholar] [CrossRef]
- Chao, W.W.; Lin, B.F. Isolation and identification of bioactive compounds in Andrographis paniculata (Chuanxinlian). Chin. Med. 2010, 5, 17. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Zhao, Y. Current evaluation of the millennium phytomedicine–ginseng (I): Etymology, pharmacognosy, phytochemistry, market and regulations. Curr. Med. Chem. 2009, 16, 2475–2484. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Yang, S.; Chen, Y.; Chen, Y.; Li, R.; Han, S.; Kamili, A.; Wu, Y.; Zhang, W. Ginsenoside Rb2 Alleviated Atherosclerosis by Inhibiting M1 Macrophages Polarization Induced by MicroRNA-216a. Front. Pharmacol. 2021, 12, 764130. [Google Scholar] [CrossRef]
- Tong, L.; Zhao, Q.; Datan, E.; Lin, G.Q.; Minn, I.; Pomper, M.G.; Yu, B.; Romo, D.; He, Q.L.; Liu, J.O. Triptolide: Reflections on two decades of research and prospects for the future. Nat. Prod. Rep. 2021, 38, 843–860. [Google Scholar] [CrossRef]
- Jiang, W.; Li, X.; Dong, S.; Zhou, W. Betulinic acid in the treatment of tumour diseases: Application and research progress. Biomed. Pharmacother. 2021, 142, 111990. [Google Scholar] [CrossRef]
- Li, K.; Ran, X.; Zeng, Y.; Li, S.; Hu, G.; Wang, X.; Li, Y.; Yang, Z.; Liu, J.; Fu, S. Maslinic acid alleviates LPS-induced mice mastitis by inhibiting inflammatory response, maintaining the integrity of the blood-milk barrier and regulating intestinal flora. Int. Immunopharmacol. 2023, 122, 110551. [Google Scholar] [CrossRef]
- Hosohata, K.; Jin, D.; Takai, S. Glaucocalyxin A Ameliorates Hypoxia/Reoxygenation-Induced Injury in Human Renal Proximal Tubular Epithelial Cell Line HK-2 Cells. Int. J. Mol. Sci. 2021, 23, 446. [Google Scholar] [CrossRef] [PubMed]
- Ye, B.; Chen, X.; Chen, Y.; Lin, W.; Xu, D.; Fang, Z.; Chattipakorn, N.; Huang, W.; Wang, X.; Wu, G.; et al. Inhibition of TAK1/TAB2 complex formation by costunolide attenuates obesity cardiomyopathy via the NF-kappaB signaling pathway. Phytomedicine 2023, 108, 154523. [Google Scholar] [CrossRef] [PubMed]
- Calvo, M.M. Lutein: A valuable ingredient of fruit and vegetables. Crit. Rev. Food Sci. Nutr. 2005, 45, 671–696. [Google Scholar] [CrossRef] [PubMed]
- Adluri, R.S.; Thirunavukkarasu, M.; Zhan, L.; Maulik, N.; Svennevig, K.; Bagchi, M.; Maulik, G. Cardioprotective efficacy of a novel antioxidant mix VitaePro against ex vivo myocardial ischemia-reperfusion injury. Cell Biochem. Biophys. 2013, 67, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Olumegbon, L.T.; Lawal, A.O.; Oluyede, D.M.; Adebimpe, M.O.; Elekofehinti, O.O.; Umar, H.I. Hesperetin protects against diesel exhaust particles-induced cardiovascular oxidative stress and inflammation in Wistar rats. Environ. Sci. Pollut. Res. Int. 2022, 29, 52574–52589. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Chen, J.; Qi, J.; Liu, M.; Zhang, M.; Xue, Y.; Li, L.; Liu, Y.; Shi, J.; Zhang, Y.; et al. Hesperetin ameliorates ischemia/hypoxia-induced myocardium injury via inhibition of oxidative stress, apoptosis, and regulation of Ca2+ homeostasis. Phytother. Res. 2023, 37, 1787–1805. [Google Scholar] [CrossRef]
- Shen, J.; Cheng, J.; Zhu, S.; Zhao, J.; Ye, Q.; Xu, Y.; Dong, H.; Zheng, X. Regulating effect of baicalin on IKK/IKB/NF-kB signaling pathway and apoptosis-related proteins in rats with ulcerative colitis. Int. Immunopharmacol. 2019, 73, 193–200. [Google Scholar] [CrossRef]
- Xu, M.; Li, X.; Song, L. Baicalin regulates macrophages polarization and alleviates myocardial ischaemia/reperfusion injury via inhibiting JAK/STAT pathway. Pharm. Biol. 2020, 58, 655–663. [Google Scholar] [CrossRef]
- Menezes da Silveira, C.C.S.; Luz, D.A.; da Silva, C.C.S.; Prediger, R.D.S.; Martins, M.D.; Martins, M.A.T.; Fontes-Junior, E.A.; Maia, C.S.F. Propolis: A useful agent on psychiatric and neurological disorders? A focus on CAPE and pinocembrin components. Med. Res. Rev. 2021, 41, 1195–1215. [Google Scholar] [CrossRef]
- Wang, Y.; Shang, C.; Zhang, Y.; Xin, L.; Jiao, L.; Xiang, M.; Shen, Z.; Chen, C.; Ding, F.; Lu, Y.; et al. Regulatory mechanism of icariin in cardiovascular and neurological diseases. Biomed. Pharmacother. 2023, 158, 114156. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, M.; Mao, C.; Zhang, C.; Ma, W.; Tang, J.; Xiang, D.; Qi, X. Icariin alleviates ferroptosis-related atherosclerosis by promoting autophagy in xo-LDL-induced vascular endothelial cell injury and atherosclerotic mice. Phytother. Res. 2023, 37, 3951–3963. [Google Scholar] [CrossRef]
- Liao, H.; Ye, J.; Gao, L.; Liu, Y. The main bioactive compounds of Scutellaria baicalensis Georgi. for alleviation of inflammatory cytokines: A comprehensive review. Biomed. Pharmacother. 2021, 133, 110917. [Google Scholar] [CrossRef] [PubMed]
- Pasari, L.P.; Khurana, A.; Anchi, P.; Aslam Saifi, M.; Annaldas, S.; Godugu, C. Visnagin attenuates acute pancreatitis via Nrf2/NFkappaB pathway and abrogates associated multiple organ dysfunction. Biomed. Pharmacother. 2019, 112, 108629. [Google Scholar] [CrossRef]
- Gu, X.; Shi, Y.; Chen, X.; Sun, Z.; Luo, W.; Hu, X.; Jin, G.; You, S.; Qian, Y.; Wu, W.; et al. Isoliquiritigenin attenuates diabetic cardiomyopathy via inhibition of hyperglycemia-induced inflammatory response and oxidative stress. Phytomedicine 2020, 78, 153319. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Shao, C.; Zhou, H.; Yu, L.; Bao, Y.; Mao, Q.; Yang, J.; Wan, H. Salvianolic acid B inhibits atherosclerosis and TNF-alpha-induced inflammation by regulating NF-kappaB/NLRP3 signaling pathway. Phytomedicine 2023, 119, 155002. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, A.D.; Huang, Y.S. Clinical non-inferiority trial on treatment of coronary heart disease angina pectoris of Xin-blood stasis syndrome type with lyophilized Salvia salt of lithospermic acid powder for injection. Chin. J. Integr. Med. 2006, 12, 12–18. [Google Scholar] [CrossRef]
- Wu, W.Y.; Wang, Y.P. Pharmacological actions and therapeutic applications of Salvia miltiorrhiza depside salt and its active components. Acta Pharmacol. Sin. 2012, 33, 1119–1130. [Google Scholar] [CrossRef] [PubMed]
- Matzinger, M.; Fischhuber, K.; Poloske, D.; Mechtler, K.; Heiss, E.H. AMPK leads to phosphorylation of the transcription factor Nrf2, tuning transactivation of selected target genes. Redox Biol. 2020, 29, 101393. [Google Scholar] [CrossRef]
- Ryu, J.H.; Ahn, H.; Lee, H.J. Inhibition of nitric oxide production on LPS-activated macrophages by kazinol B from Broussonetia kazinoki. Fitoterapia 2003, 74, 350–354. [Google Scholar] [CrossRef]
- Pignet, A.L.; Schellnegger, M.; Hecker, A.; Kohlhauser, M.; Kotzbeck, P.; Kamolz, L.P. Resveratrol-Induced Signal Transduction in Wound Healing. Int. J. Mol. Sci. 2021, 22, 12614. [Google Scholar] [CrossRef]
- Wang, H.L.; Gao, J.P.; Han, Y.L.; Xu, X.; Wu, R.; Gao, Y.; Cui, X.H. Comparative studies of polydatin and resveratrol on mutual transformation and antioxidative effect in vivo. Phytomedicine 2015, 22, 553–559. [Google Scholar] [CrossRef]
- Zhao, X.J.; Yu, H.W.; Yang, Y.Z.; Wu, W.Y.; Chen, T.Y.; Jia, K.K.; Kang, L.L.; Jiao, R.Q.; Kong, L.D. Polydatin prevents fructose-induced liver inflammation and lipid deposition through increasing miR-200a to regulate Keap1/Nrf2 pathway. Redox Biol. 2018, 18, 124–137. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.X.; Li, M.X.; Jia, Z.P. Rehmannia glutinosa: Review of botany, chemistry and pharmacology. J. Ethnopharmacol. 2008, 117, 199–214. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.J.; Ran, H.F.; Yin, Y.; Xu, X.G.; Jiang, B.X.; Yu, S.Q.; Chen, Y.J.; Ren, H.J.; Feng, S.; Zhang, J.F.; et al. Catalpol improves impaired neurovascular unit in ischemic stroke rats via enhancing VEGF-PI3K/AKT and VEGF-MEK1/2/ERK1/2 signaling. Acta Pharmacol. Sin. 2022, 43, 1670–1685. [Google Scholar] [CrossRef] [PubMed]
- Zang, Y.; Wan, J.; Zhang, Z.; Huang, S.; Liu, X.; Zhang, W. An updated role of astragaloside IV in heart failure. Biomed. Pharmacother. 2020, 126, 110012. [Google Scholar] [CrossRef]
- Liu, T.; Yu, S.; Xu, Z.; Tan, J.; Wang, B.; Liu, Y.G.; Zhu, Q. Prospects and progress on crocin biosynthetic pathway and metabolic engineering. Comput. Struct. Biotechnol. J. 2020, 18, 3278–3286. [Google Scholar] [CrossRef] [PubMed]
- Lai, Q.; Zhu, X.; Zhang, L.; Kou, J.; Liu, F.; Yu, B.; Li, F. Inhibition of OAT1/3 and CMPF uptake attenuates myocardial ischemia-induced chronic heart failure via decreasing fatty acid oxidation and the therapeutic effects of ruscogenin. Transl. Res. 2023, 261, 1–15. [Google Scholar] [CrossRef]
- Yang, B.; Xu, B.; Zhao, H.; Wang, Y.B.; Zhang, J.; Li, C.W.; Wu, Q.; Cao, Y.K.; Li, Y.; Cao, F. Dioscin protects against coronary heart disease by reducing oxidative stress and inflammation via Sirt1/Nrf2 and p38 MAPK pathways. Mol. Med. Rep. 2018, 18, 973–980. [Google Scholar] [CrossRef] [PubMed]
- Liao, L.; Tang, Y.; Li, B.; Tang, J.; Xu, H.; Zhao, K.; Zhang, X. Stachydrine, a potential drug for the treatment of cardiovascular system and central nervous system diseases. Biomed. Pharmacother. 2023, 161, 114489. [Google Scholar] [CrossRef]
- Jung, H.Y.; Seo, D.W.; Hong, C.O.; Kim, J.Y.; Yang, S.Y.; Lee, K.W. Nephroprotection of plantamajoside in rats treated with cadmium. Environ. Toxicol. Pharmacol. 2015, 39, 125–136. [Google Scholar] [CrossRef]
- Luo, W.; Lin, K.; Hua, J.; Han, J.; Zhang, Q.; Chen, L.; Khan, Z.A.; Wu, G.; Wang, Y.; Liang, G. Schisandrin B Attenuates Diabetic Cardiomyopathy by Targeting MyD88 and Inhibiting MyD88-Dependent Inflammation. Adv. Sci. 2022, 9, e2202590. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Cai, Y.; He, C.; Chen, M.; Li, H. Anticancer Properties and Pharmaceutical Applications of Plumbagin: A Review. Am. J. Chin. Med. 2017, 45, 423–441. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Fan, Y.; Loor, J.J.; Liang, Y.; Lv, H.; Sun, X.; Jia, H.; Xu, C. Aloin protects mice from diet-induced non-alcoholic steatohepatitis via activation of Nrf2/HO-1 signaling. Food Funct. 2021, 12, 696–705. [Google Scholar] [CrossRef]
- Syed, A.M.; Kundu, S.; Ram, C.; Kulhari, U.; Kumar, A.; Mugale, M.N.; Murty, U.S.; Sahu, B.D. Aloin alleviates pathological cardiac hypertrophy via modulation of the oxidative and fibrotic response. Life Sci. 2022, 288, 120159. [Google Scholar] [CrossRef] [PubMed]
- Qi, C.X.; Zhou, Q.; Yuan, Z.; Luo, Z.W.; Dai, C.; Zhu, H.C.; Chen, C.M.; Xue, Y.B.; Wang, J.P.; Wang, Y.F.; et al. Kinsenoside: A Promising Bioactive Compound from Anoectochilus Species. Curr. Med. Sci. 2018, 38, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Dong, S.; Xia, R.; Sun, M.; Sun, Y.; Ren, H.; Zhang, Y.; Xia, Z.; Yao, S.; Wang, T. Kinsenoside mitigates myocardial ischemia/reperfusion-induced ferroptosis via activation of the Akt/Nrf2/HO-1 pathway. Eur. J. Pharmacol. 2023, 956, 175985. [Google Scholar] [CrossRef]
- Wang, W.; Ma, K.; Liu, J.; Li, F. Ginkgo biloba extract may alleviate viral myocarditis by suppression of S100A4 and MMP-3. J. Med. Virol. 2019, 91, 2083–2092. [Google Scholar] [CrossRef]
| Category | Natural Product | Model | Route of Medication | Research Type | Conclusions | References |
|---|---|---|---|---|---|---|
| Terpenoids | Andrographolide | C57BL/6 mice, N = 110, H9c2 cell | Orally/Cell culture | Preclinical | Andr alleviates adverse cardiac remodeling following myocardial infarction through enhancing Nrf2 signaling pathway | [91] |
| Panaxatriol saponin | SD rats N = 60, H9c2 cell | Intragastric administration/Cell culture | Preclinical | PTS has therapeutic potential for MIRI by targeting Keap1/Nrf2 activity | [92] | |
| Ginsenoside Rd | SD rats N = 24 | Administrated intraperitoneally | Preclinical | GsRd protects against myocardial I/R injury via Nrf2/HO-1 signaling | [93] | |
| Ginsenoside Rh2 | SD rats N = 40, Neonatal rat cardiomyocytes | Intragastric administration/Cell culture | Preclinical | GRh2 could reduce oxidative stress and inflammation in cardiomyocytes after reperfusion | [94] | |
| Ginsenoside Rb2 | H9c2 cell | Cell culture | Preclinical | The underlying mechanism of ginsenoside Rb2 in H9c2 cells could be standardized to Nrf2/HO-1 signaling pathway, inhibiting cell apoptosis and regaining cell proliferation | [95] | |
| Triptolide | Wistar rats N = / | Administrated intraperitoneally | Preclinical | A novel cardioprotective effect of TPL in rats with I/R injuries, wherein the activation of Nrf2/HO-1 signaling was involved | [96] | |
| Betulinic acid | H9c2 cell | Cell culture | Preclinical | BA protects the H9c2 cells from I/RI by inhibiting oxidative stress and cell apoptosis. The cardio-protective effects were mediated by the Nrf2/HO-1, p38 and JNK pathways | [97] | |
| Maslinic acid | SD rats N = 60, H9c2 cell | Intraperitoneal injection | Preclinical | MA exerts its cardioprotective effect through regulating the crosstalk between the Nrf2 and NF-κB pathways | [98] | |
| Glaucocalyxin A | H9c2 cell | Cell culture | Preclinical | GLA protected H9c2 cells from H/R-stimulated oxidative damage, which was mediated by the Akt/Nrf2/HO-1 signaling pathway | [99] | |
| Costunolide | C57BL/6 mice N = 64, H9c2 cell | Gavage/Cell culture | Preclinical | Activation of Nrf2/Keap1 using Cos may be a therapeutic strategy for myocardial I/R injury | [100] | |
| Lutein | SD rats N = 40 | Orally | Preclinical | LU exhibits potent cardioprotective activity against ISO-induced cardiotoxicity | [101] | |
| Flavonoids | Hesperetin | Mice N = 50 | Intragastrically | Preclinical | HESP plays a protective role in ISO-induced myocardial ischemia by modulating oxidative stress, inflammation, and apoptosis via Sirt1/Nrf2 pathway activation | [102] |
| Baicalin | H9c2 cell | Cell culture | Preclinical | BI cardiomyocytes H9c2 apoptosis aroused by hypoxia might be achieved through activating Nrf2/HO-1-mediated HIF1α/BNIP3 pathway | [103] | |
| Pinocembrin | SD rats N = 56, H9c2 cell | Injected intravenously through the tail vein/Cell culture | Preclinical | PCB ameliorated cardiac functions and remodeling resulted from PIHF by ROS scavenging and Nrf2/HO-1 pathway activation which further attenuated collagen fibers deposition and apoptosis, and facilitated angiogenesis | [104,105] | |
| Icariin | H9c2 cell | Cell culture | Preclinical | ICA attenuates H/R-induced ferroptosis of cardiomyocytes by activating the Nrf2/HO-1 signaling pathway | [106] | |
| Wogonoside | C57BL/6 mice N = 35 | Intraperitoneally injected | Preclinical | WG exerted the protective role against I/R-induced myocardial injury by suppression of apoptosis, inflammation, and fibrosis via activating Nrf2/HO-1 pathway | [107] | |
| Visnagin | Wistar rats N = 36 | Orally | Preclinical | Activation of Nrf2/HO-1 signaling and PPARγ mediates the cardioprotective effect of VIS | [108] | |
| Isoliquiritigenin | C57BL/6 mice N = 50 | Intraperitoneal injection | Preclinical | Activation of Nrf2/HO-1 pathway has an essential role in ISL-induced cardiac protection by alleviating myocardial oxidative stress and inflammation response in mice with AMI | [109] | |
| Phenols | Salvianolic acid B | SD rats N = 108 | Intraperitoneal injection | Preclinical | Sal B contributed to protecting MI by inhibiting ferroptosis via activating the Nrf2 signaling pathway | [110] |
| Lithospermic acid | C57BL/6 mice N = 50, H9c2 cell | Orally/Cell culture | Preclinical | LA protects against MI/R-induced cardiac injury by promoting eNOS and Nrf2/HO-1 signaling via phosphorylation of AMPKα | [111] | |
| Kazinol B | H9c2 cell | Cell culture | Preclinical | KB prevented H/R-induced cardiomyocyte injury via modulating the AKT and AMPK-mediated Nrf2 induction | [112] | |
| Paeonol | SD rats N = 40 | Subcutaneous injection | Preclinical | Pae exerts significant cardioprotective effects against ISO-induced myocardial infarction in rats | [113] | |
| Protocatechuic acid | Wistar rats N = 35 | Orally | Preclinical | PCA as an alternative therapeutic agent to attenuate the molecular, biochemical, and histological alterations associated with MI development | [114] | |
| Resveratrol | SD rats N = 24 | Orogastric gavaged | Preclinical | Myocardial protective mechanism of RE during CIH and suggest that resveratrol treatment may be useful to counteract OSA-associated cardiac injury | [115,116] | |
| Polydatin | SD rats N = /, H9c2 cell | Intraperitoneal injection/Cell culture | Preclinical | PD effectively inhibited hypoxia- and AMI-induced myocardial damage by promotion of Nrf2/HO-1 signaling | [117] | |
| Polysaccharides and glycosides | Catalpol | C57BL/6 mice N = 30, Human cardiomyocytes AC16 cells | Intraperitoneal injection/Cell culture | Preclinical | Catalpol exerted significant cardioprotective effects following myocardial ischemia, possibly through the activation of the Nrf2/HO-1 signaling pathway | [118] |
| Astragaloside IV | SD rats N = 45, H9c2 cell | Orally/Cell culture | Preclinical | ASI prevented heart failure by counteracting oxidative stress through the Nrf2/HO-1 pathway | [119,120] | |
| Crocin | C57BL6/J mice N = 32, Neonatal mouse cardiomyocytes (NMCMs) | Orally/Cell culture | Preclinical | Crocin attenuates I/R-induced cardiomyocyte apoptosis via suppressing ER stress, which is regulated by the miR-34a/Sirt1/Nrf2 pathway | [121] | |
| Steroids | Ruscogenin | ICR mice N = 72, H9c2 cell | Intraperitoneal injection/Cell culture | Preclinical | BCAT1/BCAT2 could alleviate MI-induced ferroptosis through the activation of the Keap1/Nrf2/HO-1 pathway and RUS exerted cardioprotective effects via BCAT1/BCAT2 | [122] |
| Dioscin | SD rats N = 50, H9c2 cell | Intraperitoneal injection/Cell culture | Preclinical | Dioscin alleviated DOX-induced cardiotoxicity through modulating miR-140-5p-mediated myocardial oxidative stress | [123] | |
| Alkaloids | Neferine | H9c2 cell | Cell culture | Preclinical | NEF preconditioning attenuated H/R-induced cardiac damage via suppressing apoptosis, oxidative stress, and mitochondrial dysfunction, which may be partially ascribed to the activation of Sirt1/Nrf2 signaling pathway | [124] |
| Stachydrine | H9c2 cell | Cell culture | Preclinical | STA protects H/R injury and inhibits oxidative stress and apoptosis in cardiomyocytes by activation of the Sirt1-Nrf2 pathway | [125] | |
| Phenylpropane | Plantamajoside | H9c2 cell | Cell culture | Preclinical | PMS protected against myocardial I/R injury via attenuating oxidative stress, inflammatory response and apoptosis | [126] |
| Schizandrin B | H9c2 cell | Cell culture | Preclinical | Sch B exerts cardioprotection on H/R injury in H9c2 cells due to its antioxidant and anti-inflammatory activities via activation of the AMPK/Nrf2 pathway | [127] | |
| Quinones | Plumbagin | C57BL6/J mice N = 40 | Intraperitoneal injection | Preclinical | Protective role of PL against myocardial I/R injury by regulating antioxidant and inflammatory mechanisms | [128] |
| Aloin | H9c2 cell | Cell culture | Preclinical | Aloin may antagonize SI/R-induced cardiomyocyte injury by attenuating excessive oxidative stress and inflammation | [129] | |
| Others | Kinsenoside | C57BL6/J mice N = 88 | Orally | Preclinical | KD may exert anti-ferroptosis effect in myocardial I/R injury by decreasing mitochondrial dysfunction and increasing anti-oxidation through the Akt/Nrf2/HO-1 signaling pathway | [130] |
| Anethole | Wistar rats N = 30 | Gastric lavage | Preclinical | Anethole may retain a cardio-protective potential by controlling myocardial oxidative stress (through Nrf2 pathway) and diminishing inflammation and apoptosis via the TLR4/MYD88 pathway | [131] | |
| TFCC Clinopodium chinense (Benth.) O. Ktze | SD rats N = 120, H9c2 cell | Dosed intragastrically/Cell culture | Preclinical | TFCC protects against myocardial injury and enhances cellular antioxidant defense capacity by inducing the phosphorylation of AKT, which subsequently activated the Nrf2/HO-1 signaling pathway | [132] | |
| Ginkgo biloba extract-761 | SD rats N = 40 | Gavage | Preclinical | EGb 761 might inhibit the apoptosis of myocardial cells and protect the myocardium by activating the Akt/Nrf2 pathway, increasing the expression of HO-1, decreasing oxidative stress and repressing inflammatory reaction | [133] |
| Category | Molecular Formula | Model | Related Gene/Cytokines/Protein | Pathway | References |
|---|---|---|---|---|---|
| Terpenoids | |||||
| Andrographolide | C20H30O5 | C57BL/6 mice, H9c2 cell | ↑: SOD2, NQO1, GPX, Nrf2, HO-1 ↓: TNF-α, IL-1β, IL-6, MCP-1, p-IκBα, p-p65, p67 phox, Gp91, NOX4, TGF-β, p-smad3 | NF-κB, Nrf2/HO-1 | [91] |
| Panaxatriol saponin | C30H52O4 | SD rats, H9c2 cell | ↑: SOD1, SOD2, HO-1 ↓: cleaved caspase-3, cleaved PARP-1, Bax, Cyt-c, MB, cTn-T, CK, LDH | Keap1/Nrf2/HO-1 | [92] |
| Ginsenoside Rd | C48H82O18 | SD rats | ↑: Nrf2, HO-1 ↓: CK, LDH, cTnI | Nrf2/HO-1 | [93] |
| Ginsenoside Rh2 | C36H62O8 | SD rats, Neonatal rat cardiomyocytes | ↑: Nrf2, HO-1, SOD, GSH-Px ↓: IL-1β, IL-18, TNF-α, NLRP3, ASC, caspase-1, MDA, LDH, CK, CK-MB | Nrf2/HO-1/NLRP3 | [94] |
| Ginsenoside Rb2 | C53H90O22 | H9c2 cell | ↑: Nrf2, HO-1 ↓: CK-MB, cTn-1, LDH | Nrf2/HO-1 | [95] |
| Triptolide | C20H24O6 | Wistar rats | ↑: Nrf2, HO-1, GPX, GSH, SOD ↓: TNF-α, IL-1β, IL-6, MDA | Nrf2/HO-1 | [96] |
| Betulinic acid | C30H48O3 | H9c2 cell | ↑: Nrf2, HO-1, Bcl-2 ↓: LDH, caspase-3, Bax, p38, JNK | Nrf2/HO-1, p38, JNK | [97] |
| Maslinic acid | C30H48O4 | SD rats, H9c2 cell | ↑: GSH, SOD, p-Nrf2, HO-1, NQO1 ↓: LDH, CK-MB, MAD, Keap-1, p-IκBα, p-P65, TNF-α | Nrf2/HO-1, NF-κB | [98] |
| Glaucocalyxin A | C20H28O4 | H9c2 cell | ↑: SOD, GSH-Px, Bcl-2, p-Akt, Nrf2, HO-1 ↓: Bax, caspase-3 | Akt/Nrf2/HO-1 | [99] |
| Costunolide | C15H20O2 | C57BL/6 mice, H9c2 cell | ↑: Bcl-2, NQO1, HO-1, Nrf2 ↓: cleaved caspase-3, Bax, MDA, SOD | Keap1/Nrf2 | [100] |
| Lutein | C40H56O2 | SD rats | ↑: CAT, SOD, Nrf2, HO-1 ↓: MDA, cTnT, CK-MB, LDH, IL-1β, TNF-α, NF-κB p65, caspase-3, caspase-9 | Nrf2/HO-1 | [101] |
| Flavonoids | |||||
| Hesperetin | C16H14O6 | mice | ↑: CAT, SOD, GSH, Bcl-2, Sirt1, Nrf2, NQO1, HO-1 ↓: CK, LDH, MDA, IL-6, TNF-α, Bax, caspase-3 | Sirt1/Nrf2 | [102] |
| Baicalin | C21H18O11 | H9c2 cell | ↑: Bcl-2, HIF1α, BNIP3, Nrf2, HO-1 ↓: p53, Bax, cleaved-caspase 9, cleaved-caspase 3 | HIF1α/BNIP3, Nrf2/HO-1 | [103] |
| Pinocembrin | C15H12O4 | SD rats, H9c2 cell | ↑: Bcl-2, SOD, Nrf2, HO-1 ↓: p53, Bax, cleaved-caspase 3, MDA | Nrf2/HO-1 | [104,105] |
| Icariin | C33H40O15 | H9c2 cell | ↑: Nrf2, HO-1, GPX4, SOD, CAT ↓: LDH, ACSL4, MDA | Nrf2/HO-1 | [106] |
| Wogonoside | C22H20O11 | C57BL/6 mice | ↑: Bcl-2, Nrf2, HO-1, NQO1 ↓: Mb, CK-MB, cTnI, cleaved caspase-3, cleaved caspase-9, Bax, TNF-α, IL-6, iNOS, α-SMA, TGF-β | Nrf2/HO-1 | [107] |
| Visnagin | C13H10O4 | Wistar rats | ↑: Nrf2, HO-1, Bcl-2, PPARγ, GSH, SOD, CAT, GPX ↓: MDA, NF-κB p65, Bax, caspase 3, caspase 9, CTnI, CK-MB, LDH, TNF-α, IL-6 | Nrf2/HO-1 | [108] |
| Isoliquiritigenin | C15H12O4 | C57BL/6 mice | ↑: GSH-Px, SOD, Nrf2, HO-1 ↓: MDA, p-p65, IL-6, IL-1β, TNF-α, MIP1α, MIP2, p-IKKα/β, p-P65, p-IκBα | Nrf2/HO-1 | [109] |
| Phenols | |||||
| Salvianolic acid B | C36H30O16 | SD rats | ↑: Nrf2, HO-1, GSH, xCT, GPX4, Fth1, Fpn1 ↓: MDA, CK, CK-MB, LDH | Nrf2/HO-1 | [110] |
| Lithospermic acid | C27H22O12 | C57BL/6 mice, H9c2 cell | ↑: eNOS, Nrf2, GPX, SOD2, NQO1, Bcl-2 ↓: TnT, CK-MB, ROS, GP91, NOX4, p67 phox, p47 phox, Caspase-3, Bax | AMPK, Nrf2/HO-1 | [111] |
| Kazinol B | C25H28O4 | H9c2 cell | ↑: Bcl-2/Bax, ATP, GSH-Px, SOD, Nrf2, HO-1 ↓: caspase-3, cleaved PARP, MDA, LDH, p-AKT, p-AMPKα | AKT, AMPK, Nrf2/ARE/HO-1 | [112] |
| Paeonol | C9H10O3 | SD rats | ↑: GSH/GSSG, Bcl-2, Nrf2, HO-1, NQO1, GST, p-PI3K, p-Akt ↓: Bax, TBARS, TNF-α, Fas, caspase-8, caspases-3 | Nrf2/HO-1, PI3K/Akt | [113] |
| Protocatechuic acid | C7H6O4 | Wistar rats | ↑: CAT, SOD, GSH, GPX, GR, Nrf2, HO-1, Bcl-2, TIMP-3 ↓: CK, LDH, CTnT, MDA, NO, TNF-α, IL-1β, Bax, caspase-3, TGF-β1, MMP-9 | Nrf2/HO-1 | [114] |
| Resveratrol | C14H12O3 | SD rats | ↑: SOD, GSH, GSH-PX, p-mTOR/mTOR, SOD2, Nrf2, HO-1, p-AMPK/AMPK ↓: CK, LDH, MDA, NOX2, NOX4, p-IRE/IRE, P-PERK/PERK, GRP78, NLRP3, caspase-1, IL-1β, MPO | AMPK, Nrf2 | [115,116] |
| Polydatin | C20H22O8 | SD rats, H9c2 cell | ↑: Nrf2, HO-1, Bcl-2 ↓: caspase-3, Bax | Nrf2/HO-1 | [117] |
| Polysaccharides and glycosides | |||||
| Catalpol | C15H22O10 | C57BL/6 mice, Human cardiomyocytes AC16 cells | ↑: SOD2, GSH, Bax, Nrf2, HO-1 ↓: cTnI, CK-MB, NPPB, BNP, MDA, ROS, IL-1β, IL-6, IL-8, TNF-α, cleaved caspase 3 | Nrf2/HO-1 | [118] |
| Astragaloside IV | C41H68O14 | SD rats, H9c2 cell | ↑: CAT, GSH, SOD, Nrf2, HO-1, NQO1, SOD2, Txn-1, PI3K, p-Akt ↓: CK, Keap-1, LDH, MDA, IL-6, TNF-α, Bach1 | PI3K/Akt/Nrf2/HO-1 | [119,120] |
| Crocin | C44H64O24 | C57BL6/J mice, Neonatal mouse cardiomyocytes (NMCMs) | ↑: Bcl-2, Sirt1, Nrf2, HO-1 ↓: miR-34a, GRP78, CHOP | miR-34a/Sirt1/Nrf2 | [121] |
| Steroids | |||||
| Ruscogenin | C27H42O4 | ICR mice, H9c2 cell | ↑: GPX4, SOD, GSH, BCAT1, BCAT2, Nrf2, HO-1 ↓: ACSL4, FTL, MDA, Keap1 | Keap1/Nrf2/HO-1 | [122] |
| Dioscin | C45H72O16 | SD rats, H9c2 cell | ↑: SOD, GSH, GSH-Px, Sirt2, Nrf2, HO-1, NQO1, GST, GCLM, Sirt2, FOXO3a ↓: CK, LDH, MDA, miR-140-5p, Keap1 | miR-140-5p, Nrf2, Sirt2 | [123] |
| Alkaloids | |||||
| Neferine | C38H44N2O6 | H9c2 cell | ↑: Sirt1, Nrf2, SOD, CAT ↓: MDA, LDH | Sirt1/Nrf2 | [124] |
| Stachydrine | C7H13NO2 | H9c2 cell | ↑: SOD, Sirt1, Nrf2, HO-1 ↓: LDH, MDA, caspase-3 | Sirt1/Nrf2 | [125] |
| Phenylpropane | |||||
| Plantamajoside | C29H36O16 | H9c2 cell | ↑: SOD, CAT, GSH-Px, Bcl-2, p-Akt, Nrf2, HO-1 ↓: TNF-α, IL-6, IL-1β, Bax, caspase 3, p-IκBα, p-P65 | Akt/Nrf2/HO-1, NF-κB | [126] |
| Schizandrin B | C23H28O6 | H9c2 cell | ↑: SOD, GSH, Nrf2, NAD(P)H, NQO1, HO-1, p-AMPK ↓: LDH, MDA, TNF-α, IL-6, IL-1β, IL-8, TGF-β, IL-10 | AMPK/Nrf2 | [127] |
| Quinones | |||||
| Plumbagin | C11H8O3 | C57BL6/J mice | ↑: GSH, SOD, CAT, GPX, GST, Nrf2, HO-1, NQO1 ↓: NF-κB, COX-2, iNOS, MCP-1, IL-6, IL-8, TNF-α | Nrf2 | [128] |
| Aloin | C21H22O9 | H9c2 cell | ↑: SOD, Nrf2, HO-1 ↓: LDH, MDA, IL-6, IL-1β, TNF-α | Nrf2/HO-1 | [129] |
| Others | |||||
| Kinsenoside | C10H16O8 | C57BL6/J mice | ↑: p-Akt, Nrf2, HO-1, SOD, GSH, HO-1, GPX4 ↓: MDA, CK-MB, COX2, ACSL4, Keap1 | Akt/Nrf2/HO-1 | [130] |
| Anethole | C10H12O | Wistar rats | ↑: Nrf2, HO-1, SOD, CAT, GPX, GSH, GST, Bcl-2 ↓: Keap-1, CKMB, CK, CTnT, TNF-α, IL-1β, IL-6, NF-κB, Bax, caspase-3, caspase-9, TLR4, MYD88 | Nrf2/HO-1, TLR4/MYD88 | [131] |
| TFCC Clinopodium chinense (Benth.) O. Ktze | / | SD rats, H9c2 cell | ↑: GSH-Px, CAT, SOD, POD, HO-1, Nrf2, HO-1, AKT, Bcl-2 ↓: cleaved caspase-3, caspase 9, Bax | AKT/Nrf2/HO-1 | [132] |
| Ginkgo biloba extract-761 | / | SD rats | ↑: Bcl-2, p-Akt, HO-1, Nrf2 ↓: CK-MB, LDH, TnT, TNF-α, IL-6, IL-1β, Caspase-3, Bax | Akt/Nrf2 | [133] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Han, J.; Dmitrii, G.; Zhang, X.-a. Potential Targets of Natural Products for Improving Cardiac Ischemic Injury: The Role of Nrf2 Signaling Transduction. Molecules 2024, 29, 2005. https://doi.org/10.3390/molecules29092005
Wang H, Han J, Dmitrii G, Zhang X-a. Potential Targets of Natural Products for Improving Cardiac Ischemic Injury: The Role of Nrf2 Signaling Transduction. Molecules. 2024; 29(9):2005. https://doi.org/10.3390/molecules29092005
Chicago/Turabian StyleWang, Haixia, Juanjuan Han, Gorbachev Dmitrii, and Xin-an Zhang. 2024. "Potential Targets of Natural Products for Improving Cardiac Ischemic Injury: The Role of Nrf2 Signaling Transduction" Molecules 29, no. 9: 2005. https://doi.org/10.3390/molecules29092005
APA StyleWang, H., Han, J., Dmitrii, G., & Zhang, X.-a. (2024). Potential Targets of Natural Products for Improving Cardiac Ischemic Injury: The Role of Nrf2 Signaling Transduction. Molecules, 29(9), 2005. https://doi.org/10.3390/molecules29092005