One-Pot Synthesis of Alkyl Functionalized Reduced Graphene Oxide Nanocomposites as the Lubrication Additive Enabling Enhanced Tribological Performance
Abstract
1. Introduction
2. Results and Discussion
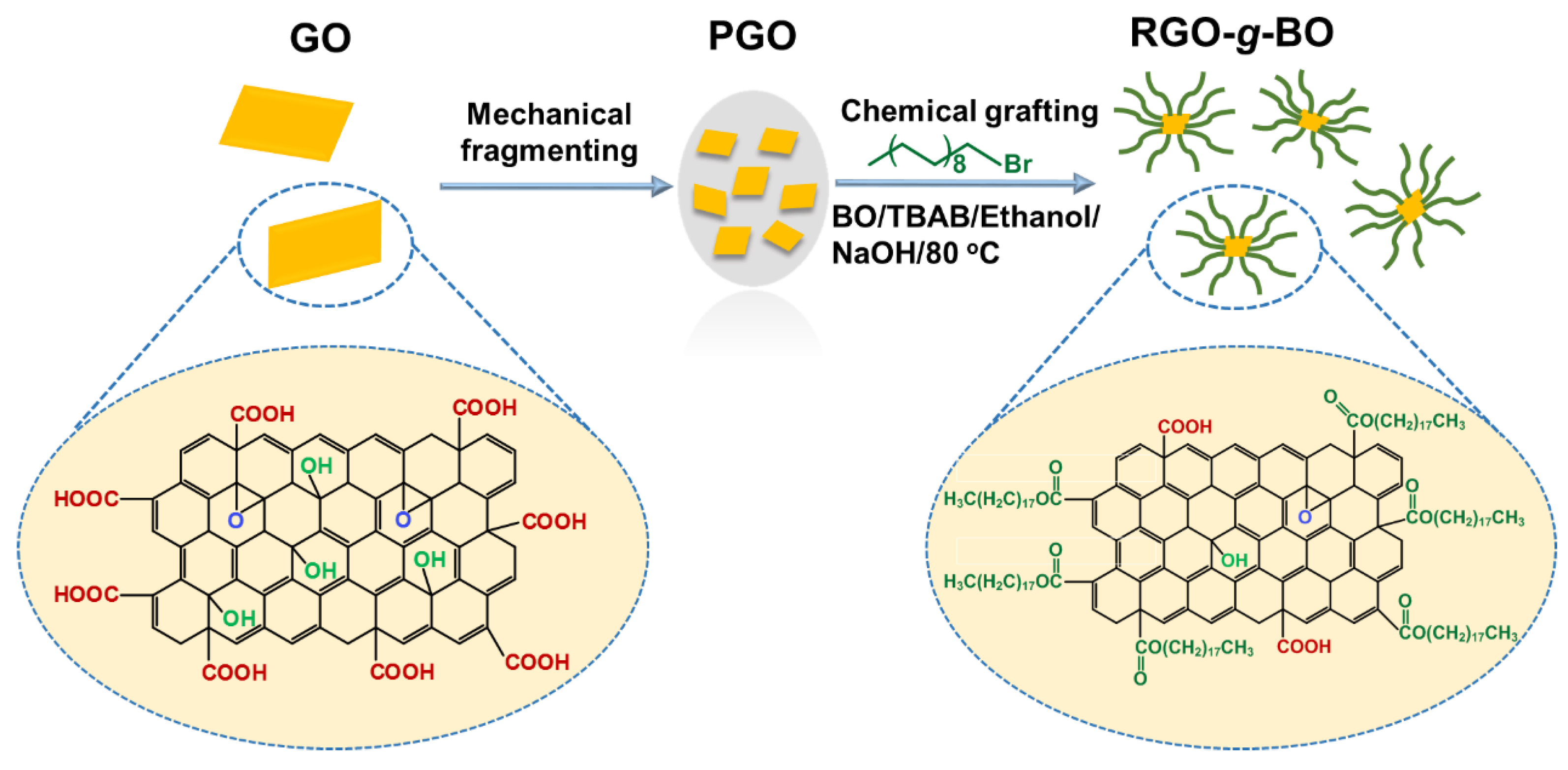
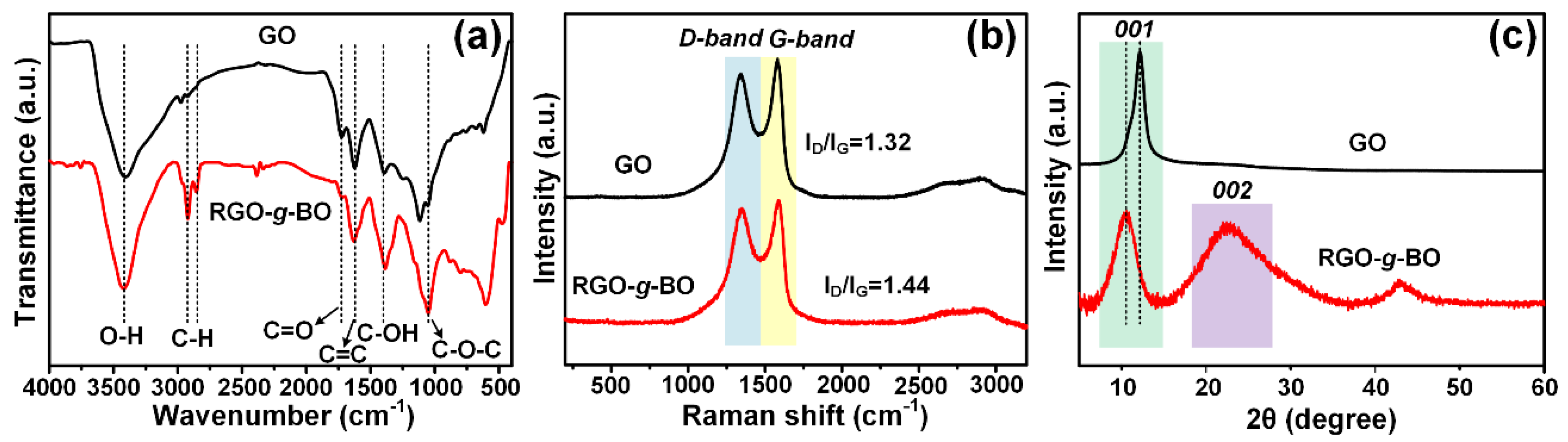
2.1. Chemical Composition and Structural Characterization
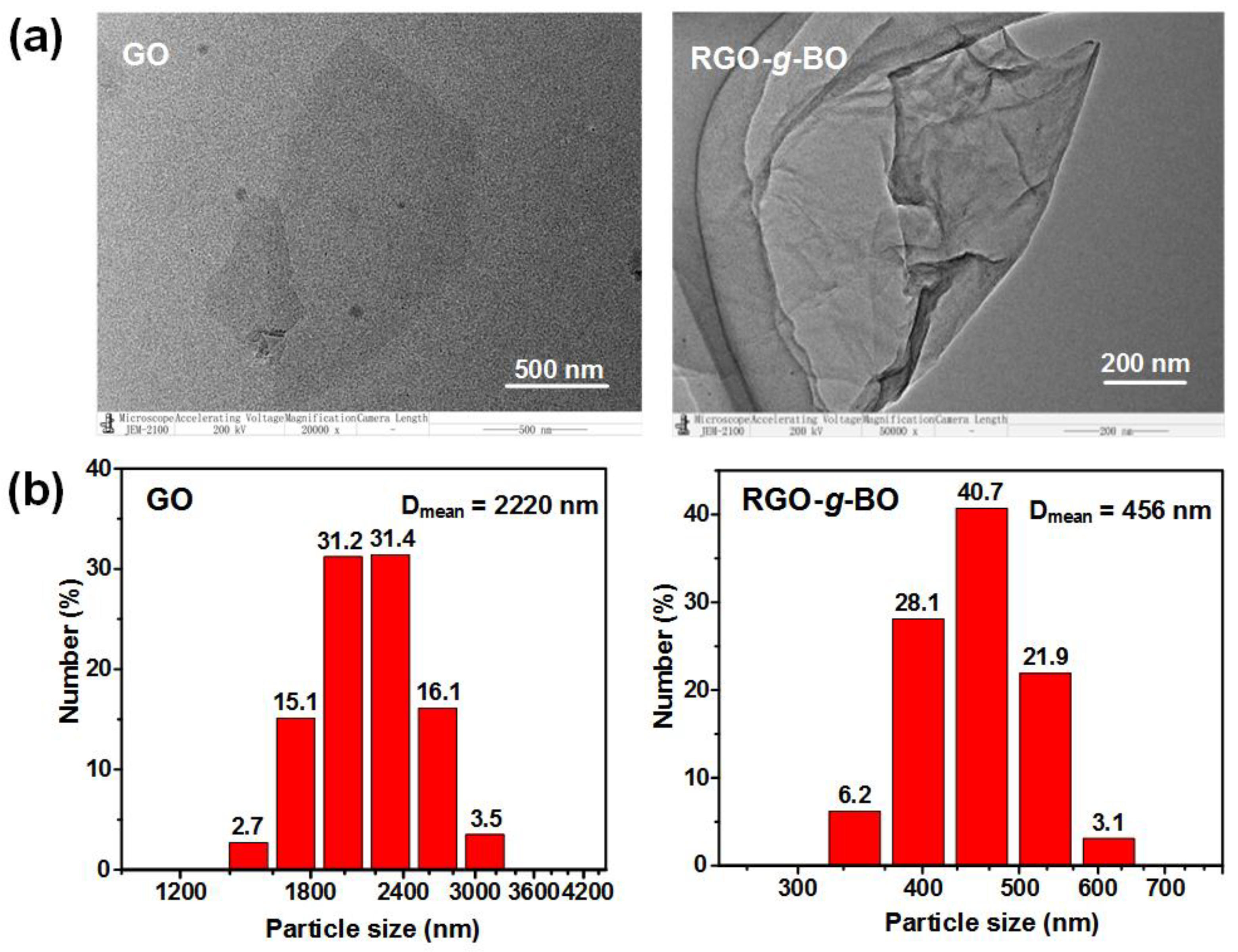
2.2. Microstructural Morphology and Particle Size Characterization
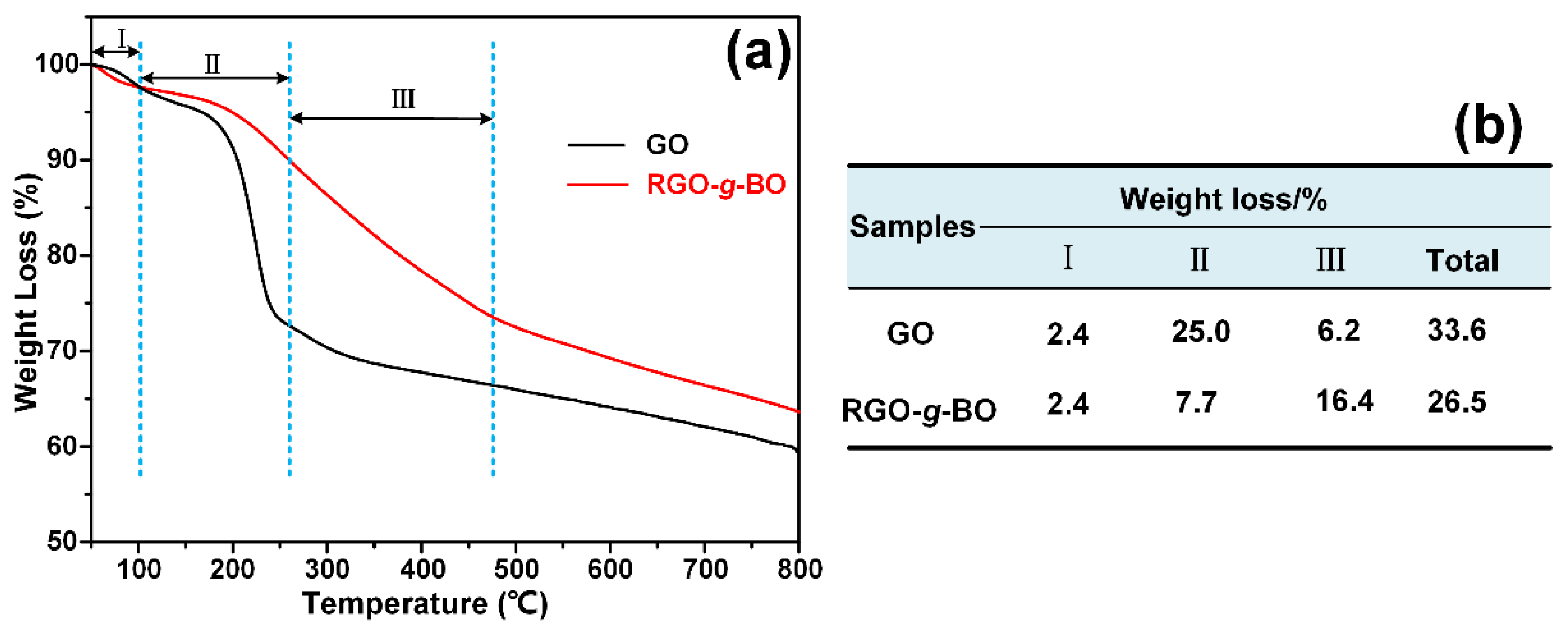
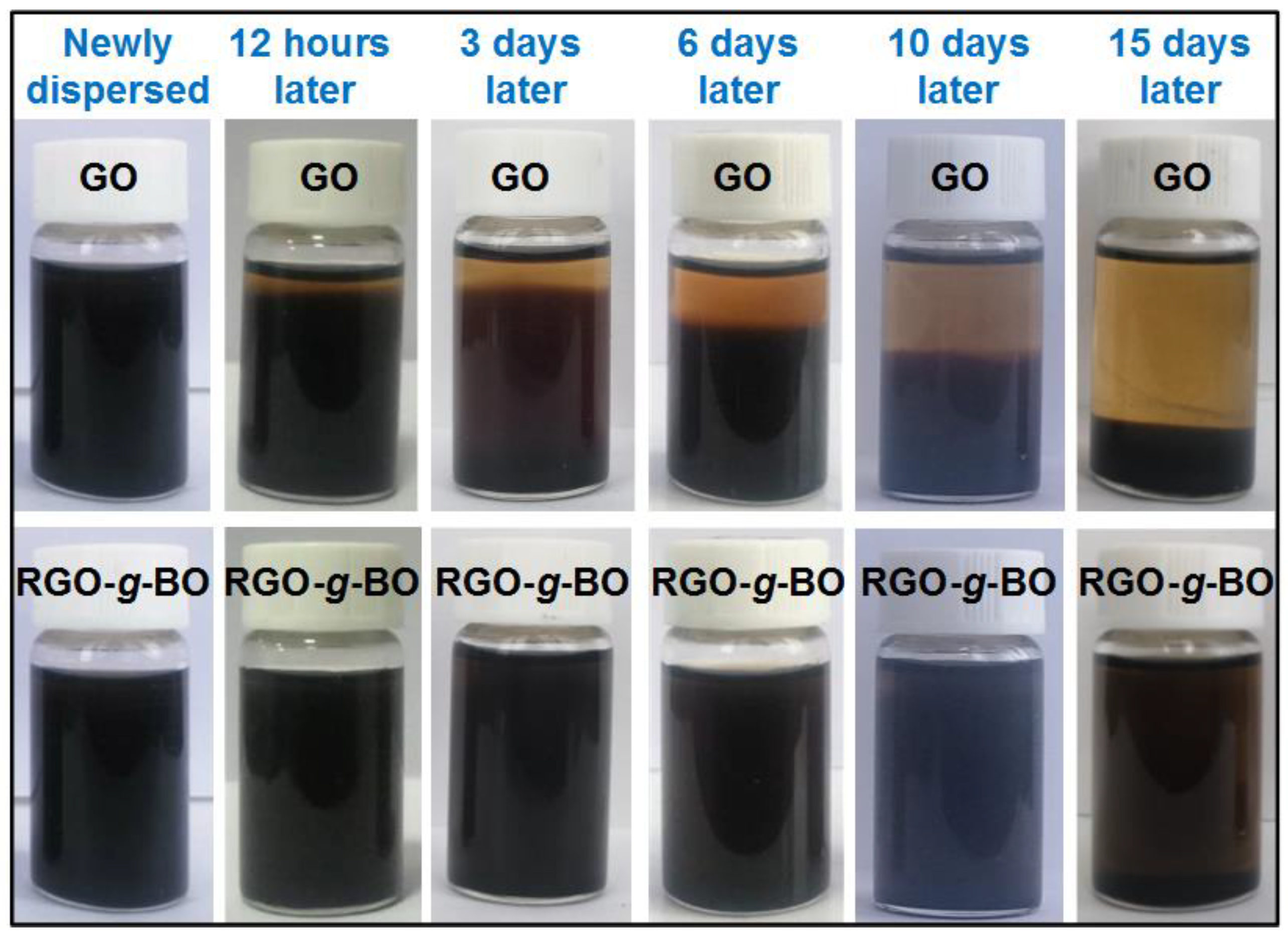
2.3. Thermal and Dispersion Stability
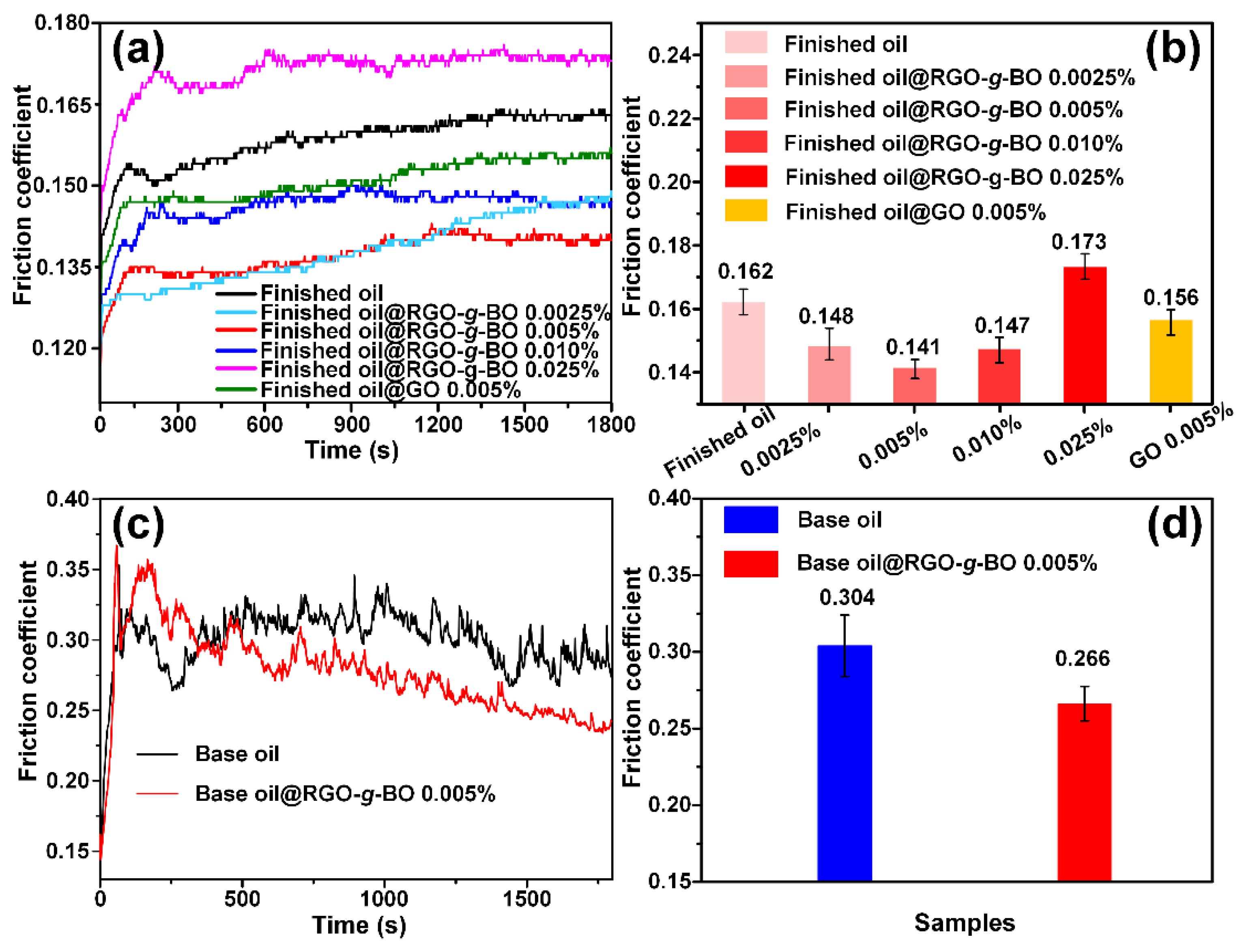
2.4. Tribological Performance Evaluation
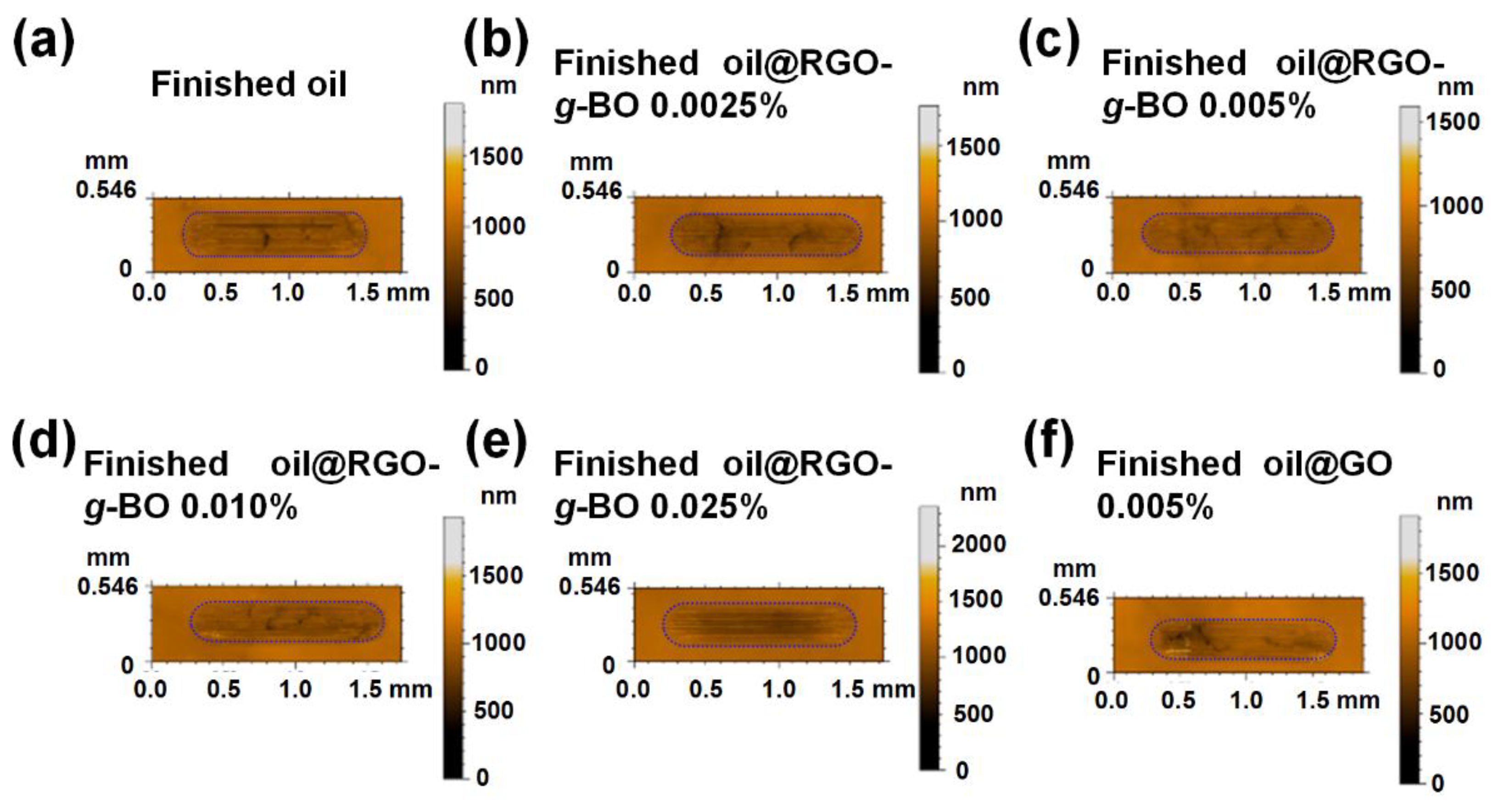
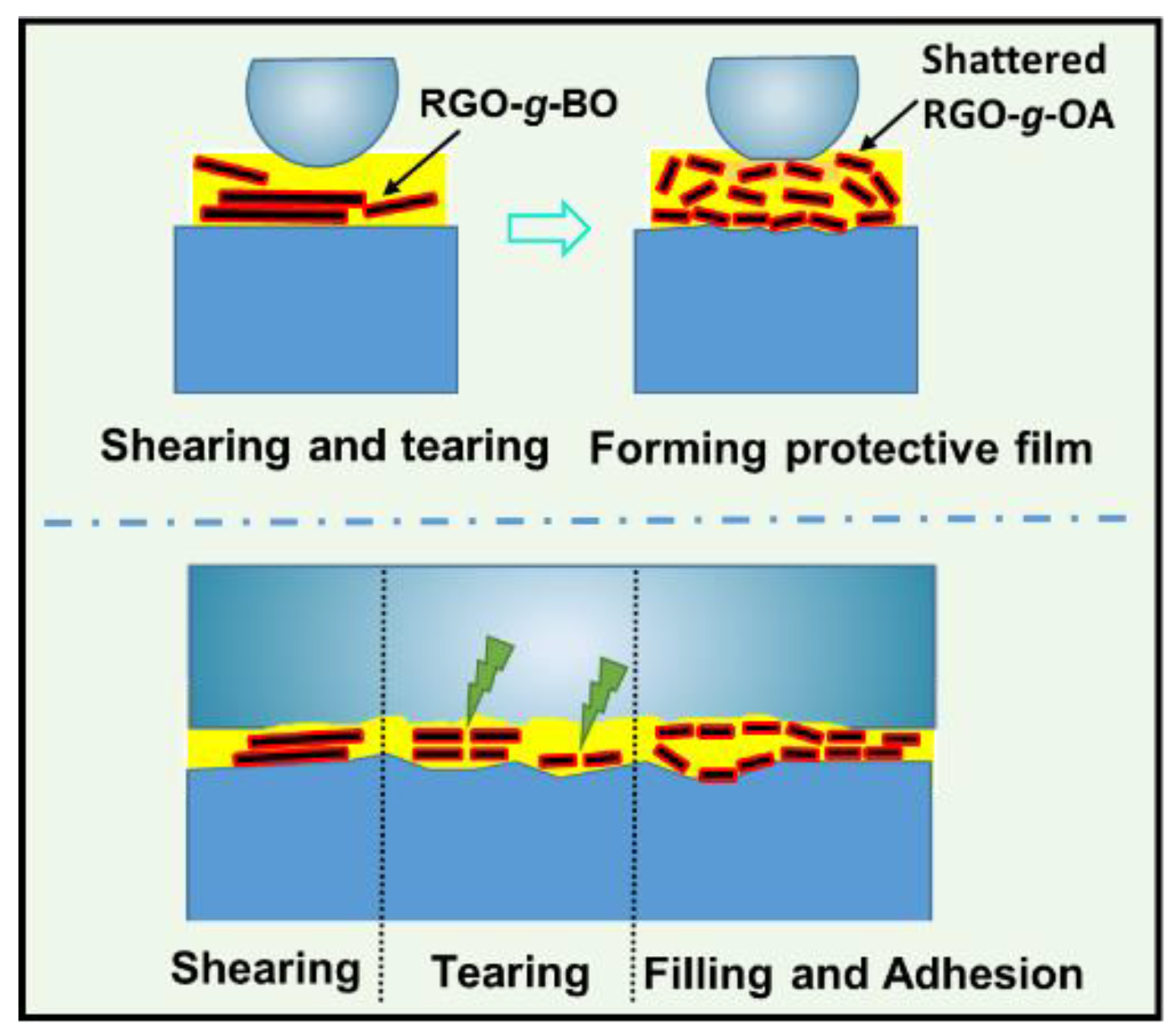
2.5. Exploration of Friction-Reducing and Antiwear Mechanism
3. Experimental Procedures
3.1. Materials
3.2. Fabrication of Chemically Functionalized Reduced Graphene Oxide (RGO-g-BO)
3.3. Preparation of Diverse Lubricant Oil Samples
3.4. Tribological Property Evaluation
3.5. Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yu, H.; Liu, X.; Zheng, Z.; Qiao, D.; Feng, D.; Gong, Z.; Dong, G. In situ graphene formation induced by tribochemical reaction for sustainable lubrication. ACS Sustain. Chem. Eng. 2023, 11, 2238–2248. [Google Scholar] [CrossRef]
- Dou, X.; Koltonow, A.R.; He, X.; Jang, H.D.; Wang, Q.; Chung, Y.-W.; Huang, J. Self-dispersed crumpled graphene balls in oil for friction and wear reduction. Proc. Natl. Acad. Sci. USA 2016, 113, 1528–1533. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; He, A.; Yun, J.-H.; Xu, X.; Jiang, Z.; Jiao, S.; Huang, H. Synergistic tribological performance of a water based lubricant using graphene oxide and alumina hybrid nanoparticles as additives. Tribol. Int. 2019, 135, 170–180. [Google Scholar] [CrossRef]
- Yu, H.; Chen, H.; Zheng, Z.; Ba, Z.; Qiao, D.; Feng, D.; Gong, Z.; Dong, G. Transformation mechanism between the frictional interface under dioctyl sebacate lubrication. Tribol. Int. 2021, 155, 106745. [Google Scholar] [CrossRef]
- Holmberg, K.; Erdemir, A. The impact of tribology on energy use and CO2 emission globally and in combustion engine and electric cars. Tribol. Int. 2019, 135, 389–396. [Google Scholar] [CrossRef]
- Kogovšek, J.; Kalin, M. Comparison of graphene as an oil additive with conventional automotive additives for the lubrication of steel and DLC-coated surfaces. Tribol. Int. 2023, 180, 108220. [Google Scholar] [CrossRef]
- Zilabi, S.; Shareei, M.; Bozorgian, A.; Ahmadpour, A.; Esmaeil, E. A review on Nanoparticle Application as an Additive in Lubricants. Adv. J. Chem. Sect. B 2022, 4, 209–221. [Google Scholar]
- Berman, D.; Erdemir, A.; Sumant, A.V. Graphene: A new emerging lubricant. Mater. Today 2014, 17, 31–42. [Google Scholar] [CrossRef]
- Zhu, C.; Yan, Y.; Wang, F.; Cui, J.; Zhao, S.; Gao, A.; Zhang, G. Facile fabrication of long-chain alkyl functionalized ultrafine reduced graphene oxide nanocomposites for enhanced tribological performance. RSC Adv. 2019, 9, 7324–7333. [Google Scholar] [CrossRef]
- Li, W.; Liu, M.; Cheng, S.; Zhang, H.; Yang, W.; Yi, Z.; Zeng, Q.; Tang, B.; Ahmad, S.; Sun, T. Polarization independent tunable bandwidth absorber based on single-layer graphene. Diam. Relat. Mater. 2024, 142, 110793. [Google Scholar] [CrossRef]
- Li, W.; Zhao, W.; Cheng, S.; Yang, W.; Yi, Z.; Li, G.; Zeng, L.; Li, H.; Wu, P.; Cai, S. Terahertz selective active electromagnetic absorption film based on single-layer graphene. Surf. Interfaces 2023, 40, 103042. [Google Scholar] [CrossRef]
- Ma, J.; Wu, P.; Li, W.; Liang, S.; Shangguan, Q.; Cheng, S.; Tian, Y.; Fu, J.; Zhang, L. A five-peaks graphene absorber with multiple adjustable and high sensitivity in the far infrared band. Diam. Relat. Mater. 2023, 136, 109960. [Google Scholar] [CrossRef]
- Shangguan, Q.; Zhao, Y.; Song, Z.; Wang, J.; Yang, H.; Chen, J.; Liu, C.; Cheng, S.; Yang, W.; Yi, Z. High sensitivity active adjustable graphene absorber for refractive index sensing applications. Diam. Relat. Mater. 2022, 128, 109273. [Google Scholar] [CrossRef]
- Li, S.; Li, Q.; Carpick, R.W.; Gumbsch, P.; Liu, X.Z.; Ding, X.; Sun, J.; Li, J. The evolving quality of frictional contact with graphene. Nature 2016, 539, 541–545. [Google Scholar] [CrossRef] [PubMed]
- La, D.D.; Truong, T.N.; Pham, T.Q.; Vo, H.T.; Tran, N.T.; Nguyen, T.A.; Nadda, A.K.; Nguyen, T.T.; Chang, S.W.; Chung, W.J.; et al. Scalable fabrication of modified graphene nanoplatelets as an effective additive for engine lubricant oil. Nanomaterials 2020, 10, 877. [Google Scholar] [CrossRef] [PubMed]
- Franzosi, D.; de Queiroz, J.C.; Tomanik, E.; Christinelli, W.; Profito, F.J.; Souza, R.M. Exploring the potential of graphene nanoplatelets as a lubricant additive: Topography evolution and performance under boundary lubrication conditions. Tribol. Int. 2024, 192, 109282. [Google Scholar] [CrossRef]
- Fan, K.; Chen, X.; Wang, X.; Liu, X.; Liu, Y.; Lai, W.; Liu, X. Toward excellent tribological performance as Oil-Based lubricant additive: Particular tribological behavior of fluorinated graphene. ACS Appl. Mater. Inter. 2018, 10, 28828–28838. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Peng, Y.; Yu, M.; Lang, H.; Cao, X.; Zou, K. Dynamic sliding enhancement on the friction and adhesion of graphene, graphene oxide, and fluorinated graphene. ACS Appl. Mater. Inter. 2018, 10, 8214–8224. [Google Scholar] [CrossRef]
- Guo, Y.-F.; Zhang, H.-T.; Liu, Y.-W.; Zhou, X.-F.; Liu, Z.-P. Molecular-scale grinding of uniform small-size graphene flakes for use as lubricating oil additives. New Carbon Mater. 2023, 38, 954–963. [Google Scholar] [CrossRef]
- Wang, L.; Gong, P.; Li, W.; Luo, T.; Cao, B. Mono-dispersed Ag/Graphene nanocomposite as lubricant additive to reduce friction and wear. Tribol. Int. 2020, 146, 106228. [Google Scholar] [CrossRef]
- Rao, X.; Sheng, C.; Hou, X.; Wei, Y.; Dai, L. Preparation and properties of graphene composite lubricants additive used for cylinder liner in marine diesel burning low sulfur fuel oil. Wear 2023, 528–529, 204994. [Google Scholar] [CrossRef]
- del Río, J.M.L.; Pérez, G.A.; Martínez, A.; Peña, D.; Fernández, J. Tribological improvement of potential lubricants for electric vehicles using double functionalized graphene oxide as additives. Tribol. Int. 2024, 193, 109402. [Google Scholar] [CrossRef]
- Lin, J.; Wang, L.; Chen, G. Modification of graphene platelets and their tribological properties as a lubricant additive. Tribol. Lett. 2011, 41, 209–215. [Google Scholar] [CrossRef]
- Wu, P.; Chen, X.; Zhang, C.; Zhang, J.; Luo, J.; Zhang, J. Modified graphene as novel lubricating additive with high dispersion stability in oil. Friction 2021, 9, 143–154. [Google Scholar] [CrossRef]
- Paul, G.; Hirani, H.; Kuila, T.; Murmu, N.C. Nanolubricants dispersed with graphene and its derivatives: An assessment and review of the tribological performance. Nanoscale 2019, 11, 3458–3483. [Google Scholar] [CrossRef] [PubMed]
- Georgakilas, V.; Otyepka, M.; Bourlinos, A.B.; Chandra, V.; Kim, N.; Kemp, K.C.; Hobza, P.; Zboril, R.; Kim, K.S. Functionalization of graphene: Covalent and Non-Covalent approaches, derivatives and applications. Chem. Rev. 2012, 112, 6156–6214. [Google Scholar] [CrossRef] [PubMed]
- Chouhan, A.; Mungse, H.P.; Khatri, O.P. Surface chemistry of graphene and graphene oxide: A versatile route for their dispersion and tribological applications. Adv. Colloid Interface Sci. 2020, 283, 102215. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.; Zhao, J.; Wang, W.; He, Y.; Luo, J. Influence of the micromorphology of reduced graphene oxide sheets on lubrication properties as a lubrication additive. Tribol. Int. 2018, 119, 614–621. [Google Scholar] [CrossRef]
- Zhao, J.; Li, Y.; Wang, Y.; Mao, J.; He, Y.; Luo, J. Mild thermal reduction of graphene oxide as a lubrication additive for friction and wear reduction. RSC Adv. 2017, 7, 1177–1766. [Google Scholar] [CrossRef]
- Yu, B.; Wang, K.; Pang, X.; Wu, G.; Pu, J.; Zhao, H. Tribological properties of alkylated reduced graphene oxide as lubricant additive. Tribol. Int. 2022, 165, 107273. [Google Scholar] [CrossRef]
- Xiao, H.; Liu, S. 2D nanomaterials as lubricant additive: A review. Mater. Des. 2017, 135, 319–332. [Google Scholar] [CrossRef]
- Mungse, H.P.; Gupta, K.; Singh, R.; Sharma, O.P.; Sugimura, H.; Khatri, O.P. Alkylated graphene oxide and reduced graphene oxide: Grafting density, dispersion stability to enhancement of lubrication properties. J. Colloid Interface Sci. 2019, 541, 150–162. [Google Scholar] [CrossRef] [PubMed]
- Gupta, B.; Kumar, N.; Panda, K.; Kanan, V.; Joshi, S.; Visoly-Fisher, I. Role of oxygen functional groups in reduced graphene oxide for lubrication. Sci. Rep. 2017, 7, 45030. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Wang, Z.; Yang, J.; Jia, X.; Zhang, Z. Facile synthesis of copper/polydopamine functionalized graphene oxide nanocomposites with enhanced tribological performance. Chem. Eng. J. 2017, 324, 51–62. [Google Scholar] [CrossRef]
- Chouhan, A.; Mungse, H.P.; Sharma, O.P.; Singh, R.K.; Khatri, O.P. Chemically functionalized graphene for lubricant applications: Microscopic and spectroscopic studies of contact interfaces to probe the role of graphene for enhanced tribo-performance. J. Colloid Interface Sci. 2018, 513, 666–676. [Google Scholar] [CrossRef]
- Wolk, A.; Rosenthal, M.; Neuhaus, S.; Huber, K.; Brassat, K.; Lindner, J.K.N.; Grothe, R.; Grundmeier, G.; Bremser, W.; Wilhelm, R. A novel lubricant based on covalent functionalized graphene oxide quantum dots. Sci. Rep. 2018, 8, 5843. [Google Scholar] [CrossRef] [PubMed]
- Ismail, N.A.; Zulkifli, N.W.M.; Chowdhury, Z.Z.; Johan, M.R. Grafting of straight alkyl chain improved the hydrophobicity and tribological performance of graphene oxide in oil as lubricant. J. Mol. Liq. 2020, 319, 114276. [Google Scholar] [CrossRef]
- Zhang, L.; He, Y.; Zhu, L.; Jiao, Z.; Deng, W.; Pu, C.; Han, C.; Tang, S. Alkyl phosphate modified graphene oxide as friction and wear reduction additives in oil. J. Mater. Sci. 2019, 54, 4626–4636. [Google Scholar] [CrossRef]
- Liu, Z.; Nørgaard, K.; Overgaard, M.H.; Ceccato, M.; Mackenzie, D.M.; Stenger, N.; Stipp, S.L.; Hassenkam, T. Direct observation of oxygen configuration on individual graphene oxide sheets. Carbon 2018, 127, 141–148. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, G.; Gao, A.; Cui, J.; Zhao, S.; Yan, Y. Robust Graphene/Poly(vinyl alcohol) janus aerogels with a hierarchical architecture for highly efficient switchable separation of Oil/Water emulsions. ACS Appl. Mater. Interfaces 2019, 11, 36638–36648. [Google Scholar] [CrossRef]
- Zhao, S.; Yan, Y.; Gao, A.; Zhao, S.; Cui, J.; Zhang, G. Flexible polydimethylsilane nanocomposites enhanced with a Three-Dimensional Graphene/Carbon nanotube bicontinuous framework for High-Performance electromagnetic interference shielding. ACS Appl. Mater. Interfaces 2018, 10, 26723–26732. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.Y.; Yang, K.; Anh, N.D.; Park, C.; Lee, S.M.; Lee, T.G.; Jeong, M.S. Raman study of D* band in graphene oxide and its correlation with reduction. Appl. Surf. Sci. 2021, 536, 147990. [Google Scholar] [CrossRef]
- Pope, C.G. X-ray diffraction and the bragg equation. J. Chem. Educ. 1997, 74, 129. [Google Scholar] [CrossRef]
| Samples | Wear Volume (μm3) | Increment |
|---|---|---|
| Finished oil | 52,151 | 0% |
| Finished oil@RGO-g-BO 0.0025% | 29,949 | −43% |
| Finished oil@RGO-g-BO 0.005% | 24,391 | −53% |
| Finished oil@RGO-g-BO 0.010% | 36,326 | −30% |
| Finished oil@RGO-g-BO 0.025% | 58,509 | +12% |
| Finished oil@GO 0.005% | 37,720 | −28% |
| Samples | Surface Roughness (μm) | Increment |
|---|---|---|
| Finished oil | 0.074 | 0% |
| Finished oil@RGO-g-BO 0.0025% | 0.055 | −26% |
| Finished oil@RGO-g-BO 0.005% | 0.049 | −34% |
| Finished oil@RGO-g-BO 0.010% | 0.068 | −8% |
| Finished oil@RGO-g-BO 0.025% | 0.085 | +15% |
| Finished oil@GO 0.005% | 0.070 | −5% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, G.; Zhu, C.; Yan, Y.; Cui, J.; Jiang, J. One-Pot Synthesis of Alkyl Functionalized Reduced Graphene Oxide Nanocomposites as the Lubrication Additive Enabling Enhanced Tribological Performance. Molecules 2024, 29, 2004. https://doi.org/10.3390/molecules29092004
Zhang G, Zhu C, Yan Y, Cui J, Jiang J. One-Pot Synthesis of Alkyl Functionalized Reduced Graphene Oxide Nanocomposites as the Lubrication Additive Enabling Enhanced Tribological Performance. Molecules. 2024; 29(9):2004. https://doi.org/10.3390/molecules29092004
Chicago/Turabian StyleZhang, Guangfa, Chao Zhu, Yehai Yan, Jian Cui, and Jingxian Jiang. 2024. "One-Pot Synthesis of Alkyl Functionalized Reduced Graphene Oxide Nanocomposites as the Lubrication Additive Enabling Enhanced Tribological Performance" Molecules 29, no. 9: 2004. https://doi.org/10.3390/molecules29092004
APA StyleZhang, G., Zhu, C., Yan, Y., Cui, J., & Jiang, J. (2024). One-Pot Synthesis of Alkyl Functionalized Reduced Graphene Oxide Nanocomposites as the Lubrication Additive Enabling Enhanced Tribological Performance. Molecules, 29(9), 2004. https://doi.org/10.3390/molecules29092004








